GLYCOGEN METABOLISM 1. Glycogen Structure Most of the glucose residues in glycogen are linked by ...
-
Upload
dwight-bruce -
Category
Documents
-
view
216 -
download
0
Transcript of GLYCOGEN METABOLISM 1. Glycogen Structure Most of the glucose residues in glycogen are linked by ...

GLYCOGEN METABOLISM
1

Glycogen Structure
• Most of the glucose residues in glycogen are linked by -1,4-glycosidic bonds.
• Branches at about every tenth residue are created by -1,6-glycosidic bonds.
2

Glycogen is an important fuel reserve for several reasons
• Glycogen serves as a buffer to maintain blood-glucose levels– Especially important because glucose is virtually
the only fuel used by the brain.– Is good source of energy for sudden, strenuous
activity
• Unlike fatty acids, it can provide energy in the absence of oxygen
3

The major sites of glycogen storage
1. liver 2. The skeletal muscles
• Glycogen is present in the cytosol in the form of granules ranging in diameter from 10 to 40 nm
4

Glycogen Metabolism
Glycogenesis• Addition of α1-4
linkages to non-reducing ends.
• 1 ATP per linkage.• Branching enzymes.• Inactivated by cAMP
Glycogenolysis• Cleave α1-4 linkages at
non-reducing ends.• Phosphorolysis (Pi in place
of H2O.
• Debranching enzymes.• Activated by cAMP
5

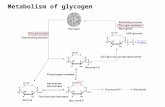
Glycogen degradation
Consists of three steps e 1. release of G1-P from glycogen2. The remodeling of the glycogen substrate to permit
further degradation3. The conversion of G1-P into G6-P.
It is the initial substrate for glycolysis1. it can be processed by the pentose phosphate pathway
to yield NADPH and ribose derivatives2. it can be converted into free glucose for release into the
bloodstream.
6

Glycogen degradation
7

Regulation of Glycogen metabolism
• Allosterically:– By adjustment of enzyme activity to meet the needs of
the cell.
• Hormones stimulate cascades that lead to reversible phosphorylation of the enzymes, which alters their kinetic properties.– Regulation by hormones allows glycogen metabolism to
adjust to the needs of the entire organism.
8

Glycogen Breakdown Requires the Interplay of Several Enzymes
• Four enzyme activities:– one to degrade glycogen,– two to remodel glycogen so that it remains a
substrate for degradation– one to convert the product of glycogen
breakdown into a form suitable for further metabolism.
9

Glycogen Phosphorylase: A key enzyme
• Cleaves its substrate by the addition of orthophosphate (Pi) to yield G1-P (phosphorolysis)
• Catalyzes the sequential removal of glycosyl residues from the nonreducing ends of the glycogen molecule (the ends with a free 4-OH groups)
10

• G°´ for this reaction is small because a glycosidic bond is replaced by a phosphoryl ester bond that has a nearly equal transfer potential.
• Phosphorolysis proceeds far in the direction of glycogen breakdown in vivo because the [Pi]/[G6-P] ratio is usually >100, substantially favoring phosphorolysis.
• The phosphorolytic cleavage of glycogen is energetically advantageous because the released sugar is already phosphorylated
11

Two Remodeling Enzymes
• Transferase:– Shifts a block of three glycosyl residues from one
outer branch to the other
• -1,6-glucosidase (debranching enzyme)– Hydrolyzes the -1, 6-glycosidic bond, resulting in
the release of a free glucose molecule.– Glucose is phosphorylated by hixokinase
(glycolysis)
12

13

• This paves the way for further cleavage by phosphorylase.
• In eukaryotes, the transferase and the -1,6-glucosidase activities are present in a single polypeptide chain, in a bifunctional enzyme
14

Phosphoglucomutase • G1-P formed in the phosphorolytic cleavage of glycogen
must be converted into G6-P to enter the metabolic mainstream.
• This enzyme is also used in galactose metabolism
15

