Free-radical polymerization of dioxolane and dioxane derivatives: Effect of fluorine substituents on...
-
Upload
weihong-liu -
Category
Documents
-
view
215 -
download
3
Transcript of Free-radical polymerization of dioxolane and dioxane derivatives: Effect of fluorine substituents on...

Free-Radical Polymerization of Dioxolane and DioxaneDerivatives: Effect of Fluorine Substituents on the RingOpening Polymerization
WEIHONG LIU,1 FRANTISEK MIKES,1 YINZHONG GUO,1 YASUHIRO KOIKE,2,3 YOSHI OKAMOTO1*
1Polytechnic University, Polymer Research Institute, Six Metrotech Center, Brooklyn, New York 11201
2Keio University, Faculty of Science and Technology, Yokohama 223-8522, Japan
3Exploratory Research for Advanced Technology Organizer (ERATO), Koike Photonics Polymer Project,K2 Town Campus, 144-8 Ogura Saiwai-ku, Kawasaki 212-0054, Japan
Received 8 April 2004; accepted 14 May 2004DOI: 10.1002/pola.20309Published online in Wiley InterScience (www.interscience.wiley.com).
ABSTRACT: Partially fluorinated and perfluorinated dioxolane and dioxane derivativeshave been prepared to investigate the effect of fluorine substituents on their free-radical polymerization products. The partially fluorinated monomer 2-difluoromethyl-ene-1,3-dioxolane (I) was readily polymerized with free-radical initiators azobisisobu-tyronitrile or tri(n-butyl)borane–air and yielded a vinyl addition product. However, thehydrocarbon analogue, 2-methylene-1,3-dioxolane (II), produced as much as 50% ringopening product at 60 °C by free-radical polymerization. 2-Difluoromethylene-4-meth-yl-1,3-dioxolane (III) was synthesized and its free-radical polymerization yielded ringopening products: 28% at 60 °C, decreasing to 7 and 4% at 0 °C and �78 °C, respec-tively. All the fluorine-substituted, perfluoro-2-methylene-4-methyl-1,3-dioxolane (IV)produced only a vinyl addition product with perfluorobenzoylperoxide as an initiator.The six-membered ring monomer, 2-methylene-1,3-dioxane (V), caused more than 50%ring opening during free-radical polymerization. However, the partially fluorinatedanalogue, 2-difluoromethylene-1,3-dioxane (VI), produced only 22% ring opening prod-uct with free-radical polymerization and the perfluorinated compound, perfluoro-2-methylene-1,3-dioxane (VII), yielded only the vinyl addition polymer. The ring openingreaction and the vinyl addition steps during the free-radical polymerization of thesemonomers are competitive reactions. We discuss the reaction mechanism of the ringopening and vinyl addition polymerizations of these partially fluorinated and perflu-orinated dioxolane and dioxane derivatives. © 2004 Wiley Periodicals, Inc. J Polym Sci PartA: Polym Chem 42: 5180–5188, 2004Keywords: fluorinated 2-methylene-1,3-dioxolane; fluorinated 2-methylene-1,3-diox-ane; radical polymerization; ring opening polymerization; fluoropolymers
INTRODUCTION
Fluorinated polymers are of increasing interest inthe development of advanced materials with su-
perior thermal and chemical stability, possessingexcellent electrical as well as unique optical prop-erties.1–3 Recently, we have found that the par-tially fluorinated monomer, 2-difluoromethylene-1,3-dioxolane (I), readily polymerized with the free-radical initiators azobisisobutyronitrile (AIBN) ortri(n-butyl)borane–air.4 The polymer (I–P) ob-tained was not soluble in common organic sol-
Correspondence to: Y. Okamoto (E-mail: [email protected])Journal of Polymer Science: Part A: Polymer Chemistry, Vol. 42, 5180–5188 (2004)© 2004 Wiley Periodicals, Inc.
