Building the Foundation: Overview of Hemophilia Novel ......CSL Behring, Alnylam, Novo Nordisk, HEMA...
Transcript of Building the Foundation: Overview of Hemophilia Novel ......CSL Behring, Alnylam, Novo Nordisk, HEMA...

Building the Foundation:Overview of Hemophilia Novel
Therapies Pipeline Steven Pipe, MD
University of MichiganAnn Arbor, MI, USA
caNEXTions05 November 2019

Disclosures
• Contract research: Siemens• Steering Committee (clinical trials): Biomarin, uniQure, Bayer• Scientific Advisory Board: Sangamo• Consultant: Shire, Pfizer, Biomarin, uniQure, Bayer, Roche/Genentech,
CSL Behring, Alnylam, Novo Nordisk, HEMA Biologics, Bioverativ, Catalyst Biosciences, DNArx, Spark Therapeutics
• Board Member: Chair – Medical and Scientific Advisory Council to the National Hemophilia Foundation

Potentialgene therapy
Hemophilia: current and future approaches to care
Pipe SW. Hematology Am Soc Hematol Educ Program. 2016;2016(1):650-656.
Pre-replacementtherapy
Replacementtherapy
Non-replacementtherapy
On demand Prophylaxis
Standardhalf-life
Extendedhalf-life
Mimetics/agonistsSubstitution therapy
AntagonistsHemostatic rebalancing

Potentialgene therapy
Hemophilia: current and future approaches to carePre-replacement
therapyReplacement
therapyNon-replacement
therapy
On demand Prophylaxis
Standardhalf-life
Extendedhalf-life
Mimetics/agonistsSubstitution therapy
AntagonistsHemostatic rebalancing
Tool
s of
our t
rade
Plasma-derivedclotting factors
Recombinantclotting factors
Unmodified Bioengineered
Supportivecare only
Bispecific antibodies
siRNA knockdown
mAb inhibitors
Bioengineered serpins
Gene addition
Gene editing
Cellular therapy
Pipe SW. Hematology Am Soc Hematol Educ Program. 2016;2016(1):650-656.

Joint Bleeding and Hemophilia Severity
• Patients with severe hemophilia (FVIII <1%) experience more joint bleeding than do those with moderate (FVIII 1-5%) or mild (FVIII >5%) disease
Adapted from: Den Uijl et al. Haemophilia. 2011;17(6):849-853.
5

Prophylaxis across the US HTC Network
Manco-Johnson MJ et al. Blood. 2017;129(17):2368-2374.

Bleed rates and range of motion across the US HTC Network
Prophylaxis in 1999
Prophylaxis in 2010
No prophylaxis in 1999
No prophylaxis in 2010
Patient treatment by year:
Manco-Johnson MJ et al. Blood. 2017;129(17):2368-2374.

The Challenge with Traditional Clotting Factor Replacement Therapy
Mahdi et al., Br J Haematol (2015)

Trends in modern hemophilia therapeutics
• Shift from “minimally effective” prophylaxis to “optimized/personalized prophylaxis”• Emphasis on higher trough levels through:
• More intensive prophylaxis• Utilization of extended half-life clotting factors
• Bioengineered molecules with enhanced properties• Steady-state prophylaxis rather than “peaks and troughs”• Subcutaneous delivery over intravenous• Cross-segment therapeutics
• Efficacy in presence and absence of inhibitors• Efficacy across a number of bleeding disorders

EHL Factors and Trough Levels• Esperoct (N8-GP)
– Prophylaxis regimen of 40 IU/kg q4d– t1/2 = 18.4 hours; mean trough of 8%– ABR (median) = 1.3
• Rebinyn– Prophylaxis regimen of 10 IU/kg or 40 IU/kg weekly– t1/2 = 110 hours, mean trough of 9.8% and 21.3%, respectively– 40 IU/kg weekly: two-thirds reported complete resolution of
target joints
• Idelvion– Prophylaxis regimen of 40 IU/kg weekly or 75 IU/kg q 2 weeks– t1/2 = 102 hours, mean trough of 20% and 12.4%, respectively– 100% resolution of target joints
10
Ehrenforth et al., Haemophilia. 2014;20(suppl 3):188. Young G et al., Thromb Res. 2016;141:69-76. Santagostino E et al., Blood. 2016;127(14):1761-1769.

