Early Brain Damage Development Behavior Children: Clues...
Transcript of Early Brain Damage Development Behavior Children: Clues...

NEURAL PLASTICITY VOLUME 8, NOS. 1-2, 2001
Early Brain Damage and the Development of Motor Behaviorin Children: Clues Therapeutic Intel ention?
Mijna Hadders-Algra
Department ofNeurology, University ofGroningen, Groningen, The Netherlands*
ABSTRACT
The Neuronal Group Selection Theory(NGST) could offer new insights into themechanisms directing motor disorders, such ascerebral palsy and developmental coordinationdisorder. According to NGST, normal motordevelopment is characterized by two phases ofvariability. Variation is not at random butdetermined by criteria set by genetic information.Development starts with the phase of primaryvariability, during which variation in motorbehavior is not geared to external conditions. Atfunction-specific ages secondary variability starts,during which motor performance can be adaptedto specific situations. In both forms, of variability,selection on the basis of afferent information
plays a significant role. From the NGST pointof view, children with pre- or perinatallyacquired brain damage, such as children withcerebral palsy and part of the children withdevelopmental coordination disorder, sufferfrom stereotyped motor behavior, produced bya limited repertoire or primary (sub)corticalneuronal networks. These children also haveproblems in selecting the most efficient neuronalactivity, due to deficits in the processing ofsensory information. Therefore, NGST suggests
*Correspondence to:Developmental NeurologyCMC-IV, 3ra floor, Hanzeplein9713 KZ Groningen, the Netherlandstel: +31 50 3614247; fax: +31 50 3636905e-mail: [email protected]
that intervention in these children at early ageshould aim at an enlargement of the primaryneuronal networks. With increasing age, theemphasis of intervention could shift to theprovision of ample opportunities for activepractice, which might form a compensation forthe impaired selection.
KEYWORDS
Neuronal Group Selection Theory, variability,selection, developmental coordination disorder,cerebral palsy
INTRODUCTION
During the last century, knowledge on motorcontrol rapidly increased, an expansion ofknowledge which was associated with changes inthe concepts on the organization of motor behavior.Motor behavior is no longer explained in terms ofreflex mechanisms (Sherrington, 1906; Magnus &De Kleijn, 1912). On the contrary, motility isnowadays regarded as the net result of the activityof complex spinal or brainstem machineries, whichare subtly modulated by segmental afferentinformation and ingeniously controlled by supra-spinal networks (Schomburg, 1990; Grillner et al.,1995). For instance, it is assumed that motorcontrol of rhythmical move-ments like locomotion,respiration, sucking, and mastication is based on
so-called Central Pattern Generators (CPGs). CPGs
(C) Freund & Pettman, U.K., 2001 31

32 M. HADDERS-ALGRA
are neuronal networks which can generate complexbasic activation patterns of the muscles without anysensory signals. Yet, sensory information of themovement is important in adapting the movementto the environment.
The activity of the networks, which are usuallythought to be located in the spinal cord or brainstem, is controlled from supraspinal areas viadescending motor pathways (Grillner et al., 1995).The supraspinal activity itself is also organized innetworks, large-scale ones, in which cortical areasare functionally connected through direct recursiveinteraction or through intermediary cortical orsubcortical (striatal, cerebellar) structures (Bressler,1995; Hikosaka et al., 1999, Liu et al., 1999). Thesupraspinal motor networks are the circuitrieswhich expanded in particular during phylogenyand which determine, to a large extent, humanmotor ontogeny.
Research in the area of human motor develop-ment is characterized by an ongoing debate on therole of endogenous and exogenous factors. In thepresent paper, it is argued that this ’nature-nurture’controversy could be eliminated by the applicationof a new perspective, i.e., the perspective of theNeuronal Group Selection Theory (NGST). Thepaper presents an outline of NGST and describesthe significance ofNGST for understanding normaland abnormal motor development. The paperconcludes with suggestions for therapeuticalinterventions in children who acquired a brainlesion at early agesuggestions that are based onthe function-specific plasticity windows indicatedby NGST.
THEORIES ON MOTOR DEVELOPMENT
Neural-Maturationist Theories. These theoriessuggest that motor development is based on a
gradual unfolding of predetermined patterns in thecentral nervous system and an increasing corticalcontrol over lower reflexes (McGraw, 1943; Gesell& Amatruda, 1947; Peiper, 1963). According to theNeural-Maturationist Theories, motor developmentfollows distinct rules, such as the cephalo-caudaland central-to-distal sequences of development.The theories leave only little room for develop-mental modifications by means of environmentalfactors and experience.
A more recently developed theory, theDynamic Systems Theory, considers such a virtualneglect of a contribution of external factors tomotor development as incompatible with reality(Thelen, 1995). According to the Dynamic SystemsTheory, motor development is regarded as a dynamicsystem, i.e., a complex system which changes overtime due to the interaction of multiple components.The components consist of intrinsic factors, suchas muscle strength, body weight, postural support,the infant’s mood and brain development; andextrinsic factors, such as the environmentalcondition and specific task requirements. TheDynamic Systems Theory postulates that motorbehavior spontaneously adopts specific, temporarilyattractive states of organization. Behavior changesand develops in a non-linear way, i.e., by means oftransitions, due to changes of the characteristics ofthe intrinsic or extrinsic component parts (Thelen,1985, 1995; Thelen et al., 1993; Ulrich, 1997). Inother words, the Dynamic Systems Theory and theNeural-Maturationist Theories differ, especially intheir view on the role of the nervous system inmotor development. The Neural-MaturationistTheories consider the endogenously driven
Neural-Maturationst/Dynamic System Theories
For many years, normal motor developmenthas been interpreted within the framework of the
Among the Neural-Maturationists, McGraw has an atypicalposition. Even though she considered endogenous maturationalprocesses the main driving forces of development, sheacknowledged that experience during particular time-windowscould modify motor development (McGraw, 1935, 1943).

MOTOR DEVELOPMENT OF CHILDREN WITH BRAIN LESIONS 33
maturational state of the nervous system the mainconstraint for developmental progress, whereas inthe Dynamic Systems Theory, the make-up of theneural substrate plays a subordinate role only.
Neuronal Group Selection Theory
Recently, Gerald M. Edelman developed a newtheoretical concept on neural development: theNeuronal Group Selection Theory (NGST;Edelman, 1989, 1993; Spores & Edelman, 1993).This theory could offer the golden mean betweenthe Neuro-Maturationist and Dynamic Systemstheories and might facilitate the understanding ofthe effects of brain damage at early age (Hadders-Algra, 2000a,b). According to NGST, the brainor more specifically, the ensemble of cortical andsubcortical systemsis dynamically organizedinto variable networks, the structure and functionof which are selected by development andbehavior. The units of selection are collections ofhundreds to thousands of strongly interconnectedneurons, called neuronal groups. These units act asfunctional units dealing, for instance, with aspecific type of motor behavior or informationfrom a specific sensory modality. NGST states thatdevelopment starts with primary neuronalrepertoires, with each repertoire consisting ofmultiple neuronal groups (Fig. 1). The cells andthe crudely specified connectivity of the primaryrepertoires are determined by evolution. In otherwords, genetic information plays a substantial rolein the primary determination of brain development.For instance, it is thought that the area-specificcharacteristics of the neo-cortex is partially basedon properties laid down at the time ofneurogenesis (O’Leary, 1989).
Another indication that genetic informationcontributes significantly to brain development isthe fact that at least 50% of tissue-specific humangenes are expressed in the brain (Evans, 1998). Aprimary genetic determination does, however, not
preclude variation as primary determination is onlythe starting point for epigenetic cascades allowingfor interaction with the environment. The latterresults in a dynamic regulation of cell division,adhesion, migration, death, and neurite extensionand retraction (Changeux & Danchin 1976; Rakic,1988; O’Leary, 1989; Changeux, 1997). In theinitial assembly of the brain, synaptic activity mostprobably plays a role. Still, a permanent andcomplete loss of synaptic transmission does notprevent a normal assembly, including a normalformation of layered structures, fiber pathways,and morphologically defined synapses. Synapticactivity is, however, needed for the maintenance ofneuronal structures and connections (Verhage etal., 2000).
When the primary neuronal groups have beenformed, development proceeds with selection onthe basis of afferent information produced bybehavior and experience (Fig. 1). The selectionprocess is thought to be mediated by changes insynaptic strength of intra- and inter-groupconnections, in which the topology of the cells(Nelson et al., 1993) and the presence or absenceof coincident electrical activity in pre- and post-synaptic neurons plays a role (Hebb, 1949; Changeux& Danchin, 1976). When the selection has justbeen accomplished, behavioral variation is slightlyreduced. Soon, however, abundant variation returns
because the organism and its populations ofneuronsis constantly exposed to a multitude of experiences.The experiential afferent information inducesmodifications in the strength of the synapticconnections within and between the neuronal groups,resulting in the variable secondary repertoire (Fig. 1).The changed and changing connectivity within thesecondary repertoire allows for a situation- specificselection of neuronal groups. Thus, the secondaryneuronal repertoires and their associated selectionmechanisms form the basis of mature variablebehavior, which can be adapted to environmentalconstraints (Edelman & Tononi, 2000).