5180

vents such as chloroform and dimethyl sulfoxide(DMSO), but was soluble in fluorinated acidic sol-vents such as hexafluoroisopropanol (HFIP). I–Pwas semicrystalline and melted at 356 °C. Thethermogravimetric analysis of I–P indicated thatthe polymer was thermally stable under an airatmosphere; the onset of decomposition began at414 °C. The polymer was also found to be stable inconcentrated acidic and alkaline solutions. IR andNMR measurements indicated that there was noring opening product and the polymerization pro-ceeded through vinyl addition. However, the hy-drocarbon analogue, 2-methylene-1,3-dioxolane(II), has been reported to yield 50% ring openingat 60 °C, reaching 83% at 125 °C with radicalpolymerization.5 Bailey,6 Klemm,7,8 and cowork-ers extensively investigated the free-radical poly-merization of 2-methylene-1,3-dioxolane and2-methylene-1,3-dioxane derivatives. They ob-served that the radical polymerization of thesemonomers yielded mostly copolymers of the ringopening and vinyl addition components. The ratioof the ring opening and vinyl addition productswas found to be dependent on their substituentsand on the reaction conditions.
Recently, we have been interested in fluorine-containing polymers that are potentially usefulfor plastic optical fiber materials. This has there-fore prompted us to investigate the effect of fluo-rine substituents in dioxolane and dioxane mono-mers on the free-radical polymerization productsand on the polymerization mechanism.
EXPERIMENTAL
Materials
Solvents and starting materials were purchasedfrom Aldrich Chemical Company. CClF2CH(OH)2and hexafluoropropylene oxide were purchasedfrom SynQuest Fluorochemicals Laboratory. Allreagents were used without further purification.
Measurements
The 1H, 19F, and 13C NMR spectra were obtainedwith a Bruker ACF 300 spectrometer. NMR spec-tra for these perfluoropolymers were measured inhexafluorobenzene with deuterated chloroform asan internal locking solvent. Fourier transform in-frared (FTIR) spectra were obtained with aPerkinElmer FTIR-1600 spectrometer. Gas chro-matography/mass spectrometry (GC–MS) analy-
sis was accomplished on an HP 5890 gas chro-matograph and an HP 5970B mass spectrograph.Size exclusion chromatography (SEC) analysiswas accomplished on a system with a Waters 510pump in line with TSK gel HMXL and H5000columns, and with dual detectors: a Waters 440UV absorbance detector and a Waters R401 dif-ferential refractometer. Chloroform was used asan eluting solvent, with a flow rate of 1.0 mL/minat 30 °C. The molecular weight of the polymerswas calibrated with polystyrene (PS) standards.Differential scanning calorimetry (DSC) mea-surements were performed on a DSC 2920 modulein conjunction with a TA Instrument 5100 systemat a heating rate of 10 °C/min under a nitrogenatmosphere. The midpoint of the heat capacitytransition was taken as the glass-transition tem-perature (Tg). Thermogravimetric analysis (TGA)was performed on a Hi-Res modulated TGA 2950thermogravimetric analyzer under nitrogen at aheating rate of 10 °C/min.
Synthesis of 2-Difluoromethylene-1,3-dioxolane (I)
To a 1 L flask with a condenser, 185 g ofCClF2CH(OH)2 (�1.4 mol), 197 g of 2-bromoetha-nol (1.5 mol), and 20 g of CaCl2 were added. Themixture was heated to 90 °C for several hours andwas then cooled to room temperature. The upperlayer was decanted to a flask. Two liters of ace-tone and 386 g of K2CO3 (2.8 mol) were added tothe flask. After the reaction was kept at 50 °C for3 days, the solid was filtered out, and the solventwas removed by distillation. 2-Chlorodifluoro-methyl-1,3-dioxolane produced was purified bydistillation. Yield 88% (195 g), bp: 62 °C/30mmHg. 1H NMR (CDCl3, �, ppm): 5.24 ppm (t, 1H,OCHO), 3.9–4.3 ppm (m, 4H, OOCH2O). 19FNMR (CDCl3, �, ppm): �70.56 ppm (2F,OCF2O).
To a flask, 50 g (0.31 mol) of 2-chlorodifluoro-methyl-1,3-dioxolane and 800 mL of tetrahydro-furan (THF) were added. The flask was cooled inan ice–water bath. tBuOK (37 g, 0.33 mol) wasslowly added. The reaction was monitored bymeasuring 19F NMR. The signal at �70.56 ppm,ascribed to 2-chlorodifluoromethyl-1,3-dioxolane,decreased and the signal at �136.75 ppm, as-cribed to the product, appeared and increasedwith time. The conversion was calculated basedon the peak areas of the two signals. After theelimination of HCl exceeded 85%, the monomerproduced was collected in a cold trap (�78 °C)with THF, under reduced pressure. The productwas found to be readily polymerized upon heat-
FREE-RADICAL POLYMERIZATION 5181

ing. Thus, after removing some of THF under areduced pressure at 0 °C, the resulting THF so-lution containing the monomer (22.7 g) was usedfor the radical polymerization. 1H NMR (CDCl3, �,ppm): 4.36 (s, 4H); 19F NMR (CDCl3, �, ppm):�136.04 (s, CF2A).