Higher Trough Levels and Bleed Prevention
•PROPEL Study (Adynovate, Shire/Takeda)– use pharmacokinetic-guided prophylaxis targeting two different FVIII trough levels in severe hemophilia A
– Low trough (1-3%) vs High trough (8-12%)– assessed proportion with annualized bleed rate = 0
– high trough (66%) vs low trough (39%)
Klamroth R et al., Presented at EAHAD, Feb 2019

Chhabra et al., Managed Care (2018)
Switches to EHL Factor VIII Associated with Increased Utilization and Expenditures

Tortella et al., J Manag Care Spec Pharm (2018)
Switches to EHL Factor IX Associated with Increased Utilization and Expenditures

What Are the Unmet Needs that Remain for Hemophilia A?
• There would still be attraction for once-weekly dosing for some patients• Particularly for moderate severity, mild severity who need prophylaxis (even if for short
courses)
• Inhibitor prevention remains highest priority• Some optimism that product-related immunogenicity may be low with these EHLs
• Inhibitor eradication may actually be higher with EHLs1-3
• Biologic rationale for improved immunomodulatory impact of Fc• Anecdotal evidence of improved ITI with rFVIIIFc• Unknown with PEGylation, but also biologic rationale for reduced immunogenicity
• Need to show improved outcomes with EHLs to justify price
1. Janbain M, Pipe S. Hematology Am Soc Hematol Educ Program. 2016;2016(1):648-649.2. Carcao M...Pipe SW. Haemophilia. 2018;24:245–252 3. Blumberg et al. Blood. 2018; in press 14

Novel approaches to hemophilia therapy
Arruda VR et al. Blood. 2017;130:2251-2256

Procoagulants Anticoagulants
More Clotting More Bleeding
Normal Hemostatic Balance
Callaghan, Sidonio and Pipe. Blood (2018)

Procoagulants Anticoagulants
More Clotting More Bleeding
Hemophilia
Callaghan, Sidonio and Pipe. Blood (2018)

Procoagulants Anticoagulants
More Clotting More Bleeding
Hemophilia plus procoagulants
aPCCrFVIIa
FVIII/FIX
Emicizumab
Callaghan, Sidonio and Pipe. Blood (2018)

Background: Emicizumab
Humanised bispecific monoclonal antibody
Bridges activated factor IX (FIXa) and FX to restore function of missing FVIIIa
No structural homology to FVIII (not expected to induce FVIII inhibitors or be affected by presence of FVIII inhibitors)
Long half-life of ~30 days
Administered subcutaneously
Approved in several countries for once-weekly prophylaxis in persons with haemophilia A with inhibitors of all ages
19
Emicizumab
Factor IXaFactor X
Shima S, et al. N Engl J Med 2016;374:2044–53.Yoneyama K, et al. Clin Pharmacokinet 2017 Epub.HEMLIBRA (emicizumab-kxwh) [prescribing information]. 2017.
HEMLIBRA (emicizumab) [summary of product characteristics]. 2018.Oldenburg J, et al. N Engl J Med 2017; 377(9):809–18.

Emicizumab clinical trials
20
ABR, annualized bleeding rate; BPA, bypassing agent; PwHA, persons with Haemophilia A; PK, pharmacokinetics; Q2W, every 2 weeks; Q4W, every 4 weeks; QW, once weekly.
Clinical trial Population
ABR, treated bleeds:emicizumab prophylaxis vs noprophylaxis
% patients with zero treated bleeds
ABR, treated bleeds: emicizumab prophylaxis vs prior prophylaxis in NIS
HAVEN 1 (NCT02622321)
PwHA ≥12 years with FVIII inhibitors
87% reduction (QW)* 63% (QW), 6% (no prophylaxis)
79% reduction with emicizumab QW vs prior BPA prophylaxis
HAVEN 2 (NCT02795767)
PwHA <12 years with FVIII inhibitors
N/A (no comparator) 87% (QW) 99% reduction with
emicizumab QW vs prior BPA prophylaxis
HAVEN 3 (NCT02847637)
PwHA ≥12 years without FVIII inhibitors
96% reduction (QW) 97% reduction (Q2W)
56% (QW), 60% (Q2W), 0% (no prophylaxis)
68% reduction with emicizumab QW vs prior FVIII prophylaxis
HAVEN 4 (NCT03020160)
PwHA ≥12 years with or without FVIII inhibitors
Primary analyses evaluating emicizumab Q4W prophylaxis on bleeding rate, safety, PK
Oldenburg J, et al. N Engl J Med 2017;377:809–18. Mancucso, ME, et al. Blood 2017;130:1071.Young G, et al. Blood 2017;130:85.
Genentech Press Release. Nov 19, 2017.Mahlangu J, et al. Presented at WFH 2018. Abstract 854.
*Improved bleeding rate observed in subsequent 24-week periods beyond initial 24-weeks.

