Corrosion 2
description
Transcript of Corrosion 2

CAUSE AND FORM OF CORROSION
1- INTRODUCTION
2- Corrosion Forms
The term "forms" of corrosion is generally well known from one of the most enduring books on corrosion engineering [1]. The different forms of corrosion represent corrosion phenomena categorized according to their appearance. Dillon [2] considered Fontana's basic forms of corrosion and divided them into three groups, based on their ease of identification. The three categories used were:
Group 1 - readily identifiable by ordinary visual examination Uniform corrosion
Pitting
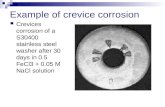
Crevice corrosion
Galvanic corrosion
Group 2 - may require supplementary means of examination Erosion corrosion
Cavitation
Fretting corrosion
Intergranular corrosion
Group 3 - verification is usually required by microscopy (optical, electron microscopy etc.)
Exfoliation
Dealloying (selective leaching)
Stress Corrosion Cracking
Corrosion fatigue
However, the corrosion of metals and alloys almost never follows a single law nor is governed by a single mode. Even the simplest situations can lead to the corrosion of a material by a combination of modes driven by intertwined mechanisms.
34 من 1 صفحة

Group 1 - readily identifiable by ordinary visual examination1- Uniform Corrosion
Uniform corrosion is characterized by corrosive attack proceeding evenly over the entire surface area, or a large fraction of the total area. General thinning takes place until failure. On the basis of tonnage wasted, this is the most important form of corrosion. However, uniform corrosion is relatively easily measured and predicted, making disastrous failures relatively rare. In many cases, it is objectionable only from an appearance standpoint. In other cases uniform corrosion adds color and appeal to to a surface. Two classics in this respect are the patina created by naturally tarnishing copper roofs and the rust hues produced on weathering steels.
Artistic rust formed on weathering steelThe breakdown of protective coating systems on structures often leads to this form of corrosion. Dulling of a bright or polished surface, etching by acid cleaners, or oxidation (discoloration) of steel are examples of surface corrosion.Corrosion resistant alloys and stainless steels can become tarnished or oxidized in corrosive environments. Surface corrosion can indicate a breakdown in the protective coating system, however, and should be examined closely for more advanced attack. If surface corrosion is permitted to continue, the surface may become rough and surface corrosion can lead to more serious types of corrosion. An example of uniform corrosion damage on a rocket assisted artillery projectile is shown here.
2- Pitting corrosion
Pitting corrosion is a localized form of corrosion by which cavities or "holes" are produced in the material. Pitting is considered to be more dangerous than
uniform corrosion damage because it is more difficult to detect, predict and design against. Corrosion products often cover the pits.
A small, narrow pit with minimal overall metal loss can lead to the failure of an entire engineering system. Pitting corrosion, which, for example, is almost a common denominator of all types of localized corrosion attack, may assume different shapes. Apart from the localized loss of thickness, corrosion pits can also be harmful by acting as stress risers. Fatigue and stress corrosion cracking may initiate at the base of corrosion pits.
34 من 2 صفحة

The microstructure of metals and alloys is made up of grains, separated by grain boundaries. Intergranular corrosion is localized attack along the grain boundaries, or immediately adjacent to grain boundaries, while the bulk of the grains remain cooling cycle. Thereby this zone is left prone to sensitization if the alloy is subsequently reheated in a temperature range where grain boundary chromium carbides are formed.Reheating a welded component for stress relieving is a common cause of this problem. In the absence of the reheating step, the alloy would not be prone to intergranular attack. Exfoliation corrosion is a further form of intergranular corrosion associated with high strength aluminum alloys. Alloys that have been extruded or otherwise worked heavily, with a microstructure of elongated, flattened grains, are particularly prone to this damage.Corrosion products building up along these grain boundaries exert pressure between the grains and the end result is a lifting or leafing effect. The damage often initiates at end grains encountered in machined edges, holes or grooves and can subsequently progress through an entire section.
Some definitions
Pitting: corrosion of a metal surface, confined to a point or small area, that takes the form of cavities.
Pitting factor: ratio of the depth of the deepest pit resulting from corrosion divided by the average penetration as calculated from weight loss.
Pitting resistance equivalent number (PREN): an empirical relationship to predict the pitting resistance of austenitic and duplex stainless steels. It is expressed as PREN = Cr + 3.3 (Mo + 0.5 W) + 16N.
3- Crevice Corrosion
Crevice corrosion is a localized form of corrosion usually associated with a stagnant solution on the micro-environmental level. Such stagnant microenvironments tend to occur in crevices (shielded areas) such as those formed under gaskets, washers, insulation material, fastener heads, surface deposits, disbonded coatings, threads, lap joints and clamps.As oxygen diffusion into the crevice is restricted, a differential aeration cell tends to be set up between crevice (microenvironment) and the external surface (bulk environment). The chronology of the aggravating factors leading to a full blown crevice is illustrated here. The cathodic oxygen reduction reaction cannot be sustained in the crevice area, giving it an anodic character in the concentration cell. This anodic imbalance can lead to the creation of highly corrosive micro-environmental conditions in the crevice, conducive to further
34 من 3 صفحة

metal dissolution. This results in the formation of an acidic micro-environment, together with a high chloride ion concentration.All forms of concentration cell corrosion can be very aggressive, and all result from environmental differences at the surface of a metal. Even the most benign atmospheric environments can become extremely aggressive as illustrated in this example of aircraft corrosion. This advanced form of crevice corrosion is called 'pillowing'. The most common form is oxygen differential cell corrosion. This occurs because moisture has a lower oxygen content when it lies in a crevice than when it lies on a surface. The lower oxygen content in the crevice forms an anode at the metal surface. The metal surface in contact with the portion of the moisture film exposed to air forms a cathode.
4- Galvanic Corrosion
Galvanic corrosion (also called dissimilar metal corrosion) refers to corrosion damage induced when two dissimilar materials are coupled in a corrosive electrolyte. The driving force for corrosion is a potential difference between the different materials. The bimetallic driving force was discovered in the late part of the eighteenth century by Luigi Galvani in a series of experiments with the exposed muscles and nerves of a frog that contracted when connected to a bimetallic conductor. The principle was later put into a practical application by Alessandro Volta who built, in 1800, the first electrical pile, or battery: a series of metal disks of two kinds, separated by cardboard disks soaked with acid or salt solutions. This is the basis of all modern wet-cell batteries, and it was a tremendously important scientific discovery, because it was the first method found for the generation of a sustained electrical current.The principle was also engineered into the useful protection of metallic structures by Sir Humphry Davy and Michael Faraday in the early part of the nineteenth century. The sacrificial corrosion of one metal such as zinc, magnesium or aluminum is a widespread method of cathodically protecting metallic structures.In a bimetallic couple, the less noble material will become the anode of this corrosion cell and tend to corrode at an accelerated rate, compared with the uncoupled condition. The more noble material will act as the cathode in the corrosion cell. Galvanic corrosion can be one of the most common forms of corrosion as well as one of the most destructive.
Galvanic corrosion: stainless screw v cadmium plated steel washer
Galvanic corrosion inside horizontal stabilizer
Cadmium plated locknut
The relative nobility of a material can be predicted by measuring its corrosion potential. The well known galvanic series lists the relative nobility of certain materials in sea water. A small anode/cathode area ratio is highly undesirable. In this case, the galvanic current is concentrated onto a small anodic area. Rapid thickness loss of the dissolving anode tends to occur under these conditions. Galvanic corrosion problems should be solved by designing to avoid
34 من 4 صفحة

these problems in the first place. Galvanic corrosion cells can be set up on the macroscopic level or on the microscopic level. On the microstructural level, different phases or other microstructural features can be subject to galvanic currents.
34 من 5 صفحة

