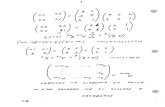FE Thermo Review
description
Transcript of FE Thermo Review

UNIVERSITY OF
FLORIDA Fundamentals Exam
Thermodynamics Review
Fundamentals Exam

UNIVERSITY OF
FLORIDA
Fundamentals Exam
I assume you have applied?!?
Morning Session:
General – 120 questions, ½ pt each
Afternoon Session:
General or Discipline Specific- 60 questions, 1 pt each

UNIVERSITY OF
FLORIDA
Fundamentals Exam
What to do in the afternoon?
Your call...pass/fail is about the same.
Preparation easier for general.

UNIVERSITY OF
FLORIDA
Fundamentals Exam
Morning Session:
11 out of 120 thermo questions
Afternoon General Session:
6 out of 60 thermo questions

UNIVERSITY OF
FLORIDA
Fundamentals Exam
NCEES Reference Handbook
Have you got it?
Why not?
How do you get it?
How do you use it?
www.ncees.org

UNIVERSITY OF
FLORIDA
Fundamentals Exam
Morning session
Generally unrelated. About 2 minutes per question.
Fast recall essential.
Use a marking system to keep track of your progress.
Afternoon: 4 minutes per question.

UNIVERSITY OF
FLORIDA
Fundamentals Exam
Process of Elimination
Cross out wrong answers first.
Wrong answers are sometimes easier to find than right ones! Units on
answer is sometimes a clue.
Answers are seldom given with more than 3 sig figs, your choice should be
the closest to your solution.

UNIVERSITY OF
FLORIDA
Fundamentals Exam
Guessing
No penalty for guessing.
Leave 10 minutes for each session for “educated” guessing.

UNIVERSITY OF
FLORIDA
Fundamentals Exam
Hint:Write correct answer in the margin of your test booklet beside the question and wait to you get to the end of the
page before transferring to the answer key.
Lookout for those long, drawn out questions… questions with four
paragraphs for answers!!

UNIVERSITY OF
FLORIDA
Fundamentals ExamTry working the following problem (you have two minutes for each type problem like this):If a sample experiencing a change of temperature from 23 deg C to 46 deg C also experiences a change in specific enthalpy of 120 kJ/kg, of what material is the sample most likely to be composed? You can use the data in the NCEES Supplied Reference Handbook or the tables in the back of your book. Better to practice these problems with your NCEES handbook…..remember to be at one with this book!!
Did you get Helium?There are sample tests on the NCEES website. Try these out. There are also sample tests in books like Barron’s How to Prepare for the Fundamentals of Engineering FE/EIT Exam. The bookstores have books like this to help you review for the exam.

UNIVERSITY OF
FLORIDA
Fundamentals Exam
Select the best response for an isolated system.
a. The entropy of the system remains constant
b. The heat transfer equals the work done
c. The heat transfer equals the internal energy change
d. The heat transfer is zero.

UNIVERSITY OF
FLORIDA
Fundamentals Exam
Select the best response for an isolated system.a. The entropy of the system remains constantb. The heat transfer equals the work donec. The heat transfer equals the internal energy
changed. The heat transfer is zero.For a closed thermodynamic system:Q – w = U + KE + PE, isolated implies Q = W = 0, (d) is the answer, (b) is close but not
complete

UNIVERSITY OF
FLORIDA
Fundamentals Exam
Two kilograms of air are contained in a cylinder. If 80 kJ of heat are added to the air, estimate the temperature rise if the pressure is held constant. Cp = 1.0 kJ/kgK, Cv = 0.716kJ/kgK and k = 1.4.
a. 56 deg Cb. 40 deg Cc. 33 deg Cd. 28 deg C

UNIVERSITY OF
FLORIDA
Fundamentals Exam
Two kilograms of air are contained in a cylinder. If 80 kJ of heat are added to the air, estimate the temperature rise if the pressure is held constant. Cp = 1.0 kJ/kgK, Cv = 0.716kJ/kgK and k = 1.4.
a. 56 deg Cb. 40 deg Cc. 33 deg Cd. 28 deg CAnswer is (b) Q = mh = m CpT, 80 =
2*1.0*T, T= 40 deg C