HAVEN 1–4 long-term efficacy: treated bleeds over time• Across HAVEN 1─4, the model-based ABR* for treated bleeds was 1.5 (95% CI, 1.20; 1.84)
over the median 83-week duration of exposure
*Calculated using negative binomial regression; †based on calculated ABRs. ABR, annualized bleed rate.Callaghan M et al. Presented at ISTH; July 6‒10, 2019; Melbourne, Australia.
Treated bleed ABRs† over consecutive24-week treatment intervals (N=400)
Proportion of participants with 0 or 1–3 treated bleeds over time (N=400)
0
1
2
3
4
5
6
7
1–24 weeks 25–48 weeks 49–72 weeks 73–96 weeks
Calc
ulat
ed A
BR
n 391 354 284 114
1.9
0.8 0.80.30 0 0 0
Mean ABR (95% CI) Median ABR (IQR)
70.879.4 82.7
88.622.5
18.6 14.4 11.4
0102030405060708090
100
1–24 weeks 25–48 weeks 49–72 weeks 73–96 weeks
Perc
enta
ge o
f par
ticip
ants
0 bleeds 1–3 bleeds
n 391 354 284 114

PK and efficacy modelling for different emicizumab dosing regimens
1.5 mg/kg QW
Mod
elle
d AB
R
3 mg/kg Q2W 6 mg/kg Q4W
PK m
odel
ling
All 3 regimens were expected to achieve clinically efficacious concentrations and provide similar efficacy
All dosing regimens begin with loading period of 3 mg/kg/week for 4 weeks, followed by maintenance dose as indicated
5
Yoneyama K, et al. Clin Pharmacokinet 2017 Epub.
120
90
Plas
ma
emic
izum
abco
ncen
tratio
n (µ
g/m
L)
60
30
0
0 4 8 12 16 20 24
60
40
50
Prop
ortio
n of
pat
ient
s w
ith e
ach
annu
al b
leed
ing
rate
(%)
30
20
10
00 1 32 4 5 76 8
60
40
50
Prop
ortio
n of
pat
ient
s w
ith e
ach
annu
al b
leed
ing
rate
(%)
30
20
10
00 1 32 4 5 76 8
60
40
50
Prop
ortio
n of
pat
ient
s w
ith e
ach
annu
al b
leed
ing
rate
(%)
Annual bleeding rate
30
20
10
00 1 32 4 5 76 8
120
90
Plas
ma
emic
izum
abco
ncen
tratio
n (µ
g/m
L)
60
30
0
0 4 8 12 16 20 24
120
90
Plas
ma
emic
izum
abco
ncen
tratio
n (µ
g/m
L)
Time after starting emicizumab (week)
60
30
0
0 4 8 12 16 20 24
Annual bleeding rate
Time after starting emicizumab (week)
Annual bleeding rate
Time after starting emicizumab (week)

Procoagulants Anticoagulants
More Clotting More Bleeding
Hemophilia
Callaghan, Sidonio and Pipe. Blood (2018)

Procoagulants Anticoagulants
More Clotting More Bleeding
Hemophilia minus anticoagulants
Callaghan, Sidonio and Pipe. Blood (2018)

New Targets
Achneck et al Circulation 2010;122:2068–2077
TF VIIa
X Xa
IXa IX
XVa
thrombin
XIa XI
fibrinformation
XIIaExtrinsic pathway
Intrinsic pathway
Common pathway
VIIIa VIIITFPI
Anti-thrombin
PF-06741086 Concizumab
Fitusiran
Emicizumab
APCSerpinPC

26Machin and Ragni (2018)
Potential advantages of RNAi technology for the treatment of haemophilia
Therapeutic hypothesis
Individuals with haemophilia who have co-inherited thrombophilia mutations – such as AT deficiency –have milder bleeding phenotypes: thus AT lowering represents a rational therapeutic strategy for bleed control in haemophilia
Fitusiran is a nonbiologic therapy with an N-acetylgalactosamine(GalNAc) ligand for targeted delivery to the liver, site of antithrombin (AT) synthesis

