Factors affecting rate of chemical reactions
-
Upload
abigail-sapico -
Category
Education
-
view
312 -
download
2
Transcript of Factors affecting rate of chemical reactions
Factors Affecting Rate of Chemical Reactions
Experiment No. 4Factors Affecting Rate of Chemical Reactions
Objectives:To show the effects of the different factors in the rate of a given chemical reaction
Procedure:Nature of ReactantsCompare the speeds of the following sets of reaction and write the corresponding equations:
Experiment No. 11 mL of dilute H2SO4a drop of BaCl2 solution
test tube 11 mL of dilute magnesia mixture
few drops of disodium phosphate solutiontest tube 2
Experiment No. 2dilute KmnO4dilute H2SO4
test tube 1test tube 2acidified withdilute KmnO4acidified withdilute H2SO41 mL of hydrogen peroxide
Experiment No. 33 mL 0f 6N HCltest tube 1
test tube 3test tube 23 mL 0f 6N HCl3 mL 0f 6N HCl
Experiment No. 3
test tube 13 mL 0f 6N HCl
3 mL 0f 6N HCl3 mL 0f 6N HCltest tube 2test tube 3
7
Experiment No. 3
test tube 13 mL 0f 6N HCl
3 mL 0f 6N HCl3 mL 0f 6N HCltest tube 2test tube 3
gram of Zn granules
gram of Al
1 piece of magnesium riboonRecord the time for Al and Magnesium to be consumed.
8
Procedure:Catalyst
Experiment No. 12 mL hydrogen peroxidea pinch of manganese dioxide
test tube 1test tube 22 mL hydrogen peroxideCompare the rate at which decomposition of hydrogen peroxide takes place in the two test tubes.
Experiment No. 25 mL of 6N HCl3 mL of 1% MnSO4
test tube 1
3 mL H2Otest tube 25 mL of 6N HCl
Experiment No. 25 mL of 6N HCl3 mL of 1% MnSO4
test tube 13 mL H2Otest tube 25 mL of 6N HCl
Experiment No. 25 mL of 6N HCl3 mL of 1% MnSO4
test tube 1
3 mL H2Otest tube 25 mL of 6N HCl
strip of magnesium ribbonstrip of magnesium ribbon
Procedure:Concentration of Reactants
Experiment No. 13 mL 0f 3N HCltest tube 1
test tube 3test tube 23 mL 0f 6N HCl3 mL 0f 12N HCl
Experiment No. 13 mL 0f 3N HCltest tube 1
test tube 3test tube 23 mL 0f 6N HCl3 mL 0f 12N HCl
a piece of magnesium ribbona piece of magnesium ribbon
a piece of magnesium ribbon
Procedure:Temperature
3 mL of 0.1 N sodium oxalate3 mL of KmnO4
test tube 1test tube 23 mL of 0.1 N sodium oxalateacidified withfew drops of dilute H2SO4
3 mL of 0.1N sodium oxalate
test tube 1
75 C
19
Procedure:Surface area of Reactants
3 m L of 6N HCl
test tube 1test tube 23 mL of 6N HCl
folded magnesium ribbonflat strip magnesium ribbon
3 m L of 6N HCl
test tube 1test tube 23 mL of 6N HCl
folded magnesium ribbon
flat strip magnesium ribbon
Observation and Results:Nature of ReactantsCompare the speeds of the following sets of reaction:ExperimentNo. 1
The solution in the second test tube has no reaction after adding a few drops of disodium phosphateThe solution in the first test tube has the faster reaction after adding a drop of BaCl2
Observation and Results:Nature of ReactantsCompare the speeds of the following sets of reaction:ExperimentNo. 2
The reaction in the first test tube occur faster upon adding hydrogen peroxide.
The reaction in the second test tube occur fast.
Observation and Results:Nature of ReactantsCompare the speeds of the following sets of reaction:ExperimentNo. 3The fastest reaction is in the 2nd test tube with a piece of magnesium ribbon
The reaction in the 3rd test tube is slow upon adding gm of Al.In the third test tube with Zn, the reaction is faster than in the 3rd test tube.
Observation and Results:CatalystDetermine the rate of decomposition of H2O2 that takes place into the two test tubes. This maybe determined from the rate of evolution of oxygen gas.ExperimentNo. 1The decomposition in the 2nd test tube is faster upon adding manganese dioxide.
Observation and Results:CatalystTime the reaction.ExperimentNo. 2in the 1st test tube, the magnesium ribbon was consumed more rapidly which occured in only 1min.in the 2nd test tube, the magnesium ribbon was consumed after 3mins.
Observation and Results:Concentration of ReactantsCompare the reaction rates by noting the rate of evolution of hydrogen gasExperimentNo. 1
The fastest reaction is in the 2nd test tube with a piece of magnesium ribbon which reacted in less than 1min.The reaction in the third test tube is slow and it begun after 5mins.The magnesium in the 1st test tube reacted after 3mins. The reaction is moderate
Observation and Results:TemperatureObserve the change in color and record the time of the reaction.ExperimentNo. 1
In 2nd test tube, the color also changed from violet to colorless for only 3mins. upon heating at 75C. In the 1st test tube, the color suddenly change into violet upon adding the potasium permanganate solution.
Observation and Results:Surface Area of the ReactantsRecord the time of the reaction.ExperimentNo. 1The flat strip of magnesium ribbon reacted in less than 1minthe coiled strip reacted after 1min.
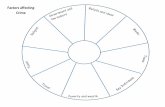
Generalization:There are five factors that effects the rate of chemical reaction:1. Nature of Reactants. There are chemicals that are more active than others. These can change the speed of the reaction. 2. Presence of Catalyst. Adding catalyst in the solution increases the rate of reaction but the speed depends on the solution where the catalyst is added.
Generalization:There are five factors that effects the rate of chemical reaction:3. Concentration of Reactants. Increasing the concentration of reactants also increases the rate of reaction because the no. of possible collisions also increases.4. Temperature As the temperature rises, the collision frequency and the collision efficiency increases.
Generalization:There are five factors that effects the rate of chemical reaction:5. Surface Area of Reactants Increase in surface will increase reaction rate because greater no. of reactant will come in contact with each other.
Prepared and presented by:Abigail M. Sapico