Current Directions in Deep Brain Stimulation for … › content › pdf › 10.1007 ›...
Transcript of Current Directions in Deep Brain Stimulation for … › content › pdf › 10.1007 ›...

REVIEW
Current Directions in Deep Brain Stimulationfor Parkinson’s Disease—Directing Currentto Maximize Clinical Benefit
Aristide Merola . Alberto Romagnolo . Vibhor Krishna . Srivatsan Pallavaram .
Stephen Carcieri . Steven Goetz . George Mandybur . Andrew P. Duker . Brian Dalm .
John D. Rolston . Alfonso Fasano . Leo Verhagen
Received: January 6, 2020 / Published online: March 9, 2020� The Author(s) 2020
ABSTRACT
Several single-center studies and one largemulticenter clinical trial demonstrated thatdirectional deep brain stimulation (DBS) couldoptimize the volume of tissue activated (VTA)based on the individual placement of the leadin relation to the target. The ability to generateaxially asymmetric fields of stimulation trans-lates into a broader therapeutic window (TW)compared to conventional DBS. However,
changing the shape and surface of stimulatingelectrodes (directional segmented vs. conven-tional ring-shaped) also demands a revision ofthe programming strategies employed for DBSprogramming. Model-based approaches havebeen used to predict the shape of the VTA,which can be visualized on standardized neu-roimaging atlases or individual magnetic reso-nance imaging. While potentially useful foroptimizing clinical care, these systems remainlimited by factors such as patient-specificanatomical variability, postsurgical lead migra-tions, and inability to account for individualcontact impedances and orientation of the sys-tems of fibers surrounding the electrode. Alter-native programming tools based on thefunctional assessment of stimulation-inducedclinical benefits and side effects allow one tocollect and analyze data from each electrode ofthe DBS system and provide an action plan ofranked alternatives for therapeutic settingsbased on the selection of optimal directional
Aristide Merola and Alberto Romagnolo contributedequally to the manuscript and shared co-firstauthorship.
Enhanced digital features To view enhanced digitalfeatures for this article go to https://doi.org/10.6084/m9.figshare.11864961.
Electronic supplementary material The onlineversion of this article (https://doi.org/10.1007/s40120-020-00181-9) contains supplementary material, which isavailable to authorized users.
A. Merola (&)Department of Neurology, Ohio State UniversityWexner Medical Center, Columbus, OH, USAe-mail: [email protected]
A. RomagnoloDepartment of Neuroscience ‘‘Rita Levi Montalcini’’,University of Turin, Turin, Italy
V. Krishna � B. DalmDepartment of Neurosurgery, Ohio State WexnerMedical Center, Columbus, OH, USA
S. PallavaramNeuromodulation Division, Abbott Laboratories,Austin, TX, USA
S. CarcieriBoston Scientific Neuromodulation, Valencia, CA,USA
S. GoetzMedtronic PLC Brain Modulation, Minneapolis,MN, USA
Neurol Ther (2020) 9:25–41
https://doi.org/10.1007/s40120-020-00181-9

contacts. Overall, an increasing amount of datasupports the use of directional DBS. It is con-ceivable that the use of directionality mayreduce the need for complex programmingparadigms such as bipolar configurations, fre-quency or pulse width modulation, or inter-leaving. At a minimum, stimulation throughdirectional electrodes can be considered asanother tool to improve the benefit/side effectratio. At a maximum, directionality maybecome the preferred way to program becauseof its larger TW and lower energy consumption.
Keywords: Contact; Deep brain stimulation;Directionality; Lead; Parkinson disease;Programming
Key Summary Points
Directional deep brain stimulation (DBS)has the potential to minimizestimulation-induced side effects andmaximize clinical benefits
To maximize the opportunity ofdirectionality, the surgical planningshould be based on the DBS lead levelcontaining segmented directionalelectrodes
Visualization software platforms can assistprogramming by estimating the volumeof tissue activated by conventional ordirectional DBS electrodes
Functional software platforms can supportdirectional programming by creating anaction plan of ranked alternatives thatmay be needed over time
Directional DBS may avoid the need forcomplex stimulation protocols, such asbipolar stimulation, frequency or pulsewidth modulation, or interleaving
INTRODUCTION
Over the last three decades, the long-term effi-cacy of deep brain stimulation (DBS) in themanagement of Parkinson disease (PD) has beenwell established, with 60% improvement oflevodopa-related motor complications [1, 2],40–60% amelioration in quality of life [3], and asignificant increase in quality-adjusted lifeexpectancy [4]. Despite these remarkableresults, not all patients experience maximalbenefit from DBS. Unintentional spread ofstimulation outside the target and into adjacentareas may result in dose-limiting motor or non-motor side effects, preventing an optimal out-come [5, 6].
G. MandyburMayfield Brain and Spine, Cincinnati, OH, USA
A. P. DukerDepartment of Neurology, Gardner Family Centerfor Parkinson’s Disease and Movement Disorders,University of Cincinnati, Cincinnati, OH, USA
J. D. RolstonDepartment of Neurosurgery, University of Utah,Salt Lake City, UT, USA
J. D. RolstonDepartment of Biomedical Engineering, Universityof Utah, Salt Lake City, UT, USA
A. FasanoEdmond J. Safra Program in Parkinson’s Disease,Morton and Gloria Shulman Movement DisordersClinic, Toronto Western Hospital, UHN, Toronto,ON, Canada
A. FasanoDivision of Neurology, University of Toronto,Toronto, ON, Canada
A. FasanoKrembil Brain Institute, Toronto, ON, Canada
A. FasanoCenteR for Advancing NeurotechnologicalInnovation to Application (CRANIA), Toronto, ON,Canada
L. VerhagenDepartment of Neurological Sciences, MovementDisorder Section, Rush University, Chicago, IL, USA
26 Neurol Ther (2020) 9:25–41

