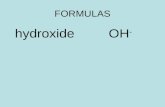
Determining Chemical Formulas Read pp. 107-120 Extension Questions p. 120 #1, 5-7.
-
Upload
augusta-reynolds -
Category
Documents
-
view
214 -
download
0
Transcript of Determining Chemical Formulas Read pp. 107-120 Extension Questions p. 120 #1, 5-7.

Determining Chemical Formulas
Read pp. 107-120
Extension Questions p. 120 #1, 5-7

Law of Constant Composition
“A compound contains elements in certain fixed proportions (ratios) and in no other combinations, regardless of how the compound is prepared or where it is found in nature.”

How do we determine chemical formulas?
1. Mass Spectrometer• Finds the molar mass of a
compound• A small sample is vapourized into
a gas phase and then ionized (becomes charged) into ions.
• The detector sorts and separates the ions according to their mass and charge.
• Results portrayed in a bar graph that tells you the composition of the compound.
DO NOT COPY!

How do we determine chemical formulas?
2. Combustion Analyzer
• Finds the % of C, H, O and maybe N in a compound
• Sample of compound is burned using oxygen gas at 980 Celsius. Products are now CO2 and H2O masses are weighed to determine % composition.
DO NOT COPY!

Percent Composition
The percentage (by mass) of EACH element in a compound.

Empirical Formula
A formula that gives you the lowest ratio of atoms in a compound. However, it does not tell you the exact number of each type of atom.

Example 1
What is the empirical formula of a compound with 39.99% C, 6.727% H and 53.28% O?
1. Find masses in 100 g sample
mC = 39.99 x 100 g mH = 6.727 x 100 g
100 100
= 39.99 g = 6.727 g
mO = 53.28 x 100 g
100
= 53.28 g

2. Find moles for each element
nC = 39.99 g nH = 6.727 g nO = 53.28 g
12.01 g/mol 1.01 g/mol 16.00 g/mol
= 3.33 mol = 6.66 mol = 3.33 mol
3. State the ratio, then divide by lowest ratio
nC : nH : nO
3.33 : 6.66 : 3.33
1 : 2 : 1
The empirical formula is CH2O

Example 2
The percentage composition of a compound is 69.9% iron and 30.1% oxygen. What is the empirical formula of the compound?
1. Find mass (in 100 g sample)
mFe = 69.9 x 100 g mO = 30.1 x 100 g
100 100
= 69.9 g = 30.1 g
2. Find moles for each element
nFe = 69.9 g nO = 30.1 g
55.85 g/mol 16.00 g/mol
= 1.25 mol = 1.88 mol

3. State ratio then divide by lowest ratio
nFe : nO
1.25 : 1.88
1 : 1.5 (you need WHOLE #s, so multiply both #s by 2 in order to get whole #s)
2 : 3
Therefore, the empirical formula is Fe2O3.

Molecular Formula
A formula that tells you the exact number of atoms in one molecule of the compound. It may be equal to its empirical formula or a multiple of that formula.
To find this, divide the molar mass of the known sample by the empirical mass.

Example 3 From Example 1, the sample was known to have a molecular mass of 180.18 g/mol. What is the molecular formula of this compound?
Empirical formula: CH2O (from Example 1 slide)
1. Find molar mass of EF
M = 1(12.01 g/mol) + 2(1.01 g/mol) + 1(16.00 g/mol)
= 30.03 g/mol
2. Find ratio of Mactual : MEF then divide by lowest #
180.18 g/mol : 30.03 g/mol 6 : 1 (so the molar mass of the sample is 6 times greater than the
molar mass of the EF sample)

3. Calculate MF using ratio
MF = 6 (EF)
= 6(CH2O)
= C6H12O6
The molecular formula of the compound is C6H12O6.

Example 4 (when EF is not given)
A combustion analyzer found that the % composition of a compound was 32.0% C, 6.70% H, 42.6% O, and 18.7% N. The mass spectrometer found the molar mass to be 75.8 g/mol. What is the MF of the compound?
1. Calculate mass of each element in 100 g sample
mC = 32.0 x 100 g mH = 6.70 x 100 g mO = 42.6 x 100 g
100 100 100
mN = 18.7 x 100 g
100

2. Find moles of each element
nC = 32.0 g nH = 6.70 g nO = 42.6 g
12.01 g/mol 1.01 g/mol 16.00 g/mol
= 2.66 mol = 6.63 mol = 2.66 mol
nN = 18.7 g
14.01 g/mol
= 1.33 mol
3. State ratio (and divide by lowest #)
nC : nH : nO : nN
2.66 : 6.63 : 2.66 : 1.33
2 : 5 : 2 : 1
Empirical formula is C2H5O2N

4. Find molar mass of EF
M = 2(12.01 g/mol) + 5(1.01 g/mol) + 2(16.00 g/mol) + 1(14.01 g/mol)
M = 75.00 g/mol
5. Find ratio of Mactual : MEF and divide by lowest #
75.8 g/mol : 75.00 g/mol
1 : 1
6. Calculate MF using ratio
MF = 1(EF)
= 1(C2H5O2N)
= C2H5O2N
The molecular formula is C2H5O2N.