UNIVERSITY OF
FLORIDA
Fundamentals Exam
Steam at high temperature and pressure passes through a half open globe valve. Select the property that remains constant through the valve.
a. enthalpy
b. temperature
c. pressure
d. entropy

UNIVERSITY OF
FLORIDA
Fundamentals Exam
Steam at high temperature and pressure passes through a half open globe valve. Select the property that remains constant through the valve.
a. enthalpyb. temperaturec. pressured. entropyAnswer is (a), energy equation q-ws = h + pe +
ke, q = 0, ws = 0, pe = 0 therefore h = 0, an isenthalpic process, enthalpy is constant

UNIVERSITY OF
FLORIDA
Fundamentals Exam
For an isentropic process of an ideal gas (k= 1.4), with an initial pressure of 50 pounds per square inch absolute, an initial specific volume of 8.2 cubic feet per pound mass, and a final pressure of 120 psia, what is the final value of the specific volume?
a. 8.2 cubic feet/lbmb. 3.42 cubic feet/lbmc. 19.7 cubic feet/lbmd. 4.39 cubic feet/lbm

UNIVERSITY OF
FLORIDA
Fundamentals ExamFor an isentropic process of an ideal gas (k= 1.4), with an
initial pressure of 50 pounds per square inch absolute, an initial specific volume of 8.2 cubic feet per pound mass, and a final pressure of 120 psia, what is the final value of the specific volume?
a. 8.2 cubic feet/lbmb. 3.42 cubic feet/lbmc. 19.7 cubic feet/lbmd. 4.39 cubic feet/lbmAnswer is (d). P1v1
k = P2v2k, v2 = v1(P1/P2)1/k
= (8.2)*(50/120)1/1.4 = 4.39 ft3/lbm

UNIVERSITY OF
FLORIDA
Fundamentals Exam
How much energy must be transferred through heat interaction to raise the temperature of a 4 kilogram sample of methane in a closed system from 15 deg C to 35 deg C?
a. 34.8 kJ
b. 45 kJ
c. 139 kJ
d. 180 kJ

UNIVERSITY OF
FLORIDA
Fundamentals Exam
How much energy must be transferred through heat interaction to raise the temperature of a 4 kilogram sample of methane in a closed system from 15 deg C to 35 deg C?
a. 34.8 kJb. 45 kJc. 139 kJd. 180 kJAnswer is ( c). Q- w = U + KE + PE Closed
system. Q = U = mu = mCvT = (4kg)*(1.74kJ/kg K)*(35-15 deg C)= 139.2 kJ

UNIVERSITY OF
FLORIDA
Fundamentals Exam
A tank contains 0.02m3 of liquid and 1.98 m3 of vapor. If the density of the liquid is 960 kg/m3 and that of the vapor is 0.5kg/m3, what is the quality of the mixture?
a. 5.2%
b. 4.9%
c. 2.04%
d. 1.01%

UNIVERSITY OF
FLORIDA
Fundamentals Exam
A tank contains 0.02m3 of liquid and 1.98 m3 of vapor. If the density of the liquid is 960 kg/m3 and that of the vapor is 0.5kg/m3, what is the quality of the mixture?
a. 5.2%b. 4.9%c. 2.04%d. 1.01%Answer is (b). x = mg/(mg + mf) =
(1.98)*(.5)/((1.98*0.5)+(0.02*960)) = 0.049 or 4.9%

UNIVERSITY OF
FLORIDA
Fundamentals Exam
Which of the following is an intensive property?
a. Pressureb. Entropyc. Internal Energyd. Enthalpy
Answer is (a) Pressure does not depend on mass , extensive properties are proportional to mass

