CMO Easter Europe
Transcript of CMO Easter Europe
-
7/31/2019 CMO Easter Europe
1/10
GBIHC041MR / Published OCT 2010
Page 1
Contract Manufacturing in Eastern Europe - InconsistentDemand and Competition from Asia Raises Concerns
GBI Research. This is a licensed product and is not to be photocopied
Contract Manufacturing in Eastern Europe - InconsistentDemand and Competition from Asia Raises Concerns
Reference Code: GBIHC041MR Publication Date: October 2010
GBI Research predicts that the Eastern European contract manufacturing market is poised to growat a h ealthy Compound Annual Growth Rate (CAGR) of XX% to re ach $XX billion by 2016, from$XX bi llion in 2009. The factors driv ing t his growth are the ava ilability of lo w-cost ski lled l abobetter infrastru cture and g overnment polic ies, better Intellectua l Propert y Rights (IPR) protectionand an incr easing uptake of bio logics and ge nerics. However, com petition from lo w-cost AsianCMOs is a m ajor challenge for Eastern European CMOs. The report highlights Poland and CzecRepublic as major CMO d estinations in Eastern Europe and also analyzes key events and trendaffecting CMOs.
Eastern European CMO Market is Poised for Impressive Growth
Contract Manufacturing in Eastern Europe, Eastern Europe CMO Market Size, $bn,2007-2016
2007 2008 2009 2010 2011 2012 2013 2014 2015 2016
Revenue($bn)
CAGR (2012-2016) XX%
CAGR (2007-2012) XX%
Factors driving the growth are increased
generics and biosimilars uptake stimulatedby patent expiries for major blockbusterdrugs, likely regulatory approval forbiosimilars in the US and increase inbiologics manufacturing in Eastern Europe
Source: GBI Research, WHO, FDA, Contract Pharma
The CMO market in the Eastern Europe is expected to do well in the coming few years. The markeis likely to grow at a rate fas ter than t hat for the overall global CMO mar ket. GBI Rese arch find
that the CMO market in Eastern Europe will grow at a CAGR of XX% over the period 2009-2016The factors driving the gr owth are ava ilability of lo w-cost skille d la bor, b etter infrastruc ture angovernment support for CMO market, increase in the uptake of generics, superior IPR protectioand increased uptake of biologics and biosimilars.
Eastern Europeancontract manufacturingmarket is valued at $XXbillion as of 2009. It isestimated to reach $XXbillion by 2016, growing atthe rate of XX% annually.
-
7/31/2019 CMO Easter Europe
2/10
GBIHC041MR / Published OCT 2010
Page 3
Contract Manufacturing in Eastern Europe - InconsistentDemand and Competition from Asia Raises Concerns
GBI Research. This is a licensed product and is not to be photocopied
1 Table of Contents
1 Table of Contents ...................................................................................................................1.1 List of Tables................................................................................................................1.2 List of Figures ..............................................................................................................
2 Introduction.............................................................................................................................2.1
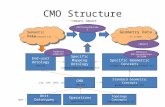
GBI Research Guide.....................................................................................................3 Overview of the CMO Industry.................................................................................................
3.1 Current Status of Pharmaceutical Industry ....................................................................3.2 The Need to Outsource for Pharmaceutical Companies.................................................
3.2.1 Reduction in Physical Assets..................................................................................3.2.2 Restructuring of Product Portfolio...........................................................................3.2.3 Downsizing Manufacturing .....................................................................................3.2.4 Reduction in R&D Spending...................................................................................
3.3 What CMOs are and Reasons for Outsourcing to Them.................................................3.3.1 Different Types of CMOs and the Range of Services Provided by Them................13.3.2 How Does Outsourcing to CMOs Take Place?........................................................3.3.3 Key Outsourcing Models ........................................................................................3.3.4 Key Benefits of Outsourcing...................................................................................
4 Contract Manufacturing in Eastern Europe - Market Characterization.......................................4.1 Market Forecasts .........................................................................................................5 Geographical Landscape.......................................................................................................
