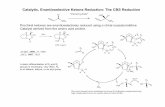
Harmful Gas reduction catalytic
-
Upload
shashank-yadav -
Category
Documents
-
view
218 -
download
0
Transcript of Harmful Gas reduction catalytic
-
8/11/2019 Harmful Gas reduction catalytic
1/24
http://localhost/var/www/apps/conversion/tmp/scratch_1/dx.doi.org/10.1016/j.jphotochemrev.2012.08.002mailto:[email protected]://www.elsevier.com/locate/jphotochemrevhttp://www.sciencedirect.com/science/journal/13895567http://localhost/var/www/apps/conversion/tmp/scratch_1/dx.doi.org/10.1016/j.jphotochemrev.2012.08.002 -
8/11/2019 Harmful Gas reduction catalytic
2/24
-
8/11/2019 Harmful Gas reduction catalytic
3/24
-
8/11/2019 Harmful Gas reduction catalytic
4/24
32 J. Lasek et al. / Journal of Photochemistry and PhotobiologyC: Photochemistry Reviews 14 (2013) 2952
Fig. 1. Themethods of NO x removal.
NO can be oxidized by injecting ozone into exhaust gas. The NOoxidation rate is very fast in this method because O 3 decomposes
into O 2 and a highly reactive O radical [43] . The created NO 2 canbe subsequently reduced to N 2 by Na 2 SO3 or Na 2 S. An examplereaction of this process is shown below:
2NO2 + Na2 S N2 + Na2 SO4 (2.13)
The deNO x process can be carried out simultaneously with thede-SO 2 process. Using this combined process, 95% and 100% ef-ciencies were achieved forthe removal of NO x andSO 2 , respectively[44] .
3. Photocatalytic-NO x removal
Photocatalysis is an innovative andpromising technique for theremoval of poisonous or undesirable compounds such as volatileorganiccompounds [45,46] ornitrogenoxides.Thissectionisthere-fore focused on methods of photo-NO x removal. NO removal byphotocatalyticreactions is described, as arethe types of photocata-lysts used for these processes and the applications of photo-deNO xreactionsin real cases.Finally,the futureof photo-deNO x processesis also discussed.
Three methods for NO x removal by photocatalysis have beenpresented in the literature: photo-decomposition, photo-oxidationand photo-SCR. These methods have advantages and disadvan-tages that will be described in detail in the following sections.Photo-decomposition andphoto-SCR belong to reductionmethods.
The main aim of these deNO x processes is thus transformation of this pollutant into N 2 and other harmless compounds. The photo-oxidation of NO x will lead to nitric acid formation, which must beremoved from the photocatalyst surface. The removal of NO byphotocatalysis was rst reported by Pichat et al. [47] and Cour-bon and Pichat [48] . They investigated not only NO decompositionon TiO 2 (Degussa P25) under UV light irradiation but also butanoloxidation using NO as oxidant (i.e., photo-SCR). The researchersobserved transformation of NO into N 2 and N 2 O. Later, Yokomichiet al. [49] proposed a photocatalyst based on zeolite Cu-ZSM-5as an active material for NO x decomposition. Many past stud-ies attempted to develop or improve photocatalytic techniquesincreased NO x removal efciency.
In thefollowing sections,the summaryof photo-decomposition,
photo-oxidation and photo-SCR are listed in Tables 13 .
The
efciency of NO abatement (second column from the right in thetables) was calculated from the equation:
Eff = 1 NO xafterNO xbefore
100% (3.0)
where NO xbefore represents the NO molar fraction measured beforethe photoreactor (inlet) and NO xafter is the NO molar fraction mea-sured in the outlet of the photoreactor.
3.1. Photo-selective catalytic reduction
Photo-selective catalytic reduction (photo-SCR) is a very attrac-tive way to remove NO x because it allows the transformation of pollutants into harmless gaseous compounds, such as N 2 , withouthigh temperatures. This process occurs on a photocatalyst surfaceand involves the reduction of NO x in the presence of a reducingagent under light irradiation. NH 3 or hydrocarbons are typicallyselected as reducing agents, with the choice dependent on the pro-cess conditions. Photo-SCR by hydrocarbons or carbon monoxideis preferred because the use of NH 3 as a reducing agent can berisky due to secondary emission. In this case, an accurate materialbalance must be determined. Nevertheless, application of photo-SCR with hydrocarbons or CO in real scenarios is not easy. One of the major difculties is the inuence of water and oxygen, whichwill be discussed later. The oxidation of hydrocarbons into CO 2mustbe considered. Another problem is thatundesired compounds(e.g.,N 2 O) canappear as intermediateby-products.Fortunately,thereduction of N 2 O photocatalytically is possible and examples havebeen described in the literature [5053] .
Table 1 summarizes photo-SCR and includes basic informa-tion such as photocatalyst used, conditions, and common reducing
agents. Thecolumn Max. eff. or selectivitycontainsmainly deNO xefciency with selectivity appearing in brackets.
3.1.1. Reduction mechanism in the presence of CO or hydrocarbons
The mechanisms of photo-SCR are not well known and remainunder intense investigation. Bowering et al. [58] proposed a mech-anism for NO reductionon pure TiO 2 (Degussa P25) in the presenceof CO. The main reactions occurring on the photocatalyst surfaceare:
O(ads) + CO(ads) CO2(g) (3.1)
2NO (ads) + CO(ads) N2 O(ads) + CO2(ads) (3.2)
N2 O(ads) + CO(ads) N2(g) + CO2(ads) (3.3)
-
8/11/2019 Harmful Gas reduction catalytic
5/24
-
8/11/2019 Harmful Gas reduction catalytic
6/24
-
8/11/2019 Harmful Gas reduction catalytic
7/24
-
8/11/2019 Harmful Gas reduction catalytic
8/24
-
8/11/2019 Harmful Gas reduction catalytic
9/24
-
8/11/2019 Harmful Gas reduction catalytic
10/24
-
8/11/2019 Harmful Gas reduction catalytic
11/24
J. Lasek et al. / Journal of Photochemistry and Photobiology C: Photochemistry Reviews 14 (2013) 2952 39
N(ads) + CO NCO(ads) (3.4)
Eq. (3.3) is desired as the last step of NO photo-reduction in thepresence of CO.
The mechanism of photo-SCR using CO as a reducing gas overother photocatalysts was described in the literature. Anpo et al.[69] proposed NO reduction in the presence of CO on a Mo-MCM-41 surface. They also concluded that the presence of this reducingagent strongly enhanced NO reduction. Roy et al. [55] presentedanother mechanism for NO photo-reduction on Pd ion-substitutednano-TiO 2 (Ti1 xPd xO2 , where x= 0.050.3). Bowering et al. [57]explained the function of silver loading on P-25 Degussa for thedecomposition and reductionof NO in thepresenceof CO under UVlight irradiation. Thampi et al. [61] proposed photo-NO reductionover TiO 2 modied by Ru, Rh and Cu. In general, loading of metalson the TiO 2 surface inuenced the by-products (e.g., N 2 O) formedand the N 2 selectivity.
The mechanism of photo-SCR of NO in the presence of hydro-carbons is still poorly understood. Stoichiometric mass balancereactions were proposed for the photo-SCR of NO in the presenceof propane [60] :
10NO + C3 H8 5N2 + 3CO2 + 4H2 O (3.5)
12NO + C3 H8 2N2 O + 3CO2 + 4H2 O + 4 N2 (3.6)Some researchers have not shown a detailed mechanism but
have giveninformation on by-products and possible reaction path-ways. For example, Matsuoka et al. [59] explained that one of the products of NO reduction on a vanadium silicate-1 surfacein the presence of propane under UV irradiation was CH3 CHO. Inanother photocatalyst system (Mo-MCM-41) presented by Anpoet al. [69] , CH3 COCH3 was created under UV irradiation. In thiscase, the following reaction mechanism was proposed: the inter-mediate species formed between NO and the hydrocarbon radicalsthat are formed by H abstraction of the photo-excited Mo-oxidespecies from C 3 H7 subsequently reacts with NO to produce N 2as well as oxygen-containing compounds. It is also worth not-ing that propane was noticed to be a reactive reducing agent forphoto-reduction of NO over different photocatalysts (e.g., Pd/TiO 2[60] , TiO2 Hombine N [54] , VS-1, and Mo-MCM-41 [69] ). Fur-thermore, Takeuchi et al. [70] investigated photo-reactions in aC4 H8 NO2 air system and concluded that one of the by-productsof photo-reaction over ZnO under UV irradiation was HCN. Addi-tional studies were subsequently reported by Takeuchi and Ibusuki[71] , where many metal oxides (CeO 2 , CoO, Cr2 O3 , Fe2 O3 , NiO,SnO2 , TiO2 , WO3 , ZnO, and ZrO 2 ) were tested as photocatalystsunder UV irradiation. Acetaldehyde (CH 3 CHO) was observed as oneof the major products (besides CO and CO 2 ). In addition, cyanocompounds (e.g., HCN and CH 3 CN) were also observed. The gen-eration of these compounds was explained by the interaction of NO or atomic nitrogen adsorbed on the photocatalyst surface. Thecyanides were most likely produced via Eqs. (3.7) and (3.8). Theresearchers alsoobservedthat theless active photocatalysts(Cr 2 O3 ,CoO, NiO) led to greater HCN generation.