Directional leads represent an innovativetechnology designed to address this issue. Theconcept of steering current away from sideeffects and towards benefits after the lead islocked into place is appealing when consideringthe need to further improve the accuracy of DBStargeting [7]. Here, we discuss opportunities andchallenges for directional DBS and review datain support of the integration of directional leadsinto clinical practice with regards to the func-tional anatomy of DBS target structures, plan-ning strategies for optimal lead placement, anduse of software platforms to assist the selectionof the most promising stimulation settings. Thisarticle is based on previously conducted studiesand does not contain any studies with humanparticipants or animals performed by any of theauthors.
CLINICAL DATA SUPPORTINGDIRECTIONALITY
In 2014, two groups reported the results ofintraoperative studies of directional DBS leads(not permanently implanted) (Table 1). In onestudy [8], the therapeutic window (TW), definedas the range between the minimal intensity ofstimulation required to obtain meaningfulclinical benefits, or therapeutic current strength(TCS), and the intensity of stimulation at whichthe first persistent side effect occurred, or sideeffect threshold (SET), was evaluated for singledirectional and omnidirectional configurationsin 13 patients with PD or essential tremor (ET).The electrode used had two segmented contactsat the distal end, with three segments each, andtwo solid ring electrodes proximally. When teststimulation was performed, the TW was foundto be 41.3% wider using the best directionalelectrode, compared to omnidirectional stimu-lation at that same level. The TCS was 43%lower for the best directional electrode com-pared to that required for omnidirectionalstimulation, indicating a higher degree of effi-ciency with directional DBS. In the secondstudy [9], eight patients with PD were assessedintraoperatively using an experimental leadwith 32 oval disc-shaped thin-film electrodecontacts arranged on eight rows of four
contacts. The patient, the evaluating neurolo-gist, and the neurosurgeon were blind to thedirection of the steering modes. When com-pared to omnidirectional stimulation, direc-tional DBS increased the TW in all patients,with a gain ranging from 0.5 to 1.5 mA. In sevenout of eight cases, SETs could be increased by atleast 1 mA when using directional DBS.
Three single-center studies investigated USFood and Drug Administration (FDA)-approveddirectional implants (Table 1) employing a ‘‘1-3-3-1’’ lead design with the two central rows seg-mented into three individually controlled elec-trodes; each segment spaced 120� apart in theplane orthogonal to the long axis of the lead.These studies found that best directional DBShas a significantly larger median TW and higherSET [10, 11], as well as slightly lower totalelectrical energy delivered (TEED) [12]. Morerecently, a large international, multicenter,prospective, crossover study of 234 patientswith PD undergoing subthalamic nucleus (STN)DBS from 37 centers across seven countries (thePROGRESS Study, Abbott St. Jude Medical,Plano, TX, USA) confirmed the superiority ofdirectional vs. omnidirectional programming[13]. In this study, patients were conventionally(i.e., non-directionally) stimulated for 3 monthsat the optimal ring. At the 3-month primaryendpoint, the optimal ring settings were con-firmed and in the same session the best direc-tional settings were identified between the threeindividual segments and the three pairwisecombinations of segments at the best seg-mented level. The best ring and best directionalmontages were then evaluated in a randomized(based on a coin toss) and double-blinded (pa-tient and the clinician) fashion and their TWswere recorded. Study details and results of theprimary endpoint are included in Table 2. Allpatients had 12 months of follow-up and sev-eral measures were tracked in this period at 3, 6,and 12 months including Unified PD RatingScale (UPDRS)-III (motor section), UPDRS-II(activities of daily living section), clinicianpreference, patient preference, 39-item PDQuestionnaire (PDQ-39), adverse events, andothers.
Neurol Ther (2020) 9:25–41 27

Table 1 Data from single-center studies on directional DBS
Author andyear
Samplesize
Leadconfiguration
Clinicalsetting
Efficacy measures FUP Main results
Pollo et al.
2014 [8]
11 PD
(STN)
2 ET
(Vim)
2 distal
segmented
contacts (3
segments
each)
2 proximal
ring
contacts
Intraoperative
double-
blinded
evaluation
Full effect on rigidity
or good effect on
tremor
NA TW 41.3% wider and
TCS 43% lower with
directional vs.
omnidirectional
stimulation
Contarino
et al.
2014 [9]
8 PD
(STN)
32 oval disc-
shaped
contacts
Intraoperative
double-
blinded
evaluation
Full effect on rigidity NA TW wider
(0.5–1.5 mA) with
directional vs.
omnidirectional
stimulation
Steigerwald
et al.
2016 [10]
7 PD
(STN)
2 central
segmented
contacts (3
segments
each)
1 proximal
and 1 distal
ring
contacts
Retrospective
unblinded
analysis of
permanently
implanted
patients
Full effect on rigidity 3–6 months TW variations from
- 100% to ? 440%
with directional vs.
omnidirectional
stimulation
Best TW improvement
with the best
directional contact at
the less effective level
At 3–6 months, all
patients remained in
directional
stimulation
28 Neurol Ther (2020) 9:25–41

Table 1 continued
Author andyear
Samplesize
Leadconfiguration
Clinicalsetting
Efficacy measures FUP Main results
Dembek
et al.
2017 [11]
10 PD
(STN)
2 central
segmented
contacts (3
segments
each)
1 proximal
and 1 distal
ring
contacts
Prospective
double-
blinded
Improvement of at
least 1.5 points in
the UPDRS-III
composite scores of
upper limb rigidity,
finger tapping, and
hand rotation
3–6 months TW wider with
directional vs.
omnidirectional
stimulation (median
2 mA vs 1 mA)
SET higher with
directional vs.
omnidirectional
stimulation (median
4 mA vs 3 mA)
At 3–6 months, 14
leads remained in
directional
stimulation
Rebelo et al.
2018 [12]
3 PD
(Vim)
3 DT
(Vim)
2 ET
(Vim)
2 central
segmented
contacts (3
segments
each)
1 proximal
and 1 distal
ring
contacts
Retrospective
unblinded
analysis of
permanently
implanted
patients
Full effect on tremor 6 months TW wider (1.86 mA
vs. 0.97 mA) and
TCS lower (1.51 mA
vs. 2.19 mA) with
directional vs.
omnidirectional
stimulation
TEED 6–18% lower
with directional vs.
omnidirectional
stimulation
At 6 months, 9 leads
remained in
directional
stimulation
DT dystonic tremor, ET essential tremor, FUP follow-up, PD Parkinson disease, NA not applicable, SET side effectsthreshold, STN subthalamic nucleus, TCS therapeutic current strength, TEED total electrical energy delivered, TWtherapeutic window, UPDRS Unified PD Rating Scale, Vim ventral intermediate nucleus
Neurol Ther (2020) 9:25–41 29

