Human Metapneumovirus Circulation in the United States ... · Human Metapneumovirus Circulation in...
Transcript of Human Metapneumovirus Circulation in the United States ... · Human Metapneumovirus Circulation in...

ARTICLEPEDIATRICS Volume 137 , number 5 , May 2016 :e 20152927
Human Metapneumovirus Circulation in the United States, 2008 to 2014Amber K. Haynes, MPH, a Ashley L. Fowlkes, MPH, b Eileen Schneider, MD, a Jeffry D. Mutuc, MPH, a Gregory L. Armstrong, MD, c Susan I. Gerber, MDa
abstractBACKGROUND: Human metapneumovirus (HMPV) infection causes respiratory illness, including
bronchiolitis and pneumonia. However, national HMPV seasonality, as it compares with
respiratory syncytial virus (RSV) and influenza seasonality patterns, has not been well
described.
METHODS: Hospital and clinical laboratories reported weekly aggregates of specimens tested
and positive detections for HMPV, RSV, and influenza to the National Respiratory and
Enteric Virus Surveillance System from 2008 to 2014. A season was defined as consecutive
weeks with ≥3% positivity for HMPV and ≥10% positivity for RSV and influenza during
a surveillance year (June through July). For each virus, the season, onset, offset, duration,
peak, and 6-season medians were calculated.
RESULTS: Among consistently reporting laboratories, 33 583 (3.6%) specimens were positive
for HMPV, 281 581 (15.3%) for RSV, and 401 342 (18.2%) for influenza. Annually, 6 distinct
HMPV seasons occurred from 2008 to 2014, with onsets ranging from November to
February and offsets from April to July. Based on the 6-season medians, RSV, influenza,
and HMPV onsets occurred sequentially and season durations were similar at 21 to 22
weeks. HMPV demonstrated a unique biennial pattern of early and late seasonal onsets.
RSV seasons (onset, offset, peak) were most consistent and occurred before HMPV seasons.
There were no consistent patterns between HMPV and influenza circulations.
CONCLUSIONS: HMPV circulation begins in winter and lasts until spring and demonstrates
distinct seasons each year, with the onset beginning after that of RSV. HMPV, RSV, and
influenza can circulate simultaneously during the respiratory season.
Divisions of aViral Diseases, and bInfl uenza, National Center for Immunization and Respiratory Diseases, and cOffi ce of Advanced Molecular Detection, National Center for Emerging and Zoonotic Infectious Diseases,
Centers for Disease Control and Prevention, Atlanta, Georgia
Ms Haynes helped conceptualize and design the study and drafted the initial manuscript;
Mr Mutuc carried out preliminary analyses; Mr Mutuc and Dr Armstrong reviewed the
manuscript; Ms Fowlkes served as infl uenza virus subject matter expert; Ms Fowlkes and
Dr Schneider helped design the study; Ms Fowlkes and Drs Schneider and Gerber critically
reviewed the manuscript; Dr Schneider served as human metapneumovirus subject matter
expert; Dr Armstrong conceptualized the study analysis; Drs Armstrong and Gerber revised the
manuscript; Dr Gerber guided the study concept and design; and all authors approved the fi nal
manuscript as submitted.
The fi ndings and conclusions in this report are those of the authors and do not necessarily
represent the offi cial position of the Centers for Disease Control and Prevention.
DOI: 10.1542/peds.2015-2927
Accepted for publication Jan 27, 2016
Address correspondence to Amber K. Haynes, MPH, National Center for Immunization and
Respiratory Diseases, Centers for Disease Control and Prevention, 1600 Clifton Rd, NE, MS A-34,
Atlanta, GA 30329. E-mail: [email protected]
To cite: Haynes AK, Fowlkes AL, Schneider E, et al. Human Metapneumovirus
Circulation in the United States, 2008 to 2014. Pediatrics. 2016;137(5):e20152927
WHAT’S KNOWN ABOUT THIS SUBJECT: Human
metapneumovirus is a respiratory virus that causes
upper and lower respiratory infections. Clinical
presentation, populations most severely impacted,
and circulation patterns are similar to those of
respiratory syncytial virus; however, national human
metapneumovirus circulation has not been well
described.
