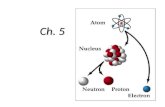
Chemistry for Biology Students. Atomic Structure Atoms are composed of ……..?
Transcript of Chemistry for Biology Students. Atomic Structure Atoms are composed of ……..?
Atomic Structure
You were right if you said:
protons
electrons and
neutrons!!
Some of these have a charge. What are they?
Atomic Structure
You were right if you said…
protons are positively charged
electrons are negatively charged
neutrons have no charge (neutral)
Atomic Structure-2• If you look at the periodic chart on the next slide,
you will see 2 numbers for each element.• The smaller number, that goes in sequence, is the
Atomic Number. • The Atomic Number is the number of protons
(positive charges) an atom has.• Since all the atoms on the periodic chart are neutral,
the atomic number also tells you the number of electrons in a neutral atom.
• Example: Chlorine (Cl) has atomic number 17. It has 17 protons and 17 electrons. Look at the periodic chart and find Chlorine to verify this.
Atomic Structure-3• The other number is a decimal number. We will
round this number to the nearest whole number.• This number is the Atomic Mass. • Most of the mass of the atom is in the nucleus,
because electrons are very, very, tiny. So, the atomic mass is the number of protons and neutrons ADDED together.
• Example: Chlorine (Cl) has an atomic mass of 35. That means if you add the protons and neutrons together, you will get 35.
Atomic Structure-4• So, if you know the atomic number and the atomic
mass of an atom, you can find the number of protons, electrons, and neutrons it has!!
• Here’s how:Number of protons = Atomic numberNumber of electrons = Atomic numberNumber of neutrons = Atomic Mass – Atomic Number
• Example: Chlorine: Atomic number is 17, atomic mass is 35Number of protons = 17Number of electrons = 17Number of neutrons = 37 – 17 = 18
Atomic Structure- 5
• Determine the protons, electrons + neutrons for the following atoms:A. Atomic number = 23, Atomic Mass = 11B. Atomic number = 9, Atomic Mass = 5C. Atomic number = 12, Atomic Mass = 6D. Atomic number = 39, Atomic Mass = 19
Check your answers on the next slide!
Atomic Structure- 6
• Answers:A. protons=11, electrons=11, neutrons=12B. protons= 5, electrons=5, neutrons= 4C. protons=6, electrons=6, neutrons=6D. protons=19, electrons=19, neutrons=20
• Did you get them all right?? If so, draw the first 20 atoms for homework. If not, go back to slide number 8, and re-do this section.
Determining Charge
• A major rule of all atoms: All atoms want their last energy level filled.
• They can have their last energy level filled by– Gaining electrons, and filling the energy level– Losing electrons, and dropping the energy level– Sharing electrons
Determining Charge-2
• To determine what an atom will do follow these steps:– Determine how many electrons the atom needs
to gain to fill its energy level (Filled would be 2 electrons for the first energy level, 8 for the 2nd, 3rd, 4th, then it gets tricky);
– Determine how many electrons the atom needs to lose to eliminate its last energy level (this will be the total number of electrons in the last energy level in a neutral atom).
Determining Charge-3
• Atoms will do the easiest of the two- in other words, which ever one you determined is the smaller number.– An atom that gains electrons, will have a negative charge,
because it will have more electrons than protons.– An atom that loses electrons, will have a positive charge,
because it will have more protons than electrons.– The charge consists of a sign and a number.– The charge is either + or - ; the number will be the number
of electrons lost or gained.
Determining Charge-4• For example: Chlorine has 7 electrons in its last energy level,
– it needs to gain 1 electron to make it full (at 8) or – it could lose all 7 electrons and only have 2 energy levels.– Chlorine will gain 1 electron, because this is the smaller number.– The charge for Chlorine after it gains one electron will be –1,
(written as: Cl- ) because it will have 1 more electron than protons. (It will have 17 protons and 18 electrons after gaining 1 electron.)
• Understand?? If not re-read slides 11-14.
Determining Charge-5
• Some atoms have ½ the number of electrons they need to fill the energy level.– This means they could gain 4 or lose 4, for example.
When these numbers are equal the atom usually does not lose or gain, but SHARES electrons with another atom.