CHALLENGES ASSOCIATEDWITH DIRECTIONALPROGRAMMING
While directional DBS systems allow fine con-trol over the parameters by which they deliverstimulation, including location, size, shape, andnature of neural activation, this increased abil-ity to provide accurate and personalized stimu-lation demands a revision of paradigmscurrently used for the placement and program-ming of DBS electrodes. In particular, theanatomical and functional connections of the
DBS targets, as well as the bioelectrical proper-ties of directional vs. conventional leads, rep-resent critical aspects to be considered for thedevelopment of innovative DBS protocols.
DBS Target Structures: Anatomicaland Functional Connections
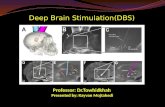
The STN is a small lens-shaped structure locatedin the anterior-lateral portion of the midbrain atthe junction of the cerebral peduncle to thetegmentum (Fig. 1). Leads are generallyimplanted following an oblique trajectory from
Table 2 Data from the PROGRESS study
Device Abbott St. Jude InfinityTM IPG system
Enrollment 234 patients with PD (157 male; 77 female)
Demographics Age 61.7 ± 8.4 years
PD duration since onset 11.7 ± 7.6 years
PD duration since diagnosis 10.2 ± 7.4 years
Number of centers 37 centers, from 7 countries
Lead configuration 2 central segmented contacts (3 segments each), 1 proximal and 1 distal ring contacts; 1-3-3-1
Clinical setting Prospective, blinded subject, blinded observer, crossover study of directional versus non-directional
stimulation
Study endpoints Primary endpoint
Superiority benchmark: at least 60% of patients at 3 months have wider TW with directional
stimulation when compared to non-directional stimulation in a randomized evaluation
Secondary endpoints
Non-inferiority benchmark: 40–60% of patients at 3 months have wider TW with directional
stimulation when compared to non-directional stimulation in a randomized evaluation
Comparison of UPDRS III at 3 months on non-directional stimulation versus at 6 months after
switching to directional stimulation
Descriptive endpoints
Patient and clinician preference at 6 months between directional and non-directional stimulation
Comparison of TW and TCS amplitudes between directional and non-directional stimulation
Device-related adverse events
Primary endpoint
results
In 90.6% of patients (183/202), TW was wider with directional stimulation as compared to non-
directional stimulation; primary endpoint superiority exceeded (p\ 0.001)
DBS deep brain stimulation, PD Parkinson disease, TCS therapeutic current strength, TW therapeutic window
30 Neurol Ther (2020) 9:25–41

a dorsal lateral entry point to a more ventralmedial endpoint in the STN. Given the highnumber of important surrounding structures,stimulation-induced side effects are importantto recognize and, if seen, can guide inferencesabout the location of the lead in relation to theplanned STN target (Table 3). Functionally, theSTN has a complex microanatomy, with largedendritic trees that are synaptically convergentbetween afferents from different functionalareas [14–16] (Table 4).
The globus pallidus pars interna (GPi) is atriangular-shaped structure forming the innerpart of the lentiform nucleus of the basal gan-glia (Fig. 1). DBS leads are generally implantedfollowing a parasagittal trajectory that traversesputamen, globus pallidus pars externa (GPe),and GPi. The target is in the posterolateral partof GPi, just medial to the medullary lamina thatseparates GPe and GPi and the tip of the elec-trode will be just dorsolateral to the optic tract.Stimulation-induced side effects, when present,can guide inferences about the location of thelead in relation to the planned target (Table 3).Functionally, the GPi is a site of convergence ofstriatal–pallidal efferents [17–28]. Together withthe substantia nigra pars reticulata (SNr), GPirepresents the main basal ganglia output to theventroanterior and ventrolateral thalamicnuclei and, eventually, to the cortex [29](Table 4).
Surgical Approach and ConsiderationsRelated to Type of DBS Lead
In currently used planning strategies, the STNmay be targeted at the anterior border of the rednucleus, 2 mm lateral to its medial border, andat 4 mm inferior to the anterior commis-sure–posterior commissure (AC–PC) plane [30].GPi may be targeted 3 mm lateral to its medialborder and one-third of its length anterior fromits posterior margin, all at the AC–PC plane [30].However, these heuristics are not absolute, andother effective targeting strategies may also beapplied. In addition, the precise targets withinthese areas are still contested by experts [31, 32].In particular, the optimal spot for stimulationwithin the STN remains debated [33].
For directional leads, targeting currentlyremains the same as for legacy quadripolarleads. However, more attention must be paid tothe depth of the electrodes. Most manufacturers(Boston Scientific and Abbott) have directionalcapabilities only in their second and thirdcontact rows, not in the first or fourth. TheAleva directSTN Acute lead, on the other hand,has directional first and second rows only,which better suit GPi because of the proximityto the internal capsule at the bottom of the lead
Fig. 1 DBS target structures. Anatomical DBS structuresof the left hemisphere in the anterior (left) and posterior(right) views. Courtesy of Abbott’s anatomical visualiza-tion educational software (StimDirect), available on the St.Jude InfinityTM clinician programmer. Subthalamicnucleus (STN): ventrally, the STN is bordered by thesubstantia nigra (SN), anterolaterally by the internalcapsule (IC), posteriorly by the medial lemniscus (ML),dorsally by the zona incerta and the fields of Forel, andmedially by the red nucleus (RN), the medial forebrainbundle, and the midbrain course of the oculomotor nerve.Globus pallidus pars interna (GPi): ventrally, the GPi isbordered by the ansa lenticularis, which separates it fromthe nucleus basalis and the amygdala, ventromedially by theoptic tract, dorsally and medially by the posterior limb ofthe internal capsule, and laterally by the internal medullarylamina of the globus pallidus which separates it from theglobus pallidus pars externa (GPe). Additionally, the GPi isdivided into an internal and external component by theincomplete medullary lamina of the globus pallidus. Ththalamus
Neurol Ther (2020) 9:25–41 31

[34]. With currently available electrodes, thedepth of the electrode should be targeted withthe intention of using a directional row (typi-cally the second or third) as the active contact.Depending on the surgeon’s practice, this mightrequire inserting the electrode deeper than witha standard quadripolar lead.
To help ensure consistent orientation of thesegments of the directional lead, manufacturershave placed a fiducial on the lead proximal tothe four electrode levels. When the fiducialpoints in the anterior direction, the three seg-ments face anteriorly, posteromedially, andposterolaterally, respectively. Nevertheless,
during implantation, the lead is subject to tor-sional movements, making it difficult to predictthe exact position of the fiducial marker and,therefore, the exact orientation of each seg-ment. If there is uncertainty regarding thedirectionality, high-resolution X-rays, fluo-roscopy, or CT scan can be used to verify therotational orientation [35].
DIRECTIONAL PROGRAMMING
The main goal of the first programming visit isto determine the TW for each of the electrode
Table 3 Stimulation-induced side effects
Direction of current spread Side effect Structures involved
STN Lateral or anterolateral Contralateral muscle contractions
Facial or tongue pulling
Dysarthria
Eyelid opening apraxia
Contralateral gaze deviation
Internal capsule
Anteromedial Autonomic changes/vegetative side effects
(nausea, heat sensation, sweating)
Lateral hypothalamic area
Medioventral Disconjugate gaze
Diplopia
Oculomotor nerve
Posterior Paresthesias Medial lemniscus
Dorsal or ventral Bradykinesia worseninga
Levodopa effect reduction
Mood changes (mania, depression, or apathy)
Zona incerta/thalamus
Substantia nigra
Scarcely localizable Impulsivity
Hypophonia
–
GPi Medial Contralateral muscle contractions Internal capsule
Posterior Bradykinesia worseninga
Dorsal Dyskinesia Globus pallidus pars externa
Ventral Phosphenes Optic tract
Lateral
Anterior
No side effects Globus pallidus pars externa
GPi globus pallidus pars interna, STN subthalamic nucleusa In spite of rigidity improvement
32 Neurol Ther (2020) 9:25–41