Free-Radical Polymerization of 2-Difluoromethylene-1,3-dioxolane (I)
The monomer THF solution (100 mL, 40 mmol)and AIBN (65 mg, 0.4 mmol) were charged in aglass tube that was then degassed and refilledwith argon in three vacuum freeze–thaw cycles.The tube was sealed and heated at 60 °C for 1 day.The polymer produced was purified by precipita-tion from an HFIP solution into methanol. Yield:3.9 g (80%).
The intrinsic viscosity [�] of the polymer ob-tained with AIBN at 60 °C was found to be 0.38dL/g in a mixed solvent of CHCl3/TFA (9/1, v/v) at25 °C.
FTIR: no carbonyl absorption.1H NMR (CDCl3/TFA, �, ppm): 4.26 (OOCH2O
on the side group).19F NMR (CDCl3/TFA, �, ppm): �116.0 ppm
(OCF2O on the main chain).13C NMR (CDCl3/TFA, �, ppm): no carbonyl
carbon peak.DSC: Tg � 125 °C, Tm � 356 °C.TGA: 427 °C at the onset of decomposition un-
der a nitrogen atmosphere.
Synthesis of 2-Difluoromethylene-4-methyl-1,3-dioxolane (III)
This compound was prepared by a similar syn-thetic route to the one described for monomer I.1-Bromo-2-propanol, rather than 2-bromoetha-nol, was reacted with CClF2CH(OH)2 and HClwas then eliminated. Yield: 60%. 1H NMR(CDCl3, �, ppm): 4.55 (d, 1H), 4.24 (d, 1H), 3.70(m, 1H), 1.30 (d, 3H); 19F NMR (CDCl3, �, ppm):�138.50 (s, CF2A).
Free-Radical Polymerization of 2-Difluoromethylene-4-methyl -1,3-dioxolane (III)
The free-radical polymerization of III was inves-tigated under various conditions. The polymeriza-tions at 60 °C with AIBN as an initiator, at 0 °Cand �78 °C with (nBu)3B–air as an initiator, pro-duced polymers containing 28% ring opening
(60% conversion), 7% ring opening (52% conver-sion), and 4% ring opening structure (31% conver-sion), respectively. The amount of ring openingstructure units were calculated based on the 19FNMR measurements.
Based on the SEC analysis, the number-aver-age molecular weights [Mns (polydispersities)] ofthe polymers obtained at 60 °C, 0 °C, and �78 °Cwere 7070 (2.10), 9040 (1.84), and 7420 (1.91),respectively.
FTIR: carbonyl absorption at 1770 cm�1.1H NMR (C6F6/CDCl3, �, ppm): 0.80–1.80 (3H,OCH3), 3.30–3.90 (1H, OOCHO),4.00–4.80 (2H, OOCH2O)
19F NMR (CDCl3, �, ppm): �110.0 (OCF2O onthe polyacetal main-chain), �115.0 to�124.0 (OCF2O on the polyester main-chain).
13C NMR (CDCl3, �, ppm): carbonyl carbonpeak at 162.0 ppm.
Synthesis of Perfluoro-2-methylene-4-methyl-1,3-dioxolane (IV)
The monomer was prepared with modified meth-ods described in a U.S. patent.9 IV was preparedin a four step synthesis, starting from hexafluoro-propylene oxide via intermediates: perfluoropyruvyl fluoride, perfluoro-oxo-3,6-dimethyl-1,4-dioxane, and perfluoro-2,4-dimethyl-2-fluorocar-bonyl-1,3-dioxolane. The decarboxylation of thedried potassium perfluoro-2,4-dimethyl-2-fluoro-carbonyl-1,3-dioxolane was attempted with aloosely packed bed of anhydrous sodium carbon-ate at 295 °C under an argon stream in the gasphase.9 Monomer was obtained in a less than 5%yield. Therefore, perfluoro-2,4-dimethyl-2-fluoro-carbonyl-1,3-dioxolane was treated with 2 equivof aqueous KOH and the resulting mixture ofpotassium perfluoro-2,4-dimethyl-1,3-dioxolane-2-carboxylate and potassium fluoride was com-pletely dried under reduced pressure at 100 °C.The dried potassium of perfluoro-2,4-dimethyl-1,3-dioxolane-2-carboxylate and potassium fluo-ride (204.3 g) was charged into a two neckedround-bottom flask equipped with a short pathdistillation head and an argon inlet. Decarboxyl-ation was carried out at 250–320 °C. The rate ofdecarboxylation can be controlled by tempera-ture; the decomposition at 260–270 °C took anaverage of 1–2 h. The decomposition productswere collected in a series of connected traps,cooled in a mixture of ethanol and dry ice, and