5.1 Comparative Analysis of Eastern European Countries ...................................................5.2 Infrastructural Facilities Railways, Roadways, Airways, Power and Overall Logistics
Support ........................................................................................................................
5.3 Labor - Availability of Skilled Labor at Affordable Rates and Labor Flexibility.................25.4 Broad Economic Indicators GDP, Availability of Credit, Taxation Policies ........... ........ 25.5 Ease of Doing Business Overall Government and Regulatory Support .......... ........... .. 2
5.5.1 Key Takeaway.......................................................................................................5.6 Individual Strengths and Weaknesses for Each Country .......... ........... .......... ........... ......
5.6.1 Poland ..................................................................................................................5.6.2 Czech Republic .....................................................................................................5.6.3 Lithuania...............................................................................................................5.6.4 Ukraine .................................................................................................................5.6.5 Hungary................................................................................................................5.6.6 Slovakia................................................................................................................5.6.7 Slovenia................................................................................................................
5.7 Analysis of Other Emerging CMO markets .......... ........... .......... .......... ........... .......... .......5.7.1 Chinese CMO Market............................................................................................5.7.2 Indian CMO Market ...............................................................................................
6 Market Drivers and Restraints .................................................................................................6.1 Drivers .........................................................................................................................
6.1.1 Availability of Low-cost Skilled Manpower ...............................................................6.1.2 Better Infrastructure and Government Policies........................................................6.1.3 Increased Consumptions of Generics.....................................................................6.1.4 Better Intellectual Property Rights Protection ..........................................................6.1.5 Increasing Uptake of Biologics and Entry of Biosimilars into Market ............ ........... 5
6.2 Restraints.....................................................................................................................6.2.1 Competition from Low-cost Asian CMOs.................................................................6.2.2 Capacity Utilization Issues Affecting the Profitability of CMOs ........... ........... ..........6.2.3 Reduced Number of New Drug Approvals...............................................................6.2.4 Complex Cost Structures in Outsourcing................................................................
7 Regulatory Landscape............................................................................................................7.1 EU GMP.......................................................................................................................
7.1.1 EU cGMP Guidelines for API Manufacturing ...........................................................
-
7/31/2019 CMO Easter Europe
3/10
GBIHC041MR / Published OCT 2010
Page 4
Contract Manufacturing in Eastern Europe - InconsistentDemand and Competition from Asia Raises Concerns
GBI Research. This is a licensed product and is not to be photocopied
7.1.2 Key Changes in EU GMP Guidelines .....................................................................7.2 PIC/S ...........................................................................................................................
8 Prominent Players ..................................................................................................................8.1 Polpharma S.A.............................................................................................................
8.1.1 Services Offered....................................................................................................8.2 Hasco-Lek S.A. ............................................................................................................
8.2.1 Services Offered....................................................................................................8.3 Sanitas AB (Sanitas Group) ..........................................................................................8.3.1 Pharmaceutical Company JELFA SA.....................................................................8.3.2 Laboratorium Farmaceutyczne Homeofarm sp. z o.o..............................................
8.4 Lonza Biotec sro ..........................................................................................................8.4.1 Services Offered....................................................................................................
8.5 Grindeks A.S................................................................................................................8.5.1 Services Offered....................................................................................................
8.6 Galilaeus Oy ................................................................................................................8.6.1 Services Offered....................................................................................................
8.7 FSP Galena .................................................................................................................8.7.1 Services Offered....................................................................................................
8.8 Noventis sro.................................................................................................................8.8.1 Services offered.....................................................................................................8.9 Zentiva N.V. .................................................................................................................8.9.1 Services Offered....................................................................................................
8.10 SVUS Pharma a.s........................................................................................................8.10.1 Services offered.....................................................................................................
8.11 Notable CMOs in Eastern Europe..................................................................................8.11.1 Nycomed ..............................................................................................................8.11.2 Polfa Tarchomin S.A..............................................................................................8.11.3 Biogened S.A.........................................................................................................8.11.4 Pliva Lachema a.s. ................................................................................................8.11.5 Aflofarm ................................................................................................................8.11.6 TAPI .....................................................................................................................