contacts [36]. Directional leads are often firsttested in ring mode, followed by directionalstimulation on individual segments if efficacyin ring mode is limited by stimulation-relatedside effects, or to identify programming strate-gies that allow a larger TW with lower energyconsumption [37]. Supporting programming
software allows for visualizations of theanatomical context along with the predictedvolume of tissue activated (VTA) and for func-tional mapping of the thresholds for clinicalefficacy and side effects. Some of these tools alsoallow for an integrated analysis of power con-sumption adjusted to each contact impedance.
Table 4 STN and GPi functional connections
Input Output
Afferents Neurotransmitter STN areainvolved
Efferents Neurotransmitter
STN Globus pallidus pars externa GABA Dorsolateral
(motor area)
Ventromedial
(limbic area)
Globus pallidus
pars interna
Glutamate
Primary motor cortex
Supplementary motor area
Premotor cortex
Glutamate Dorsolateral
(motor area)
Substantia nigra
pars reticulata
Glutamate
Prefrontal cortex
Prelimbic-medial orbital areas
Glutamate Ventromedial
(limbic area)
– –
Thalamus (parafascicular and
centromedian nuclei)
Glutamate Dorsolateral
(motor area)
Ventromedial
(limbic area)
– –
Input Output
Afferent area Neurotransmitter Efferent area Neurotransmitter
GPi Striatum (putamen and caudate) GABA Thalamus (ventroanterior and
ventrolateral nuclei)
GABA
Globus pallidus pars externa GABA Substantia nigra pars compacta GABA
Thalamus (intralaminar nucleus) Glutamate Lateral habenular nucleus GABA
STN Glutamate Pedunculopontine nucleus GABA
Pedunculopontine nucleus Glutamate and
acetylcholine
– –
Dorsal raphe nucleus Serotonin – –
Substantia nigra Dopamine – –
GABA gamma-aminobutyric acid, GPi globus pallidus pars interna, STN subthalamic nucleus
Neurol Ther (2020) 9:25–41 33

Visualization of Volume of TissueActivated
The volume of neural activation can be esti-mated using finite element techniques, whereina computer system subdivides a volumetricregion of interest and solves for a scenario byapplying appropriate mathematical relation-ships, initial conditions, and boundary condi-tions and iterating until a stable solutionresults. In this domain, this is applied by posi-tioning an appropriate model of a stimulatingelectrode, configuring it with appropriate stim-ulation parameters (polarities, stimulus ampli-tude), and determining the resulting currentflows and voltage gradients in the surroundingtissue. These electromagnetic gradients canthen be applied, in a second step, to a popula-tion of simulated neurons positioned and ori-ented preferentially around the stimulatingelectrode so as to determine points at whichneurons may be activated by a given stimula-tion regime [38]. These neurons may be mod-eled such that they respond both to theintensity of the stimulation (current amplitude)and the time-dependent parameters such as thewidth of a stimulation pulse [38]. As an exam-ple, various neuron fiber diameters may beincluded, such that the response of smallerfibers to a given stimulus pulse is different thanthat of larger fibers. The resulting neuron pop-ulation that is predicted to be activated canthen be represented visually, as a volumeenclosing the points of activation.
Model-based approaches have been shown tohave some predictive value when used in visualprogramming systems [39]. However, validatingthese models can be challenging, as it is difficultto know with certainty which neural tissuevolumes are indeed activated by stimulation.One thing that has been established is that fullyfeatured models incorporating voltage drop andcapacitance of the electrode–electrolyte inter-face, tissue encapsulation of the electrode, anddiffusion tensor-based 3D tissue anisotropy andinhomogeneity produce more realistic predic-tions than simpler models that do not accountfor these factors [40].
As new stimulation modalities are intro-duced, including the new options afforded by
directional leads, these VTA models may beused to compare the different stimulationoptions available. As examples, in bipolarstimulation, they can be used to understand thedifferences in volume between anodal andcathodal activation regions. For interleavedstimulation, they can be used to understandareas of overlap in separate pulse trains, whereintissue receives stimulation at double theunderlying frequency [41]. In directional stim-ulation, the ability to push stimulation off axis,commonly referred to as displacement of theVTA centroid, can be explored in comparison totraditional ring mode stimulation. This may beof specific interest, as the thresholds to activa-tion in directional stimulation may be differentthan in traditional rings. For fixed stimulationamplitudes, the smaller surface area of a direc-tional segment creates relatively higher gradi-ents around the electrode which may increasethe probability of neural activation [38].
Visualization Strategies: Opportunities
Patient-Specific AnatomyA preoperative magnetic resonance imaging(MRI) may be used to establish the location andboundaries of anatomical structures, eitherthrough adaptation of an anatomical atlas ordirect segmentation of structure boundariesfrom intensity information in the imagesthemselves. Further, fiber tracts may addition-ally be represented if appropriate imagingsequences (diffusion tensor imaging) or atlasesare available.
Final Lead Location and OrientationA postoperative, or in some cases an intraoper-ative, image may be used to establish the finallead location and orientation. For this purpose,images that clearly delineate the electrodepositions and the orientation of fiducial mark-ers included in the lead body via a hyper- orhypo-intense image artifact can be used.
Visualization of Predicted Stimulation ExtentA VTA or similar model may be applied to therepresentation of the stimulating electrodes.Models may be created for one or more
34 Neurol Ther (2020) 9:25–41