5182 LIU ET AL.

washed with an aqueous NaOH solution (3 wt %).After the product was dried over anhydrousMgSO4, the crude monomer (54.8 g, yield: 44.4%)was fractionally distilled. The fractions were an-alyzed by GC–MS. 50.7 g of monomer IV wereobtained (Final yield: 41.1%). Bp: 44.3 °C. 19FNMR (CDCl3, �, ppm): �80.5 to �89.0(5F,OCF3,OCF2OOO), �126.0 (4 line pattern, CF2A, 2F),�129.2 (1F, OCFO).
Free-Radical Polymerization of Perfluoro-2-methylene-4-methyl-1,3-dioxolane (IV)
Polymerization of monomer IV was carried out,for most cases, in bulk in glass ampules. Themonomer (3–4 g) containing an initiator, perflu-orobenzoylperoxide (0.1–4.0 wt %) was filteredwith a 0.4 �m poly(tetrafluoroethylene) (PTFE)disk filter into the glass ampule. The ampule wassealed after degassing and refilling with argon inthree vacuum freeze–thaw cycles. Polymerizationwas carried out with a thermostat at the desiredtemperature (50–60 °C). The polymers obtainedwere dissolved in hexafluorobenzene, filtered,precipitated into chloroform, dried in vacuo to aconstant weight, and characterized.
Poly(perfluoro-2-methylene-4-methyl-1,3-di-oxolane) is soluble in hexafluorobenzene, Fluori-nert FC-75 (3M), at ambient temperature and inchloropentafluorobenzene at 65 °C. The polymeris insoluble in common organic solvents. The in-trinsic viscosity [�] of the polymer obtained at 60°C was 0.61 dL/g in hexafluorobenzene at 25 °C.
FTIR: no carbonyl absorption.1H NMR (C6F6/CDCl3, �, ppm): none.19F NMR (C6F6/CDCl3, �, ppm): �72.0 to �83.4
(3F, OCF3), �82.0 (OOCF2O), �105.78 to�112.80 (OCF2O on the main-chain),�120.74 to �125.08 (OOCFO on the sidechain).
13C NMR (C6F6/CDCl3, �, ppm): no carbonylcarbon peak.
DSC: Tg � 134 °C.TGA: 400 °C at the onset of decomposition un-
der a nitrogen atmosphere.
Synthesis of 2-Difluoromethylene-1,3-dioxane (VI)
To a 1 L flask with a condenser, 185 g (1.4 mol)CClF2CH(OH)2, 209 g(1.5 mol) 3-bromopropanol,and 20 g of CaCl2 were added. The flask washeated to 90 °C and was then cooled to roomtemperature. The upper layer was decanted to a 5
L flask. Two liters of acetone and 386 g (2.8 mol)of K2CO3 were added to the flask. After the reac-tion was kept at 50 °C for 3 days, the solid wasfiltered out and the solvent was removed by dis-tillation. The 2-chlorodifluoromethyl-1,3-dioxaneobtained was purified by distillation. Yield: 79%(191 g), bp: 36 °C/1.5 mmHg. 1H NMR (CDCl3, �,ppm): 4.83 (t, 1H, OCHO), 3.50–4.40 (m, 4H,OOCH2O), 1.45 (d, 1H, axial H onOCH2O), 2.0–2.2 (m, 1H, equatorial H on OCH2O). 19F NMR(CDCl3, �, ppm): �69.35 (2F, OCF2O).
