DUAL PHASE LAG BEHAVIOR OF LIVING BIOLOGICAL TISSUES...
Transcript of DUAL PHASE LAG BEHAVIOR OF LIVING BIOLOGICAL TISSUES...

DUAL PHASE LAG BEHAVIOR OF LIVING BIOLOGICAL TISSUES IN LASER
HYPERTHERMIA
_______________________________________
A Thesis
presented to
the Faculty of the Graduate School
at the University of Missouri-Columbia
_______________________________________________________
In Partial Fulfillment
of the Requirements for the Degree
Master of Science
_____________________________________________________
by
NAZIA AFRIN
Dr. Yuwen Zhang, Thesis Supervisor
MAY 2011

The undersigned appointed by the Dean of the Graduate Faculty, have examined a thesis entitled
DUAL PHASE LAG BEHAVIOR OF LIVING BIOLOGICAL TISSUES IN
LASER HYPERTHERMIA
presented by NAZIA AFRIN
a candidate for the degree of Master of Science in Mechanical and Aerospace Engineering,
and hereby certify that in their opinion it is worthy of acceptance
---------------------------------------------------------------------------------
Dr. Yuwen Zhang
-------------------------------------------------------------------------------
Dr. J. K. Chen
--------------------------------------------------------------------------------
Dr. Stephen Montgomery-Smith

ii
ACKNOWLEDGEMENTS
I am highly grateful to my supervisor Professor Dr. Yuwen Zhang, Department of
Mechanical and Aerospace Engineering for his encouragement, support, patience and guidance
throughout this research work also in daily life. This dissertation would not have been possible
without guidance and help of him. He contributed and extended his valuable assistant in the
preparation nd completion of this study. I would like to thank the members of my thesis
evaluation committee, Dr. J. K. Chen and Dr. Stephen Montgomery-Smith for giving the time to
provide valuable comments and criticism. Special thanks must be extended to Dr. Stephen
Montgomery-Smith for his assistance and courage of confidence.
I would like to express my sincere thanks to Professor Dr. Robert Tzou, Chairman of
Department of Mechanical and Aerospace Engineering for all the guidance, assistance and help
throughout this study. I am very grateful to him.
I would like to thanks Marilyn Estes and Melanie Carraher for all their helps on my
graduate paperwork.
I would like to thank my all coworkers, Jianhua Zhou, Sejoong Kim, Tao Jia, Yijin Mao,
Yunpeng Ren. It is really great time to work with them and I really enjoy their company in our
lab.
Also I would like to thank my friends, Rilya Rumbayan, Roxana Mtz C, Srisharan G.
Govindarajan and Weijun Huang. It is my pleasure to spend time with such wonderful friends
and I am wishing their successful life.

iii
I would like to express my gratitude to my parents, Shamsun Nahar Islam and Late F. K.
M Aminul Islam. My mother always gives me inspirations all the time about my study although
she is far away from me. Even though my father is not alive in this world, however, still I feel his
contribution on my every success in my life.
Support for this work by the University of Missouri Research Board and U.S. National
Science Foundation (NSF) under Grant No. CBET-0730143 is gratefully acknowledged.

iv
TABLE OF CONTENTS
ACKNOWLEDGEMENT ………………………………………………………………………ii
LIST OF FIGURES…………………………………………………………………………….vii
LIST OF TABLES ……………………………………………………………………………...ix
NOMENCLATURE ………………………………………………………………………….....x
ABSTRACT …………………………………………………………………………………....xii
CHAPTER 1: INTRODUCTION………………………………………………………………..1
1.1 Background………………………………………………………………………………1
1.2 Heat Conduction Model………………………………………………………………….4
1.2.1 Pennes Bioheat Equation………………………...…………………………………4
1.2.2 Thermal Wave Model……………………………………………………………...5
1.2.3 Dual Phase Lag Model……………………………………………………………..6
1.3 Thesis Objectives…………………………………………………………………………7
CHAPTER 2: NUMERICAL SIMULATION OF THERMAL DAMAGE TO LIVING
BIOLOGICAL TISSUES INDUCED BY LASER IRRADIATION BASED ON A
GENERALIZED DUAL PHASE LAG MODEL……………………………………………....9
2.1 Tissue-laser interactions…………………………………………………………………9

v
2.2 Laser parameters…………………………………………………………………….….10
2.3 Classical DPL model…………………………………………………………………...11
2.4 Generalized DPL model……………………………………………………….…….…12
2.5 Physical model……………………………………………………………………….…17
2.6 Problem statement……………………………………………………………………....17
2.7 Calculation of the laser irradiance………………………………………………………18
2.8 Calculation of thermal damage parameter………………………………………………19
2.9 Numerical Analysis……………………………………………………………………..20
2.9.1 Discretization scheme of space…………………………………………………...20
2.9.2 Discretization of governing equation…………………………………………......20
2.10 Results and Discussions…………………………………………………………………22
2.11 Conclusion………………………………………………………………………………38
CHAPTER 3: THERMAL LAGGING IN LIVING BIOLOGICAL TISSUE BASED ON
NONEQUILIBRIUM HEAT TRANSFER BETWEEN TISSUE, ARTERIAL AND VENOUS
BLOODS………………………………………………………………………………………..40
3.1 Heat transfer in arteries, venous and solid tissue……………………………………….40
3.2 Governing equations…………………………………………………………………….41
3.3 Dual phase bioheat equation for three carriers system…………………………………..42

vi
3.4 Results and Discussion……………………………………………………………….….46
3.5 Conclusion……………………………………………………………………………….56
CHAPTER 4: SUMMERY AND CONCLUSIONS…………………………………………….59
REFERENCES…………………………………………………………………………………..60

vii
LIST OF FIGURES
Figure Page
Fig. 1 Physical model and grid system ………………………………………………………….17
Fig. 2 Temperature evolution at the irradiated surface of a highly absorbing tissue …………...23
Fig. 3 Thermal damage at the irradiated surface of a highly absorbing tissue…………………..24
Fig. 4 Temperature evolution at the irradiated surface of a scattering tissue …………………...25
Fig. 5 Thermal damage at the irradiated surface of a scattering tissue…………………………..26
Fig. 6 Temperature distribution at the irradiated surface of a scattering tissue for different τT
values…………………………………………………………………………………………… 27
Fig. 7 Thermal damage at the irradiated surface of a scattering tissue for different τT values…..28
Fig. 8 Temperature distribution at the irradiated surface of a scattering tissue calculated by the
non-equilibrium DPL model for different τq values……………………………………………..29
Fig. 9 Thermal damage at the irradiated surface of a scattering tissue calculated by the
nonequilibrium DPL model for different τq values………………………………………………30
Fig. 10 Effects of laser irradiance on temperature at the irradiated surface of a scattering
tissue……………………………………………………………………………………………..31
Fig. 11 Effects of laser irradiance on damage parameter at the irradiated surface of a scattering
tissue……………………………………………………………………………………………..32
.

viii
Fig. 12 Effects of laser exposure time on temperature at the irradiated surface of a scattering
tissue……………………………………………………………………………………………33
Fig. 13 Effects of laser exposure time on damage parameter at the irradiated surface of a
scattering tissue…………………………………………………………………………………34
Fig. 14 Effects of coupling factor on temperature at the irradiated surface of a scattering
tissue…………………………………………………………………………………………….35
Fig. 15 Effects of coupling factor on damage parameter at the irradiated surface of a scattering
tissue…………………………………………………………………………………………….36
Fig. 16 Effects of blood perfusion rate on temperature at the irradiated surface of a scattering
tissue…………………………………………………………………………………………….37
Fig. 17 Effects of blood perfusion rate on thermal damage at the irradiated surface of a scattering
tissue………………………………………………………………………………………….....38
Fig. 18 Schematic view of artery and vein surrounding by tissue………………………………40

ix
LIST OF TABLES
Table Page
Table: 1 Structure and perfusion coefficient studied in Ref [27]……………………………..47
Table: 2 Coupling factors and phase lag times………………………………………………..49
Table: 3 Phase lag times for different thermo physical properties of tissue…………………..50
Table: 4 Phase lag times for same blood perfusion rate ……………………………………...52
Table: 5 Phase lag times for the same diameter of tissue……………………………………..53
Table: 6 Phase lag time for brain……………………………………………………………...55
Table: 7 Phase lag time for muscle…………………………………………………………....56

x
NOMENCLATURES
a specific heat transfer area [m2/ m3]
c specific heat of artery [J/ (kg K)]
G coupling factor between blood and tissue [W/ (m3 K)]
h heat transfer coefficient [W/(m2 K)]
k thermal conductivity [W/(m K)]
q heat flux vector [W/m2]
r position vector [m]
QL heat source due to hyperthermia therapy [W/m3]
Qm source terms due to metabolic heating [W/m3]
t time [s]
T average temperature [K]
V intrinsic phase averaged velocity vector [m/s]
w blood perfusion rate [m3/m3 tissue]
Rd diffuse reflectance of light
A frequency factor [s-1]
R universal gas constant [J/(mol K)]
E energy of activation of denaturation reaction [J/ mol]
Nu Nusselt number
db diameter of the blood vessel [m]
S heat source due to hyperthermia therapy [W/m3]
Sm source terms due to metabolic heating [W/m3]
R vascular resistance
Greek symbols
α thermal diffusivity [m2/s]
porosity
ρ density [kg/m3]
ρa artery blood mass density [kg/m3]

xi
ρv venous blood mass density [Kg/m3]
ρs tissue density [kg/m3]
τq phase lag time of the heat flux [s]
τT phase lag of the temperature gradient [s]
τL laser exposure time
φin incident laser irradiance
µa absorption coefficient [cm-1]
µs scattering coefficient [cm-1]
φ (x) local light irradiance
δ effective penetration depth
g scattering anisotropy
Ω damage parameter
(δx)w distance between W and P (two grid points)
(δx)e distance between P and E( two grid points)
Subscripts
s solid matrix (tissue)
b blood vessel
a arterial blood
v venous blood
eff effective

xii
ABSTRACT
A Generalized dual phase lag behavior for living biological tissues are investigated for blood and
tissues and also developed a generalized dual phase model for artery, vein and tissues in this
thesis. There are two parts of this thesis:
a) A Generalized dual phase lag (DPL) bioheat model based on the non equilibrium heat
transfer in living biological tissues is applied to investigate thermal damage induced by
laser irradiation. Comparisons of the temperature responses and thermal damages
between the generalized and classical DPL bioheat model, derived from the constitutive
DPL model and Pennes bioheat equation, and as well as Fourier heat conduction model
are carried out. It is shown that the generalized DPL model could predict significantly
different temperature and thermal damage from the classical DPL model and Fourier heat
conduction model. The generalized DPL equation can reduce to the classical Pennes heat
conduction equation only when the phase lag times of temperature gradient (τT) and heat
flux vector (τq) are both zero. The effects of laser parameters such as laser exposure time,
laser irradiance, and coupling factor on the thermal damage are also studied.
b) Arterial, venous blood and solid tissue are the three energy carriers that contribute to heat
transfer in the living biological tissues. A generalized dual-phase lag mode for living
biological tissues based on nonequilibrium heat transfer between tissue, artery and
venous bloods is presented in this thesis. The phase lag times for heat flux and
temperature gradient only depend on properties of artery, vein and tissue, blood perfusion
rate and convective heat transfer rate and are estimated using the available properties
from the literature. It is found that the phase lag times for heat flux and temperature
gradient are the identical for the case that the tissue and blood have the same properties.

xiii
However, the phase lag times are different for the case that the properties of tissue and
bloods are different. The phase lag times for brain and muscles are also discussed.