CH3 + N(NO) ads HCN + otherproducts (3.7)
CH3 + HCN CH3 CN + otherproducts (3.8)
The above mechanism of NO+ CO reaction was determined ontheassumptionof absence of water andoxygen. Thus,investigationof the impact of oxidizing compounds (e.g., H 2 O and O 2 ) on photo-SCR is necessary. Water vapor, as one of the main products of fuelcombustion, exists in most exhaust gases. It should be noted thatthere are still relatively few published studies on water-assistedphoto-SCR; therefore, the particular mechanism of photo-SCR inthe presence of water and oxygen requires further investigation.
Section 5.2 briey mentions that the presence of water or/and
Fig.2. Scheme of themechanism of photo-SCRover NH 3 onTiO 2 surface [66] (n eeds
permission; directly cited).
oxygen inuences photo-SCR through the transformation of nitricoxide (NO) into NO 2 , whichis undesirable.We suggest thatincreas-ing temperature can inhibit this undesired reaction [54,63] .
3.1.2. Reduction mechanism in the presence of NH 3The overall reaction of photo-SCR with NH 3 is the same as that
of thermal SCR (Eq. (2.1) ). Teramura et al. [66] presented the mech-anism of photo-SCR in thepresence of NH 3 over TiO 2 photocatalystshown in Fig. 2. They also determined the kinetic parameters andproposed the formula in Eq. 3.9, where I represents light intensity.
r = kC NOC NH3
C O2 I (3.9)
They underscored that the intermediate product NH 2 NOcanbeeasilydecomposed by heattreatment. An important supplement tothis study would be the mechanism of NH 3 photo-oxidation in thepresence of oxygen over anatase and rutile TiO 2 .
3.1.3. Photocatalysts for photo-SCR in the presence CO or hydrocarbons
Improving photo-SCR is mainly performed by enhancing thephotocatalyst activity. Usually, the activity of a simple photocat-alyst is rst determined and then the photocatalyst is modied.Bowering et al. [58] investigated the reduction of NO over DegussaP25 in the presence of carbon monoxide. Later, the same researchgroup modied P25 with silver loading [57] and used CO as the
reducing agent. The authors observed the rst strong enhancementin N 2 selectivity (100%) for a TiO 2 -based photocatalyst withoutusing NH 3 as the reducing agent. Thampi et al. [61] modied theP-25 photocatalyst by loading Ru, Rh and Cu. Instead of photo-reaction under UV irradiation, they also investigated thermal SCR from 90 to 240 C. Unfortunately, N 2 O was observed as the majorby-product. However, the presence of Rh and Cu increased thedecomposition of N 2 O. Additionally, Su and Wu [60] observedexcellent NO photo-reduction efciency over Pd/TiO 2 in the pres-ence of propane under UV light irradiation. The efciency droppedwhenPdwas transformed intoPdO during thephoto-process (espe-ciallywhenNOandO 2 werepresent). Thus,periodic regenerationof thephotocatalyst (500 C,2hatH 2 presence) periodically is neededfor high efciency. Furthermore, Roy et al. [55] observed very good
activity for a modied Pd/TiO 2 (by impregnation) photocatalyst for
-
8/11/2019 Harmful Gas reduction catalytic
12/24
40 J. Lasek et al. / Journal of Photochemistry and PhotobiologyC: Photochemistry Reviews 14 (2013) 2952
NO abatement in the presence of carbon monoxide. They also pre-paredPd ion-substituted nano-TiO 2 (Ti1 xPd xO2 ) andthe highestactivity was observed with 1% Pd. Finally, Matsuoka et al. [59] useda vanadium silicalite-1 photocatalyst (VS-1) to run a deNO reac-tion in the presence of propane under UV irradiation. Good activitywas achieved by incorporation of VS-1 in a zeolite framework.The photocatalyst existed as isolated tetrahedrally coordinatedV-O moieties on the zeolite surface. Therefore, enhancement of photo-deNO efciency was achieved for V-containing and Mo-MCM-41 photocatalysts [72] in the presence of propane. However,the enhancement was not observed in the case of a Ti-MCM-41photocatalyst.
3.1.4. Photocatalysts for photo-SCR in the presence of NH 3A similar tendency, comparing to photo-SCR at C yHn presence,
was observed for photo-SCR in the presence of NH 3 . The deNO xefciency was improved upon optimization of the photocatalysts.These optimization studies began using TiO 2 without additives.Teramura et al. [66,73] used commercial TiO 2 (P-25 and JRC-TIO-4) as photocatalysts and observed that the presence of oxygen inthe gas mixture prevented N 2 O formation during photo-reaction.Cant and Cole [68] used TiO 2 pressed wafers (85% anatase, 15%
rutile) under UV light irradiation (photo-reaction at 30
C) andat a higher temperature (300 C) for thermal SCR. Yamazoe et al.[64,65] tested multiple metal oxides, including V, Cr, Mn, Fe, Co, Ni,Cu, Zn, Y, Zr, Nb, Mo, Ta and W, as supporting materials for TiO 2 ,but positive results were only obtained for Nb, Mo and W oxides.The best activity (92% NO conversion at GHSV= 16,000h 1 ) andthe highest N 2 selectivity (99%) were achieved for 1% WO 3 /TiO2 .The enhancement in photo-activity was explained by creation of isolated, tetrahedrally coordinated tungsten sites over the TiO 2 .Furthermore, Jinet al. [67] prepared anSi-dopedTiO 2 photocatalystby thehydrothermal methodandwovenglassfabricwas usedasthesupporting material. A 50% enhancement in photo-deNO activitywas achieved compared to Degussa P25 under UV light irradiationwith3% O 2 at50 C.
3.2. Photo-oxidation
The aim of photo-oxidation is to transform NO into HNO 3 viathe formation of HNO 2 and NO 2 . The concept is the same as thatfor traditional NO x absorption. The main advantage of this pro-cess is the higher possibility of NO conversion into NO 2 than intoHNO3 because the atmosphere contains considerable amounts of oxygen (e.g., exhaust gas 5%vs.air 21%). However,during photo-oxidation of NO, saturation of the photocatalyst surface by HNO 3will occur, requiring consistent catalyst regeneration. Additionally,photo-oxidation may lead to HNO 3 creation, the storage or utiliza-tion of which can be troublesome.
Photo-oxidation of NO provides a way to remove NO x emitted
from stationary power plant sources or vehicle engines. Table 2presents results cited in the literature. The table is divided intothe columns photocatalyst (short explanation), conditions (light,composition of gas mixture, temperature), detected compounds,main products of reaction and obtained maximum efciency. Morespecic information can be found in the literature (last column).
3.2.1. Oxidation mechanismThe NO photo-oxidation mechanism for TiO 2 photocata-
lysts undergoes three states (NO HNO2 NO2 HNO3 ) and isdescribed by the reactions shown in Eqs. 3.10-3.18 [82,83,98,99] .Wang et al. and Dalton et al. proposed additional reactions for themechanism, as shown in Eqs. (3.19)(3.22) [90,99]
TiO2
+h
eCB
+ hVB
+ (3.10)
Fig. 3. A possible mechanism of NO adsorption on TiO 2 [112] .
hVB+ + H2 Oads HOads + H+ (3.11)
hVB+ + HOads HOads (3.12)
eCB + O2ads O2ads (3.13)
NO + HOads HNO2 (3.14)
HNO2 + HOads NO2 + H2 O (3.15)
NO2 + HOads HNO3 (3.16)
NO2 + HOads HNO3 (3.17)
NO + O2ads NO3 (3.18)
HNO3 H+ + NO2 (3.19)
2NO + O2 + 3e 2NO2 (3.20)
HNO3 H+ + NO3 (3.21)
3NO2 + 2OH 2NO3 + NO + H2 O (3.22)
Eqs. (3.10)(3.13) represent typicalphotocatalytic reactionsinclud-ing charge carrier generation (Eq. (3.10) ) and the trapping of a holeand an electron to produce active hydroxyl and oxygen radicals(Eqs. (3.11)(3.13) ). The other equations represent oxidation reac-tions by hydroxyl radicals (Eqs. (3.14)(3.17) ) and oxygen radicals(Eq. (3.18) ). Additionally, Dalton [90] suggested that deNO x cycle
must be closed by HNO 3 removal (water treatment) from the pho-tocatalyst surface via the reaction in Eq. (3.23) .
HNO3ads HNO3aq (3.23)
Wu and Cheng [112] carried out detailed analysis of photocat-alytic reactions of NO on TiO 2 and transition metal-loaded M(Cu,V, and Cr)/TiO 2 using in situ FTIR spectroscopy. A possible mech-anism for NO adsorption and oxidation on the TiO 2 surface isshown in Figs. 3 and 4 . Initially, NO is adsorbed on the TiO 2 sur-face and then attacks free surface OH groups, which are oxidizedby active surface oxygen, creatingmonodentateNOHspecies.Theseare subsequently transformed into bidentate nitrite and hydroper-oxo (TiOOH) species. As shown in Fig. 4, electronhole pairs arethenphoto-generated under UV irradiation andthe peroxo (Ti(O 2 ))
is transformed into superoxo (TiOO
) by hole trapping. Because
-
8/11/2019 Harmful Gas reduction catalytic
13/24
J. Lasek et al. / Journal of Photochemistry and Photobiology C: Photochemistry Reviews 14 (2013) 2952 41
Fig. 4. A possible mechanism of NO oxidation on TiO 2 [112] .
superoxo (TiOO ) is extremely reactive with very short lifetime, itoxidizes bidentate nitrite into monodentate or bidentate nitrate.If superoxo does not react with nitrites, it transforms into oxy-gen molecules. Wu and Cheng [112] emphasized the importantrole of the OH group and oxygen vacancies in photocatalytic reac-tions. They also proposed a mechanism of photo-NO oxidation ontransition metal-loaded M(Cu, V, and Cr)/TiO 2 . However, the mostpowerful activity was observed for pure TiO 2 [112] .
