Cerebral tuberculoma in a patient receiving anti-TNF alpha (adalimumab) treatment
Click here to load reader
-
Upload
karen-lynch -
Category
Documents
-
view
217 -
download
1
Transcript of Cerebral tuberculoma in a patient receiving anti-TNF alpha (adalimumab) treatment

CASE REPORT
Cerebral tuberculoma in a patient receiving anti-TNF alpha(adalimumab) treatment
Karen Lynch & Michael Farrell
Received: 15 March 2010 /Accepted: 7 April 2010 /Published online: 26 April 2010# Clinical Rheumatology 2010
Abstract We report a case of a cerebral tuberculoma in a60-year-old woman with rheumatoid arthritis while receiv-ing the anti-tumor necrosis factor alpha monoclonalantibody, adalimumab (Humira), for active disease. MRbrain imaging for dyspraxia revealed a left parietal ring-enhancing lesion, which on resection was shown to be anecrotizing granuloma. There were no associated pulmo-nary lesions, and the patient was systemically well. Sputumand urine cultures were negative for tuberculosis. Thepatient was treated with anti-tuberculous medications andmade an excellent recovery. We consider this to be the firstdocumented case of tuberculosis involving the centralnervous system occurring in the setting of adalimumabtreatment.
Keywords Adalimumab . Anti-TNF. Cerebral .
Rheumatoid arthritis. . Tuberculoma
AbbreviationsCDC Centers for Disease ControlTNF Tumor necrosis factorZN Ziehl NielsonGFAP Glial fibrillary acidic proteinPPD Purified protein derivativeRA Rheumatoid arthritis
DMARDs Disease-modifying anti-rheumatic drugsLTBI Latent tuberculous bacilli infectionIGRA Interferon-gamma release assay.
Introduction
Rheumatoid arthritis (RA) is a chronic, progressive,systemic inflammatory disease of undetermined etiologyinvolving primarily the synovial membranes and articularstructures of joints. Clinically, it may result in significantpain, stiffness, and swelling of joints with subsequentdeformity and ankylosis in later stages of the disease. Earlyadministration of disease-modifying anti-rheumatic drugs(DMARDs), the use of non-steroidal anti-inflammatorydrugs, and glucocorticoids are all essential in the medicalmanagement of RA. With the discovery of the role ofcytokine tumor necrosis factor (TNF) alpha in the mecha-nism of inflammation in RA, anti-TNF alpha drugs, a newbiological class of DMARDs was introduced. Three ofthese medications have been commercialized since 1999 foruse in RA patients: infliximab, etanercept, and adalimumab.Since their introduction, there have been numerous studiesand meta-analysis comparing this new class of drug fromefficacy to safety profile [1]. In recent years, the emphasishas been on the risk of malignancy and serious infection(tuberculosis, histoplasmosis, listeriosis, candidiasis, andaspergillosis), with a number of comparative studies beingconducted in the US and Europe [1]. Data from thesestudies shows clearly that reactivation of latent TB infection(LTBI) has been the most commonly reported adverseeffect of anti-TNF inhibitors. This susceptibility to myco-bacterial infections, particularly reactivation of LTBI, is due
K. Lynch :M. FarrellDepartment of Neuropathology, Beaumont Hospital,Beaumont,Dublin 9, Ireland
K. Lynch (*)Department of Neurology Tufts University,St Elizabeth’s Medical Center,736 Cambridge Street,Boston, MA 02135, USAe-mail: [email protected]
Clin Rheumatol (2010) 29:1201–1204DOI 10.1007/s10067-010-1466-7

to the role that TNF alpha plays in containing and ensuringthe preservation of the granuloma structure [1, 5].
Among the TNF alpha inhibitors used in RA, there aredifferences in the risk of TB reactivation. Infliximab hasbeen shown to have a higher rate of reactivation comparedto etancercept, and data so far on adalimumab suggest thatits risk is similar to that of infliximab [9]. The increasedrisk associated with infliximab and adalimumab may be dueto the combined biological effect of these agents on bothCD4 TB-responsive cells and their known suppression ofinterferon-gamma production. Etanercept on the other handhas no great effect on these parameters.
We describe a patient with RA, who presented withcentral neurological symptoms while receiving adalimumabtreatment and on investigation was found to have a cerebraltuberculoma. There have been an unusually large numberof cases of tuberculosis, predominantly in the miliary form,reported in patients on anti-TNF alpha drugs, particularlyinfliximab, but we present the first documented case of acerebral tuberculoma without systemic involvement follow-ing adalimumab treatment.