1
CHAPTER 1
INTRODUCTION
1.1 Background
The role of lasers in medical applications has increased dramatically over the past four
decades. Laser radiation possesses unique characteristics and has been extensively used in
clinical science for diagnostic and therapeutic applications. Most of the laser medical treatments
such as surgery, angioplasty, hyperthermia of tumors and laser tissue soldering are concern with
the thermal effects.
Welch described a three-step model for predicting laser induced thermal damage in
biological tissues [1]. The laser energy deposition was described based on the light propagation
in tissue first, followed by analyzing thermal response by solving a heat conduction equation.
Finally, the damage of the tissue was determined based on protein denaturation evaluated by a
chemical rate process equation. Many researchers have adopted this approach by applying
different methods to solve the problems involved in the process. In most cases, the bioheat
conduction equation based on Fourier’s law was used to investigate laser-induced damage in
biological tissue.
A real biological tissue can be treated as a non-homogeneous fluid saturated porous
medium. Heat transfer in living biological tissues involves multiple mechanisms including
conduction in tissue, convection between blood and tissues, blood perfusion or advection and
diffusion through micro vascular beds, and metabolic heat generation [2]. To date, Pennes
bioheat equation [3] has most widely been applied to obtain temperature distribution in living
biological tissues. It is assumed that when the venous blood flows from the capillary bed to the
main supply vein, its temperature remains the same as the tissue temperature regardless the size

2
of the vessel and the flow rate. The heat conduction in biological tissue is modeled by using
Fourier’s law, which assumes thermal disturbance propagates at an infinite velocity. An infinite
speed of heat propagation implies that a thermal disturbance applied at a certain location in a
medium can be sensed immediately anywhere in the medium. There are many situations where
the assumption of infinite speed of thermal propagation could be inadequate. Parallel to Fourier’s
law, in thermal wave model (Cattaneo Vernotte wave model [4-5]), the heat flux and the
temperature gradient across a material volume are assumed to occur at different instants of time.
Although allowing for a delayed response between the heat flux and temperature gradient, the
temperature gradient is always the cause for heat flux while the heat flux is always the effect [6].
Tzou [6-8] established a DPL model which introduces two different time delays between the
temperature gradient and the heat flux. The aim of this model was to remove the precedence
assumption that was made in the thermal wave model. It allows either the temperature gradient to
precede the heat flux or the heat flux to precede the temperature gradient in a transient process.
Recently the DPL model has attracted considerable interests in the field of engineering and
medical science [8]. It has been used to interpret the non-Fourier heat conduction phenomenon in
the processed meats [9].
The transport of thermal energy in living tissue is a complex process. It involves multiple
phenomenological mechanisms including conduction in tissue, convection between blood and
tissues, blood perfusion or advection and diffusion through micro vascular beds, and metabolic
heat generation. The bioheat transfer modeling is the basis of thermotherapy and the
thermoregulation system in a human body. Variations of temperature and heat transfer in a
human body depend on the arterial and venous blood flow rates, blood perfusion rate, and
metabolic heat generation, heat conduction within the tissue, thermal properties of blood and

3
tissue, and also on the human body geometry [2]. The whole anatomical structure can be
considered as a fluid saturated porous medium as tissue can be considered as a solid matrix and
blood penetrate the porous space of the medium. So, heat transfer phenomenon can be
considered as convection heat transfer in porous medium with internal heat generation.
Pennes [3] bioheat equation is the most widely applied model for temperature distribution in
the living biological tissues. The effect of arterial blood on the heat transfer in a living tissue is
taken into account by a blood perfusion term, which is proportional to the volumetric rate of
blood perfusion and the difference between the average arterial blood and tissue temperatures.
Pennes bioheat model is valid only if when the venous blood flows from the capillary bed to the
main supply vein, its temperature remains the same as the tissue temperature regardless the size
of vessel and the flow rate. To take metabolic heat generation within the tissue and local
variation of the thermal properties of tissue into account, core and shell model [10] and four
layer model [11] were developed for the thermoregulatory application, in which temperature
changes of both arterial and venous blood flows were treated by the lumped parameter models.
The temperature variation in the axial direction is greater than that in the radial direction due
to the blood perfusion through the tissue and the countercurrent effect between the arterial and
venous blood flows [12]. The axial heat transfer and temperature gradient are not negligible
which post additional challenge in analyzing bioheat transport in living biological tissue. That
means analyzing the heat transfer phenomenon in living biological tissue should consider the
effects of direction of the blood flow. The complex vascular architecture is the fundamental
problem in heat transfer process within the human body [13], including the variation of number,
size and spacing of the vessels, the thermal interaction among arteries, veins and tissues,
metabolic heat generation, convection and blood perfusion through the capillary beds and

4
interaction with the environment in a complete model. In their series of papers, Weinbaum et al.
[14-16] proposed a bioheat equation considering the variation of the number, density and size
and flow velocity of the countercurrent arterial-venous vessels. That model was applied for the
single organ rather than the whole human body for thermoregulation.
1.2 Heat Conduction Models
1.2.1 Pennes Bioheat Equation:
The general bioheat equation considering blood perfusion and metabolic heat generation
is as follows [3]:
( )L m b b b b
Tc Q Q w c T T
t xρ ρ
∂ ∂= − + + + −
∂ ∂
q (1)
where q is heat flux; ρ and c are respectively density and specific heat of the tissue ; ρb and cb are
the density and specific heat of blood, wb is the blood perfusion rate; Tb and T are the
temperatures of blood and tissue; Qm and QL are the source term due to the metabolic heating and
hyperthermia therapy. Pennes bioheat equation was obtained by applying the classical Fourier’s
law of heat conduction in Eq. (1) and assuming the uniform blood temperature Tb throughout the
tissue. The vein temperature was assumed to be same to the tissue temperature. In addition, the
blood perfusion effect was assumed to be homogeneous and isentropic.

5
1.2.2 Thermal Wave model
With nonhomogeneous biological structures, heat flux responds to the temperature gradient
via a relaxation behavior [17]. Cattaneo [4] and Vernotte [5] simultaneously suggested a
modified heat flux model:
, , , (2)
Equation (2) assumes that the heat flux and the temperature gradient occur at different times. The
delay between the heat flux and temperature gradient is defined as the thermal relaxation time; τ.
Kaminski [18] suggested that the theoretical value of the thermal relaxation time τ for biological
tissue is in the range of 20-30 s while the experimental value was observed to be 16 s [19]. If Eq.
(2) is used in replacement of the classical Fourier’s law of heat conduction in derivation of
Pennes bioheat equation, the following bio heat equation is obtained [20-21]:
1
!"#!
!" !
(3)
where, is phase lag time for heat flux, $ is the blood perfusion rate, c is heat capacity of
tissue , %& is metabolic heat generation and % is heat source due to hyperthermia therapy. The
second order derivative of temperature with respect to time appears, and for this reason Eq. (3) is
referred to as hyperbolic bioheat equation [22]. In arrival to Eq. (3), it is assumed that the
temperature gradient is established before heat flux, which is referred to as gradient–precedence
type heat flow.

6
1.2.3 Dual Phase Lag Model
Tzou [6] established a dual phase thermal lag (DPL) model that allows either the temperature
gradient to precede heat flux vector or the heat flux vector precede temperature gradient. i.e.,
′′, ', 4
where, τq is the phase lag for the heat flux vector, and τT is the phase lag for the temperature
gradient. If the local heat flux vector results in the temperature gradient at the same location but
an early time (τq > τT ) , the heat transfer is gradient-precedence type. On the other hand, if the
temperature gradient results in the heat flux at an early time (τq < τT), the heat flow is called flux-
precedence type. The first order approximation of the Eq. (4) is
′′ ′′
(5)
If the classical Fourier’s law of conduction is replaced by Eq. (5), the bioheat equation
becomes
( ) ( )2
2 2
b s21 T T
s b b s b bq q s s T s
s s
qm m
s s s s
T w c T w cT T
t C t t C
S S S S
c c t t
τ τ α τ
τ
ρ ρ
∂ ∂ ∂ + + = ∇ + ∇ + − ∂ ∂ ∂
+ ∂ ∂ + + +
∂ ∂
(6)
Under the assumption of constant blood temperature (i.e. b b q b
s
w c T
C t
τ ∂
∂=0) and the condition
0q T
τ τ= = , Eq. (6) reduces to the classical bioheat equation. The DPL bioheat equation (6) is
the modification of the Pennes bioheat equation by considering non-Fourier effect. Because of
the lacking of appropriate theoretical model on estimation of the two phase lag model, DPL is

7
still not widely accepted by the researchers in the field. Zhang [20] developed a generalized DPL
bioheat equation based on nonequilibrium between arterial blood and tissue. The phase lag times
were expressed in terms of properties of blood and tissue, interphase convection heat transfer
coefficient, and blood perfusion coefficient. In a living biological tissue, both the arterial and
venous blood flow through the vessels and disperse through the tortuous capillary beds.
Therefore, the constituencies in the living tissue include arterial blood, venous blood and
surrounding tissues. The DPL model proposed in Ref. [20] only considered nonequilibrium
between the arterial blood and tissue while the venous blood was assumed to be in equilibrium
with the surrounding tissue. In this thesis, a new DPL model based on non-equilibrium heat
transfer in arterial blood, venous blood and living tissue will be developed. The phase lag times
for heat flux and temperature gradient under different conditions will be estimated based on the
available properties in the literature.
1.3 Thesis Objectives
The main objectives of this thesis are:
1) A Generalized dual phase lag (DPL) bioheat model based on the non equilibrium heat
transfer in living biological tissues is applied to investigate thermal damage induced by
laser irradiation.
2) Comparisons of the temperature responses and thermal damages between the generalized
and classical DPL bioheat model, derived from the constitutive DPL model and Pennes
bioheat equation, are carried out.
3) The effects of laser parameters such as laser exposure time, laser irradiance, and coupling
factor on the thermal damage are also studied.

8
4) A generalized dual-phase lag model for living biological tissues based on nonequilibrium
heat transfer between tissue, artery and venous bloods is obtained.
5) The phase lag times for temperature gradient and phase lag times for heat flux vector are
calculated for different properties and also for brain and muscles.