– They do not get a charge.– (Some atoms, like hydrogen, will share sometimes, and
at other times they will lose or gain electrons. Hydrogen sometimes loses its one electron, giving it a +1 charge: H+ )
Determining Charge-6
• Some atoms already have a full energy level! – They do not need to gain electrons.– They do not need to lose electrons.– They do not need to share electrons.– They do not need any other atom!! Therefore, they
will not react with any other atom. They do not react at all.
– They are non-reactive– inert.– Inert- atoms who never react, because their last
energy level is naturally filled.
Determining Charge- practice
• Ok, you’re ready for practice! Determine the charges for the following:A. Atomic number 20, atomic mass 40B. Atomic number 9, atomic mass 19C. Atomic number 19, atomic mass 40D. Atomic number 10, atomic mass 20E. Atomic number 6, atomic mass 12
– Answers on next slide– don’t peek until you’re done!
Determining Charge- answers• Answers:
A. +2B. –1C. +1D. inertE. Shares
Did you get them all right? If you did: GOOD!!! Now determine the charges for the first 20 atoms on the periodic chart. Use your diagrams from before.
If not, go back to slide 11 and do it again.
Reviewing Homework• When all drawings and charges have been
checked, please do the following:
• Read the chemical symbols (I have listed on the next slide).
• As you read each symbol, say the charge for that symbol.
Read these symbols in the order given and give their charges:
• H
• Li
• Na
• K
• Be
• Mg
• Ca– (continue on next slide)
Read these symbols in the order given and give their charges:
• B
• Al
• C
• Si
• N
• P– continue on next slide
Periodic Table
• Did you notice a pattern? You should have.
• The first column of the periodic chart has a +1 charge; the second column has a +2 charge; the third column has a +3 charge; the fourth column shares; the fifth column has a -3 charge; the sixth column has a -2 charge, the seventh column has a -1 charge; and the last column is inert.
Periodic Table• If you look at YOUR periodic chart, you
will see Roman Numerals at the top of the columns (actually IA, IIA etc.) . The Roman numerals indicate the number of electrons in the last energy level (except for He). This number is the GROUP NUMBER. So Ca belongs to Group 2, and O belongs to Group 6.
• Check this on the next slide.
Periodic Table• Now write the following on YOUR periodic
table as it appears on the preceding periodic table:
• Put a 1 to the left of H• Put a 2 to the left of Li• Put a 3 to the left of Na• Put a 4 to the left of K• Put a 5 to the left of Rb• Put a 6 to the left of Cs• Put a 7 to the left of Fr
Periodic Table
• These numbers are the Period Numbers- the rows on the periodic chart.
• What does the period number mean?
• Hint: Why are there only 2 elements in period 1?
Periodic Table
• You were right if you said the Period Number represents the number of energy levels an atom has! (That’s why there are only 2 elements in the 1st period- because only H and He have a single energy level!)
• So, B is in group 3, period 2. That means B has 2 energy levels, and it has 3 electrons in the 2nd energy level, & therefore, it will want to lose 3 electrons and get a +3 charge…OR
Periodic Table
• Cl is in group 7, period 3. That means Cl has 3 energy levels, and it has 7 electrons in the 3rd energy level. So, it wants to gain 1 electron, and will then have a -1 charge.
Periodic Table
• All of the atoms in Group 8, have energy levels that are full naturally. Therefore, they do not need to gain or lose any electrons-- and they don’t. They never react with other atoms, so they are called inert elements.
Definitions• Ion- any atom with a charge. You can
recognize these atoms easily: Ca++ or Ca+2, or Cl-, O-2.
• Isotope- atoms of the same element with different numbers of neutrons.
12C and 14C Both of these are Carbon atoms, but one has more neutrons.
6 6
• Inert- atoms whose outer energy level are naturally filled. They are non-reactive.
Physical Properties/Changes
• Physical Properties are the characteristics of a substance, such as size, weight, color
• A physical change can change a physical property without changing the substance itself.
Physical Properties/Changes
• For example, a piece of printer paper has the following physical properties:
• 8.5x11 inches, is white, is paper.