Case report
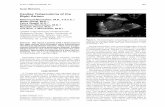
A 60-year-old woman with a 19-year history of rheumatoidarthritis was investigated for a 2-week history of left neckpain and right hand dyspraxia. She also had an associatedsensation of pressure behind the left eye, with minimalintermittent headaches. Medications included methotrexate15 mg/week for 8 years and adalimumab (Humira) 40 mltaken fortnightly for 2 years. Her arthritis activity had beenquiescent for over 2 years, and apart from raised seruminflammatory markers, she was systemically well onpresentation. Magnetic resonance imaging (MRI) of thebrain showed a left parietal ring-enhancing lesion, withsurrounding vasogenic edema (Fig. 1) and the patientproceeded to resection.
Microscopic examination of the resection specimenrevealed a large central area of necrosis surrounded byfibrocollagenous connective tissue, lymphocytes, and mul-tinucleate giant cells with reactive gliosis (Fig. 2). Immu-nocytochemistry for CD3 confirmed T-lymphocytes at theperiphery of the lesion, and ZN stain was negative fortuberculous bacilli. Appearances were consistent with acerebral tuberculoma. Further ZN stain and culture ofsputum and urine as well as polymerase chain reaction(PCR) and acid fast bacilli (AFB) staining of CSF werenegative for Mycobacterium tuberculosis. Prior to com-mencement of adalimumab, the patient had a negativescreening PPD test and chest X-ray for latent TB.
The patient was started on four antituberculosis medi-cations (rifampicin, isoniazid, ethambutol, and pyrazina-
mide) and was discharged 1 week after admission. At afollow-up visit 6 weeks later, she had resolution of herinitial neurological symptoms with significant reduction insize of the tuberculoma on MRI. She continued treatmentfor 12 months, and her rheumatoid arthritis is currentlystable.
Discussion
Adalimumab (Humira) is the first and only humanmonoclonal anti-TNF alpha antibody approved for use in
Fig. 1 Coronal T1-weighted MRI brain post-gadolinium demonstrat-ing ring-enhancing left parietal lesion
Fig. 2 Cerebral granuloma comprising of epithelioid cells, reactiveastrocytes, and fibrous gliosis surrounding a central core of caseousnecrosis
1202 Clin Rheumatol (2010) 29:1201–1204

patients with active rheumatoid arthritis that have notresponded adequately to standard anti-rheumatic therapiessuch as low dose corticosteroids, NSAIDs, and disease-modifying anti-rheumatic drugs. The immune-modulatingeffects of the three current TNF antagonists used clinically(etanercept, infliximab, and adalimumab) have been asso-ciated with various adverse effects including opportunisticinfections such as TB, demyelinating disorders, systemiclupus erythematosus-like syndrome, and lymphoprolifera-tive disorders [1].
There have been well-documented reports of TB in thesetting of patients receiving anti-TNF alpha medications, witha higher likelihood of occurring in extrapulmonary sites orbeing in the disseminated/miliary forms [2]. The AdverseEvent Reporting System (AERS), a database established bythe Food and Drug Administration (FDA) for post-marketingadverse drug event surveillance, found that over the periodof 1998–2002, rates of TB among US patients treated withinfliximab and etanercept were 54 and 28 cases/100,000,respectively. The overall rate of TB in the US over the sameperiod of time was 5.2–6.8 cases/100,000. Comparing dataworldwide over the same period of time, there were a total of70 cases of TB following treatment with infliximab, 64 ofwhich were in countries with a low background incidence ofTB. These cases occurred on average 4 months followinginfusion, and over half [3] had extrapulmonary disease, withone meningeal case described [4].
In Europe, several studies conducted in Spain andPortugal, where there is a high incidence of latent TBcompared to the US, showed substantially higher rates ofTB in patients receiving anti-TNF alpha inhibitors. Thecalculated incidence of TB with infliximab use was 1,900/100,000 patients in 2000, compared to the baselineincidence of TB in Spain of 21/100,000 inhabitants [6].Susceptibility to reactivation of LTBI cited in a later AERSdatabase [7] include co-administration of other immuno-suppressive drugs and history of latent or active TB, beingborn in or spent time in a TB endemic region. It is alsoworth noting that RA alone can be associated with thedevelopment of TB [4]; however, TNF alpha inhibitorswould appear to increase the risk beyond what would beexpected to be related to RA alone.
The above studies have limitations in assessing theimpact of these medications on TB risk because of overalldecreasing overall rates of TB worldwide, varying TBnotification rates by country, and a prescribing bias of anti-TNF alpha inhibitors. The absence of a gold standard testfor latent TB infection also complicates assessing risk ofreactivation of latent TB. We know that false positives oftuberculin skin testing (TST) and whole blood interferon-gamma release assay cannot distinguish successful immunesystem eradication or persistence of infection, and falsenegatives in patients with active RA or whilst receiving
other immunosuppressive treatments complicate the assess-ment of the true impact of TNF alpha inhibitors on TB risk.