34 M. HADDERS-ALGRA
primary neuronal repertoire
selection
secondary neuronal repertoire
constraint a constraint b constraint c constraint d
1 1 1 1selection selection selection selection
Fig. 1: Schematic representation of Edelman’s Neuronal Group Selection Theory. Each circle represents a cluster of supraspinallylocalized neurons, i.e., the neurons are localized in cortical, cortico-striato-thalamo-cortical, or cortico-cerebellar-corticalnetworks. At the upper row, neural activity is depicted at early age at four closely spaced points in time. The filled circles(= and (R)) denote neurons genetically determined to control a specific type of motor behavior, that is, they reflect aprimary neuronal repertoire. For instance, the filled circles could denote neurons controlling the motoneurones of themuscles on the ventral side of the body which are genetically determined to be in charge of postural control during abackward sway of the body (direction-specificity). The open circles (O)represent neurons genetically linked to other typesof behavior, i.e., other primary neuronal repertoires. At the four different points in time, the filled primary neural repertoireis activated in four different configurations, i.e., four different neuronal groups--denoted by the different grades of fillingof the clusters (O active; (R) inactive) This, in turn, gives rise to primary variability in behavior. Development proceeds(,I,) with selection of the neuronal group, which produces the most effective behavior applicable in a wide variation ofconditions. For instance, returning to the example of postural control, during the development of postural adjustments,selection occurs of the adjustment in which all direction specific muscles are activated (Hadders-Algra et al. 1996a,b; Fig.2). Next (,), with increasing age variation returns, giving rise to the secondary neural repertoire. The variation of thesecondary neural repertoire can best be observed in conditions lacking tight constraints. In the absence of specificconstraints, the nervous systems shows that it has access to many motor roads leading to Rome. This means that easy,unconstrained conditions allow for variation of motor behavior, also in adulthood. Yet, in conditions with constraints(lower part of the figure), a specific solution produced by the activity of a specific neuronal group is selected, the solutionbeing geared to the specifics ofthe situation.

MOTOR DEVELOPMENT OF CHILDREN WITH BRAIN LESIONS 35
NGST AND NORMAL MOTOR DEVELOPMENT
Translation of the concept of NGST to motordevelopment implies that motor development ischaracterized by two phases of variation: primaryand secondary variability (Hadders-Algra, 2000a).
Primary variability
Motor development starts during early fetal lifewith the phase of primary variability, a phase whichcontinues during infancy. Detailed studies on themotor behavior of fetuses and newborn infants haveshown that motility at early age is characterized byprofuse variation, such as variation in movement
trajectories and variation in temporal and quantita-tive aspects of motility (Minkowski, 1938; De Vrieset al., 1982; Forssberg, 1985; Vles et al., 1989;Hadders-Algra et al., 1992; Konishi et al., 1994).These variations in motor activity are not neatlytuned to environmental conditions, but the variationsthemselves constitute a fundamental developmentalphenomenon. It is conceivable that the abundantvariation in motility is brought about by activity ofthe epigenetically determined, but rather grosslyspecified, supraspinal primary neural repertoires.The system of primary repertoires presumablyexplores by means of self-generated activity, andconsequently also by means of self-generated afferentinformation, all motor possibilities available withinthe neurobiological and anthropometric constraintsset by evolution.
The properties of primary variability are wellillustrated by the general movements (GMs). GMsare the most frequently used movement pattern ofthe human fetus and newborn infant. They consistof a series of gross movements of variable speedand amplitude, which involve all parts of the bodybut lack a distinctive sequencing of the partici-pating body parts (Prechtl & Nolte, 1984; Prechtl,1990). In other words, normal GMs are veryvariable and consist of an endless exploration of
all potential movement properties, such asmovement velocities, amplitudes, and forces; andthe numerous possible combinations of actionsaround all participating joints. Likewise, themuscle coordination patterns of normal GMs aretypified by variationvariation in which musclesparticipate and variation in the timing and thequantity of muscle activation (Hadders-Algra et al.,1992, 1997). Presumably, the rich variation and thecomplexity of human GMs reflect the explorativeactivity of a widely distributed (sub)corticalnetworkthe primary neuronal repertoire--on theextensive CPG-networks of the GMs localized inthe spinal cord and brainstem (Hadders-Algra,2000a). GMs are present till about 4 months ofpost-term age. From that age onwards, they are
gradually replaced by goal-directed movements. Interms of neural networks, the gradual change fromgeneral movement activity into goal-directedbehavior could mean that the widely distributed(sub)cortical networks controlling GM-activity areflexibly rearranged by means of changed synapticconnectivity into multiple smaller networks (cf.Simmers et al., 1995). In other words, the large(sub)cortical GM-network is cut into varioussmaller networks. These smaller (sub)corticalnetworks form the primary neuronal repertoires forthe control of specific motor behaviors, such as
goal-directed motility of the arms and the legs, andpostural control. Due to the dissolution of theprimary neuronal network of GMs, thedevelopment of GMs does not include a transitionfrom a primary neuronal repertoire into a
secondary repertoire. This underscores the uniqueposition of GMs in human motor development andsupports the notion that the (sub)cortical networksinvolved in the control of GM-activity form theneural building blocks for later motor skills.
All other forms of motor behavior manifestboth phases of variability. They start with thephase of primary variability, during which motor
activity is variable and not strictly tuned to

36 M. HADDERS-ALGRA
TABLE 1
Timing of selection in the phase of primary variability
Movement pattern Period during which selection occurs Based on
Well-coordinated sucking pattem
Relatively straightly forward directedarm movement during reaching
Efficient, multi-purpose posturaladjustments (’complete’ patterns)
Diagonal gait during crawling
Heel-strike during locomotion
Before term age
Second halfyear after birth
6-10 months
6-10 months
1-11/2 years
Hadders-Algra & Dirks (2000)
Thelen et al. (1993), Konczak et al.
(1995)
Hadders-Algra et al. (1996a), Van der
Fits et al. (1999c)
Adolph et al. (1998)
Burnett & Johnson (1971), Cioni et
al. (1993)
environmental conditions. Primary variabilityoccurs during fetal life and infancy, when braindevelopment is characterized by an overproductionand subsequent pruning of neural elements (e.g.,Huttenlocher et al., 1982, Rakic et al., 1986). Therich variation in motor behavior has beendocumented for the first phases of reaching andgrasping behavior (Von Hofsten, 1991; Thelen etal., 1993; Fallang et al., 2000), crawling (Largo etal., 1985; Adolph et al., 1998), locomotor motility(Statham & Murray, 1971; Forssberg, 1985), andpostural control (Hirschfeld & Forssberg, 1994,Hadders- Algra et al., 1996a; Van der Fits et al.,1999b).
The neural systems dedicated to a specificfunction explore during the phase of primaryvariability all motor possibilities available for thatspecific function. The exploration utilizes ubiquitousinformation and results in so-called ’experience-expectant’ information storage (Greenough et al.,1987). The trial and error exploration is associatedwith a continuous processing of self-generatedafferent information, on the basis of which themost efficient movement patterns are selected. The
time of occurrence of the phase of selection andthe duration of the transition from the phase ofprimary to secondary variability is function-specific (Table 1). After the transient phase ofselection and reduced variation, the phase ofsecondary or adaptive variability starts (Touwen,1993; Hadders-Algra et al., 1998).
Transition from primary to secondary variability
The transition from primary into secondaryvariability can be illustrated with data on thedevelopment of postural adjustments. We performeda series of studies on postural adjustments inchildren who sat on a movable platform. We foundthat the early phases of the development ofpostural adjustments are characterized by extensivevariation, be it within the limits set by the primaryneuronal repertoire, i.e., the epigenetically determinedboundaries of direction specificity (Hadders-Algraet al., 1996a). Direction specificity denotes themechanism to primarily activate the muscles onthe dorsal side of the body when the body swaysforward, and to primarily activate the ventral