WHAT THIS STUDY ADDS: This study describes
national human metapneumovirus circulation
using laboratory detections reported to a national
surveillance system from 2008 to 2014. Defi ning
periods of elevated human metapneumovirus
circulation may guide virus detection and clinical
management, aiding in identifying illness and
outbreaks.
by guest on January 19, 2021www.aappublications.org/newsDownloaded from

HAYNES et al
First identified in 2001, 1 human
metapneumovirus (HMPV) is a
cause of both upper and lower
respiratory tract infections, including
bronchiolitis and pneumonia,
particularly among young children
(<5 years), the elderly, and
immunocompromised patients.2–5
Infection with HMPV has been
associated with an estimated 20 000
U.S. hospitalizations annually among
children aged <5 years.6 However,
the infrequent testing and low index
of suspicion associated with HMPV
may have limited the assessment
of temporal trends in HMPV
circulation. Also, many studies have
demonstrated that HMPV causes a
respiratory tract infection clinically
indistinguishable from infections
caused by respiratory syncytial virus
(RSV) and influenza.1–5 In contrast,
the specific prevention options and
some populations severely affected
vary for HMPV, RSV, and influenza.7
Currently there is no vaccine for
HMPV. Thus, describing HMPV
circulation in the United States in the
context of RSV and influenza may
help clinicians to prioritize diagnostic
testing, identify an etiologic agent,
manage patients clinically, and
choose appropriate prevention
strategies.
Many studies have demonstrated a
winter-to-spring circulation period
for HMPV in temperate climates, 8–10
but determination of national HMPV
trends and comparison of HMPV
seasonality to RSV and influenza
in multiple sites throughout the
United States have not yet been
done. A study conducted in 3 U.S.
sites identified HMPV circulation in
winter and spring months;6 however,
it remains unclear if this pattern
reflects trends in national HMPV
circulation. The increased availability
and use of molecular diagnostic
assays to detect respiratory viruses11
in recent years has highlighted
several HMPV-associated outbreaks
throughout the United States12 and
has enhanced the opportunity to
evaluate national trends in HMPV
circulation.
In the United States, surveillance
for several respiratory viruses is
conducted annually through the
National Respiratory and Enteric
Virus Surveillance System (NREVSS).
In this study, we describe national
HMPV circulation patterns and
compare with patterns of RSV and
influenza activity reported to NREVSS
during 6 seasons from 2008 to 2014.
METHODS
NREVSS is a passive surveillance
network established in 1984 that
collects specimen test results
for several respiratory viruses,
including HMPV, RSV, and influenza.
Approximately 300 clinical and
public health laboratories in the
United States report ≥1 specimen
test result on average for 44 weeks
in a surveillance year.13 Laboratories
report weekly aggregates of the
number of tests performed and
positive detections by antigen
detection, polymerase chain reaction
(PCR), and viral isolation. The type of
assay reported can vary depending
on the respiratory virus and year.
For RSV, we analyzed antigen
detection reports; for influenza,
we analyzed PCR reports; and for
HMPV, we analyzed both antigen
detection and PCR reports. The
NREVSS surveillance year is defined
as July of the starting year through
the end of the following June to
capture the typical national onset
and offset of several respiratory
viruses. Surveillance for RSV and
influenza through NREVSS is well
established and ongoing since 1984
and 1989, respectively. The first
HMPV diagnostic test was reported
to NREVSS in July 2005, but reports
were insufficient for robust analysis
until 2008 to 2009, when test reports
to NREVSS exceeded 70 000.
Laboratories included in this analysis
were selected based on annual
duration and volume of reported
test results. For RSV analysis, we
included laboratories reporting
≥10 RSV antigen detection tests/
week annually and ≥1 RSV antigen
detection test for 30 of 52 weeks
of the NREVSS year; for influenza
analysis, we included laboratories
reporting influenza by PCR to
the World Health Organization
collaborating laboratories;14 and
for HMPV analysis, we included
laboratories reporting ≥1 HMPV PCR
or antigen detection test for 36 of 52
weeks of the NREVSS year. These are
standard laboratory inclusion criteria
for RSV and influenza; no standard
inclusion criteria exist for HMPV.
