Echocardiographic aspects of percutaneous atrial septal defect closure in adults
-
Upload
richard-graham -
Category
Documents
-
view
212 -
download
0
Transcript of Echocardiographic aspects of percutaneous atrial septal defect closure in adults

Atrial septal defect (ASD) is the second most com-mon congenital cardiac defect in adults, account-ing for 22% of adult congenital defects.1 There
has been considerable debate regarding the benefits ofdefect closure beyond early adulthood, particularly inpatients with no or minimal symptoms.2–4 However,there is evidence of both subjective5 and objective6
improvement in exercise capacity after surgical closure.Despite the very low mortality of surgical ASD closure(<1%), percutaneous techniques offer the potentialadvantages of avoiding a thoracic scar, with shorter hos-pital stay and a better cosmetic result. This may thereforetip the balance in favour of intervention in patients witha haemodynamically significant ASD.
The first successful percutaneous closure of an ASDwas performed by King and Mills in 1974.7 However, thetechnique did not come into regular clinical use owing tothe size of the delivery catheter (22-Fr), and a cumber-some deployment system. Recently, a number of moreuser-friendly occluding systems have been developed,each with its advantages and disadvantages. All systemshave a basic design of two collapsible discs or supportedsheets joined centrally, which are deployed either side ofthe atrial septum to cover or occlude the defect. In our
institution, the Amplatzer Septal Occluder (ASO) is thefavoured device. This consists of two self-expandingdiscs of woven nitinol wire joined by a cylindrical waist.The waist plugs the defect, and is held in place by theatrial discs. The advantages of this device are the easewith which it can be repositioned and its ability to closelarger defects.
Echocardiography is now essential in the diagnosisand management of ASD. It is used to identify and selectpatients for percutaneous closure, it is central to thedevice deployment procedure, and it is an importanttool in patient follow up. This review aims to discusspercutaneous closure of ASD from an echocardiographicpoint of view. While our experience has been predomi-nantly with the ASO, the principles hold for all percuta-neous closure systems.
Diagnosis
Echocardiography can provide direct visualisation of theanatomical defect and detection of the inter-atrial shuntwith colour-flow Doppler mapping. With these tech-niques, transthoracic echocardiography (TTE) is able tovisualise 95% of septum primum and moderate–large(>5 mm diameter) secundum defects.8 Smaller (<5 mmdiameter) secundum defects, and in particular sinusvenosus defects, are more difficult to detect with TTE(sensitivity of TTE 20 and 9%, respectively), but
Echocardiographic Aspects of Percutaneous AtrialSeptal Defect Closure in Adults
Richard Graham, MB, MRCP and John Gelman, FRACP
Department of Cardiology, Monash Medical Centre, Clayton, Victoria, Australia
Echocardiography plays an integral role in the diagnosis and management of atrial septaldefects. With percutaneous closure of secundum defects becoming widespread, trans-oesophageal echocardiography in particular has assumed an important role in patient selection,as well as being central to the device deployment procedure, and an important tool in patientfollow up. (Heart, Lung and Circulation 2001; 10: 75–78)
Key words: atrial septal defect (ASD) closure device, echocardiography.
Correspondence: John Gelman, Department of Cardiology, MonashMedical Centre, 246 Clayton Road, Clayton, Victoria 3168,Australia.

Heart, Lung and Circulation 2001; 10R. Graham and J. GelmanPercutaneous ASD closure
76
transoesophageal echocardiography (TOE) has a sensi-tivity of 100%.8
In addition to direct imaging of the defect, TTE canalso provide indirect evidence of a significant left-to-rightshunt. With ratios of pulmonary : systemic flow of 1.5:1or greater, signs of right heart volume overload are seen.The right atrium and ventricle are enlarged with a dilatedpulmonary trunk. There is usually diastolic flattening ofthe interventricular septum with paradoxical motion insystole. Prominent pulmonary vein flow is seen, but leftatrial and ventricular size are normal in the absence ofother cardiac anomalies. If tricuspid regurgitation (TR) ispresent, an estimation of pulmonary artery pressure canbe made from the peak velocity of the TR jet.
If features of right heart volume overload are seen onTTE without an obvious cause, the use of intravenousechocardiographic contrast can be very helpful. Theappearance of microbubbles in the left atrium withinthree cardiac cycles of an intravenous injection of agi-tated saline is highly suggestive of an inter-atrial shunt.
Assessment of Suitability for PercutaneousClosure
While TTE imaging in children may be adequate fordetailed morphological assessment of the atrial septumnecessary to determine suitability for percutaneous clo-sure, in adult subjects, TOE is required. A TOE also pro-vides a further opportunity to exclude associated cardiacanomalies, such as partial anomalous venous drainageand pulmonary stenosis, which will influence manage-ment decisions.