9
CHAPTER 2
Numerical Simulation of Thermal Damage to Living Biological Tissues
Induced by Laser Irradiation based on a Generalized Dual Phase Lag Model
In this chapter, a generalized DPL model obtained by the performing volume average to
the local instantaneous energy equation for the blood and the tissue is used to investigate the
temperature response and thermal damage induced by laser irradiation. Comparisons of the
thermal responses and thermal damages between a generalized DPL, classical DPL model and
Fourier bioheat model are carried out also.
To study the evolution of temperature and thermal damage due to the laser irradiation, the
most important thing is the fundamental understanding of laser tissue interactions.
2.1 Tissue-laser interactions
Laser can interact with the tissue in four key ways: transmission, reflection, scattering
and absorption [23]. Transmission refers to the passing of laser through tissue without giving any
effect on that tissue or even the properties of the light. Reflection refers to the repelling of light
off the surface of the tissue without entering to the tissue. Approximately 4% to 7% of light is
reflected of skin. The amount of light reflection is proportional to the angle of incidence with the
least reflection occurring when the laser beam directed perpendicular to the tissue. The scattering
of light occurs after light has entered in the tissue. Scattering occurs is due to the heterogeneous
structure of tissue with the variations in particles size and the index of refraction between

10
different parts of tissue. Scattering spreads out the beam of light within the tissue which results n
radiation of area and then anticipated. Scattering depends on the depth of penetration because it
can be occur forward as well as backward. The amount of scattering is inversely proportional to
the wave length of the laser. Longer wave length laser thus penetrate tissue more deeply. And
laser light absorption by specific tissue targets is the fundamental goal of clinical lasers. The
absorption of the photons of light is reasonable for its effects on the tissue. The components of
the tissue that absorbed the photons depend on wavelength. These light absorbing tissue
components are known as chromophores. Absorption of energy by a chromophores results in
conversion of energy to thermal heat.
2.2 Laser parameters
1. Beam characteristics
An important feature of the light produced by a laser is how the intensity is distributed
across the beam diameter [23]. Most cutaneous lasers produce a beam with a Gaussian
profile in which the intensity peaks at the center of the beam and attenuates at the
periphery.
2. Spot Size
The spot size of a laser is equivalent to the laser beam cross section. The spot size
directly affects the fluence and the irradiance of a laser beam. Fluence and irradiance are
inversely proportional to the square of the radius of the spot size. A small spot size allows
more scattering both backwards and sideways than a larger spot size.

11
3. Pulse duration
Laser light can be delivered in a continuous wave or a pulse wave. Continuous wave
lasers emit a constant beam of light that may result in nonselective tissue injury. Pulsed
delivery of laser light allows for more selective tissue damage. The duration of time of
exposure of a laser beam determines the rate at which the laser energy is delivered. The
thermal relaxation time is generally proportional to the size of the target structure.
2.3 Classical DPL model
To capture the thermal lagging behaviors in biological tissues composing of
nonhomogeneous inner structures, the two lagging times will be include in the bioheat
conduction equation. Zhou et al. [24] proposed a DPL bioheat conduction model, together with a
broad beam irradiation method [25] and the rate process equation to investigate thermal damage
in laser-irradiated biological tissues. The temperature and damage parameter of the tissue was
compared with those obtained from classical Fourier and the hyperbolic bioheat conduction
model. It was found that the DPL heat conduction model predicted significantly different
temperature and thermal damage in tissue from hyperbolic and Fourier’s heat conduction model.
Combining Eqs. (1) and (5), while eliminating the heat flux q leads to the following DPL
bioheat equation for tissue temperature T (x, t):
2 2 3
2 2 2(1 ) ( )b b b m b b bL
q q T b
w c Q w cQT T T TT T
t c t x t x c c c
ρ ρτ τ α ατ
ρ ρ ρ ρ
∂ ∂ ∂ ∂+ + = + + + + −
∂ ∂ ∂ ∂ ∂ (7)
Alternatively, Zhou et al. [24] obtained the following bioheat conduction equation with
heat flux as unknown to simulate the DPL heat conduction in tissue:

12
2 2 3 2
2 2 2
Lq T b b b b b b T
Q T Tw c w c
t t x t x x x t xτ α ατ α α ρ α ρ τ
∂∂ ∂ ∂ ∂ ∂ ∂+ = + − + +
∂ ∂ ∂ ∂ ∂ ∂ ∂ ∂ ∂
q q q q (8)
where α is thermal diffusivity of tissue. The transport of thermal energy in living tissue involves
conduction in tissue, convection between blood and tissues, blood perfusion or advection and
diffusion through micro vascular beds and metabolic heat generation.
2.4 Generalized DPL model
The DPL bioheat equation obtained by simply modification of the fundamental Pennes
bioheat equation is not very convincing approach. The main foundation of dual phase lag
phenomena in the living biological tissue is nonequilibrium between the blood and the
surrounding tissue. Zhang [20] derived a generalized DPL model based on nonequilibrium heat
transfer [26] in living biological tissue. It was demonstrated that, the phase lag times depended
on intrinsic properties of blood and tissue, blood perfusion rate and convection heat transfer. The
values of phase lag times might vary from place to place in human body.
For heat transfer in living biological tissues, the temperatures of blood and tissue are
different and the equilibrium assumption is invalid. Although Pennes bioheat equation assumed
nonequilibrium assumption but the blood temperature is assumed to be a constant. In reality, the
convective heat transfer between blood and tissue and blood perfusion results the change of
temperatures. Xuan and Roetzel [12] obtained a two-temperature model by performing volume
average to the local instantaneous governing equation for blood and tissues. With the presence of
internal heat source by hyperthermia therapy, the following energy equations for blood and tissue
are

13
,[ . ] .( ) ( )bb b b b eff b b b s b L
Tc T k T a h T T Q
tερ ε
∂+ ∇ = ∇ ∇ + − +
∂V (9)
,(1 ) .( ) ( ) (1 ) (1 )L
ss s s eff s b b b s m
Tc k T a h T T Q Q
tε ρ ε ε
∂− = ∇ ∇ + − + − + −
∂ (10)
where ab is the specific heat transfer area, and hb is the heat transfer coefficient inside the blood
vessel, the temperatures of blood and tissue are volume averaged values; kb,eff and ks,eff are
effective thermal conductivity of blood and solid matrix tissue, respectively. Those energy
equations include significant effects from the blood flow and direction, thermal diffusion and
local nonequilibrium between blood and the surrounding tissues. The convective heat transfer
coefficient, hb and the specific area on the blood vessel in the tissue, ab accounts the effects of
vascular geometry and size of the blood vessel.
Comparing Eq. (10) and with Eq. (1) it can shown that the blood perfusion term is simply
replaced by the convective heat transfer. But the interfacial convective heat transfer and blood
perfusion are totally different processes [27]. In the presence of blood perfusion, due to the
temperature difference between blood and tissue, the convective heat transfer occurs. And on the
other hand, blood perfusion is the process of delivery the nutrition of the arterial blood to the
capillary bed in the biological tissue. Nakayama and Kuwahara [27] presented a developed
mathematical model based on volume averaging theory and the following governing equations
for bioheat transfer in tissue:
,[ . ] .( ) ( ) ( )bb b b b eff b b b s b b b s b L
Tc T k T a h T T w c T T Q
tερ ε
∂+ ∇ = ∇ ∇ + − + − +
∂V (11)
,(1 ) .( ) ( ) ( ) (1 ) (1 )L
ss s s eff s b b b s b b b s m
Tc k T a h T T w c T T Q Q
tε ρ ε ε
∂− = ∇ ∇ + − + − + − + −
∂ (12)

14
Considering not only interfacial convection heat transfer but also the blood perfusion, the
two step model can be written in the following forms [20]:
2[ . ] ( )bb b b b b s b L
Tc T k T G T T Q
tερ ε ε
∂+ ∇ = ∇ + − +
∂V (13)
2(1 ) (1 ) ( ) (1 ) (1 )L
ss s s s b s m
Tc k T G T T Q Q
tε ρ ε ε ε
∂− = − ∇ + − + − + −
∂ (14)
where ε is a proportional rate, subscript s is referred to tissue, and G is coupling factor between
blood and tissue and can be expressed as follows:
b b b bG a h w c= + (15)
It is evident from Eq. (15) that the coupling factor depends upon convection heat transfer
and blood perfusion rate.
Dual phase lag bioheat equation can be obtained by eliminating either tissue or blood
temperature from the two temperature model. Adding Eqs. (13) and (14) the following equation
can be obtained:
2 2(1 ) . (1 ) (1 )L
b sb b s s b b b b b s s m
T Tc c c T k T k T Q Q
t tερ ε ρ ερ ε ε ε
∂ ∂+ − + ∇ = ∇ + − ∇ + − +
∂ ∂V (16)
Minkowycz at al [28] assumed the assumption that before onset of equilibrium, the
temperature of blood undergoes a transient process. This assumption can be written by this
following equation:
( )bb b s b
Tc G T T
tερ
∂= −
∂ (17)

15
The rearrange form of this equation is
b b bs b
c TT T
G t
ερ ∂= +
∂ (18)
Substituting Eq. (18) into Eq. (16), the following equation with the blood temperature as
sole unknown is obtained:
22 2
2
(1 ). [ ( )]
( ) ( )L
mb b b bq b eff b T b
eff eff
Q QT T cT T T
t t c t c
εερτ α τ
ρ ρ
− +∂ ∂ ∂+ + ∇ = ∇ + ∇ +
∂ ∂ ∂V (19)
where the phase lags for heat flux and temperature gradient are
(1 )
( )
b b s sq
eff
c c
G c
ε ε ρ ρτ
ρ
−= (20)
(1 ) b b sT
eff
c k
Gk
ε ε ρτ
−= (21)
where
( ) (1 )eff b b s s
c c cρ ερ ε ρ= + − (22)
(1 )eff b s
k k kε ε= + − (23)
( )
eff
eff
eff
k
cα
ρ= (24)
To obtained the equation with tissue temperature as sole unknown, Eq. (19) can be
substitute on Eq. (18) and the final bioheat equation is

16
22 2
2
(1 )[ ( )] [(1 )
( ) ( ) ( )
]
s s b b m L b b mq eff s T s
eff eff eff
L
T T c Q Q c QT T
t t c t c G c t
Q
t
ερ ε ερτ α τ ε
ρ ρ ρ
∂ ∂ − + ∂∂+ + = ∇ + ∇ + + −
∂ ∂ ∂ ∂
∂+
∂
(25)
The contribution of blood flow on the temperature distribution is represented by the third
term on the left –hand side of Eq. (25) or the second term on the right –hand side of Eq. (14).
These two terms represent same physical phenomenon, one can write [29]
. ( )b b s b sc T G T Tερ ∇ ≈ −V (26)
Substituting Eq. (26) into Eq. (25), Zhang obtained the following equation with tissue
temperature as sole unknown [20]
22 2
2
(1 )[ ( )] ( )
( ) ( )
[(1 ) ]( )
s s m Lq eff s T s b s
eff eff
b b m L
eff
T T Q QGT T T T
t t t c c
c Q Q
G c t t
ετ α τ
ρ ρ
ερε
ρ
∂ ∂ − +∂+ = ∇ + ∇ + − +
∂ ∂ ∂
∂ ∂+ − +
∂ ∂
(27)
This is the generalized dual phase lag (DPL) bioheat equation based on the non
equilibrium heat transfer in living biological tissues.
The objective of this paper is to investigate temperature response and thermal damage
induced by laser irradiation using the generalized dual phase lag (DPL) bioheat model based on
the non equilibrium heat transfer in living biological tissues. A generalized DPL model in terms
of heat flux can be obtained from the two step model and the Dual phase lag model as follows:
2 2 3 2
2 2 2 (1 ) (1 )
α α ττ α α τ α
ε ε
∂∂ ∂ ∂ ∂ ∂ ∂+ = + − + +
∂ ∂ ∂ ∂ ∂ ∂ − ∂ − ∂ ∂s s TL
q s s T s
G GQq q q q T T
t t x t x x x t x (28)