• Tearing the paper diagonally, will change the size (a physical property) but it still remains paper.
Chemical properties/changes
• Chemical properties are characteristics that make the substance what it is.
• Chemical properties include: melting and boiling points, density
• Chemical changes change the substance itself-- it is no longer the same substance, and has different chemical properties.
Chemical properties/changes
• For example, the white printer paper when burned (this is a chemical change) no longer remains paper. It is ash.
• Chemical changes rearrange atoms, so the substances that result are different.
Physical/Chemical Properties
• So, when ice changes to water, and water changes to steam, are these physical or chemical changes?
Physical/Chemical Changes
• You were right if you said physical changes!
• These are called phase changes. They are simply different phases of matter.
Chemical Bonds
• Look at the drawing below. The first atom is Sodium- it has one electron in its last energy level. It wants to lose that electron
Chemical Bonds
• The second atom is Fluorine. It has 7 electrons in its last energy level. It wants to gain one electron.
Chemical Bonds
• So… Sodium gives up its electron to Fluorine. The electron will now rotate around Fluorine’s nucleus. It essentially “belongs” to Fluorine.
Chemical Bonds
• Since Sodium has lost an electron, it gets a +1 charge
• Since Fluorine has gained an electron, it gets a -1 charge
• The attraction of opposite charges holds the atoms together.
• It is now Na-F; the “-” indicates a bond.
Chemical Bonds
• This type of bond is an ionic bond: a chemical bond due to the loss and gain of electrons, where the atoms are held together by the attraction of opposite charges.
Chemical Bonds
• Carbon has 4 electrons in its outer energy level, and hydrogen has one. Instead of losing or gaining electrons, these atoms will share their electrons.
Chemical Bonds
• The outer energy levels for H and C overlap, but the electrons continue to rotate around their own nucleus.
Chemical Bonds
• Chemical Bonds due to the sharing of electrons are called covalent bonds.
• Water, H2O, has covalent bonds too.
Molecules/Compounds
• Molecule: 2 or more atoms held together by chemical bonds (this is a VERY LOOSE definition of molecule, but it will suffice for us).
• Compound: 2 or more Different atoms held together by chemical bonds.
• All compounds are molecules, but not all molecules are compounds.
Molecules/Compounds• Determine if the following
are molecules, compounds or both.
• H2O
• CH4
• O2
• CO2
• H2
Molecules/Compounds• H2O is both a molecule and a compound
• CH4 is both a molecule and a compound
• O2 is a molecule, but NOT a compound
• CO2 is both a molecule and a compound
• H2 is a molecule, but NOT a compound
• Understand?? (If there is more than one element, it is a compound and a molecule; but if there is only one element, it is only a molecule.)
Molecular Formulas
Molecular formulas tell us 3 things:
1. The kind of atom (element) in the molecule- indicated by the chemical symbols:– 6H2O
– The elements in this expression are H and O
Molecular Formulas
2. The number of each kind of atom in the molecule- indicated by the subscript number that follows (1 is understood, it is not written)– 6H2O
– There are 2 H and 1 O in each molecule of H2O.
Molecular Formulas
3. The number of molecules- indicated by the large number in front of the molecular formula.– 6H2O
– There are 6 molecules of H2O in this expression
– Remember this number refers to the entire molecule as if it were written like this:
– 6 (H2O)
Molecular Formulas
In the Molecular Formula, 5H2SO4
What is the total number of atoms in this expression?
Molecular Formulas
• You were right if you said 35!
• In the Molecular Formula, 5H2SO4 , how many atoms are in one molecule?
Molecular Formulas
• You were right if you said 7 atoms!
• In each molecule of H2SO4 there are
– 2 atoms of H– 1 atom of S– 4 atoms of O
Chemical Reactions
• Chemical reactions are written like this:
H2 + O2 --> H2O
• Everything at the back of the arrow is called a Reactant; the reactants here are H2 and O2
• Everything the arrow points to is called a Product; the product here is H2O.
Chemical reactions
• Sometimes the arrow is reversed:
H2 + O2 <-- H2O
This reaction says that water is being broken down into hydrogen and oxygen. The reactant is H2O and the products are H2
and O2.