Group 2 - may require supplementary means of examination
1- Erosion Corrosion
Materials selection plays an important role in minimizing erosion corrosion damage. Caution is in order when predicting erosion corrosion behavior on the basis of hardness. High hardness in a material does not necessarily guarantee a high degree of resistance to erosion corrosion. Design features are also particularly important.It is generally desirable to reduce the fluid velocity and promote laminar flow; increased pipe diameters are useful in this context. Rough surfaces are generally undesirable. Designs creating turbulence, flow restrictions and obstructions are undesirable. Abrupt changes in flow direction should be avoided. Tank inlet pipes should be directed away from the tank walls, towards the center. Welded and flanged pipe sections should always be carefully aligned. Impingement plates of baffles designed to bear the brunt of the damage should be easily replaceable.
Erosion corrosion of a brass tube in flowing seawater The thickness of vulnerable areas should be increased. Replaceable ferrules, with a tapered end, can be inserted into the inlet side of heat exchanger tubes, to prevent damage to the actual tubes. Several environmental modifications can be implemented to minimize the risk of erosion corrosion. Abrasive particles in fluids can be removed by filtration or settling, while water traps can be used in steam and compressed air systems to decrease the risk of impingement by droplets. De-aeration and corrosion inhibitors are additional measures that can be taken. Cathodic protection and the application of protective coatings may also reduce the rate of attack.
2- Cavitation Corrosion
For minimizing cavitation damage specifically, steps that can be taken include the minimization of hydrodynamic pressure gradients, designing to avoid pressure drops below the vapor pressure of the liquid, the prevention of air ingress, the use of resilient coatings and cathodic protection.
34 من 6 صفحة

3- Fretting corrosion
Fretting corrosion refers to corrosion damage at the asperities of contact surfaces. This damage is induced under load and in the presence of repeated relative surface motion, as induced for example by vibration. Pits or grooves and oxide debris characterize this damage, typically found in machinery, bolted assemblies and ball or roller bearings. Contact surfaces exposed to vibration during transportation are exposed to the risk of fretting corrosion.Damage can occur at the interface of two highly loaded surfaces which are not designed to move against each other. The most common type of fretting is caused by vibration. The protective film on the metal surfaces is removed by the rubbing action and exposes fresh, active metal to the corrosive action of the atmosphere. Notice, in this second example, the corrosion byproduct around the two rivets below the crack indicating the presence of fretting corrosion.
4- Intergranular Corrosion
The microstructure of metals and alloys is made up of grains, separated by grain boundaries. Intergranular corrosion is localized attack along the grain boundaries, or immediately adjacent to grain boundaries, while the bulk of the grains remain largely unaffected.This form of corrosion is usually associated with chemical segregation effects (impurities have a tendency to be enriched at grain boundaries) or specific phases precipitated on the grain boundaries. Such precipitation can produce zones of reduced corrosion resistance in the immediate vicinity. A classic example is the sensitization of stainless steels. Chromium-rich grain boundary precipitates lead to a local depletion of Cr immediately adjacent to these precipitates, leaving these areas vulnerable to corrosive attack in certain electrolytes. Reheating a welded component for stress relieving is a common cause of this problem. In the absence of the reheating step, the alloy would not be prone to intergranular attack.Intergranular corrosion is also often associated with high strength aluminum alloys. Alloys that have been extruded or otherwise worked heavily, with a microstructure of elongated, flattened grains, are particularly prone to this damage.
34 من 7 صفحة

Group 3 - verification is usually required by microscopy (optical, electron microscopy etc.)
1- Exfoliation
Exfoliation corrosion is a particular form of intergranular corrosion associated with high strength aluminum alloys. Alloys that have been extruded or otherwise worked heavily, with a microstructure of elongated, flattened grains, are particularly prone to this damage.Corrosion products building up along these grain boundaries exert pressure between the grains and the end result is a lifting or leafing effect. The damage often initiates at end grains encountered in machined edges, holes or grooves and can subsequently progress through an entire section.
2- Dealloying (selective leaching)
Dealloying orselective leaching refers to the selective removal of one element from an alloy by corrosion processes. A common example is the dezincification of unstabilized brass, whereby a weakened, porous copper structure is produced. The selective removal of zinc can proceed in a uniform manner or on a localized (plug-type) scale. It is difficult to rationalize dezincification in terms of preferential Zn dissolution out of the brass lattice structure. Rather, it is believed that brass dissolves with Zn remaining in solution and Cu replating out of the solution. Graphitization of gray cast iron, whereby a brittle graphite skeleton remains following preferential iron dissolution is a further example of selective leaching.
3- Stress Corrosion Cracking
Stress corrosion cracking (SCC) is the cracking induced from the combined influence of tensile stress and a corrosive medium. The impact of SCC on a material usually falls between dry cracking and the fatigue threshold of that material. The required tensile stresses may be in the form of directly applied stresses or in the form of residual stresses.
34 من 8 صفحة

Cold deformation and forming, welding, heat treatment, machining and grinding can introduce residual stresses. The magnitude and importance of such stresses is often underestimated. The residual stresses set up as a result of welding operations tend to approach the yield strength. The build-up of corrosion products in confined spaces can also generate significant stresses and should not be overlooked. SCC usually occurs in certain specific alloy-environment-stress combinations.Usually, most of the surface remains unattacked, but with fine cracks penetrating into the material. In the microstructure, these cracks can have an intergranular or a transgranular morphology. Macroscopically, SCC fractures have a brittle appearance. SCC is classified as a catastrophic form of corrosion, as the detection of such fine cracks can be very difficult and the damage not easily predicted. Experimental SCC data is notorious for a wide range of scatter. A disastrous failure may occur unexpectedly, with minimal overall material loss.
4- Corrosion fatigue
The combined effect of corrosion and cyclic loading have been shown to produce cracks from corrosion pits and pits have frequently been the source of cracks on aircraft components operating in fleets. Once the pit or group of pits form, the rate of pit growth is dependent mainly on the material, environmental conditions, and type and state of stress. Therefore, to estimate the total corrosion fatigue life of a component, it is of great importance to develop realistic models to establish the component life in these situations and to formulate methods by which designers and operators know likely sources of pitting early in the design and fleet operation. Therefore, to understand this phenomena, some models based on pitting corrosion fatigue (PCF) mechanisms and understanding have been proposed in the past and new ones are emerging.It is important to note that both pitting theory and crack growth theory have been used in pitting corrosion fatigue model development. The first known conceptual (notional) model was presented in 1971 and subsequently the pit growth rate theory proposed by Godard was combined with fatigue crack growth concepts. Following this basic idea, a few models have been proposed.This paper presents some examples of critical pitting corrosion fatigue situations in aircraft, discusses the framework of the PCF models to date, presents some applications of the models, and discusses current work underway. Additionally, some recommendations are made related to future work needed to enhance structural integrity and degradation of aircraft from this failure mechanism.
34 من 9 صفحة