To a flask, 55 g (0.32 mol) of 2-chlorodifluoro-methyl-1,3-dioxane and 500 mL of THF wereadded. The flask was cooled in an ice–water bath.tBuOK (37 g, 0.33 mol) was slowly added. Thereaction was monitored by measuring 19F NMR.The signal at �69.35 ppm, ascribed to 2-chlorodi-fluoromethyl-1,3-dioxolane, decreased and thesignal at �126.16 ppm, ascribed to the VI, ap-peared and increased with time. The conversionwas calculated based on the peak areas of the twosignals. After the elimination of HCl exceeded85%, the monomer produced was collected in acold trap (�78 °C) with THF under reduced pres-sure. VI was found to be readily polymerized uponheating and, hence, the isolation of the monomerfrom the THF failed. The THF solution was con-centrated by removal of the THF in vacuo at 0 °C.The obtained monomer solution of 225 mL in THF(ca. 15.0 g of monomer) was used for the radicalpolymerization. 1H NMR (CDCl3, �, ppm): 4.12and 4.76 (t, 4H, OOCH2O); 1.45 (m, 1H, axial HonOCH2O), 2.03 (m, 1H, equatorial H onOCH2O). 19F NMR (CDCl3, �, ppm): �126.31 (s,CF2A).
Free-Radical Polymerization of 2-Difluoromethylene-1,3-dioxane (VI)
The monomer solution (100 mL, ca. 50 mmol) andAIBN (65 mg, 0.40 mmol) were charged in a glasstube that was then degassed and refilled withargon in three vacuum freeze–thaw cycles. Thetube was sealed and heated at 60 °C for 1 day.After removal of most of the THF, the polymerwas obtained by precipitation into methanol.Yield: 2.4 g (35%).
Based on SEC analysis, the Mn and polydisper-sity of the polymer obtained with AIBN at 60 °Cwas 8220 and 1.72, respectively.
FTIR: carbonyl absorption at 1767 cm�1.1H NMR (CDCl3, �, ppm): 4.175 (OOCH2O in
the polyacetal portion), 4.412 and 3.601
FREE-RADICAL POLYMERIZATION 5183

(OOCH2O in the polyester portion), 1.80(OCH2O).
19F NMR (CDCl3, �, ppm): �104.82 (Mainpeak, OCF2O on the polyacetal main-chain), �106.0 to �116.0 (Small peaks,OCF2O on the polyester main-chain).
DSC: Tg � 44 °C.
Synthesis of Perfluoro-2-methylene-1,3-dioxane(VII)
1,3-Propanediol (2.0 mol), methyl pyruvate (2.0mol), DOWEX� ion-exchange resin (10 g), andabsolute benzene (1 L), were refluxed until theevolution of water ceased, in a flask fitted with aDean–Stark trap. The 2-methyl-2-methoxycar-bonyl-1,3-dioxane obtained was purified by frac-tional distillation. Yield: 60%. Bp: 45 °C/1.0mmHg; 19F NMR (CDCl3, �, ppm): 1.33–1.42 (d,1H, axial H), 2.01–2.19 (m, 1H, equatorial H),1.50 (s, 3H, OCH3), 3.90 (s, 3H, OCH3), 3.5–4.4(m, 2H, OOCH2O).
2-Methyl-2-methoxycarbonyl-1,3-dioxane (530g) was fluorinated with fluorine gas diluted withnitrogen in Fluorinert FC-75 at Exfluor ResearchCompany. After the reaction was terminated, themixture was treated with aq. KOH solution toform organic and water phases. The product inthe aq. phase was separated. A portion of thewater was removed under reduced pressure. Thepotassium salt of perfluoro-2-methyl-1,3-dioxane-2-carboxylic acid was obtained by filtration. Yield:90%. No mp. The onset of decomposition under anitrogen atmosphere was found to be 202 °C,based on TGA measurement. 1H NMR (CDCl3, �,ppm): none. 19F NMR (CDCl3, �, ppm): �81.53 (s,3F,OCF3), �87.47, �88.01, �93.83, �94. 48 (4F,OCF2), �129.36, 130.28, �142.81, �143.73 (2F,OCF2O);
The potassium salt (100 g, 0.289 mol) was de-composed at 250 °C to yield the product, whichwas collected in a trap cooled to �78 °C. Themonomer was purified by fractional distillation.Yield 42 g, 0.173 mol (60%). Bp: 38 °C. 1H NMR(CDCl3, �, ppm): none. 19F NMR (CDCl3, �, ppm):�110.10 (s, 2F,ACF2), �135.66 (m, 2F,OCF2O),�92.34 (t, 4F, OOCF2O); GC–MS: m/e 244 (M�)with the expected pattern.
