Chrome Migration
Transcript of Chrome Migration
-
7/30/2019 Chrome Migration
1/3
English Espaol Franais Deutsch Italiano Portugus Svenska Nederlands Polski esky Romn Magyar Suomi Trke
The Worlds Most Comprehensive METALS DatabaseLogin
Order nowHome
How It Helps
Unique Features
Fact Sheet
FAQ
Arti cles
News and Updates
Free Demo
Experience the
full power ofKEY to METALSfor FREE
Contact us
Order Now
KEY to METALS Resource Center Articles Article
Corrosion and Corrosion Properties ofStainless Steels: Part One
Corrosion and Corrosion Properties of
Stainless Steels: Part Two
Corrosion and Corrosion Properties of
Stainless Steels: Part Three
Finding corrosion resistant
materials in the KEY to METALSdatabase
The KEY to METALS database contains many
corrosion resistant materials across a large
range of countries and standards.
Where available, full property information can
be viewed for materials including chemical
composition, mechanical properties, physical
properties, advanced property data and much
more.
Using the Advanced Search page, it is possible
to search for materials by their key descriptive
words detailed in the standard title by using the
Standard Description function of Advanced
Search.
It maybe that you need to further narrow the
search criteria by using the other fields in the
Advanced Search page e.g. Country/Standard.
Then click Submit.
A list of materials will then be generated for you
to choose from.
This article belongs to a series of
articles. You can click the links
below to read more on this topic.
Search Knowledge BaseEnter a phrase to search for:
Search byFull text
Keywords
Headings
Abstracts
Click on image to enlarge
Corrosion and Corrosion Properties of StainlessSteels: Part Three
Abstract :Intergranular corrosion, also called intercrystalline corrosion, occurs on or adjacent to thegrain boundaries of a metal. It is caused by microsegregation of impurities and alloyingelements on the grain boundaries.The driving force of intergranular corrosion is the difference between the electrodepotentials of the grain boundary and the grain itself, which form a galvanic cell in presenceof an electrolyte.
Intergranular corrosion of stainless steels
The microstructure of metals and alloys consists of a granular composition. Grains are small
crystals whose surfaces join the surfaces of other grains to form grain boundaries. Grain
boundaries separate the grains. Intergranular corrosion, also called intercrystalline corrosion,
occurs on or adjacent to the grain boundaries of a metal. Some causes of intergranular
corrosion are welding, stress annealing, improper heat treating or overheating in service.
Inspectors have difficulty in detecting the early stages of intergranular corrosion. This form of
corrosion results in a loss of strength in metal parts where the grains have fallen out.
Intergranular corrosion is caused by microsegregation of impurities and alloying elements on
the grain boundaries. The driving force of intergranular corrosion is the difference between the
electrode potentials of the grain boundary and the grain itself, which form a galvanic cell in
presence of an electrolyte.
If the phases segregated at the grain boundaries have lower value of electrode potential they
will oxidize (anodic reaction) and the grain metal having higher value of electrode potential will
provide cathodic reaction (reduction). Dissolution of anodic grain boundaries starts from the
surface and advances along the grains interfaces. The process results in deterioration of the
bonding between the grains and drop of mechanical properties.
If the precipitates at the grain boundaries have higher electrode potential the grains willdissolve (anodic reaction). In this case the grain boundaries will not be attacked. Figure 1
shows the intergranular corrosion.
Figure 1: Intergranular corrosion.
Stainless steel has a very thin and stable oxide film rich in chrome. This film reforms rapidly by
reaction with the atmosphere if damaged. If s tainless steel is not adequately protected f rom the
atmosphere during welding or is subject to very heavy grinding operations, a very thick oxide
layer will form. This thick oxide layer, distinguished by its blue tint, will have a chrome-depleted
layer under it, which will impair corrosion resistance. Both the oxide film and depleted layer
must be removed, either mechanically (grinding with a fine grit is recommended, wire brushing
and shot blasting will have less effect ), or chemically (acid pickle with a mixture of nitric and
hydrofluoric acid). Once cleaned, the surface can be chemically passivated to enhance
corrosion resistance, (passivation reduces the anodic reaction involved in the corrosion
process).
Carbon steel tools, also supports or even sparks from grinding carbon steel, can embed
fragments into the surface of the stainless steel. These fragments can then rust if moistened.
Therefore it is recommended that stainless steel fabrication be carried out in a separate
designated area and special stainless steel tools used where possible.