potential sets of stimulating parameters (i.e.,different active contacts, different current orvoltage amplitudes, monopolar vs. bipolarstimulation, directional vs. conventional ringstimulation, etc.) such that the overlap of theresulting stimulation field to patient anatomycan be assessed.
Integration of Ancillary InformationIn addition to anatomy, lead location, andstimulation extent, visualization solutions mayadditionally allow for the addition of otherinformation of potential use in identifyingoptimal stimulation settings. For example,intra- or postoperative electrophysiologicalmeasures may be visualized in the patient-specific anatomical context. In addition, aggre-gated historical information about stimulationoutcome in the form of a statistical outcome orside effect map may be visualized in the patient-specific anatomical context to further informprogramming decisions.
Visualization Strategies: Challenges
Limitations of Anatomical ModelsCurrently used anatomical models on whichthe lead is visualized are usually not patient-specific, and even when patient-specific, theyare based on presurgical MRI images that do notaccount for procedural brain shift and postsur-gical anatomical changes reported at up to4 mm in the deep brain [42–46].
Deviation of DBS LeadDBS leads show large deviations from theirintended implanted orientation: more than 30�rotation in 42% of the leads and more than 60�rotation in 11% of the leads [47]. Thus, theorientation of the individual segmented con-tacts might be no longer valid relative to theunderlying anatomy in presurgical MRI pre-sented during programming. Furthermore, sig-nificant lead migration (greater than 3 mm)along the ventrodorsal axis or upward dis-placement from immediate to delayed CT hasbeen reported in over 12% of leads placed at anexpert center [48, 49].
Variability in Tissue Impedanceand AnisotropyValidating VTA models is challenging as it isdifficult to know with certainty which neuraltissue volumes are stimulated [50]. VTA modelsmade available for programming are based ongeneric homogeneous models that do notaccount for tissue inhomogeneities, for instancepermittivity and conductivity of brain struc-tures, which may alter VTA predictions from- 44% to 174% [51]. Also, VTA models availablein programming platforms do not account forpatient-specific tissue anisotropy that can atbest only be modeled using tractography fromhigh-resolution patient-specific diffusion tensorimaging (DTI) [52, 53]. Other real-world factors,not usually modeled, that influence the accu-racy, shape, and extent of the VTA includephysiology and pathophysiology [50], brainpulsation and patient hydration [54], glial scarformation around the electrode [55], and localfluid retention [56].
Software Platforms to Assist DBSProgramming
SureTuneTM (Supplementary Fig. S1) is a pro-gramming visualization tool available for clini-cal use in many regions and close to beingreleased also in the USA. It incorporates theBardinet–Yelnick anatomic atlas and registra-tion algorithm [57], and further allows a user tomanually adjust structure size, shape, andlocation to allow for valid representation of apatient’s specific anatomical variation. It alsosupports intensity-based segmentation of MRIvisible structures. Within the existing software,leads can be placed according to stereotacticcoordinates or aligned via postoperative images.Anatomic representations of structures can bevalidated by co-registration of microelectroderecordings to confirm anatomical boundaries ifadditional confidence is desired. Stimulationmodeling is available for common stimulationconfigurations, and stimulation plans can becreated for use in the clinical setting. Finally, viaa service offering, statistical maps of outcome orside effects can be created which can then beprospectively visualized to inform future
Neurol Ther (2020) 9:25–41 35

clinical decision-making or research applica-tions: a recent study [58] showed that suchstatistical maps correlated well with best clinicalprogramming, demonstrating predictive utilityin prospective cohorts for GPi stimulation indystonia.
While potentially useful for optimizingclinical care, SureTuneTM has several limita-tions. Its atlas is a single brain histologicallybased example that may not account for subjectto subject variation in anatomy. While itsapproach of algorithmic fitting plus humanadjustment can be highly accurate, it is alsodependent on image quality and human judg-ment to achieve a good patient-specific fit.Finally, it uses homogeneous and isotropicassumptions in its VTA methodology, whichmay introduce errors in the visualization due tovariation of tissue properties within a specificpatient’s brain.
SureTuneTM is tightly integrated with otherMedtronic surgical tools, allowing import ofsurgical information from StealthTM navigationand planning software and the documentationof intraoperative electrophysiology which mayfurther validate a patient-specific visualization.Medtronic’s current programming offeringallows visualization of the VTA created by thedevice and the annotation of clinical observa-tions. Future updates to SureTuneTM will extendinteroperability, allowing anatomical and elec-trophysiological information to appear on theprogramming tablet along with the stimulationvolumes.
Boston Scientific GUIDETM XTThe Boston Scientific neuromodulation stimu-lators use a system that includes both VTAmodeling and functional testing. Visualizationof VTAs with the Boston Scientific system isavailable through a software tool (GUIDETM
XT). GUIDETM XT is compatible with theBrainLab Elements (Munich, Germany) surgicalplanning software, available for clinical use inmany countries. The software enables 3D mod-eling and visualization of the VTA relative tothe patient’s anatomical structures. To generatea model of lead location in the brain, a simu-lated DBS lead from a patient’s postoperative CTscan is registered to an anatomical atlas based
on segmentation of a patient’s preoperativeMRI. Clinical stimulation parameters can thenbe programmed onto the simulated lead togenerate the associated VTA. In cases of subop-timal electrode placement or clinical response,additional options may be needed, and currentsteering between directional segments may beuseful to expand the TW (SupplementaryFig. S2) [10]. Visualization tools may be usefulfor understanding these nuances of directionalDBS and adjusting stimulation appropriately.For example, the VTA created by coactivation oftwo segments has a different shape than theVTA created by activation of a single segment,with the former having a lesser radial extent ofactivation than the latter at the same ampli-tudes. The VerciseTM system also includes theNeural Navigator software, intended to aid infunctional testing of different stimulator set-tings by mapping the resulting clinical effects.Therapeutic effects and side effects associatedwith monopolar stimulation on both ring anddirectional electrodes are represented on a 2Dclinical effects map (Supplementary Figs. S3 andS4), and effects may be recorded on all leads forall stimulation configurations. VTAs for bothstandard DBS leads and directional DBS leadsare also visualized within the Neural Navigatorsoftware, which can import the anatomicalstructures from the BrainLab platform.
Abbott InformityTM
While the Abbott InfinityTM clinician program-mer does not have a VTA visualization tool, itincludes the InformityTM programming soft-ware designed primarily for simplifying func-tional programming. The software guides theuser through directional programming usingvisual representations of stimulation responses,occurrences of stimulation-induced symptomrelief and side effects, to create an action plan ofranked electrode montage alternatives that maybe needed over time during therapy. The layoutand workflow of the InformityTM programmingsoftware enable ‘‘event markers’’ to documentthe amplitudes that produce symptom reliefand side effects (Supplementary Figs. S5, S6, andS7). After monopolar survey of ring and seg-mented electrodes, the InformityTM softwareallows clinicians to rank the investigated
36 Neurol Ther (2020) 9:25–41