Equation (28) will be used as the governing equation in this study.
2.5 Physical Model
A finite slab of a biological tissue with a thickness ‘L’ and initial temperature T
considered. A flat-top laser beam is applied normally to the left
(Fig. 1). A 1-D model will be sufficient to analyzing the thermal response of the heated medium
when the spot size of the broad beam laser is much
effected zone for the time peri
thermally insulated (q = 0) while the boundary condition at the left
light absorption and scattering of
Fig. 1
2.6 Problem Statements
For highly absorbed tissues, the laser heating is approximated as boundary condition of
second kind. The laser volumetric heat source or laser irradiance, Q
conditions are given by [24]:
(1 )φ= −in dq R for x
17
Equation (28) will be used as the governing equation in this study.
finite slab of a biological tissue with a thickness ‘L’ and initial temperature T
laser beam is applied normally to the left surface of the slab at time t
D model will be sufficient to analyzing the thermal response of the heated medium
when the spot size of the broad beam laser is much larger than the thickness of the thermally
effected zone for the time period of interest. The right boundary surface is assumed
while the boundary condition at the left surface depends on the laser
of tissues.
Fig. 1 Physical model and grid system
Problem Statements
For highly absorbed tissues, the laser heating is approximated as boundary condition of
he laser volumetric heat source or laser irradiance, QL, is zero and the boundary
or x = 0 when 0 < t < Lτ
finite slab of a biological tissue with a thickness ‘L’ and initial temperature T0 is
surface of the slab at time t = 0+
D model will be sufficient to analyzing the thermal response of the heated medium
than the thickness of the thermally
is assumed to be
surface depends on the laser
For highly absorbed tissues, the laser heating is approximated as boundary condition of
is zero and the boundary
(29)

18
0=q for x = L when 0 < t < Lτ (30)
where τL is the laser exposure time, φin is the incident laser irradiance and Rd is the diffuse
reflectance of light at the irradiated surface.
For strongly scattering tissues, laser heating is considered as a body heat source (QL ≠ 0)
but the irradiated surface is thermally insulated. The boundary conditions in this case can be
represented as:
q = 0 for x = 0 when 0 < t < Lτ
(31)
q = 0 for x = L when 0 < t < Lτ (32)
The initial conditions for both cases are:
q = 0 and 0∂
=∂
q
t for 0 < x < L, t = 0 (33)
2.7 Calculation of the laser irradiance (QL)
When the laser light irradiation is absorbed within a very small depth of tissue (~1 µm),
the laser heating can be predicated by considering the laser irradiation as a surface heat flux on
the irradiated surface (see Eq. (29)). When the scattering is considerable over the visible and near
infrared wavelength [30], heat flux boundary condition is not enough to describe the laser
deposition into a tissue. Rather, the laser light attenuation depends on the properties of laser light
and its propagation. The absorbed laser is considered as a body heat source. The laser volumetric
heat source can be determined as follows:
( ) ( )L aQ x xµ φ= (34)

19
where aµ is the absorption coefficient, and ( )xφ is the local light irradiance varying with depth
of the tissue.
To calculate light distribution in scattering tissue, a broad beam laser method [25] is
adopted and the light distribution can be determined by the following relation:
1 1 2 2( ) [ exp( / ) exp( / )]inx C k z C k zφ φ δ δ= − − − (35)
where δ is the effective penetration depth; C1, C2, k1 and k2 are determined by Monte Carlo
solutions, depending on the diffuse reflectance, Rd; the effective penetration depth δ can be
obtained from the diffusion theory as
1
3 [ (1 )]a a s
gδ
µ µ µ=
+ − (36)
where µs is the scattering coefficient and g is the scattering anisotropy. Equation (36) is valid
when 0.1≤ ( )
s
a s
µµ µ+
≤ 0.999 and 0.7 ≤ g ≤ 0.9 [25].
2.8 Calculation of thermal damage parameter (Ω)
The damage parameter is evaluated according to the Arrenius equation [1, 31]:
0
exp( )ft
t
EA dt
RTΩ = −∫ (37)
where A is the frequency factor, 3.1×1098 s-1 [1]; E is the energy of activation of denaturation
reaction, 6.28×105 J/mol [1]; R is the universal gas constant, 8.314 J/ (mol. K); T is the absolute

20
temperature of the tissue at the location where thermal damage is evaluated; t0 is the time at
onset of laser exposure; and tf is the time of thermal damage evaluation. When Ω = 1.0, the tissue
is assumed irreversibly damaged which causes the denaturation of 63% of the molecules.
2.9 Numerical Analysis
2.9.1 Discretization scheme of space
The total thickness L is divided into (L1-1) equal width control volumes (Fig. 1). The
grid points are located at the geometric center of each control volume. w and e denote the faces
of the control volume where P is located, and W and E are the adjacent grid points. ∆x is the
width of each control volume. (δx)w and (δx)e are the distance of the two adjacent grid points
measured from the point P, respectively.
2.9.2 Discretization of governing equation
The finite volume method [32] is employed to discretize the governing equation (28) and
the boundary conditions. Performing integration of Eq. (28) over the control volume of grid point
P (Fig. 1) and over the time step from t to t+∆t leads to:
2 2 3
2 2 2
2
( ) ((1 )
)(1 )
ατ α α τ α
ε
α τ
ε
+∆ +∆∂∂ ∂ ∂ ∂ ∂
+ = + − +∂ ∂ ∂ ∂ ∂ ∂ − ∂
∂+
− ∂ ∂
∫ ∫ ∫ ∫e t t e t t
sLq s s T s
w t w t
s T
GQq q q q Tdtdx
t t x t x x x
G Tdtdx
t x
(38)
Applying backward difference in time and piecewise-linear profile in space, the
following algebraic equation for heat flux can be obtained from Eq. (38):
+∆ +∆ +∆= + +t t t t t t
P P E E W Wa q a q a q b (39)

21
where
q
P w e
xa a a x
t
τ ∆= + + + ∆
∆ (40)
( ) ( )
s s TE
e
ta
x e x
α α τ
δ δ
∆= + (41)
( ) ( )
s s TW
w w
ta
x x
α α τ
δ δ
∆= + (42)
1 1 2 2
2[ ]
( ) ( ) ( ) ( )
[ [ / ] [ / ]]
[ ](1 ) 2 (1 ) 2 2
q qt t t t ts T s T s T s T
P e w P
e w e w
s a in
P
t t t t t t t t
s E W s T E W E W
x xb x q q q q
t x x x x t
x t C Exp k x C Exp k xx x
G T T G T T T Tt
τ τα τ α τ α τ α τ
δ δ δ δ
α µ φ δ δ
α α τ
ε ε
−∆
+∆ +∆
∆ ∆= + ∆ + + − − −
∆ ∆
∂ ∂− ∆ ∆ − − −
∂ ∂
− − −+ ∆ + −
− −
(43)
Following the general procedures are described in Ref. [32], the discretization equation
for the boundary grid points can also be obtained. This Discretization of the present bioheat
transfer model involves three time instants, i.e. t-∆t, t and t+∆t. The current time at which the
heat flux needs to be solved is t+∆t. After replacing the values of the temperature-involved terms
into Eq. (43) for the source term b, the discretization Eq. (39) becomes a linear system of
algebraic equations and can be solved by TDMA (Tri-diagonal matrix algorithm). Once the heat
flux at the grid point P is determined, the temperature can be computed from the discretization
form of the bioheat transfer as below
[ ( )]2 (1 )
t t t t
t t t tE w
P P a P m b P
s s
q qt GT T Q T T
c xµ ϕ
ρ ε
+∆ +∆+∆ − −∆
= + + + + −∆ −
(44)

22
Equation (43) involves the value of T at the current time t t+ ∆ , so an iterative solution
between Eqs. (39) and (44) is required in each time step until convergence of the value of T is
met.
2.10 Results and Discussion
The following properties of a living biological tissue are used for this analysis.
Thermophysical properties of tissues [33]: ρ =1000 kg/m3, k = 0.628 W/ (m K), c = 4187 J/(kg
K); thermo-physical properties of blood vessel: bρ = 1060 kg/m3, cb = 3860 J/(kg K), wb =
1.87)10-3 m3/ (m3 tissue s): optical properties [34]: sµ = 120.0 cm-1, a
µ = 0.4 cm-1, g = 0.9 ;
blood temperature: Tb = 37oC; metabolic heat generation: Qm = 1.19×103 W/m3 [33]. The
thickness of the slab of tissue is L = 5 cm, and the initial temperature is T0 = 37˚C. The diffuse
reflectance Rd = 0.05 is used for the laser light distribution of scattering tissue. Two laser
irradiances are considered, φin= 2 W/cm2 and 30 W/cm2. The laser duration time τL is 5s. After
the model convergence test, a total of 120 grid points and a time step (∆t) of 0.001s are
employed. Three different values of the coupling factor are taken based on the blood perfusion
rate. According to the blood perfusion rate wb= 1.87×10-3 m3/ (m3 tissue s), the values of ε are
0.0079, 0.025 and 0.0845 [20] and the coupling factors are 67435, 55078 and 47488 W/m3K [20,
35].
The first case studied is that the laser light is highly absorbed by the tissue. As stated
earlier, the heat flux boundary condition Eq. (29) is applied at the laser irradiated surface. Figure
2 compares the temperature responses at the irradiated surface obtained from the Fourier heat
conduction, constitutive DPL model and generalized DPL model, and Fig. 3 displays the change
of the resulting thermal damage parameters. The laser irradiance is taken as 2W/cm2 for all the

23
three cases. The lag times used in this computation are τq =16 s and τT = 0.05 s for the
generalized DPL model and also the constitutive DPL model [36-38], and τq = τT = 0 for the
Fourier heat conduction model. As shown in Fig. 2, the generalized DPL model predicts lower
temperature response compared to the classical DPL model, especially after laser pulse is off.
Fig. 2 Temperature evolution at the irradiated surface of a highly absorbing tissue
The reason is that the coupling factor between blood and the tissue in the generalized
DPL model includes not only blood perfusion but also convection heat transfer in the tissue,
whereas the constitutive DPL model allows only the blood perfusion effects. On the other hand,
the classical Fourier’s heat conduction model predicts the lowest temperature rise. This is
because the Fourier heat conduction model predicts the infinite propagation speed of heat. When
the laser light impinges onto tissue surface, heat is transferred into deeper part of the tissue
without any delay.

24
Fig. 3 Thermal damage at the irradiated surface of a highly absorbing tissue
For those case of laser light highly absorbed by the tissue, the predicted thermal damage
response at the irradiated surface shown in Fig. 3 indicates that the constitutive DPL model
results in the highest irreversible tissue damage compared to the generalized DPL model. On the
other hand, Fourier heat conduction shows the mildest thermal damage. When the two phase lags
are present, the generalized DPL model can be used for photothermal reaction for laser irradiated
biological tissue.
Figure 4 illustrates the temperature response of a scattering tissue at the irradiated
surface. In a scattering tissue, the laser irradiation is considered as a volumetric heat source that
is determined by the light propagation. The phase lags times used are the same as those in the
previous case (Figs. 2 and 3); but, the laser irradiance is increased to 30 W/cm2. The results
obtained from the generalized DPL model are significantly different from the constitutive DPL

25
model and the Fourier heat conduction model for the later time. After the laser is turned off, the
temperature dropped more significantly in generalized DPL model than others.
Fig. 4 Temperature evolution at the irradiated surface of a scattering tissue
Figure 5 illustrates the thermal damage transient in the scattering tissue. It is shown from
Fig. 5 that the generalized DPL model predicts mildest thermal damage compared to classical
DPL model and Fourier heat conduction.

