Covalent bonds
-
Upload
gary-johnston -
Category
Education
-
view
121 -
download
4
description
Transcript of Covalent bonds

Covalent (Molecular) Bonds Grade 7 ChemistryMr. JohnstonSaigon South International School

Guiding Questions
1. How are atoms held together in a covalent (molecular) bond?
2. What are the properties of molecular (covalent) bond?
3. How do bonded atoms become partially charged?

Key Vocabulary
Covalent BondsMoleculeDouble BondTriple BondMolecular CompoundNon-Polar BondPolar Bond

Comparing Ionic and Covalent Bonds
Additional Readings on Covalent Bonds

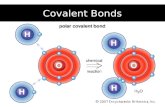
COVALENT BONDbond
formed by the sharing of electrons between 2 non-metals



Part 1: How are atoms held together in a covalent bond?

Covalent bonds- Two atoms share one or more pairs of outer-shell electrons.
Oxygen Atom Oxygen Atom
Oxygen Molecule (O
2)


What are the properties of of Molecular compounds?
Unlike ionic compounds, molecular (covalent) compounds do not usually conduct electricity, when melted or dissolved in water. Also, compared to ionic compounds, molecular compounds generally have lower melting points and boiling points”

when electrons are shared equally
NONPOLAR COVALENT BONDS
H2 or Cl2

when electrons are shared but shared
unequally
POLAR COVALENT BONDS
H2O

Way to remember a polar bond...
The stronger, more stable atom pulls harder!