2- Causes of Corrosion2-1- Introduction to Corrosion FailuresThe identification of the factors associated with the forms of corrosion can guide failure investigators. A listing of the most important factors would ensure that engineers with little or no corrosion training are made aware of the complexity and multitude of variables involved. Inexperienced investigators would be reminded of critical variables that may otherwise be overlooked.
2-2- Causes of CorrosionA point of view proposed by professor Staehle is that all engineering materials are reactive chemically and that the strength of materials depends totally upon the extent to which environments influence the reactivity and subsequent degradation of these materials. In order to define the strength of an engineering material it is essential to define the nature of the environments affecting the material over time.
Material factorBulk chemical compositionMicrostructureGrain boundary compositionSurface condition
Environment FactorNominal environment definition
TypeChemistry ConcentrationPhaseConductivity
Local environment definitionVelocitythin layer wettingwetting and drying cyclesheat transfer boilingwear and frettingdeposits
Stress FactorStress definition
mean stressmaximum stressminimum stressconstant load/constant strainstrain rateplane stress/plane strainmodes I, II, IIbiaxialcyclic frequencywave shape
Sources of stressIntentionalResidualcorrosion wedgingthermal cycling
34 من 10 صفحة

Geometry FactorDiscontinuities which intensify stressGeneration of galvanic potentialsChemical crevicesSettling of solidsRestricted geometries leading to concentration cells
Temperature Factor
Time FactorChanges in GB chemistryChanges in microstructureChanges in surface deposits, chemistry or/and heat transfer resistanceDevelopment of surface defects, pitting or/and erosionDevelopment of occluded cells
3-1 Material Factor in Corrosion Failure
The material factor controlling the probability of a corrosion failureThe choice of a material is the result of several compromises. For example, the technical appraisal of an alloy will generally be a compromise between corrosion resistance and some other properties such as strength and weldability. And the final selection will be a compromise between technical competence and economic factors. In specifying a material, the task usually requires three stages:
Listing the requirements
Selecting and evaluating the candidate materials
Choosing the most economical material.
The materials selection process is also influenced by the fact that the materials are either considered for the construction of a new system, or for the modification or repairs in an existing facility. For the construction of new equipment, the selection procedure should begin as soon as possible and before the design is finalized. The optimum design for corrosion resistance will often vary with the material used.In a repair application, there is usually less opportunity for redesign, and the principal decision factors will be centered on delivery time and ease of fabrication in the field. It is also advisable to estimate the remaining life of the
34 من 11 صفحة

equipment so that the repair is not over-designed in terms of the corrosion allowance.
3-2 Environment Factor in Corrosion Failure
The environment factor controlling the probability of a corrosion failureAccording to the basic materials degradation model, the principal features underlying the environment factor consist in a long list of elements describing the chemical makeup of the environment as well as the aggravating contributors that can be part of operating conditions as schematically illustrated.A testing program that would investigate only the nominal condition without consideration for effects such as flow, pH cells, deposits, and other galvanic effects is useless for lifetime prediction. An exact and complete environmental definition must include a description of the microenvironment actually in contact with a metallic surface. However, the circumstances producing this microenvironment are also important. Processes such as wetting and drying, build-up of deposits, and changes in flow patterns greatly influence the chemistry of a surface.
3-4Corrosion FrameworkHumans are used to working with imprecise information. They naturally accept vague use of language, making continuous interpretations of the information they receive based upon context. This section introduces a generic framework linking mechanistic principles leading to corrosion damage with the observable signs of a corrosion attack.Sixteen recognized corrosion experts accepted to complete an opinion poll listing the main sub-factors and the common forms of corrosion. The responses were then analyzed.
34 من 12 صفحة

The most important factors leading to different forms of corrosion failures according to an expert survey
An obvious application of these results would be to help optimize the number of fields required for the development of an efficient knowledge based system.
34 من 13 صفحة

Basic Principles - Kinetics1- IntroductionThermodynamic principles can help explain a corrosion situation in terms of the stability of chemical species and reactions associated with corrosion processes. However, thermodynamic calculations cannot be use to predict corrosion rates. When two metals are put in contact, they can produce a voltage as in a battery or electrochemical cell.The material lower in what has been called the 'galvanic series' will tend to become the anode and corrode while the material higher in the series will tend to support a cathodic reaction. Iron or aluminum, for example, will have a tendency to corrode when connected to graphite or platinum. What the series cannot predict is the rate at which these metals corrode. Electrode kinetic principles have to be used to estimate these rates.
2- The Exchange CurrentThe exchange current, Io, is a fundamental characteristic of electrode behavior that can be defined as the rate of oxidation or reduction at an equilibrium electrode expressed in terms of current. Exchange current, in fact, is a misnomer since there is no net current flow. It is merely a convenient way of representing the rates of oxidation and reduction of a given single electrode at equilibrium, when no loss or gain is experience by the electrode material.For the specific reaction describing the corrosion of iron this would imply that the exchange current is related to current of each direction of a reversible reaction, i.e. an anodic current (Ia) and a cathodic branch (Ic).
anodic reaction ... Fe Fe2+ + 2e-<O:P</O:P
reversed anodic reaction ... Fe Fe2+ + 2e-
Since the net current is zero at equilibrium, it implies that the resultant current of these two reactions is zero.There is no theoretical way of accurately determining the exchange current for any given system. This must be determined experimentally. For the characterization of electrochemical processes it is always preferable to normalize the value of the current by the surface area of the electrode and use the current density often expressed as a small i, i.e. i = I/surface area. The magnitude of exchange current density is a function the following main variables:
Metal composition
Exchange current density depends upon the composition of the metal or alloy and the solution. For redox reactions, the exchange current density would depend on the composition of the metal supporting an equilibrium reaction.
Surface roughness
The exchange current density is usually expressed in terms of projected or geometric surface area and depends upon the surface roughness. The higher exchange current density for the H+/H2 system equilibrium on platinized platinum (10-2 A cm-2) compared to bright platinum (10-3 A cm-2) is a result of larger specific surface area of the former.
Soluble species concentration
The exchange current is also a complex function of the concentration of both the reactants and products involved in the specific reaction
34 من 14 صفحة

described by the exchange current. This function is particularly dependent on the shape of the charge transfer barrier () across the electrochemical interface.
Surface impurities
Impurities adsorbed on the metal surface usually affect its exchange current density. Exchange current density for the H+/H2 system is markedly reduced by the presence of trace impurities like arsenic, sulfur and antimony.
3- Polarization EffectsMetallic surfaces can be polarized by the application of an external voltage or by the spontaneous production of a voltage away from equilibrium. This deviation from equilibrium potential is called polarization. The magnitude of polarization is usually described as an overvoltage () which is a measure of polarization with respect to the equilibrium potential (Eeq) of an electrode.This polarization is said to be either anodic, when the anodic processes on the electrode are accelerated by changing the specimen potential in the positive (noble) direction or cathodic when the cathodic processes are accelerated by moving the potential in the negative (active) direction. There are three distinct types of polarization in any electrochemical cell, the total polarization across an electrochemical cell being the summation of the individual elements:E(applied) - Eeq = total = act +conc +iRwhere
act is the activation overpotential, a complex function describing the charge transfer kinetics of the electrochemical processes. act is predominant at small polarization currents or voltages.conc is the concentration overpotential, a function describing the mass transport limitations associated with electrochemical processes. act is predominant at large polarization currents or voltages.iR is often called the ohmic drop. iR follows Ohm's law and describes the polarization that occurs when a current passes through an electrolyte or through any other interface such as surface film, connectors ...
Galvanic SeriesA galvanic series has been drawn up for metals and alloys in seawater, which shows their relative nobility. The series is based on corrosion potential measurements in seawater only. The relative position of the materials can change in other environments. In general, the further apart the materials are in
34 من 15 صفحة

