Gamifant - 1au3b422k9zdqzddw3my51gg-wpengine.netdna-ssl.com · Introduction Brand name: ... (HLH)...
Transcript of Gamifant - 1au3b422k9zdqzddw3my51gg-wpengine.netdna-ssl.com · Introduction Brand name: ... (HLH)...

NEW PRODUCT SLIDESHOW
Gamifant (emapalumab-lzsg)

Introduction
Brand name: Gamifant
Generic name: Emapalumab-lzsg
Pharmacological class: Interferon gamma
blocking antibody
Strength and Formulation: Emapalumab-lzsg
10mg/2mL, 50mg/10mL; soln for IV infusion
after dilution; preservative-free
Manufacturer: Sobi, Inc.
How supplied: Single-dose vial—1
Legal Classification: Rx

Gamifant

Indication
Primary hemophagocytic
lymphohistiocytosis (HLH) in adults and
children (newborn and older) with
refractory, recurrent or progressive
disease or intolerance with conventional
HLH therapy

Dosage & Administration
Give prophylaxis for herpes zoster,
Pneumocystis jirovecii, and fungal
infections prior to infusion
Begin dexamethasone ≥5–10mg/m2 daily
the day before starting Gamifant; dose can
be tapered as appropriate

Dosage & Administration
Give by IV infusion over 1hr
Initially 1mg/kg twice weekly (every 3–4
days) until HSCT performed or
unacceptable toxicity
Subsequent doses may be increased
based on response
Dose modification: see full labeling

Considerations for Special
Populations
Pregnancy: No available data to inform
drug-associated risk
Nursing mothers: Consider benefits of
breastfeeding with potential adverse effects
on child
Elderly: Insufficient number studied

Warnings/Precautions
Monitor closely for serious infections (eg,
sepsis, pneumonia, bacteremia); and those
caused by specific pathogens favored by IFN-
gamma neutralization (including
mycobacteria, herpes zoster, histoplasma
capsulatum)

Warnings/Precautions
Evaluate for TB risk factors and test for
latent TB prior to treatment start
Give TB prophylaxis if patient at risk, has (+)
PPD test result or (+) IFN-gamma release
assay
Monitor for TB, adenovirus, EBV, and CMV
every 2 weeks and as clinically indicated
Monitor for infusion-related reactions

Interactions
Avoid concomitant live or live attenuated
vaccines for at least 4 weeks after the last
dose
May reduce efficacy of CYP450
substrates: monitor and adjust dose of
these drugs

Adverse Reactions
Infections
Hypertension
Infusion-related
reactions
Pyrexia
Hypokalemia
Constipation
Rash
Abdominal pain
Diarrhea
Lymphocytosis
Cough
Irritability
Tachycardia
Tachypnea

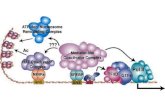
Mechanism of Action
Emapalumab-lzsg is a monoclonal antibody
that binds to and neutralizes interferon
gamma (IFNγ)
Nonclinical data suggest that IFNγ plays a
pivotal role in the pathogenesis of HLH by
being hypersecreted

Clinical Studies
Gamifant was evaluated in a multicenter,
open-label, single-arm trial (NI-0501-04) in 27
pediatric patients with suspected or confirmed
primary HLH with either refractory, recurrent,
or progressive disease during conventional
HLH therapy or who were intolerant of
conventional HLH therapy

Clinical Studies
Study treatment duration was up to 8 weeks
after which patients could continue
treatment on the extension study
Of the 27 patients, 22 enrolled onto the
open-label extension study which monitored
patients for up to 1 year after HSCT or after
the last infusion (NI-0501-05)
All patients received background
dexamethasone

Clinical Studies
Efficacy was based upon overall response
rate (ORR) at the end of treatment, defined
as achievement of either a complete or
partial response or HLH improvement
HLH improvement was defined as ≥3 HLH
abnormalities improved by ≥50% from
baseline

Clinical Studies
The data showed an ORR of 63% (95% CI,
0.42, 0.81; P =.013)
Complete response was seen in 26% of
patients
Partial response was seen in 30% of patients
HLH improvement was seen in 7.4% of
patients

Clinical Studies
Median duration of first response (defined as time from achievement of first response to loss of first response) was not reached
Range: 4 to 56+ days
70% of patients proceeded to HSCT
For more clinical trial data, see full labeling

New Product Monograph
For more information view the product
monograph available at:
https://www.empr.com/drug/gamifant/