Chemistry of Life. Basic Structures of Life Matter: Matter: Has mass and occupies space Element:...
-
Upload
egbert-murphy -
Category
Documents
-
view
214 -
download
0
Transcript of Chemistry of Life. Basic Structures of Life Matter: Matter: Has mass and occupies space Element:...
Chapter 1
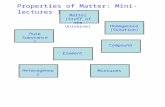
Chapter 2Chemistry of Life1Basic Structures of LifeMatter: Has mass and occupies spaceElement: Pure substanceCompound: Chemical combination of two or more substances
2Protons carry a positive charge.Electrons carry a negative charge.Neutrons are electrically neutral.
Protons, Electrons, and Neutrons3 View animation on All About Atoms
NucleusAtoms4Atomic Number and WeightEach element carries a unique number of protons.Atomic number: The number of protons in the nucleusAtomic weight: The number of protons + the number of neutrons5QuestionWhich of the following is a pure substance that cannot be broken down into two or more substances?
CompoundElementMatterAtom6Chemical BondsIonicCovalentHydrogen
7Ionic Bonds
Ionic bonds occur when one atom transfers an electron to another atom8
Ionic Bonds9Ionic BondsElectrically charged atoms are called ions. positive = cationsnegative = anions
Compounds that ionize in water and create such a solution are called electrolytes.
10Covalent BondsView animation on Chemical Bonds: Ionic and Covalent
Covalent bonds occur when two atoms share one or more pairs of electrons.11Covalent BondsCovalent bonds are stronger than ionic bonds; they are used to create many of the chemical structures of the body.12Hydrogen Bonds
A hydrogen bond is a weak attraction between a slightly positive hydrogen atom in one molecule and a slightly negative oxygen or nitrogen atom in another. 13View animation on Hydrogen Bonds
Hydrogen Bonds14Hydrogen BondsUnpaired electrons give water a partial negative charge near the oxygen atom and the two hydrogen atoms create a slight positive charge on the other side. Water is electrically neutral but has an uneven distribution of electrons making it a polar molecule.15
QuestionWhat bond occurs when one atom transfers an electron to another atom?
HydrogenChemicalIonicCovalent16To function, the body needsENERGY17EnergyEnergy is stored in the bonds of molecules. This is potential energy.Chemical reactions release the energy. Energy in motion is kinetic energy.All of the chemical reactions in the body are called metabolism.18EnergyCatabolism breaking down complex compounds into simpler ones. Catabolic reactions release energy.Anabolism building larger and more complex chemical molecules from smaller subunits. Anabolic reactions require energy.19
Organic and Inorganic CompoundsMost of the molecules of the body form organic compounds.Organic compounds contain carbon.Inorganic compounds include water, oxygen, carbon dioxide, and acids and bases.
20Types of Chemical ReactionsSynthesis: A + B ABDecomposition: AB A + B Exchange: AB + CD AC + BD
All three of these chemical reactions can go in either direction: They are reversible reactions.
21WaterWater comprises 50% of an adults body weight.Fluids in the body consist of chemicals dissolved in water.Water has unique characteristicsIt is a solvent.It is a lubricant.It changes temperature slowly.22AcidsStrong acid
Weak acid
Acids and bases are among the most important chemicals in the body.Acids release hydrogen when dissolved in water. 23BasesOH ions . . .
. . . accept H+ ions
View animation on Acids and Bases 24pH Scale
25pH ScaleAcidity or alkalinity is expressed in terms of pHpH scale ranges from 0 to 14. pH greater than 7 = basic (alkaline); higher pH = more OH ionspH of 7 = neutral; equal numbers of H+ and OH ionspH less than 7 = acidic; lower pH value = more H+ ionsNormal pH range of the human body is extremely narrow: ranging from 7.35 to 7.45.
26Organic CompoundsCarbohydratesLipidsProteinsNucleic acids27Organic CompoundsThe term organic is used to describe the vast array of compounds that contain carbon.Carbon serves as the basis for thousands of molecules of varying size and shape. 28CarbohydratesCarbohydrates are the bodys main energy source.Glucose is the primary form of sugar used by cells.Glycogen is the stored form of glucose.29LipidsA reserve energy supplyProvide structure to cellsInsulate nervesServe as vitaminsCushion organs30ProteinsProteins are the most abundant and important organic compound in the body.Proteins consist of amino acids.
31Amino Acid
32QuestionWhat is the bodys primary energy source?
CarbohydratesProteinsLipidsWater33Nucleic AcidsNucleic acids include DNA and RNA.They consist of thousands of nucleotides.Nucleotides consist of a five-carbon sugar, a phosphate group, and one of several nitrogen bases.34Nucleic AcidsIn DNA, the sugar is deoxyribose; in RNA, the sugar is ribose.DNA is the largest molecule in the body.DNA carries the genetic code.RNA copies the genetic code of DNA to direct protein synthesis.35Adenosine Triphosphate Adenosine triphosphate (ATP) is a nucleotide that stores energy.Adenosine triphosphate (ATP) consists of a base, a sugar, and a phosphate group.The phosphate groups are connected with high-energy bonds.
36ATP: Releasing Energy
When one of these bonds is broken through a chemical reaction, energy is released that can be used for work.After the bond is broken through a chemical reaction, adenosine triphosphate (ATP) becomes adenosine diphosphate (ADP) and a single phosphate.
37ATP: Repairing Bonds
View animation on Breakdown of ATP The cell uses some of the energy released from the breakdown of the nutrients in food to reattach the third phosphate to the ADP, again forming ATP.
38
QuestionWhich statement is true?
DNA and RNA are nucleotides.ATP is a nucleic acid.Nucleic acids are inorganic compounds.Nucleotides consist of a sugar, a phosphate group, and a nitrogen base.
39