this series, the higher the risk of galvanic corrosion. However, the series does not provide any information on the rate of galvanic corrosion and thus serves as basic qualitative guide only.
BASIC PRINCIPLES - THERMODYNAMICS1- IntroductionOne can use thermodynamics, e.g. Pourbaix or E-pH diagrams, to evaluate the theoretical activity of a given metal or alloy provided the chemical make-up of the environment is known. But for practical situations, it is important to realize that the environment is a variable that can change with time and conditions. It is also important to realize that the environment that actually affects a metal corresponds to the micro-environmental conditions this metal really 'sees', i.e. the local environment at the surface of the metal.It is indeed the reactivity of this local environment that will determine the real corrosion damage. Thus, an experiment that would investigate only the nominal environmental condition without consideration for local effects such as flow, pH cells, deposits, and galvanic effects is useless for lifetime prediction2- Free Energy and Electrochemical ReactionsElectrical work is the product of charge moved Q times the cell potential (E) through which it is moved. If the work done is that of an electrochemical cell in which the potential difference is E, and the charge is that of one mole of reaction in which n moles of electrons are transferred, then the electrical work -w done by the cell must be nE. In this relationship, the Faraday constant F is necessary to obtain coulombs from moles of electrons.In an electrochemical cell operating reversibly, no current flows and:G = -nFEUnder standard conditions, the standard free energy of the cell reaction G0 is directly related to the standard potential difference across the cell, E0:G0 = -nFE0
Electrode potentials can be combined algebraically to give cell potential. For a galvanic cell, which operates spontaneously, a positive cell voltage will be obtained if the difference is taken in the usual way, as:Ecell = Ecathode - Eanode
The free energy change in a galvanic cell, or in a spontaneous cell reaction, is negative and the cell voltage positive. In electrolytic cells, the reaction is driven in the non-spontaneous direction by an external electrical force. The free energy change in an electrolytic cell, or in a non-spontaneous cell reaction, is therefore positive and the cell voltage negative.Other thermodynamic quantities can be derived from electrochemical
measurements. For example, the entropy change (S) in the cell reaction is given by the temperature dependence of G:henceand finally
34 من 16 صفحة

The equilibrium constant (Keq) for the same reaction can be obtained with the following equation:RT ln Keq = -DG0 = nFE0
3- Standard Electrode PotentialsStandard potential differences are the actual cell potential differences measured in reversible cells under standard conditions. For solid or liquid compounds or elements, standard conditions are the pure compound or element; for gases they are 100 kPa pressure; and for solutes they are the ideal 1 molar (mol/liter) concentration.Tables of standard electrode potentials can be obtained if any one electrode, operated under standard conditions, is designated as the standard electrode or standard reference electrode with which all other electrodes will be compared. This electrode is called the standard hydrogen electrode, abbreviated SHE The potential difference across a reversible cell made up of any electrode and a SHE is called the reversible potential of that electrode, E. If this other electrode is also being operated under standard conditions of pressure and concentration, then the reversible potential difference across the cell is the standard electrode potential E0 of that electrode. In many practical potential measurements, the standard hydrogen electrode cannot be used because hydrogen reacts with other substances in the cell or because other substances in the cell react with the catalytic platinum electrode surface upon which the H+/H2 potential is established.It is often much more convenient to use alternative electrodes whose potentials are precisely known with respect to the SHE Two of the electrodes most commonly used for this purpose are the AgCl/Ag electrode, //AgCl(s),Cl-/Ag(c) at E0 = +0.2224 V vs. SHE, and the saturated calomel electrode (SCE) at 0.241 V vs. SHE The effect of changing the reference electrode is to change the zero of a potential scale while leaving the relative positions of all of the potentials unchanged.Equilibrium reaction of the main reference electrodes used in corrosion, at 25oC
Name Equilibrium reactionHydrogen 2 H+ + 2 e- = H2
Silver chloride AgCl + e- = Ag + Cl-
Calomel Hg2Cl2 + 2 e- = 2 Hg + 2 Cl-
Mercurous sulfate Hg2SO4 + 2 e- = 2 Hg + SO42-
Mercuric oxide HgO + 2 e- + 2 H+ = Hg + H2OCopper sulfate Cu2+ + 2 e- = Cu (sulfate solution)<O:PThe potential corresponding to these half-reactions can be calculated from basic thermodynamic data by following the steps described here
4- Stability of IronBuilding a Pourbaix or E-pH diagram to represent the stability of a metal or an alloy in a given environment is not an insurmountable task. However it could take a few hours of your precious time to produce what is commonly a universally accepted tool to discuss the expected behavior of metals, many Thanks to the well known Belgium scientist that gave his name to these diagrams. The process of building these diagrams should always follow the following steps:
34 من 17 صفحة

1. Study background reference material on the metal/environment of choice. For the Iron-water system we found four acceptable references.
2. Decide on the species that will be considered. For the Iron-water system the data representing the species considered is abundantly available.
3. Decide on the target state of the species considered. For many metals and alloys there are different levels of hydration in the scale of stability. The Iron-water system is typically described in two states of hydration, i.e. wet and dry. The addition of extraneous soluble specious such the commonly present chloride and sulfate ions can greatly complicate the thermodynamic picture.
4. Write down the equations interrelating the chemical species corresponding to the state chosen. The twenty three equations representing the wet state of iron species in pure water are shown here.
5. Well, now that the easy part is done, one has to go over a few sleepless nights to come to the diagram presented here.
Fortunately a few software systems are available to compute E-pH diagrams. KTS Thermo, for example, can provide basic diagrams for the most commonly used metallic elements in water. KTS Thermo provides a simple interface to choose the system and species to be considered and the temperature of interest.
34 من 18 صفحة

Corrosion of Historical Landmarks1- IntroductionHistorical Landmarks are sites, buildings, features, or events that are of significance to a Society and have anthropological, cultural, military, political, architectural, economic, scientific or technical, religious, experimental, or other value. The quality of life of a Society can be correlated to its efforts in promoting the appreciation, preservation and appropriate use of historic structures and landscapes. The destruction or degradation of these landmarks indicates a shift in this quality of life. Such landmarks can have nationwide significance or belong to a very specific segment of a Nation, a State, a City, or any community. Corrosion is not necessarily the only factor at play as is shown here.
2- The Normandy Bridge The Normandy Bridge has been inaugurated on January 20 1995. Built on the river Seine, near Le Havre (France), the construction period has spread from 1988 to 1994. At the inauguration date, it was the world record of the cable-stayed bridges with a 856-m span and a total length of 2141-m. The two towers are the world highest bridge towers made of prestressed concrete and weigh 20,000-tons each. The deck is at 52-m over the highest water.The wire cables that support the span are critical structural elements, and protecting them against corrosion is especially important. Each cable (the bridge has 184 in all) consists of a bundle of wires with seven 15-mm strands each. These strands are hot-galvanized before being formed into wires, and the wires are then clad in a high-density polyethylene sheath before they leave the factory. A petroleum-based wax is used to fill the spaces between the strands, as well as between the wires and the sheath. The same protection is provided for the secondary cables that connect the primary cables to each other.Many other parts of the Normandy Bridge have been protected by hot galvanization, sometimes supplemented with a coat of paint. The following requirements were specified:
For parts to be galvanized but not painted: 80 micron coating
For parts to be both galvanized and painted: same specification as above, plus a 200 micron coating of powder paint
Thus not all of the galvanized steel used in the Normandy Bridge is visible. Some of it is covered with other materials, such as the polyethylene sheaths on the cables, or paint. Other galvanized components are hidden beneath the roadway they support. In all cases, however, the galvanized coating performs a direct, essential, protective role, by providing a physical barrier that prevents any oxidation of the steel, because of the electrochemical properties of the zinc.Galvanized steel is used in a tremendous variety of structures on the bridge: guardrails, walkways, ladders, platforms, road supports, cable paths, storm water drains, pylon access doors, crash barriers, light standards, and tubes to protect the bridge cables from vandalism, all have been galvanized and painted.
3- The Eiffel Tower The Eiffel Tower is built of cast iron, a material that will last virtually forever if it is painted regularly. Since it was built, the tower has been painted once every seven years. The application of an anticorrosion treatment lasts a good year, so that the tower can stay open and continue to greet visitors.
34 من 19 صفحة

