Identification of zona- and fucoidan-binding proteins in ... · Electrophoresis and protein...
Transcript of Identification of zona- and fucoidan-binding proteins in ... · Electrophoresis and protein...

Development 109, 41-50 (1990)Printed in Great Britain © T h e Company of Biologists Limited 1990
41
Identification of zona- and fucoidan-binding proteins in guinea-pig
spermatozoa and mechanism of recognition
ROY JONES and RACHEL M. WILLIAMS
Department of Molecular Embryology, AFRC Institute of Animal Physiology and Genetics Research, Babraham, Cambridge CB2 4AT, UK
Summary
Binding of guinea-pig spermatozoa to the zona pellucidaof homologous eggs has been reported to involve 'recep-tors' on the inner acrosomal membrane (Huang et al.1981). These receptors can be blocked by sulphatedpolysaccharides such as fucoidan (Huang and Yanagi-machi, 1984). The aims of the present investigation wereto identify these putative zona receptors using I25I-fucoidan as a probe and examine their mechanism ofrecognition. Results show that l25I-fucoidan binds toseveral proteins extracted from guinea-pig spermatozoawith molecular masses of 95, 60, 48, 34, 30 and18-20X103 (K) on SDS-PAGE. The 48K, 34K and 30Kcomponents represent proacrosin and two forms ofacrosin, respectively. I-zona pellucida glycoproteinsalso bound strongly to the 48K, 34K and 30K spermproteins. The other high and low mass binding proteins
were not positively identified but cytochemicaJ exper-iments with fluoresceinamine-fucoidan and FITC-soybean trypsin inhibitor indicate that they are intra-acrosomal. The mechanism of binding of 125I-fucoidan toproacrosin/acrosin (and also the 95K, 60K and18K-20K components) involves multiple sulphategroups on the polysaccharide in a specific orientation toallow them to interact with basic residues on the protein.It is suggested that guinea-pig spermatozoa retain suf-ficient proacrosin/acrosin bound to the inner acrosomalmembrane after the acrosome reaction to mediate bind-ing to the zona pellucida and that functionally proacro-sin is analogous to sea urchin bindin.
Key words: spermatozoa, fertilization, zona receptors,sulphated polysaccharides, proacrosin, acrosome reaction.
Introduction
Identification and characterization of receptor mol-ecules that mediate gamete recognition and fusion is animportant step towards understanding the early stagesof fertilization in mammals. Based on extensive work inthe mouse, Wassarman and co-workers have suggestedthat sperm receptor activity on the egg is associatedwith <9-linked carbohydrate moieties on one specificzona pellucida glycoprotein, ZP3 (reviewed by Wassar-man, 1987). The implication of these experiments isthat sperm-egg recognition involves a protein-carbohydrate interaction, a conclusion that is supportedby the competitive effects of a variety of mono- andpolysaccharides on fertilization in vitro, (Huang et al.1982; Peterson et al. 1984; Shalgi et al. 1986; Boldt et al.1989) and the adverse consequences of exposing thezona to exoglycosidases (Shalgi et al. 1986; Wassarman,1987).
With spermatozoa, however, identification and local-ization of the complementary ligand molecules for thezona has proved more problematic. Unlike the zonawhose components are uniformly distributed through-out the matrix, sperm antigens are polarized intodistinct regional domains (Koehler, 1978; Myles et al.1981; Gaunt et al. 1983). Current evidence indicates
that different antigens are involved in different facets ofthe fertilization process and that these antigens resideon more than one type of membrane. It would seemthat, in the majority of species (mouse, rat, sheep,hamster), capacitated spermatozoa attach to the zonasurface with intact or vesiculating acrosomes (Yanagi-machi, 1988), from which it has to be concluded that atleast some recognition molecules must be located onthe plasma membrane overlying the anterior spermhead (Saling and Storey, 1979; Shalgi et al. 1989). Thisconcurs to a considerable extent with what is knownabout the distribution of several putative zona adhesionmolecules such as galactosyltransferase and proteinaseinhibitor binding sites in the mouse (Shur and Hall,1982; Benau and Storey, 1987) and several antigensidentified by monoclonal antibodies on hamster, rabbitand rat spermatozoa (Moore and Hartman, 1984;O'Rand et al. 1988; Jones et al. 1990).
However, a different situation prevails in the guineapig. In this species, evidence indicates that acrosome-reacted rather than acrosome-intact spermatozoa bindpreferentially to the zona pellucida in vitro (Huang etal. 1981). Furthermore, fucoidan, a fucose-rich polysac-charide that is very potent in blocking fertilization inguinea pigs and most mammalian species binds specifi-cally to the inner acrosomal membrane/equatorial

42 R. Jones and R. M. Williams
segment region of acrosome reacted but not acrosomeintact spermatozoa (Huang et al. 1982; Huang andYanagimachi, 1984). The simplest explanation of theseobservations is that in the guinea-pig, putative zonarecognition molecules are not normally present on theplasma membrane but are contained within the acro-some, either as part of the matrix or bound to acro-somal membranes. Only when the acrosome reactiontakes place are these molecules exposed and only thencan the sperm head bind firmly to the egg.
Recently, a soluble component of the acrosome,proacrosin, has been shown to behave as a ligand forfucoidan and zona pellucida glycoproteins in bull, boar,ram and human spermatozoa (Jones, 1989). In thiscommunication, we have extended our observations tothe guinea pig with the following objectives; (a) toidentify and characterize major fucoidan binding pro-teins on spermatozoa in this species; (b) to investigatethe cellular location and behaviour of these proteinsbefore and after release from the acrosome; (c) toelucidate the mechanism of interaction between fucoi-dan and its binding proteins.