Among the TNF alpha inhibitors, there are differences inthe risk of TB reactivation, with infliximab showing ahigher risk compared to etancercept, and data so far onadalimumab suggest that its risk is similar to that ofinfliximab [9]. After the approval of adalimumab in 2003by the European Medicines Agency and the initiation ofroutine TB screening prior to commencement of treatment,a total of 23 TB reactivation cases were reported (0.33/100PYs) over a 3-year period in the drugs’ post marketingsurveillance data [5, 10]. Other than the case we presenthere, there have been to date no documented cases ofisolated cerebral tuberculoma with adalimumab and justtwo reports of a cerebral and cerebellar tuberculomafollowing infliximab [1, 8].
CNS tuberculosis overall accounts for almost 1% of TBcases worldwide, manifesting as tuberculous meningitis,and less commonly as tuberculous encephalitis, tuber-culoma, and tuberculous brain abscess. Groups susceptibleinclude children and HIV-infected patients, and riskfactors include alcoholism, malignancy, and the concom-itant use of immunosuppressive agents. Even withtreatment, the mortality rate of CNS tuberculosis can varyfrom 20% to 80% depending on the time of presentation[12]. Deaths from CNS tuberculoma alone are moredifficult to quantify probably due to the relative rarity ofcases worldwide and lack of registries in the underdevel-oped or developing countries where the majority of thecases occur.
The relative rarity of cases worldwide has meant thatCNS TB remains a difficult diagnostic challenge. Tradi-tional CSF analysis identifying AFB on smear and cultureis the most widely used method for diagnosis; however,sensitivity is highly variable, in part due to the typicalscarcity of AFB in CNS tuberculosis, their relative absencein tuberculoma cases, the volume of CSF collected, andtime taken examining the sample. Molecular techniquesused such as nucleic acid amplification (NAA) and otherPCR-based methods, which accurately and rapidly diagnoseTB in respiratory specimens, are being evaluated now fortheir applicability in the diagnosis of CNS tuberculosis. Thetypical lack of bacilli present in CSF in CNS tuberculosisand tuberculomas in particular, as well as the presence ofamplification inhibitors in CSF, means that NAA and PCRmethods have been shown to have a poor sensitivity of 56%and are not ideal for ruling out CNS tuberculosis [13]. Onestudy confirmed this by comparing PCR assays in thediagnosis of tuberculomas. They demonstrated that immu-nohistochemical techniques outperformed PCR on surgical-ly resected tuberculomas [14].
Clinical judgment remains paramount in the diagnosis ofCNS tuberculosis even with negative diagnostic tests.
Clin Rheumatol (2010) 29:1201–1204 1203

Molecular techniques may not the best method in thediagnosis, but may be useful as an adjunct to traditionalhistology and culture or in monitoring response totreatment. For tuberculomas, MRI can further improve thediagnostic accuracy. Typical findings include solitary ormultiple, round “nodules,” often located in the frontal orparietal lobes, with irregular walls and showing ringenhancement after contrast. Standard treatment of CNStuberculosis is 2 months of isoniazid, rifampin, pyrazina-mide, and ethambutol followed by 7–10 months ofisoniazid and rifampin. The use of dexamethasone inaddition is relatively controversial with some mortalitybenefit among children and non-HIV-infected adults [15]
It is assumed that most cases of TB in patients on anti-TNF inhibitors or other immunosuppressives are due toreactivation of latent infection and would therefore bepreventable with treatment for LTBI. However, patientsliving or born in TB endemic countries could be also at riskfor newly acquired infection. At present, there are limitedcase reports on the incidence of new TB due to TNF alphainhibitors, and recent data from the AERS database wouldsuggest that as more patients undergo TST and are treatedfor LTBI, it is possible that the difference in rates betweeninfliximab and etanercept will be reduced in regions withhigh TB prevalence and that time to TB onset aftertreatment initiation will increase [7].