0
20
40
60
80
100
120
0 3 6 9 12 15 18 21 24 27 30 33 36
Antit
hrom
bin
(%)
Months since first dose
AT lowering with monthly fitusiran1,*
Fitusiran Phase 2 OLE Interim Results:Antithrombin Levels and Thrombin Generation
Fitusiran is an investigational medicine. Its safety and efficacy have not been established by any health authorities.Data cutoff: May 2, 2019. *Data indicate mean AT or mean TG peak height ± SE. Data include period of dosing resumption after the clinical hold and exclude period during the clinical hold. Month 0 indicates baseline values. †HVs with AT lowering <25%.2‡n=12 for month 1 and n=11 for month 2 (50 mg); n=21 for month 1 and n=19 for month 2 (80 mg). §n=10 for months 1 and 2 (50 mg); n=20 for month 1 and n=19 for month 2 (80 mg).AT, antithrombin; HV, healthy volunteer; OLE, open-label extension; SE, standard error; TG, thrombin generation.1. Sanofi Genzyme. Data on file. 2019. 2. Pasi KJ et al. N Engl J Med 2017;377:819–828. 27
Fitusiran 50 mg monthly (n=12)Fitusiran 80 mg monthly (n=22)
n‡= 12 11 11 10 7 7 2 1 2 3 7 5 5n‡= 22 18 14 12 11 5 5 3 4 12 11 8 4
HVrange†
n§= 12 11 10 9 6 5 1 1 2 2 7 5 5n§= 22 17 14 10 9 3 5 3 4 10 11 9 4
0
20
40
60
80
100
120
140
0 3 6 9 12 15 18 21 24 27 30 33 36Thro
mbi
n ge
nera
tion
peak
hei
ght,
nmol
/L
Months since first dose
TG with monthly fitusiran1,*
Fitusiran 50 mg monthly (n=12)Fitusiran 80 mg monthly (n=22)

0
4
8
12
16
28
Fitusiran is an investigational medicine. Its safety and efficacy have not been established by any health authorities.Data transfer: May 2, 2019. ABR and duration represent pooled data from Phase 1 and Phase 2 OLE studies. Phase 1 data are included if gap between studies was ≤56 days. Only subjects with 28 days of follow-up during the observation period are included in this analysis.
Fitusiran Phase 2 OLE Interim Results:Exploratory Analysis of Bleeding Events
ABR in subjects with inhibitors
Median duration in observation period: 25 months (range: 5–33 months)
Median duration in observation period: 18 months (range: 7–25 months)
Med
ian
ABR
2.0
12.0
1.08
Pre-study
Prophylaxis (n=7)
On demand(n=12) Observation
period (n=19)
05
101520253035404550
Med
ian
ABR
42.0
1.04
Observation period (n=14)
Pre-study(n=14)
ABR in subjects without inhibitors
Overall median ABR of 1.08

29
These data are from an ongoing clinical program and results may be subject to change.Fitusiran is an investigational medicine. Its safety and efficacy have not been established by any health authorities.Data cutoff: May 2, 2019. AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; HCV, hepatitis C virus; OLE, open-label extension; ULN, upper limit of normal.
Fitusiran Phase 2 OLE Interim Results: Overview of Safety and Tolerability• AEs were reported in 33 of 34 subjects
‒ The majority of AEs were mild or moderate in severity and unrelated to the study drug‒ The most common AEs (occurring in ≥15% of subjects overall) were: ALT increased (32% of subjects); headache
(24%); injection-site erythema (21%); arthralgia (18%)
• Increases in ALT >3× ULN were observed in 14 subjects (all confirmed HCV antibody positive at baseline)
‒ All asymptomatic and resolving, with no elevations of bilirubin >2x ULN
• No instances of anti-drug antibody formation• A total of 14 serious adverse events (SAEs) were reported in 8 of 34 subjects
‒ 3 with SAEs considered possibly related to study drug ‒ Asymptomatic ALT and AST elevation in subject with chronic HCV infection; led to discontinuation
‒ Seizures in subject with history of seizure disorder (CT and MRI negative for bleed and thrombosis)
‒ Fatal cerebral venous sinus thrombosis (CVST)
• Since implementation of the new bleed management guidelines, developed to mitigate the risk of thrombosis, there have been no related thrombotic events