Recently, Hunger et al. [78] and Ballari et al. [77] proposedkinetic models for photo-removal of NO x underUV-A irradiation inconcrete pavement containing TiO 2 . The kinetic parameters weredetermined as a function of NO and NO 2 concentration, ow rate,humidity, and UV-A irradiance. The external mass transfer of thegaseous compounds and the internal molecular processes (diffu-sion, reaction) were also taken into account. The models wereevaluated experimentally and model predictions showed goodagreement with experimental results. Yu et al. [113] performeda kinetic study of photo-NO oxidation under visible light irradia-tion over commercial carbon-doped TiO 2 (Kronos, Germany). Themodel included the process parameters of volumetric ow rate of the pollutant, relative humidity, irradiance, pollutant concentra-tion, reactor size and dose of the photocatalyst.
An important issue for effective photo-oxidation of NO ismaintenanceof process parameters (e.g., lightintensity,lightwave-
length, residence time of NO x in reaction zone, humidity and typeof catalyst) at optimum levels. NO 2 (intermediate) can easily bereleased from the TiO 2 surface to the atmosphere if the processparameters are not maintained at a proper level. According to theliterature, the relative humidity seems to be the most importantfactor, as a high concentration of hydroxyl radicals contributes toeasier NO 2 transformation into HNO 3 . The mechanism of photo-oxidation of NO to NO 3 via NO2 creation was recently conrmedby Folli et al. [114] , who underscored the role of water in photo-oxidation on nano-sized TiO 2 (100% anatase). Hydroxyl radicals(OH ) play an important role in photo-reactions are created via theoverall reaction:
H2 O + 12 O2 2OH (3.24)
Hence, the photo-oxidation of NO depends strongly on thepresence of water in thereaction. Thestrong dependency of photo-oxidationefciency on relative humiditywas alsoobserved by Chinet al. [115] .
Very recently Wu et al. [116] revealed the role of PtO andPtO2 deposits in the photocatalytic oxidation of NO over a Pt-modied TiO 2 catalyst. A portion of PtO 2 on the surface of thePt/TiO 2 was observed by in situ XPS to be reduced to PtO underUV irradiation. Thus, the PtO 2 was an electron trap. Photocatalyticactivity was improved as a result. The photocatalytic reaction wastested in a continuous ow photoreactor under UV light irradia-tion ( = 300400nm, 125W Hg Philips lamp) with a gas mixturecontaining 100ppmv NO in air (relative humidity 75%). PtO2particles dispersed on the TiO 2 surface were populated with the
electrons generated during the photo-reaction. As a result, PtO 2
was transformed into PtO and O , which could be oxidized intomolecular oxygen via the reactions:
PtO2 + eCB PtO + O (3.25)
2O + 2h VB+ O2 (3.26)
Palladium sites contributed to the adsorption of NO. Accordingto the authors explanation, formation of Pt 0 -NOnitrosyls and Pt n+-NOmononitrosyls was possible.The irradiation of thissysteminthe
presence of OH and O 2 led to the preferential oxidationof nitro-syls to nitro compounds. Based on this explanation,the mechanismof NO photo-oxidation on Pt/TiO 2 was proposed as follows:
Ptn+ + NOadsorpt ion on TiO 2 Ptn+ -nitrosyls
OHandO 2
groups Nitrocompounds (3.27)
3.2.2. PhotocatalystsMany investigators have tried to improve photo-NO x oxidation
by increasing the efciency of the deNO x process. The main aim of the photo-oxidation of NO x is to apply the technique to real sys-tems (e.g., industrial or other commercial systems). Efforts havefocused on improving the photocatalyst, the experimental condi-tions (e.g., type of light, light intensity, gas mixture composition,andresidence time) andthe type of reactor. Many researchers usedcommercial TiO 2 (e.g., Degussa P25) as a reference photocatalystagainst improved photocatalysts. For example, Ao and Lee [95] andAo et al. [94] used commercial Degussa P25 and TiO 2 immobi-lized on an activated carbon lter. They elucidated the inuence of humidity, residence time and VOCs on the efciency of the photo-deNO x process. They then focused on the simultaneous removalof NO x and VOCs (benzene, toluene, ethylbenzene and o-xylene)at various humidities. Toma et al. [96,97,100103] tried differentplasma and thermal spraying processes for increasing the removalefciencyof NO x. Thehighest deNO x efciency was 52% (calculatedfor NO) using a photocatalyst prepared as a ne particle coat-ing. Plasma techniques (e.g., atmospheric plasma spraying (APS) orsuspension plasma spraying (SPS)) and high-velocity oxygen fuelspray (HVOF) were also used [103] . Furthermore, TiO 2 was mixedwithAl tocreate a TiO 2 Al composite coating, which was expectedto improve the charge separation and enhance the photocatalyticactivity [100] .
Signicant improvement of pollutant removal was observed fora TiO2 /C photocatalyst. Hashimoto et al. [117] investigated theinuence of calcination temperature, crystal size and specic sur-face area on photo-NO oxidation efciency of TiO 2 . Decreasingcalcination temperature or crystal size and increasing specic sur-face area improved activity. This result was reasonable becausehigher calcination temperatures lead to increased crystal size.The researchers also determined the qualitative and quantitativedependence of this phenomenon. In addition, superior activity wasobtained when TiO 2 was loaded with palladium [8183] . Wuet al.and Sheng et al. improved not only the NO conversion (72% higherthan P25) but also decreased deactivation of the photocatalyst in
comparison to commercial TiO 2 . The investigators explained thisresult with charge trapping on the Pd surface. The proposed mech-anism for NO oxidation on TiO 2 /Pd is shown in Fig. 5.
Hashimoto et al. [104] mixed (mechanically) HyCOM(hydrothermal crystallization in organic media) TiO 2 with Y-and A-type zeolites. They concluded that for a mixing ratio of A zeolite:TiO 2 = 3.7, the activity of NO x photo-oxidation wasenhanced 3 fold in comparison to bare TiO 2 . In addition, Ballariet al. [77] tested commercial concrete paving stones containingTiO2 . The experiments were carried out using a laminar ow pho-toreactor under UV-A (345 nm) irradiation. They then proposed akinetic model for photo-NO x oxidation and determined the kineticcoefcients for these chemical reactions.
The big challenge for researchers is improving the visible-
light response of photocatalysts used for NO x removal. Yin et al.
-
8/11/2019 Harmful Gas reduction catalytic
14/24
42 J. Lasek et al. / Journal of Photochemistry and PhotobiologyC: Photochemistry Reviews 14 (2013) 2952
Fig. 5. Proposed mechanism of photocatalytic oxidation of NO over Pd 2+ modiedTiO2 . (1) band gap excitation (e ( , h +) under UV illumination, (2) trapping of e byPd4+ ions, (3) trapping of h + at photocatalytic surface, (4) reduction of Pd 4+ toPd 2+ ,(5)oxidation of H 2 OtoOH ( , (6) oxidation ofNO toNO 2 , HNO2 andNO 3 [83] (needspermission; directly cited).
[75,85,86] attempted to obtain visible responses for photocatalystsby doping TiO 2 with nitrogen and iron and platinum. The reac-tions [85] were carried out under visible light irradiation usingan LED light source (red 627nm, green 530nm, blue 445nm)or a high-pressure mercury lamp with lters (cutting
-
8/11/2019 Harmful Gas reduction catalytic
15/24
J. Lasek et al. / Journal of Photochemistry and Photobiology C: Photochemistry Reviews 14 (2013) 2952 43
Fig. 7. Scheme of annular diffusion scrubber used by Komazaki et al. [91] . Speci-cation of the reactor and experimental conditions: Outer tube: Pyrex-glass tubecoated with TiO 2 and hydroxyapatite (21mm i.d., 25mm o.d.); Inner tube: Quartztube (16.5mm i.d., 19.5mm o.d.); UV Blacklight (FL8BL, 8 W, NEC, Japan), Effectivelength: 25cm; Air ow rate: 0.25.0 l/min (needs permission; directly cited).
Toma et al. [94,96,97,100103] designed an experimental setupwhere the NO x mixture was prepared in situ by chemical reactionbetween copper powder and nitric acid. The researchers also cre-ateda neural computational model to predict experimental results.The input data were the photocatalyst powder mass, the exposuretime andthe exposed surface area. The output results were NO andNO x removal efciencies [97] . Wu and Cheng [112] used a special
reactor for in situ FTIR investigation of the reactions occurring onthe photocatalyst surface in photo-NO oxidation. The concentra-tions of nitrogen compounds on TiO 2 and transition metal-loadedM(Cu, V, and Cr)/TiO 2 were measured under dynamic conditions.This system can also be utilized for investigating photo-processesat high temperature. The scheme of this reactor setup is shown inFig. 8.