G1-P G6-P
G1,6-BP
Serine
Serine
16

• You may recall that the enzyme glucose-6-phosphatase (G6Pase) catalyzes the last step of gluconeogenesis - conversion of G6P to glucose + phosphate.
• This enzyme necessary also for release of glucose into the bloodstream from glycogen metabolism (glycogen -> G1P -> G6P -> Glucose).
• It is interesting to note that G6Pase is ABSENT FROM MUSCLE.
• This is because muscle does NOT export glucose. the liver, on the other hand, DOES export glucose and thus has abundant supplies of the enzyme.
17

Liver Contains G6-Pase; a hydrolytic enzyme absent from Muscle
A major function of the liver is to maintain a near constant level of glucose in the blood.
The liver G6-Pase, cleaves the phosphoryl group to form free glucose and orthophosphate.
This G6-Pase, is located on the lumenal side of the smooth endoplasmic reticulum membrane
18

Glycogen Phosphorylase
Pyridoxal Phosphate integral group of the Enzyme
The Pi substrate binding site
19

• In human, liver phosphorylase and muscle phosphorylase are approximately 90% identical in amino acid sequence.
• The differences result in important shifts in the stability of various forms (isozymes) of the enzyme.
20

Phosphorylase exists in two states
The R state, catalytic site is more accessible and a binding site for orthophosphate is well organized.
The T state is less active because the catalytic site is
partly blocked.
21

Phosphorylase Is Regulated by:• Allosteric Interactions:
– By several allosteric effectors that signal the energy state of the cell
• Reversible Phosphorylation:– responsive to hormones such as:
• Insulin• Epinephrine• Glucagon
• The glycogen metabolism regulation differs in muscle than in liver because:– The muscle uses glucose to produce energy for itself,
whereas the liver maintains glucose homeostasis of the organism as a whole
22

phosphorylase
B
usually inactive
ATP A
usually active
P
Phosphorylase kinase
Phosphorylase a differs from b by a phosphoryl group on each subunit
23

Depending oncellular conditions
The equilibrium for phosphorylase a, favors the R-state
The equilibrium for phosphorylase b, favors the T-state
The R and T states of each of the a or b forms are in equilibrium
24

In muscles-Posphorylase b• High AMP, binds to a nucleotide-binding site and stabilizes
the conformation of phosphorylase b in the R-state. • ATP acts as a negative allosteric effector by competing with
AMP and so favors the T-state. • G6-P also favors the T-state of phosphorylase b, an example
of feedback inhibition
25

• Under most physiological conditions, phosphorylase b is inactive because of the inhibitory effects of ATP and G6-P.
• In contrast, phosphorylase a is fully active, regardless of the levels of AMP, ATP, and G6-P.
26

Liver Phosphorylase Produces Glucose for Use by Other Tissues
• In contrast with the muscle enzyme, liver phosphorylase a but not b exhibits the most responsive T-to-R transition.
• The binding of glucose shifts the allosteric equilibrium of the a form from the R to the T state, deactivating the enzyme
• Unlike the enzyme in muscle, the liver phosphorylase is insensitive to regulation by AMP because the liver does not undergo the dramatic changes in energy charge seen in a contracting muscle
27

28

Phosphorylase kinase
29

Phosphorylase kinase in the skeletal muscle: is (
is catalytic areregultoryis calmodulin
30

Epinephrine and Glucagon Signal the Need for Glycogen Breakdown
• Muscular activity or its anticipation leads to the release of epinephrine (adrenaline),from the adrenal medulla.
• Epinephrine markedly stimulates glycogen breakdown in muscle and, to a lesser extent, in the liver.
• The liver is more responsive to glucagon, a polypeptide hormone that is secreted by the cells of the pancreas when the blood-sugar level is low.
31

• Epinephrine binds to the -adrenergic receptor in muscle, whereas glucagon binds to the glucagon receptor in liver.
• These binding events activate the subunit of the heteromeric Gs protein.
• A specific external signal is transmitted into the cell32

Glycogen Is Synthesized and Degraded by Different Pathways
• glycogen is synthesized by a pathway that utilizes uridine diphosphate glucose (UDP-glucose) rather than G1-P as the activated glucose donor.
33

UDP-Glucose Is an Activated Form of Glucose
• UDP-glucose, the glucose donor in the biosynthesis of glycogen, is an activated form of glucose.
• The C-1 carbon atom of the glucosyl unit of UDP-glucose is activated because its hydroxyl group is esterified to the diphosphate moiety of UDP.
34