Protocol Factor VIII Factor IX (SHL) Factor IX (EHL) aPCC rFVIIaRecommended single dose 10 IU/kg 20 IU/kg 20 IU/kg 30 U/kg ≤45 μg/kg
Single dose should not exceed 20 IU/kg 30 IU/kg 30 IU/kg 50 U/kg 45 μg/kg
Repeat dosing
Must call clinical study center before second dose*; evaluation and treatment at clinical study center should be considered
Must call clinical study center
before third dose†
Should not repeat in <24 hours Should not repeat in <5–7 days
Should not repeat in <24 hours
Should not repeat in <2 hours
For situations requiring higher doses, more frequent administration, or multiple repeat doses, discussion with study medical monitor and clinical advisor is recommended, and AT replacement should be considered
Antifibrinolytics should not be used in combination with replacement factor or bypassing agent
Fitusiran is an investigational medicine. Its safety and efficacy have not been established by any health authorities.These bleed management recommendations were implemented after the lift of the Phase 2 OLE clinical hold.*Should be seen at site within 48–72 hours if >2 doses are required. †Should be seen at site within 48–72 hours if >3 doses are required.aPCC, activated prothrombin complex concentrate; AT, antithrombin; EHL, extended half-life; OLE, open-label extension; rFVIIa, recombinant activated factor VII; SHL, standard half-life. Sanofi Genzyme. ALN-AT3SC-002 Clinical Study Protocol Amendment 5. May 31, 2018.
Recommendations for Bleed Management During Fitusiran Prophylaxis
30

Management of Bleeds in Phase 2 OLE with New Recommendations15 subjects had ≥ 1 bleed, and 12 of these subjects (80%) were compliant with the recommendationsa
Treatment of Bleeds
Factor VIII(n=6)
Factor IX(n=2)
aPCC(n=2)
rFVIIa(n=4)
Total treated bleeds 26 4 25 54
Administrations per bleed, mean (range) 1.3 (1-4) 2.0 (1-3) 1.2 (1-2) 2.0 (1-5)
Mean dose per injection (range)
13.5 IU/kg(5 - 25 IU/kg)
11.5 IU/kg(8 - 21 IU/kg)
32.5 U/kg(27 - 57 IU/kg)
70.9 μg/kg(34 – 105
IU/Kg)
Bleed Causality1
Spontaneous, %Traumatic, %
4654
0100
7228
6731
Bleed Location2
Joint, %Muscle, %Other, %
9280
502525
9280
9622
a3 subjects considered non-compliant: 1 subject had higher than recommended doses of BPA to control multiple bleeds; 1 subject had repeat doses of BPA prior to the recommended 24-hour interval; and 1 subject had a higher than recommended dose of BPA to control a single bleed. Additional training was provided for the personnel and subjects at these centers.OLE, open-label extension; aPCC, activated prothrombin complex concentrate; BPA, bypassing agentPasi J et al., ISTH 2019 Poster Presentation (PB0324)
11 other cause of bleed was an operation (1 bleed, rFVIIa)2Other bleed locations included skin/mucosa (1 bleed, factor IX) and operative site (1 bleed, rFVIIa).
*Of 109 treated bleeds, 2 were not accessed at time of data transfer (November 19, 2018) Note: of the 13 bleeds that required repeat dosing and were treated non-compliantly: 9 occurred in 1 subject, and 4 in a second subject

Conclusions• Monthly fitusiran dosing demonstrated a durable therapeutic effect,
including sustained AT lowering, increased TG, and low ABR• Effect of fitusiran on AT lowering is reversible upon dose interruption and can be
restored with resumption of dosing• Lower doses of factor or BPA may provide adequate haemostatic
protection against bleeds for people receiving fitusiran prophylaxis • Promising investigational approach for rebalancing hemostasis in patients with
hemophilia A or B, with or without inhibitors• The safety and efficacy of fitusiran is now being evaluated in the ongoing
global Phase 3 ATLAS program*
32
*ATLAS trials: NCT03417102, NCT03417245, NCT03549871.Fitusiran is an investigational medicine. Its safety and efficacy have not been established by any health authorities