3.3. Photo-decomposition
The assumptionfor photo-decomposition of NO x is thatthe pro-cess occurs via reaction on the photocatalyst surface (Eq. (3.28) ).The development of a photocatalyst for NO x photo-decompositionis focused on increasing the activity and selectivity towards
N2 production. Table 3 details selected studies on the photo-decompositionof NO x.MostoftheresultshavebeendonebyAnposresearch group. More information about their investigations can befound in literature [69,118120] and reference therein.
2NO N2 + O2 (3.28)
3.3.1. Decomposition mechanismPossible mechanisms for NO decomposition on TiO 2 (Degussa
P25) are listed in Eqs. (3.25)(3.32) [58] . The dominant reaction isEq. (3.31) , where N 2 O is generated as the major product.
TiO2 + h eCB + hVB+ (3.29)
NO(ads) + eCB N(ads) + O(ads) (3.30)
NO(ads) + N(ads) N2 O(ads) (3.31)
Fig.8. Schemeof photoreactorusedforin situ FTIR investigationsby Wuand Cheng[112,187] (needs permission, directly cited).
NO(ads) + O(ads) NO2(ads) (3.32)
2O (ads) O2(ads) (3.33)
2 N (ads) N2(g) (3.34)
2NO (ads) N2(g) + O2(g) (3.35)
N2 O(ads) N2(g) + O2(ads) (3.36)
Courbon andPichat [48] andCantand Cole [68] proposed similar
mechanisms for NO photo-decomposition on TiO 2 :TiO2 + h eCB + hVB+ (3.37)
O2 (cus) + hVB+ O (cus) (3.38)
NO(ads) + eCB NO (ads) (3.39)
NO (ads) + O (cus) N(ads) + O(ads) + O2 (ads) (3.40)
where the abbreviation cus is coordinatively unsaturated oxideions. Gaseous compounds, such as N 2 , O2 ,andN 2 O, are formed viathe following reactions:
N(ads) + N(ads) N2(ads) N2(gas) (3.41)
N(ads) + NO(ads) N2 O(ads) N2 O(gas) (3.42)
O(ads) + O(ads) O2(ads) O2(gas) (3.43)
The nitrates are formed via the following reaction:
NO(ads) + O(ads) NO2(ads) nitrates (3.44)
The rate of the reaction in Eq. (3.44) increases in the presence of high concentrations of gaseous oxidant compounds (O 2 , H2 O). Thisphenomenon plays a more important role in photo-decompositionprocesses than photo-oxidation processes (see Section 3.2 ).
NO can also be transformed into N 2 O or NO 2 depending onthe reaction conditions (e.g., pressure, photocatalyst, light wave-length and intensity). Anpo and Takeuchi et al. [69,120] observedstrong dependence for the coordination number of the Ti-oxidespecies and N 2 formation selectivity. TiO 2 can exist as highly dis-
persed and isolated tetrahedral species (e.g., Ti-oxide/Y-zeolite
-
8/11/2019 Harmful Gas reduction catalytic
16/24
44 J. Lasek et al. / Journal of Photochemistry and PhotobiologyC: Photochemistry Reviews 14 (2013) 2952
Fig. 9. Relationship between the coordination number of Ti-oxide species and theselectivity for N 2 formation in the photocatalytic decomposition of NO on varioustitanium oxide photocatalysts [69] (needs permission; directly cited).
photocatalysts prepared by ion exchange techniques) or as aggre-gatedoctahedrallycoordinated Ti-oxidespecies (Ti-oxide/Y-zeolitephotocatalysts prepared by impregnation). High efciency andselectivity are obtained for Ti-oxide species with lower coordina-tion number. The relationship between coordination number andselectivity forN 2 formationin NO decomposition is shown in Fig. 9.Additionally, titanium oxide species are excited by UV irradiationduring photocatalytic reactions (Eq. (3.45) ) to form exited com-plexes (Ti 3+ -O )*:
(Ti4+ -O2 )h
h
(Ti3+ -O )
(3.45)
A tetrahedrally coordinated Ti oxide was conrmed by X-rayabsorption near edge structure (XANES) and extended X-rayabsorption ne structure (EXAFS) analysis technique. The behaviorof the tetrahedrally coordinated Ti-oxide could be explainedby themechanism of NO reduction. The NO species is adsorbed onto theTi-oxide as weak ligands to form the reactive precursors. Under UVirradiation, charge transfer excited complexes of the oxides, (Ti 3+ -O )*, are formed. Within their lifetimes, electron transfer fromthe trapped electron center, Ti 3+ , into the -antibonding orbital of NO takes place and electron transfer from the -bonding orbitalof another NO into the trapped hole center, O , occur simulta-neously. These electron transfers lead to direct decomposition of two molecules of NO on (Ti 3+ O )* into N 2 and O 2 even at 275K.
Such a mechanism provides high selectivity for N 2 formation andinhibits formation of undesired N 2 O and NO 2 [69] .The lifetime of created holes andelectrons on theisolated tetra-
hedral Ti-oxide species are longer than that on the aggregatedoctahedrally coordinated Ti-oxide species (aggregated or bulk TiO 2photocatalysts). Thus, theconversion of NO into N 2 is preferable onthe isolated tetrahedral Ti-oxide species. In this case, the formationof N2 O and NO 2 can be prevented.
3.3.2. PhotocatalystsThe development of newor improved photocatalysts for photo-
deNO x is a big challenge for scientists and material engineers.Commercial TiO 2 (Degussa or others) is often used as a referencematerial. Improvements are evaluated by checking photo-activity
withsimple, powdered photocatalysts. Detailed information about
preparation and activity of different photocatalysts canbe found inthe literature [69,119,120,122,128] .
Optimum properties were rst investigated for powdered TiO 2[47,48] . Courbon and Pichat [48] obtained approximately 50%selectivity for N 2 formation on TiO 2 powder (Degussa) under UVirradiation. The rest of the photo-decomposition product was N2 O.One of the major contributions to the development of photocata-lysis was made by Anpo et al., who have improved many types of photocatalysts using the following objectives [69] :
obtaining a more active tetrahedral structure (Ti- or Mo- or V- orCr-oxide)
increasing the selectivity for N 2 and the lifetime of the activestructure
increasing the deNO x efciency, and obtaining a photocatalyst that is active under visible light irradi-
ation.
Yamashita et al. [118] and Anpo et al. [69] reported that in thecase of powdered TiO 2 , the selectivity for N 2 was only 25% (75%for N 2 O). Anpo et al. [69] improved the photocatalyst activity byincreasing the dispersion of Ti-oxide species and anchoring thephotocatalyst onto silica glass or zeolite support. The ion exchangeor impregnation methods were applied to obtain highly activephotocatalysts. They noticed a high correlation between prepara-tion method and Ti content for NO x decomposition activity and N 2selectivity. The best result (91% selectivity for N 2 with the high-est activity) was obtained for a photocatalyst with 1.1wt.% TiO 2prepared by ion exchange. Titanium oxide was anchored on Al 2 O3or SiO 2 to give a binary photocatalyst. Anpo and Takeuchi [120]observed increased selectivity for N 2 when the Ti content in thebinary photocatalyst was decreased.They also emphasized the roleof tetrahedral Ti-oxide for improving the efciency of NO decom-position.
Anpos group has tried different preparation methods (e.g.,impregnation,ion exchange,and advanced metal ion implantation)and different supporting materials (e.g., silica glass, zeolites suchas Ti-HMS, and Ti-MCM-41) for obtaining visible light responsivephotocatalysts with high selectivity for N 2 [69] . Moreover, theyalso adopted the advanced metal ion implantation technique forobtaining visible light response photocatalysts. Anpo and Takeuchi[120] prepared V ion implanted Ti-HMS and Ti-MCM-41 photo-catalysts and applied them to the decomposition of NO into N 2 andO2 under visible light irradiation ( > 420 nm). This technique canalso be applied to bulk TiO 2 photocatalysts. The un-implanted orun-doped photocatalyst was not active under visible light irradia-tion. However, this technique requires the use of high acceleratingenergy to allow ions to be implanted into the oxide structure andobtainthe desired effects. The catalyst prepared with lower energy
will not generate the visible light active structure. The scheme forthis advanced metal ion implantation method is shown in Fig. 10.
Anpos research group also tried other photocatalysts based ona zeolite structure. For a Cr-HMS photocatalyst, the photo-activityundervisible light irradiation was worsethan underUV irradiation.However, the selectivity for N 2 was very high (97%) under visiblelight and only 45% under UV light. For V oxide species constructedwithin the framework of a zeolite structure (V/SiO 2 photocatalyst)andthe Mo-MCM-41photocatalyst, signicant enhancement in NOreduction was observed in the presence of hydrocarbons (C 3 H8 ,CH4 , and C 2 H6 ) or carbon monoxide. Therefore, these photocata-lysts can alternatively be applied to photo-SCR (see Section 3.1 ).Other studies performedby Anpos research group used Cu +/ZSM-5zeolite photocatalysts at 275 K [123,135] , vanadium silicate (VS-
2) [134] , Ag+/ZSM-5 zeolite [123] , highly dispersed titanium oxide
-
8/11/2019 Harmful Gas reduction catalytic
17/24
J. Lasek et al. / Journal of Photochemistry and Photobiology C: Photochemistry Reviews 14 (2013) 2952 45
Fig. 10. Schematic diagram of an advanced metal ion implantation method [132]
(needs permission; directly cited).
species [120] , and transition metal oxides (e.g., Ti, V, Mo, and Cr)incorporated within the framework of zeolites [69] .