For each virus, we calculated the
weekly proportion-positive. To
define the season for RSV13 and
influenza, 14 we used the widely
accepted 10% weekly proportion-
positive threshold. Specifically,
the RSV or influenza seasons were
defined as the first of 2 consecutive
weeks when the proportion of
positive weekly aggregates exceeded
10% positivity, and the season
offset, as the last of 2 consecutive
weeks when the proportion of
weekly aggregates exceeded 10%
positivity. To define the season for
HMPV, we selected a 3% weekly
proportion-positive threshold. We
defined the HMPV season onset
as the first of 2 consecutive weeks
when the proportion of positive
weekly aggregates exceeded 3%
positivity, and the season offset,
as the last of 2 consecutive weeks
when the proportion of weekly
aggregates exceeded 3% positivity.
At a threshold of 3%, 84% to 94%
of HMPV detections by antigen
detection tests were captured
each year, and 80% to 92% of
HMPV detections by PCR tests
were captured. For each virus, we
calculated the onset, offset, peak,
and duration (onset to offset) for
each individual season and the
median for the 6 seasons. A 4-season
median rather than a 6-season
median was calculated for influenza;
2 by guest on January 19, 2021www.aappublications.org/newsDownloaded from

PEDIATRICS Volume 137 , number 5 , May 2016
2 seasons (2008 to 2009, 2009 to
2010) were excluded because of the
unprecedented occurrence of the
H1N1 pandemic. An early season was
defined as a season with an earlier
onset than the 6-season median and
higher peak proportion-positive
than the 6-season median, and a late
season was defined as a later onset
than the 6-season median and lower
peak proportion-positive than the
6-season median.
RESULTS
Among all laboratories reporting
HMPV tests results to NREVSS from
July 2008 to June 2014, 1 065 742
HMPV test results were reported
and 38 160 (3.6%) were positive
(25 409 [3.9%] by PCR; 12 751
[3.0%] by antigen detection). Among
consistently reporting laboratories
included in our analysis, 945 836
tests were reported from July 2008
to June 2014, and 33 583 (3.6%)
were positive (21 972 [3.9%] by PCR,
11 611 [3.1%] by antigen detection)
for HMPV, 1 839 877 tests were
reported and 281 581 (15.3%) were
positive for RSV, and 2 206 654 tests
were reported (including specimens
from the H1N1 influenza pandemic)
and 401 342 (18.2%) were positive
for influenza. During the study
period, laboratories consistently
reporting HMPV comprised general
hospitals (60.3%), children’s
hospitals/facilities (28.9%), public
health facilities (9.6%), and reference
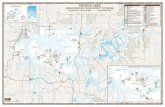
laboratories (1.2%). The number of
laboratories reporting HMPV tests
increased from 21 laboratories
representing 16 states in 2008 to
2009 to 60 laboratories representing
30 states in 2013 to 2014 (Table 1,
Fig 1). The number of HMPV tests
remained relatively constant over
the 6 surveillance years, but the
most prevalent diagnostic method
reported fluctuated from year to
year. In 2008 to 2009 and 2009 to
2010, 77% and 52%, respectively,
of HMPV diagnostic tests reported
were antigen detection; however,
from 4 seasons that spanned 2010 to
2014, 52% to 85% of diagnostic tests
reported were PCR. Similarly, the
number of laboratories consistently
reporting HMPV by antigen detection
decreased from the 2008 to 2009 to
2013 to 2014 season (median 21,
range 15 to 25), and the number
of laboratories reporting by PCR
increased from the 2008 to 2009 to
the 2013 to 2014 season (median 34,
range 9 to 49) (Table 1). Reporting
both test methods for HMPV by
laboratories was infrequent but
increased from 3 in 2008 to 2009 to 9
in 2013 to 2014.
The weekly proportion of specimens
positive by antigen detection or PCR
methods for HMPV show definitive
seasonal patterns each year (Fig 2A).