Free-radical Polymerization of Perfluoro-2-methylene-1, 3-dioxane (VII)
The monomer (2 g) and perfluorobenzoylperoxide(13 mg) were charged in a glass tube that was
then degassed and refilled with argon in threevacuum freeze–thaw cycles. The tube was sealedin vacuo and heated at 80 °C for 1 week; a solidpolymer was obtained (0.10 g). Yield: 5.0%.
Based on end-group analysis with 19F NMR,the product was found to be an oligomer and theMn of the oligomer obtained at 80 °C was 4310.
FTIR: no carbonyl absorption between 1700and 1800 cm�1.
1H NMR (C6F6/CDCl3, �, ppm): none.19F NMR (C6F6/CDCl3, �, ppm): �82.0
(OOCF2O), �104.0 to �120.0 (OCF2O onthe main-chain), �130.0 to �138.7 (OCF2Oon the side chain).
13C NMR (C6F6/CDCl3, �, ppm): no carbonylcarbon peak.
DSC: Tg � 44 °C.
RESULTS AND DISCUSSION
Monomer Synthesis
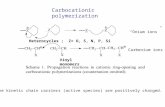
We have prepared several fluorinated dioxolaneand dioxane derivatives. The chemical structuresof the monomers along with their known hydro-carbon analogues are shown in Scheme 1.
Monomer I was prepared with the syntheticroutes shown in Scheme 2, as previously re-ported.4 Here, we report the details as explainedin the Experimental section. A new monomer,2-difluoromethylene-4-methyl-1,3-dioxolane (III),was also prepared by a synthetic route similar tothe one described for monomer I, with 1-bromo-2-propanol rather than 2-bromoethanol. Theperfluoro-2-methylene-4-methyl-1,3-dioxolane IVwas synthesized by the modified procedure re-
Scheme 1. Monomer structures.
5184 LIU ET AL.

ported in a U.S. patent.9 2-Difluoromethylene-1,3-dioxane VI was similarly synthesized by themethod shown in Scheme 2, with 3-bromopropa-nol rather than 2-bromoethanol. The detailedpreparation was also described in the Experimen-tal section. A new monomer, perfluoro-2-methyl-ene-1,3-dioxane VII, was synthesized with directfluorination in a liquid phase, according toScheme 3 (see details in the Experimental sec-tion).
Polymerization and Mechanism
Monomer I was found to be readily polymerizedupon heating. Thus, the monomer produced wascollected in a cold trap (�78 °C) with THF. Thesolution was used for radical polymerization withAIBN at 60 °C and also with tri(n-butyl)borane–air at �78 °C. The polymer obtained is not solublein common solvents such as THF, DMSO, andchloroform, but it is soluble in fluorinated acidicsolvents such as HFIP and trifluoroacetic acid(TFA) and also in a mixed solvent of chloroformand TFA. The intrinsic viscosity [�] of the poly-mer obtained with AIBN at 60 °C was found to be0.38 dL/g in a mixed solvent of CHCl3/TFA (9/1,v/v) at 25 °C. The 19F NMR spectrum indicatedthat no vinylidene fluorines were present. Thepeak at �116.0 ppm was assigned to theOCF2Oof the main chain. Only one peak in the 1H NMRspectrum at 4.26 ppm was detected and it wasassigned to the protons of the dioxolane ring. IRand NMR measurements indicated there was noring opening product, and the polymerization pro-ceeded through vinyl addition. However, its hy-drocarbon analogue, monomer II, has been re-ported to yield 50% ring opening product at 60 °Cwith radical polymerization. These results are
cited in Table 1, along with the results of otherdioxolane and dioxane derivatives.
The polymerization mechanism of 2-methyl-ene-1,3-dioxolane and their various substitutedderivatives has been widely investigated byBailey,6 Klemm,7,8 and their coworkers. The rad-ical initiator attacks the active carbon–carbondouble bond at the tail position and the resultingfree-radical can undergo vinyl addition polymer-ization or ring opening, as shown in Scheme 4.The ring opening isomerization and the vinyl ad-dition step are competitive reactions. The hydro-carbon analogue, monomer II, produced 50% ringopening polymers, indicating that the rates of thering opening isomerization and propagation of thevinyl addition polymerization were almost thesame. However, in the case of monomer I, thevinyl group is partially substituted with fluorineatoms on the outer position of the methylene andthe vinylidene group is then polarized. Therefore,the vinyl addition in the polymerization of I maybe more favorable when compared with the hy-drocarbon analogue (II). The transition state mayinvolve a relatively electrophilic radical speciesand a relatively nucleophilic double bond. Conse-quently, the propagation reaction through vinyladdition is appreciably faster than the isomeriza-tion reaction and results in a polymer without aring opening product.