Materials and methods
Collection and extraction of spermatozoaSpermatozoa were collected from the cauda epididymidis ofadult male guinea pigs (Hartley strain) and proteins extractedby 2 different procedures. For solubilization with detergents,spermatozoa were extruded into phosphate-buffered saline(PBS) containing 2mM p-aminobenzamidine (pAB), washedonce by centrifugation at 600g for lOmin and pellets resus-pended in 3 volumes of PBS containing 1 % Triton X-100,1 %sodium deoxycholate (DOC), lmM pAB, lmM phenylmeth-ylsulphonylfluoride (PMSF), 10/igmP1 aprotinin and10j*gml leupeptin (Arboleda and Gerton, 1987). Afterstanding for 30min at 4°C, the suspension was centrifuged at10000g for 15 min and supernatants stored frozen. These willbe referred to as detergent extracts. Alternatively, the caudaepididymidis was minced directly into 0.25 N H2SO4/50mMbenzamidine HC1 at 4°C, shaken to disperse spermatozoawhich were then aspirated away from tissue fragments (Hardyet al. 1987). Suspensions were stirred at 4°C for 60 min,centrifuged at 10000 g for 30min and supernatants dialysedextensively (24h, 2 changes) against lmM HC1 pH3 at 4°C.These will be referred to as acid extracts. Total protein wasmeasured by the dye binding method of Bradford (1976) usingbovine serum albumin (BSA) as standard.
Electrophoresis and protein blottingExtracted proteins were separated on 8-15 % non-reducingSDS-polyacrylamide gels (SDS-PAGE) according toLaemmli (1970) and either stained directly with CoomassieBlue R-250 or transferred onto nitrocellulose paper by West-ern blotting (Towbin et al. 1979). Blots were blocked with 5 %BSA in PBS for 120 min at 20°C and probed with 1/40 dilutedrabbit antiserum to boar proacrosin (Jones, 1987) or 125I-labelled fucoidan (~200000ctsmin~' ml"1 in PBS) or 125I-labelled soybean trypsin inhibitor (SBTI;~100000ctsmin~1mrl in PBS) as described previously(Jones et al. 1988). Fucoidan was purified from crude material(Sigma) by /S-elimination with NaBHt/NaOH, extensivepronase digestion followed by gel filtration on Sephadex G75
(DeAngelis and Glabe, 1987). A compositional analysis of thepurified material revealed 74% fucose with the remainderconsisting primarily of xylose, galactose and uronic acid (R.M. Williams and R. Jones, unpublished observations). Fucoi-dan was conjugated with fluoresceinamine (Glabe et al. 1983)in PBS and iodinated by the "Iodogen' procedure (Markwelland Fox, 1978). SBTI suspended in PBS (lmgrnP1) was alsoiodinated with 'Iodogen'. Radioactivity on blots was detectedby autoradiography using preflashed X-ray film (Fuji Ltd) andquantified by densitometry on a Joyce-Loebl integratingdensitometer. In competition experiments, blots were incu-bated with a variety of saccharides and synthetic polymers(see Results) for 60 min immediately after blocking with 5 %BSA but before addition of labelled probe. Proteinase activitywas detected in 10% polyacrylamide gels containing 0.1%SDS and 0.1% gelatin as described by Siegel and Polakoski(1985) and presence of glycoprotein by staining gels withperiodic acid SchifFs (PAS) reagent (Dubray and Bezard,1982).
Isolation and iodination of guinea-pig zonae pellucidaeOvaries from adult female guinea pigs were minced inPBS/2 mM pAB/5 mM EDTA and released eggs recovered bymicropipettes. Zonae pellucidae were isolated by repeatedlysucking eggs into narrow-bore pipettes. Zonae (~200) in 50 /Awere mixed with 50 /d 0.1M borate buffer pH8.5 containing100 /iCi 125I-Bolton and Hunter iodination reagent (Amer-sham). After 20 min at 4°C zonae were washed in 5 changes of0.1M borate pH8.5 to remove unbound reagent, pelleted bycentrifugation and solubilized by heating at 70°C for 30 min in100/d 5mM sodium carbonate pH9.2. Suitable aliquots con-taining —200 000 cts min"1 ml were made up in PBS andused to probe Western blots of sperm proteins as describedabove.
Fluorescence microscopySpermatozoa were extruded from the cauda epididymidis/vasdeferens into 5 ml of Ca2+-free modified Tyrode's solution(mT) (Huang et al. 1981). Spermatozoa were washed once bycentrifugation at 600 g for 10min, resuspended to approxi-mately 106ml~' in mT and 50;tl aliquots mixed with 50 ;dfluoresceinamine-fucoidan (O.lmgml"' in PBS) or fluor-escein isothiocyanate-conjugated SBTI (FITC-SBTI) at0.5mgmr' in PBS. The FITC-SBTI was prepared using10% FITC on Celite as described by Kinderknecht (1962).Suspensions were incubated for 30 min at room temperature,washed twice in mT and viewed unfixed by epifluorescenceillumination. Spermatozoa in mT were permeabilized by coldshocking to 2°C (Mann, 1964). Alternatively, the acrosomereaction was induced by the addition of 2 mM CaCl2 to mT andincubating at 37°C for 4-6h. For competition assays, mono-,di-, tri- or polysaccharides (see Results) were added to spermsuspensions for 30 min before fucoidan-fluoresceinamine andthen incubated as described above.
Results
Identification of zona- and fucoidan-binding proteinsin detergent and acid extracts of guinea-pigspermatozoa by Western blottingPreliminary experiments showed that the method ofextraction of proteins from guinea-pig spermatozoa hadsome influence on the molecular mass of receptors for
-zona glycoproteins and 125I-fucoidan. In the exper-125J

Zona receptors on guinea-pig spermatozoa 43
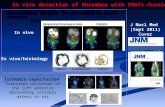
I II I II I I I I I I VFig. 1. SDS-PAGE under non-reducing conditions of proteinsextracted from guinea-pigspermatozoa with (i) 1% TritonX-100/1% DOC containing 1 mMpAB, IITIM PMSF, 10/igmr1
aprotinin and lOj/gmlleupeptin, or (ii) 0.25 N H2SO4containing 50 mM benzamidine.Panel (a), proteins stained withCoomassie Blue R-250. Panel (b),autoradiograph from a Westernblot of a parallel gel to (a) probedwith 125I-fucoidan. Panel (c),autoradiograph from a Westernblot of a parallel gel to (a) probedwith IZ5I-zona glycoproteins. Panel(d), autoradiograph of the i25l-zona glycoprotein probe undernon-reducing (iii) and reducing(iv) conditions. Values shown areapparent molecular mass ofknown protein standards(Pharmacia). *=weakly PASpositive, **=strongly PASpositive.
iments described, we have compared two procedures:first, a mixture of detergents to solubilize a wide rangeof proteins from surface and intracellular membranes,and second, acidic conditions to extract basic proteins.