MOTOR DEVELOPMENT OF CHILDREN WITH BRAIN LESIONS 37
muscles when the body is swaying in the oppositedirection (Forssberg & Hirschfeld, 1994). Indeed,already before infants can sit independently, i.e., at5 to 6 months, the postural activity of neck andtrunk muscles is direction specific. At this age, therepertoire of direction-specific postural adjustmentsto large perturbations of equilibrium is variable andconsists of adjustments in which one, or two, ormore than two postural muscles are activated in anyconceivable combination. The selection of themost efficient postural adjustment, in which alldirection-specific neck, trunk, and proximal legmuscles are activated, occurs during the thirdpostnatal trimester and is guided by information onthe stability of the head in space (Hadders-Algra etal., 1996a; Fig. 2). The selection can be acceleratedsubstantially by daily balance training, a findingwhich underscores the significance of activeexperience in the selection process (Hadders-Algraet al., 1996b).
The selection induces a transient phase withreduced variation. In the development of posturaladjustments, the phase of reduced variation lastsrelatively long, i.e., from 9 months until about 21/2
years of age. The protracted presence of decreasedvariability in the development of postural controlis presumably related to the difficulty of the taskof balancing the body during the first phases ofstanding and walking (Hadders-Algra et al., 1998).In the development of the majority of motorfunctions, the phase of reduced variation is shortso short that the phase of primary variabilityimperceptibly passes into the phase of secondaryvariability (Adolph et al., 1998).
Secondary variability
When the secondary variability is formed, thebrain is characterized by extensive synapserearrangement, the net result of synapse formationand synapse elimination (Purves, 1994). In addition,processing times become increasingly shorter, which
in part can be attributed to ongoing myelination(Jemigan et al., 1991; Mtiller et al., 1994). Duringthis phase, a variable movement repertoire is createdwith an efficient motor solution for each specificsituation. The development of situation-specificmotor strategies is guided by active trial-and-errorlearning, based on experiences which are unique tothe individual (Greenough et al., 1987: ’experience-dependent’ information storage). Between the agesof 2 and 3 years, the secondary motor variationstarts to bloom, but it lasts until adolescence beforethe motor repertoire is mature. In the mature situation,subjects can adapt movements exactly and efficientlyto task specific conditions or, in the absence oftight constraints, generate a repertoire of motorsolutions for a single motor task (e.g., Diener et al.,1983; Van der Fits et al., 1998; Forssberg et al.,1999b).
NGST AND MOTOR DEVELOPMENT AFTERBRAIN LESION AT EARLY AGE
Motor disorders after brain lesion at early age
The outcome after brain damage acquired inthe pre-, peri-, or neonatal period is heterogeneous.Some children recover completely, whereas otherssuffer from severe handicapping conditions (Costelloet al., 1988; Ford et al., 1989). The developmentalsequelae are to some extent related to the size andthe site of the lesion and the timing of the insult.The size of the lesion predicts outcome best.Lesions involving multiple cortical areas, especiallythose including subcortical damage, almost alwaysresult in clearly handicapping conditions, such asmoderate to severe forms of CP, whereas restrictedfocal lesions have motor outcomes which varybetween a normal condition, clumsiness, and mild tomoderate forms of CP (Fawer et al., 1987; Fazzi etal., 1994; Hadders-Algra et al., 1999a; Forssberg et al.,

38 M. HADDERS-ALGRA
NON-TRAINED5-6 MONTHS
INFANTABCDEFGH.IJK0 25 50 75
NR. TRIALS121116241616142591828
100%
INFANTLMNOPQ
RST0
TRAINED
25 50 75
NR. TRIALS26151629161982728
100%
INFANTABCDEFGH
JK0 25 50 75
9-10 MONTHS
NR. TRIALS446553255310
100%
INFANT NR. TRIALSL 8M 3N 3O 6p 6Q 4R 2S 4T 3
0 25 50 75 100%
E-INH NF RA RF
Fig. 2: Developmental changes in postural adjustments during sitting in 20 healthy infants. The balancing abilities in nine infantswas trained by their parents by presenting the infants attractive toys, sidewards, and semi-backwards in the borderzone ofreaching without falling (’trained’ group). Training was performed three times a day for five minutes for a period of threemonths after the first assessment of the postural adjustments. The postural adjustments were assessed at the ages of 5-6 and9-10 months during slow translations of a moving platform which induced a backward sway of the body of the sittinginfant. In each horizontal bar, the distribution of response patterns of the direction specific postural adjustments for one
subject is represented. The diagram on the right supplies the hatching codes of the response patterns used in the left part ofthe figure. In this diagram the shading of the squares indicate: square number 1" inhibition of one or more extensormuscles, square number 2: activation of NF neck flexor muscle, square number 3" activation of RA rectus abdominismuscle, square number 4: activation of RF rectus femoris muscle. NF, RA and RF are the direction specific musclesactivated to prevent a fall of the body in backward direction. Note the decrease in variation of response patterns with
increasing age, a process which is significantly enhanced by daily balance training, and which results in selection of thepattern in which all direction specific muscles are activated. Adapted from Hadders-Algra et al. 1996b.