26
Fig. 5 Thermal damage at the irradiated surface of a scattering tissue
Figure 6 shows the temperature response for different phase lag times (τT) for
temperature gradient while the phase lag time for heat flux (τq) is kept constant, 16 s. The
incident irradiance of laser beam is set as before, 30W/cm2. It can be seen from Fig. 6 that the
temperature variations during the laser irradiation time period (5s) are almost same for different
τT values, although the temperature becomes diverse after the laser is turned off.

27
Fig. 6 Temperature distribution at the irradiated surface of a scattering tissue for different
τT values
As expected, this predicts a different damage progress and in turn, results in different
final damage extents in the biological tissues (Fig. 7). The longer the phase lag τT, the larger the
saturated value of damage parameter. The saturated damage parameter predicted with τT = 32s is
about 2.6 times that predicted by the Fourier’s law of heat conduction.

28
Fig. 7 Thermal damage at the irradiated surface of a scattering tissue for different τT values
To further investigate the condition under which the DPL results approach the prediction
by Fourier’s law, the simulations are performed for constant τT (0.05 s) and different values of τq
(32 to 0.05s) and the results are shown in Figs. 8 and 9. It tends to induce more thermal effects as
τq increased. Figures 8 and 9 illustrate the effect of τq on the evolution of irradiated surface
temperature and thermal damage. It can be observed that the longer the delay time τq, the higher
the temperature rise and the larger the saturated values of damage parameter. As τq decrease to
0.05s, the curve almost overlaps with that obtained from the Fourier’s law.

29
Fig. 8 Temperature distribution at the irradiated surface of a scattering tissue calculated by the
nonequilibrium DPL model for different τq values

30
Fig. 9 Thermal damage at the irradiated surface of a scattering tissue calculated by the
nonequilibrium DPL model for different τq values
Comparing the temperature responses Figs. 6 and 8 shows that for laser irradiated
biological tissues τq has more impact on the temperature in the early time while τT has more
impact on the temperature in the later time. The generalized DPL model will be close to classical
Fourier’s heat conduction when the phase lags are very small. Otherwise, the heat conduction
described by the generalized DPL bioheat transfer model would differ from the classical
Fourier’s heat conduction even if τT = τq = 0.
The next investigation of this study is to illustrate the effects of laser parameters and the
coupling factor on the thermal damage in the tissue using the generalized DPL bioheat model.

31
Figures 10 and 11 show the effects of laser irradiance on temperature and damage
parameter. As expected, the higher the laser irradiance, the higher the temperature and the
earlier, steeper and greater the damage parameter. The higher denaturation process resulting from
the higher irradiance prolongs due to the fact that it takes longer time to cool down the tissue.
For the laser exposure time 5 s, the minimum irradiance that causes the irreversible thermal
damage is found to be in between 20-25 W/cm2.
Fig. 10 Effects of laser irradiance on temperature at the irradiated surface of a scattering
tissue

32
Fig. 11 Effects of laser irradiance on damage parameter at the irradiated surface of a scattering
tissue
Figures 12 and 13 present the effects of the laser exposure time on the temperature and
the resulting thermal damage. It is shows that the effects of the laser exposure time are smaller to
those of the laser irradiance. The longer the laser exposure time is, the higher the temperature
rises and more the thermal damage is induced in the irradiated surface of the tissue. From Fig.
13, it can be observed that the tissue would be more irreversibly damaged (Ω≥1.0) when the
laser exposure time is more than 4 s.

33
Fig. 12 Effects of laser exposure time on temperature at the irradiated surface of a scattering
tissue

34
Fig. 13 Effects of laser exposure time on damage parameter at the irradiated surface of a
scattering tissue
The effects of the coupling factor on temperature and thermal damage is shown in
Figs.14 and 15. The coupling factor indicates the energy exchange between the blood and the
tissues. Both the blood perfusion and the convective heat transfer have effects on the coupling
factor. In this study, the blood perfusion rate is assumed to be constant 1.87 ×10-3 m3/ (m3s
tissue). Thus, the coupling factor change only depends upon the change of the blood vessel [20]
diameter. As can be seen from Figs. 14 and 15, the higher the coupling factors, the more the
temperature decrease. The thermal damage is increased with the decrease of blood tissue
coupling factor.

35
Fig. 14 Effects of coupling factor on temperature at the irradiated surface of a scattering tissue

36
Fig. 15 Effects of coupling factor on damage parameter at the irradiated surface of a scattering
tissue
Blood perfusion rate depends on the location of the tissue. Convection cooling effect of
the blood flow plays a significant role in an optimized treatment procedure in laser-induced
thermotherapy. Figure 16 shows the effects of blood perfusion rate on the temperature. The
higher the blood perfusion rate the stronger the convection heat loss due to the faster blood flow.
It is shown in Fig. 17 that as the blood perfusion increase, the less extent of thermal damage is
caused with the consequence of decrease temperature.

37
Fig. 16 Effects of blood perfusion rate on temperature at the irradiated surface of a scattering
tissue

38
Fig. 17 Effects of blood perfusion rate on thermal damage at the irradiated surface of a
scattering tissue
2.11 Conclusion
The treatment efficiency as well as safety is a primary concern in the laser medical
applications. Therefore, the most important issue is to understand and accurately assess the laser
induced thermal damage in the biological tissue. DPL model obtained by performing volume
average to the local instantaneous energy equations for the blood and the tissue is used to
investigate the thermal response of the laser irradiated biological tissues in this thesis. The broad
beam laser irradiation method based on Monte Carlo simulation is used to determine the laser

39
light propagation in the biological tissue. The generalized DPL bioheat model based on
nonequilibrium heat transfer and the classical DPL model are compared with Fourier’s heat
conduction model. It is shown that the generalized DPL model predicts significantly different
temperature and thermal damage in the irradiated surface of tissue. It is also found that for the
laser irradiated biological tissues the phase lag time of heat flux (τq) has more impact on the
temperature in the early time while the phase lag time of temperature gradient (τT) has more
impact on the temperature in the later time. The generalized DPL model reduces to the Fourier’s
heat conduction model only when τq = τT = 0. The influences of laser exposure time and
irradiance, blood perfusion, and the coupling factor on temperature and thermal damage are also
studied. The result shows that the overall effects of the laser parameters on the temperature and
damage parameter are similar to those of the time delay τT.

40
CHAPTER 3
Thermal Lagging in Living Biological Tissue based on Nonequilibrium Heat
Transfer between Tissue, Arterial and Venous Blood
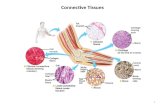
3.1 Heat Transfer in Arteries, Venous and Solid Tissue
The heat transfer in the whole biological tissue involves heat conduction in the tissue,
convection heat transfer between tissue and blood in artery and vein, as well as blood perfusion.
The tissue is treated as a solid matrix part of the saturated porous medium, and the blood
permeate in the pore space of the porous medium [39, 27] (see Fig. 18).
Fig. 18 Schematic view of artery and vein surrounding by tissue.

41
3.2 Governing Equations
In the human body, blood flows in artery and vein through the compound matrix of tissue.
The blood flow rate and direction are totally different in artery and vein because the vein is much
narrower than the artery. Therefore, thermal equilibrium between different carriers do not exist.
In addition, Pennes bioheat equation assumes the constant blood temperature but in anatomical
human structure, convective heat transfer between the blood and the tissue causes a constant
change of the blood temperature. The governing equations for three carriers in a human body
[26] are:
*+* ,-*- .* . *0 *** 1* * *% 45
3+3 ,-3- .3 . 30 333 13 3 3% 46
1 + -- 1 1** 133 1 %& 1 % 47
where the blood (arterial and venous) and tissue temperature are volume averaged values; the
second term of the right hand side of the equation (45) and (46) and second and third term of the
eq. (47) represents the contribution of blood perfusion on the energy balance in blood and tissue
and G is referred to as a coupling factor between the blood (arterial and venous) and the tissue;
ka *, kv 3 and ks(1-ε) are the effective thermal conductivities of arterial blood, venous blood
and tissue, respectively. Those three equations include significant effects of the blood flow and
direction, thermal diffusivity and local thermal nonequilibrium between the blood and the

42
surrounding tissue. The two coupling factors are a measure of combined convection and
perfusion [20]. The porosity of the porous media is equal to summation of volume fractions of
arterial and venous blood, i.e.,
a vε ε ε= + (48)
If the sufficient information about the thermal and anatomical properties are available and
also if the blood velocities and directions are known, Eqs. (45) - (47) can be used to determine
average temperature distributions. The second term on the left hand side of Eqs (45) and (46)
expresses the counter current effect between the arterial and venous blood flows. In Eq. (47), the
effect of metabolites heat generation is also taken into account. The main way to control and
regulate the temperature of the human body is via heat exchange as well as the metabolites heat
generation between the blood and the solid matrix.
3.3 Dual Phase Bioheat Equation for Three Carriers System
The dual phase lag bioheat equation can be obtained by eliminate either blood (arterial or
venous) or tissue temperature from the multi-temperature model equation. In this thesis, operator
method is used to obtain one equation with tissue temperature as sole unknown. The total energy
equation can be established by adding individual energy equations for artery, vein and tissue.
Adding Eqs. (45)- (47), the following energy equation can be obtained:

43
*+* -*- 3+3 -3- 1 + -- *+*6*. * 3+363. 3 *** 333 1 *% 3% 1 %& 1 % 49
Under a rapid heating condition, the tissue and blood (both artery and venous) are not at
the same temperature at a local level. With the assumption taken by Minkowycz et al. [28] that it
is hypothesized that before the onset of equilibrium, the blood temperatures for both artery and
vein undergo transient processes can be obtained by:
*+* -*- 1* * 50
3+3 -3- 13 3 51
Substituting Eqs. (50) and (51) into Eq. (49), the bioheat equation with tissue temperature as
a sole unknown can be expressed as:
9 --
-- :;* +*+<== .* ;3+3+<== .3 > 1 *+3+*+3 -?
-?
<== @ -- A 3 +313*+* .3 *+*1*3+3.*1*13+<==--
*+*3+3 -%
- 1 *+*3+3 -%& - 1 *3+*+31*13B<==
--
C% 1 %&D+<== 13*+* 1*3+3 -%- 13*+* 1*3+31 -%& - 52
where the phase lags for heat flux and temperature gradient are given by:

44
9 *+*1*3+3 3+313*+* 1 +13*+* 1 +31*+31*13+<== 53
*G*1*3+3 3G313*+* 1 G13*+* 1 3 1*+3G1*13G<== 54
with the effective properties being defined as,
+<== *+* 3+3 1 + 55 <== ** 33 1 56
<== <==+<== 57 Equation (52) represents the DPL bioheat equation with average tissue temperature as
a sole unknown and the phase lags times for heat flux and temperature are functions of the
properties of artery, vein and solid tissue and the coupling factors between the three carriers. In
Eq. (52), the third order time derivative term indicates the effect of three carriers (artery, vein
and tissue) system. Although Eq. (52) is more complex than Eq. (6), it is more accurate because
it is based on nonequilibrium between different energy carriers. In addition, Eq. (52) has only
average tissue temperature as a sole unknown and no arterial and venous temperatures are
involved. It accounted for the conduction and convection (blood perfusion) effects in the arterial
and venous blood. The directions of the blood flow in arteries and veins can be accurately
accounted by the convection terms on the left hand side of the equation. Equation (52) is distinct
from Eq. (6) by another fact that the phase lag times can be easily obtained from Eqs. (53) and
(54).

