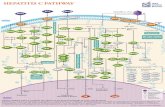
Heterofermentative Pathway Uses part of the pentose phosphate pathway Only one pyruvate is made Have...
-
Upload
ilene-rice -
Category
Documents
-
view
215 -
download
0
description
Transcript of Heterofermentative Pathway Uses part of the pentose phosphate pathway Only one pyruvate is made Have...
Heterofermentative Pathway Uses part of the pentose phosphate pathway Only one pyruvate is made Have a decarboxylation and C-C cleavage to give a C 3 and a C 2 Overview: Activation-use 1 ATP Two oxidations done to make -carbonyl C-C bond cleavage G3P to pyruvate like in Streptococcus Less ATP because more ox/red reactions phosphotransacetylase acetaldehide dehydrogenase alcohol dehydrogenase PGALD dehydrogenase PGA kinase enolase PGA mutase pyruvate kinase lactate dehydrogenase R5P epimerase G6P dehydrogenase 6PG dehydrogenase phosphoketolase hexokinase Glucose Glucose-6-P ADP ATP [6-P-Gluconolactone] 6-P-gluconate Ribulose-5-P CO 2 H 2 O NADPH NADP + NADPH NADP + Xylulose-5-P Lactate NADH NAD + Acetyl-P Acetaldehyde Ethanol Acetate NADP + + NADPH ATP ADP ATP yield used 1 ATP made 2 ATP net yield= 1 ATP Additional oxidation/reduction reactions decrease potential ATP yield. Small amount What would happen if the organism could divert electrons away from ethanol production? Acetyl-CoA CoA P i Glyceraldehyde-3-P 1,3-bisphosphoglycerate 3-phophoglycerate 2-phosphoglycerate phosphoenolpyruvate pyruvate ADP ATP ADP ATP NAD + NADH H 2 O Heterofermentative Pathway in Leuconostoc sp. G6P dehydrogenase 6PG dehydrogenase CO 2 H 2 HCOH PO 3 = HCOH HOCH HC OH HC O O H 2 CO HCOH PO 3 = HCOH HOCH HC O COOH H 2 CO HCOH PO 3 = HCOH HOCH HCOH O COOH H 2 CO HCOH PO 3 = HCOH C O H 2 CO HCOH PO 3 = H 2 COH HCOH C H 2 O NADP + NADPH NADP + Glucose-6-P 6-P-glucono- lactone (enzyme-bound) 6-P-gluconate 3-keto-6-P- gluconate (enzyme-bound) Ribulose-5-P Mechanism of beta-decarboxylation 1) Carbonyl accepts electrons from C-C between the 1 and 2 carbons. 2) Carbon dioxide and an enolate are formed. 3) Re-shifting of the electrons forms the keto sugar O H 2 CO HOCH PO 3 = H 2 COH HCOH C H 2 CO HC PO 3 = HCOH O O-PO 3 = O CH 3 C O-PO 3 = + Phosphoketolase Reaction Xylulose-5-P Acetyl-Phosphate Glyceraldehyde-3-P E-TPP- CH 2 OH E-TPP C CH 2 OH O-PO 3 = Acetyl-Phosphate Enzyme contains Thiamin pyrophosphate (TPP) as cofactor the function here is transketolation. Conclusion Heterofermentative organisms use a pathway with a greater number of redox reactions than Streptococcus. Make very oxidized and very reduced compounds. More NAD(P)H to be reoxidized constrains ATP synthesis, high energy intermediate used as an electron acceptor. Vitamins: essential portions of cofactors that organism can not make de novo Fermentation Analysis In order to understand how an organism makes its energy or what biochemical pathways are present, one must first know what the products of metabolism are. First Law of Thermodynamics: mass is conserved must account for all of the carbon and electrons originally present in the substrate. Fermentation analysis From this, we can then figure out the pathways and amount of ATP made. Also, inspection of the products will allow us to make predictions about the cells metabolism. Initially, we will look at glucose consumption in rich medium Growth factors from media supply cell carbon Most of glucose goes to products, only 5-10% incorporated into cells. In industry, one must also account for cell mass. Experimental set up Take time zero and time final samples and measure Glucose and product formation. Example: Leuconostoc brevis Compd.Amount (mmol) # of Csmmol of C Glucose Lactate Glycerol Ethanol Acetate CO Have we detected all of the products? carbon Calculate the carbon recovery by multiplying the amount detected by the number of carbon atoms for each compound, then sum up all of the carbon in the products. Carbon in glucose = 6 X 100 mmoles =600 mmoles Carbon in products = ( ) mmoles Carbon in products = mmoles % C recovery = (584.7 mmol/600 mmol) * 100 % C recovery = 97.4% Have detected all of the electrons? In a fermentation, electrons removed from glucose are added back to a compound derived from glucose. Thus, the ratio of oxidized products to reduced products must equal 1. Since glucose (C 6 H 12 O 6 ) has 2 Hs for every O, products with more than 