Fluid Electrolyte Notes
-
Upload
smilechance8 -
Category
Documents
-
view
212 -
download
0
Transcript of Fluid Electrolyte Notes
-
8/22/2019 Fluid Electrolyte Notes
1/3
Cations transmit nerve impulses to muscles and contract skeletal and smooth muscles (K, Na,Ca, Mg), positive charge (t is like the plus sign)
Anions attached to cations (CL, HCO3, PO4, SO4), negative charge (n reminds you ofnegative)
Osmolality concentration of body fluid, normal is 275 295, if less is hypo-osmolar (result ofexcess water intake or fluid overload caused by an inability to excrete excess water, if more ishyperosmolar (caused by severe diarrhea, increased salt and solutes (protein) intake, inadequatewater intake, diabetes, ketoacidosis or sweating)
Sodium main extracellular electrolyte, major fxn is to regulate body fluids
Isotonic solutions D5W, NS, LR, Ringers solution, very similar to extracellular and intracellularfluids, used with fluid volume loss; D5W when given rapidly or continuously will become hypotonicbecause dextrose is rapidly metabolized into water and carbon dioxide
Crystalloids dextrose, saline, LR, used for replacement and maintenance fluid therapy
Colloids volume expanders, dextran solutions, amino acids, hetastarch, plasmanate, dextran is
not a substitute for whole blood because it doesnt have any products that can carry oxygen,hetastarch is isotonic and can decrease platelet and hematocrit counts and is contraindicated inbleeding disorders, CHF, renal dysfunction, plasmanate can be used instead of plasma oralbumin to replace body protein
Blood and blood products whole blood, packed RBCs, plasma, albumin
Lipids fat emulsion solutions, indicated when IV therapy lasts longer than 5 days
Daily water need 2000 ml/day average, 15 ml/pound, increase by 15% if pt has fever
Potassium 20 times more prevalent in cells than in vessels, 3.5 5.3, narrow normal range, toolittle or too much can lead to cardiac arrest, poorly stored in the body so give it daily, bananas
and dried fruits are higher than oranges and fruit juices, necessary for transmission andconduction of nerve impulses and for contraction of skeletal, cardiac and smooth muscles; givenwith an anion (ie. Chloride or bicarb), extremely irritating to the GI and intestinal tract so give withglass of fluid, IV K must be diluted in IV fluids, cannot be given as a bolus or push, always mustbe diluted
Hypokalemia when cells are damaged from trauma, injury, surgery or shock, potassium leaksfrom the cells into the intravascular fluid and is excreted by the kidneys, with cellular loss ofpotassium it shifts from the blood plasma into the cell to restore the cellular potassium balanceleading to hypokalemia, vomiting and diarrhea also decreases K levels; s/s nausea, vomiting,dysrhythmias, abdominal distention, soft flabby muscles; for low levels encourage foods high inpotassium (ie. fruit juice, citrus fruits, dried fruits, bananas, nuts, veggies); certain drugs promoteK loss hydrochlorothiazide, Lasix (potassium-wasting diuretics), cortisone preparations; if they
take these drugs they need to eat more foods with potassium; use potassium cautiously in ptswith renal insufficiency, be cautious is urine output is less than 600 ml/day
Drugs there are potassium-wasting diuretics (excrete K, Na, Cl in the urine) and potassium-sparing diuretics (retain K but excrete Na, Cl in the urine); laxatives, corticosteroids, antibiotics,potassium-wasting diuretics are the major drug groups that cause hypokalemia; oral and IVpotassium salts, CNS agents, potassium-sparing diuretics can cause hyperkalemia
Hyperkalemia if the kidneys shut down or are diseased, K accumulates in the intravascularfluid, caused by renal insufficiency or administration of large doses of K over time, for mild
-
8/22/2019 Fluid Electrolyte Notes
2/3
elevation restrict foods high in K, to immediately decrease K levels use sodium bicarbonate,calcium gluconate or insulin and glucose or Kayexalate with sorbitol (this drug therapy exchangesa NA ion for a K ion in the body and is a more permanent means of correcting hyperkalemia), s/s
nausea, abdominal cramps, oliguria, tachycardia or late bradycardia, weakness, numbness ortingling in the extremities