montages according to various clinically rele-vant criteria. With traditional omnidirectionalprogramming, amongst montages with compa-rable outcomes some clinicians prioritize max-imizing the TW while others prioritizeminimizing the TCS (power consumption). Tothat end, the decision support tool availablewithin the InformityTM software not onlyallows ranking the montages on the basis ofpower (microwatts) and TW (milliamps) butalso on the basis of a measure referred to as thetherapeutic window percentage (TW%). TW%,which is TW expressed as a percentage of TCS, isa means to balance the trade-off between max-imizing TW and minimizing TCS and givesclinicians a means to optimize gains in bothTCS and TW simultaneously. A final sortingoption, balanced threshold, gives clinicians anadditional level of optimization to balancegains in TW% while minimizing power con-sumption as per clinician preference (Supple-mentary Fig. S7). The software allows completecustomization of symptom relief and side effectlists and enables the export of the entire func-tional programming session in the form of aPDF report that can be exported to electronicmedical records for future review.
CONCLUSIONS
Increasing clinical evidence supports the supe-riority of directional over omnidirectionalstimulation. It is conceivable that directionalleads, by providing asymmetric stimulation,may avoid the need for complex programmingparadigms such as bipolar stimulation, fre-quency or pulse width modulation, or inter-leaving. At a minimum, stimulation throughdirectional electrodes can be considered asanother tool to improve the benefit/side effectratio. At a maximum, directional stimulationmay become the preferred way to program, evenwhen omnidirectional stimulation causes noside effects, because of its larger TW and lowerenergy consumption.
However, as new DBS systems increase intheir capability to control the shape, size, andlocation of stimulated neural tissue, the com-plexity of optimizing the configuration of such
systems grows as well. The use of simple pro-gramming protocols, such as the activation of asingle directional segment, has proved to beeffective in the vast majority of cases [13].Directional multiple-electrode programmingconfigurations, whether using independentcurrent controllers or interleaving, may provideadditional nuances in programming strategies,but may also result in increased programmingcomplexity [59], changes to battery powerconsumption, and reduction in the laterality ofthe volume of activation [60]. To assist withDBS programming, DBS producers developedinnovative software platforms for functionalmapping, as well as tools for visualization of thevolume of neural activation. Still, these modelsare constrained by simplifying assumptions,such as using homogeneous and isotropic tissueenvironments and arbitrarily positioned popu-lations of generally linear axons. Also, no studyhas so far established if commercially availablesoftware platforms are superior to standardprogramming.
In conclusion, while directionality offers aunique opportunity to improve the functionaloutcomes of DBS, it also requires definition ofunified strategies for functional mapping andcommon standards for VTA visualization sincesuboptimal programming remains one of themajor causes for DBS failure [6, 61]. Here, weadvocate for intensive collaboration betweenDBS manufacturers and academic centers todevelop and test in large multicenter clinicaltrials innovative programming algorithms aim-ing at maximizing the benefits of directionalDBS.
ACKNOWLEDGEMENTS
Funding. No funding or sponsorship wasreceived for this study or publication of thisarticle.
Authorship. All named authors (Dr. Merola,Dr. Romagnolo, Dr. Krishna, Dr. Pallavaram, Dr.Carcieri, Dr. Goetz, Dr. Mandybur, Dr. Duker,Dr. Dalm, Dr. Rolston, Dr. Fasano, and Dr.Verhagen Metman) meet the International
Neurol Ther (2020) 9:25–41 37

Committee of Medical Journal Editors (ICMJE)criteria for authorship for this article, takeresponsibility for the integrity of the work as awhole, and have given their approval for thisversion to be published.
Disclosures. Aristide Merola is supported byNIH (KL2 TR001426) and has received speakerhonoraria from CSL Behring, Abbvie, andCynapsus Therapeutics. He has received Grantsupport from Lundbeck. Alberto Romagnolo hasreceived Grant support and speaker honorariafrom AbbVie, speaker honoraria from ChiesiFarmaceutici and travel Grants from Lusofar-maco, Chiesi Farmaceutici, Medtronic, and UCBPharma. Vibhor Krishna has received researchGrant from Medtronic, Boston Scientific, andAbbott. Srivatsan Pallavaram is a Medical Sci-ence Advisor (Medical Affairs) at Abbott Labsand receives salary for his services. StephenCarcieri is an employee at Boston Scientific andreceives salary for his services. Steven Goetz isan employee at Medtronic and receives salaryfor his services. George Mandybur has receivedconsulting agreement with Boston Scientificand Abbott, as well as research Grants fromBoston Scientific. Andrew P. Duker and BrianDalm have nothing to declare. John D. Rolstonhas consulting agreements with NeuroPace andMedtronic, and stock in Axion Biosystems. Hereceived funding from the NIH (NCATS KL2TR002539 and NINDS K23 NS114178). AlfonsoFasano sits in the advisory board of Evotion,Inbrain Neuroelectronics and Cortics, receivedhonoraria for consultancies from Apple, Abbvie,Abbott, BrainLab, Boston Scientific, Chiesi far-maceutici, Ipsen, Medtronic, Sunovion, andUCB; honoraria for participation in advisoryboards from Abbvie, Boston Scientific, andIpsen; research Grants from Abbvie, BostonScientific, Cummings Foundation, DystoniaMedical Research Foundation Canada, MichaelJ. Fox Foundation, Medtronic, and University ofToronto. Leo Verhagen sits in the advisoryboards of Abbott Neuromodulation, AbbVie Inc,Biogen Inc. He is in the editorial board of Neu-rology and Therapy and Brain Sciences. He hasreceived consultancies from Abbott, AbbVie Inc,and Boston Scientific, and research supportfrom Medtronic, Boston Scientific, Abbott,
AbbVie, Neuroderm, Biogen Inc, and Prileniatherapeutics. He has received NIH funding (R01NS40902) as a site-PI. Leo Verhagen is a memberof the journal’s Editorial Board.
Compliance with Ethics Guidelines. Thisarticle is based on previously conducted studiesand does not contain any studies with humanparticipants or animals performed by any of theauthors.
Data availability. Data sharing is notapplicable to this article as no datasets weregenerated or analyzed during the current study.
Open Access. This article is licensed under aCreative Commons Attribution-NonCommer-cial 4.0 International License, which permitsany non-commercial use, sharing, adaptation,distribution and reproduction in any mediumor format, as long as you give appropriate creditto the original author(s) and the source, providea link to the Creative Commons licence, andindicate if changes were made. The images orother third party material in this article areincluded in the article’s Creative Commonslicence, unless indicated otherwise in a creditline to the material. If material is not includedin the article’s Creative Commons licence andyour intended use is not permitted by statutoryregulation or exceeds the permitted use, youwill need to obtain permission directly from thecopyright holder. To view a copy of this licence,visit http://creativecommons.org/licenses/by-nc/4.0/.
REFERENCES
1. Limousin P, Foltynie T. Long-term outcomes ofdeep brain stimulation in Parkinson disease. NatRev Neurol. 2019;15:234–42.
2. Zibetti M, Merola A, Rizzi L, et al. Beyond nine yearsof continuous subthalamic nucleus deep brainstimulation in Parkinson’s disease. Mov Disord.2011;26:2327–34.
3. Gorecka-Mazur A, Furgala A, Krygowska-Wajs A,Pietraszko W, Kwinta B, Gil K. Activities of dailyliving and their relationship to health-related
38 Neurol Ther (2020) 9:25–41