Screening for latent TB prior to anti-TNF treatment andthe initiation of prophylaxis (isoniazid for 9 months) whereindicated is paramount in reducing the overall numbers ofreactivation cases. Currently, the CDC recommends that aninduration of ≥5 mm diameter be considered a positive PPDresult in patients with RA. Together with a careful historyand chest X-ray, the effectiveness of pre-treatment screen-ing can be maximized and prophylaxis can be initiated forlatent TB cases where appropriate. Benefits in screening forLTBI were demonstrated in the Spanish registry BIOBA-DASER [11], after recommendations were implemented in2002. They showed a 74% reduction in TB cases in patientswith RA on infliximab, but still demonstrated an overallincreased risk compared to the general population. As ourcase highlights, despite a negative screening test, it isalways necessary to consider TB in patients being treatedwith TNF alpha inhibitors as screening may be complicatedby a number of factors. These include the lack of a goldstandard test for latent TB, false negatives in patients withactive TB or the immunocompromised RA patient, and thedifficulty in distinguishing between reactivation and pro-gression of a newly acquired TB infection. As we continueto see more widespread use of TNF alpha inhibitors in thetreatment of RA, further work will be needed on improvingthe accuracy of diagnostic screening techniques for LTBIand prevent the potential devastating manifestation of CNStuberculosis.
Disclosures The following case report is authored by Karen LynchMD and Michael Farrell MD. It adheres to ethical guidelines andstandards, and there was no financial or other conflict of interestsassociated with this case or the authors.
References
1. Alonso-Ruiz A, Pijoan JI, Ansuategui E, Urkaregi A, CalabozoM, Quintana A (2008) Tumor necrosis factor alpha drugs inrheumatoid arthritis: systemic review and metanalysis of efficacyand safety. BMC Musculoskelet Disord 17(9):52, Review
2. Dimakou K, Papaioannides D, Latsi P, Katsimboula S, Koran-tzopoulos P, Orphanidou D (2004) Disseminated tuberculosiscomplicating anti-TNF-α treatment. Int J Clin Pract 58(11):1052–1055
3. Askling J, Fored CM, Brandt L et al (2005) Risk and casecharacteristics of tuberculosis in rheumatoid arthritis associatedwith tumor necrosis factor antagonists in Sweden. ArthritisRheum 52:1986–1992
4. Bongartz T, Sutton AJ, Sweeting MJ et al (2006) Anti-TNFantibody therapy in rheumatoid arthritis and the risk of seriousinfections and malignancies: systematic review and meta-analysisof rare harmful effects in randomized controlled trials. JAMA 295(19):2275–2285
5. Furst DE, Schiff MH, Fleischmann RM et al (2003) Adalimumab, afully human anti tumor necrosis factor-alpha monoclonal antibody,and concomitant standard antirheumatic therapy for the treatment ofrheumatoid arthritis: results of STAR (Safety Trial of Adalimumab inRheumatoid Arthritis). J Rheumatol 30(12):2563–2571
6. Keane J, Gershon S et al (2001) Tuberculosis associated withinfliximab, a tumor necrosis factor-neutralizing agent. N Engl JMed 345:1098–1104
7. Raval A, Akhavan-Toyserkani G et al (2007) Brief communica-tion: characteristics of spontaneous cases of tuberculosis associ-ated with infliximab. Ann Intern Med 147:699
8. Ubukata M et al (2005) Pulmonary TB with cerebellar lesion in apatient after anti-TNF alpha (infliximab) treatment for rheumatoidarthritis. Nihon Kokyuki Gakkai Zasshi 43(4):247–51, Article inJapanese
9. Fonscea JE, Canhao H, Silva C et al (2006) Tuberculosis inrheumatic patients treated with tumor necrosis alpha antagonists:the Portuguese experience. Acta Reumatol Port 31:247–253
10. Schiff et al (2006) Safety analyses of adalimumab (Humira) inglobal clinical trials and US postmarketing surveillance of patientswith rheumatoid arthritis. Ann Rheum Dis 65:889–894
11. Carmona L, Gomez-Reino JJ, Rodriguez-Valverde V et al (2005)Effectiveness of recommendations to prevent reactivation of latenttuberculosis infection in patients treated with tumor necrosis factorantagonists. Arthritis Rheum 52:1766–1772
12. CDC. Extrapulmonary tuberculosis cases and percentages of siteof disease: reporting areas, 2005. Centers of Disease Control andPrevention, Atlanta, Georgia. www.cdc.gov/tb/surv/surv2005/PDF/table27.pdf
13. Pai M, Flores L et al (2003) Diagnostic accuracy of nucleic acidamplification tests for tuberculous meningitis: a systematic reviewand meta-analysis. Lancet Infect Dis 3:633–643
14. Sumi MG, Mathai A et al (2001) Diagnostic utility of polymerasechain reaction and immunohistochemical techniques for thelaboratory diagnosis of intracranial tuberculoma. Clin Neuro-pathol 20:176–180
15. Thwaites GE, Nguyen DB et al (2004) Dexamethasone for thetreatment of tuberculous meningitis in adolescents and adults. NEngl J Med 351:1741–1751
1204 Clin Rheumatol (2010) 29:1201–1204