The two main determinants of suitability for percuta-neous closure are: (i) absolute ASD size; and (ii) the extentof the supporting rim of tissue around the defect.9 TheASO can close defects up to a diameter of 36 mm,although other devices are more limited. All closuredevices are dependent on an adequate rim of septal tissuearound the defect, to ensure stability of the device and toavoid encroachment on surrounding structures. Thisrequirement precludes sinus venosus defects, which havean inadequate rim towards the adjacent vena cava, andseptum primum defects, which usually involve the atrio-ventricular valves, from percutaneous closure. The precisesize of rim required is specific to device type: the ASO hasa left atrial disc diameter 7 mm greater than the devicewaist, which is therefore the minimum clearance neces-sary. The entire defect rim should be imaged, but particu-lar attention should be paid to the distance from themitral valve, coronary sinus, right upper pulmonary veinand vena cavae. Initially, it was recommended that thereshould also be adequate rim supero-anteriorly, towardsthe aortic root, but the ASO is able to spreadeagle
around the aorta and remain stable even in the absenceof a septal rim in this location.
Other features of the atrial septum that influencepercutaneous closure are the degree of mobility oraneurysm of the septum, and the presence of multipledefects or fenestrations. With an aneurysmal septum,there is a tendency for the thin, mobile tissue to stretcharound a device, which may result in undersizing of thedefect. Multiple defects can be closed successfully by apercutaneous technique.10 Two ASO devices can bedeployed if the defects are separated by 7 mm of tissue,with the atrial discs overlapping. In our experience,another approach which can be used for a fenestratedseptum is to deploy a single large Amplatzer PFOOccluder (AGA Medical, Golden Valley, MN, USA)across a central fenestration. This device has a spindle-like waist, with large right and left atrial discs which cancover additional defects.
Procedure
Although recent reports suggest that deployment of apercutaneous ASD closure device can be done safelyunder echocardiographic guidance only,11 the procedureis usually carried out under both fluoroscopic and TOEguidance. TOE assists in accurate defect sizing to ensurethe correct size of device is used, as well as guidingdevice deployment into the optimal position.
Currently, the stretched diameter of the ASD is usedto select the appropriate size of device. This is assessedby withdrawing an inflated balloon catheter from the leftatrium across the defect. The minimum balloon diameterthat occludes the shunt can then be measured in a pre-calibrated sizing plate. It is recognised that direct mea-surement of the defect diameter on TOE imaging tendsto underestimate defect size. This occurs for severalreasons. As previously mentioned, the defect marginsmay be comprised of thin, mobile septal tissue that isreadily compressed by the balloon (and also the closuredevice). Defects may be ovoid or irregular in shaperather than circular, resulting in the maximal diameterbeing missed. Finally, 3-D TOE has demonstrated thedynamic morphology of ASD, with the size changingthroughout the cardiac cycle.12 The ability of 3-D TOE toimage the entire defect and its margins in a single viewindicates that it will become an important technique inASD closure, particularly for complex and multipledefects. At present, however, imaging cannot be per-formed in real time, instead requiring a lengthy acquisi-tion followed by computer reconstruction. It is not yetwidely available.
Once the device is selected, TOE facilitates deviceplacement across the defect, ensuring parallel alignment

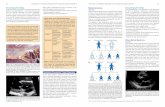
with the atrial septum and complete expansion of eachdisc within each atrium (Fig. 1). The absence of a signifi-cant shunt should also be confirmed before devicerelease, although it is common to see minor flow acrossthe defect superiorly at this stage, as the catheter exertsdownward traction on the device. TOE monitoringshould also ensure that the delivery system is free fromthe left atrial appendage and mitral valve before deploy-ing the left atrial disc. Entanglement of the deliverycatheter with the Chiari network has been described.13
Following device release, an assessment for the pres-ence and severity of residual shunt is made. The ASOinevitably has a small shunt through the central mesh,until it thromboses. More important is evidence of shuntaround the device on Doppler colour-flow mapping,which needs to be documented for comparison with fol-low-up studies.
Follow Up
Most centres perform serial TTE at 1 day, 1 month and3–6 months post procedure. The aim of these is to con-firm stable position of the device and to reassess residualshunt. Regression of right heart dilatation should also belooked for. In our centre, in addition to TTE follow up, aTOE is done at 1 month post procedure. This has greatersensitivity than TTE for detecting shunt, and can alsodetect other rarer complications such as thrombus.14,15
With the ASO, residual shunt as assessed by TTE hasbeen documented in 86, 15, 8 and 1% of patients atimplantation, 24 h, 1 month and 3 months, respectively.16
The long-term residual shunt rate is therefore compara-ble to that seen with surgical closure.17
Conclusion
Percutaneous closure for ASD is becoming morewidespread as experience in the technique grows, andthe devices become easier to deploy. Echocardiographyplays an integral role in all stages of the procedure.Physicians involved in the management of patients withan ASD need to be aware of both the technique and theechocardiographic aspects of percutaneous closure toallow appropriate patient selection and follow up.