Evolving trends with modern therapeutics for haemophilia A
Factor replacement therapy• Breaking the VWF ceiling – FVIII divorced
from VWF
• Once weekly dosing now possible
• SQ vs IV
• PK-directed therapy
• Flexibility – “normalize” hemostasis as needed
• Routine monitoring available
• Prophylaxis and breakthrough bleeding may be treated
Non-factor replacement therapy• Fixed dosing – weekly to monthly
• Subnormal to “normalized” hemostasis
• No routine monitoring
• Limited to no personalization for optimization of prophylaxis
• Liberation from routinely “having to think about their hemophilia”
• Advantage of steady-state correction
• Continued reliance on factor replacement therapy

Drivers for choice/preference of current therapeutics
Factor replacement therapy• Physician-driven
• Familiarity and flexibility
• Addresses the physiologic defect
• Concern regarding potential FVIII functions not corrected with non-replacement therapies
• Monitoring for clinical management
• Patient-driven• Inertia related to legacy treatment
• Comfort with peak correction as needed
• No perceived medical need to change
• Concern with observed or unknown AEs
Non-replacement therapy• Physician-driven
• Medically-driven necessity• Better bleed control, adherence
• Venous access challenges
• Experience suggesting a new standard for prophylaxis
• Opportunity for inhibitor avoidance?
• Patient-driven• Lower frequency of treatment
• Route of administration easier
• Worry about bleeds less
• Satisfaction with improved bleed control

Current FVIII Treatments Are Subject to a VWF-Imposed t1/2 Ceiling1
Increasing the half-life of rFVIII is ultimately dependentupon decoupling FVIII and endogenous VWF
35
EHL, extended half-life; FVIII, factor VIII; PEG, polyethylene glycol; rFVIIIFc, recombinant factor VIII Fc fusion protein; t1/2, elimination half-life; VWF, von Willebrand factor.1. Pipe SW. Am J Hematol. 2012;87(Suppl 1):S33-S39. 2. Mahlangu J, et al. Blood. 2014;123(3):317-325. 3. Tiede A, et al. J Thromb Haemost. 2013;11(4):670-678. 4. Coyle TE, et al. J Thromb Haemost. 2014;12(4):488-496. 5. Konkle BA, et al. Blood. 2015;126(9):1078-1085.
Technology for FVIII EHL t1/2Conventional FVIII
comparator t1/2
Fc fusion (rFVIIIFc) 19.0 h2 12.4 h
Glyco-PEGylation (N8-GP) 19.0 h3 11.7 h
Cys variant-PEGylation (BAY 94-9027) 18.7 h4 13.0 h
Amino group-PEGylation (BAX 855) 14.3 h5 10.4 h
VWF binding to FVIII
VWF monomer
VWF
mul
timer

BIVV001: The First FVIII Therapy Designed to Break the VWF-Imposed t1/2 Ceiling
36BIVV001 is an investigational product that has not been proven to be safe or effective; XTEN technology in-licensed from Amunix Operating, Inc. FVIII, factor VIII; rFVIIIFc, recombinant factor VIII Fc fusion protein; t1/2, elimination half-life; VWF, von Willebrand factor.
C1 C2
D’D3
Fc
A1
A2
A3
C1 C2
D’D3
Fc
Fc
rFVIIIFc-VWF-XTEN
BIVV001
VWF D’D3 domain to decouple FVIII from
VWF
XTEN insertionsto increase t1/2
C1 C2
D’D3
Fc
A1
A2
A3
C1 C2
D’D3
Fc
Fc C1 C2
D’D3
Fc
A1
A2
A3
C1 C2
Fc
Fc
Activated rFVIIIFc
Activated BIVV001 EqualsEffects of Activated Thrombin
rFVIIIFc VWF XTEN