Three major fucoidan-binding proteins were detectedin detergent extracts with molecular masses 48K, 60Kand a diffuse band at approximately 95K (K=xlO3)(Fig. lb). The diffuse nature of the signal from the 95Kprotein is probably caused by the presence of carbo-hydrate as a strong reaction was obtained on thiscomponent with the PAS stain (Fig. la). In somedetergent extracts, the 48K component appeared as adoublet. A weak signal was also obtained over somelow mass components at 18K-20K. Acid extracts on theother hand give a slightly different pattern; the threehigh molecular mass proteins were very weakly labelledand instead the probe bound strongly to components at34K, 30K and 18K-20K (Fig. lb).
A similar pattern was obtained with the zona glyco-protein probe. When this probe was analysed bySDS-PAGE under non-reducing conditions, some highmass material remained on the top of the gel with abroad diffuse area between 69K and 150K (Fig. Id).Under reducing conditions the high mass materialdisappeared and only the 69K to 150K componentswere detectable (Fig. Id). Western blots containingacid extracted sperm proteins bound I25I-zona glyco-proteins strongly over two components at 34K and 30K
•and weakly at 18K-20K (Fig. lc). In detergent extractsthe probe recognized sperm proteins at 95K and 48Kwith very weak binding to 18K proteins (Fig. lc).
Detection of proacrosin/acrosin in acid and detergentextracts of guinea-pig spermatozoaThe acrosome of mammalian spermatozoa is wellknown to contain proteinases, notably the proacrosin/acrosin family (Green, 1978; Hedrick et al. 1988). Toidentify proacrosin and some of the active forms ofacrosin in detergent and acid extracts of guinea-pigspermatozoa, parallel blots to those described abovewere reacted with a rabbit polyclonal antibody pre-pared to boar proacrosin (Jones, 1987). As shown inFig. 2a, a strong reaction was obtained over a 48Kantigen in detergent extracts and over the 30K-34Kproteins in acid extracts. A weak but positive signal wasalso produced by the 18K-20K components in acidextracts. No significant reaction above preimmuneserum was observed on any other antigens (Fig. 2b).
To substantiate the identification of proacrosin/acrosin antigens, proteinase activity against gelatin assubstrate was detected using the SDS-PAGE system ofSiegel and Polakoski (1985). As shown in Fig. 3, strongproteolysis was present in the 30K-34K components inboth detergent and acid extracts with less activity in theregion of 48K. Together with cross-reactivity of theanti-boar proacrosin antibody, therefore, it is reason-able to presume that the 48K protein represents proac-rosin and the 30K-34K proteins two species of activeacrosin. One explanation why the latter antigens werenot observed on Western blots of detergent extractsprobed with antibody or 125I-fucoidan may be becausethey were present in such small amounts that they werebeyond the limits of detection but, having high protein-

44 R. Jones and R. M. Williams
a b
ii i II
x10 - 3
48 —
34-^30 —
20-
I II
Hx10 - 3
48
30-
Fig. 2. Western blots of guinea-pig sperm proteins in (i)detergent or (ii) acid extracts probed with (a) 1/40 dilutedrabbit anti-boar proacrosin antiserum, or (b) 1/40 dilutedpre-immune serum, followed by 1/400 diluted peroxidase-conjugated goat anti-rabbit IgG. Bound antibody wasvisualized with 4-chloronaphthol dye. Apparent molecularmasses are as indicated. Proteins were separated by non-reducing SDS-PAGE.
ase activity, they were demonstrable on SDS-gelatingels.
Cytochemical distribution of fucoidan-binding proteinson guinea-pig spermatozoaFreshly washed caudal spermatozoa suspended in mTmedium contained a variable number (8-28 %, depen-ding on the animal) of immotile cells that had either losttheir acrosomes completely or had obvious damage tothe acrosomal cap. Weak fluorescence was associatedwith the postacrosomal region and tail of these cellsafter staining with fluoresceinamine-fucoidan(Fig. 4B). However, motile spermatozoa or immotilespermatozoa with intact acrosomes did not bind theprobe and did not fluoresce (Fig. 4A). These resultswere consistent from sample to sample suggesting thateither fucoidan-binding proteins are not normally pres-ent on the outer surface of the plasma membrane or,alternatively, that they are too few in number to bedetectable by fluorescent tracing techniques.
Following permeabilization by cold shock on theother hand, spermatozoa-bound fluoresceinamine-fucoidan strongly, the pattern and intensity of stainingbeing influenced to an appreciable extent by the pres-ence of 2mM pAB in the medium. In the absence of2IDM pAB, most spermatozoa lost their acrosomescompletely (92% without acrosomes) andfluoresceinamine-fucoidan bound weakly to the post-acrosomal and tail regions (Fig. 4C). Weak fluor-escence was also present over the anterior denudedhead of some, but not all, spermatozoa. However,when spermatozoa were cold-shocked in the presenceof 2ITIM pAB, loss of the acrosomal cap was consider-
Fig. 3. Proteinase activity in (i) detergent and (ii) acidextracts of guinea-pig spermatozoa as detected followingSDS-PAGE under non-reducing conditions. The gelcontained 10% polyacrylamide and 0.1% gelatin. Afterincubation at 37°C for 16h (Siegel and Polakoski, 1985) thegel was stained with Coomassie Blue R-250 to detect clearzones of gelatin hydrolysis.
ably reduced (35 % without acrosomes) although therewere clear signs of damage to acrosomal membranes.The latter spermatozoa showed strong fluorescenceover the retained acrosomal cap as well as variablestaining on the postacrosomal and tail domains(Fig. 4D). It was noticeable that spermatozoa that hadlost their acrosomal caps completely still retained sig-nificant remnants of membranes over the anterior headand that these remnants were fluorescent. The presenceof unlabelled fucoidan (2mgml~') in suspensions ofpermeabilized spermatozoa before staining withfluoresceinamine-fucoidan blocked uptake of theprobe completely (Fig. 4F).
