Growth Kinetics
Transcript of Growth Kinetics

Outline
Quantifying growth kinetics• Overview of models that describe cell growth• Unstructured nonsegregated models• Substrate-limited models• Models with growth inhibitors

Models for Describing Cell Growth

Models for Describing Cell Growth
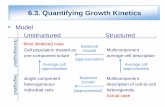
Unstructured models assume fixed cell composition, which is equivalent to assuming balanced growth:• Empirical (i.e. based on observations)• Do not consider stoichiometry• Do not consider organelles
Structured models look at cells microscopically, including internal kinetics and regulation:• Experimental and modeled• Do consider stoichiometry• Do consider organelles

Describing Cell Growth: Unstructured Models
nX
Volume of interest: e.g. a group of cells in a bioreactordS/dt;dP/dt;dX/dt
O2
outputsinputs ΣS
H+ΣP
The overall reaction:
Substrates + Cells byproducts + more cells
ΣS + X ΣP + nXµ

Models for Describing Cell Growth
Nonsegregated models treat the entire culture as homogeneous:• Assume uniformity of cells in the culture• Consider the culture as a “black box”/cell
factorySegregated models examine the cells as discrete units:• Assume the cells may differ from one another• Consider individual cells

Models for Describing Cell GrowthStructured segregated models are the most realistic, but are computationally complexUnstructured nonsegregated models are simple and applicable to many situations of practical interest• However, they are generally only valid in
single-stage, steady-state continuous culture or the exponential phase of batch culture; they fail during any transient condition

Using Unstructured NonsegregatedModels to Predict Specific Growth Rate
Substrate-limited growth:• The relationship between substrate concentration and
specific growth rate often assumes the form of saturation kinetics
• For cellular systems, these kinetics are described by the Monod equation:
• Where µm is the maximum specific growth rate when S>>Ks
SKS
s
mg +=
µµ (6.30)

Effect of Nutrient Concentration on the Specific Growth Rate of E. Coli

Substrate-Limited Growth KineticsThe Monod equation adequately describes substrate-limited growth only when growth is slow, and the population density is low• If substrate consumption is rapid, then the release of
toxic waste products is more likely• At high population levels, the build-up of toxic
metabolites becomes more significant• The following rate expression has been proposed for
rapidly-growing, dense cultures:
SSKS
s
mg +=
00
µµ (6.31)

Alternatives to the Monod Equation
The Blackman equation:
This expression often fits data better than the Monod equation, but the discontinuity in the equation can be problematic
mg µµ =
s
mg K
S2µµ =
If S ≥ 2Ks
If S < 2Ks

Alternatives to the Monod EquationThe Tessier equation:
The Moser equation:
The Moser equation is the most general form of all the substrate-limited growth equations; when n=1 it is equivalent to the Monod equation
( )KSmg e−−= 1µµ
( ) 11 −−+=+
= nsmn
s
nm
g SKSK
S µµµ
(6.34)
(6.35)

Alternatives to the Monod Equation
The Contois equation:
The Contois equation has a saturation constant proportional to cell concentration that describes substrate-limited growth at high cell densities• According to this equation, the specific growth rate
decrease with decreasing substrate concentrations, and eventually becomes inversely proportional to the cell concentration in the medium, i.e.:
SXKS
SX
mg +=
µµ
Xg1
∝µ
(6.36)

The Generalized Specific Growth Rate Equation
These four equations can be described by a single differential equation:
Where υ = µg/µm, S is the rate-limiting substrate concentration, and K, a, and bare constants• The values of these constants are different for
each equation
( )baKdSd υυυ
−= 1 (6.37)

Constants of the Generalized Specific Growth Rate Equation
Table 6.2, Shuler & Kargi

Models with Growth Inhibitors
Substrate inhibition:• As with enzyme kinetics, substrate inhibition may be
competitive or non-competitiveFor noncompetitive substrate inhibition:
If KI >> Ks, then:
+
+
=
I
s
mg
KS
SK 11
µµ (6.39)
(6.40)Is
mg KSSK
S2++
=µµ

Substrate Inhibition
For competitive substrate inhibition:
Substrate inhibition in any form may be alleviated and/or prevented by slow, intermittent addition of the substrate to the growth medium
SKSK
S
Is
mg
+
+
=1
µµ (6.41)

Substrate Inhibition

Models with Growth InhibitorsProduct inhibition:• High concentrations of product can be inhibitory to
microbial growth• For competitive product inhibition:
• For non-competitive product inhibition:
SKPK
S
ps
mg
+
+
=
1
µµ
+
+
=s
mg
PK 11
µµ
(6.42)
(6.43)
pKS

Product Inhibition
Ethanol fermentation from glucose by yeasts is an example of noncompetitive product inhibition• Ethanol is an inhibitor at concentrations above ~5% (v/v)• Rate expressions specifically for ethanol inhibition are:
n
ms
mg P
P
SK
−
+
= 11
µµ
pKP
s
mg e
SK
−
+
=1
µµ
where Pm is the product concentration at which growth stops
,
where Kp is the product inhibition constant
,

Models with Growth InhibitorsInhibition by toxic compounds:• The presence of toxic compounds in the growth
medium can inhibit the growth of cells, or even lead to cell death
• For competitive inhibition:
• For noncompetitive inhibition:
SKIK
S
Is
mg
+
+
=1
µµ
+
+
=
I
s
mg
KI
SK 11
µµ

Inhibition by Toxic CompoundsUncompetitive inhibition:
In some cases, toxic compounds can cause the inactivation of cells or cell death:
Where is the death rate constant (h-1)
+
+
+
=
I
I
s
mg
KIS
KI
K
S
11
µµ
'd
s
mg k
SKS
−+
=µµ
'dk
(6.48)
(6.49)