I. Waves & Particles (p. 134-145)
-
Upload
hamilton-klein -
Category
Documents
-
view
34 -
download
2
description
Transcript of I. Waves & Particles (p. 134-145)

Ch. 5- Electrons in AtomsUnit 7 Targets: The Electronic Structure of Atoms (Chap 5) •I CAN Utilize appropriate scientific vocabulary to explain scientific concepts.•I CAN Perform calculations involving the energy, wavelength and frequency of electromagnetic waves.•I CAN Perform calculations to determine the de Broglie wavelength of any object.•I CAN Compare and contrast Bohr’s solar system model with Schrodinger’s wave mechanical model.•I CAN Predict the movement of electrons from the ground state to an excited state, back to the ground state.•I CAN Recognize elements based on their emission spectra. (Flame lab).•I CAN Generate electron configurations for elements: full electron configuration, noble gas configuration, orbital diagram.•I CAN Differentiate relationships between orbitals, sublevels and energy levels.•I CAN Correlate the relationship between electrons in a single orbital or single sublevel.

Electromagnetic radiation- a form of energy that exhibits wavelike behavior.
• Wavelength () - length of one complete wave
• Frequency () - # of waves that pass a point during a certain time period SI UNIT for frequency is hertz (Hz) = 1 wave / s
• Amplitude (A) - distance from the origin to the trough or crest

Agreater
amplitude
(intensity)
greater frequency
(color)
crest
origin
trough
A

•To understand the electronic structure of
atoms we must understand light and how it is
emitted or absorbed by substances. •We will examine visible light a type of
Electromagnetic Radiation (EM) which carries (radiant) energy through space (speed of light) and exhibits wavelike behavior.
•Also need to think of light as particle, to help understand how EM radiation and atoms interact

LOW
ENERGY
HIGH
ENERGY

Move through a vacuum at the ‘speed of light’
3.00 x 108 m/sBehaves like waves that move through
water, which are the result of a transfer of
energy to the water (from a stone),
expressed as up and down movement of
water
Both electric and magnetic properties

LOW
ENERGY
HIGH
ENERGY
R O Y G. B I V
red orange yellow green blue indigo violet

Wave Speed = (distance between wave peaks) x (frequency)
= (wavelength) x (frequency)
EM radiation moves through a vacuum at the “speed of light”
3.00 x 108 m/s also called c. A lower energy wave (infrared and red) has a longer
wavelength() and lower frequency(f)
A higher energy wave (blue - violet) has a shorter
wavelength() and higher frequency(f).

Frequency & wavelength are inversely proportional
c = c: speed of light (3.00 108 m/s): wavelength (m, nm, etc.): frequency (Hz)

EX: Find the frequency of a photon with a wavelength of 434 nm.
GIVEN:
= ?
= 434 nm = 4.34 10-7 m
c = 3.00 108 m/s
WORK: = c
= 3.00 108 m/s 4.34 10-7 m
= 6.91 1014 Hz

Planck (1900)
•Observed - emission of light from hot objects
•Concluded - energy is emitted (absorbed or released) in small, specific amounts (quanta)
•Quantum - smallest energy packet that can be emitted or absorbed as EM radiation by an atom.

E: energy (J, joules)h: Planck’s constant (6.6262 10-34 J·s): frequency (Hz)
E = h
Planck proposed that the energy, E, of a single quantum energy packet equals a constant (h) times its frequency
The energy of a photon is proportional to its frequency.

EX: Find the energy of a red photon with a frequency of 4.57 1014 Hz.
GIVEN:
E = ? = 4.57 1014 Hzh = 6.6262 10-34 J·s
WORK:
E = h
E = (6.6262 10-34 J·s)(4.57 1014 Hz)
E = 3.03 10-19 J

Planck (1900)
•Observed - emission of light from hot objects
•Concluded - energy is emitted (absorbed or released) in small, specific amounts (quanta)
•Quantum - smallest energy packet that can be emitted or absorbed as EM radiation by an atom.

Planck (1900)
vs.
Classical Theory Quantum Theory

Energy is always emitted or absorbed in whole number multiples of hv, such as hv, 2 hv, 3 hv, 4hv, …. The allowed energies are quantized, that is their values are restricted to certain quantities.
The notion of quantized rather than continuous energies is strange. Consider a ramp and a staircase, on a ramp you can vary the length your steps and energy used on the walk up. When walking up steps you must exert exactly the specific amount of energy needed to reach the next step. Your steps on steps are quantized, you cannot step between them.

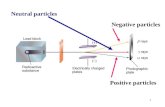
Einstein (1905)• Observed - photoelectric effect

Einstein (1905)
Concluded - light has properties of both waves and particles (photons)
“wave-particle duality”
Photon - particle of light that carries a quantum of energy
Used planck’s quantum theory to deduced that: Ephoton = hv


ground state
excited state
ENERGY IN PHOTON OUT
DEFINITION: A set of frequencies of EM waves emitted by atoms of the element.
EX. neon light absorb electrical energy, e- get excited, become somewhat unstable and
release energy in the form of light, a prism separates the light into an atomic emission
spectrum.

e- exist only in orbits with specific amounts of energy called energy levels
Therefore…
• e- can only gain or lose certain amounts of energy
• only certain photons are produced

Energy of photon depends on the difference in energy levels
Bohr’s calculated energies matched the IR, visible, and UV lines for the H atom
1
23
456

Each element has a unique bright-line emission spectrum.• “Atomic Fingerprint”
Helium
Bohr’s calculations only worked for hydrogen!

Examples:• Iron
Now, we can calculate for all elements and their electrons – next section


Louis de Broglie (1924)• Applied wave-particle theory to e-
• e- exhibit wave properties
EVIDENCE: DIFFRACTION PATTERNS
ELECTRONSVISIBLE LIGHT

Heisenberg Uncertainty Principle• Impossible to know both the velocity and
position of an electron at the same time

Schrödinger Wave Equation (1926)• finite # of solutions quantized energy levels
• defines probability of finding an e-
Wavelength = plank’s constant/mass X velocitymvh

Orbital (“electron cloud”)• Region in space where there is 90%
probability of finding an e-
Radial Distribution CurveOrbital

Four Quantum Numbers:• Specify the “address” of each electron in an
atom
UPPER LEVEL

1. Principal Quantum Number ( n )
• Main energy level occupied the e-
• Size of the orbital
• n2 = # of orbitals in the energy level

2. Angular Momentum Quantum # ( l )• Energy sublevel
• Shape of the orbital
s p d f

n = # of sublevels per level
n2 = # of orbitals per level
Sublevel sets: 1 s, 3 p, 5 d, 7 f

3. Magnetic Quantum Number ( ml )
• Orientation of orbital around the nucleus
• Specifies the exact orbitalwithin each sublevel

px py pz

Orbitals combine to form a spherical shape.
2s
2pz2py
2px

4. Spin Quantum Number ( ms )
• Electron spin +½ or -½
• An orbital can hold 2 electrons that spin in opposite directions.

Pauli Exclusion Principle• No two electrons in an atom can have the
same 4 quantum numbers.
• Each e- has a unique “address”:
1. Principal #
2. Ang. Mom. #
3. Magnetic #
4. Spin #
energy level
sublevel (s,p,d,f)
orientation
electron

Read Section 4-2!