2 Hs per O have been reduced, and products with less than 2 Hs per O have been oxidized. OR value of a compound To calculate the OR value of a compound, give a numerical score of +1 for every O and -1 for every 2 Hs. Examples: Glucose (C 6 H 12 O 6 ): 6O is +6, 12 H's is -6, 6-6=0 Lactate (C 3 H 6 O 3 ): 3O is +3, 6H's is -3, 3-3=0 Acetate (C 2 H 4 O 2 ): 2O is +2, 4H's is -2, 2-2=0 Glycerol (C 3 H 8 O 3 ): 3O is +3, 8 H's is -4, 3-4 = -1 Ethanol (C 2 H 6 O): 1O is +1, 6 H is -3, 1-3= -2 Carbon dioxide (CO 2 ): 2 O's = +2 Example: Leuconostoc brevis CompdAmount (mmol) OR value mmol (ox) mmol (red) Glucose Lactate Glycerol Ethanol Acetate CO O/R ratio of the fermentation Once the OR value of the compound is determined this is multiplied by the amount detected (see Table) Calculate the O/R ratio OR ratio = |178.6/(-171.8)+(-6.8)| OR ratio = 178.6/178.6 = 1.0 Ratios close to 1 mean all of the electrons have been accounted for. C 1 to C 2 ratio A common C-C cleavage reaction is C 3 --> C 1 + C 2 usually indicating pyruvate is an intermediate. If this occurs in your organism, then expect a C 1 /C 2 ratio of 1. C 1 = 89.3 mmoles C 2 = 85.9 mmoles mmoles = 93.2 mmoles C 1 to C 2 ratio = 89.3 mmoles/ 93.2 mmoles C 1 to C 2 ratio = 0.96 Value is close to one so probably have pyruvate cleavage. Conclusion Fermentation balance is the first step in understanding the metabolism of an organism Must have C recovery close to 100% and an O/R ratio close to 1. C 1 /C 2 ratio indicates pyruvate cleavage You can use the above information in the lab to determine what analyses are needed to complete the balance. What happens if an alternate electron acceptor is present in a fermentation? Electron flow dictates carbon flow and energy yield Alternate electron acceptors provide fermentative bacteria a choice The result will be less lactate and ethanol and more acetate and ATP are made. We will study the effect of oxygen on the metabolism of lactic acid bacteria De Felipe et al., J. Bacteriol. vol 180, p 3804, 1998 Utilization of oxygen by facultative lactic acid bacteria. Some lactic acid bacteria possess enzymes that reoxidize NADH (and NADPH) by reducing oxygen to water (Dolins enzymes) Oxidase NAD(P)H + H + + O 2 --> H 2 O 2 + NAD(P) + Peroxidase NAD(P)H + H + + H 2 O 2 --> 2 H 2 O + NAD(P) + What happens when oxygen is present? When oxygen and Dolins enzymes are present, NAD(P)H is reoxidized by reducing oxygen to water rather than pyruvate to lactate or acetyl-P to ethanol. More acetate and ATP, less ethanol and lactate, are made. Make more ATP Acetate kinase acetyl-P + ADP --> acetate + ATP For every acetate made, one ATP is made by substrate- level phosphorylation by this reaction. When Dolins enzymes and oxygen are present, 1) acetyl-P goes to acetate and ATP rather than to ethanol, and 2) pyruvate is metabolized to acetate and CO 2 rather than to lactate. Streptococcus sp. and Dolins enzymes Summary If there is an alternate electron acceptor, less lactate, more acetate, CO 2, and ATP Bifidobacterium sp. Bifid or 2 lobes Gram positive, curved rod found in the feces of breast-fed infants, Requires many growth factors, including N-acetylglucosamine Makes 2 lactate and 3 acetate from 2 glucose Makes high-energy intermediate by phosphoketolase reaction rather than ox/red. Schell, M. A. et. al. (2002). The genome sequence of Bifidobecterium longum reflects its adaptation to the human gastrointestinal tract. PNAS V99 N22 p Outline of pathway Activation: 2 glucose to 2 glucose-6-P uses 2 ATP Make 2 G3P and 3 acetyl-P from 2 glucose by transketolase, transaldolase, and phsophoketolase reactions 2 G3P to 2 lactate by reactions seen in Streptococcus 3 acetyl-P to 3 acetate and 3 ATP by acetate kinase Synthesis of 2 G-3-P and 3 C2 units from 2 glucose. Uses these enzymes to interconvert hexoses and pentoses. C C C C C C O C C C C CH 3 CO O-PO 3 P i Phosphoketolase: C 6 (or C 5 ) + P i -> C 4 (or C 3 ) + acetyl-P C C C C C C O C C C C + C C C O C C C C C C C + Transaldolase: C 6 + C 4 -> C 7 + C 3 C C C O C C C C C C C + C C C C C C C C C C + O O Transketolase C 7 + C 3 -> C 5 + C 5 Summary Make acetyl-P by phosphoketolase rather than by ox/red reaction Dont have to use acetyl-P as electron acceptor ATP yield higher than other anaerobes for this reason. Avoidance of ox/red leads to higher ATP gain.