UNIVERSITY OF
FLORIDA
Fundamentals ExamWhich of the following devices is possible?a. A cyclic machine that will experience no other interaction than to produce energy
through a work interaction, while transferring energy from a high-temperature reservoir to a low-temperature reservoir through heat interactions.
b. A cyclic machine that will experience no other interaction than to transfer to a thermal reservoir an amount of energy equal to the amount of energy it receives from a work interaction.
c. A device that will change the thermodynamic state of a material from on equilibrium state to another without experiencing a change in the amount of energy contained in the material, in the amount of material, or in the external forces placed on the material.
d. A cyclic machine that will experience no other interaction than to accept from a heat interaction with a high-temperature reservoir an amount of energy equal to the amount of energy it receives from a work interaction.
Answer is (a). Note that this problem takes about a minute to read!! You better understand this one as you read it or you won’t do this in 2 minutes!! (b) is not right because entropy decreases continuously, (c) is not right because you can’t be in two equilibrium states, (d) is not correct because energy is increasing continuously

UNIVERSITY OF
FLORIDA
Fundamentals ExamEnergy is added in the amount of 50 kJ in a heat interaction
to a closed system while 30 kJ of work is done by the system. The change of the internal energy of the system is:
a. 80 kJb. 20 kJc. –20kJd. –80kJ
Answer is (b). E = Q-W = +50kJ-(+30kJ) = 20 kJThis is an example of how you need to know the first law and know the correct sign conventions for work and energy. Q into a system is + W done by a system is + (remember it is a positive thing to
get work out of a student!!)

UNIVERSITY OF
FLORIDA
Fundamentals Exam
Devices:TurbinesCompressorsDiffusersNozzlesThrottling DevicesHeat Exchangers

UNIVERSITY OF
FLORIDA
Fundamentals Exam
Devices:TurbinesCompressors
mi’(hi +Vi2/2 + gzi) –me’ (he +Ve
2/2 + gze)+Q’ –W’ = 0
Assume well insulated, assume Vi=Ve
and steady flow mi’ = me’,and zi = ze
Then: m’(hi – he) = W’Compressors: W’ is –Turbines: W’ is +

UNIVERSITY OF
FLORIDA
Fundamentals Exam
Devices:Nozzles and Diffusers
mi’(hi +Vi2/2 + gzi) –me’ (he +Ve
2/2 + gze)+Q’ –W’ = 0
Assume well insulated, no shaft work, and steady flow mi’ = me’,and zi = ze
Then: (hi – he +Vi2/2 -Ve
2/2 ) = 0
Ve > Vi for nozzles and Ve<Vi for diffusers

UNIVERSITY OF
FLORIDA
Fundamentals Exam
Devices:Throttling Device
mi’(hi +Vi2/2 + gzi) –me’ (he +Ve
2/2 + gze)+Q’ –W’ = 0
Assume well insulated, assume Vi=Ve
and steady flow mi’ = me’, zi = ze and no shaft work
Then: (hi – he) = 0 isenthalpic

UNIVERSITY OF
FLORIDA
Fundamentals Exam
Devices:Heat Exchangers
mi’(hi +Vi2/2 + gzi) –me’ (he +Ve
2/2 + gze)+Q’ –W’ = 0
Assume well insulated, assume Vi=Ve
and steady flow mi’ = me’, no shaft work and zi = ze
Then: m’(hi – he) = Q’

UNIVERSITY OF
FLORIDA
Fundamentals Exam
Cycles:CarnotReversed CarnotOttoRankineRefrigerationAfternoon: if mechanical: two stage refrig
cycle, air refrigeration, psychrometric cycles, Brayton cycle, Brayton cycle with regeneration, etc.