The addition of 2 ITIM CaCl2 to mT medium followedby incubation at 37°C for 4-6h stimulated a differentresponse. In these samples, three types of spermatozoawere observed; motile spermatozoa with intact acro-somes that did not stain with fluoresceinamine-fucoidan (similar to Fig. 4A), immotile spermatozoathat had lost their acrosomes completely and showedweak fluorescence on the equatorial segment/post-acrosomal domains (similar to Fig. 4C) and immotilespermatozoa that had lost their acrosomes but whichfluoresced strongly over the anterior head (Fig. 4E). Inview of the results obtained above with 2 ITIM pAB in themedium, we consider it likely that the latter spermato-zoa had initiated an acrosome reaction but still retainedsignificant amounts of their acrosomal contents/membranes.

Zona receptors on guinea-pig spermatozoa 45
Fig. 4. Paired fluorescence andphase-contrast photographs ofguinea-pig spermatozoa stainedwith fluoresceinamine-fucoidan (A-F) or FITC-SBTI(G-H). (A) Acrosome intactsperm; (B) dead sperm thathas lost its acrosome;(C) sperm cold shocked in mTmedium alone; (D) sperm coldshocked in mT+2mM pAB;(E) sperm incubated 6h at37°C in mT+2mM CaCl2;(F) sperm cold shocked inmT+2mM pAB and stained inpresence of 2mgml~1 'cold'fucoidan; (G) acrosome intactsperm stained with FITC-SBTI; (H) sperm cold shockedin mT and stained with FITC-SBTI. Note that in all casesacrosome intact spermatozoado not stain whereaspermeabilized spermatozoabind the probes, the patterndepending on the extent towhich the acrosomal contentsare dispersed. Bar=5jnn.
Inhibition ofI2S I-fucoidan uptake bymonosaccharid.es, polysaccharides and syntheticpolymersFucoidan is a branched sulphated polysaccharide (Med-calf and Larsen, 1977) with a molecular mass ofapproximately 100K (DeAngelis and Glabe, 1987). To
assess the properties of 125I-fucoidan that are importantfor recognition of guinea-pig proacrosin/acrosin (e.g.type of sugar, length of polymer, degree of sulphation,etc.), Western blots from electrophoretic separation ofdetergent or acid-extracted proteins were incubatedwith a variety of monosaccharides, polysaccharides and

46 R. Jones and R. M. Williams
a b c d e f g h i j k l m n o
Mrx1CT3
95 —e MM » IMI60—
4 8 - r
Hx10 - 3
34 —30"*
20 —
II
9HVI
ilrf iili
48 —
34 —30 —
Fig. 5. Autoradiographs taken from Western blotscontaining detergent- (upper panel) or acid- (lower panel)extracted proteins that has been blocked with variousmono-, tri-, tetra- and polysaccharides before addition of125I-fucoidan. Blocking agents were as follows, (a) Nothing(control), (b) 50^M 'cold' fucoidan. (c) 50fiM galactan. (d)714 HM mannan. (e) 625 J/M chondroitin sulphate A. (f)625//M chondroitin sulphate C. (g) 20 /.IM dextran. (h) 20 J/Mdextran sulphate (500K). (i) 20 ̂ M dextran sulphate (5K).(j) 606 JAM heparin. (k) 100 J/M polyvinylsulphate. (1) 0.25 MD(+) fucose. (m) 0.25M D(+) galactose. (n) 0.20M stachyose.(o) 0.125 M raffinose. >90% inhibition over all proteins wasobserved with 'cold' fucoidan, galactan, dextran sulphate(500K), polyvinylsulphate. Mannan caused ~40% inhibitionon the 95K, 34K, 30K, 20K and 18K proteins. Heparin anddextran sulphate (5K) inhibited the 95K, 34K, 30K, 20Kand 18K proteins by ~70%. Inhibition by fucose, galactose,stachyose and raffinose was ~15% and was not consideredsignificant as solutions of these sugars were viscous anddifficult to mix thoroughly at the high concentrations used.
sulphated compounds before addition of the labelledprobe. As shown in Fig. 5, uptake of 125I-fucoidan ontothe 30K-34K and 18K-20K antigens in acid extractswas inhibited >90% by 'cold' fucoidan (50 ̂ M), galac-tan (50 HM), dextran sulphate 500K (20 /m) and polyvi-nylsulphate (PVS; 100 JIM). Some inhibition was ob-served with dextran sulphate 5K (20 fm), mannan(714 JIM) and heparin (606 fiM) but no effect was foundwith chondroitin sulphates A and C (625 JIM), dextran506K (20/J.M), D(+) fucose (0.25 M), D(+) galactose(0.25M), raffinose (0.125M) or stachyose (0.20M).
Similar pattern of inhibition was obtained against the48K, 60K and 95K components in detergent extracts(Fig. 5), the only noticeable discrepancy being theinability of heparin and dextran sulphate 5K to blockbinding of 125I-fucoidan to the doublet at 48K.