The weekly proportion of positive
HMPV tests ranged from a low of
<1% between July and November to
a maximum of 6% to 16% between
March and April. We observed a
slight biennial pattern with early
and late seasons for HMPV based on
the median weekly proportion-PCR
positive tests with early (NREVSS
surveillance years: 2009 to 2010,
2011 to 2012, and 2013 to 2014)
and late (NREVSS surveillance years:
2008 to 2009, 2010 to 2011, and
2012 to 2013) seasons (Fig 2B).
Nationally, the HMPV 6-season
median onset occurred in early
January (week 1), and individual
season onsets occurred from late
November to late February within
5 weeks of the 6-season median
3
TABLE 1 HMPV Diagnostic Tests Reported to NREVSS by Test Type and Year, United States, 2008 to 2014
NREVSS Year Antigen Detection PCR Totala
Laboratories
Reporting, n
States
Represented, n
Tests, n (%) Laboratories
Reporting, n
States
Represented, n
Tests, n (%) Laboratories
Reporting, n
States
Represented, n
2008 to 2009 15 14 69 698 (77) 9 8 20 353 (23) 21 16
2009 to 2010 20 17 83 033 (52) 20 12 77 342 (48) 33 21
2010 to 2011 21 17 76 710 (48) 32 17 83 155 (52) 46 25
2011 to 2012 25 19 59 935 (42) 35 18 83 241 (58) 53 28
2012 to 2013 24 18 60 573 (30) 43 21 141 709 (70) 62 31
2013 to 2014 20 15 28 881 (15) 49 28 161 206 (85) 60 30
A total of 945 836 test results were reported for HMPV (antigen detection, 378 830 [40%]; PCR, 567 006 [60%]).a Total includes those qualifying laboratories reporting HMPV test results by antigen detection, PCR, or both diagnostic test methods.
FIGURE 1Geographic distribution of states with laboratories consistently reporting HMPV diagnostic test results by test type, United States, 2008 to 2014. n = number of states with qualifying laboratories consistently reporting HMPV test results for the specifi ed NREVSS Year. Laboratories reporting HMPV test results by antigen detection, PCR, or both diagnostic methods.
by guest on January 19, 2021www.aappublications.org/newsDownloaded from

HAYNES et al
onset (Table 2). The HMPV 6-season
median offset occurred in mid-May
(week 20) ranging from late April
to early July within 7 weeks of
the individual season offsets. The
6-season median peak occurred in
late March (week 12) and ranged
from late February (week 7) to early
May (week 17) for individual season
peaks. The HMPV 6-season duration
was 21 weeks (individual season
duration range 19 to 25 weeks). Only
minor variations in the occurrence
of the HMPV season onset, peak, and
offset were observed between PCR
and antigen detection methods.
The RSV seasons were most
consistent, with very little change
in onset, offset, and peak, unlike
HMPV and influenza (Fig 3). The
HMPV season onset, offset, and
peak occurred after RSV for all 6
seasons. Year-to-year patterns in
the sequential occurrence of offset
and peak were similar among RSV
and HMPV, but not influenza (Table
2). The 6-season median onset for
RSV, influenza, and HMPV occurred
in sequential order (Table 2). The
first respiratory virus season median
onset to occur was RSV, which had
a 6-season median onset in early
November (individual season onset
range late October to late November).
The influenza 4-season median
onset occurred in early December,
4
FIGURE 2Human metapneumovirus reporting by diagnostic test type (A) and early and late seasons (B), United States, 2008 to 2014. *Three-week moving average (average of the previous, current, and following week proportion of positive tests for each testing method) of the median weekly proportion-PCR positive tests and antigen positive tests. †Early seasons: 2009 to 2010, 2011 to 2012, 2013 to 2014; late seasons: 2008 to 2009, 2010 to 2011, 2012 to 2013.