3.3.3. ProcessesOther ways of improving the deNO x process include nd-
ing the best experimental conditions and proper reactors.This process is predominantly carried out in batch reactors[118,121,122,127,128,131,133135] and sometimes in ow reac-tors [80,125,129,133] . Lim et al. [125] used two types of annularow photoreactors, two serially connected reactors with the pho-tocatalyst coated on a quartz reactor tube and a two-dimensionaluidized bed reactor. They investigated the inuence of process
conditions onNO x decompositionoverTiO 2 particles(DegussaP25)under UV irradiation. The authors investigated initial NO concen-tration, light intensity, process temperature, TiO 2 /silica ratio andsupercial gas velocity. The scheme of the uidized bed reactoremployed is shown in Fig. 11 .
3.4. Section summary
Photocatalysis can be a very efcient method for NO x abate-ment. In this section, we presented three methods of NO xremoval by photocatalytic reaction: photo-SCR, photo-oxidationandphoto-decomposition.The mostpromising method seemsto bephoto-SCR, butit is also themostdifcultprocess to employfor realapplications. All the described photo-deNO x processes have their
advantages and weaknesses ( Table 4 ). Nevertheless, it is believed
Fig.11. Two-dimensioneduidizedbed reactor used byLim etal. [125] . E xperimen-tal conditions : 0.12g TiO 2 /silica (Degussa P25, silica gel 202355 m, Merck, ratio:
0.070.33), ow rate 88826ml/min,106 ppmv NO/He,four uorescent black lightlamps(8 W, Sankyo Denki, Japan,F8T8 max intensityat 365 nm)and fourgermicidalwhitelightlamps(8 W, Sankyo Denki, Japan,G8T8, max intensityat 254nm) (needspermission; directly cited).
thatthe limitations canbe eliminated throughcontinuous scienticinvestigation.
4. Applications
In Section 3, we showed the results from investigation of photo-deNO x processes. The research was carried out on laboratory scalefor better understanding of the NO x reduction mechanism under
UV and visible light. Furthermore, the researchers tried to improvephotocatalyst activity and optimize experimental conditions forNO x removal efciency. The nal goal of developing photo-deNO xprocesses is to apply them to commercial systems. It is worth not-ing that research on photo-deNO x was carried out on a large scale(204 m 3 ) at the European photoreactor (EUPHORE) in Valencia,Spain ( Fig. 12). The EUPHORE is equipped with several useful ana-lytical instruments including an in situ FTIR spectrometer, a GC, anHPLC, a GCMS, analyzers for numerous gases (e.g., NO/NO x/NO2 ,ozone, CO, CO 2 , total hydrocarbons (THC), O 2 ), light intensityradiometers and a modern diesel engine (Lynx V277 90 PS FordFocus engine) for the supply of exhaust. The synergetic reactionsofNO x, HC,ozoneand other gaseous compoundswere investigatedunder solar-light irradiation [137140] .
Table 4Advantages and limitations of photocatalytic processes for NO x removal.
Advantages Limitations
Useof renewable and accessible sourceof energy
Catalyst sensitivity and possibilityof catalyst damage (e.g., at SO 2presence)
Savings of energy and natural sources(fossil fuels)
Lower reaction rate in comparisonto thermal catalysis
Prevention of CO 2 emission Usually, faster catalyst deactivationApplied green chemistry Rather UV light is preferableClean and prospective technologyPossibility of co-existence with
traditional deNO x methods (primaryand secondary)
-
8/11/2019 Harmful Gas reduction catalytic
18/24
46 J. Lasek et al. / Journal of Photochemistry and PhotobiologyC: Photochemistry Reviews 14 (2013) 2952
Fig.12. TheEuropeanPhotoreactorin Valencia,Spain [139,140] (n eeds permission;directly cited).
In this section, the application of photocatalytic materials andthe results are given for real cases. New photocatalytic materi-als or processes very often appear in patent applications because
photocatalysishas beena veryattractive eldfor commercialappli-cations. It should be mentioned that NO x can be produced from theatmosphere(environmental NO x) and from uegas generatedfromcombustion (end-pipe NO x).
4.1. Investigation of photocatalysts for real applications
Investigation of new photocatalysts eventually ends with realapplications. For this purpose researchers attempt to create realor quasi -real conditions for testing new materials. Before utiliza-tion, a new material should be tested to see if it can be used incommercial applications. For example, Hunger et al. [78] used con-crete paving blocks for NO x removal in European cities. The mainresearch aim was to determine the kinetic coefcient of the photo-deNO x process. They concluded that the kinetic parameters couldbe used to model real applications using CFD methods. Maggoset al. [141] investigated two types of photo-active paints (contain-ing 10% TiO 2 ): mineral silicate paint and styrene acrylic paint. Theexperiments were carried out in a real-scale environmental cham-ber (30m 3 ) under UV light irradiation. The initial concentrationof NO was 220 ppb and the process was photo-oxidation of NO.The impact of relative humidity (RH) on deNO x efciency was alsoinvestigated. Increasing RH from 20% to 50% signicantly inhibitedNO x removal.The maximumefciencyfor themineral silicate paintand the styrene acrylic paint were 74% NO, 27% NO 2 and 91% NO,71% NO2 , respectively.
Similar investigations [142] w ere carried out in a real-scale cargarage (322 m 2 covered by white acrylic paint including 10% TiO 2 ).
The real exhaust gas from a car engine was introduced into thegarage. Photo-oxidation of NO x was then carried outunderUV irra-diation. The researchers noticed that increasing CO concentrationsincreased NO x removal, but the presence of VOCs inhibited thisprocess. The maximum efciency was 19% for NO in this system.In addition, Maggos et al. [143] tested building materials (contain-ing 3% TiO2 ) for NO x removal in pilot-scale street canyons (threestreets, width: 2m; height: 5.2 m; length: 18.2 m). The exhaustgas containing nitrogen oxides was introduced into the canyonby a pipe distributor. The gaseous compound (NO x, SO2 , CO, CO2 ,and TVOCs) concentrations were then continuously measured. Theexperiments were carried out under solar irradiation (continu-ously monitored) and meteorological parameters, such as winddirection, wind speed, temperature, and relative humidity, were
measured. The NO x concentrations measured in the TiO 2 canyon
Fig.13. TheDives in Misericordia Church, Rome,Italy.Outside surface of thechurchwas coated by cementincluding TiO 2 [145] (needs permission; directly cited).
were 36.782.0% lower than the ones recorded from the referencecanyon.
Chen et al. [144] designed and investigated photocatalytic coat-ing elements on road pavements for NO x removal. They tested acement concrete slab containing a TiO 2 photocatalyst under arti-
cial daylight irradiation (3 50W daylight lamps, Philips, Model# F36T12/D/HO, irradiation density of 250W/m 2 , inlet NO concen-tration of 2500ppb) and obtained more than 90% NO conversion(photo-oxidation). The authors also tested the impact of inlet NOconcentration on NO x removal efciency, and high NO removalefciency was obtained for residence times between 5 and 20min.
4.2. Photo-deNO x processes and materials applied in realapplications
Photocatalysts for NO x abatement are used not only in pilot-scale reactors but also in real commercial objects, particularlybuilding materials. For example, Guerrini and Corazza [145]claimed that the rst application of cement containing TiO 2 wasin 1996 within the three large symbolic sails of the Dives in Mis-ericordia Church (Rome, Italy; see Fig. 13).
Guo et al. [146] collected interesting examples of TiO 2 -basedbuilding materials for removal of poisonous compounds (includingNO x) from air. Inthis paper,manyexamples of photo-activemateri-als were described (exterior: paints, tiles, glass, plastics, aluminumpanels; interior: wall paper, paints, window blinds; and road con-struction: soundproof walls, tunnel walls). Taoda [147] claimedthat photo-active materials not only have good properties for theremoval of NO x or other pollutants,but are also recognized as morepractical and esthetic. Interesting examples of pedestrian side-walks constructed with TiO 2 -containing permeable blocks werealso included, as were TiO 2 -containing adsorbents as decorativeelements ( Fig. 14). As shown in Fig. 14(c), the decorative element
can function well if it is situated in open-spaces where UV light(from sunlight) is available, such as balconies and terraces.
Anpo [133] gave the example of soundproof highway wallscoated with TiO 2 photocatalysts for the abatement of NO x con-structed in Osaka in April 1999. Hamada et al. [148] showed deNO xconstruction applied in airports including Tokyo International Air-port (Haneda Airport) and Ishigaki Domestic Airport (Okinawaprefecture). TiO 2 was used as the photocatalyst in these cases and1030% NO x reduction was obtained. Examples of materials andtechnologies based on TiO 2 photocatalysts for deNO x processes(e.g., tiles, porous ceramics lters coating trafc tunnels, pavedroads and buildings with TiO 2 , indoor air cleaners) are describedin the reviews of Fujishima and Zhang [149] and Carp [150] . Fur-thermore,Venturini andBacchi [151] gaveexamples(Rogoredoand
Monza, Italy) of substances (including TiO 2 ) sprayed onto asphalt
-
8/11/2019 Harmful Gas reduction catalytic
19/24
J. Lasek et al. / Journal of Photochemistry and Photobiology C: Photochemistry Reviews 14 (2013) 2952 47
Fig. 14. Application of photo-materials including TiO2. Comparison of traditional
permeable blocks, (a) with antifouling surface including photocatalyst (b) and pho-tocatalytic functional adsorbent as decorative element c) [147] (needs permission;directly cited).
pavement surfaces for the removal of NO x and other pollutants inair.