If any part of stainless-steel is heated in the range 900-1400F (482-760C) for any reasonable
time there is a risk that the chrome will form chrome carbides Cr23C6 with any carbon present
Key Benefits Product Overview Resource Center Demo Contact
Page 1of 3Corrosion and Corrosion Properties of Stainless Steels: Part Three :: KEY to METALS A...
4/16/2013http://www.keytometals.com/page.aspx?ID=CheckArticle&site=kts&NM=239
-
7/30/2019 Chrome Migration
2/3
After clicking a material from the resulting list, a
list of subgroups derived from standard
specifications appears.
From here it is possible to view specific propertydata for the selected material and also to view
similar and equivalent materials in our powerful
cross reference tables.
For example, by clicking on the chemical
composition link on the subgroup page it is
possible to view chemical composition data for
the material.
For youre a chance to take a test drive of the
KEY to METALS database, we invite you to join
a community of over 150,000 registered users
through the KEY to METALS Free Demo.
Click on image to enlarge
Click on image to enlarge
Click on image to enlarge
in the steel along the austenite grains. This causes depletion of chromium from the austenitic
grains resulting in decreasing the corrosion protective passive film.
This effect is called sensitization. It is also called weld decay since it usually happens during
welding process when the zone around the weld is heated.
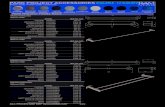
Figure 2 shows migration of chromium during heating of stainless steels.
Figure 2: Migration of chromium during heating of stainless steels.
To ensure good corrosion resistance of the weld root, it must be protected from the
atmosphere by an inert gas shield during welding and subsequent cooling. The gas shield
should be contained around the root of the weld by a suitable dam, which must permit a
continuous gas flow through the area.
Welding should not commence until sufficient time has elapsed to allow the volume of purging
gas flowing through the dam to equal at least the 6 times the volume contained in the dam.
Once purging is complete, the purge flow rate should be reduced so that it only exerts a small
positive pressure, sufficient to exclude air. I f good corrosion resistance of the root is required,
the oxygen level in the dam should not exceed 0.1% (1000 ppm); for extreme corrosion
resistance this should be reduced to 0.015% (150 ppm).
Backing gasses are typically argon or helium; nitrogen is often used as an economic
alternative where corrosion resistance is not critical, nitrogen + 10% helium is better. A wide
variety of proprietary pastes and backing materials are available than can be use to protect the
root instead of a gas shield. In some applications where corrosion and oxide coking of the weld
root is not important, such as large stainless steel ducting, no gas backing is used.
Figure 3 shows two microstructures of type 304 stainless steel. The figure on the left is the
normalized microstructure and the one on the right is the "sensitized" structure and is
susceptible to intergranular corrosion or intergranular stress corrosion cracking.
Figure 3: Microstructure of stainless steel type 304.
Means of preventing sensitization:
Solution heat treatment: heating to a temperature above 1900F (1040C) followed by
Page 2of 3Corrosion and Corrosion Properties of Stainless Steels: Part Three :: KEY to METALS A...
4/16/2013http://www.keytometals.com/page.aspx?ID=CheckArticle&site=kts&NM=239
-
7/30/2019 Chrome Migration
3/3
How it Helps | Unique Features | Fact Sheet |Articles | News and Updates | Terms of Use | Site map
2013 Key to Metals AG. All Rights Reserved.
quenching (rapid cooling) in water or quenching oils. During the heating stage the carbides
dissolve and their formation is suppressed by fast cooling.
Lowering concentration of carbon. Sensitization is depressed in low carbon (max. 0.03%)
stainless steels, designated with the suffix L (304L, 316L).
Stabilization by carbide forming elements. Formation of chromium carbides is avoided in
stabilized austenitic stainless steels (321, 347) containing carbide forming elements like
titanium, niobium, tantalum, zirconium. Stabilization heat treatment of such steels results in
preferred formation of carbides of the stabilizing elements instead of chromium carbides.
Date Published: Dec-2008
The KEY to METALS database brings gl obal metal properties together into one
integrated and searchable database. Quick and easy access to the mechanical
properties, chemical composition, cross-reference tables, and more provide users with
an unprecedented wealth of information. Click the butt ons below to learn more from the
Guided Tour or to test drive the KEY to METALS database.
Guided Tour
Click here to viewKEY to METALSGuided Tour
Try Out FREE Demo
ExperienceKEY to METALSwith full accessto over 600 alloys.
Page 3of 3Corrosion and Corrosion Properties of Stainless Steels: Part Three :: KEY to METALS A...
4/16/2013http://www keytometalscom/pageaspx?ID=CheckArticle&site=kts&NM=239