TABL
E 2
Sea
son
On
set,
Off
set,
Pea
k, a
nd
Du
rati
on f
or R
SV,
Infl
uen
za, a
and
HM
PV
for
Ind
ivid
ual
Sea
son
s an
d t
he
6-S
easo
n M
edia
n, U
nit
ed S
tate
s, 2
008
to 2
014
NR
EVS
S Y
ear
Mon
th O
nse
t O
ccu
rred
(M
MW
R W
eek)
Mon
th O
ffse
t O
ccu
rred
(M
MW
R W
eek)
Mon
th P
eak
Occ
urr
ed (
MM
WR
Wee
k)S
easo
n D
ura
tion
, Wee
ks
RS
VIn
fl u
enza
HM
PV
RS
VIn
fl u
enza
HM
PV
RS
VIn
fl u
enza
HM
PV
RS
VIn
fl u
enza
HM
PV
2008
to
2009
Nov
(44
)Ja
n (
02)
Jan
(02
)M
ar (
11)
Apr
(14)
May
(20
)Ja
n (
3)Fe
b (
6)M
ar (
12)
2013
19
2009
to
2010
Nov
(45
)Ap
r (1
7)D
ec (
52)
Mar
(12
)D
ec (
48)
May
(19
)Ja
n (
3)Ju
n (
24)
Feb
(9)
2032
20
2010
to
2011
Nov
(46
)N
ov (
47)
Jan
(04
)Ap
r (1
4)Ap
r (1
4)Ju
l (27
)Fe
b (
5)Fe
b (
5)M
ay (
17)
2120
24
2011
to
2012
Nov
(46
)Fe
b (
05)
Dec
(48
)Ap
r (1
4)Ju
n (
24)
Apr
(16)
Jan
(4)
Mar
(10
)Fe
b (
7)21
2021
2012
to
2013
Oct
(43
)N
ov (
45)
Feb
(07
)M
ar (
13)
Apr
(15)
Jul (
27)
Jan
(1)
Dec
(52
)Ap
r (1
4)23
2321
2013
to
2014
Nov
(45
)D
ec (
48)
Nov
(48
)M
ar (
13)
May
(20
)M
ay (
20)
Dec
(52
)D
ec (
52)
Mar
(12
)21
2525
6-S
easo
n M
edia
na
Nov
(45
)D
ec (
48)a
Jan
(01
)M
ar (
13)
May
(18
)aM
ay (
20)
Jan
(3)
Jan
(3)
aM
ar (
12)
21b
22a,
b21
b
a In
fl u
enza
6-s
easo
n m
edia
ns
excl
ud
e th
e 20
08 t
o 20
09 a
nd
200
9 to
201
0 se
ason
s b
ecau
se o
f th
e H
1N1
pan
dem
ic.
b T
he
6-se
ason
med
ian
for
sea
son
du
rati
on is
bas
ed o
n t
he
med
ian
of
the
seas
on d
ura
tion
for
th
e 6
seas
ons
(4 s
easo
ns
for
infl
uen
za)
and
not
th
e d
iffe
ren
ce o
f th
e 6-
seas
on m
edia
n f
or t
he
onse
t an
d o
ffse
t.
by guest on January 19, 2021www.aappublications.org/newsDownloaded from

PEDIATRICS Volume 137 , number 5 , May 2016
(individual season onset range
late November to early February).
The HMPV 6-season median onset
occurred in early January (individual
season onset range late November to
late February). The first respiratory
virus season offset to occur was RSV.
The 6-season median offset for RSV
occurred in late March (individual
season offset range mid-March to
early April), followed by influenza in
early May (individual season offset
range early April to early June) and
HMPV in mid-May (individual season
offset range late April to early July).
The 6-season median peak occurred
in the following sequential order:
RSV (late January, week 3), influenza
(late January, week 3), and HMPV
(late March, week 12). The 6-season
median onset and peak for HMPV
occurred 8 and 9 weeks, respectively,
after RSV and 5 and 9 weeks after
influenza, respectively (Table 2). The
6-season median duration for all 3
viruses were very similar at 21 to 22
weeks.
DISCUSSION
This is the first published summary
of HMPV national data from NREVSS
and demonstrates several unique
features. From 2008 to 2014, the
national HMPV data suggest that
HMPV seasons occur later than RSV
seasons, and based on the 6-season
median onset, the RSV season
occurred first, followed by influenza
and then HMPV. The unprecedented
H1N1 influenza pandemic that
affected the 2008 to 2009 and 2009
to 2010 seasons made comparison
during these seasons difficult
to interpret. In addition, HMPV
demonstrated a biennial pattern of
early and late seasons. There were
no distinct differences in HMPV
seasonality determined by antigen
detection and PCR, and PCR was the
most prevalent diagnostic method
used to identify HMPV in the last 4
years of analysis.