MOTOR DEVELOPMENT OF CHILDREN WITH BRAIN LESIONS 39
1999a). The other two factors, the site of the lesionand the timing of the insult, are interdependent.Lesions acquired prior to 36 weeks post-menstrualage (PMA) typically occur in the periventricularregions, whereas lesions acquired around term agein general are located in the cortical areaswithor without involvement of subcortical areas and/orthe brain stem (Volpe, 1995). In preterm infants, ithas been reported that frontally located lesions areassociated with better outcomes than lesions locatedparietally or occipitally (Fawer et al., 1987; Fazzi etal., 1994).
Clinically, two groups of motor disorders areattributed to a lesion of the brain at early age"cerebraEDl palsy and clumsiness. Cerebral palsy(CP) is an umbrella term covering a group of non-progressive, but often changing, motor-impairmentsyndromes secondary to lesions or anomalies ofthe brain arising in the early stages of development(Mutch et al., 1992). This means that CPbydefinitionis caused by damage of the brain atearly age, even though the abnormalities of thebrain cannot always be visualized with imagingtechniques. CP affects about in 500 live bornchildren (Hagberg et al., 1996). Clumsy childrennowadays are classified according to DSM-IV asDevelopmental Coordination Disorder (DCD), aterm in general denoting children who have such apoor motor coordination that it affects dailyactivities at home and at school, notwithstandingthe presence of a normal intelligence and theabsence of evident neurological pathology (AmericanPsychiatric Association, 1994). The prevalence ofDCD is about. 10% (6% to 13%; Hadders-Algra, inpress). In children with DCD, the connectionbetween structural abnormalities of the brain andmotor dysfunctions is rather ambiguous. RecentlyHadders-Algra and Touwen (in press) argued thatindications for pre- and perinatal brain damage canbe found in only one-third of the children withminor motor dysfunctions. The motor disorder ofthe latter children could be regarded as a border-
line form of cerebral palsy. The motor problems ofthe remaining majority of clumsy children mightbe based on dysfunctions at the microscopic levelof the nervous system, such as abnormalities in theneurotransmitter or receptor systems (Hadders-Algra& Groothuis, 1999; Hadders-Algra et al., 1999a; Vander Fits et al., 1999a).
NGST and developmental motor disorders
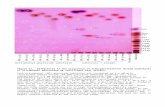
Extending NGST to the domain of abnormalmotor development offers an interesting perspectivefor the sequelae of brain lesions. Following thelines ofthought ofNGST, it can be surmised that alesion of the brain at early age results in (a) a lossor a reduction of neuronal repertoires and(b) impaired selection (Hadders-Algra, 2000b). Largelesions of the brain would induce a complete lossof primary neuronal repertoires, resulting in failureto develop specific functions. Recent data on thedevelopment of postural control support thissuggestion. We found that children with severespastic tetraplegia, who did not develop the abilityto sit independently, did not possess the primarydirection-specific repertoires of postural adjustments(Hadders-Algra et al., 1999a,b). Less extensivelesions would result in a reduction of the primaryneuronal repertoires and a reduced variation inmotor behavior (Fig. 3). Indeed, one of the majorsigns of infants with brain damage is stereotypedmotility, which at early age is expressed in theform of stereotyped GMs. It has been wellestablished that lesions of the brain, resulting inthe development of CP, induce GMs which aredevoid of variation and complexity (Hadders-Algra et al., 1997; Prechtl et al., 1997; Fig. 3). Thelack of variation can also be observed in the musclecoordination patterns of these abnormal GMs. Thepatterns either show a synchronous activation of allparticipating muscles or a stereotyped reciprocalactivity (Hadders-Algra et al., 1997).

40 M. HADDERS-ALGRA
A
B
Fig. 3: Representation of videofragments of GMs of two infants aged 3 months post term. The fragments start at the upper lefthand comer and should be read as the lines in a book. The interval between the frames is 8.16 s. The infant in the upperpanel (A) was born at term. She shows normal, variable, and complex GMs. The variation is illustrated by the differentpostures of the limbs in the different frames. Movement complexity is exemplified by the movement of the left leg on thethird row: the movement is not a simple flexion-extension movement, but a flexion-extension combined with asimultaneous abduction at the hip and an endorotation of the foot. The infant in the lower panel (B) is born prematurely ata gestational age of 28 weeks. She has definitely abnormal GMs. The deviant character of the movements is expressed bythe lack of variation: the frames have a high degree of similarity. The frames give the false impression that the infant didnot move at all, but she moved equally much as infant A. (Figure published with permission of the parents and theNederlands Tijdschrift voor Geneeskunde where the figure was published originally [Hadders-Algra 1997, 141, p. 817]).

MOTOR DEVELOPMENT OF CHILDREN WITH BRAIN LESIONS 41
It is common clinical knowledge that reducedvariation continues to be the hallmark of motorbehavior of infants with CP: they show little variationin spontaneous posture and motility and in variousinfantile reactions and responses (Ingram, 1966;Bobath, 1966; Touwen, 1978). Also at older age,the motor behavior of children with CP ischaracterized by stereotypy (Bobath, 1966; Aicardi& Bax, 1998). For instance, the typical posture ofthe most affected arm in children with spastichemiplegia is a posture with the shoulder inadduction, flexion, and endorotation, the elbow inflexion and pronation, and the wrist and fingers in(semi)flexion (Ingrain, 1966). Recently, these clinicalobservations were confirmed in experimental studies.Studies, in which the postural abilities of childrenwith borderline, mild, and moderate forms of CPwere assessed with the help of perturbation experi-ments, revealed which the repertoire of direction-specific postural adjustments in these children wassignificantly reduced (Brogren et al., 1998; Hadders-Algra et al., 1999a). Likewise, a study on thespatial-temporal organization of spontaneous legmovements during the first half year of lifedemonstrated that the kicking movements ofinfants with CP, due to lesions of the peri-ventricular white matter, were characterized byreduced variation (Vaal et al., in press).
It is conceivable that children with borderlineto moderate forms of CP not only suffer from a
limited motor repertoire but also from deficienciesin the processes of selection. Selection can behampered by impairments in the processing ofproprioceptive, tactile, or visual information, dys-functions which are frequently encountered in
children with CP (Nashner et al., 1983; Yekutiel et
al., 1994; Cioni et al., 1996). Recent data on thedevelopment of postural adjustments during reachingin infants with CP corroborate this suggestion.Healthy infants select the most efficient posturaladjustments to compensate for the posturalperturbation of a reaching movement between the
ages of 12 and 18 months, whereas in childrenwith CP, moderate variation in postural adjustmentsduring reaching persists beyond the age of 18months. This suggests that the selection of the mostefficient postural adjustment has not occurred bythat age (Van der Fits et al., 1999c; Hadders-Algraet al., 1999b).
Presumably, children with borderline to moderateforms of CP do reach the phase of secondaryvariability, be it with some delay. It is likely thatalso at the level of the secondary repertoires, theimpaired sensory processing interferes with theprocess of selection, i.e., the selection of the bestmotor solution for specific motor tasks. Thiswould imply that children with borderline tomoderate forms of CP have difficulties in adaptingtheir motor behavior accurately to specificconditions because of the double problem of a
hampered selection out of a limited repertoire (Fig.4). Recent data confirm this suggestion. Childrenwith mild to moderate forms of CP have problemsin adjusting the forces of their fingers duringobject manipulation (Eliasson et al., 1992, 1995;Gordon & Duff, 1999; Gordon et al., 1999; Eliasson& Gordon, 2000). They also have difficulties inadapting their postural adjustments to specificconditions, such as the velocity of a reachingmovement or the degree of pelvis-tilt while sitting(Hadders-Algra et al., 1999a,b). An inappropriateselection of the best motor solution inducesvariation in the fine-tuning of motor behavior:variations in the timing of motor events and in thescaling of the forces employed (Eliasson et al.,1992, 1995; Valvano & Newell, 1998; Gordon &Duff, 1999; Gordon et al., 1999; Eliasson & Gordon,2000). Recent studies support the idea that deficits in
sensory processing contribute to the variation inthe scaling of motor output of children with mildto moderate forms of CP. The studies showed thatpractice, implying repetition of self-generated sensoryinput (Valvano & Newell, 1998; Gordon & Duff,1999; Gordon et al., 1999), and augmentation of

42 M. HADDERS-ALGRA
Fig. 4:
expansion of the reduced primary neuronal repertoire
-.impaired selection
o? qo
secondary neuronal repertoire
o9#- _O,o o##(R)..qo .o_
constraint a constraint b constraint c constraint d"-k ". ".
impaired selection impaired selection impaired selection impaired selection
o_O# qoo%1
Schematic diagram of the putative mechanisms of intervention after brain lesion at early age, based on theprinciples of NGST. The diagram is a twin partner of Fig. (see legends of Fig. 1). The lower grey areadenotes a lesion of the brain at early age. The lesion of the brain resulted in a reduction of the primaryrepertoire of the filled clusters; only four of the originally nine participating clusters were le (cf, Fig. 1).NGST suggests that at early age intervention should focus on augmentation of the primary repertoires. This isillustrated at the upper row of the diagram. Plastic changes induced a functional change of three neighboringclusters. This is indicated by the three clusters, which were non-filled in Fig. 1, but are depicted here with filledcircles with double margins. Thus, the reorganization resulted in a restoration of a part of the lost variation.NGST suggests that at older ages, the focus of intervention should be on the provision of ample opportunitiesfor active practice, as a richness in practice might form a compensation for the impaired selection processes(point of focus indicated by (-k) in the diagram).