45
While Eq. (52) is in the form that can be directly used to obtain the tissue temperature if the
arterial and venous blood velocities are known, it would be helpful if it can be casted in the form
that is similar to the Pennes bioheat equation. The contribution of blood flow on the temperature
distribution is represented by the third term on the left-hand side of Eq. (52) or the second and
third terms on the right-hand side of Eq. (47). Since both of these two terms represents the same
physical phenomenon, one can expect that [29]:
;* +*6* ;3+363 H 1** 133 58
which converts the effect of blood flow on the tissue temperature to coupling between blood
temperature to the tissue temperature. Obviously, the information about the effect of directions
of blood flow on the tissue temperature has been, in theory, included in the coupling factor Ga
and Gv. This approximation is only valid when the blood temperature is not equal to the tissue
temperature and the direction of the blood flow is taken into account in consideration in bioheat
equation.
Substituting Eq. (58) into Eq. (52), the following DPL bioheat equation is obtained
9 --
--
1 *+3+*+31*13+<==-?-? <== @ -- A
3 +313*+* .3 *+*1*3+3.*1*13+<==--
1 *3+*+31*13B<==--
C1* * 133 D +<== C% 1 %&D+<== *+*3+3 -%
-
1 *+*3+3 -%& - 13*+* 1*3+3 -%-
13*+* 1*3+31 -%& - 59

46
The difference between the present DPL bioheat equation (59) and the classical DPL
bioheat equation (6) is that the latter considers heat conduction in tissue only but Eq. (59)
considers the contributions to conduction by both tissue and blood.
3.4 Results and Discussion
Equation (52) or (59) conveys one of the most important advantages of the present DPL
bioheat equation over the classical DPL bioheat equation (6). The phase lag times for heat flux
and temperature gradient can be obtained as functions of known quantities such as the properties
of blood and tissue, interphase convection heat transfer coefficient and blood perfusion rate. The
present DPL model also reveals that the root of dual phase lag is the nonequilibrium thermal
transport between blood and tissue. It is evidence from Eqs. (53) and (54) that the phase lag
times are governed by the coupling factor (G), porosity of the medium, and heat capacities of
blood and tissues. The coupling factor (G) describes the energy exchange between the (arterial
and venous) bloods and their surrounding tissues. It is an important property for analyzing the
biological system. The coupling factor depends upon convection heat transfer and blood
perfusion rate:
1* J*K* $*B* (60)
13 J3K3 $3B3 (61)
For the bundle of vascular artery and veins with diameters da and dv, the respective
coupling factors are [20]:

47
1* 4*G*L*
MN $*B* 62
13 43G3L3
MN $3B3 63 where the Nusselt number is approximately Nu = 4.93 [40, 41].
With the assumptions of uniform distribution of blood vessel in the tissue [42], different
diameters of blood vessels, porosity ( 2( / )ε =a a sd d ), and blood perfusion rate are investigated
and listed in Table 1. The thermo physical properties of blood and tissue are assumed to be
identical: * 3 0.5 W/m K, O* O3 O 1050 kg/m3 and B* B3 B 3770
J/kg K.
Table 1: Structure and perfusion coefficient studied in Ref [41]
Case ds
(mm)
da
(mm)
dv
(mm) * 3
wa
(kg/m3
sec)
wv
(kg/m3
sec)
1 17.83 1.14 1.254 0.004 0.005 1 -1.43
2 12.85 1.14 1.254 0.008 0.01 2 -2.86
3 10.75 1.14 1.254 0.01 0.014 3 -4.29
4 9.7 1.14 1.254 0.014 0.017 4 -5.71
5 8.65 1.14 1.254 0.017 0.021 5 -7.14
6 19.82 2.28 2.508 0.013 0.02 1 -1.43
7 14.42 2.28 2.508 0.025 0.03 2 -2..86
8 12.06 2.28 2.508 0.036 0.043 3 -4.29
9 10.48 2.28 2.508 0.05 0.06 4 -5.71
10 9.92 2.28 2.508 0.053 0.064 5 -7.14

48
11 20.98 4.56 5.016 0.05 0.057 1 -1.43
12 15.73 4.56 5.016 0.164 0.102 2 -2.86
13 13.58 4.56 5.016 0.11 0.14 3 -4.29
14 12.06 4.56 5.016 0.143 0.173 4 -5.71
15 11.27 4.56 5.016 0.164 0.2 5 -7.14
The perfusion rate is defined as, the mean pressure difference between artery and vein
divided by the vascular resistance [35], i.e.,
$ P* P3Q 64
According to Poiseuille-Hagen formula [35], the relation between the volumetric flow
rate in a long narrow tube, the viscosity of the fluid and the radius of the tube is expressed
mathematically as follows:
R P* P3 S81TUVW 65
where, F, T, U and W are the volumetric flow rate, viscosity, radius and length of the tube. Since
the volumetric flow rate is pressure difference divided by resistance, the vascular resistance is
expressed as
Q 8TWSUV 66
From the above three equations (64) - (66), it can be seen that the arterial and venous
blood perfusion rate are only functions of diameters. The approximate values of lumen diameter
and the wall thickness for artery are 4 mm and 1mm, respectively. The approximate values of
lumen diameter and wall thickness of vein are 5 mm and 0.5 mm, respectively [35]. Using these

49
approximate values, a relationship established between the diameter for artery and vein is that
the diameter of vein is 1.1 times the diameter of artery. And the relation between the two blood
perfusion rate is 1.43v aw w= − . Those two relations are used to calculate different properties of
venous blood such as porosity, diameter and blood perfusion rate that are summarized in Table 1.
The contributions of convection heat transfer and blood perfusion rate along with the
above properties in the coupling factor, the phase lag times are listed in Table 2. For all cases in
Table 2, the phase lag times for heat flux and temperature gradient are exactly the same because
=sa sak C and =sv svk C where, /=sa s ak k k and /=sa s aC C C . Comparison between the phase lag
times shows that the DPL phenomena is more pronounced when the blood vessel diameter is
large because the phase lag times significantly increase when the diameter of the blood vessel
increases.
Table 2: Coupling factors and phase lag times (* 3 0.5 W/m K, O* O3 O=
1050 Kg/m3 and B* B3 B 3770 J/Kg K)
Case
aaha
(W/m3
K)
avhv
(W/m3
K)
cawa
(W/m3
K)
cvwv
(W/m3
K)
Ga
(W/m3 K)
Gv
(W/m3 K)
9
(sec)
(sec)
1 30,348 31,351 3,770 -5,391 34,118 25,960 1.221 1.221
2 60,696 62,702 7,540 -10,782 68,236 51,920 1.216 1.216
3 83,456 87,783 11,310 -16,173 94766 71,610 1.218 1.218
4 106,217 106,594 15,080 -21,527 121,297 85,065 1.229 1.229
5 128,978 131,674 18,850 -26,918 147,828 104,757 1.223 1.223
6 24,657 31,351 3,770 -5,391 28,428 25,960 4.793 4.793
7 47,418 47,027 7,540 -10,782 54,959 36,244 4.932 4.932
8 68,283 67,405 11,310 -16,173 79,593 51,231 4.906 4.906

50
9 94,837 94,053 15,080 -21,527 109,917 72,526 4.815 4.815
10 100,527 100,323 18,850 -26,918 119,377 73,406 4.896 4.896
11 23,709 22,338 3,770 -5,391 27,479 16,947 19.449 19.449
12 39,832 39,973 7,540 -10,782 47,372 29,190 18.856 18.856
13 52,160 54,864 11,310 -16,173 63,470 38,691 18.439 18.439
14 67,808 67,797 15,080 -21,527 82,889 46,270 18.094 18.094
15 77,766 78,378 18,850 -26,918 96,616 51,460 17.964 17.964
The thermo-physical properties of tissue depend on the type and location of the tissue in
the body. Therefore, the assumption of the same thermo-physical properties for blood and tissue
is not always valid. The DPL phenomena are then investigated for the case that the properties of
tissues and blood differ: ρs = 1000 kg/m3, ks = 0.628 W/m K, cs = 4187 J/ kg K, ρa= ρv =1060
kg/m3 and ca = cv = 3860 J/kg K. The thermal conductivity of blood used by Yuan [42] was kb=
0.5W/m K and this value agrees with other sources [20]. The thermal conductivities of artery and
vein are taken as the same value, i.e., ka= kv = 0.5 W/m K. Table 3 shows the contributions of
convection heat transfer and blood perfusion, in the coupling factors and phase lag times.
Table 3: Phase lag times for different thermo physical properties of tissue (ρs=1000 kg/ m3,
ka = 0.5 W/m K, ks= 0.628 W/m K, cs= 4187 J/ kg K, ρa= ρv= 1060 kg/m3, ca= cv = 3860 J/ kg K)
Case
aaha
(W/m3
K)
avhv
(W/m3
K)
cawa
(W/m3
K)
cvwv
(W/m3
K)
Ga
(W/m3
K)
Gv
(W/m3
K)
9
(sec)
(sec)
1 30,348 31,351 3,860 -5,520 34,208 25,831 1.265 1.267
2 60,696 62,702 7,720 -11,040 68,416 51,662 1.259 1.261
3 75,870 87,783 11,580 -16,559 95,036 71,223 1.262 1.265
4 106,217 106,594 15,440 -22,041 121,257 84,553 1.273 1.277

51
5 128,978 131,674 19,300 -27,560 148,278 104,114 1.269 1.274
6 24,657 31,351 3,860 -5,520 28,518 25,831 4.948 4.963
7 47,418 47,027 7,720 -11,040 55,138 35,987 5.121 5.146
8 68,283 67,405 11,580 -16,559 79,863 50,845 5.094 5.131
9 94,837 94,053 15,440 -22,041 110,277 72,013 4.975 5.023
10 100,527 100,323 19,300 -27,560 119,827 72,763 5.089 5.143
11 23,709 22,338 3,860 -5,520 27,569 16,818 20.153 20.345
12 39,832 39,973 7,720 -11,040 39,831 28,933 20.899 21.236
13 52,160 54,864 11,580 -16,559 63,740 38,305 19.197 19.609
14 67,808 67,797 15,440 -22,041 83,248 45,756 18.875 19.366
15 77,766 78,378 19,300 -27,560 97,066 50,817 18.735 19.280
The phase lag times for heat flux and temperature gradient shown in Table 3 are not
exactly the same, but the differences are small from 0.07% to 3.0%. With the same properties,
phase lag time for heat flux is less than the phase lag time for temperature gradient. When the
effect of different thermo physical properties are considered, the phase lag times for the
temperature gradient are increased by 3% to 12% compared to the values in Table 2 with the
same porosity and blood perfusion rate. The difference between the results in Table 2 and 3 is
more significant compared to the similar study by Zhang [20] because in this case the venous
blood is accounted as an additional carrier. When the diameter of arterial blood vessel increases
from 1.14 mm to 4.56 mm, the highest difference from its previous value for τq is over 11%
(Case 12). Although the thermal conductivity, specific heats of arterial and venous blood are
different from those of the tissue in Table 3, the phase lag times for heat flux and temperature
gradient are approximately close to each other although the maximum difference is 7%. Since
the fluctuating behavior of phase lag times with the same diameter are shown in this case, Table
4 is generated to show the continuous decreasing phase lag times. It can be seen from Table 4