The above results were also confirmed cytochemi-cally. When cold-shocked spermatozoa (in the presenceof 2mM pAB) were preincubated with galactan (50 ̂ M)or dextran sulphate 500K (20 ̂ M) or PVS (100 fm) for30min before addition of fluoresceinamine-fucoidan,there was complete inhibition of binding relating topositive controls. Results were similar to that shown inFig. 4 for 'cold' fucoidan. However, the presence of
Fig. 6. Autoradiograph taken from Western blot of proteinsin (i) detergent or (ii) acid extracts of guinea-pigspermatozoa probed with 125I-SBT1. Proteins wereseparated on non-reducing SDS-PAGE. Apparentmolecular masses are as indicated.
fucose (0.25 M) or D(+) galactose (0.25 M) or dextran506K (20 ̂ M) did not prevent uptake of the probe andfluorescence was indistinguishable from controls.
Binding of soybean trypsin inhibitor to guinea-pigspermatozoaAs a corollary to the preceding experiments on fucoidanbinding, we also investigated the interaction betweenSBTI and guinea-pig spermatozoa. In the mouse andboar, several lines of evidence indicate that a trypsin-like receptor on spermatozoa is involved in recognitionand/or binding to the zona pellucida (Saling, 1981;Benau and Storey, 1987; Jones et al. 1988). WhenWestern blots containing guinea-pig sperm proteinswere probed with 125I-SBT1, a similar pattern emergedto that observed earlier with 125I-fucoidan (Fig. 6), theprincipal difference being that the 95K and 60K com-ponents in detergent extracts did not bind I25I-SBTI.Thus, the proteinase inhibitor recognizes only antigensthat we have identified as proacrosin/acrosin and poss-ibly some low molecular mass autolysis products ofacrosin.
Also in keeping with the fucoidan results, detectableamounts of FITC-SBTI did not bind to acrosome intactguinea-pig spermatozoa (Fig. 4G). However, after coldshock and loss of the acrosomal cap, moderate fluor-escence was present on the equatorial segment, postac-rosomal region and tail (Fig. 4H). The addition of 'cold'SBTI (lmgmF1) to sperm suspensions before FITC-SBTI inhibited uptake of the probe completely (cf.Fig. 4G).

Discussion
Huang et al. (1981) first reported that only acrosome-reacted guinea-pig spermatozoa could attach firmly tothe zona pellucida of homologous eggs, that this processcould be inhibited by fucoidan (Huang et al. 1982) andthat when fluorescein-labelled fucoidan was used as aprobe it bound to specific 'receptors' on the inneracrosomal membrane/equatorial segment regions(Huang and Yanagimachi, 1984). By inference, it wasreasoned that there was coincidence between fucoidanand zona receptors on guinea-pig sperm. The exper-iments described in this communication were designedto identify these putative receptors and their mechan-ism of interaction with fucoidan. Results indicate thatboth fucoidan- and zona-binding proteins in guinea-pigspermatozoa are intra-acrosomal and that one of themajor receptors is the proacrosin/acrosin system. Rec-ognition is dependent to a large extent on the orien-tation of charged sulphate groups on fucoidan but isindependent of the composition of the polysaccharidebackbone. Collectively, they support the hypothesisthat mammalian sperm proacrosin/acrosin is involvedin secondary or tight binding of spermatozoa to thezona and that it is functionally analogous to sea urchin'bindin'.
The validity of using fucoidan as a probe to detectputative zona receptors on spermatozoa is based onseveral established precedents and criteria. First, fucoi-dan blocks fertilization in a wide variety of mammalianspecies (hamster, human, boar, rat and mouse; Huangetal. 1982; Peterson etal. 1984; Shalgi et al. 1986; Boldtet al. 1989) and is especially effective in inhibitingbinding of acrosome-reacted guinea-pig spermatozoa tohomologous eggs (Huang et al. 1982). Second, fucoidanhas been used to identify adhesion molecules on differ-ent cell types (Hoylaerts et al. 1984), most notablybindin from sea urchin spermatozoa (Vacquier, 1986).Third, similar molecules are recognised by fucoidan andzona glycoproteins when they are used to probe West-ern blots containing detergent- or acid-extracted pro-teins from guinea-pig (Fig. lc) and boar spermatozoa(Jones et al. 1988). Thus, there are good reasons tobelieve that interaction of fucoidan with spermatozoamimics that of the natural substrate (i.e. the zonapellucida) and that the mechanism of binding is similar.
The cytochemical distribution of fucoidan-bindingproteins on guinea-pig spermatozoa as reported here isvery close to that described by Huang and Yanagimachi(1984). These authors found that a biotinylated-fucoidan/FITC-avidin probe did not bind to intactmotile spermatozoa but localized over the postacroso-mal and tail regions of immotile cells that had lost theiracrosomes completely, considered by them to be deadspermatozoa. After induction of an acrosome reactionon motile spermatozoa however, the inner acrosomalmembrane and equatorial segment became fluorescent.We also found that intact, motile spermatozoa did notstain but after permeabilizing them by cold shock theyreadily bound the probe, the pattern depending on theextent to which the acrosomal contents were dispersed.
Zona receptors on guinea-pig spermatozoa 47
In the presence of 2mM pAB, which inhibits loss ofacrosomal matrix components (Shams-Borhan andHarrison, 1981), fluorescence was strongest over theacrosomal cap with a variable amount on the equatorialsegment and postacrosomal regions. Without 2mMpAB in the medium, acrosomal caps were lost and theprobe bound to postacrosomal and tail domains. Ifspermatozoa were incubated in the presence of 2 ITIMCaCl2 then fluorescence was present over the anteriorhead of 22-33 % of cells. We regard these spermatozoaas undergoing an acrosome reaction and that the probebound to exposed inner acrosomal membrane/remnants of acrosomal contents. Unlike Huang andYanagimachi (1984) however, we found these sperma-tozoa were immotile and their numbers varied duringincubation. We attribute this to our failure to synchro-nize the acrosome reaction properly and that spermato-zoa were continuously moving into, and out of, thevesiculated acrosome-reacted state. Nonetheless, weconclude from these observations that fucoidan-bindingproteins from guinea-pig spermatozoa are locatedwithin the acrosome, either on the acrosomal mem-branes or as part of the acrosomal matrix. Staining ofthe postacrosomal and tail domains of permeabilizedspermatozoa would be caused by rebinding of dispersedacrosomal contents from the medium, i.e. we have noevidence that it is the reverse situation to PH20 antigenwhich has been shown to migrate from the postacroso-mal plasma membrane to the inner acrosomal mem-brane after the acrosome reaction (Myles and Primak-off, 1984). In the latter context, it is of interest thatendoproteolytic cleavage of PH20 antigen takes placeconcomitant with its migration. If one of the acrosomalcomponents that relocates onto the postacrosomal re-gion was a proteinase (and our evidence suggests thatthis is the case, see below), then it suggests a hithertounsuspected connection between the acrosome reac-tion, behaviour of surface membrane antigens andability of spermatozoa to bind to the zona.