Single 65 IU/kg Dose of BIVV001 Extends FVIII t1/2 to 43 Hours
37AUC0–inf, total area under the curve from time zero to infinity; CL, clearance; Cmax, maximum concentration; FVIII, factor VIII; IR, incremental recovery; MRT, mean residence time; PK, pharmacokinetic; rFVIII, recombinant factor VIII; SD, standard deviation; t1/2, elimination half-life.
The mean (SD) FVIII activity post-infusion at 5 days was 38% (10) and at 7 days was 17% (5)
1
1 0 0
D a y s a fte r e n d o f in fu s io n
Me
an
pla
sma
FV
III
act
ivity
(IU
/dL
)
10 2 3 4 5 7 1 0
5 %
3 %
1 %
1 2 1 4
1 01 0 %
6 5 IU /k g rF V III (n = 9 )
6 5 IU /k g B IV V 0 0 1 (n = 8 )
2 0 %1 7 %
3 8 %
PK parameter 65 IU/kg BIVV001 (n=8)a
65 IU/kg rFVIII(n=9)a Mean ratiob
t1/2, h42.85
(38.28–48.55)13.15
(9.96–18.23)3.24 (2.76–3.79)
P<0.001
Cmax, IU/dL 159(117–198)
138(93–173)
1.17 (1.09–1.25)P<0.001
AUC0–inf, h × IU/dL 12,800(9440–17,100)
1960(1440–2550)
6.54 (5.89–7.27)P<0.001
MRT, h 68.1(56.9–75.7)
15.7(13.2–19.2)
4.32 (3.96–4.72)P<0.001
CL, mL/h/kg 0.51(0.38–0.69)
3.31(2.56–4.49)
0.15 (0.14–0.17)P<0.001
IR, IU/dL per IU/kg 2.45(1.80–3.04)
2.11(1.42–2.66)
1.18 (1.10–1.26)P<0.001
FVIII activity determined by the one-stage activated partial thromboplastin clotting assay.aValues are geometric means (range).bValues are mean ratio (95% confidence interval).

Novel approaches to hemophilia therapy
Arruda VR et al. Blood. 2017;130:2251-2256

P. ClementPEN May 2017L.A. Kelly Communications Inc.

Pre-clinicalDevelopment
First Waveof Clinical Trials
Proof of Conceptin Hemophilia B
Current Waveof
Clinical Trials
Pre-2000’s 1996-2006 2010-2014 2014-present
More Pre-clinical Studies

Overview of adeno-associate virus (AAV)-mediated liver-directed gene therapy
Doshi and Arruda, Ther Adv Hematol (2018)

0
10
20
30
40
50
1 11 21 31 41 51 61 71 81 91 101 111 121 131 141 151 161 171 181
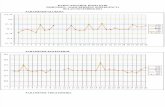
FIX
activ
ity (I
U/d
L)
1 (7.2) 2 (5.3) 3 (1.5) 4 (8.2) 5 (3.5)
0
10
20
30
40
50
0 10 20 30 40 50 60 70 80 90 100 110 120 130 140 150
FIX
activ
ity (I
U/d
L)
6 (11.2) 7 (7.1) 8 (8.4) 9 (3.9) 10 (6.7)
Sustained dose-dependent increases in FIX activity
Values in parentheses represent mean FIX activity over time. Only values at least 10 days after last FIX concentrate administration are included. FIX prophylaxis was continued after AMT-060 and tapered between Weeks 6 and 12 *Patient retrospectively tested positive for AAV5 neutralizing antibodies using the luciferase-based assay. 3 patients were presumed cross-reactive matter positive. FIX, factor IX; CI, confidence interval; IU, international units 42
Cohort 1Steady state mean FIX activity (95%CI):
5.1 (1.7 – 8.5)
Cohort 2Steady state mean FIX activity (95%CI):
7.5 (4.1 – 10.8)
Weeks following AMT-060 treatment
FIX activity levels correlated approximately 1:1 with FIX protein expression
* * *

Reductions in FIX use and bleeds sustained over long term follow up (Cohort 1)
Mean FIX consumption excludes surgical procedures 43
4.0
1.50.5 0.8
0
2
4
6
8
10
12
14
16
Cohort 2
Mea
n an
nual
ized
tota
l ble
eds
(n)
Pretreatment Year 1 Year 2
Annualized Bleed Rate (Cohort 2)Annualized Bleed Rate (Cohort 2)
14.4
7.6
2.8
6.2
1.7
0
2
4
6
8
10
12
14
16
Cohort 1
Mea
n an
nual
ized
tota
l ble
eds
(n)
Annualized Bleed Rate (Cohort 1)
Pretreatment Year 1 Year 2 Year 3
354,800
64,00031,700
60,84223,817
0
50,000
100,000
150,000
200,000
250,000
300,000
350,000
400,000
Mea
n to
tal a
nnua
lized
FIX
repl
acem
ent (
IU)
Mean FIX consumption (Cohort 1)
Reduction relative to pre-AMT-060 FIX use Bleeds
Year 1 82% 47%Year 2 91% 81%Year 3 83% 57%Year 4 93% 88%
Year 4 Pretreatment Year 1 Year 2 Year 3 Year 4