MOTOR DEVELOPMENT OF CHILDREN WITH BRAIN LESIONS 43
movement-related afferent information (Hadders-Algra et al., 1999b) result in a decrease of variationin motor output and thus in a better task-specificadaptation ofmotor behavior.
NGST offers especially a framework for theunderstanding of the so-called ’negative’: signs ofCP, i.e., the paresis and central dyscoordination.Most likely, these ’negative’ movement disordersare more disabling for persons with CP than the’positive’ problems of CP, such as spasticity,musculoskeletal malformations, dyskinesia, andpersistent infantile reactions (Forssberg & Hadders-Algra, in press; cfi, Landau, 1974). Still, clinicalcare mainly focuses on the latter phenomena, withparesis and dyscoordination receiving relativelylittle attention. Of course, the treatment of’positive’ problems should not be discarded. Butneurohabilitation of children with CP could gainsubstantially by including interventions which aimat a reduction of sensorimotor dyscoordination. Inthis respect, the framework ofNGST could offer ahelping hand.
NGST: STRATEGIES FOR INTERVENTIONAFTER A BRAIN LESION AT EARLY AGE
Brain damage at early age is followed byconsiderable plastic changes. These changes, whichare regarded as mediators ofat least a part offunctional recovery, vary with the age of the insultand the size of the lesion (Kolb & Whishaw, 1989;Kolb, 1995). For instance, plasticity and recoveryare relatively large when the lesion is small andwhen the lesion occurs after the completion ofneuronal migration during the period when the
2The neurologist Jackson divided neurological symptoms intotwo categories: negative ones, which denote a deficit of normalbehavior due to destruction of neural tissue, and positive ones,which indicate exaggerated or distorted forms of behavior due toaction of neurons released from their normal integrativerelationship with other neural structures (Walshe, 1961 ).
processes of dendritic outgrowth and synapseformation are highly active (Kolb, 1995; Villa-blance & Hovda, 2000). The latter means that inthe human, considerable plasticity can be expectedwhen lesions occur between 2 and 3 months beforeand 6 and 8 months after term age. In general,plasticity does not involve the generation of newneurons, but a change in functional destination ofexisting neurons (Kolb, 1995; Kujala et al., 2000).An exception to this general rule is the recentfinding that midline frontal cortex lesions inneonatal rats can be followed by the regenerationof cortical tissue, the degree of regeneration beingrelated to the degree of functional recovery (Kolbet al., 1998). But usually, plasticity implies areprogramming of spared neural tissue, i.e., a
reorganization of the remaining cortical-sub-cortical networks and their descending projections(Carr et al., 1993; Cao et al., 1994; Chu et al., 2000).In terms of NGST, plasticity could mean that theneurons neighboring a lesionedand thus reducedprimary neuronal repertoire change function andget incorporated into the affected repertoire (Fig.4). This results in a recovery of the lesionedfunction in the form of a less reduced primaryrepertoire. Yet, the price of this reorganization canbe a moderate reduction of multiple primary neuronalrepertoires, including those not directly affected bythe lesion. A price, which clinically can be expressedby multiple dysfunctions and an overall drop in IQ(Vargha-Khadem et al., 1992; Kolb, 1995).
Notwithstanding the possible costs of re-
organization, the net results of plastic changesoccurring after a lesion of the brain at early age are
usually positive. From the NGST point of view,this could mean that early intervention after brainlesion should attempt to increase the primaryrepertoires. Presumably this could be achieved byproviding the infant with variable experiences.Variation in motor experience could, for instance,be obtained by varying the infant’s posture, as
posture is the basis for motility (Massion, 1998). The

44 M. HADDERS-ALGRA
question whether or not an increase in primaryvariability can be achieved is a subject for futureresearch. At present, the body of literature onintervention in young infants has neglected thelong-term effect of intervention on motordevelopment. But in analogy to the beneficial effectsof the early stimulation of cognitive developmentin infants biologically at risk because of pretermbirth (Infant Health and Development Program,1990), it can be hypothesized that well-definedearly sensorimotor intervention might have asimilar positive effect on motor development.
In addition to the focus on variable experiencesin order to increase the primary repertoires, NGSTsuggests which intervention at early age shouldaim at facilitating selection. Studies on normal motordevelopment indicated that frequent experience withtrial and error enhances the process of selection(Hadders-Algra et al., 1996b; Vereijken & Thelen;1997). Other studies on motor development inhealthy infants showett that the effect of training isspecific and does not generalize to other motorfunctions (Super, 1976; Zelazo et al., 1993). Possibly,training is most effective when the infant indicatesthat a specific motor skill is in developmentalfocus (McGraw, 1935; Super, 1976). It can besurmised that the process of selection in infantswith deficits in the processing of sensory informationon the basis of a brain lesion requires considerablymore repetition of trial and error experiences thanthe selection in typically developing infants does.In other words, infants with neurological dysfunctionmight benefit from ample opportunities to activelytry developing motor skills.
In older children with borderline to moderateforms of CP, dysfunctions in the secondaryvariability are most prominent. NGST suggeststhat children with these types of dysfunctions willbenefit from active practice, which will enhancethe processes of selection and thereby the productionof better adapted motor behavior. Indeed, experi-mental studies which evaluated the effect of
training on specific motor skills in children withCP indicated that active experience improves motorfunction (Valvano & Newell, 1998; Gordon & Duff,1999; Gordon et al., 1999). In clinical practice,children with CP are seldom treated according tostandardized programs. For instance, the frequentlyused NeuroDevelopmental Treatment consists of amixture of the application of handling techniquesand an encouragement of active movementwitheach therapist creating her/his own mixture ofmethods (DeGangi & Royeen, 1994). The findingthat the programs with the highest frequencies oftreatment (5-7 times per week) have the best resultssupports the notion of NGST that ample practicecan promote motor development in children with CP(Bower & McLellan, 1992; Bower et al., 1996).
CONCLUDING REMARKS
From the point of view of NGST, interventiontherapies for children with motor dysfunctions atearly age should focus on provision of variablesensorimotor experiences. The latter might beachieved by means of the application of variablepostures which counteract the infant’s propensityto produce stereotyped activity. With increasingage, the emphasis of intervention shifts to theprovision of ample opportunities for active practice,as plentiful practice might form a compensationfor the impaired selection. In children with spastichemiplegia, the technique of prolonged restraint ofthe relatively unaffected arm might be helpful.This technique, which successfully has been appliedin subjects with chronic motor impairment afterstroke, induces a forced use of the affected arm byblocking the use of the unaffected arm (Wolf et al.,1989; Taub et al., 1993). In addition, it is important torealize that children with brain dysfunction needmore practice than their non-affected peers.Therefore, it is essential to reinforce the child’smotivation by creating an ecological, playful