9 Major Events and Trends in the CMO Industry.........................................................................9.1 US Healthcare Reforms in 2010 Will Affect CMOs Positively due to Increased Demandfor Medicines in the US.................................................................................................9.2 Upcoming Blockbuster Drugs will Create a Positive Impact on the CMO Industry..........89.3 Increased Focus on Emerging Markets by Major Pharmaceutical Companies will Affect
CMOs Positively............................................................................................................
9.4 Developments Leading to an Anti-counterfeiting Trade Agreement will Affect CMOsPositively......................................................................................................................
9.5 Increased M&A Activity During 2009-10 is Likely to Affect the CMO Business Positively....................................................................................................................................
9.6 Tightening of GMP Guidelines by the FDA and the EU in Near Future will Increase Costsfor the CMOs and will Negatively Affect their Profitability. ..............................................
9.7 Lower Capacity Utilization in the Biologics Manufacturing Industry is Negatively Affectingthe Profitability of the CMOs .........................................................................................
9.8 Drug Price Cuts Across Western Europe will Negatively Affect the CMOs........... .......... 910Appendix ........... .......... .......... ........... .......... ........... .......... .......... ........... .......... ........... .............
10.1 Market Definitions .........................................................................................................10.2 Abbreviations ........... .......... .......... ........... .......... ........... .......... .......... ........... .......... ........10.3 Research Methodology.................................................................................................
10.3.1 Coverage..............................................................................................................10.3.2 Secondary Research .............................................................................................10.3.3 Primary Research..................................................................................................10.3.4 Expert Panel Validation .........................................................................................
10.4 Contact Us ...................................................................................................................
-
7/31/2019 CMO Easter Europe
4/10
GBIHC041MR / Published OCT 2010
Page 5
Contract Manufacturing in Eastern Europe - InconsistentDemand and Competition from Asia Raises Concerns
GBI Research. This is a licensed product and is not to be photocopied
10.5 Disclaimer ....................................................................................................................10.6 Sources........................................................................................................................
1.1 List of Tables
Table 1: Contract Manufacturing in Eastern Europe, Market Characterization, Eastern EuropeCMO Market Size, $bn, 2007-2016...............................................................................
Table 2: Market Drivers, All Employees: Indexes of Hourly Compensation Costs (United States =100).............................................................................................................................
Table 3: Market Drivers, Better Government Policies Supporting Business Operations in EasternEurope ........................................................................................................................
Table 4: Market Drivers, Major Drug Patent Expirations, 2010-2016 ........... .......... .......... ........... .Table 5: PIC/S Members from Eastern Europe...........................................................................Table 6: Promising Biologic Drugs likely to get Approved by FDA in 2010 .......... ........... ........... .. 8
-
7/31/2019 CMO Easter Europe
5/10
GBIHC041MR / Published OCT 2010
Page 6
Contract Manufacturing in Eastern Europe - InconsistentDemand and Competition from Asia Raises Concerns
GBI Research. This is a licensed product and is not to be photocopied
1.2 List of Figures
Figure 1: Cost Optimization Steps in the Pharmaceutical Industry................................................Figure 2: Impact Analysis of Various Cost Optimization Methods .......... ........... .......... ........... .......Figure 3: Global CMO Industry Structure .....................................................................................Figure 4: Process for Selection of CMO and Management of Outsourcing Project.......................1Figure 5: Key Benefits of Outsourcing to CMOs...........................................................................Figure 6: Increasing Share of CMOs in Overall Pharmaceutical Manufacturing............................1Figure 7: Revenue Split of Global CMO Market by Geography.....................................................Figure 8: Break-up of Global CMO Market by Services Provided .................................................2Figure 9: Contract Manufacturing in Eastern Europe, Market Characterization, Eastern Europe