This legendary structure comprises 220 000 m2 of surfaces that have to be maintained and repainted (7 300 tonnes of structural metal, and 18 000 metal parts held together by 2 500 000 rivets). Some of these surfaces are very difficult to reach. The budget for the job totals 20 million francs.The 17th time the Eiffel Tower was to be repainted, there were three new requirements:
The monument had to be cleaned completely, with better preparation of the corroded areas before they were paintedThe work had to be inspected
Greater safety precautions had to be taken on the site
The 25 painters who work on the Eiffel Tower still use the traditional methods from the time when Gustave Eiffel designed it. The paint must be applied only manually, with brushes; rollers and paint guns are not allowed.Special paint systems usedThe paints applied to the Eiffel Tower are formulated for this specific purpose. For this 17th paint job, a paint using new, lead-free pigments was developed, tested on 200 m2 of surface, and subjected to reactivity tests by the SNTE (Société nationale de la Tour Eiffel). To emphasize the shape of the tower as seen from the ground, it was painted in three shades of brown specified by the SNTE's architect -the lightest shade at the top, and the darkest at the bottom.Preparation of corroded areas and complete cleaning of towerAll corroded areas were stripped, sanded, and ground, then received two coats of an oleoglycerophthalic primer containing lead silicate-chromate pigment, which affords excellent resistance to corrosion while maintaining flexibility for the paints applied on top of it. (The areas that were determined not to be corroded were cleaned with high-pressure steam.)The general finishing coat was applied with an oleoglycerophthalic paint containing basic lead silicate-chromate pigment. The colors were Eiffel Tower brown numbers 1, 2, and 3 (one for each level of the tower). This paint has the following properties: highly aesthetic appearance; perfect compatibility with the existing, older paints; excellent corrosion resistance; and complete suitability for the Eiffel Tower.In total, 60 tonnes of paint (including 10 tonnes of primer) were applied in the course of this job. It was estimated that about 15 tonnes of paint had eroded since the last time the tower was painted.
4- The Statue of LibertyThe Statue of Liberty was first assembled in Paris in 1884, then disassembled and reassembled in the United States at what is now called Liberty Island. Beginning in July 1981, a series of inspections was conducted by the National Park Service and by an International team of engineers and architects. Over a dozen problem areas needing attention were identified:
Rust stains on the exterior copper skin
Severe corrosion of some small areas of the copper skin
Deterioration of the torch
Degradation in the crown area and spikes
Structural concerns in the shoulder of the torch arm
Corrosion of the iron armature
Paint peeling on the interior copper skin and support structure
34 من 20 صفحة

The problem that most necessitated the restoration of the Statue of Liberty was galvanic corrosion of the iron armature in contact with the copper skin. Galvanic corrosion occurs when dissimilar metals are in electrical contact in the same electrolyte. The difference in electrochemical potential between the dissimilar metals (iron and copper) is the driving force for electrolysis, whereby the armature, forming horizontal anodes at an accelerated rate. The iron armature, forming horizontal and vertical ribs against the copper skin, and the attachment mechanism, whereby copper saddles (which are flush riveted to the copper skin) surround the iron armature, provided a configuration conducive to galvanic corrosion. Since all of the iron armature was interconnected, only one location of physical contact to the copper skin was necessary to provide electrical continuity to the copper throughout the Statue. The designers of the Statue had tried to prevent this galvanic coupling by insulating the two materials with asbestos cloths soaked in shellac. This was only a temporary barrier that became porous with age and provided electrolytic continuity by wicking and capillary action
5- Restoration of Christ the RedeemerMajor restoration work was done in the winter of 2000 on the 38 meters tall statue of Christ the Redeemer that overlooks Rio de Janeiro. The statue has been deteriorating over the years because of climatic effects. The project is the initiative of the Municipality and the Archdiocese of Rio. The project was funded by the Brazilian Environmental Institute, the newspaper "O Globo," and Banco Real.This statue, often called the greatest architectural monument of its time, was restored through a project that includes protection against corrosion, cleaning of the statue, new lighting and a mechanical access system. Since its completion in 1931, the monument has been exposed to strong winds, extreme humidity, and abrupt temperature changes causing severe structural wear.An impressed current cathodic protection (ICCP) system was installed to control further corrosion of the steel reinforced concrete that forms the statue. This ICCP system forces a small direct current from the titanium anode mesh, with a design life of more than 75 years and embedded in the mortar, to the reinforcing steel in the mantel. This is not the first time the statue has undergone restoration. However, the most advanced techniques have been used in this project.
34 من 21 صفحة

PORTRAIT OF Corrosion Engineers
Attributes of a Corrosion Engineers
1- Knowledge of corrosion: IT SEEMS OBVIOUS that before anyone can undertake anything but trial and error or empirical methods of solving corrosion problems, he must have basic information as to the nature of corrosion and the mechanics of corrosion processes. With this knowledge he can make a faster and more accurate diagnosis or analysis of any problem that arises and will be in a much better position to reason from one experience to another. It will also permit him to appraise the information presented to him, plan research to uncover new information, and interpret and apply results of investigations when they have been completed.
2- Knowledge of corrosion resistant characteristics of materialsNo one can expect to choose the proper material to overcome a corrosion problem unless he knows what materials are available and something about their corrosion resistant qualities-advantages and limitations. He does not need to keep all of this in his head, but he should know where to find information when he needs it. This may be located in books or other publications in his library or files or he may know where the information is most likely to be secured elsewhere by telephone, telegram, mail, or direct consultation.
3- Knowledge of corrosive characteristics of chemicalsIn dealing with a corrosion problem, the nature of the corrosive medium is obviously as important as the properties of the material itself. It is therefore necessary to know a good deal about the corrosive characteristics of the chemical or chemicals involved and how these are affected by such factors as concentration, temperature, velocity, aeration, or the presence of oxidizing or reducing substances or special contaminants. This will obviously influence the choice of material or indicate what changes in important environmental factors may be expected to have a favorable effect in reducing corrosion of existing equipment.
4- Information on physical and mechanical properties of materialsCorrosion resistance alone is not sufficient to qualify a material for a particular service. It also must be strong enough to sustain the mechanical stresses that may be encountered. Thermal conductivity may be important where high rates of heat transfer are required, or the thermal expansion should be such as to permit association with some other material without intolerable distortion or the development of excessive thermal stresses.
5- Information on availability and costRegardless of how attractive a material may be from any other point of view, it is of no use for a particular purpose if it cannot be secured in the required form. Filter cloth cannot be woven from an alloy available only as castings. Several materials may possess the corrosion resistant and mechanical properties required for a job, but many of them may be too expensive to be considered.
34 من 22 صفحة