HMPV season durations occurred
between November and July
(6-season median 21 weeks). Weekly
HMPV positivity fluctuated between
nearly zero and ≥6% every 12
months, showing a distinguishable
seasonal pattern. Similar to RSV
and influenza, HMPV seasons
occurred during winter and spring,
as previously described.8–10 The
weekly HMPV percent-positivity
in this analysis never reached zero
over the 6 years of surveillance,
confirming previous reports of low
but continuous HMPV circulation
beyond winter and spring.15 We also
observed a biennial pattern for HMPV
of alternating early and late season
from 2008 to 2014, which was not
seen for RSV or influenza.16, 17
The most prevalent HMPV diagnostic
method reported shifted from
antigen detection to PCR during 2008
to 2014. The shift toward increased
reporting of PCR tests likely reflects
changes in conventional diagnostic
testing among participating NREVSS
laboratories. Within any given season
during this time period, a 3% weekly
positivity measure captured ≥80%
of PCR HMPV detections reported
by qualifying institutions. The 3%
weekly proportion is comparatively
lower than the 10% positivity used to
indicate elevated influenza and RSV
circulations. However, as in other
studies, we determined that a smaller
proportion of diagnostic tests were
positive for HMPV compared with
other respiratory pathogens.18–21
The data analyzed were not robust
enough to assess regional trends in
HMPV circulation.
The cocirculation analysis suggest
that HMPV, RSV, and influenza
cocirculate, as previously described
by Esper et al.15 Although the
circulations of influenza, RSV, and
HMPV overlap, the populations
susceptible to severe infection and
the management of these infections
differ.22–24
Therefore, clinicians can use
surveillance data, such as NREVSS,
to help identify HMPV seasonality
and help prioritize HMPV testing in
patients with respiratory symptoms.
As laboratory recruitment into
NREVSS and PCR use continue to
increase throughout the United States,
future NREVSS data should be able to
more reliably allow for more detailed
analyses, including regional HMPV
trends and outbreak occurrence,
similar to those for RSV and influenza.
Our study had several limitations.
Our findings are based on NREVSS,
which is a passive and voluntary
surveillance system in which (1)
5
FIGURE 3Onset, offset, peak, and duration by season and 6-season median for HMPV, RSV, and infl uenza, United States, 2008 to 2014. 2008 to 2009 and 2009 to 2010 infl uenza seasons not included in the 4-season median because of H1N1 pandemic.
by guest on January 19, 2021www.aappublications.org/newsDownloaded from

HAYNES et al
participating laboratories can differ
from season to season and may
report different respiratory viruses;
(2) HMPV test reporting is relatively
new and does not garner the same
test reporting volume or regional
representation as more established
respiratory viruses, such as RSV; (3)
patient age and specific specimen
information are not collected; and
(4) duplication is a possibility if
antigen detection and PCR testing
are performed and reported on the
same specimen or if >1 specimen is
reported from a patient during the
same illness episode. Because HMPV
is a recently recognized respiratory
virus, health professionals may not
routinely consider or test for HMPV.
Finally, we selected a low positivity
threshold to define HMPV seasonality
because fewer detections were
positive for HMPV compared with
other respiratory viruses monitored
by NREVSS.
CONCLUSIONS
In the Unites States, HMPV circulates
in distinct annual seasons with
biennial patterns of early and late
seasons. Our findings suggest that
RSV onset occurs the earliest during
the fall/winter respiratory virus
season, followed by influenza and
then HMPV. To distinguish HMPV
from other cocirculating viruses,
health professionals should consider
HMPV testing during the respiratory
season, especially when HMPV is the
predominant virus circulating.
REFERENCES
1. Van den Hoogen BG, de Jong JC,
Groen J, et al. A newly discovered
human pneumovirus isolated from
young children with respiratory tract
disease. Nat Med. 2001;7(6):719–724
2. Boivin G, De Serres G, Hamelin
ME, et al. An outbreak of severe
respiratory tract infection due to
human metapneumovirus in a long-
term care facility. Clin Infect Dis.