CMO Market Size ($bn), 2007-2016 .............................................................................Figure 10:Parameters Considered for Ranking of Different Eastern European Nations.................2Figure 11:Ranking on Infrastructure of Different Eastern European Nations .................................2Figure 12:Rankings for Attractiveness of Labor of Different Eastern European Nations.......... ...... 2Figure 13:Rankings for Broad Economic Indicators of Different Eastern European Nations..........2Figure 14:Rankings for Ease of Doing Business for Different Eastern European Nations ........... .. 2Figure 15:Ranks for Overall Business Attractiveness ...................................................................Figure 16:Geographical Landscape, Strengths and Weaknesses, Poland ....................................2Figure 17:Geographical Landscape, Strengths and Weaknesses, Czech Republic.......................3Figure 18:Geographical Landscape, Strengths and Weaknesses, Lithuania ........... ........... ........... 3Figure 19:Geographical Landscape, Strengths and Weaknesses, Ukraine .......... .......... ........... .... 3Figure 20:Geographical Landscape, Strengths and Weaknesses, Hungary .......... ........... ........... .. 3Figure 21:Geographical Landscape, Strengths and Weaknesses, Slovakia .......... ........... ........... .. 3Figure 22:Geographical Landscape, Strengths and Weaknesses, Slovenia..................................3Figure 23:Contract Manufacturing in Eastern Europe, Market Driver and Restraints.....................4Figure 24:Market Drivers, Difference in Hourly Wage Costs in Euro Area v/s Eastern Europe......44Figure 25:Market Drivers, Percentage of Tertiary Graduates by Disciplines, Eastern Europe v/s
Developed Nations.......................................................................................................Figure 26:Market Drivers, Increasing Generics Opportunities Due to Patent Expiries for Major
Drugs ..........................................................................................................................Figure 27:Market Drivers, Market Opportunity for CMOs Due to Patent Expiry for Blockbuster
Drugs ..........................................................................................................................Figure 28:Market Drivers, Increased Opportunities for CMOs Due to Generics Manufacturing ..... 5Figure 29:Market Drivers, IPR Protection Eastern European v/s Asian CMO Markets .......... ........ 5Figure 30:Market Drivers, Growth in Global Biologics Market, 2009-2016.....................................5Figure 31:Market Drivers, Analysis of Increasing Opportunities for Biologics Manufacturing for
CMOs..........................................................................................................................Figure 32:Market Drivers, Increased Biologics Manufacturing Opportunities for CMOs.................5Figure 33:Market Restraints, Cost Comparison Between Eastern European and Asian CMOs.....5Figure 34:Market Restraints, Reduction in Drug Approval Rates by FDA Affecting CMOs ............ 5Figure 35:Market Restraints, Opportunities Lost by CMOs Due to Reduced Approval Rates by FDA
...................................................................................................................................Figure 36:Regulatory Landscape, Manufacturing Regulatory Framework in Eastern Europe........6Figure 37:Steps in API Manufacturing Governed by EU GMP Guidelines .....................................6Figure 38:Prominent Players, Polpharma S.A...............................................................................Figure 39:Prominent Players, Hasco-Lek S.A. ..............................................................................Figure 40:Prominent Players, Sanitas Group................................................................................Figure 41:Prominent Players, Lonza Biotec sro ............................................................................Figure 42:Prominent Players, Grindeks A.S..................................................................................Figure 43:Prominent Players, Galilaeus Oy ..................................................................................Figure 44:
Prominent Players. FSP Galena ...................................................................................Figure 45:Prominent Players, Noventis sro..................................................................................
Figure 46:Prominent Players, Zentiva N.V. ..................................................................................Figure 47:Prominent Players, SVUS Pharma a.s..........................................................................Figure 48:Comparison of CMOs...................................................................................................Figure 49:Major Trends and Events and Their Impact on the CMO Industry .......... ........... ........... . 8
-
7/31/2019 CMO Easter Europe
6/10
GBIHC041MR / Published OCT 2010
Page 7
Contract Manufacturing in Eastern Europe - InconsistentDemand and Competition from Asia Raises Concerns
GBI Research. This is a licensed product and is not to be photocopied
2 Introduction
Pharmaceutical contract man ufacturing has witnessed im pressive gro wth over the last decad eContract ma nufacturers acr oss the globe hav e i ncreasingly pr ovided cost-effecti ve drudevelopment and man ufacturing so lutions to ph armaceutical a nd biotech comp anies. T hpharmaceutical companies are dr iven to outsource their manufacturing by benefits su ch as l owcosts, less time to de liver products to mark et and the increased focus o n profitability arising fromoutsourcing.