For example, silver might be somewhat better than nickel for tubes in an evaporator to concentrate caustic soda to 50%, but it would not be enough to justify the extra cost involved; steel might be a better choice economically overall for handling dilute caustic under less stringent conditions. Steel is the economically practical material for tank cars for shipping concentrated sulfuric acid, although there are many strong complex alloys that are corroded by sulfuric acid much less than steel under the conditions that may be encountered in tank cars.
6- Information on fabrication techniquesA corrosion engineer must not only be familiar with all of the common practices in the working and fabrication of metals, but also know which materials are amenable to fabrication by the procedure that is to be used. It is useless to choose a metal that cannot be welded for a vessel that cannot be fabricated in any other way. It is equallY as useless to specify an alloy that is difficult to machine for a complicated part that must be finished to close tolerances and be capable of being turned out in quantity on automatic machines at a reasonable cost.
7- Knowledge of special requirement of what is being producedIt is often necessary to choose materials in such a way as to avoid any chance that significant amounts of particular metal compounds may be picked up by the product that would impair important properties such as clarity, color, flavor, strength, or keeping quality, or otherwise restrict the opportunity for its use and sale in some important market.
8- Proficiency in planning, executing, and interpreting test programsExisting information must frequently be supplemented by developing new data needed to solve a particular corrosion problem. It is necessary to be familiar with the several types of corrosion tests that may be made and the advantages and disadvantages of each so as to choose the best approach. The techniques of carrying out the required tests should be understood so that the tests may be conducted properly. The limitations of the tests must be appreciated so that the results of tests may be appraised properly and applied intelligently.
9- Ability to get along with othersThe work of a corrosion engineer will bring him into frequent contact with responsible people in many other branches of his organization:
He should work closely with the engineering staff in working out new designs or modifying existing ones to reduce the opportunity for corrosion.He should remain in close contact with the maintenance engineers so that the details of difficulties and their probable causes may be ascertained; suggestions may than be made as to the best way to cope with them in making repairs of avoid them altogether through preventative maintenance.He should work with the production department to be aware of their particular requirements and needs for improvement with respect either to more dependable operation of equipment or better quality of product as these may be affected by corrosion.He will require the help of (and can be helpful to) the accounting department in establishing the actual cost of corrosion in each case and the savings that may be expected by reducing losses from this source.
34 من 23 صفحة

He must work closely with the purchasing department in guiding the choice of materials for particular purposes in working out specifications for materials, equipment, and fabrication procedures and in arranging for such inspection as required. He should assist the sales department by helping discover any deficiencies of the product that might be corrected by a better corrosion control and by demonstrating the sales value of the improvements that result from his activities.He must keep management informed of his need and his accomplishments so that he may be provided with the support he will require to be effective.
10- Common senseNo comment is required beyond the reminder that all of the other attributes will be greatly increased in value if they are always tempered with common sense.
34 من 24 صفحة

CORROSION ECONOMY
1 Introduction to Corrosion EconomicsAny engineering project undertaken by a profit motivated organization has the underlying aim of enhancing the wealth of its owners (shareholders). Management in industry ultimately bases its decisions on this principle, including those related to corrosion control. The selection of optimal projects from the viewpoint of owners wealth lies in the financial domain of capital budgeting techniques. These techniques determine how capital should be invested in the long term. Five key motives can be identified for making capital investments (expenditures):
Expansion for increasing the scope and output of operations
Replacement for obsolete or run-down assets
Renewal for life-extension of assets, as an alternative to replacement
Investment in non-tangible assets such as advertising, research, information, management consulting etc.
The formal steps in the capital budgeting process in sequential order are:1. proposal generation, 2. review and analysis of the proposals, 3. decision making, 4. implementation, 5. monitoring of results, to compare the actual project outcome with the
predictions. This module will focus on the second step, namely how to evaluate the economic viability of corrosion control investments.
2- Net Present ValueThe net present value is a summation of the present value of all cash inflows and outflows minus the initial project cost (C0). To include the effects of taxation (essentially a business expense), all actual cash flows for tax-paying organizations are reduced by the formula given by the amount after taxes = C·(1 -Tx).Depreciation allowances are an excellent example of where such tax savings are possible, they are treated similarly to income. All expenses allowed to be charged against income for tax purposes, but not representing actual cash flow, are modified by the following formula: Cash flow = non-cash expense charge (Tx)The PV of the tax savings cash flow from a depreciation expense series (DES) of an original cost is given by: PVDES = C0(Q, i, NQ)·Tx
where Q is the present value factor for a cash flow stream and
NQ is the time span of the depreciation expense stream
Combining these definitions, one can obtain the fundamental NPV equation that includes the tax effects:
3-Cash Flows and Capital Budgeting TechniquesEvery corrosion engineering project will have a certain cash flow pattern over time. Usually, there is an initial outflow of cash, when a new asset fitted with a certain corrosion control system is acquired. Subsequently there are inflows of cash, resulting from
34 من 25 صفحة

operations and further cash outflows required for maintenance, corrosion control upgrades, running costs etc. In capital budgeting techniques the different cash flows involved in the project are identified, estimated and analyzed, with a view to maximizing owners wealth.Such cash flows can be complex, if all the financial implications of project options are investigated in detail. Invariably in corrosion economics calculations a compromise has to be made between two opposing needs, i.e. the need for precision and the need for simplicity. For example, the present costs or investment in two alternative anti-corrosion methods may be known with a high degree of certainty. However, the service lives or future maintenance costs or operating costs may be estimates with only a limited degree of certainty. The need for stringent risk assessment required of many modern engineering systems may also add to the complexity of estimating useful life and cost estimates.When considering the above cash inflows and outflows over time, the time value of money has to be considered. This concept implies that money has a value that varies depending on when it is received or disbursed. Readers will have gained first-hand knowledge of this principle from any loans they have taken out with financial institutions. A loan received "now" has to be repaid with interest charges in the future. The following is a generalized formula between the present value and future value of cash flows:
which states that present value (PV) of a future cash flow (Fn) after (n) time periods equals the future amount (Cn) discounted to zero date at some interest rate (i). The value of "n" is usually specified in years and "i" as the annual interest rate. Several capital budgeting techniques exist that are based on the time value of money.
4- Examples of corrosion economicsExample 1
A new heat exchanger is required in conjunction with a rearrangement of existing facilities. Because of corrosion, the expected life of a 90-10 copper-nickel heat exchanger is 7 years. The installed cost is $9,700. An alternative to this heat exchanger is a unit fabricated of 70-30 Cu-Ni, with an installed cost of $16,500 and an estimated life of 12 years to be written of in 10 years. An expected interest rate over the next ten years, is 8%, with a tax rate of 44% and the depreciation method is straight-line. Determine which unit would be more economical based on annual costs.
Solution
Materials selection for a heat exchanger
90/10 70/30P $9,700 $16,500{t(P-S)/n}(P/A, I%, n) $3,174 $4,872-(1-t)(X)(P/A, i%, n) 0 0S(P/F, i%, n) 0 0PW -$6,526 -$11,628A -$1,253 -$1,733
The 90/10 copper-nickel alloy is thus a more cost economical solution
34 من 26 صفحة

Example 2
Because of its lower corrosion resistance you know that the 90-10 Cu-Ni heat exchanger in the first problem will require the use of an extra dose of oxygen scavenger and you know that this will cost you a minimum of $1,400 per year. Determine if the choice established in the first problem would be modified.
Solution
Example 3
A paint system originally cost $5.12 per square meter to apply and has totally failed after 4 years. Assume an interest rate of 8%; a tax rate of 44% and straight line depreciation to answer the following questions:
1. If the paint system is renewed twice at the same cost for a total life of 12 years, what is the annual cost, assuming the first application is capitalized and those in the 4th and 8th considered to be expenses ?
2. Total maintenance could be avoided by biennial touchup (wire brush, spot primer, and topcoat). What is the most that can be spent on this preventative maintenance ?
Solution
Example 4
A plant storage tank requires either a) a replacement to last 4 years with no maintenance, at a cost of $7,200, or b) a coating system on the existing tank to achieve the same life. How much can be spent now for coating installation if annual coating maintenance costs are 10% of the installation costs. Assume an interest rate of 8%; a tax rate of 44% and straight line depreciation.
Solution
5- Straight Line DepreciationVerink has developed a generalized equation that is particularly adapted to corrosion engineering problems. This equation takes into account the influence of taxes, straight-line depreciation, operating expenses, and salvage value in the calculation of present worth and annual cost. Using this equation, a problem can be solved merely by entering data into the equation with the assistance of compound interest data.
where: A represents the annual end-of-period cash flow
34 من 27 صفحة