52
that with the same blood perfusion rate, the phase lag times are identical to each other and
gradually decreasing when the arterial diameter is kept the same (da = 1.14 mm) and tissue
diameter varies.
Table 4: Phase lag times for same blood perfusion rate (ρs= 1000 kg/ m3, ks= 0.628 W/m K,
cs=4187 J/ kg K, ρa= ρv = 1060 kg/ m3, ca= cv = 3860 J/ kg K)
wa wv εa εv aaha avhv Ga Gv τq τT
1 -1.42857 0.0041 0.005 31,015 31,015 34,785 25,629 1.224 1.224
1 -1.42857 0.008 0.010 59,713 59,713 63,483 54,328 1.174 1.174
1 -1.42857 0.011 0.014 85,322 85,322 89,092 79,936 1.159 1.159
1 -1.42857 0.014 0.017 104,793 104,793 108,563 99,408 1.151 1.151
1 -1.42857 0.017 0.021 131,779 131,779 135,549 126,393 1.143 1.143
2 -2.8571 0.004 0.005 31,015 31,015 38,555 20,244 1.380 1.380
2 -2.8571 0.008 0.010 59,713 59,713 67,253 48,942 1.223 1.223
2 -2.8571 0.011 0.014 85,322 85,322 92,862 74,550 1.187 1.187
2 -2.8571 0.014 0.017 104,793 104,793 112,333 94,022 1.172 1.172
2 -2.8571 0.017 0.021 131,779 131,779 139,319 121,007 1.158 1.158
3 -4.2857 0.004 0.005 31,015 31,015 42,325 14,858 1.692 1.692
3 -4.2857 0.008 0.010 59,713 59,713 71,023 43,556 1.292 1.292
3 -4.2857 0.011 0.014 85,322 85,322 96,632 69,165 1.224 1.224
3 -4.2857 0.014 0.017 104,793 104,793 116,103 88,636 1.198 1.198
3 -4.2857 0.017 0.021 131,779 131,779 143,089 115,621 1.177 1.177
4 -5.7143 0.004 0.005 31,015 31,015 46,095 9,472 2.407 2.407
4 -5.7143 0.008 0.010 59,713 59,713 74,793 38,170 1.391 1.391
4 -5.7143 0.011 0.014 85,322 85,322 100,402 63,779 1.271 1.271
4 -5.7143 0.014 0.017 104,793 104,793 119,873 83,250 1.231 1.231
4 -5.7143 0.017 0.021 131,779 131,779 146,859 110,236 1.199 1.199
5 -7.1429 0.004 0.005 31,015 31,015 49,865 4,087 5.091 5.091

53
5 -7.1429 0.008 0.010 59,713 59,713 78,563 32,785 1.532 1.532
5 -7.1429 0.011 0.014 85,322 85,322 104,172 58,393 1.332 1.332
5 -7.1429 0.014 0.017 104,793 104,793 123,643 77,865 1.272 1.272
5 -7.1429 0.017 0.021 131,779 131,779 150,629 104,850 1.225 1.225
With the same diameter of tissue (ds = 17.83 mm), the effect of blood perfusion rate on the
phase lag times are shown in Table 5. It is seen that the phase lag times for heat flux and
temperature gradient are identical for all cases and gradually increase from its previous value
with the increasing blood perfusion rate.
Table 5: Phase lag times for the same diameter of tissue (ρs=1000 kg/ m3, ks=0.628 W/m K,
cs=4187 J/ kg K, ρa= ρv= 1060 kg/m3, ca= cv=3860 J/ kg K)
wa wv εa εv aaha avhv cawa cvwv Ga Gv τq τT
1 -1.429 0.0041 0.005 31,015 31,015 3,770 -5,386 34,785 25,630 1.224 1.224
2 -2.857 0.0041 0.005 31,015 31,015 7,540 -10,771 38,555 20,244 1.381 1.381
3 -4.286 0.0041 0.005 31,015 31,015 11,310 -16,157 42,325 14,858 1.692 1.692
4 -5.714 0.0041 0.005 31,015 31,015 15,080 -21,543 46,095 9,472 2.407 2.407
5 -7.143 0.0041 0.005 31,015 31,015 18,850 -26,929 49,865 4,087 5.091 5.091
1 -1.429 0.0041 0.005 31,015 31,015 3,770 -5,386 34,785 25,630 1.224 1.224
2 -2.857 0.0041 0.005 31,015 31,015 7,540 -10,771 38,555 20,244 1.380 1.380
3 -4.286 0.0041 0.005 31,015 31,015 11,310 -16,157 42,325 14,858 1.692 1.692
4 -5.714 0.0041 0.005 31,015 31,015 15,080 -21,543 46,095 9,472 2.407 2.407
5 -7.143 0.0041 0.005 31,015 31,015 18,850 -26,929 49,865 4,047 5.091 5.091
1 -1.429 0.0041 0.005 31,015 31,015 3,770 --5,386 34,785 25,630 1.224 1.224
2 -2.857 0.0041 0.005 31,015 31,015 7,540 -10,771 38,555 20,243 1.380 1.380
3 -4.286 0.0041 0.005 31,015 31,015 11,310 -16,157 42,325 14,858 1.692 1.692
4 -5.714 0.0041 0.005 31,015 31,015 15,080 -21,543 46,095 9,472 2.407 2.407
5 -7.143 0.0041 0.005 31,015 31,015 18,850 -26,929 49,865 4,086 5.091 5.091

54
Fourier’s law can be considered as a special case of the dual phase heat conduction model
only when the phase lag times for temperature gradient and heat flux are zero. Under the
assumption of Fourier’s law, there is no time lag between the heat flux and temperature gradient.
In this thesis, DPL bioheat equation with a sole unknown average tissue temperature is obtained
for the three carrier systems. By solving the continuity and momentum equations in porous
medium, the intrinsic averaged velocity vector for artery and vein in the solid matrix can be
obtained, which is needed to solve the Eq. (52) or (59). The intrinsic averaged velocity is much
higher in artery than vein, i.e., Va > Vv. The only way that the DPL bioheat equation can be
simplified to the Pennes’ bioheat equation is only if the there are no lagging in the medium (i.e.,
τq= τT = 0). This is different from the case when no internal or external source is present in the
energy equation in which the DPL model is reduced to the classical parabolic energy equation
when the phase lag times for heat flux and temperature gradient are equal to each other, even if
they are not zero.
The same procedure is done to investigate the convection heat transfer, blood perfusion,
coupling factors and phase lag times for brain and muscle and the results are listed in Table 6 and
Table 7. In the case of brain, the phase lag time for heat flux is always less than the phase lag
time for temperature gradient as previous. The maximum difference between the phase lag time
for heat flux and phase lag time for temperature gradient is 3.011 sec (Case 12).

55
Table 6: Phase lag time for brain: (ρs=1030 kg/m3, ks=0.5395 W/K m, $s=9.33 kg/m3 sec,
cs=3680 J/kg K, ρa=ρv=1060 kg/m3,ka=kv=0.5 W/m K and ca=cv=3860J/kg K)
Case aaha
(W/m3 K)
avhv
(W/m3 K)
cawa
(W/m3 K)
cvwv
(W/m3 K)
Ga
(W/m3 K)
Gv
(W/m3 K)
9
(sec)
(sec)
1 30,348 31,351 3,860 -5,520 34,208 25,831 1.290 1.472
2 60,696 62,702 7,720 -11,040 68,416 51,662 1.284 1.464
3 75,870 87,783 11,580 -16,559 95,036 71,223 1.286 1.467
4 106,217 106,594 15,440 -22,041 121,257 84,553 1.297 1.480
5 128,978 131,674 19,300 -27,560 148,278 104,114 1.293 1.476
6 24,657 31,351 3,860 -5,520 28,518 25,831 5.040 5.756
7 47,418 47,027 7,720 -11,040 55,138 35,987 5.209 5.752
8 68,283 67,405 11,580 -16,559 79,863 50,845 5.174 5.947
9 94,837 94,053 15,440 -22,041 110,277 72,013 5.044 5.763
10 100,527 100,323 19,300 -27,560 119,827 72,763 5.158 5.894
11 23,709 22,338 3,860 -5,520 27,569 16,818 20.438 23.35
12 39,832 39,973 7,720 -11,040 39,831 28,933 21.100 24.111
13 52,160 54,864 11,580 -16,559 63,740 38,305 19.315 22.068
14 67,808 67,797 15,440 -22,041 83,248 45,756 18.933 21.590
15 77,766 78,378 19,300 -27,560 97,066 50,817 18.752 21.349
In the case of muscle (Table 7), the same pattern of phase lag times has been observed as
the brain. In this case, the maximum difference of two phase lag times is 5.011 sec.

56
Table 7: Phase lag time for muscle: (ρs=1040 kg/m3, ks= 0.4935 W/K m, $s= 0.63 kg/m3 sec, cs=
3720 J/kg K, ρa= ρv= 1060 kg/m3, ka= kv = 0.5 W/m K and ca= cv= 3860J/kg K)
Case aaha
(W/m3 K)
avhv
(W/m3 K)
cawa
(W/m3 K)
cvwv
(W/m3 K)
Ga
(W/m3 K)
Gv
(W/m3 K)
9
(sec)
(sec)
1 30,348 31,351 3,860 -5,520 34,208 25,831 1.264 1.627
2 60,696 62,702 7,720 -11,040 68,416 51,662 1.258 1.586
3 75,870 87,783 11,580 -16,559 95,036 71,223 1.260 1.588
4 106,217 106,594 15,440 -22,041 121,257 84,553 1.272 1.602
5 128,978 131,674 19,300 -27,560 148,278 104,114 1.268 1.596
6 24,657 31,351 3,860 -5,520 28,518 25,831 4.941 6.222
7 47,418 47,027 7,720 -11,040 55,138 35,987 5.109 6.422
8 68,283 67,405 11,580 -16,559 79,863 50,845 5.078 6.370
9 94,837 94,053 15,440 -22,041 110,277 72,013 4.953 6.196
10 100,527 100,323 19,300 -27,560 119,827 72,763 5.066 6.333
11 23,709 22,338 3,860 -5,520 27,569 16,818 20.070 25.110
12 39,832 39,973 7,720 -11,040 39,831 28,933 20.756 25.767
13 52,160 54,864 11,580 -16,559 63,740 38,305 19.025 23.464
14 67,808 67,797 15,440 -22,041 83,248 45,756 18.675 22.835
15 77,766 78,378 19,300 -27,560 97,066 50,817 18.515 22.495
The values of the phase lag times for brain (Table 6) and muscles (Table 7) are much
higher than the values with same thermo physical properties (Table 2) as well as different thermo
physical properties (Table 3). Also for same diameter, the difference of the values of phase lag
times is much higher for brain and muscles than the different properties of tissue and blood. The
values of phase lag times for heat flux and temperature gradient with the effect of vein (shown in
Table 3 and 4) are more than those without the effect of vein in the biological tissue [20].
3.5 Conclusions
For the three-carrier system (artery, venous and tissue) in a living biological system, a
dual phase lag bioheat equation with tissue temperature as the sole unknown can be obtained by

57
analyzing non-equilibrium heat transfer and by eliminating the temperature of arterial and
venous blood. The phase lag times for heat flux and temperature gradient are expressed as
functions of thermo physical properties of blood and surrounding tissue, interphase convection
heat transfer, and the blood perfusion rate in biological system. The novelty of this paper is that
DPL model is derived from nonequilibrium between arterial blood, venous blood, which was not
accounted in classical Pennes bioheat equation, is taken into account. If the densities, specific
heats and thermal conductivities of arterial and venous blood are similar to the tissue, the phase
lag times for heat flux and temperature gradient are identical. When the densities are different
and the specific heat and thermal conductivities of blood and tissues are considered, the two
phase lag times are still similar. The phase lag times studied in this thesis range from 1 to 21 sec.
The non-Fourier thermal behavior (DPL effect) allows us to study the microstructure interactions
with heat transport. Due to the presence of blood perfusion in living tissue, the DPL bioheat
equation can reduce to Pennes bioheat equation only when both phase lag times are equal to
zero.