The pharmac eutical co ntract manufactur ing i ndustry i s larg ely dominated b y players fromdeveloped nations due to their wide service portfolio, better technology and the h igh level of IPRprotection in th ose countries. But in recent years CMOs from emergin g markets such as EasterEurope, China and India have been catching up quickly because of the l ow labor and raw materiacosts, improvi ng serv ice portfolios and increasing l evels of IPR protecti on i n those re gions. T hCMO markets in these re gions are growing at a rate higher than the gl obal CMO marke t, and arexpected to gain a significant share in the overall CMO market in the near future.
This report focuses on the contract manufacturing market in Eastern Europe, the factors affectinthe growth of t his market, prominent C ontract Manufacturing Org anizations (CMOs) o perating ithe regions, and trends and events affecting the i ndustry. The report als o provides a f orecast fothe Eastern European CMO market.
2.1 GBI Research Guide
The report starts with an executive summary of the ke y points regarding the CMO market inEastern Europe.
The Industry Overview chapter gives a bri ef explanation about the ne ed for outsourcing in thpharmaceutical industry, CMOs and the key benefits of outsourcing to CMOs.
The Market Character ization chapter provides i nformation ab out the increasing n umber oCMOs in pha rmaceutical manufactur ing a nd the bre ak-up of glo bal CMO market, bgeography and service. The section also gives the size of Eastern European CMO market andthe forecast for the period 2009-2016.
The Geograp hical Landscape chapter ra nks diffe rent E astern European countri es on seleccriteria for their suitability for CMO business and assesses their strengths and weaknesses iregards to the CMO market. It also provi des a comparison of the CM O market in the r egiowith that from emerging Asian nations.
The Market Driver and Restraints chapter deals with the f actors driving and holding back thgrowth in the CMO market in Eastern Europe.
The Regulat ory L andscape chapter cov ers t he regul atory mech anism exist ent in E asterEurope and key r egulatory guidelines for manufacturing of Active Pharmaceutical Ingredient(API).
The Prominent Players chapt er gives infor mation about key CMOs op erating in the regi ontheir brief profiles and a list of the services provided by them.
The final chapt er, titled Event s/Trends and Their Impact on the CMO Industr y, describes keevents or tre nds taking pl ace gl obally and t heir im pact on the CMO i ndustry es pecially oCMOs in Eastern Europe.
-
7/31/2019 CMO Easter Europe
7/10
GBIHC041MR / Published OCT 2010
Page 44
Contract Manufacturing in Eastern Europe - InconsistentDemand and Competition from Asia Raises Concerns
GBI Research. This is a licensed product and is not to be photocopied
6.1 Drivers
6.1.1 Availability of Low-cost Skilled Manpower
A major driv er for the Eastern Europ e CMO market is the avai lability of skilled manpower at lo wcost. Costs of labor in East ern Euro pe on an av erage a re four to ten times less co mpared tcountries in the Western Europe and in the US. Testimony to this fact is found in the data from thIndex of hour ly compensation costs publ ished by the Bu reau of La bor Statistics of the Unite
States Department of Labor. The data clearly shows the difference between labor costs of the USWestern European nations and those of the Eastern European nations.