F represents a future sum of money
i% represents the interest rate
n is the number of years
PW is the present worth referred also as Net Present Value (NPV)
P is the cost of the system at time 0
S represents salvage value
t is the tax rate expressed as a decimal
X represents the operating expenses
First Term [-P]: This term represents the initial project expense, at time zero. As an expense, it is assigned a negative value. There is no need to translate this value to a future value in time, as the PW approach discounts all money values to the present (time zero).Second Term [{t(P-S)/n}(P/A, i%, n)]: the second term in this equation describes the depreciation of a system. The portion enclosed in braces expresses the annual amount of tax credit permitted by this method of straight-line depreciation. The portion in parentheses translates these as equal amounts back to time zero by converting them to present worth.Third Term [-(1-t)(X)(P/A, i%, n)]: the third term in the generalized equation consists of two terms. One is (X)(P/A, i%, n) that represents the cost of items properly chargeable as expenses, such as the cost of maintenance, insurance, and the cost of inhibitors. Because this term involves expenditure of money, it also comes with a negative sign. The second part, t(X)(P/A, i%, n), accounts for the tax credit associated with this business expense and because it represents a saving it is associated with a positive sign.Fourth Term [S(P/F, i%, n)]: the fourth term translates the future value of salvage to present value. This is a one time event rather than a uniform series and therefore it involves the single payment present worth factors. Many corrosion measures, such as coatings and other repetitive maintenance measures, have no salvage value, in which cases this term is zero.
Present Worth (PW) can be converted to equivalent annual cost (A) by using the following formula:
A = (PW)(A/P, i%, n)
34 من 28 صفحة

Atmospheric Corrosion
1- Introduction Components exposed to the elements will inevitably experience damage due to atmospheric corrosion. The severity of the corrosion and the rate at which corrosion will take place are dependant primarily upon the properties of the surface formed electrolytes, which in turn are dependant upon factors such as the humidity and pollution levels in the atmosphere.Assessing the corrosivity of a particular atmosphere can be relatively simple. Two methods have been developed to deal with this problem:
The first method involves the exposure of metallic coupons to the environment and classifying the resultant corrosion, e.g. the coupon based method (CLIMAT) based on the wire on bolt ASTM standard..The second method involves measuring several atmospheric parameters and classifying the atmosphere according to standardized measurements, e.g. ISO specification 9223.
This module contains some examples describing CLIMAT results and some weather data estimations according to the ISO methodology for locations typical of marine and rural environments.
2- MechanismAtmospheric corrosion is an electrochemical process, requiring the presence of an electrolyte. Thin film "invisible" electrolytes tend to form on metallic surfaces under atmospheric corrosion conditions, when a certain critical humidity level is reached. For iron, this level is around 60%, in unpolluted atmospheres. The critical humidity level is not a constant - it depends on the corroding material, the hygroscopic nature of corrosion products and surface deposits and the presence of atmospheric pollutants.
In the presence of thin film electrolytes, atmospheric corrosion proceeds by balancing anodic and cathodic reactions. The anodic oxidation reaction involves the dissolution of the metal in the electrolyte, while the cathodic reaction is often assumed to be the oxygen reduction reaction. Oxygen from the atmosphere is readily supplied to the electrolyte, under thin film corrosion conditions.
34 من 29 صفحة

3- Exposure TestingThere are many factors to consider when selecting a weathering test station to conduct a test program. These can be summarized into two categories:
Location: An ideal test site should be located in a clean pollution free area, if pollution is not deemed to be a parameter, within the geo-climatic region to be used. This is important for the prevention of unnatural effects on the specimens. Within the local area chosen, there must be no isolated sources of pollution or deleterious atmospheric contamination.Maintenance: The exposure maintenance program followed by the test site will also play a major role in determining the accuracy of testing. It is important for the specimens on the test racks to be correctly maintained. This involves ensuring the correct mounting method, and constant follow-up attention to maintain the quality.
For direct exposure the specimen is mounted on the exposure frame open backed or solid backed, and subject to all atmospheric effects. This type can be used at a number of exposure angles. The standard angles used are 45, 5, and 90 degrees, these angles being referenced from a horizontal angle of 0 degree. The angle chosen should be one that matches as closely as possible the position of the end use of the material. The racks should be cleaned on a regular basis to remove mildew and algae if these contaminate producers are present on the test site.To see a picture of the rural marine atmosphere exposure rack at Dockyard Laboratory (Pacific), 'click here'.
4- MicroenvironmentsThe corrosivity due to atmospheric conditions can be greatly affected by local conditions such as wind speed and direction, dust, debris, humidity, condensation and electrolytic species. These local conditions can change greatly within a few meters, depending on patterns in air turbulence. One extreme example of local variations due to the corrosivity of a seawater environment is the top deck of an aircraft carrier, where waves and seawater mist are abundant. An example of local seasonal differences often exist in countries where deicing salts are used in the winter months. The following results have been obtained by exposing CLIMAT coupons in the vicinity of two important highways in Eastern Ontario.
Highway 2 (trans Canada Highway)
Highway 401
The transport and deposition of aerosols are subject to mass transport laws such as convection and turbulent diffusion. Based on these principles, the pattern of aerosol surface deposition can be modeled near obstacles. In such a modeling experiment, it was found that aerosol deposition rates onto and near an obstacle have a very localized structure due to variations in wind speed, wind speed gradients and turbulence.
34 من 30 صفحة

Atmospheric Corrosion of Metals
Corrosion of metals used in buildings was discussed in a general way in CBD 20. Metals for exterior applications should be considered as individual cases of atmospheric corrosion, for such effects may be modified by the particular elements of a design. This Digest will offer guidelines and basic data for estimating atmospheric corrosion in given situations and is designed to assist in the selection and application of materials. The Division of Building Research has published the results of an atmospheric corrosion test program initiated by the Associate Committee on Corrosion Research and Prevention.1,2 The first report covers the major architectural metals and alloys of aluminum, magnesium, stainless steel, low carbon and low alloy steels, copper and zinc. The second gives the results for metal-coated steel, including cadmium and zinc electroplated, hot-dip galvanized, zinc and aluminum metallized sheet samples and metal-coated and sealed steel with organic coatings. The results are applicable in the first instance to conditions of exposure simulating those at eight corrosion exposure sites in Canada. Similar results for a large number of metals and alloys have been collected and published by committees of the American Society for Testing and Materials for exposure sites in the USA. Fortunately, the conditions of exposure at the eight Canadian sites have been reasonably well established by measurements of temperature, time-of-wetness (or RH conditions), and extent of pollution by SO2. It should therefore be possible to apply the results of these studies to designs where the conditions of exposure will be similar, although there remains the problem of how to predict exactly what these conditions will be. Time-of-Wetness In normal atmospheres containing pollutants, metals begin to corrode at an accelerated rate when the relative humidity of the air layer next to the surface exceeds about 75 per cent. This condition occurs when the air is cooled or when the surface itself is cooled, as happens when a surface radiates to a clear sky at night. The decrease in surface temperature can be as much as 5 F deg (2.8 C deg) below ambient. Such a decrease may cause the surface temperature to drop below the dew-point for an ambient relative humidity of about 85 per cent. This condition is affected by design features such as size, orientation, and surface characteristics; for example, one may observe dew formation on the rooftop of a car when there is none on its sides. Time-of-wetness is the most important factor in atmospheric corrosion and can account for many unexplained variations in observed results. It can be measured by means of an instrument developed at DBR/NRC, and corresponds to the film of moisture on a surface that will produce a potential of over 0.2 volt between two electrodes of platinum and zinc. Table I presents the measured values of time-of-wetness for a number of sites and the critical value of RH derived from meteorological records. The duration of the condition of relative humidity above this value corresponds to the measured time-of-wetness. It follows that the average value of time-of-wetness can be determined for any locality for which records of RH have been collected. This has been done for a 10-year period (1957-66) for the 112 meteorological stations in Canada. A plot of a selection of these data is given in Figure 1. They can be grouped in two categories: coastal and inland. Times-of-wetness, as indicated by the duration of a given range in relative humidity, show a spread of about 20 per cent, on the average, between the two types of location.
Figure 1. Duration of relative humidity ranges in the atmosphere. Table I. Percentage Time-of-Wetness For Canadian Exposure Sites As Measured By The Dew Detector Period Halifax Halifax Ottawa Saskatoon Esquimalt
34 من 31 صفحة