quality of life in patients with Parkinson diseaseafter subthalamic nucleus deep brain stimulation.World Neurosurg. 2019;125:e552–62.
4. Espay AJ, Vaughan JE, Marras C, Fowler R, EckmanMH. Early versus delayed bilateral subthalamic deepbrain stimulation for parkinson’s disease: a decisionanalysis. Mov Disord. 2010;25:1456–63.
5. Castrioto A, Lhommee E, Moro E, Krack P. Moodand behavioural effects of subthalamic stimulationin Parkinson’s disease. Lancet Neurol. 2014;13:287–305.
6. Okun MS, Tagliati M, Pourfar M, et al. Managementof referred deep brain stimulation failures: a retro-spective analysis from 2 movement disorders cen-ters. Arch Neurol. 2005;62:1250–5.
7. Sheehy JP, Chen T, Bohl MA, Mooney MA, Mirza-deh Z, Ponce FA. Accuracy in deep brain stimula-tion electrode placement: a single-surgeonretrospective analysis of sterotactic error in over-lapping and non-overlapping surgical cases.Stereotact Funct Neurosurg. 2019;97:37–43.
8. Pollo C, Kaelin-Lang A, Oertel MF, et al. Directionaldeep brain stimulation: an intraoperative double-blind pilot study. Brain. 2014;137:2015–26.
9. Contarino MF, Bour LJ, Verhagen R, et al. Direc-tional steering: a novel approach to deep brainstimulation. Neurology. 2014;83:1163–9.
10. Steigerwald F, Muller L, Johannes S, Matthies C,Volkmann J. Directional deep brain stimulation ofthe subthalamic nucleus: a pilot study using a novelneurostimulation device. Mov Disord. 2016;31:1240–3.
11. Dembek TA, Reker P, Visser-Vandewalle V, et al.Directional DBS increases side-effect thresholds—aprospective, double-blind trial. Mov Disord.2017;32:1380–8.
12. Rebelo P, Green AL, Aziz TZ, et al. Thalamic direc-tional deep brain stimulation for tremor: spend less,get more. Brain Stimul. 2018;11:600–6.
13. Schnitzler AS, Mir PM, Brodsky MB, et al. Direc-tional versus omnidirectional deep brain stimula-tion for Parkinson’s disease: results of a prospective,blinded, multi center, single-arm crossover study[abstract]. Mov Disord. 2019;34(suppl 2).
14. Hamani C, Saint-Cyr JA, Fraser J, Kaplitt M, LozanoAM. The subthalamic nucleus in the context ofmovement disorders. Brain. 2004;127:4–20.
15. Hartmann-von Monakow K, Akert K, Kunzle H.Projections of the precentral motor cortex andother cortical areas of the frontal lobe to the
subthalamic nucleus in the monkey. Exp Brain Res.1978;33:395–403.
16. Mathai A, Pare J, Jenkins S, Smith Y, editors. Glu-tamatergic inputs to the subthalamic nucleus: aquantitative analysis of the synaptic microcircuitryof vGluT1-and vGluT2-containing terminals innormal and Parkinsonian nonhuman primates. In:Xth triennial meeting of the international BasalGanglia Society Long Branch, NJ; 2010.
17. Goldberg J, Bergman H. Computational physiologyof the neural networks of the primate globus pal-lidus: function and dysfunction. Neuroscience.2011;198:171–92.
18. Yelnik J. Functional anatomy of the basal ganglia.Mov Disord. 2002;17:S15–21.
19. Kim R, Nakano K, Jayaraman A, Carpenter MB.Projections of the globus pallidus and adjacentstructures: an autoradiographic study in the mon-key. J Comp Neurol. 1976;169:263–89.
20. Kuo JS, Carpenter MB. Organization of pallidotha-lamic projections in the rhesus monkey. J CompNeurol. 1973;151:201–35.
21. Parent A, De Bellefeuille L. The pallidointralaminarand pallidonigral projections in primate as studiedby retrograde doublelabeling method. Brain Res.1983;278:111–27.
22. Parent A, Gravel S, Boucher R. The origin of fore-brain afferents to the habenula in rat, cat andmonkey. Brain Res Bull. 1981;6:23–38.
23. Shink E, Smith Y. Differential synaptic innervationof neurons in the internal and external segments ofthe globus pallidus by the GABA-and glutamate-containing terminals in the squirrel monkey.J Comp Neurol. 1995;358:119–41.
24. Nambu A. Globus pallidus internal segment. ProgBrain Res. 2007;160:135–50.
25. Smith Y, Wichmann T, DeLong M. Synaptic inner-vation of neurons in the internal pallidal segmentby the subthalamic nucleus and the external pal-lidum in monkeys. J Comp Neurol. 1994;343:297–318.
26. Lavoie B, Parent A. Immunohistochemical study ofthe serotoninergic innervation of the basal gangliain the squirrel monkey. J Comp Neurol. 1990;299:1–16.
27. Lavoie B, Parent A. Pedunculopontine nucleus inthe squirrel monkey: projections to the basal gan-glia as revealed by anterograde tract-tracing meth-ods. J Comp Neurol. 1994;344:210–31.
Neurol Ther (2020) 9:25–41 39

