Ebola virus disease (EVD)
-
Upload
harshit-jadav -
Category
Health & Medicine
-
view
228 -
download
2
Transcript of Ebola virus disease (EVD)

Ebola Virus Disease
By
Harshit Jadav
NIPER-Gandhinagar
Harshit Jadav 1

Ebola Virus Disease (EVD)
• Ebola virus disease is a severe, fatal, zoonoticfilovirus infection
• The current outbreak in west Africa, (first casenotified in 1976)
• Fatality rates are between 80% and 100%
• This filovirus is classified as a biological class 4pathogen
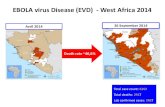
• The most severely affected countries areGuinea, Sierra Leone and Liberia
Harshit Jadav 2

Harshit Jadav 3

Ebola virus
• Lipid-enveloped, non-segmented, negatively stranded RNA virus
• Appears to have “spikes” due to Glycoprotein membrane
• Belongs to the viral family Filoviridae
• The natural reservoir of the virus is unknown
• CDC found that it is a zoonotic virus mostly found in bats
Harshit Jadav 4

Species of Ebolavirus
• The virus family Filoviridae includes 3 genera: Cuevavirus, Marburgvirus, and Ebolavirus
• There are five species of Ebola virus
Zaire ebolavirus
Sudan ebolavirus
Taï Forest ebolavirus
Bundibugyo ebolavirus
Reston ebolavirus
Harshit Jadav 5

– Order: Mononegavirales
– Family: Filoviridae
– Genes: Ebola virus
– Species: Zaire ebolavirus
• Zaire ebolavirus is responsible for the current outbreak in west Africa
Harshit Jadav 6

Outbreak (in small population)(eg. Ebola in West Africa, MERS in Soudi Arabia )
Epidemic (in specific community or region)(eg. Cholera in India, yellow fever in Africa)
Pandemic (in very large population)(eg. SARS , Influenza)
Harshit Jadav 7

Article information
• Journal: The New England Journal Of Medicine
• Author: WHO Ebola Response team
• Volume: 365 (16)
• Date of publication: 16th October, 2014
• Impact factor: 54.42Harshit Jadav 8

Epidemiology
• Since December 2013 and as of 19 October 2014, WHO has reported 9936 cases of Ebola virus disease (EVD) in West Africa including 7877 deaths
• 42% healthcare workers were infected with EVD
Harshit Jadav 9

• Ebola hemorrhagic fever (EHF) is an acute viral syndrome that presents with fever and an ensuing bleeding diathesis that is marked by high mortality in human and nonhuman primates
Harshit Jadav 10

Transmission
• The virus is thought to be initially acquired by exposure to body fluids or tissue from infected animals, such as bats and non-human primates
• Human to human transmission occurs through contact with body fluids from infected patients
• Most cases result from close physical contact or contact with body fluids (such as sweat, blood, faeces, vomit, saliva, genital secretions, urine, and breast milk) of infected patients.
• The incubation period after infection is usually 5-9 days
Harshit Jadav 11

Pathophysiology • Most of the studies were performed in non-
human primate and rodent models
• The virus genome consists of a single 19 kb strand of negative sense RNA with seven viral genes that are transcribed by the viral RNA dependent RNA polymerase present in the virion.
• single strand of RNA is covered by helically arranged viral nucleoproteins NP and VP30, which are linked by matrix proteins VP24 and VP4 to the lipid bilayer that coats the virion
Harshit Jadav 12

Pathophysiology (cont’d)
• Tissue invasion occurs through infected fluid coming into contact with breaks in the mucosa or skin.
• This can occur with animal to human or human to human transmission.
• Monocytes, macrophages, and dendritic cells are the preferred replication sites for filoviruses on initial infection.
• Infected cells migrate to the regional lymph nodes, liver, and spleen, thereby disseminating the infection.
Harshit Jadav 13

Who are at risk?
• Contacts of infected patients (includinghealthcare workers and household contacts) -if they were exposed to the patient’s bodyfluids without protective equipment withinthe past 21 days.
• Persons involved in Burial ceremonies ofinfected patients
• Health-care workers
• Household persons of infected patients
Harshit Jadav 14

A contact
• A contact is defined by WHO as someone who has slept in the same household as a patient;
– had direct physical contact with the patient during the illness or at the funeral;
– touched the patient’s body fluids, clothes, or bed linens during the illness; or
– been breast fed by the patient
Harshit Jadav 15

Phase of EVD
• There are typically three phases of illness
1. A few days of non-specific fever, headache, and myalgia (5-7 days)
2. A gastrointestinal phase in which diarrhoea and vomiting, abdominal symptoms, and dehydration are prominent. In the second week, the patient may recover or deteriorate (8-15 days)
3. Collapse, neurological manifestations, and bleeding, shock, coma, death (16-21 days)
Harshit Jadav 16

Typical symptoms
• Fever ≥101°F• Fatigue• Nausea or vomiting• Diarrhoea• Headache• Abdominal pain• Myalgia• Prostration• Sore throat• Unexplained bleeding or bruising• Spontaneous abortion or miscarriage• Hiccups• Rash
All the symptoms are common so it mislead to flu or
malaria
Harshit Jadav 17

Clinical features
The most common symptoms reported were
fever (87.1%),fatigue (76.4%), loss of appetite (64.5%), vomiting (67.6%),diarrhoea (65.6%), headache (53.4%), abdominal pain (44.3%),unexplained bleeding (18%)
Harshit Jadav 18