Heart, Lung and Circulation 2001; 10 R. Graham and J. GelmanPercutaneous ASD closure
77
Figure 1. (a) Excellent alignment of the Amplatzer SeptalOccluder device and inter-atrial septum in percutaneous atrialseptal defect closure. (b) Device perpendicular to the atrialseptal defect. (c) Device entirely within the left atrium.

References1. Fuster V, Brendenburg RO, McGoon DC, Giuliani ER. Clinical
approach and management of congenital heart disease in theadolescent and adult. Cardiovasc. Clin. 1980; 10: 161–97.
2. Ward C. Secundum atrial septal defect: routine surgical closureis not of proven benefit. Br. Heart J. 1994; 71: 219–23.
3. Shah D, Azhar M, Oakley CM, Cleland JGF, NihoyannopoulosP. Natural history of secundum atrial septal defect in adultsafter medical or surgical treatment: a historical prospectivestudy. Br. Heart J. 1994; 71: 224–8.
4. Konstantinides S, Geibel A, Olschewski M et al. A comparison ofsurgical and medical therapy for atrial septal defects in adults.N. Engl. J. Med. 1995; 333: 469–73.
5. Gatzoulis MA, Redington AN, Somerville J, Shore DF. Shouldatrial septal defects in adults be closed? Ann. Thorac. Surg. 1996;61: 657–9.
6. Helber U, Baumann R, Sebolt H et al. Atrial septal defect inadults: cardiopulmonary exercise capacity before and 4 monthsand 10 years after defect closure. J. Am. Coll. Cardiol. 1997; 29:1345–50.
7. King TD, Mills NL. Nonoperative closure of atrial septaldefects. Surgery 1974; 75: 383–8.
8. Hausman D, Daniel WG, Mugge A, Ziemer G, Pearlman A.Value of transoesophageal colour Doppler echocardiographyfor detection of different types of atrial septal defect in adults.J. Am. Soc. Echocardiogr. 1992; 5: 481–8.
9. Reddy SCB, Rao PS, Ewenko J et al. Echocardiographic pred-ictors of success of catheter closure of atrial septal defect withthe buttoned device. Am. Heart J. 1995; 129: 76–82.
10. Cao QL, Radtke W, Berger F, Zhu W, Hijazi ZM. Transcatheterclosure of multiple atrial septal defects. Initial results and valueof two- and three-dimensional transoesophageal echocardi-ography. Eur. Heart J. 2000; 21: 941–7.
11. Ewert P, Berger F, Daehnert I et al. Transcatheter closure of atrialseptal defects without fluoroscopy. Feasibility of a new method.Circulation 2000; 101: 847–9.
12. Maeno YV, Benson LN, McLaughlin PR, Boutin C. Dynamicmorphology of the secundum atrial septal defect evaluated bythree dimensional transoesophageal echocardiography. Heart2000; 83: 673–7.
13. Cooke JC, Gelman JS, Harper RW. Chiari network entanglementand herniation into the left atrium by an atrial septal defectoccluder device. J. Am. Soc. Echocardiogr. 1999; 12: 601–3.
14. Gastmann O, Werner GS, Babic UU, Figulla HR. Thrombusformation on transcatheter ASD occluder device in a patientwith coagulation factor XII deficiency. Cathet. Cardiovasc. Diagn.1998; 43: 81–3.
15. Cooke JC, Gelman JS, Menahem S, Harper RW. Thrombus on anASD closure device: a call for caution. Heart, Lung, Circ. 2000; 9:30–31.
16. Chan KC, Godman MJ, Walsh K, Wilson N, Redington A, GibbsJL. Transcatheter closure of atrial septal defect and interatrialcommunications with a new self expanding nitinol double discdevice (Amplatzer septal occluder): multicentre UK experience.Heart 1999; 82: 300–306.
17. Berger F, Vogel M, Alexi-Meskishvili V, Lange PE. Comparisonof results and complications of surgical and Amplatzer deviceclosure of atrial septal defects. J. Thorac. Cardiovasc. Surg. 1999;118: 674–80.
Heart, Lung and Circulation 2001; 10R. Graham and J. GelmanPercutaneous ASD closure
78