Identification of the 48K fucoidan-binding protein asproacrosin and the 32K-34K components as forms ofactive acrosin is based on the following points, (a)Coincidence of molecular mass with those reported byArboleda and Gerton (1987) and Adekunle etal. (1987)for guinea-pig proacrosin and acrosin using the SDS-PAGE system of Laemmli (1970). Hardy et al. (1987),on the other hand, found two forms of proacrosin inextracts of guinea-pig spermatozoa with molecular massof 43K and 54/56K. As pointed out by Gerton andcolleagues, this discrepancy may depend on whether ornot protein standards are reduced or non-reduced. Apuzzling feature of the results presented here is whyacid extracts contained active forms of acrosin. Whenthe same protocol was applied to boar, bull and ramspermatozoa, proacrosin was extracted very efficientlyand did not activate (Jones, 1989). We do not know ifactivation took place during dialysis or electrophoresisbut the phenomenon was encountered only with acidextracts; detergent extracts made in the presence ofproteinase inhibitors were enriched in proacrosin. It isof interest that acid extracts also contained a higher

48 R. Jones and R. M. Williams
proportion of low molecular mass fucoidan-bindingproteins than detergent extracts supporting speculationthat the 18K-20K components represent autolysisproducts of active acrosin. (b) Immunoreactivity with apolyclonal antibody to boar proacrosin. Antibodies toboar acrosin have been reported to cross-react withguinea-pig acrosin (Arboleda and Gerton, 1987).Although our antibody was prepared to purified boarproacrosin, it also recognises ram and bull proacrosin(Jones, 1989). Since mammalian proacrosin is a highlyconserved molecule (boar and human proacrosin show~70% linear sequence homology; Adham et al. 1989;Baba et al. 1989) cross-reactivity of this nature is notunexpected. The weak reactivity by the 18K-20Kproteins together with their ability to bind 125I-SBTIalso suggests that they are proteinases, but furtherbiochemical characterization is necessary before a defi-nite relationship to proacrosin can be demonstrated, (c)Proteinase activity against gelatin substrate was associ-ated with the 48K and 30K-34K proteins. These resultsare identical to those obtained by Arboleda and Gerton(1987). If activation of proacrosin to acrosin took placeduring electrophoresis, it would account for proteinaseactivity in the detergent extracts at 30K-34K since evensmall amounts of protein would have high activity.
On the basis of the above evidence, we conclude thatthe 48K antigen represents a form of proacrosin and the30K-34K antigens two forms of active acrosin.
The identity of the 60K and 95K fucoidan-bindingproteins is more problematic. Neither showed protein-ase activity against gelatin nor were they recognised bythe anti-proacrosin antibody or 125I-SBTI. High mol-ecular mass forms of proacrosin ('pre-proacrosin') havebeen reported in rabbit and boar spermatozoa (Polak-oski and Parrish, 1977; Mukerji and Meizel, 1979;Berruti, 1985) and in acid extracts of guinea-pig testis(Hardy etal. 1987). During spermiogenesis and epididy-mal maturation, these pre-proacrosins are 'processed'to lower molecular mass forms (Arboleda and Gerton,1988). Thus, there is circumstantial evidence that the60K and 95K fucoidan-binding components may be pre-proacrosins. Whatever the case, we have to concludethat they are intracrosomal since the pattern of stainingon permeabilized spermatozoa was similar withfluoresceinamine-fucoidan (binds to three proteins onWestern blots at 48K, 60K and 95K) and FITC-SBTI(binds to one protein at 48K). It is possible that the 60Kand 95K proteins also have a role in sperm-zonaadhesion but to date we have no information on theirbehaviour during the acrosome reaction or whether,like proacrosin/acrosin, they behave as 'sticky' mol-ecules and relocate onto different areas of the spermsurface.
The ability of a variety of suiphated, but not unsul-phated, polysaccharides and polymers to inhibit bindingof 125I-fucoidan to proacrosin/acrosin indicates thatmolecular size and sulphate ester groups are importantparameters in the recognition process. However, it isnot just the presence or absence of sulphate groups thatis critical but their spatial orientation and alignmentalong the polysaccharide backbone. This would explain
why chondroitin sulphates A and C have poor inhibi-tory ability despite having a charge density greater thanthat of fucoidan (DeAngelis and Glabe, 1987). The veryhigh sulphate content of polyvinylsulphate probablymakes it likely that several groups will be in the correctposition for binding to basic residues on the protein.These properties have been described in other systems;for example, sulphation of C-6 and C-3 positions inglucosamine have been shown to be critical determi-nants in the binding of heparin to anti-thrombin III(Atha et al. 1985) and interaction of fucans with bindinfrom sea urchin spermatozoa depends on complemen-tary orientation between sulphate groups and arginineresidues on the protein (DeAngelis and Glabe, 1988).Similar properties have been described recently forRSA glycoproteins from rabbit spermatozoa (O'Randet al. 1988). Both bindin and RSA glycoproteins ag-glutinate trypsinized rabbit erythrocytes but we have noevidence at present that mammalian sperm proacrosinshave this capacity. It is worth noting that pig zonapellucida glycoproteins are suiphated (Dunbar et al.1980) and their binding to proacrosin/acrosin can beinhibited by only certain kinds of suiphated polymers(Jones et al. 1988).