• UDP-glucose is synthesized from G1-P and (UTP) in a reaction catalyzed by UDP-glucose pyrophosphorylase.
35

• This reaction is readily reversible.• Pyrophosphate is rapidly hydrolyzed in vivo to
orthophosphate by an inorganic pyrophosphatase.• The essentially irreversible hydrolysis of
pyrophosphate drives the synthesis of UDP-glucose.
36

Glycogen Synthase Catalyzes the Transfer of Glucose from UDP-Glucose to a Growing Chain
37

• glycogen synthase, is the key regulatory enzyme in glycogen synthesis.
• It can add glucosyl residues only if the polysaccharide chain already contains more than four residues.
• Thus, glycogen synthesis requires a primer.– This priming function is carried out by glycogenin, a protein
composed of two identical 37-kd subunits, each bearing an oligosaccharide of -1,4-glucose units.
– C1 of the first unit of this chain, the reducing end, is covalently attached to the phenolic hydroxyl group of a specific tyrosine in each glycogenin subunit.
38

How is this chain formed?
• Each subunit of glycogenin catalyzes the addition of eight glucose units to its partner in the glycogenin dimer.
• UDP-glucose is the donor in this autoglycosylation.• At this point, glycogen synthase takes over to extend
the glycogen molecule
39

A Branching Enzyme Forms -1,6 Linkages
• Branching occurs after a number of glucosyl residues are joined in -1,4 linkage by glycogen synthase.
• A branch is created by the breaking of an -1,4 link and the formation of an -1,6 link.
• A block of residues, typically 7 in number, is transferred to a more interior site.
• The block of 7 or so residues must include the nonreducing terminus and come from a chain at least 11 residues long.
• The new branch point must be at least 4 residues away from a preexisting one.
40

Branching is important because it increases the solubility of glycogen.
Branching creates a large number of terminal residues, the sites of action of glycogen phosphorylase and synthase.
Thus, branching increases the rate of glycogen synthesis and degradation.
41

Glycogen Synthase Is the Key Regulatory Enzyme in Glycogen Synthesis
• Glycogen synthase is phosphorylated at multiple sites by protein kinase A (PKA) and several other kinases.
• The resulting alteration of the charges in the protein lead to its inactivation
• Phosphorylation has opposite effects on the enzymatic activities of glycogen synthase and phosphorylase
42
Net charge after posphorylation

• Phosphorylation converts the active a form of the synthase into inactive b form.
• The phosphorylated b form requires a high level of the allosteric activator G6-P for activity
• The a form is active whether or not G6-P is present
43

Glycogen Is an Efficient Storage Form of Glucose
• One ATP is hydrolyzed incorporating glucose 6-phosphate into glycogen
44
-ATP

Glycogen
Glucose
G6-P
G1-P
Pyruvate
-1 ATP
10%branch
90%
+31 ATP
G1-P -1 ATP
The complete oxidation of glucose 6-phosphate yields about 31 molecules of ATP.Storage consumes slightly more than one molecule of ATP per molecule of glucose 6-phosphate; so the overall efficiency of storage is nearly 97%.
45

Glycogen Breakdown and Synthesis Are Reciprocally Regulated
• By a hormone-triggered cAMP cascade acting through protein kinase A
46

Insulin Stimulates Glycogen Synthesis by Activating Protein Phosphatase 1
• When blood-glucose levels are high, insulin stimulates the synthesis of glycogen by triggering a pathway that activates protein phosphatase 1
Different site from PKA phosphorylation in response
to epinepfrine
47

Glycogen Metabolism in the Liver Regulates the Blood-Glucose Level
• After a meal rich in carbohydrates, blood-glucose levels rises, leading to an increase in glycogen synthesis in the liver
• Insulin is the primary signal for glycogen synthesis• The liver senses the concentration of glucose in the
blood, (~80 to 120 mg/100ml).• The liver takes up or releases glucose accordingly.
48

• The amount of liver phosphorylase a decreases rapidly when glucose is infused.
• After a lag period, the amount of glycogen synthase a increases, which results in the synthesis of glycogen.
49