The polymerizations of monomer III were per-formed under different conditions. The resultingpolymers were soluble in normal organic solventssuch as THF and chloroform. Hence, the molecu-lar weight and polydispersity can be determinedby SEC analysis; the data are listed in the Exper-imental section. The 19F NMR spectrum for thepolymers indicated that the vinylidene fluorinesat �138.50 ppm disappeared. The peak at �110.0
Scheme 2. Synthesis of 2-difluoromethylene-1,3-dioxolane, I.
Scheme 3. Synthesis of perfluoro-2-methylene-1,3-dioxane, VII.
FREE-RADICAL POLYMERIZATION 5185

ppm was assigned to the OCF2O on the polyac-etal main-chain and the signals between �115.0and �124.0 ppm were ascribed to theOCF2O onthe polyester main-chain. The ring opening con-tent can be calculated based on the 19F NMRspectrum. It was found that the polymer obtainedat 60 °C contains 28% ring opening units. Thismay be because when the methyl group was sub-stituted at the 4-position of I, the free-radicalintermediate produced by the ring opening step
may have had a resonance stabilization with themethyl group. Hence, the rate of the ring openingisomerization could compete with that of the vinyladdition reaction, resulting in a copolymer con-taining polyacetal and polyester units. It was alsonoted that a lower reaction temperature led to alower ring opening content. The mechanism isalso described in Scheme 4 (R4 � OCH3).
When all the hydrogens of III were substitutedby fluorine atoms, as in monomer IV, the free-
Table 1. Free-Radical Polymerization Products of Dioxolaneand Dioxane Derivatives
Monomers Initiator and Reaction ConditionsRing OpeningContent (%)
I AIBN (60 °C) None4
I Tri(n-butyl)borane–air (�78 °C) None4
II AIBN (60 °C) 505
II Di-t-butylperoxide (125 °C) 835
III AIBN (60 °C) 28a
III Tri(n-butyl)borane–air (0 °C) 7a
III Tri(n-butyl)borane–air (�78 °C) 4a
IV Perfluoro benzoyl peroxide (60 °C) Nonea
V Di-t-butylperoxide (130 °C) 855
VI AIBN (60 °C) 224
VII Perfluoro benzoyl peroxide (80 °C) Nonea
a Present work.
Scheme 4. Free-radical polymerization mechanism of the dioxolanes with or withoutring opening.
5186 LIU ET AL.

radical initiators such as AIBN and benzoyl per-oxide were not miscible with the perfluorinatedmonomer. Thus, the free-radical polymerizationof IV was performed with perfluorobenzoylperox-ide as the initiator. The 19F NMR spectrum forthe polymer indicated that the vinylidene fluo-rines at �126.0 ppm disappeared. The peak at�77.70 ppm was assigned to theOCF3 in the sidegroup. The peak between �105.78 and �112.80was assigned to the OCF2O on the main-chainand the peak between �120.74 and �125.08 ppmwas ascribed to the OOCFO in the side group.FTIR and 13C NMR spectra indicated that thepolymer contained only polyacetal units. Thismay be because the fluorinated ether bond,OCF2OOO, is known to be very strong10 and didnot cleave during the free-radical polymerization.
The free-radical polymerization of the six-membered ring monomer, 2-methylene 1,3-diox-ane V, has been reported by Bailey et al.5 Accord-ing to their report, V could be performed in pyri-dine with di-t-butyl peroxide at 130 °C, producing85% ring opening, thus, its behavior is not verydifferent from II. As a comparison to this series,the radical polymerization of 2-difluoromethyl-ene-1,3-dioxane VI was also polymerized at 60 °C,with AIBN as an initiator. The resulting polymerwas soluble in chloroform and THF. The FTIRspectrum of the polymer showed a sharp band at1760 cm�1, corresponding to the ester absorption.In the fluorine NMR, the sharp peak at �104.82ppm was assigned to the fluorines (OCF2O) onthe main-chain of the segment without a ringopening. The small peaks between �106.0 and�116.0 ppm were ascribed to the fluorines on thepolyester portion. The ring opening content of thepolymer was calculated based on the 19F NMRspectrum. The free-radical polymerization of VIproduced a 22% ring opening product at 60 °C, aspreviously reported.4
The exact cause for the partial ring openingprocess of VI is not clear at present. However, wemay postulate that the free-radical intermediateof I may experience significantly faster propaga-tion for the addition polymerization, whereas theradical intermediate of VI may allow more timefor the ring opening reaction. Thus, the ring open-ing isomerization step is competing with the vinyladdition propagation and results in the formationof a 22% ring opening product.