Chemical reactions
This reaction is a synthesis reaction, because it is building up into a larger molecule: H2 + O2 --> H2O
Synthesis reactions are called anabolic reactions, and usually require energy.
Chemical Reactions
This reaction is breaking down a larger molecule into smaller ones : H2O --> H2 + O2
Reactions which break down large molecules are called catabolic reactions, and usually release energy.
Chemical Reactions
• All chemical reactions require energy to get started, this is called Activation energy.
• Reactions that require more energy, are called endergonic reactions
• Reactions that release energy after getting started, are called exergonic reactions.
Chemical Reactions
• Hints to help you remember:
• EXERgonic reactions have energy Exiting
• ENDergonic reactions have energy entering
Chemical Reactions
• In summary,
• anabolic reactions are usually endergonic
• catabolic reactions are usually exergonic
Balancing equations
• The law of conservation of matter says that matter can neither be created nor destroyed.
• Therefore, whenever a chemical reaction occurs, we must be able to show what happens to each atom of matter
Balancing equations If you count the atoms of reactants in the
following equation, you will see that it is different than the number of atoms in the products:
H2 + O2 --> H2O
There are 2 H atoms as reactants and 2 H atoms as products.
There are 2 O atoms as reactants but only 1 O atom in the product.
Balancing Equations
This reaction is said to be unbalanced:
H2 + O2 --> H2O
In order to balance an equation, you can change the number of molecules of each compound, but you may not change any of the small subscript numbers. (Changing the small numbers changes the reaction. For example, changing H2O to H2O2, as most students want to do, changes the reaction to say that H2 and O2 combine to form hydrogen peroxide-- not water)
Balancing Equations __H2 + __O2 --> __H2O
Therefore numbers can only be put in the blanks above.
__H2 + __ O2 --> 2H2O
By putting a 2 in front of H2O, the O atoms are now balanced… but now the H atoms are not balanced.
2H2 + __ O2 --> 2H2O
By putting a 2 in front of H2, the H atoms are now balanced also, and the entire equation is balanced.
Balancing Equations- Practice
• Take a piece of loose-leaf and balance the equations on the next few slides.
• The answers are at the end.
Balancing Equations- Practice 1. H2 + Cl2 --> HCl
2. Na + Cl2 --> NaCl
3. Mg + F2 --> MgF2
4. K + O2 --> K2O
5. H2 + F2 --> HF
6. Ca + O2 --> CaO
7. Al + Cl2 --> AlCl3
Check your answers on the next slide when you are done.
Balancing Equations- Answers
1. H2 + Cl2 --> 2 HCl
2. 2Na + Cl2 --> 2NaCl
3. Mg + F2 --> MgF2
4. 4K + O2 --> 2K2O
5. H2 + F2 --> 2HF
6. 2Ca + O2 --> 2CaO
7. 2Al + 3Cl2 --> 2AlCl3
pH
• Water, H2O, is normally ionized.
This means that the molecule, which normally would be H-O-H, breaks into H+ and OH- ; because one H gives up its electron (and becomes + charged), while the OH gains an electron and becomes - charged.
pH
• H+ is called the hydrogen ion
• OH- is called the hydroxide ion
• For each water molecule that breaks down, there will be ONE hydrogen ion, and ONE hydroxide ion.
pH• The pH scale measures the relative
concentrations of hydrogen and hydroxide ions.
• The pH scale goes from 0 to 14.
• pH 0 to pH 7, not including pH 7, is an acid
• pH 7 to pH 14, not including pH 7, is a base (also called alkaline)
• pH 7 is neutral
pH
• At pH 7, there are equal concentrations of both hydrogen and hydroxide ions
• Between pH 0 and pH 7, there is a greater H+ concentration than OH- concentration
• Between pH 7 and pH 14, there is a greater OH- concentration than H+ concentration.
pH
• The farther away from pH 7 you go, the greater the differences between the H+ and OH- concentrations will be.
• Conversely, the closer the pH is to pH 7, the closer the H+ and OH- concentrations will be.
pH
• Therefore, the farther away from pH 7, the stronger the ACID will be.