MOTOR DEVELOPMENT OF CHILDREN WITH BRAIN LESIONS 45
setting with positive feedback (Harter,Graves, 1995; Sims et al., 1996).
1978;
ACKNOWLEDGEMENTS
I thank Eva Brogren and Tineke Dirks for theircritical and valuable remarks on a previous draft ofthis manuscript. Jolanda Schaap is gratefullyacknowledged for technical assistance in thepreparation ofthe figures.
REFERENCES
Adolph KE, Vereijken B, Denny MA. 1998. Learningto crawl. Child Dev 69:1299-1312.
Aicardi J, Bax M. 1998. Cerebral palsy. In" Aicardi J,ed, Diseases of the Nervous System in Childhood,2nd edition. Clinics in Developmental MedicineNo. 115/118, Cambridge, UK: Mac Keith Press;210-239.
American Psychiatric Association. 1994. Diagnostic andStatistical Manual of Mental Disorders, 4 edition.Washington, DC, USA: American PsychiatricAssociation; 358.
Bobath K. 1966. The motor deficit in patients withcerebral palsy. Clinics in Developmental MedicineNo. 23. London, UK: William Heinemann MedBooks; 54.
Bower E, McLellan DL. 1992. Effect of increasedexposure to physiotherapy on skill acquisition ofchildren with cerebral palsy. Dev Med Child Neurol34: 25-39.
Bower E, McLellan DL, Amey J, Campbell MJ. 1996.A randomized controlled trial of different intensitiesof physiotherapy and different goals settingprocedures in 44 children with cerebral palsy.Dev Med Child Neurol 38: 226-237.
Bressler SL. 1995. Large scale cortical networks andcognition. Brain Res Rev 20: 288-304.
Brogren E, Hadders-Algra M, Forssberg H. 1998.Postural control in sitting children with spasticdiplegia. Neurosci Biobehav Rev 22:591-596.
Burnett CN, Johnson EW. 1971. Development of gaitin childhood: Part II. Dev Med Child Neurol 13"207-215.
Cao Y, Vikingstad EM, Huttenlocher PR, Towle V1,Levin DL. 1994. Functional magnetic resonancestudies of the reorganization of the human handsensorimotor area after unilateral brain injury inthe perinatal period. Proc Nat Acad Sci USA 91:9612-9616.
Carr LJ, Harrison LM, Evans AL, Stephens JA. 1993.Patterns of central motor reorganization in hemi-plegic cerebral palsy. Brain 116: 1223-1247.
Changeux J-P. 1997. Variation and selection in neuralfunction. Trends Neurosci 20:291-293.
Changeux J-P, Danchin A. 1976. Selective stabilisationof developing synapses as a mechanism for thespecification of neuronal networks. Nature 264:705-712.
Chu D, Huttenlocher PR, Levin DN, Towle VL. 2000.Reorganization of the hand somatosensory cortexfollowing perinatal unilateral brain injury. Neuro-pediatrics 31"63-69.
Cioni G, Duchini F, Milianti B, Paolicelli PB, SicolaE, Boldrini A, et al. 1993. Differences andvariations in the patterns of early independentwalking. Early Hum Dev 35" 193-205.
Cioni G, Fazzi B, Ipata AE, Canapicchi R, Van Hof-Van Duin J. 1996. Correlation between cerebralvisual impairment and magnetic resonance imagingin children with neonatal encephalopathy. DevMed Child Neurol 38: 120-132.
Costello AM deL, Hamilton PA, Baudin J, Townsend J,Bradford BC, Stewart AL, et al. 1988. Predictionof neurodevelopmental impairment at four yearsfrom brain ultrasound appearance of very preterminfants. Dev Med Child Neuro130:711-722.
DeGangi GA, Royeen CB. 1994. Current practiceamong NeuroDevelopmental Treatment Assoc-iation members. Am J Occup Therapy 48" 803-809.
De Vries JIP, Visser GHA, Prechtl HFR. 1982. Theemergence of fetal behavior. I. Qualitative aspects.Early Hum Dev 7:301-322.
Diener HC, Bootz F, Dichgans J, Bruzek W. 1983.Variability of postural reflexes in humans. ExpBrain Res 52: 423-428.
Edelman GM. 1989. Neural Darwinism. The Theoryof Neuronal Group Selection. Oxford, UK: OxfordUniversity Press; 371.
Edelman GM. 1993. Neural Darwinism: Selection andreentrant signaling in higher brain function.Neuron 10:115-125.

46 M. HADDERS-ALGRA
Edelman GM, Tononi G. 2000. Consciousness" HowMatter Becomes Imagination. London, UK: AllenLane, The Penguin Press; 274.
Eliasson AC, Gordon AM. 2000. Impaired forcecoordination during object release in childrenwith hemiplegic cerebral palsy. Dev Med ChildNeuro142: 228-234.
Eliasson AC, Gordon AM, Forssberg H. 1992.Impaired anticipatory control of isometric forcesduring grasping by children with cerebral palsy.Dev Med Child Neurol 34" 216-225.
Eliasson AC, Gordon AM, Forssberg H. 1995. Tactilecontrol of isometric finger forces during graspingin children with cerebral palsy. Dev Med ChildNeurol 37: 72-84.
Evans GA. 1998. The human genome project. ArchNeurol 55: 1287-1290.
Fallang B, Saugstad OD, Hadders-Algra M. 2000.Goal directed reaching and postural control insupine position in healthy infants. Behav BrainRes 115: 9-18.
Fawer CL, Diebold P, Calame A. 1987. Periventricularleucomalacia and neurodevelopmental outcome inpreterm infants. Arch Dis Childhood 62" 30-36.
Fazzi E, Orcesi S, Caffi L, Ometto A, Rondini G,Telesca C, et al. 1994. Neurodevelopmental outcomeat 5-7 years in preterm infants with periventri-cular leukomalacia. Neuropediatrics 25:134-136.
Ford LM, Steichen J, Steichen PA, Babcock D,Fogelson MH. 1989. Neurologic status and intra-cranial hemorrhage in very-low-birthweight pre-term infants. Outcome at year and 5 years. Am JDis Children 143" 1186-1190.
Forssberg H. 1985. Ontogeny of human locomotorcontrol. I. Infant stepping, supported locomotionand transition to independent locomotion. ExpBrain Res 57: 480-493.
Forssberg H, Hadders-Algra M. In press. Pathophysi-ology of movement disorders in cerebral palsy. In:Velcikovic Perat M, Neville B, eds, Cerebral Palsy.Amsterdam, the Netherlands: Elsevier Science Publ.
Forssberg H, Hirschfeld H. 1994. Postural adjustmentsin sitting humans following external perturbations:Muscle activity and kinematics. Exp Brain Res97:515-527.
Forssberg H, Eliasson A-C, Redon-Zouitenn C,Mercuri E, Dubowitz L. 1999a. Impaired grip-liftsynergy in children with unilateral brain lesions.Brain 122:1157-1168.
Forssberg H, Juaite A, Hadders-Algra M. 1999b. Sharedmemory representations for programming oflifting movements and associated whole bodypostural adjustments. Neurosci Lett 273: 9-12.
Gesell A, Amatruda CS. 1947. Developmental diagnosis.Normal and Abnormal Child Development, 2nd
edition. New York, NY, USA: Harper& Row; 496.Gordon AM, Charles J, Duff SV. 1999. Fingertip
forces during object manipulation in children withhemiplegic cerebral palsy. II. Bilateral coordination.Dev Med Child Neurol 41" 176-185.
Gordon AM, Duff SV. 1999. Fingertip forces duringobject manipulation in children with hemiplegiccerebral palsy. I. Anticipatory scaling. Dev MedChild Neurol 41:166-175.
Graves P. 1995. Therapy methods for cerebral palsy. JPaediatric Child Health 31: 24-28.
Greenough WT, Black JE, Wallace CS. 1987. Experienceand brain development. Child Dev 58: 539-559.
Grillner S, Deliagina T, Ekeberg O, El Manira A, HillRH, Lansner A, et al. 1995. Neural networkswhich coordinate locomotion and body orientationin lamprey. Trends Neurosci 18: 270-279.
Hadders-Algra M. 2000a. The Neuronal GroupSelection Theory: An attractive framework toexplain variation in normal, motor development.Dev Med Child Neuro142: 566-572.
Hadders-Algra M. 2000b. The Neuronal GroupSelection Theory: promising principles for theunderstanding and treatment of motor disorders.Dev Med Child Neuro142:707-715.
Hadders-Algra M. In press. The clumsy child--at theborder of cerebral palsy? In: Velcikovic Perat M,Neville B, eds, Cerebral Palsy. Amsterdam, TheNetherlands: Elsevier Science Publ.
Hadders-Algra M, Brogren E, Forssberg H. 1996a.Ontogeny of postural adjustments during sitting ininfancy: Variation, selection and modulation. JPhysio1493: 273-288.
Hadders-Algra M, Brogren E, Forssberg H. 1996b.Training affects the development of posturaladjustments in siring infants. J Physiol 493: 289-298.
Hadders-Algra M, Brogren E, Forssberg H. 1998.Postural adjustments during sitting at pre-schoolage: The presence of a transient toddling phase.Dev Med Child Neuro140: 436-447.
Hadders-Algra M, Brogren E, Katz-Salamon M,Forssberg H. 1999a. Periventricular leukomalacia