2007;44(9):1152–1158
3. Jartti T, van den Hoogen B, Garofalo
RP, Osterhaus AD, Ruuskanen
O. Metapneumovirus and acute
wheezing in children. Lancet.
2002;360(9343):1393–1394
4. Madhi SA, Ludewick H, Abed Y, Klugman
KP, Boivin G. Human metapneumovirus-
associated lower respiratory tract
infections among hospitalized human
immunodefi ciency virus type 1 (HIV-
1)-infected and HIV-1-uninfected
African infants. Clin Infect Dis.
2003;37(12):1705–1710
5. Viazov S, Ratjen F, Scheidhauer
R, Fiedler M, Roggendorf M.
High prevalence of human
metapneumovirus infection in young
children and genetic heterogeneity
of the viral isolates. J Clin Microbiol.
2003;41(7):3043–3045
6. Edwards KM, Zhu Y, Griffi n MR, et al;
New Vaccine Surveillance Network.
Burden of human metapneumovirus
infection in young children. N Engl J
Med. 2013;368(7):633–643
7. Wolf DG, Greenberg D, Kalkstein
D, et al. Comparison of human
metapneumovirus, respiratory
syncytial virus and infl uenza A virus
lower respiratory tract infections in
hospitalized young children. Pediatr
Infect Dis J. 2006;25(4):320–324
8. Kahn JS. Epidemiology of human
metapneumovirus. Clin Microbiol Rev.
2006;19(3):546–557
9. Anderson EJ, Simões EA, Buttery JP,
et al. Prevalence and characteristics
of human metapneumovirus infection
among hospitalized children at high
risk for severe lower respiratory tract
infection. J Pediatric Infect Dis Soc.
2012;1(3):212–222
10. Williams JV, Harris PA, Tollefson SJ,
et al. Human metapneumovirus and
lower respiratory tract disease in
otherwise healthy infants and children.
N Engl J Med. 2004;350(5):443–450
11. Mahony JB, Petrich A, Smieja M.
Molecular diagnosis of respiratory
virus infections. Crit Rev Clin Lab Sci.
2011;48(5-6):217–249
12. Centers for Disease Control and
Prevention (CDC). Outbreaks of human
metapneumovirus in two skilled
nursing facilities—West Virginia and
Idaho, 2011-2012. MMWR Morb Mortal
Wkly Rep. 2013;62(46):909–913
13. Centers for Disease Control and
Prevention (CDC). Respiratory syncytial
virus activity—United States, July
2011-January 2013. MMWR Morb
Mortal Wkly Rep. 2013;62(8):141–144
14. Centers for Disease Control and
Prevention (CDC). Update: infl uenza
activity—United States and worldwide,
6
ABBREVIATIONS
HMPV: human metapneumovirus
NREVSS: National Respiratory
and Enteric Virus
Surveillance System
PCR: polymerase chain reaction
RSV: respiratory syncytial virus
PEDIATRICS (ISSN Numbers: Print, 0031-4005; Online, 1098-4275).
Copyright © 2016 by the American Academy of Pediatrics
FINANCIAL DISCLOSURE: The authors have indicated they have no fi nancial relationships relevant to this article to disclose.
FUNDING: Supported by the Centers for Disease Control and Prevention, National Center for Immunization and Respiratory Diseases, Division of Viral Diseases
and Infl uenza.
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential confl icts of interest to disclose.
by guest on January 19, 2021www.aappublications.org/newsDownloaded from

PEDIATRICS Volume 137 , number 5 , May 2016
2003-04 season, and composition of the
2004-05 infl uenza vaccine. MMWR Morb
Mortal Wkly Rep. 2004;53(25):547–552
15. Esper F, Martinello RA, Boucher
D, et al. A 1-year experience with
human metapneumovirus in
children aged <5 years. J Infect Dis.
2004;189(8):1388–1396
16. Reiche J, Jacobsen S, Neubauer K,
et al. Human metapneumovirus:
insights from a ten-year molecular and
epidemiological analysis in Germany.