Reductions in FIX use and bleeds sustained over long term follow up (Cohort 2)
44
4.0
1.50.5 0.8
0
2
4
6
8
10
12
14
16
Cohort 2
Mea
n an
nual
ized
tota
l ble
eds
(n)
Pretreatment Year 1 Year 2 Year 3
Annualized Bleed Rate (Cohort 2)Annualized Bleed Rate (Cohort 2)
4.0
1.40.6 0.7
0
2
4
6
8
10
12
14
16
Cohort 2
Mea
n an
nual
ized
tota
l ble
eds
(n)
Pretreatment Year 1 Year 2 Year 3
Annualized Bleed Rate (Cohort 2)
173,200
38,60014,600 7,278
0
50,000
100,000
150,000
200,000
250,000
300,000
350,000
400,000
Mea
n to
tal a
nnua
lized
FIX
repl
acem
ent (
IU)
Pretreatment Year 1 Year 2 Year 3
Mean FIX consumption (Cohort 2)
Reduction relative to pre-AMT-060
FIX use Bleeds
Year 1 78% 65%Year 2 92% 85%
Year 3 96% 83%
Mean FIX consumption excludes surgical procedures

Introduction: gene therapy for hemophilia B: AMT-060/AMT-061
AAV5 capsid Liver-specific promoter & human FIX gene
AMT-060 – wildtypeClinically demonstrated safe and durable3 increases in FIX
activity with meaningful improvements in clinical outcomes36,7
AMT-061 – Padua variant (expected 6- to 7-fold increase in activity)
• Low prevalence of pre-existing neutralizing antibodies able to impact clinical outcomes1,4
• Previously tested in humans without sign of cellular immune activation2
AMT-061AGG to CTG in gene resulting in R338L in protein
1. Boutin et al, Human Gen Ther 2010; 21(6):704-12. 2. D’Avola et al, Journal of Hepatology 2016; doi: http://dx.doi.org/10.1016/j.jhep.2016.05.012. 3. Nathwani et al. NEJM 2014; 371:1994-2004. 4. Majowicz et al, ASGCT 2018

54.1
30.1
50.9
0
10
20
30
40
50
60
70
0 2 4 6 8 10 12 14 16 18 20 22 24 26 28 30 32 34 36
FIX
activ
ity o
ne-s
tage
aPT
T (%
of n
orm
al)
Week
Participant 1Participant 2Participant 3
AMT-061 Efficacy: FIX activity at 36 weeks post-treatment
46
Mean FIX activity at 36 weeks: 45.0%
*
aPTT, FIX, Factor IX. No immunosuppression required. *May include activity from exogenous FIX replacement. activated partial thromboplastin time;
*

AMT-061 Phase 2b: Conclusions and next steps
AE, adverse event; FIX, Factor IX; NAbs, neutralizing antibodies. 47
AMT-061 was generally well-tolerated with no serious AEs related to treatment All participants achieved clinically meaningful FIX activity:
FIX activity increased by week 1-2 Mean 45% of normal at week 36 FIX activity in the normal range for two of the three participants
No bleeds or associated use of factor replacement therapy No clinically significant liver enzyme elevations No loss of FIX activity or requirement for immunosuppression
The Phase 3 HOPE-B AMT-061 study (NCT03569891) is enrolling First patient treated early 2019 Expected to enroll approximately 55 participants with severe hemophilia B Those with pre-existing AAV5 NAbs will not be excluded





85% reduction in mean ABR from baseline where all patients were on standard of care prophylaxis
95% reduction in mean FVIII usage annualized after week 5


New paradigm of current and potential treatmentsSubstitution & hemostatic rebalancing therapies
Pros• SQ delivery, low burden• Steady state hemostasis• Pediatric and adult application• Inhibitor/non-inhibitor efficacy
Cons• Likely not achieving “normal”
but may be “curative”• Thrombotic risk• Assay issues• Managing peak bleeding risk events• Annual expense
Investigational gene therapy
Pros• “One and done”• Steady state hemostasis• “curative” levels if not even “normal”• Annual cost savings
Cons• Eligibility
• Not for pediatric or inhibitors (yet)• Pre-existing immunity
• Known/unknown risks• Immunologic, cellular stress, integration risk?
• Uncertain durability, ability for redosing• High initial costs