• Or…Acids with LOWER pH’s are stronger
pH
• Therefore, the farther away from pH 7, the stronger the BASE will be.
• Or…Bases with HIGHER pH’s are stronger
pH
• In summary,
• pH 0-7 is acidic, and [H+] > [OH-]
• pH 7 is neutral and [H+] = [OH-]
• pH 7-14 is basic and [OH-] > [H+]
pH Quiz
Answer the questions on the following slides to see if you understand pH.
Write your answers in your notebook. You will be able to check your answers at the end.
pH Quiz
• 2. Which has the greatest H+ concentration, pH 2 or pH 5?
• 3. Which has the greatest OH- concentration, pH 8 or pH 12?
• 4. Which is the strongest acid, pH 3 or pH5?
• 5. Which is the strongest base, pH 8 or pH 10?
Atomic Models- Introduction
You have learned about ionic and covalent bonds. For each electron lost, gained or shared there is one chemical bond. So, if Na loses one electron to F, it has one chemical bond with F. Conversely, if F gains one electron from Na, it has one chemical bond with Na.
Structural Formulas
• The compound formed when Na and F bond is NaF
• To draw this compound, a bond is drawn between the Na and F symbols, like this: Na-F
Structural Formulas
• This is called a structural formula: Na-F• Structural formulas tell us everything a
molecular formula tells us, but they also tell us the arrangement of the atoms in the compound.
• A chemical symbol is written for each atom in the compound, and lines are drawn to show each bond between the atoms.
• There must be a chemical symbol at the end of every line drawn.
Structural Formulas
• Look in the box you have been given.
• The sticks and springs represent the bonds. You will use sticks most of the time.
• Springs are only used for double or triple bonds, so you can’t use just one spring.
Structural Formulas
• The colored balls are specific atoms. For example, the red ones are oxygen.
• Look carefully at the red balls.
• They have 2 holes, because oxygen has 6 electrons in its last energy level and needs to gain 2 electrons. Therefore, oxygen will have 2 bonds.
Structural Formulas
• The black balls are carbon.
• Look carefully at the black balls.
• Carbon shares 4 electrons, therefore it has 4 bonds.
• So, the carbon balls have 4 holes.
• Understand???
Procedure
• Take a piece of loose-leaf and make three columns with the following headings:
• Molecular formula
• Name
• Structural formula
Procedure
• I will give you a list of molecular formulas.• Write the molecular formula in the correct
column on your loose-leaf.• Determine the names of compounds I do not
give you- try to figure them out.• Then build the molecule.• Then write the structural formula by looking
at your model.
Procedure/Hints
• Do not draw the balls- substitute the chemical symbols for the colored balls
• Show the sticks (or springs) by drawing short lines from one chemical symbol to another.
Procedure/Hints• How can you tell if your model is correct?
– You should have the correct number of each kind of atom
– There should not be any empty holes in any of the colored balls
– All sticks/springs should have a ball on each end.– Have your lab partner count the atoms to double
check and make sure you have exactly the right number-- no more and no less.
Procedure
• We will do the first one together.
• I have tried to put the answers at the end of this powerpoint. Don’t peek until you are finished-- or until you are really, really stuck.
KEY: Red=oxygen; black= carbon; green= chlorine; yellow= hydrogen
• Molecular formula: H2
• Write this on your loose-leaf• Write the name of the molecule. (If you guessed
“hydrogen”, you would be correct!)
• Build this molecule
• Write the structural formula for this molecule.
• If you drew H-H, you are correct!
KEY: Red=oxygen; black= carbon; green= chlorine; yellow= hydrogen
• Repeat this procedure for the following compounds.
• Make sure you do these in order-- don’t skip.
• Remember, use sticks unless they don’t fit, and if you use springs, you must use 2 of them.
• Ready…..?
KEY: Red=oxygen; black= carbon; green= chlorine; yellow= hydrogen
2. HCl
3. CH4 methane
4. H2O
5. C2H6 ethane
6. C3H5OH propanol
7. C3H6O omit name
8. O2 (This is tricky! Keep thinking and you’ll get it. Be sure there are no
empty holes.)
9. CO2
10. C2H5COOH propanoic acid
Some answers are on the next slide