HR 653 & S 305 National Childhood Brain Tumor Prevention Network Act of 2009 Lloyd Morgan, Brain...
-
Upload
kristian-tyler -
Category
Documents
-
view
212 -
download
0
Transcript of HR 653 & S 305 National Childhood Brain Tumor Prevention Network Act of 2009 Lloyd Morgan, Brain...

HR 653 & S 305National Childhood Brain Tumor Prevention Network Act of 2009
Lloyd Morgan, Brain Tumor Survivor ([email protected]) Dr. Tarik Tihan, Pediatric Neuro-pathologist

2
Why Is This Bill Needed?Almost nothing is known about the causes of Childhood Brain Tumor (CBT)
No single institutions can possibly do a CBT study on their own
There has never been a comprehensive investigation into the cause of CBT
IF WE DON’T LOOK, WE WILL NEVER KNOW

3
Childhood Brain Tumor FactsLeading cause of death from solid tumors in US children
1st most common form of solid tumor in US children
2nd most common malignancy among US children
Still considered an orphan disease

4
CBT is an Orphan Disease with a costly profile
Surgery only (30%) $136,531 per child
Surgery & Radiation (30%) $216,531 per child
Surgery, Radiation & Chemotherapy (40%)
$296,367 per child
AVERAGE COST$ 224,429 per
child
Cost of Initial Treatment per child; calculated for the first line of treatment options.
Source :California Childhood Brain Tumor Consortium Study. Dr. Paul Fisher, Stanford University, 2006

5
CBT results in loss of many years of potential life
Source :Average years of Potential Life Lost for Childhood Brain Tumors. Thuppal et al. Neuroepidemiology. 2006;27(1):22-7.

6
The incidence of the most common childhood glioma is increasing.
Childhood (0-19 yr) Age Adjusted Incidence Rates for Pilocytic Astrocytoma
0.0
0.2
0.4
0.6
0.8
1.0
1986
1988
1990
1992
1994
1996
1998
2000
2002
2004
Year of Diagnosis
Brain tumors per 100,000 children
Source: SEER 9 Registries 1986-2005

7
There are numerous types of CBTs and some have been recently defined
Newly Described Tumor Types by WHO (2007)Angiocentric GliomaPilomyxoid AstrocytomaPapillary Glioneuronal TumorRosette-forming Glioneuronal Tumor of the 4th Ventricle (RGNT)Papillary Tumor of the Pineal RegionPituicytomaSpindle Cell Oncocytoma

8
Causes of Childhood Brain TumorsTherapeutic X-ray to the head <2%
A small fraction of all childhood tumors.
Genetic diseases <5%Tumor predisposition syndromes such as neurofibromatosis, tuberous sclerosis, nevoid basal cell carcinoma syndrome, Turcot syndrome, Li-Fraumeni syndrome<5% of childhood brain tumors
UNKNOWN >90%
IF WE DON’T LOOK, WE CANNOT LEARN

9
The current research process
Each study is a piece of a 5,000 piece jig-saw puzzle32 studies of childhood ependymomas funded
32 pieces of the puzzle • 1990-2005• 1,444 children• No common protocol
What does the jig-saw picture look like?Impossible to see the picture

10
Challenges to Studies on Causes and Risk Factors for Childhood Brain Tumors
Insufficient number of children to study risk factorsThe causes/risk factors are likely to be multiple with complex interactionsWithout common protocol results cannot be combined
Example: 32 studies of ependymomas Number of children in studies
• Minimum= 11 children ;maximum=92 childrenNo study use same protocolSingle factor studied: prognostic factor
• 32 studies; little to nothing learned!

11
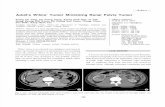
32 Studies: What Is The Picture?

12
The RationaleChildhood Brain Tumor (CBT) is an Orphan Disease with a costly profile.The incidence of the most common type of CBT is increasingThere are new histological types of CBT in the new WHO 2007No single institution can accrue sufficient number of “similar” CBT patients in a reasonable periodCausal associations are not likely to be direct All aspects of CBT needs to be studied together
Genetics, epigenetics, environment, nutrition, pathology, viruses, and clinical

13
Study Design-National Consortium
Regions with sufficient patient population for epidemiological studyStudy the “whole picture” rather than one part at a timeAll relevant disciplines (multiple experts/resources )
Environmental exposure analysis (Is there an environmental cause?)
Nutritional analysis (Is there something in the diet?)
Genetic/Genomic analysis (What role do genes play?)
Epigenetic analysis (What turns genes on or off?)
Pathological evaluation/archival information (Are CBT types changing?)
Guthrie cards/perinatal information (Has the child’s DNA mutated since birth?)
Correlation with clinical treatment groups (What are prognostic factors and which treatment can address the causative events?)

14
CBT Study PlanCase-Control design (1:2 or greater ratio)Regional Consortia: possibly 5 (>2,500 children)
California/NorthwestTexas/SouthwestMidwestFlorida/SoutheastNew York/Northeast
Same procedures and analyses Designated Central Laboratories for specialized testingCentral Data Repository to collect all research data
Available to all researchers whether or not a study investigator
Provisions for consensus reporting

15
Study Coordination
Funding is additional money to NIH budgetConsortia and study coordination by NCI
Awards grants to regional consortiumCoordinates consortiaCoordinates central statistics and data management
Existing NIH Research PriorityPlan and support a multicenter case-control study of the etiology of childhood brain cancer through the Brain Tumor Epidemiology Consortium (BTEC)• NIH Research Plan for Children’s Brain Tumors, 2008

Legislative Strategy for House and Senate
Based on prior experience in passing the Benign Brain Tumor Cancer Registries Amendment Act

17
2009 (111th Congress)
Contact members of House of Representatives
Request they co-sponsor HR 653
Contact member of SenateRequest they co-sponsor S 305
Work with House and Senate Committees
Host briefingsHold meetings

18
2009-2010 (111th Congress)Early January
Introduce House and Senate Bills • DONE
FebruaryMeet with NIH/NCI LeadershipHold Congressional briefings
Push for passage in sub-committeeMeet with sub-committee members and staffersIdentify sub-committee champions
Push for passage in CommitteeMeet with sub-committee members and staffersIdentify sub-committee champions
Push for passage in House and SenateCelebrate

19
Need More Information?Want to Help?
Lloyd Morgan, “Chief Cheerleader”510 528-5302; [email protected]




![[PPT]TUMOR TRAKTUS UROGENITAL - FK UWKS 2012 C | … · Web viewTUMOR TRAKTUS UROGENITAL I. Tumor Ginjal A. Tumor Grawitz B. Tumor Wilms II. Tumor Urotel III. Tumor Testis IV. Karsinoma](https://static.fdocuments.in/doc/165x107/5ade93b87f8b9ad66b8bb718/ppttumor-traktus-urogenital-fk-uwks-2012-c-viewtumor-traktus-urogenital.jpg)






![L. Lloyd Morgan [bilovsky@aol.com]1 Interphone Studies To Date An Examination of Poor Study Design Resulting in an UNDER-ESTIMATION of the Risk of Brain.](https://static.fdocuments.in/doc/165x107/56649e585503460f94b51b68/l-lloyd-morgan-bilovskyaolcom1-interphone-studies-to-date-an-examination.jpg)