UNIVERSITY OF
FLORIDA
Fundamentals Exam
Review state functions, specifically the concept of quality, x
u = xug + (1-x)uf
h = xhg + (1-x)hf
s = xsg + (1-x)sf
v = xvg +(1-x)vf
Look at Rankine cycle, steam quality out of a turbine

UNIVERSITY OF
FLORIDA
Thermodynamics Review
A Carnot engine operates between 300°C and 40 °C.What is the efficiency of the engine?
(A) 87%(B) 65%(C) 45%(D) 30%

UNIVERSITY OF
FLORIDA
Thermodynamics Review
Solution: (C)
See Page 49 in Reference Handbook
Carnot Cycle efficiency:
%4545.)273300(
)27340(1
/1/)(
K
K
TTTTT
c
HLHLHc
Not (A): If you got (A) you didn’t use the absolute temperature units.
“When in doubt use absolute.”
%45%7.868666.300
401
C
Co
o
c

UNIVERSITY OF
FLORIDA
Thermodynamics Review
Refrigerant 134a is isentropically compressed in a compressor from a saturated vapor state at 0.4 MPa pressure to 2 MPa pressure. The work required to run the compressor is:
(A)130 kJ/kg
(B) -100 kJ/kg
(C) 100kJ/kg
(D)-60kJ/kg

UNIVERSITY OF
FLORIDA
Thermodynamics Review
Solution: (B)
See Pages 48 and 55 in Reference Handbook for First Law (energy balance) and p-h table for Refrigerant 134aUse the Chart:The question states that the refrigerant is in a saturated vapor state, therefore the enthalpy can be obtained by finding the intersection line for the given pressures and the right side of the “dome” respectively.
and
The exit enthalpy has the same s as the inlet but at a higher P.
Energy balance:
The answer is negative because work is put into the system.
)/(100)/(430)/(330 kgkJkgkJkgkJhhW ei
)/(330 kgkJhi )/(430 kgkJhe

UNIVERSITY OF
FLORIDA
Thermodynamics
The Air Standard Assumptions
• for gas powered cycles
• for Otto cycle, diesel cycle and Brayton cycle
• the working fluid is air which continuously circulates through the system, acts as an ideal gas
• all processes are internally reversible
• combustion process is approximated by a heat addition process from external source
• exhaust process is approximated by a heat rejection process

UNIVERSITY OF
FLORIDA
Thermodynamics
The Cold Air Standard Assumptions
• all of above assumptions
• assumes specific heats are constant and are evaluated at 25 deg C or 77 deg F
• all of the above assumptions are made to simplify a very complex cycle

UNIVERSITY OF
FLORIDA
Thermodynamics
The Otto Cycle, aka SIE, Spark Ignition Engine
• This cycle applies to two stroke and four stoke engines. It is an ideal representation of the process.
1-2: isentropic compression (compression stroke)
2-3: constant volume heat addition (power stroke)
3-4: isentropic expansion (exhaust stroke)
4-1: constant volume heat rejection (intake stroke)

UNIVERSITY OF
FLORIDA
Thermodynamics

UNIVERSITY OF
FLORIDA
ThermodynamicsModel this as a closed system. No major changes in kinetic or potential energy.
Energy equation becomes:
(qin-qout)+(win-wout) = u
But more importantly, let’s look at the processes from 2 to 3 and 4 to 1:
qin = u3-u2 = Cv(T3-T2)
qout = u4 – u1 = Cv(T4 – T1)
(no work done during these processes)
th,Otto = wnet/qin
Looking at the first law, in = out
wnet = qin – qout
Therefore,
th,Otto = wnet/qin = (qin-qout)/qout

UNIVERSITY OF
FLORIDA
Thermodynamicsth,Otto = wnet/qin = (qin-qout)/qout
Substituting for qin and qout
th,Otto = 1 – ((T4-T1)/(T3-T2)
Since 1 to2 and 3 to 4 are isentropic processes, we can substitute our isentropic relationships for T and v.
Therefore, (see derivation on page 360)
1
11
kthOtto r
Where the compression ratio r = V1/V2 = v1/v2
And k = specific heat ratio = Cp/Cv

UNIVERSITY OF
FLORIDA
Thermodynamics
Let’s look at an example:
Assume a compression ratio of 8.
Assume air at the beginning of the process is at 17 deg C and 100KPa.
Assume 800 kJ/kg of heat is added in the heat addition process (this would be equivalent to the fuel added to the cycle).
Assume constant specific heats.
The efficiency of this cycle would equal:
%5.56565.08
11
11
)14.1(1or
r kthOtto