Figure 24: Market Drivers, Difference in Hourly Wage Costs in Euro Area v/s EasternEurope
2004 2005 2006 2007 2008 2009
IndexesofHourlyCompensation
Costs(UnitedStates=100)
Euro Area Eastern Europe
This indicates the labor cost savings/hr for
Eastern Europe as compared to the Euro
Area
Source: GBI Research, US Department of LaborNote: Data for 2008 and 2009 has been estimated through the CAGR for period 1998-2007
Table 2: Market Drivers, All Employees: Indexes of Hourly Compensation Costs (UnitedStates = 100)
Country or Area 1998 2000 2002 2004 2005 2006 2007
Euro Area (1)
Eastern Europe (2)
(1) Euro Area refers to European Union member countries that have adopted the Euro as the commoncurrency as of January 1, 2009.
(2) Eastern Europe refers to the Czech Republic, Hungary, Poland, and Slovakia.
Source: GBI Research, US Department of Labor
Avalability of low-costskilled manpower is amajor driver for the CMOindustry in Eastern
Europe.
-
7/31/2019 CMO Easter Europe
8/10
GBIHC041MR / Published OCT 2010
Page 92
Contract Manufacturing in Eastern Europe - InconsistentDemand and Competition from Asia Raises Concerns
GBI Research. This is a licensed product and is not to be photocopied
10 Appendix
10.1 M arket Definitions
CMO - A C ontract manufacturing Organization (CMO) or contract manufacturer is a company thaoffers manufacturing and packaging services, with volume capabilities ranging from small amountfor preclinical R&D to larger volumes necessary for clinical trials purposes and commercialization.
Biologics - Biologics are dr ugs and drug ingredients derived from hum an, animal and microbia
sources using biotechnology. Biologics are considered to b e highly target specific, less toxic anhighly effective.
Blockbuster - Blockbuster drugs are drugs with annual revenues of more than $1 billion.
EU -5 - EU-5 constitutes five nations of Eu rope i ncluding The UK, F rance, Germany, Italy anSpain
Generic Drug - A gener ic drug is a dru g that is man ufactured and marketed without p atenprotection. The price of generic drugs is lower as compared to the patented drugs.
IPR - IPR is the right to possess or control the us e of intellect ual property such as tra demarkscopyrights, patents and trade secrets, granted by a national or supra-national authority.
10.2 Abbreviations
ACTA - Anti-counterfeiting Trade Agreement
API - Active Pharmaceutical Ingredients
BLA - Biologics License Application
cGMP - Current Good Manufacturing Practice
CMO - Contract Manufacturing Organization
CNS - Central Nervous System
CRO - Contract Research Organization
CSO - Contract Sales Organization
EC - European Commission
EMEA - European Medicines Agency
EPO - European Patent Organisation
EU - The European Union
EU-5 - Five developed nations in the EU including The UK, France, Germany, Italy and Spain
FDA - Food and Drug Administration
FDI - Foreign Direct Investment
FIPCO - Fully Integrated pharmaceutical company
GCI - Global Competitiveness Index developed by the World Bank
GDP - Gross Domestic Product
GMP - Good Manufacturing Practice
HAPI - High-potency Active Pharmaceutical IngredientsIFC - International Finance Corporation
IPQ - International Pharmaceutical Quality
IP - Intellectual Property
IPR - Intellectual Property Rights
IPAB - Intellectual Property Appellate Board of India
ISO - International Organization for Standardization
LPI - Logistics Performance Index by the World Bank
-
7/31/2019 CMO Easter Europe
9/10
GBIHC041MR / Published OCT 2010
Page 93
Contract Manufacturing in Eastern Europe - InconsistentDemand and Competition from Asia Raises Concerns
GBI Research. This is a licensed product and is not to be photocopied
M&A - Merger and Acquisition
NME - New Molecular Entities
OTC - Over-the-counter drugs
PIC/S - T he Pharmaceutical Inspection Convention and Pharmaceutical Inspection Co-operatioScheme
QA Quality Assurance
QC - Quality Control
OECD - Organization for Economic Co-operation and Development
R&D Research and Development
RFP - Request for proposal
SEZ - Special Economic Zone
SFDA - State Food and Drug Administration, China
SME Small and Medium Enterprises
SOW - Statement of work
TRIPS - Trade Related Aspects of Intellectual Property Rights
USTR - Office of the United States Trade Representative
WIPO - World Intellectual Property Organization
WEF - World Economic Forum
WHO World Health Organization
WTO - World Trade Organization
10.3 Research Methodology
GBI Research dedicated Research and Analysis Teams consists of experienced professionals wita pedigree in marketing, market research, consulting background in the medical devices industrand advanced statistical expertise.