28. Lavoie B, Smith Y, Parent A. Dopaminergic inner-vation of the basal ganglia in the squirrel monkeyas revealed by tyrosine hydroxylase immunohisto-chemistry. J Comp Neurol. 1989;289:36–52.
29. Lanciego J, Luquin N, Obeso J. Functional neu-roanatomy of the basal ganglia. Cold Spring HarbPerspect Med. 2012;2:a009621.
30. Panov FE, Larson P, Martin A, Starr P. Deep brainstimulation for Parkinson’s disease. In: Winn HR,editor. Youmans and Winn neurological surgery.7th ed. Philadelphia: Elsevier; 2017.
31. Hamel W, Koppen JA, Alesch F, et al. Targeting ofthe subthalamic nucleus for deep brain stimulation:a survey among parkinson disease specialists. WorldNeurosurg. 2017;99:41–6.
32. Nestor KA, Jones JD, Butson CR, et al. Coordinate-based lead location does not predict Parkinson’sdisease deep brain stimulation outcome. PLoS One.2014;9:e93524.
33. Bot M, Schuurman PR, Odekerken VJJ, et al. Deepbrain stimulation for PD: defining the optimallocation within the subthalamic nucleus. J NeurolNeurosurg Psychiatry. 2018;89:493–8.
34. Fasano A, Lozano AM, Cubo E. New neurosurgicalapproaches for tremor and Parkinson’s disease. CurrOpin Neurol. 2017;30:435–46.
35. Sitz A, Hoevels M, Hellerbach A, et al. Determiningthe orientation angle of directional leads for deepbrain stimulation using computed tomography anddigital X-ray imaging: a phantom study. Med Phys.2017;44:4463–73.
36. Dowsey-Limousin P. Postoperative management ofVim DBS for tremor. Mov Disord. 2002;17:S208–11.
37. Schupbach WMM, Chabardes S, Matthies C, et al.Directional leads for deep brain stimulation:opportunities and challenges. Mov Disord. 2017;32:1371–5.
38. Anderson CJ, Anderson DN, Pulst SM, Butson CR,Dorval AD. Neural selectivity, efficiency, and doseequivalence in deep brain stimulation throughpulse width tuning and segmented electrodes.bioRxiv. 2019. https://doi.org/10.1101/613133.
39. Frankenmolle AM, Wu J, Noecker AM, et al. Rev-ersing cognitive–motor impairments in Parkinson’sdisease patients using a computational modellingapproach to deep brain stimulation programming.Brain. 2010;133:746–61.
40. Chaturvedi A, Butson CR, Lempka SF, Cooper SE,McIntyre CC. Patient-specific models of deep brainstimulation: influence of field model complexity on
neural activation predictions. Brain Stimul. 2010;3:65–7.
41. Karl JA, Ouyang B, Verhagen Metman L. A noveldual-frequency deep brain stimulation paradigm forParkinson’s disease. Neurol Ther. 2019;8:483–9.
42. Halpern CH, Danish SF, Baltuch GH, Jaggi JL. Brainshift during deep brain stimulation surgery forParkinson’s disease. Stereotact Funct Neurosurg.2008;86:37–43.
43. Pallavaram S, Dawant BM, Remple MS, et al. Effectof brain shift on the creation of functional atlasesfor deep brain stimulation surgery. Int J ComputAssist Radiol Surg. 2010;5:221–8.
44. Khan MF, Mewes K, Gross RE, Skrinjar O. Assess-ment of brain shift related to deep brain stimula-tion surgery. Stereotact Funct Neurosurg. 2008;86:44–53.
45. Pallavaram S, Dawant BM, Li R, et al. A method tocorrect for brain shift when building electrophysi-ological atlases for deep brain stimulation (DBS)surgery. Med Image Comput Comput Assist Interv.2009;12:557–64.
46. Matias CM, Frizon LA, Asfahan F, Uribe JD,Machado AG. Brain shift and pneumocephalusassessment during frame-based deep brain stimula-tion implantation with intraoperative magneticresonance imaging. Oper Neurosurg (Hagerstown).2018;14:668–74.
47. Dembek TA, Hoevels M, Hellerbach A, et al. Direc-tional DBS leads show large deviations from theirintended implantation orientation. ParkinsonismRelat Disord. 2019;67:117–21.
48. Morishita T, Hilliard JD, Okun MS, et al. Postoper-ative lead migration in deep brain stimulation sur-gery: incidence, risk factors, and clinical impact.PLoS One. 2017;12:e0183711.
49. van den Munckhof P, Contarino MF, Bour LJ,Speelman JD, de Bie RM, Schuurman PR. Postoper-ative curving and upward displacement of deepbrain stimulation electrodes caused by brain shift.Neurosurgery. 2010;67:49–53.
50. Ineichen C, Shepherd NR, Surucu O. Understandingthe effects and adverse reactions of deep brainstimulation: is it time for a paradigm shift toward afocus on heterogenous biophysical tissue propertiesinstead of electrode design only? Front Hum Neu-rosci. 2018;12:468.
51. Howell B, McIntyre CC. Role of soft-tissue hetero-geneity in computational models of deep brainstimulation. Brain Stimul. 2017;10:46–50.
40 Neurol Ther (2020) 9:25–41

52. Howell B, McIntyre CC. Analyzing the tradeoffbetween electrical complexity and accuracy inpatient-specific computational models of deepbrain stimulation. J Neural Eng. 2016;13:036023.
53. Walckiers G, Fuchs B, Thiran JP, Mosig JR, Pollo C.Influence of the implanted pulse generator as ref-erence electrode in finite element model ofmonopolar deep brain stimulation. J NeurosciMethods. 2010;186:90–6.
54. Yousif N, Bayford R, Bain PG, Liu X. The peri-elec-trode space is a significant element of the elec-trode–brain interface in deep brain stimulation: acomputational study. Brain Res Bull. 2007;74:361–8.
55. Howell B, Huynh B, Grill WM. Design and in vivoevaluation of more efficient and selective deepbrain stimulation electrodes. J Neural Eng. 2015;12:046030.
56. Astrom M, Johansson JD, Hariz MI, Eriksson O,Wardell K. The effect of cystic cavities on deep brainstimulation in the basal ganglia: a simulation-basedstudy. J Neural Eng. 2006;3:132–8.
57. Yelnik J, Bardinet E, Dormont D, et al. A three-di-mensional, histological and deformable atlas of thehuman basal ganglia. Neuroimage. 2007;34:618–38.
58. Reich MM, Horn A, Lange F, et al. Probabilisticmapping of the antidystonic effect of pallidal neu-rostimulation: a multicentre imaging study. Brain.2019;142:1386–98.
59. Ten Brinke TR, Odekerken VJJ, Dijk JM, van denMunckhof P, Schuurman PR, de Bie RMA. Direc-tional deep brain stimulation: first experiences incenters across the globe. Brain Stimul. 2018;11:949–50.
60. Zhang S, Silburn P, Pouratian N, et al. Comparingcurrent steering technologies for directional deepbrain stimulation using a computational modelthat incorporates heterogeneous tissue properties.Neuromodulation. 2019. https://doi.org/10.1111/ner.13031.
61. Wagle Shukla A, Zeilman P, Fernandez H, Bajwa JA,Mehanna R. DBS programming: an evolvingapproach for patients with Parkinson’s disease.Parkinsons Dis. 2017;2017:8492619.
Neurol Ther (2020) 9:25–41 41