MOTOR DEVELOPMENT OF CHILDREN WITH BRAIN LESIONS 47
and preterm birth have a different detrimental effecton postural adjustments. Brain 122: 727-740.
Hadders-Algra M, Dirks T. 2000. De motorischeontwikkeling van de zuigeling: variren, selecterenen leren adapteren. Houten: Bohn, Stafleu & VanLoghum; 117.
Hadders-Algra M, Groothuis AMC. 1999. Quality ofgeneral movements in infancy is related to thedevelopment of neurological dysfunction, attentiondeficit hyperactivity disorder and aggressivebehavior. Dev Med Child Neurol 41:381-391.
Hadders-Algra M, Klip-Van den Nieuwendijk AWJ,Martijn A, Van Eykem LA. 1997. Assessment ofgeneral movements: Towards a better understandingof a sensitive method to evaluate brain function inyoung infants. Dev Med Child Neurol 39: 88-98.
Hadders-Algra M, Touwen BCL. In press. Perinatalevents and soft neurological signs in neuro-behavioral outcome studies. Dev Neuropsychol.
Hadders-Algra M, Van der Fits IBM, Stremmelaar EF,Touwen BCL. 1999b. Development of posturaladjustments during reaching in infants with cerebralpalsy. Dev Med Child Neurol 41: 766-776.
Hadders-Algra M, Van Eykem LA, Klip-van denNieuwendijk AWJ, Prechtl HFR. 1992. Develop-mental course of general movements in earlyinfancy. II. EMG correlates. Early Hum Dev 28"231-252.
Hagberg B, Hagberg G, Olow I, Von Wendt L. 1996.The changing panorama of cerebral palsy inSweden. VII. Prevalence and origin in the birthyear period 1987-1990. Acta Paediatrica 85"954-60.
Harter S. 1978. Effectance motivation reconsidered.Toward a developmental model. Hum Dev 21:34-64.
Hebb DO. 1949. The organization of behavior. NewYork, NY, USA: Wiley; 303.
Hikosaka O, Nakahara H, Rand MK, Sakai K, Lu X,Nakamura K, et al. 1999. Parallel neural networksfor learning sequential procedures. Trends Neurosci22:464-471.
Hirschfeld H, Forssberg, H. 1994. Epigenetic develop-ment of postural responses for siring duringinfancy. Exp Brain Res 97: 528-540.
Huttenlocher PR, DeCourten C, Garey LJ, Van derLoos H. 1982. Synaptogenesis in human visualcortex- evidence for synapse elimination duringnormal development. Neurosci Lett 33" 247-252.
Infant Health & Development Program. 1990. Enhancingthe outcomes of low-birth-weight, prematureinfants. J Am Med Assoc 263" 3035-3042.
Ingram TTS. 1966. The neurology of cerebral palsy.Arch Dis Childhood 41" 337-357.
Jemigan TL, Trauner DA, Hesselink JR, Talla PA.1991. Maturation of the human cerebrumobserved ’in vivo’ during adolescence. Brain 114:2037-2049.
Kolb B. 1995. Brain Plasticity and Behavior. Mahwah,New Jersey, USA: Lawrence Erlbaum Assoc.; 183.
Kolb B, Gibb R, Gomy G, Whishaw IQ. 1998.Possible regeneration of rat medial frontal cortexfollowing neonatal frontal lesions. Behav BrainRes 91: 127-141.
Kolb B, Whishaw IQ. 1989. Plasticity in theneocortex: Mechanisms underlying recovery fromearly brain damage. Prog Neurobiol 32: 235-276.
Konczak J, Borutta M, Topka H, Dichgans J. 1995.The development of goal-directed reaching ininfants: Hand trajectory formation and joint torquecontrol. Exp Brain Res 106:156-168.
Konishi Y, Takaya R, Kimura K, Konishi K, Fujii Y,Saito M, et al. 1994. Development of posture inprone and supine position during the pretermperiod in low risk preterm infants. Arch DisChildhood 70: F188-F191.
Kujala T, Alho K, Na/ttanen R. 2000. Cross-modalreorganization of human cortical functions. TrendsNeurosci 23" 115-120.
Landau WM. 1974. Spasticity: The fable of a neuro-logical demon and the emperor’s new therapy.Arch Neurol 31: 217-219.
Largo RH, Molinari L, Weber M, Comenale Pinto L,Duc G. 1985. Early development of locomotion:Significance of prematurity, cerebral palsy andsex. Dev Med Child Neuro127:183-191.
Liu Y, Gao J-H, Liotti M, Pu Y, Fox PT. 1999.Temporal dissociation of parallel processing in thehuman subcortical outputs. Nature 400: 364-367.
Magnus R, De Kleijn A. 1912. Die abh/tngigkeit desTonus der Extremit/ttenmuskeln von der Kopf-stellung. Pfltlger’s Archiv 145" 455-548.
Massion J. 1998. Postural control systems in develop-mental perspective. Neurosci Biobehav Rev 22:465-472.
McGraw M. 1935. Growth: A study of Johnny andJimmy. New York, NY, USA: Appleton-Century;316.

48 M. HADDERS-ALGRA
McGraw MB. 1943. The neuromuscular maturation ofthe human infant. (reprinted in: Classics inDevelopmental Medicine, no. 4, London, UK:Mac Keith Press, 1989; 117).
Minkowski M. 1938. Neurobiologische Studien ammenschlichen Foetus. In: Abderhalden E, ed,Handbuch der biologischen Arbeitsmethoden.Abt. V: Methoden zum Studium der Funktionender einzelne Organe im Tierischen Organismus.Teil 5B. Berlin, Germany: Urban & Schwarzenberg;511-619.
Mailer K, Ebner B, HOmberg V. 1994. Maturation offastest afferent and efferent central and peripheralpathways: No evidence for a constancy of centralconduction delay. Neurosci Lett 166: 9-12.
Mutch L, Alberman E, Hagberg B, Kodama K,Velickovic Perat M. 1992. Cerebral palsyepidemiology: Where are we now and where arewe going? Dev Med Child Neurol 34: 547-555.
Nashner LM, Shumway-Cook A, Marin O. 1983.Stance posture control in a select group ofchildren with cerebral palsy: Deficits in sensoryorganization and muscular coordination. ExpBrain Res 49: 393-409.
Nelson PG, Fields RD, Yu C, Liu Y. 1993. Synapseelimination fi’om the mouse neuromuscular junctionin vitro: A non-Hebbian activity dependent process. JNeurobio124: 1517-1530.
O’Leary DDM. 1989. Do cortical areas emerge from aprotocortex? Trends Neurosci 12: 400-406.
Peiper A. 1963. Cerebral function in infancy andchildhood, 3rd edition. New York, NY, USA:Consultants Bureau; 683.
Prechtl HFR. 1990. Qualitative changes of spontaneousmovements in fetus and preterm infant are a markerof neurological dysfunction. Early Human Dev23: 151-158.
Prechtl HFR, Einspieler C, Cioni G, Bos A, Ferrari F,Sontheimer D. 1997. An early marker ofdeveloping neurological handicap after perinatalbrain lesions. The Lancet 339" 1361-1363.
Prechtl HFR, Nolte R. 1984. Motor behavior ofpreterm infants. In: Prechtl HFR, ed, Continuity ofNeural Functions From Prenatal to Postnatal Life.Clinics in Developmental Medicine No. 94, Oxford,UK: Blackwell Scientific Publications; 79-92.
Purves D. 1994. Neural activity and the growth of thebrain. Cambridge, UK: Cambridge UniversityPress; 106.
Rakic P. 1988. Specification of cerebral cortical areas.Science 241" 170-176.
Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N,Goldman-Rakic PS. 1986. Concurrent over-production of synapses in diverse regions of theprimate cerebral cortex. Science 232: 232-235.
Schomburg ED. 1990. Spinal sensorimotor systemsand their supraspinal control. Neurosci Res 7:265-340.
Sherrington CS. 1906. The physiological position anddominance of the brain. In: Sherrington CS, ed,The Integrative Action of the Nervous System.London, UK: Constable & Comp.; 308-353.
Sims K, Henderson SE, Morton J, Hulme C. 1996.The remediation of clumsiness. II Is kinaesthesisthe answer? Dev Med Child Neurol 38: 988-997.
Simmers J, Meyran P, Moulins M. 1995. Modulationand dynamic specification of motor rhythm-generating circuits in crustacea. J Physiol (Paris)89:195-208.
Spores O, Edelman GM. 1993. Solving Bemstein’sproblem: A proposal for the development ofcoordinated movement by selection. Child Dev64:960-981.
Statham L, Murray MP. 1971. Early walking patternsof normal children. Clin Orthopedics Related Res79: 8-24.
Super CM. 1976: Environmental effects on motordevelopment: The case of ’African Infant Precocity’.Dev Med Child Neurol 18:561-567.
Taub E, Miller N, Novack TA, Cook III EW, FlemingWC, Nepomuceno CS, et al. 1993. Technique toimprove chronic motor deficit atter stroke. ArchPhys Med Rehab 74: 347-354.
Thelen E. 1985. Developmental origins of motorcoordination: Leg movements in human infants.Dev Psychobiol 18: 1-22.
Thelen E. 1995. Motor development. A new synthesis.Am Psychologist 50: 79-95.
Thelen E, Corbetta D, Kamm K, Spencer JP,Schneider K, Zemicke RF. 1993. The transition toreaching: Mapping intention and intrinsic dynamics.Child Dev 64" 1058-1098.
Touwen BCL. 1975. Variability and stereotypy in normaland deviant development. In: Apley J, ed, Care ofthe Handicapped Child. Clinics Dev Med No. 67,London, UK: Heinemann Medical Books; 99-110.
Touwen BCL. 1993. How normal is variable, or howvariable is normal? Early Human Dev 34: 1-12.