GBI Research adheres to the Codes of Practice of the Market Research Society (www.mrs.org.uk
and the Society of Competitive Intelligence Professionals (www.scip.org).All GBI Res earch d atabases are co ntinuously updated and revis ed. T he follo wing rese arcmethodology is followed for all databases and reports.
10.3.1 Cov erage
The objective of updating GBI Research coverage is to ensure that it r epresents the most up tdate vision of the industry possible.
Changes to th e ind ustry ta xonomy are b uilt on the basi s of extensiv e research of compa nyassociation and competitor sources.
Company cov erage is b ased on thr ee ke y factor s: mar ket capita lization, reve nues and m ediattention/innovation/ market potential.
An exhaustive search of 56 member exchanges is conducted and companies are prioritized o
the basis of their market capitalization;
The estimated revenu es of all maj or com panies, incl uding privat e an d gover nmental, aregathered and used to prioritize coverage; and
Companies which are maki ng th e n ews, or which ar e of p articular interest due to theinnovative approach are prioritized.
GBI Research aims to cover all major news events and deals in the medical industry, updated on adaily basis.
The coverag e is further stre amlined and strength ened with additional inputs from GBI ResearcExpert Panel (see below).
-
7/31/2019 CMO Easter Europe
10/10
GBIHC041MR / Published OCT 2010
Page 94
Contract Manufacturing in Eastern Europe - InconsistentDemand and Competition from Asia Raises Concerns
10.3.2 Seconda ry Research
The research process begins with exhaustive secondary research on internal and external sourcebeing carried out to source qualitative and quantitative information relating to each market.
The secondary research sources that are typically referred to include, but are not limited to:
Company websites, ann ual r eports, fina ncial rep orts, bro ker reports, in vestor prese ntationand SEC Filings;
Industry trade journals, scientific journals and other technical literature;
Internal and external proprietary databases;
Relevant patent and regulatory databases;
National government documents, statistical databases and market reports;
Procedure registries; and
News articl es, press releas es and web-casts specific to the compa nies op erating in thmarket.
10.3.3 Primar y Research
GBI Research cond ucts hu ndreds of prim ary interviews a year with industry p articipants a n
commentators in or der to v alidate its data and an alysis. A typical research i nterview fulfills thfollowing functions:
It provides first-hand information on the market size, market trends, growth trends, competitivlandscape, future outlook ;
Helps in validating and strengthening the secondary research findings; and
Further develops the Analysis Teams expertise and market understanding.
Primary r esearch involv es E-mail inter actions, tele phonic intervie ws as well as fa ce-to-facinterviews for each market, category, segment and sub-segment across geographies.
The participants who typically take part in such a process include, but are not limited to:
Industry participants: CEOs, VPs, marketin g/product managers, market i ntelligence managerand national sales managers;
Hospital stores, laboratories, pharmacies, distributors and para-medics;
Outside e xperts: Investment Bankers, Va luation E xperts, Rese arch An alysts spec ializing ispecific medical equipment markets; and
Key Opi nion Leaders: Ph ysicians an d s urgeons sp ecializing in differe nt thera peutic areacorresponding to different kinds of medical equipment.
10.3.4 Expert Panel Validation
GBI Research uses a panel of experts to cross verify its databases and forecasts.
GBI Research expert pa nel comprises of market ing man agers, product specia lists, in ternationasales managers from medical device companies; academics from research universities, KOLs fromhospitals, consultants from v enture c apital funds a nd distributors/suppliers of medica l equipmenand supplies .
Historic data and forecasts are relayed to GBI Research Expert Panel for feedback and adjusted iaccordance with their feedback.
Details of the make u p of th e e xpert pa nel can b e vie wed throu gh gbiresearch.com, and aravailable to clients on request.