Form Builder: Hopkins-wide Data Collection Tool for Research Projects
-
Upload
carla-medina -
Category
Documents
-
view
27 -
download
1
description
Transcript of Form Builder: Hopkins-wide Data Collection Tool for Research Projects
Form Builder: Hopkins-wide Data Collection
Tool for Research Projects
Joseph Finkelstein, MD, PhDChronic Disease Informatics Program
Johns Hopkins University
CRMS is a web-based tool designed to organize and streamline clinical research management
CRMS is supported by The Johns Hopkins Institute for Clinical and Translational Research (ICTR)
It is designed to improve communication among study team members, store subject enrollment information in a secure location, and run real-time reports
CRMS modules◦ Subject Recruitment, Case Report Forms (Form Builder),
Subject & Protocol Registry, Specimen Tracking, Protocol Schema & Patient Calendar, Data Warehouse, Financial Management
Clinical Research Management System (CRMS)
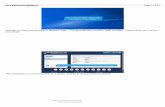
Form Builder is one component of CRMS
This web-based tool allows researchers to build forms that are scientifically rigorous and user-friendly
Form Builder
Form Level◦ Form creation, storage, modification, deletion◦ Data collection using web-based CRF◦ Embedded CRF instructions◦ Assigning multiple categories to the forms◦ Form search
Research Protocol Level◦ Support main requirements for controlled randomized trials which
include: Double entry, Blinding, Scoring, Reporting, Alerting, and Exporting operations with CRF for each trial
◦ Ability to limit access to their CRF by members of other research protocols
Enterprise Level◦ Maintaining and warehousing of research data coming from different
research protocols◦ Implement industry standards for naming and transmission of research
data◦ Ability to operate and store CRF in Hopkins data warehouse on an
aggregate level
Basic Functionality
http://cdip.med.jhmi.edu/intro/Investigators/investigators_main.aspx
Email [email protected]
Any questions