Recently, Laufs et al. [152] presented a study of photocatalyticoxidation realized using commercial TiO 2 -doped facade paints(white and blue). A commercial material (Kronos Int.: vlp 7000)containing a carbon-doped titanium dioxide in anatase modica-tion (photo-activity under UV and visible (
-
8/11/2019 Harmful Gas reduction catalytic
20/24
48 J. Lasek et al. / Journal of Photochemistry and PhotobiologyC: Photochemistry Reviews 14 (2013) 2952
Aido [160] proposed a method for preparing panel-shapedceramics that contained TiO 2 for effective removal of NO x from theatmosphere. These ceramics could be used as sidewall construc-tion material.In addition,Ji et al. [161] disclosed a photocatalystforthe removal of formaldehyde, toluene, VOCs and NO x from air. Thephotocatalyst was a mixture of tourmanilite micro-particles (core)and titanium oxide nano-particles doped with Re metal and/orother transition metals. Han [162] presented a method for prepar-ing eco-friendly paint that contained TiO
2 (310wt.%) and could
be used for purication purposes (e.g., antibacterial agent, ster-ilization, decomposition of organic contaminants, NO x removal).Furthermore, Huder [163] presented a porous photocatalyst thatwas structured to allow penetration of light into the photocata-lyst. The extremely large surface area signicantly enhanced thephotocatalytic activity of the reaction.
4.3.2. Installations and applicationsThe utilization of developed photo-materials in processes,
installations or objects is obviously the next step after patentapplication. Researchers attempt to show new installations forNO x removal using different methods, such as process parame-ters and co-application with another installation. These examplescan be divided into two main groups: end-pipe deNO x andenvironmental deNO x processes. The end-pipe processes includethe application of deNO x concepts in a closed, determined sys-tem such as reaction chambers, reactors, and installations whereprocess gases (e.g., exhaust gas) are cleaned. EnvironmentaldeNO x processes describe the application of deNO x processesfor air cleaning (indoor and outdoor) using photo-active cat-alysts contained in decorative elements, soundproof walls andothers.
4.3.2.1. End-pipe deNO x processes. Taodaetal. [164,165] proposedaphoto-installationfor NO x andother pollutantremoval (e.g., VOCs).The installation was connected to the ue duct of a large-scaleincineration furnace. The pellets coated with TiO 2 and loaded withdifferent metals (e.g., platinum, rhodium, ruthenium, palladium,iron, silver, copper andzinc) were used for photo-reactions carriedout at room temperature under UV light irradiation. Kagitani et al.[166] claimed an installation that included a photoreactor for theremoval of NO x emitted fromautomobile engines. The NO x removalwas carried out under UV irradiation (low voltage mercury lamp)for air-mixed exhaust gas with ammonia (NH 3 :NO x ratio rangedfrom 1.5 to 2:1). The photo-reaction of NO x and NH 3 , as well asthephoto-oxidation of hydrocarbons andCO, was occurred.Particulatematerials, such as oxide products, and ammonium present in thereactor were removed with a wet type electric dust collector. Wu[167] disclosed a combined wet absorption and photo-oxidation(under UV irradiation and in the presence of TiO 2 ) process for theremoval ofNO x containedin smoke. Theprocess exhibitedhigh NO xremoval efciency(6090%), non-poisonousnal productsandlow
cost. This technology can be utilized for end-pipe NO x abatement.
4.3.2.2. Environmental deNO x processes. Kawai and Tatsumi [168]constructed a lter that contained a porous light emittingsemiconductor (containing gadolinium). Titanium isopropoxide(Ti(OC2 H5 )4 ) was dissolved in ethanol and sprayed onto the sur-face of the GaN whiskers of the sample, after which it was heatedfor 1 h ata temperature of 500 C in air to coat the GaN surface withactive TiO 2 . UV irradiation was generated upon applying a voltage.The main function of the lter was to decompose organic matter,bacteria and viruses. However, the researchers noticed that theini-tial concentration of NO x dropped from 100ppm to zero during therst 2 h of operation of the lter. They attributed this phenomenonto photocatalytic NO x removal. When voltage was applied, UV light
with a wavelength of 365 nm was emitted. The lter could be used
for the decomposition and removal of other poisonous or undesir-able substances, such as SO x, CO, diesel particulates, pollen, dust,mites and other contaminants from air.
Hokkirogawa [169,170] disclosed an air-purifying, soundproof-ing wall that was recommended for photo-removal of NO x emittedonhighways. A singleelementof thewallwas50 cm 50cm 2 cmand a 100W black light lamp was used as the UV source. Thewall included TiO 2 and its photocatalytic properties were tested inatmosphericair that contained10 ppmof NO
xat a relativehumidity
of 70%. The 10ppm NO x was completely removed in 100minutes.Additionally, Schwaag [171] disclosed a decorative screen for theremoval of NO x in air. The TiO 2 photocatalyst was coated or dis-persed on the base material.The active surface of the photocatalystwas increased using different shapes (e.g., ns, ridges, dimples,holes) or by making it into different forms (e.g., foams, aggregates,webs, weaves or felts). Theauthorsonly emphasized comparison of thedecorationqualityand thede-pollutionperformance. However,the authors did not report the reaction conditions and the deNO xrate.
Saito et al. [172] disclosed a soundproong wall with good per-formance in NO x and SO x removal. The photo-active layer of thewallcontained TiO 2 and pollutants such as NO x and SO x were trans-formedintoHNO 3 and H 2 SO4 by reaction with water found in airorrainwater.Similar results werereported by Shigaya [173] . The pho-tocatalyst was adhered on a honeycomb-shaped metal plate. Thesoundproong wall was transparent because synthetic resin wasused as the supporting material. Thus, the drivers and passengerswere able to see the scenery outside. The NO x and SO x were trans-formed into HNO 3 and H 2 SO4 by photo-reaction in the presence of water.
5. Future trends and challenges in photo-deNO x
Many photo-assisted processes are utilized in real applications.Photo-oxidation and photo-decomposition are the commonlyapplied methods in commercial processes. The most importantgoal in these applications is to search optimal conditions andphotocatalysts for maximum NO x removal.Additionally, results forphoto-SCR under real conditions (e.g., exhaust gas) are difcult toobtain. The main problem is that water favors the transformationof NO into NO 2 and will decrease photocatalyst activity. Negativeresults can also be caused by the presence of SO 2 , halogens anddust.
5.1. Proposed combination of photo-deNO x with other primary or secondary methods
We believe that it is possible to combine primary methods (e.g.,reburning and EGR), secondary methods (SCR) and photo-SCR intoa high efciency de-pollution system. Fig. 15 shows a simpliedscheme for an industrial boiler system using photo-SCR. We con-sider three main zones where the photoreactor could be placed inthe boiler system:
A. atthe uegas recycle system(co-existencewithan EGR system) \B. at the traditional SCR system, andC. inside the convective chimney draught system.
The main advantage of this combined process is increaseddeNO x efciency through incorporation of traditional NO x abate-ment methods with photo-processes. The reaction processes Aand B in the proposition include photo-SCR in the presence of CO and hydrocarbons (Eqs. (3.1)(3.6) in Section 3.1 ). The reducingagent could be commercial LPG gas or others. The most promising
but more difcult to control location for the photoreactor is the
-
8/11/2019 Harmful Gas reduction catalytic
21/24
J. Lasek et al. / Journal of Photochemistry and Photobiology C: Photochemistry Reviews 14 (2013) 2952 49
Fig. 15. The simplied schemeof photo-SCR applying in industrial boilersystem: Awith EGR system, B at traditional SCR system, Cinside chimney draught system.
ue gas recycle system (zone A in Fig. 15). The process wouldadopt a primary deNO x process andcreate a newtechnology that isattractive andadvanced. Excessreducing agentcould be introducedto obtain high NO x removal efciency [60] . The addition of excesshydrocarbons will increase the removal efciency of NO x, but theunreactedfuelwillnotbelostbecauseitisburntinthemainfurnacecombustion zone. The gas mixture (original exhaust +reducingagent) will return back to the combustion zone. The temperatureof the exhaust gas must be decreased because high temperatures(above 200 C) are not desirable in photo-processes. This is why aheat exchanger should be placed before the photoreactor.
Decreased photo-activity with increasing temperature is not ageneral rule. It is often observed that an optimal temperature win-dowfor photo-processes exists. Forexample,Laseket al.foundthatthe optimal temperature window was 70100 C for photo-SCR of NO x [63] . Further increasing the temperature decreaseddeNO x ef-ciency. In addition, Brief description of the temperature windowindifferent photo-processes can be found in their work. Chen andChu concluded that as temperature increased, the reaction rate (inthe photo-oxidation of NO x over a concrete road surface containing
TiO2 ) decreased [153] . However, they explained that higher envi-ronment temperatures decrease the adsorption afnity of waterrather than that of NOx on activated concrete road surface, whichresults in the higher efciencies for NOx decontamination. In otherwords, as thetemperatureincreased, theafnityof water decreasedfaster than NO x.