ARL*1961-1972
York Redoubt1962-1968 1961-1970 1962-1970 1961-1969
Yearly Average
39.1 44.1 35.3 30.3 42.5
SD** 4.9 3.9 4.9 4.5 4.7Max 48.4 48.9 41.8 39.5 52.8Min 30.7 37.3 29.6 25.4 35.7RH Equiv***1955-1966 89.0 87.0 80.0 83.0 87.0MonthlyAverage(Dec.)
44.4 52.0 51.6 47.5 61.5
SD 11.6 8.5 9.6 18.0 9.5Max 72.3 67.4 71.5 74.8 71.5Min 29.1 43.0 38.5 26.2 43.8MonthlyAverage(June)
29.5 36.2 26.8 21.4 23.2
SD 6.8 7.6 11.7 7.0 7.4Max 47.6 50.4 52.2 29.2 29.5Min 21.6 24.4 13.7 8.6 7.0
*Atlantic Regional Laboratory, DBR/NRC.**Standard deviation.***RH value derived from meteorological records (Figure 1); duration of the condition for which the relative humidity is above this value corresponds to the measured time-of-wetness.
Measurements carried out on a galvanized sheet wall cladding and roof of a storage building indicated that the time-of-wetness for the roof overhang was twice that for the vertical walls. It can be expected that the rate of corrosion of the roof overhang will also be twice the rate for the walls. The time-of-wetness in the vent space of the wall of a three-storey building, behind precast concrete exterior panels, has been found to be similar, on the average, to the time-of-wetness outside. There was a noticeable difference, however, between the values at the top and bottom of the building. The influence of moist air leaking from the building and greater wetting from rain near the top contributes to an increase of about 10 per cent in the time-of-wetness in that zone. This indicates the necessity for better corrosion protection for metal hangars, etc., in the zone near the top of the building than is required near the bottom. It should be apparent that surfaces which trap and pond water or cool by radiation so that water vapour condenses as dew can experience a time-of-wetness two or more times the atmospheric average for the locality. It should also be apparent that they will experience an increase in corrosion rate in the same proportion. Good design practice can avoid much of this difficulty. Pollution Much has been said about pollution in the last few years, but in the context of atmospheric corrosion only sulphur dioxide has been shown to accelerate corrosion in a predictable way. Particulate matter such as sea salts, soot and soil has not been directly correlated with the rate of corrosion, although it has been demonstrated that particulates deposited on a metal surface can be responsible for the initiation of corrosion.
34 من 32 صفحة

Tests of corrosion of architectural metals have been made over a 10-year period at eight exposure sites, and the corresponding levels of sulphur dioxide pollution have been measured and recorded. Since 1970 three levels of government have participated in measuring pollution by SO2 in most large urban areas. As a result, data are available for estimating the SO2 concentration of the environment where major structures are planned. The Federal Department of the Environment, Air Pollution Control Directorate, publishes monthly and annual National Air Pollution Surveillance Reports. Some of the data collected by air pollution agencies are given as parts per million (ppm), parts per 100 million, or µg/m³, when the method involves a continuous sampling of the air to obtain an hourly value. These can be converted to units of MDD (Milligrams SO3/dm²/day) by the following conversions: 1 MDD = 0.028 ppm1 MDD = 80 µg/m³ It should be remembered that whereas pollution varies hourly, daily and seasonally, corrosion is a long-term process and only average, monthly, or yearly values should be used for predicting it. For this reason it is best to obtain yearly values from measurements taken monthly of total sulphation. Such data are now available for some major cities (Figure 2).
Figure 2. Levels of pollution by SO2 in some major cities in Canada. Finally, SO2 levels can be greatly influenced by local pollution whose source can even be the chimney of the building under consideration. Roof flashings, gutters, metal roofs and metal cladding can be greatly influenced by SO2 released close to the top of the building. Any prediction of corrosion rate must take such situations into account. Sea Salts Atmospheric corrosion of metals in an environment defined as marine is greatly accelerated, but this condition exists only where the exposure is subject to direct sea spray. It has been found that the corrosivity of a marine atmosphere is reduced several fold only 1 to 2 km (1/2 to 1 mile) inland. At one exposure site in Kure Beach, N.C., the corrosion rate of steel at the 80-ft site is about 10 times higher than that at the 800-ft site, showing that chloride concentration decays rapidly with distance from the coast. Temperature There is evidence that temperature is a factor in atmospheric corrosion. In Canada, however, most of the densely populated section of the country lies in a narrow belt of nearly constant latitude and the mean annual temperature varies by no more than about 10 F deg (5.5 C deg). In view of this, temperature as a factor in corrosion need be considered only for the Far North. The very low corrosion rate observed for Norman Wells is partly attributable to the low average temperature as well as to low pollution. How To Use Available Information When the question of corrosion of a metal proposed for use in a certain location is to be considered, the over-all or average environmental conditions of time-of-wetness, temperature, and concentration of SO2 and chlorides should first be established. In the second phase of the detailed design, an assessment should be made of the corrosion rate of each metal to be exposed by identifying test data from a site where the conditions of time-of-wetness and pollution are similar. If available data do not correspond because the conditions of exposure are different, then estimates can be made by assuming a linear relation of corrosion and time-of-wetness. For some metals such as Alcan 57S alloy and zinc the linear relation seems to extend to SO2 pollution also, but unfortunately there does not seem to be a simple correlation for most others. Correlations to date are applicable for short-term corrosion, especially for the first month or even year, but not for the long term. In most instances long-term corrosion is greatly affected by corrosion products, but their influence has not yet been determined sufficiently to enable a quantitative prediction. Of all the metals tested only zinc was found to have a
34 من 33 صفحة

constant rate of corrosion, independent of length of exposure, at three of the sites (Ottawa, Montreal, Trail). Thus, its long-term performance is predictable from short-term tests. For other sites its rate of corrosion decreased with time of exposure, so that the 10-year value was about half the one-year value. If the corrosion rates of two metals are known at one location and the rate for one of them is known at another location, it will not be possible to predict the corrosion rate of the second metal from the ratio of the two rates at the original site because this ratio does not remain constant. It must be concluded that the designer has to rely heavily on experience in estimating atmospheric corrosion of metals. This Digest should provide guidance in making judgements; and further information is available in a more detailed publication.3
34 من 34 صفحة