Lastly, in the context of fertilization in the guinea pig,we suggest as a working hypothesis that the acrosomereaction of the fertilizing sperm takes place on or closeto the zona pellucida. Although most of the acrosomalcontents disperse, some matrix material containingproacrosin/acrosin remains attached to the inner acro-somal membrane from where it mediates firm bindingto the zona pellucida by cross-linking to suiphatedpolysaccharides. Binding may then be enhanced bylocalized proteolytic activity of acrosin on the zona in amanner analogous to the requirement for trypsinizationof erythrocytes before they can be agglutinated bycertain lectins. Proteolysis of this kind is thought tomake carbohydrate moieties of cell surface glyco-proteins more accessible to cross-linking reactions. Itshould be emphasized that this hypothesis does notexclude the possibility that primary zona receptors arepresent on the plasma membrane overlying the guinea-pig sperm head but rather it helps to explain howsecondary zona receptors appear dominant in thisspecies.
The authors are grateful to Mrs L. Notton for preparationof the typescript.
References
ADEKUNLE, A. O., ARBOLEDA, C. E., ZERVOS, P. H., GERTON, G.L. AND TEUSCHER, C. (1987). Purification and initialcharacterization of guinea-pig testicular acrosin. Biol. Reprod.37, 201-210.
ADHAM, I. M., KLEMM, U., MAIER, W-M., HOYER-FENDER, S.,TSAOUSIDOU, S. AND ENGEL, W. (1989). Molecular cloning ofpreproacrosin and analysis of its expression pattern inspermatogenesis. Eur. J. Biochem. 182, 563-568.
ARBOLEDA, C. E. AND GERTON, G. L. (1987). Studies of threemajor proteases associated with guinea-pig sperm acrosomes. J.exp. Zool. 244, 277-287.

Zona receptors on guinea-pig spermatozoa 49
ARBOLEDA, C. E. AND GERTON, G. L. (1988). Proacrosin/acrosinduring guinea pig spermatogenesis. Devi Biol. 125, 217-225.
ATHA, D. H., LORMEAU, J - C , PETTTOU, M., ROSENBERG, R. D. AND
CHOAY, J. (1985). Contribution of monosaccharide residues inheparin binding to Antithrombin III. Biochemistry 24,6723-6729.
BABA, T., WATANABE, K., KASHIWABARA, S. AND ARAI, Y. (1989).
Primary structure of human proacrosin deduced from its cDNAsequence. FEBS Letts. 244, 296-300.
BENAU, D. A. AND STOREY, B. T. (1987). Characterization of the
mouse sperm plasma membrane zona-binding site sensitive totrypsin inhibitors. Biol. Reprod. 36, 282-292.
BERRUTI, G. (1985). Evidence of a high molecular weight form ofacrosin in boar acrosomal extract. Int. J. Biochem. 17, 87-94.
BOLDT, J., HOWE, A. M., PARKERSON, J. B., GUNTER, L. E. AND
KUEHN, E. (1989). Carbohydrate involvement in sperm-eggfusion in mice. Biol. Reprod. 40, 887-8%.
BRADFORD, M. M. (1976). A rapid and sensitive method for thequantitation of microgram quantities of protein utilizing theprinciple of protein-dye binding. Analyt. Biochem. 72, 248-254.
DEANGEUS, P. L. AND GLABE, C. G. (1987). Polysaccharidestructural features that are critical for the binding of sulphatedfucans to bindin, the adhesive protein from sea urchin sperm. J.biol. Chem. 262, 13 946-13952.
DEANGEUS, P. L. AND GLABE, C. G. (1988). Role of basic aminoacids in the interaction of bindin with sulphated fucans.Biochemistry 27, 8189-8194.
DUBRAY, G. AND BEZARD, G. (1982). A highly sensitive periodicacid-silver stain for 1,2,-diol groups of glycoproteins andpolysaccharides in polyacrylamide gels. Analyt. Biochem. 119,325-329.
DUNBAR, B. S., WARDRIP, N. J. AND HEDRICK, J. K. (1980).
Isolation, physiochemical properties, and macromolecularcomposition of zona pellucida from porcine oocytes.Biochemistry 19, 356-365.
GAUNT, S. J., BROWN, C. R. AND JONES, R. (1983). Identification
of mobile and fixed antigens on the plasma membrane ofspermatozoa using monoclonal antibodies. Expl Cell Res. 144,275-284.
GLABE, C. G., HARTY, P. K. AND ROSEN, S. D. (1983). Preparation
and properties of fluorescent polysaccharides. Analyt. Biochem.130, 287-294.
GREEN, D. P. L. (1978). The activation of proteolysis in theacrosome reaction of guinea-pig sperm. J. Cell Sci. 32, 153-164.
HARDY, D. M., WILD, G. C. AND TUNG, K. S. K. (1987).
Purification and initial characterization of proacrosins fromguinea-pig testes and epididymal spermatozoa. Biol. Reprod. 37,189-199.
HEDRJCK, J. L., URCH, U. A. AND HARDY, D. M. (1988). The
structure-function properties of the sperm enzyme acrosin. In'Enzymes in Agricultural Biotechnology' (ed. S. Shoemaker, S.Sonnet and J. Whitaker) pp. 55-73, ACS Books, Washington,D.C.
HOYLAERTS, M. , OWEN, W. G. AND COLLEN, D. (1984).Involvement of heparin chain length in the heparin-catalyzedinhibition of thrombin by antithrombin III. J. biol. Chem. 259,5670-5677.
HUANG, T. T. F. JR, FLEMING, A. D. AND YANAGIMACHI, R. (1981).
Only acrosome-reacted spermatozoa can bind to and penetratezona pellucida: a study using the guinea-pig. J. exp. Zool. 217,287-290.
HUANG, T. T. F. JR, OHZU, E. AND YANAGIMACHI, R. (1982).
Evidence suggesting that L-fucose is part of a recognition signalfor sperm-zona pellucida attachment in mammals. Gamete Res.5, 355-361.