UNIVERSITY OF
FLORIDA
ThermodynamicsLet’s find the temperature at 3. This would be the maximum temperature our equipment would have to tolerate.
qin= 800kJ/kg = Cv(T3-T2)=0.718*(T3 – T2)
We need T2 to solve for T3.
From the isentropic relationships,
T1/T2 = (V2/v1)k-1
Note that r = v1/v2,
Therefore,
T1/T2 = (1/r)k-1
And T2 = 290K/(1/8) (1.4-1)
T2 = 666 K
So T3 = (800/0.718)+666 = 1780 K or 2745deg F
Can also do this assuming variable specific heats.

UNIVERSITY OF
FLORIDA
ThermodynamicsLet’s find the pressure at 3. This would be the maximum pressure our equipment would have to tolerate.
P1v1/T1 = P2v2/T2
Therefore P2 = P1v1T2/v2 T1
P2 = 100kPa(666K/290K)(8) = 1837 kPa
And
P2v2/T2 = P3v3/T3
P3 = P2T3v2/T2v3
P3 = (1837kPa)(1780K/666K)(1)
Note v2 = v3
P3 = 4910 kPa or 712 psia!!

UNIVERSITY OF
FLORIDA
ThermodynamicsThe Brayton Cycle
• Used to analyze gas turbines (where compression and expansion occur using rotating machinery)
• Gas Turbines usually operate as an open cycle
• Used in aircraft propulsion and electric power generation
Exhaust propels craft or used to generate steam.
http://travel.howstuffworks.com/turbine3.htm

UNIVERSITY OF
FLORIDA
ThermodynamicsSimple Ideal Brayton Cycle
1-2: isentropic compression
2-3: constant pressure heat addition
3-4: isentropic expansion
4-1: constant pressure heat rejection
Modeled as a closed cycle. Air standard assumptions are applied. Air is the working fluid.

UNIVERSITY OF
FLORIDA
ThermodynamicsModel this as a closed system. No major changes in kinetic or potential energy.
qin = h3 – h2 = Cp (T3 – T2)
-qout = h1 – h4 = Cp(T1 – T4)
Or qout = Cp(T4 – T1)
th,Brayton = wnet/qin
Looking at the first law, in = out
wnet = qin – qout
Therefore,
th,Brayton = wnet/qin = (qin-qout)/qin
th,Brayton = 1 – T1(T4/T1-1)/T2(T3/T2-1)
Substituting the isentropic relationships for T as a function of P and realizing that P2=P3 and P1 = P4, th,Brayton = 1 – 1/rp
(k-1)/k
where rp = pressure ratio = P2/P1

UNIVERSITY OF
FLORIDA
Thermodynamics
• For gas turbine engines, the rp ranges from 5 t0 20.
• Since some of the turbine work goes to run the compressor, there is another term used to describe this cycle:
the back work ratio = Compressor work/turbine work.
• Usually more than half the turbine work goes to run the compressor.
• The back work ratio for steam power plants is very low in comparison.
• Gas turbines used in power plants can be brought on line very quickly whereas the Rankine cycle steam cycles take a lot of time to bring up to speed. This is why gas turbine engines are used as “peaking” units.
• With improvements in firing temperature, turbomachinery efficiency and heat recovery, the gas turbine power generating systems are now comparable to steam plants in performance, especially when the waste heat is combined with a Rankine cycle plant (bottoming cycle).

UNIVERSITY OF
FLORIDA
Thermodynamics
Let’s look at an example:
Assume a pressure ratio of 8.
Assume the air at the compressor inlet (pt 1)is at 300K (room temperature).
Assume the air at the turbine inlet(pt 3) is at 1300 K. (1880 deg F)
Find the gas temperatures at the exits of the turbine and compressor.
Find the back work ratio.
Find the thermal efficiency.