Serial connection of a photoreactor with a traditional SCR (zoneB in Fig. 15) is more practical and cost-effective. The two-reactorsystem can be combined to work at higher temperatures. The reac-tion process is the same as process A, but the reducing agent isnot used in excess. Therefore, the efciency cannot be as high asA, but the thermal SCR should be enough to remove most of theNO x.
Photocatalysts can also be placed in the convective chimney
draught system (see C), but photo-oxidation will occur in thisarrangement. In this case, the internal chimney surface (coveredby photo-active paint) should be regenerated periodically becausetheactivityof thephotocatalyst willdecline.However, thisarrange-mentseemstobethesimplestanditcanbeappliedmoreeasilythanA or B. Usually, an exhaust gas monitoring system is placed onthe platform surrounding the chimney and gas samples are takendirectly from the chimney zone using a special slot. Thus, an activephoto-surface and a light source can be placed under the monitor-ing slots.
5.2. The inuence of water and oxygen in photo-SCR
As mentioned, an important problem for photo-SCR application
is the presence of water and oxygen in real exhaust gases. The
surface of TiO 2 becomes superhydrophilic under UV light irradi-ation [174,175] . If water is adsorbed on the TiO 2 surface, it createsa very thin layer of water that inhibits photo-SCR. The transport of reactants and the products from the photocatalyst surface into thegaszone is also resisted by the formationof a waterlayer. Addition-ally, the presence of water, an oxidizer, will induce the formationof harmful NO 2 . Hoffman et al. [176] thoroughly investigated theinuence of OH groups on NO 2 formation in the presence of hydro-carbons. They used an excimer laser (248nm) and an Ar + -laseras light sources. The OH radicals were thus produced from H 2 O2photolysis. Muto and Takizawa [177] investigated homogenousphotolysis of eight hydrocarbons ( n-hexane, toluene, n-octane,o-xylene, m -xylene, propene, isobutene, and 2-methyl-2-butene;30ppmv or 10ppmv) in the presence of NO, NO2 , O2 , H2 O andC2 H5 ONO under UV light (360nm) irradiation. The creation of NO 2is not desirable for photo-SCR because the nal products should benon-poisonous.
Similar results can be observed for gases with high concentra-tions of oxygen. Poulston et al. [54] tried to resolve this problemby increasing the reaction temperature. They observed that under
UV irradiation at higher temperature (150 C), NO was transformedinto N 2 or N 2 O, even in the presence of signicant amounts of oxygen (12%). They tested TiO 2 as the photocatalyst and severalhydrocarbons (C 4 H10 , C3 H8 , C3 H6 , C2 H6 , and C 2 H4 ) as reducingagents, concluding thatNO was transformed intomostly NO 2 whenthe process temperature was below 42 C. Moreover, Takeuchi andIbusuki [71] testedphotochemical reactionson gaseous mixtures of C3 H6 NO2 dry air under UV light irradiation over different metaloxides (CeO 2 , CoO, Cr2 O3 , Fe2 O3 , NiO, SnO2 , TiO2 , WO3 , ZnO, andZrO2 ). It was noteworthy that they used y ash samples obtainedfrom the electrostatic precipitation of coal-red power plants asthe photocatalyst. Ibusuki and Takeuchi [178] f urther investigatedtoluene oxidation in the presence of O 2 , NO2 and H 2 O under UVlightirradiationover TiO 2 .TheyobservedthatNOwasoneoftheby-
products andrelativehumiditystronglyinuencedthe NO 2 andNOconcentrations in the gas mixture. At 30% relative humidity, mini-mal NO was produced from the reaction between NO 2 and toluene.
Takagiet al. [179] considered the mechanismof o-xylene photo-oxidation (homogeneous) in the presence of NO and water. Anoptimal concentration of water was found for the photo-SCR system. However, the water problem should be considered if photo-SCR is applied in real industrial systems. The main issue isthereforedetermining the optimal conditions for photo-SCRof NO xin the presence of water and oxygen. From a literature review, itcan be concluded that important factors for improving deNO x ef-ciency are temperature and the concentrations of water, oxygenand reducing agent.
We attempted to nd the optimal conditions for photo-SCR under humid conditions using literature data. For example, a
-
8/11/2019 Harmful Gas reduction catalytic
22/24
50 J. Lasek et al. / Journal of Photochemistry and PhotobiologyC: Photochemistry Reviews 14 (2013) 2952
Fig. 16. Creation of NO 2 duringphoto-reactionat water presencein function of temperature;(a) lower water concentration below 0.7vol.%, at high ( , 163 8),medium ( ,56 5), and low ( , 11 5) C3 H8 /NO ratio; (b) higher water concentration above 1.2vol.%, at high ( , 145 12), medium ( , 84 4), and low ( , 20 5) C3 H8 /NO ratio. Datafrom Ref. [63] .
positive inuence for temperature on photo-SCR in the presenceof water was recently conrmed by our research group.The photo-SCR was carried out under UV light irradiation over a PdO/TiO 2catalyst. Signicant inhibition of NO 2 generation was observedduring the experiment at higher temperatures. These results arepresented in Fig. 16 . The left (a) and right (b) portions of the gurerepresent the results obtained at lower and higher concentrationsof water, respectively. Increasing the temperature from30 to 100 Cdecreased NO 2 from approximately 40 to 5 ppm. Increases in tem-perature caused inhibition of NO 2 generation in every experiment,but the maximum deNO x efciency was observed in the range70100 C. Further increasing the temperature decreased deNO xefciency. Thus,the temperature window for achievingbest deNO xefciency was determined to be 70100 C. This phenomenon wasexplained by heterogeneous catalysis. More detailed informationis available elsewhere [63] .
6. Summary and remarks
The photo-deNO x processes presentedin thisreview are dividedinto three groups: photo-decomposition, photo-SCR and photo-oxidation. The photo-oxidation and photo-decomposition of NO xwere observed to behave better than photo-SCR. However, thelater is still not a mature eld of research capable of indus-trial applications. In addition, photo-SCR is more desirable thanphoto-oxidation because the nal product of SCR is harmless N 2 .Photo-decomposition is an ideal process, but it has not yet beeninvestigatedin thepresenceof other compounds, especially O 2 andH2 O. For this reason, we paid more attention to the inuence of water and oxygen. Photo-SCR and photo-decomposition can likelybe employed simultaneously, butthe rst oneis undoubtedly morefeasible for the treatment of exhaust gases. Nevertheless, somephoto-NO x removal processes have already been applied in realcases.
This reviewof photo-deNO x processes alsocompares traditional
and photo-assisted NO x abatement methods. Although the rsttype of method is better known and applied more commonly incommercial systems, the photo-deNO x method is a very attractivealternative. Photo-assisted processes are a very intensively devel-oping area of science. The development of new deNO x processesis an urgent task. This area of research is not only interesting toscientists, but is also critical for the development of green envi-ronmental processes.
The combination of thermal (traditional) and photocatalyticdeNO x canbe synergistic andshould be explored for the abatementof poisonous substance emissions. We believe that photocatalysiscan enhance these efforts. One of the main challenges for sci-entists is to obtain a stable photocatalyst that is active undervisible light. As a result, intensive investigations in this aspect
are still ongoing. The development of new photocatalysts that are
active under visible light will make the entire process more effec-tive and affordable because sunlight is an abundant and powerfulsource of energy.
7. Sources of further information
Photocatalytic removal of NO x can often be found in com-mercial air cleaning processes. Commercial markets offer manyproducts for the photo-oxidation of NO x and others pollutants[180] . Additionally, commercial laboratories specialize in the test-ing of photocatalysts used for deNO x. The Photocatalyst Section atthe Environmental Technical Laboratory Ltd, Japandetails the testsused to assess photocatalytic materials in nitrogen oxide removal[181] . Some companies recommend TiO 2 as a good photocatalystfor NO x and other pollutants removal (e.g., Belgian Road ResearchCentre [182] , TitanPE Technologies Inc. [183] , Bio Shield, Inc. [184] ,and Gra zd ze CEMENT SA, Poland [185] ). Photocatalytic deNO xprocesses are also prominently featured on quasi-science websites(e.g., [186] ).
Acknowledgements
This work was nancially supported by the National ScienceCouncil of Taiwan under project NSC98-2911-I-002-002.We wouldlike to thank Raymond Liao for help with writing. Janusz Lasekwould like to thank Prof. Jeffrey Chi-Sheng Wu for the invitationand supervision of a one-year post-doctoral project at the NationalTaiwan University.
References
[1] J.-T. Lin, M.B. McElroy, K.F. Boersma, Atmos.Chem. Phys. 10 (2010) 6378.[2] F. Normann, K. Andersson, B. Leckner, F. Johnsson,Prog.Energy Combust. Sci.
35 (2009) 385397.[3] S. Roy, M.S. Hegde, G. Madras, Appl. Energy 86 (2009) 22832297.[4] M. Danesh Miah,M. Farhad HossainMasum, M. Koike, Energy Policy38 (2010)
46434651.[5] K.Skalska,J.S. Miller, S.Ledakowicz, Sci.Total Environ. 408(2010) 39763989.[6] Y. Sadanaga, J. Matsumoto, Y. Kajii , J. Photochem. Photobiol . C 4 (2003)
85104.[7] B. Chen, C. Hong, H. Kan, Toxicology 198 (2004) 291300.[8] P.E. Morrow, J. Toxicol. Environ. Health13 (1984) 205227.[9] J.A.Last, W.M. Sun,H. Witschi,Environ.Health Perspect.102 (1994)179184.