Diagnostic test of EVD
• Antigen capture enzyme linked immunosorbent assay(ELISA) testing
A useful diagnostic test with high specificity
Can be used to confirm the diagnosis along with a positivereverse transcriptase-polymerase chain reaction result
• Full blood count
Decreasing platelet count with low haemoglobin count
• Coagulation studies
Prolonged prothrombin time or activated partialthromboplastin
Harshit Jadav 19

Diagnostic test of EVD
• Blood culturesNegative blood cultures are helpful
• Ebola specific IgM and IgG antibodiesIgM antibodies can appear in serum as early as
day 2 after infection but results are variable up to day 9.
They become negative between 30 and 168 days after symptom onset
IgG response develops between days 6 and 18 and can persist for several years
Harshit Jadav 20

Management of EVD
• There is no any specific vaccine or treatment for EVD.
• Patients just get symptomatic treatment
• Isolation of patients is necessary to patients as well as other personals
Harshit Jadav 21

Symptomatic treatment
• Fever and pain—Fever and pain should be treated with paracetamol first. Opioid analgesics (such as morphine) are preferable for more severe pain
• Nausea and vomiting—Oral or intravenous antiemetics (such as ondansetron and metoclopramide) are recommended
• Heartburn, dysphagia, and upper abdominal pain—proton pump inhibitor (such as omeprazole) may be beneficial
Harshit Jadav 22

• Seizures - Although uncommon, seizures can beseen in advanced disease, A benzodiazepine canbe used to abort the seizure and can be givenintramuscularly
• Agitation - haloperidol or a benzodiazepine
• Sepsis and septic shock – broad spectrumantibiotics (such as ceftriaxone, piperacillin-tazobactam, or meropenem) in the first hour
• Malaria should be tested for and treated withappropriate antimalarial therapy
Symptomatic treatment (cont’d)
Harshit Jadav 23

Infection control measures for healthcare workers
• Wear protective clothing
• Practise proper infection control and sterilisation measures
• Isolate suspected patients from each other
• Avoid direct contact with bodies of people who have died from Ebola, or suspected Ebola
Harshit Jadav 24

Emerging treatments • Convalescent whole blood or plasma:
transfusion of blood from convalescent patientsshowed beneficial effect in acute phase ofinfection.
Harshit Jadav 25

Zmapp:
• The best known emerging treatment so far,ZMapp, is a combination of three humanisedmonoclonal antibodies targeted at three Ebolavirus glycoprotein epitopes and is engineered forexpression in tobacco plants
• ZMapp had proved protective when given to non-human primates 24-48 hours after infection.
• It has not yet been tested in humans for safety or efficacy
• The whole study is supported by US govt.
Harshit Jadav 26

TKM-Ebola
• TKM-Ebola consists of a combination of smallinterfering RNAs that target Ebola virus RNApolymerase L, formulated with lipid nanoparticletechnology.
• It has been shown to be protective in non-humanprimates (guinea pigs and monkeys)
• The US Food and Drug Administration hasgranted expanded access to this drug under anInvestigational New Drug application (INDA)
• Under emergency protocols, it had given to asmall number of patients in Zaire.
Harshit Jadav 27

Brincidofovir
• Formerly known as CMX-001, brincidofovir iscurrently undergoing phase III trials for thetreatment of cytomegalovirus and adenovirus.
• It also shows activity against Ebola virus invitro.
• The drug has been used in patients with Ebolavirus infection in the US under EmergencyInvestigational New Drug applicationsapproved by the FDA.
Harshit Jadav 28

Favipiravir
• Formerly known as T-705, favipiravirselectively inhibits viral RNA dependent RNApolymerase
• It is active against influenza viruses, West Nilevirus, yellow fever virus, foot and mouthdisease virus, as well as other flavivirusesarenaviruses, bunyaviruses
• It is effective against Ebola virus in mousemodels
• Human trials are started in west AfricaHarshit Jadav 29

BCX-4430
• BCX-4430 is an adenosine analogue that is active against Ebola virus in rodents
• It is thought to act through the inhibition of viral RNA dependent RNA polymerase
Harshit Jadav 30

AVI-7537
• AVI-7537 consists of antisense phosphorodiamidate morpholino oligomers (PMOs) that target the Ebola virus VP24 gene
• It confers a survival benefit to Ebola virus infected non-human primates
• AV-7537 undergone phase I clinical studies.
Harshit Jadav 31

Other agents
• Some drugs approved for other indications, which have known safety profiles at clinically used doses, including chloroquine and imatinib, have shown activity against EV in vitro and, in some cases, in rodent models.
• They are also used in the treatment of EHF in west Africa by some hospitals
Harshit Jadav 32

Vaccine
• cAd3-ZEBOV (also known as the NIAID/GSK Ebola vaccine or cAd3-EBO Z) is an experimental vaccine for two ebolaviruses, Ebola virus and Sudan virus, developed by scientists at GlaxoSmithKline (GSK) and tested by National Institute of Allergy and Infectious Disease (NIAID).
• The vaccine is derived from Chimpanzee adenovirus, chimp adenovirus type 3(chAd3) genetically engineered express glycoprotein from the Zaire
Harshit Jadav 33

Vaccine (cont’d)
• rVSV-EBOV is an experimental vaccine for the Ebola filovirus, developed by scientists at the Canadian National Microbiology Laboratory
• rVSV-ZEBOV is an attenuated vesicular stomatitis virus with one of its genes replaced by an Ebola virus gene.
• Human trials have started in the US
Harshit Jadav 34