UNIVERSITY OF
FLORIDA
ThermodynamicsUsing the cold air standard assumptions and assuming negligible changes in kinetic and potential energy:
R air = 0.3704 psia ft3/lbm R
Cp = 0.24 Btu/lbm R
K = 1.4
th,Brayton = 1 – 1/r (k-1)/k
th,Brayton = 1 – 1/(8) (1.4-1)/1.4
th,Brayton = 0.448 or 44.8%
T2/T1 = (8) (1.4-1)/1.4= 1.811
T2 = (300)( 1.811 ) = 543.4K
And
T4/T3 = (1/8) (1.4-1)/1.4 = .552
T4 = (1300)(0.522) = 717.6K

UNIVERSITY OF
FLORIDA
Thermodynamics
qin = h3 – h2 = Cp (T3 – T2)
qin = ( 1.005 kJ/kg K)(1300-543.4)
qout = Cp(T4 – T1) = (1.005 kJ/kg K)(717.6-300)
th,Brayton = (qin-qout)/qin =
0.448 or 44.8%
Back work ratio = Cp(T2-T1)/Cp(T3-T4) =
(543.5-300)/(1300-717.6) =
0.42

UNIVERSITY OF
FLORIDA
Thermodynamics
Using air standard assumptions (see text):
Back work ratio = 0.402
Thermal efficiency = 42.6%
T2 = 540K
T4 = 770 K
Our cold air standard assumptions worked well.

UNIVERSITY OF
FLORIDA
ThermodynamicsThe Rankine or Vapor Power Cycle
• Used to steam power plant operations

UNIVERSITY OF
FLORIDA
ThermodynamicsSimple Ideal Rankine Cycle
1-2 isentropic compression with a pump
3-4 constant pressure heat addition in a boiler
5-6 isentropic expansion in a turbine
6-1 constant pressure heat rejection in
condenser
Other points can be used to
describe pipe
Losses (thermal and pressure)

UNIVERSITY OF
FLORIDA
ThermodynamicsWe will consider water(steam) as our motive fluid.
The steam leaving the boiler (pts. 4 and 5) is usually superheated steam.
The steam leaving the turbine (pt 6) is
usually high quality steam. Remember
that s5 = s6
The water leaving the condenser is either
saturated liquid or subcooled liquid water.
We usually assume that h2 = h1.
Or we can estimate h2 = h1 + v(P2-P1).
Let’s work an example problem to
illustrate how this works.

UNIVERSITY OF
FLORIDA
Thermodynamics
P
h
Consider a steam power plant and assume an ideal Rankine cycle to model the system.
The steam enters the turbine at 3MPa and 350 C
Condenser pressure is at 75 kPa.
What is the thermal efficiency for this cycle?
th,Rankine = wnet/qin= (qin – qout)/qin
qin = h1 – h4
qout = h3-h2
h1 = h of superheated steam = 3115.3 kJ/kg
s1 = s2 = 6.7428 kJ/kg K
s2 = sf75kPa + x sfg75kPa = 6.7428 kJ/kg K
x = (6.7428-1.213)/6.2434 = 0.88
Using x and hf75kPa and hfg75kPa, we can calc.
h2 = 384.39+(0.88)(2278.6)= 2402.6 kJ/kg
1
23
4

UNIVERSITY OF
FLORIDA
Thermodynamics
P
h
h3 = hf75kPa = 384.39 kJ/kg
h4 = h3 approx.
qin = h1 – h4= 3115.3 – 384.39 kJ/kg = 2730.9 kJ/kg
qout = h3-h2 = 384.39 - 2402.6 = -2018.2 kJ/kg
th,Rankine = (2730.9-2018.2)/2730.9 =
26%
Note that I calculated the same efficiency neglecting any change in h across the pump. I assumed the pump was isenthalpic instead of isentropic.
If we do consider the pumph = 0, then the pump work is approx. zero and the back work ratio = 0.
If the work of the pump is calculated we find that the back work ratio is 0.004 or 0.4%. Compare to the 0.42 for the Brayton cycle.
1
23
4