58
CHAPTER 4 SUMMERY AND CONCLUSIONS
To ensure the treatment efficiency and personal safety in laser application in medical
sector, one of the most important issues is to understand and accurately access laser-induced
thermal damage to the living biological tissue. In this thesis, in chapter 2, a generalized DPL
model for blood and surrounding tissues is applied to investigate the temperature response and
thermal damage of laser irradiated biological tissue. The laser light propagation in tissues is
determined by Monte Carlo simulation. The thermally induced damage in tissue is evaluated by
using the rate process equation for protein denaturation process. For comparison, the results are
also computed with the classical DPL model and Fourier heat conduction model. It is shown that
the present approach predicts significantly different temperature and thermal damage in tissue
than the classical DPL model and Fourier heat conduction model. It is also found that for the
laser irradiated biological tissues the phase lag time of heat flux (τq) has more impact on the
temperature in the early time while the phase lag time of temperature gradient (τT) has more
impact on the temperature in the later time. The generalized DPL model reduces to the Fourier’s
heat conduction model only when τq = τT = 0. The influences of laser exposure time and
irradiance, blood perfusion, and the coupling factor on temperature and thermal damage are also
studied. The result shows that the overall effects of the laser parameters on the temperature and
damage parameter are similar to those of the time delay τT.
For the three-carrier system (artery, venous and tissue) in a living biological system, a
generalized dual phase lag bioheat equation with tissue temperature as the sole unknown can be
obtained by analyzing non-equilibrium heat transfer and by eliminating the temperature of

59
arterial and venous blood. The phase lags times are expressed as functions of the thermo-
physical properties of arterial and venous blood and surrounding tissues, interphase convective
heat transfer, and the blood perfusion rate in the biological system. In addition, the convection of
blood is taken into account to derive this model where it was not accounted in classical Pennes
bioheat equation. From this result, it is shown that if the densities, specific heat and thermal
conductivities of artery and venous blood are similar to the tissue, the phase lag times are
identical to each other. When the densities are different and specific heat and thermal
conductivity are considered then the phase lag times are also identical to each others. The phase
lag times for brain and muscle are also calculated in this chapter. The range of the phase lag time
for heat flux vector and phase lag time for temperature gradient studied in this chapter from 1 to
21 s. From results, it is shown that due to presence of blood perfusion in surrounding tissue, the
generalized DPL model can reduce to Fourier heat conduction model only when both phase lag
times are equal to zero.

60
References
[1] A. J. Welch, The thermal response of laser irradiated tissue, IEEE Journal of Quantum
Electronics 20 (12) (1984) 1471-1481.
[2] N. Afrin, Yuwen Zhang, J.K. Chen, Thermal lagging in living biological tissue based on
non-equilibrium heat transfer in a three-carrier system, International Journal of Heat and
Mass Transfer, Vol 54 (2011) 2419-2426.
[3] H.H. Pennes, Analysis of tissue and arterial blood temperatures in the resting forearm, J.
Appl. Physiol. 1 (1948) 93–122.
[4] C. Catteneo, A form of heat conduction equation which eliminates the paradox 503 of
instantaneous propagation, Compes Rendu 247 (1958) 431–433.
[5] P. Vernotte, Les paradoxes de la théorie continue de l´équation de la chaleur, 505 Comptes
Rendus 246 (1958) 3154–3155.
[6] D. Y. Tzou, Macro to Microscale Heat Transfer: The Lagging Behavior, Taylor & Francis,
Washington, DC, 1997.
[7] D. T. Tzou, A Unified field approach for heat conduction from macro –to microscales,
ASME Journal of Heat Transfer 117 (1995) 8-16.
[8] D.Y. Tzou, Weizhong Dai, Thermal lagging in multi-carrier systems, International Journal
of Heat and Mass Transfer 52 (2009) 1206-1213.

61
[9] P. J. Antaki, New interpretation of non-Fourier heat conduction in processed meat, ASME
Journal of Heat Transfer 127 (2005) 189-193.
[10] J. Werner and P. Webb, Cylinder model of human thermoregulation for general use on
personal computers. The Annals of Physiological Anthropology, 1993, 12, 123-134.
[11] H., Arkin and A., Shitzer, Model of thermoregulation in the human body, Part I: The heat
transfer model (the ‘passive model’). Department of Mechanical Engineering, Technion.
Haifa, Israel, 1984.
[12] Y. Xuan and W. Roetzel, Bioheat equation of the human thermal system, Chem. Eng.
Technol. 20 (1997) 268-276.
[13] S. Weinbaum, and L. M. Jiji, A two phase theory for the influence of circulation on the
heat transfer in surface tissue. In Advances in Bioengineering, M .K. Wells. ASME, New
York, 1979, 179-182.
[14] S. Weinbaum, L. M. Jiji and Lemons, D.E theory and experiment for the effect of vascular
microstructure on surface tissue heat transfer – Part I anatomical foundation and model
conceptuation, Journal of Biomechanical Engineering, 1984, 106, 321-330.
[15] L. M. Weinbaum S. Jiji, and D. E. Lemons, Theory and experiment for the effect of
vascular microstructure on surface tissue heat transfer. Part II model formulation and
solution, Journal of Biochemical Engineering, 1984, 106, 331-341.

62
[16] W J, Weinbaum, Song, S and L. M. Jiji, A theoretical model for peripheral tissue heat
transfer using the bioheat equation of Weinbaum and Jiji. Journal of Biomechanical
Engineering, 1987, 109, 72-78.
[17] M. A. Hader, MA Al-Nimr, Abu Nabah BA. The dual-phase-lag heat conduction model in
thin slabs under a fluctuating volumetric thermal disturbance. International Journal of
thermo physics. 2002, 23, 1669–1680.
[18] W. Kaminski, Hyperbolic heat conduction equation for materials with a nonhomogeneous
inner structure, ASME. Heat Transfer 112(1990) 555-560.
[19] K. Mitra, S. Kumar, A. Vedavarz, M. K. Moallemi, Experimental evidence of hyperbolic
heat conduction in processed meat, ASME J. Heat Transfer 117 (1995) 568-573.
[20] Y. Zhang, Generalized dual-phase lag bioheat equations based on non equilibrium heat
transfer in living biological tissues, International Journal of Heat and Mass Transfer,
Volume 52, Issues 21-22, October 2009, Pages 4829-4834.
[21] J. Zhou, Y. Zhang and J. K. Chen. An axistmmetric dual-phase bioheat model for laser
heating of living tissues. International Journal of Thermal Science. Volume 48(2009) 1477-
1485.
[22] J. Zhou, Y. Zhang and J.K. Chen, Non-Fourier heat conduction effect on laser induced
thermal damage in biological tissues, Numerical heat transfer, Part A: volume 54 (2008) 1-
19.

63
[23] Lisa Carroll, Tatyana r. Humphreys, LASER- tissue interactions, Clinics in Dermatology
24 (2006), 2-7.
[24] J. Zhou, J. K. Chen, Y. Zhang, Dual Phase lag effects on thermal damage to biological
tissues caused by laser irradiation, Computers in Biology and Medicine 39 (2009) 286-293.
[25] C. M Gardner, S.L. Jacques, A. J. Welch, Light transport in tissue: accurate, heuristic for
one dimensional fluence rate and escape function based upon Monte Carlo simulations,
lasers in Surgery and Medicine 18 (1996) 129-138.
[26] Y. Xuan and W. Roetzel, Bioheat equation of the human thermal system, Chem. Eng.
Technol. 20 (1997) 268-276.
[27] A. Nakayama, F. Kuwahara, A general bioheat transfer model based on the theory of
porous medium, Int. Heat Mass Transfer 51(2008) 3190-3199.
[28] W. J. Minkowycz, A. Haji-Seikh, K. Vafai, On departure from local thermal equilibrium in
porous medium due to rapid changing heat source; the sparrow number, Int, J. Heat and
Mass Transfer 42 (1999) 3373-3885.
[29] A.R.A Khaled, K. Vafai, The role of porous media in modeling flow and heat transfer in
biological tissues, Int. J .Heat transfer 46 (2003) 4989-5003.
[30] A.J. Welch, M.J.C van Gemert, Optical-Thermal Response of Laser-Irradiated Tissue,
Plenum Press, New York, 1995.
[31] F. Xu, T. J. Lu, K. A. Seffen, and E. Y. K. Ng, Mathematical Modeling of Skin Bioheat
Transfer, ASME Applied Mechanics Reviews 62, 050801 (2009).

64
[32] S. V. Patankar, Numerical Heat Transfer and Fluid Flow, Hemisphere, New York, 1980.
[33] Y. Yamada, T. Tien, M. Ohla, Theoretical analysis of temperature variation of biological
tissues irradiated by light, ASME/JSME Thermal Engineering Conference 4 (1995) 575-
581.
[34] T.N. Glenn, S. Rastegar, S.L. Jacques, Finite element analysis of temperature controlled
coagulation in laser irradiated tissue, IEEE Transactions on Biomedical Engineering 43 (1)
(1996) 79-87.
[35] W. F.Ganong, Review of Medical Physiology, Eighteenth Edition, 537.
[36] W. Kaminski, Hyperbolic heat conduction equation for materials with a nonhomogeneous
inner structure, ASME Journal of Heat Transfer 112 (1990) 555-560.
[37] K.Mitra, S.Kumar, A.Vedavarz, M.K. Moallemi, Experimental evidence of hyperbolic heat
conduction in processed meat, ASME Journal of Heat Transfer 117 (1995) 568-573.
[38] P.J. Antaki, New interpretation of non-Fourier heat conduction in processed meat, ASME
Journal of Heat Transfer 127 (2005) 189-193.
[39] A. Faghri, Y. Zhang, Transport Phenomena in Multiphase System, Elsevier, Burlington,
MA, 2006.
[40] W. Roetzel, Y. Xuan, Transient response of the human limb to an external stimulus,
International Journal Heat and Mass Transfer 41(1998) 229-239.

65
[41] C.L. Tien, K. Vafai. Convection and radiation heat transfer in porous media, in: J. W.
Hutchinson. T.Y Wu (Eds), Advances in applied Mechanics, volume 27. Academic Press,
Boston, MA, 1989, 225-281.
[42] P. Yuan, Numerical analysis of an equivalent heat transfer coefficient in a porous model for
simulating a biological tissue in a hyperthermia therapy, Int. J. Heat Mass transfer 52(2009)
1734-1740.