Sodium major cation in the ECF, normals 135-145, regulates body fluids, promotes thetransmission and conduction of nerve impulses, part of the sodium/potassium pump that causescellular activity; Na shifts into cells as K shifts out of cells repeatedly to maintain water balanceand neuromuscular activity, Na combines readily with Cl or HCO3 to promote acid-base balance
Hyponatremia can result from vomiting, diarrhea, surgery, potent diuretics; s/s muscleweakness, headaches, abdominal cramps, nausea, vomiting; can give NS to increase sodiumcontent in the vascular fluid
Hypernatremia requires sodium restriction; s/s flushed skin, elevated body temp, elevated BP,rough dry tongue; can result from consuming certain drugs (cortisone, cough meds, someantibiotics)
Water essential nutrient, more important to life than any other nutrient, body needs more water
each day than any other nutrient, can survive only a few days without water, minerals help thebody maintain an appropriate balance and distribution of water, 60% of an adults body weight,more in a child
Carries nutrients and waste productsMaintains the structure of large moleculesParticipates in metabolic reactionsServes as a solvent for minerals, vitamins, amino acids, glucose
Acts as a lubricant and cushion around joints, eyes, spinal cordAids in regulation of normal body tempMaintains blood volume
Water intake when the blood becomes concentrated (having lost water but not the dissolvedsubstances in it) the mouth gets dry and the hypothalamus initiates drinking behavior, thirst lags
behind the bodys need, first sign of dehydration is thirst, if you cant get fluid or dont perceive thethirst message you get dehydrated rapidly; water intoxication leads to hyponatremia
Water sources water itself and other beverages, fruits and veggies have up to 90% water,meats and cheeses contain 50%, water is also generated during metabolism, caffeine can becounted towards total intake, alcohol acts as a diuretic and dehydrates you
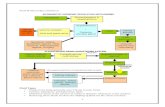
Fluids maintain blood volume which, in turn, influences blood pressure, central to the regulation ofblood volume and BP are the kidneys, instructions on whether to retain or release substances orwater comes from ADH, rennin, angiotensin and aldosterone (see Figure 12-3, pg 401 Nutrition)
ADH retains water; (also called vasopressin) whenever BP or blood volume falls too low orextracellular fluid becomes too concentrated they hypothalamus signals the pituitary gland to
release ADH, this is a water conserving hormone that stimulates the kidneys to reabsorb water,so the more water you need the less your kidneys excrete, this also triggers thirst
Renin retains sodium; cells in the kidney release renin in response to low blood pressurecausing the kidneys to reabsorb sodium, sodium reabsorption is always accompanied by waterretention which restores blood volume and BP
Angiotensin constricts blood vessels; renin also activates angiotensin which is a powerfulvasoconstrictor which raises BP
-
8/22/2019 Fluid Electrolyte Notes
3/3
Aldosterone retains sodium; angiotensin causes the release of aldosterone from the adrenalglands, it signals the kidneys to retain more sodium and water, the effect is that when more wateris needed less is excreted
Cells must maintain a balance of 2/3 body fluids inside the cells and 1/3 body fluids outsidecells, if too much water enters then the cell can rupture, if too much water leaves cells cancollapse; to control the movement of water, the cells direct the movement of the major minerals
If an anion enters the fluid, a cation must accompany it or another anion must leave so thatelectrical neutrality is maintained, its a good bet that whenever Na and K ions are moving, theyare going in opposite directions
Electrolytes attract water, some electrolytes reside outside cells (sodium, chloride) and someinside cells (K, Mg, PO4, SO4), cell membranes are selectively permeable (they allow passage ofsome molecules but not others), whenever electrolytes move across the membrane water follows,proteins attract water and help to regulate fluid movement, regulation occurs mainly in the GI tractand the kidneys
Na and Cl are the most easily lost because they are the primary extracellular electrolytes,sweating, bleeding or excretion
Start at pg 404, Acid-Base balance