[10] S.A. Cormier, S. Lomnicki, W. Backes, B. Dellinger, Environ. Health Perspect.114 (2006).
[11] V. Mohsenin, Toxicology 89 (1994) 301312.[12] R. de Richter, S. Caillol, J. Photochem. Photobiol. C 12 (2011) 119.[13] P. Glarborg, A.D. Jensen, J.E. Johnsson, Prog. Energy Combust. Sci. 29 (2003)
89113.[14] B.Grado n, Zeszyty naukowe Politechniki Slaskiej, Hutnictwo, 67, 2003.[15] Y.B. Zeldovich, Acta Physicochem. USSR. 21 (1946) 577628.[16] J. Wolfrum, Chemie Ingenieur Technik CIT 44 (1972) 656659.[17] P.C. Malte,D.T. Pratt, Combust. Sci. Technol. 9 (1974) 221231.[18] J. Tomeczek,B. Grado n, Combust. Flame 133 (2003) 311322.[19] J. Tomeczek,B. Grado n, Combust. Sci. Technol. 125 (1997) 159180.
[20] C.P. Fenimore, Proceedings of theCombustion Institute, 1971, pp. 373380.
-
8/11/2019 Harmful Gas reduction catalytic
23/24
-
8/11/2019 Harmful Gas reduction catalytic
24/24
52 J. Lasek et al. / Journal of Photochemistry and PhotobiologyC: Photochemistry Reviews 14 (2013) 2952
[132] M. Anpo, M. Takeuchi, Int. J. Photoenergy 3 (2001) 8994.[133] M. Anpo, Pure Appl. Chem. 72 (2000) 12651270.[134] M. Anpo,S.G. Zhang, S. Higashimoto, M. Matsuoka,H. Yamashita,Y. Ichihashi,
Y. Matsumura, Y. Souma, J. Phys. Chem. B 103 (1999) 92959301.[135] M. Anpo, Sol. Energy Mater.Sol. Cells 38 (1995) 221238.[136] S. Zhang, N. Fujii, Y. Nosaka, J. Mol. Catal. A: Chem. 129 (1998) 219224.[137] B. Klotz, Summaryof Presentationsat theUS/German Environmental Cham-
ber Workshop, Riverside, CA, October 46, 1999, pp. 113.[138] J. Zdor, T. Turnyi, K. Wirtz, M. Pilling, J. Atmos.Chem. 55 (2006) 147166.[139] B. Zielinska, J. Sagebiel, W. Stockwell, J. McDonald, J. Seagrave, P. Wiesen,
K. Wirtz, Investigation of Atmospheric Transformations of Diesel Emissions
in the European Photoreactor (EUPHORE), in: I. Barnes, K. Rudzinski (Eds.),Environmental Simulation Chambers: Application to Atmospheric ChemicalProcesses, Springer, Netherlands, 2006, pp. 279284.
[140] B. Zielinska, Exp. Toxicol. Pathol. 57 (2005) 3142.[141] T. Maggos, J.G. Bartzis, P. Leva, D. Kotzias, Appl. Phys. A 89 (2007) 8184.[142] T. Maggos, J.G. Bartzis, M. Liakou, C. Gobin, J. Hazard. Mater. 146 (2007)
668673.[143] T. Maggos, A. Plassais, J. Bartzis, C. Vasilakos, N. Moussiopoulos, L. Bonafous,
Environ. Monit. Assess. 136 (2008) 3544.[144] D.H. Chen, K. Li, R. Yuan, Annual Project Report Submit ted to Houston
Advanced Research Center and Ofce of Air Quality Planning and Standards.U.S. Environmental Protection Agency. Research Triangle Park, NC 27711,2007, pp. 117.
[145] G.L. Guerrini, F. Corazza, First Arab International Conference and Exhibitionon TheUses of White Cement, Cairo, Egypt, 2830 April 2008.
[146] S.Guo, Z.Wu, W. Zhao, Chin. Sci. Bull. 54 (2009) 11371142.[147] H. Taoda, Synthesiology 1 (2008) 287295(translation from Japanese).[148] H. Hamada, K.-i. Komure, R. Takahashi, T. Yamaji, RILEM International
Symposium on Environment-Consious Materials and Systems for Sus-tainable Development, September 67, 2004, Koriyama, Japan, 2004,pp. 361366.
[149] A. Fujishima, X. Zhang, Comptes Rendus Chimie9 (2006) 750760.[150] O. Carp, C.L. Huisman, A. Reller, Prog. Solid State Chem. 32 (2004) 33177.[151] L. Venturini, M. Bacchi, II International Conference Environmentally Friendly
Roads Enviroad, Warsaw, Poland October 1516, 2009.[152] S. Laufs, G. Burgeth, W. Duttlinger, R. Kurtenbach, M. Maban, C. Thomas, P.
Wiesen, J. Kleffmann, Atmos. Environ. 44 (2010) 23412349.[153] M. Chen, J.-W. Chu, J. Cleaner Product. 19 (2011) 12661272.[154] Y. Paz, Appl. Catal. B 99 (2010) 448460.[155] M. Hayakawa, M. Chikuni, T. Watanabe, United States Patent 6,191,062
(2001).[156] K. Tsujimichi, H. Hasuo, H. Kobayashi, UnitedStates Patent0036897 (2001).
[157] M. Ohmori, H. Nakamura, N. Murase, N. Uotani, T. Ohkubo, United StatesPatent 6,337,301 (2002).
[158] I.C. Gambarelli, G. Pozzi, United StatesPatent 7,608,297 (2009).[159] S. Sugihara, UnitedStates Patent 6,908,881 (2005).[160] Y. Aido, Japan Patent924 846 6 (1997).[161] Z.J. Ji, X.W.Yan,J. Wang, China Patent 1597091 (2005).[162] S.H. Han, KoreanPatent 0007840 (2009).[163] K. Huder, German Patent4110227 (1992).[164] H. Taoda, Y., Yamada, K. Aizawa, UnitedStates Patent6,508,992 (2003).[165] H. Taoda, Y. Yamada, K. Aizawa, United StatesPatent 6,838,059 (2005).[166] T. Kagitani, K. Doi, T. Azuma, Japan Patent55,015,637 (1980).
[167] Z.W. Wu, China Patent 1,883,775 (2006).[168] C.Kawai, M. Tatsumi, United States Patent 7,468,529 (2008).[169] K. Hokkirigawa, N. Yoshimura, J. Hirose, United States Patent 0031642 A1
(2004).[170] K. Hokkirigawa, N. Yoshimura,J. Hirose, European Patent 1369532 A2 (2003).[171] D. Schwaag, Great Britain Patent 2,441,171 (2008).[172] T. Saito,T. Ogawa, A. Kushibe, S. Ando, Y. Ito, Japan Patent10331116 (1998).[173] N. Shigaya, Japan Patent254,322 (2001).[174] M. Miyauchi, A. Nakajima,K. Hashimoto, T. Watanabe,Adv. Mater.12 (2000)
19231927.[175] M. Miyauchi,A. Nakajima,T. Watanabe,K. Hashimoto, Chem. Mater. 14 (2002)
28122816.[176] A. Hoffmann, J. Grossmann, R. Zellner, J. Photochem. Photobiol. A 176 (2005)
260269.[177] H. Muto, Y. Takizawa, Chemosphere 21 (1990) 14231428.[178] T. Ibusuki, K. Takeuchi,Atmos. Environ. (1967) 20 (1986) 17111715.[179] H. Takagi, N. Washida, H. Akimoto, K. Nagasawa, Y. Usui, M. Okuda, J. Phys.
Chem. 84 (1980) 478483.[180] http://www.alibaba.com/products/tio2/613.html?noddp=Y , website
31.05.2010.[181] http://www.etlabo.co.jp/en/image/photocatalytic materials.pd f , Website
31.05.2010.[182] A. Beeldens, http://www.brrc.be/pdf/tra/tra beeldens txt.p df . Website
31.05.2010.[183] http://www.tipe.com.cn/index.htm.[184] http://www.teamenviroclean.com/chemistry of tio2 nanotechnolo gy, web-
site 31.05.2010.[185] http://www.gorazdze.pl , website 31.05.2010.[186] http://odpady.org.pl/plugins/content/content.php?content.2134.1 , website
31.05.2010.[187] I.T.A. Harrick ScienticProducts,2nd Floor, Pleasantville, NY 10570,U.S. (Ed.),
2003, pp. 918.
http://www.alibaba.com/products/tio2/613.html?noddp=Yhttp://www.alibaba.com/products/tio2/613.html?noddp=Yhttp://www.etlabo.co.jp/en/image/photocatalytic_materials.pdfhttp://www.brrc.be/pdf/tra/tra_beeldens_txt.pdfhttp://www.teamenviroclean.com/chemistry_of_tio2_nanotechnologyhttp://www.gorazdze.pl/http://odpady.org.pl/plugins/content/content.php?content.2134.1http://odpady.org.pl/plugins/content/content.php?content.2134.1http://odpady.org.pl/plugins/content/content.php?content.2134.1http://www.gorazdze.pl/http://www.teamenviroclean.com/chemistry_of_tio2_nanotechnologyhttp://www.brrc.be/pdf/tra/tra_beeldens_txt.pdfhttp://www.etlabo.co.jp/en/image/photocatalytic_materials.pdfhttp://www.alibaba.com/products/tio2/613.html?noddp=Y