MOTOR DEVELOPMENT OF CHILDREN WITH BRAIN LESIONS 49
Ulrich BD. 1997. Dynamic systems theory and skilldevelopment in infants and children. In: ConnollyKJ, Forssberg H, eds, Neurophysiology andNeuropsychology of Motor Development. Clinicsin Developmental Medicine No. 143-144,London, UK: Mac Keith Press; 319-345.
Vaal J, Van Soest AJ, Hopkins B, Sie LTL, Van derKnaap MS. 2000. Development of spontaneousleg movements in infants with and withoutperiventricular leukomalacia. Exp Brain Res 135"94-105.
Valvano J, Newell KM. 1998. Practice of a precisionisometric grip-force task by children with spasticcerebral palsy. Dev Med Child Neurol 40: 464-473.
Vargha-Khadem F, Isaacs E, Van der Werf S, Robb S,Wilson J. 1992. Development of intelligence andmemory in children with hemiplegic cerebralpalsy. Brain 115:315-329.
Van der Fits IBM, Klip AWJ, Van Eykem LA,Hadders-Algra M. 1998. Postural adjustmentsaccompanying fast pointing movements in standing,siring and lying adults. Exp Brain Res 120: 202-216.
Van der Fits IBM, Flikweert ER, Stremmelaar EF,Martijn A, Hadders-Algra M. 1999a. Develop-ment of postural adjustments during reaching inpreterm infants. Pediatr Res 46: 1-7.
Van der Fits IBM, Klip AWJ, Van Eykem LA,Hadders-Algra M. 1999b. Postural adjustmentsduring spontaneous and goal-directed arm move-ments in the first half year of life. Behav BrainRes 106, 75-90.
Van der Fits IBM, Otten E, Klip AWJ, Van EykemLA, Hadders-Algra M. 1999c. The developmentof postural adjustments during reaching in 6 to 18
months old infants: Evidence for two transitions.Exp Brain Res 126:517-528.
Vereijken B, Thelen E. 1997. Training infant treadmillstepping: The role of individual pattern stability.Dev Psychobiol 30: 89-102.
Verhage M, Maia AS, Plomp JJ, Brussaard AB,Heeroma JH, Vermeer H, et al. 2000. Synapticassembly of the brain in the absence of neuro-transmitter secretion. Science 287: 864-868.
Villablance JR, Hovda DA. 2000. Developmentalneuroplasticity in a model of cerebral hemi-spherectomy and stroke. Neuroscience 95: 625-637.
Vies JSH, Kingma H, Caberg H, Daniels H, Casaer P.1989. Posture oflow-risk preterm infants between 32and 36 weeks postmenstrual age. Dev Med ChildNeurol 31: 191-195.
Volpe JJ. 1995. Neurology ofthe Newborn, 3rd edition.Philadelphia, Pennsylvania, USA: Saunders; 876.
Von Hofsten C. 1991. Structuring of early reachingmovements: A longitudinal study. J Motor Behav23: 280-292.
Walshe FMR. 1961. Contributions of John HuglingsJackson to neurology: A brief introduction to histeachings. Arch Neurol 5" 119-131.
Wolf SL, Lecraw DE, Barton LA, Jann BB. 1989.Forced use of hemiplegic upper extremities toreverse the effect of learned nonuse amongchronic stroke and head-injured patients. ExpNeurol 104: 125-132.
Yekutiel M, Jariwalda M, Stretch P. 1994. Sensorydeficit in the hands of children with cerebralpalsy: A new look at assessment and prevalence.Dev Med Child Neuro136:619-624.
Zelazo NA, Zelazo PR, Cohen KM, Zelazo PD. 1993.Specificity of practice effects on elementaryneuromotor patterns. Dev Psychobiol 29: 686-691.

Submit your manuscripts athttp://www.hindawi.com
Neurology Research International
Hindawi Publishing Corporationhttp://www.hindawi.com Volume 2014
Alzheimer’s DiseaseHindawi Publishing Corporationhttp://www.hindawi.com Volume 2014
International Journal of
ScientificaHindawi Publishing Corporationhttp://www.hindawi.com Volume 2014
Hindawi Publishing Corporationhttp://www.hindawi.com Volume 2014
BioMed Research International
Hindawi Publishing Corporationhttp://www.hindawi.com Volume 2014
Research and TreatmentSchizophrenia
The Scientific World JournalHindawi Publishing Corporation http://www.hindawi.com Volume 2014
Hindawi Publishing Corporationhttp://www.hindawi.com Volume 2014
Neural Plasticity
Hindawi Publishing Corporationhttp://www.hindawi.com Volume 2014
Parkinson’s Disease
Hindawi Publishing Corporationhttp://www.hindawi.com Volume 2014
Research and TreatmentAutism
Sleep DisordersHindawi Publishing Corporationhttp://www.hindawi.com Volume 2014
Hindawi Publishing Corporationhttp://www.hindawi.com Volume 2014
Neuroscience Journal
Epilepsy Research and TreatmentHindawi Publishing Corporationhttp://www.hindawi.com Volume 2014
Hindawi Publishing Corporationhttp://www.hindawi.com Volume 2014
Psychiatry Journal
Hindawi Publishing Corporationhttp://www.hindawi.com Volume 2014
Computational and Mathematical Methods in Medicine
Depression Research and TreatmentHindawi Publishing Corporationhttp://www.hindawi.com Volume 2014
Hindawi Publishing Corporationhttp://www.hindawi.com Volume 2014
Brain ScienceInternational Journal of
StrokeResearch and TreatmentHindawi Publishing Corporationhttp://www.hindawi.com Volume 2014
Neurodegenerative Diseases
Hindawi Publishing Corporationhttp://www.hindawi.com Volume 2014
Journal of
Cardiovascular Psychiatry and NeurologyHindawi Publishing Corporationhttp://www.hindawi.com Volume 2014