The free-radical polymerization of perfluoro-2-methylene-1,3-dioxane VII was performed inbulk, with perfluorobenzoylperoxide as the initi-ator. The polymerization rate of VII was very slow
and only 5% polymer was obtained after 1 week at80 °C. The polymer is soluble in fluorinated sol-vents such as hexafluorobenzene. In the 19F NMRspectrum, the peak at �82.0 ppm was assigned tothe OOCF2O in the side group. The signals be-tween �104.0 and �120.0 were assigned to theOCF2O on the main-chain and the broad peakbetween �130.0 and �138.7 ppm was ascribed tothe OCF2O in the side group. Furthermore, twosharp peaks at �139.4 ppm and 148.9 ppm alsoappeared and were ascribed to the ortho- andpara-fluorines in the fluorinated phenyl endgroup. The fluorine peak at the meta-positionoverlapped with the solvent peak. Thus, the Mncan be calculated based on end-group analysis,assuming termination by recombination. TheFTIR and the 13C NMR spectra indicated that thepolymer contained only the vinyl addition prod-uct. This may also be because of the higher bondstrength of OOCF2, which did not cleave in thefree-radical polymerization process.
CONCLUSIONS
The partially fluorinated and perfluorinated dioxo-lane and dioxane monomers were synthesized. Thefree-radical polymerizations were performed tostudy the polymerization mechanism. We foundthat the monomers with fluoro-substituents haveless tendency toward ring opening compared totheir hydrocarbon analogues. The polymerizationsof the fluorinated monomers proceeded primarilythrough vinyl addition and resulted in chemicallyand thermally stable polymers that are soluble influorinated solvents. These polymers may havepotential applications as plastic optical fibers,waveguides, coatings, and photoresist materials.
This work was supported in part by the Japan Scienceand Technology Corporation through a Grant fromERATO–Photonic Polymer.
REFERENCES AND NOTES
1. Feiring, A. F. In Organofluorine Chemistry Princi-ples and Commercial Applications; Banks, R. E.;Smart, B. E.; Tatlow, J. C., Eds.; Plenum Press:New York, 1994; Chapter 15, p 339.
2. Smart, B. E.; Feiring, A. E.; Krespan, C. G.; Yang,Z. Y.; Hung, M. H.; Resnick, P. R.; Dolbier, W. R.,Jr.; Rong, X. X. Macromol Symp 1995, 98, 753–767.
3. Hung, M. H.; Resnick, P. R.; Smart, B. E.; Buck,W. H. In Polymeric Materials Encyclopedia; Salam-
FREE-RADICAL POLYMERIZATION 5187

one, J. C., Ed.; CRC Press: Boca Raton, 1996; pp2466–2476.
4. Liu, W.; Guo, Y.; Koike, Y.; Okamoto, Y. Macromol-ecules 2004, 37, 254–255.
5. Bailey, W. J.; Ni, Z; Wu, S. J Polym Sci PolymChem Ed 1982, 20, 3021–3030.
6. Bailey, W. J. In Ring Opening Polymerization;McGrath, J. E., Ed.; ACS Symposium Series 286;American Chemical Society: Washington, DC,1985; p 47.
7. Schulze, Th.; Letsch, J.; Klemm, E. J Polym SciPart A: Polym Chem 1996, 34, 81–87.
8. Klemm, E.; Schulze, Th. Acta Polym 1999, 30,1–19.
9. Selman, S.; Squire, E. N. U.S. Patent 3,308,107,1967.
10. Lagow, R. J.; Bierchenk, T. R.; Juhlke, T. J.; Kawa,H. In Synthetic Fluorine Chemistry; Olah, G. A.;Chambers R. D.; Prakash, G. K. S., Eds.; Wiley:New York, 1992; Chapter 5.
5188 LIU ET AL.