HUANG, T. T. F. JR AND YANAGIMACHI, R. (1984). Fucoidaninhibits attachment of guinea pig spermatozoa to the zonapellucida through binding to the inner acrosomal membrane andequatorial domains. Expl Cell Res. 153, 363-373.
JONES, R. (1987). Evidence of boar sperm proacrosin as acarbohydrate binding protein. Cell Biol. Intern. Rep. 11, 833.
JONES, R. (1989). Identification of carbohydrate-binding proteins inmammalian spermatozoa (human, bull, boar, ram, stallion and
hamster) using 125I-fucoidan and 125I-neoglycoprotein probes.Human Reprod. 4, 550-557.
JONES, R., BROWN, C. R. AND LANCASTER, R. T. (1988).
Carbohydrate-binding properties of boar sperm proacrosin andassessment of its role in sperm-egg recognition and adhesionduring fertilization. Development 102, 781-792.
JONES, R., SHALGI, R., HOYLAND, J. AND PHILLIPS, D. M. (1990).
Topographical rearrangement of a plasma membrane antigenduring capacitation of rat spermatozoa in vitro. Dev. Biol. (inpress).
KINDERKNECHT, H. (1962). Ultra-rapid fluorescent labelling ofproteins. Nature, Lond. 193, 167-168.
KOEHLER, J. K. (1978). The mammalian sperm surface: studies withspecific labelling techniques. Int. Rev. Cytol. 54, 73-108.
LAEMMLI, U. K. (1970). Cleavage of structural proteins during theassembly of the head of bacteriophage T4. Nature, Lond. 227,680-685.
MANN, T. (1964). In 'Biochemistry of Semen and of the MaleReproductive Tract'. Methuen, London.
MARKWELL, M. A. K. AND Fort, C. F. (1978). Surface-specificiodination of membrane proteins of viruses and eucaryotic cellsusing l,3,4,6-tetrachloro-3a', 6a^diphenylglycouril. Biochemistry17, 4807-4817.
MEDCALF, D. G. AND LARSEN, B. (1977). Fucose-containingpolysaccharides in the brown algae Ascophyllum nodosum andFucus vesiculosus. Carbohydr. Res. 59, 531-537.
MOORE, H. D. M. AND HARTMAN, T. D. (1984). Localization bymonoclonal antibodies of various surface antigens of hamsterspermatozoa and the effect of antibody on fertilization in vitro. J.Reprod. Fen. 70, 175-183.
MUKERJI, S. K. AND MEIZEL, S. (1979). Rabbit testis proacrosin:purification, molecular weight estimation and amino acid andcarbohydrate composition of the molecule. / . biol. Chem. 254,11721-11728.
MYLES, D. G. AND PRIMAKOFF, P. (1984). Localized surfaceantigens of guinea pig sperm migrate to new regions prior tofertilization. J. Cell Biol. 99, 1634-1641.
MYLES, D. G., PRIMAKOFF, P. AND BELLVE, A. R. (1981). Surface
domains of the guinea pig sperm defined with monoclonalantibodies. Cell 23, 433-439.
O 'RAND, M. G., WIDGREN, E. E. AND FISHER, S. J. (1988).
Characterization of the rabbit sperm membrane autoantigen,RSA, as a lectin-like zona binding protein. Devi Biol. 129,231-240.
PETERSON, R. N., RUSSELL, L. D. AND HUNT, W. P. (1984).
Evidence for specific binding of uncapacitated boar spermatozoato porcine zonae pellucidae in vitro. J. exp. Zool. 231, 137-147.
POLAKOSKI, K. L. AND PARRISH, R. F. (1977). Boar proacrosin. J.biol. Chem. 252, 1888-1894.
SAUNG, P. M. (1981). Involvement of trypsin-like activity inbinding of mouse spermatozoa to zonae pellucidae. Proc. natn.Acad. Sci. U.S.A. 78, 6231-6235.
SAUNG, P. M. AND STOREY, B. T. (1979). Mouse gameteinteractions during fertilization in vitro: chlortetracycline as afluorescent probe for the mouse sperm acrosome reaction. J. CellBiol. 83, 544-555.
SHALGI, R., MATTTYAHU, A. AND NEBEL, L. (1986). The role of
carbohydrates in sperm-egg interaction in rats. Biol. Reprod. 34,446-452.
SHALGI, R., PHILUPS, D. M. AND JONES, R. (1989). Status of the
rat acrosome during sperm-zona pellucida interactions. GameteRes. 22, 1-13.
SHAMS-BORHAN, G. AND HARRISON, R. A. P. (1981). Production,characterization, and use of ionophore-induced, calcium-dependent acrosome reaction in ram spermatozoa. Gamete Res.4, 407-432.
SHUR, B. D. AND HALL, N. G. (1982). A role for mouse spermsurface galactosyltransferase in sperm binding to the egg zonapellucida. J. Cell Biol. 95, 574-579.
SIEGEL, M. S. AND POLAKOSKI, K. L. (1985). Evaluation of thehuman sperm proacrosin-acrosin system using gelatin-sodiumdodecyl sulphate-polyacrylamide gel electrophoresis. Biol.Reprod. 32, 713-720.

50 R. Jones and R. M. Williams
TOWBIN, H., STAEHEUN, T. T. AND GORDON, J. (1979). WASSARMAN, P. M. (19S7). The biology and chemistry ofElectrophoretic transfer of proteins from polyacrylamide gels to fertilization. Science 235, 554-560.nitrocellulose sheets: procedure and some applications. Proc. YANAGIMACHI, R. (1988). Mammalian fertilization. In The Physiologynatn. Acad. Sci. U.S.A. 76, 4350-4354. of Reproduction, vol. 1 (ed. E. Knobil and J. Neil), pp. 135-185,
VACQUIER, V. D. (1986). Activation of sea urchin spermatozoa Raven Press, Ltd., New York,during fertilization. TIBS 11, 77-81. (Accepted 12 February 1990)