PLoS One. 2014;9(2):e88342
17. Aberle SW, Aberle JH, Sandhofer
MJ, Pracher E, Popow-Kraupp T.
Biennial spring activity of human
metapneumovirus in Austria. Pediatr
Infect Dis J. 2008;27(12):1065–1068
18. Boivin G, De Serres G, Côté S, et al.
Human metapneumovirus infections in
hospitalized children. Emerg Infect Dis.
2003;9(6):634–640
19. Mullins JA, Lamonte AC, Bresee JS,
Anderson LJ. Substantial variability in
community respiratory syncytial virus
season timing. Pediatr Infect Dis J.
2003;22(10):857–862
20. Williams JV, Wang CK, Yang CF,
et al. The role of human
metapneumovirus in upper
respiratory tract infections in
children: a 20-year experience. J
Infect Dis. 2006;193(3):387–395
21. Chano F, Rousseau C, Laferrière C,
Couillard M, Charest H. Epidemiological
survey of human metapneumovirus
infection in a large pediatric tertiary
care center. J Clin Microbiol.
2005;43(11):5520–5525
22. Glezen WP, Taber LH, Frank AL,
Kasel JA. Risk of primary infection
and reinfection with respiratory
syncytial virus. Am J Dis Child.
1986;140(6):543–546
23. Centers for Disease Control and
Prevention. Prevention and control
of seasonal infl uenza with vaccines:
Recommendations of the advisory
committee on immunization
practices—United States, 2013-
2014. MMWR Morb Mortal Wkly Rep.
2013;62(RR07):1–43
24. Bastien N, Ward D, Van Caeseele P, et
al. Human metapneumovirus infection
in the Canadian population. J Clin
Microbiol. 2003;41(10):4642–4646
7 by guest on January 19, 2021www.aappublications.org/newsDownloaded from

DOI: 10.1542/peds.2015-2927 originally published online April 4, 2016; 2016;137;Pediatrics
Armstrong and Susan I. GerberAmber K. Haynes, Ashley L. Fowlkes, Eileen Schneider, Jeffry D. Mutuc, Gregory L.
Human Metapneumovirus Circulation in the United States, 2008 to 2014
ServicesUpdated Information &
http://pediatrics.aappublications.org/content/137/5/e20152927including high resolution figures, can be found at:
Referenceshttp://pediatrics.aappublications.org/content/137/5/e20152927#BIBLThis article cites 23 articles, 4 of which you can access for free at:
Subspecialty Collections
http://www.aappublications.org/cgi/collection/bronchiolitis_subBronchiolitishttp://www.aappublications.org/cgi/collection/pulmonology_subPulmonologybhttp://www.aappublications.org/cgi/collection/infectious_diseases_suInfectious Diseasefollowing collection(s): This article, along with others on similar topics, appears in the
Permissions & Licensing
http://www.aappublications.org/site/misc/Permissions.xhtmlin its entirety can be found online at: Information about reproducing this article in parts (figures, tables) or
Reprintshttp://www.aappublications.org/site/misc/reprints.xhtmlInformation about ordering reprints can be found online:
by guest on January 19, 2021www.aappublications.org/newsDownloaded from

DOI: 10.1542/peds.2015-2927 originally published online April 4, 2016; 2016;137;Pediatrics
Armstrong and Susan I. GerberAmber K. Haynes, Ashley L. Fowlkes, Eileen Schneider, Jeffry D. Mutuc, Gregory L.
Human Metapneumovirus Circulation in the United States, 2008 to 2014
http://pediatrics.aappublications.org/content/137/5/e20152927located on the World Wide Web at:
The online version of this article, along with updated information and services, is
by the American Academy of Pediatrics. All rights reserved. Print ISSN: 1073-0397. the American Academy of Pediatrics, 345 Park Avenue, Itasca, Illinois, 60143. Copyright © 2016has been published continuously since 1948. Pediatrics is owned, published, and trademarked by Pediatrics is the official journal of the American Academy of Pediatrics. A monthly publication, it
by guest on January 19, 2021www.aappublications.org/newsDownloaded from