Bioremediation of Commercial Polychlorinated Biphenyl Mixture … · 2018-11-13 · ii...
Transcript of Bioremediation of Commercial Polychlorinated Biphenyl Mixture … · 2018-11-13 · ii...

Bioremediation of Commercial
Polychlorinated Biphenyl Mixture
Aroclor 1260 by Naturally Occurring
Microorganisms
Pathiraja Mudiyanselage Gathanayana Pathiraja
B.Sc. (Honours) in Microbiology
M.Sc. in Environmental Science and Technology
Submitted in Fulfilment of the Requirements for the Award of the
Degree of Doctor of Philosophy
Science and Engineering Faculty
Queensland University of
Technology
2018

Bioremediation of Commercial Polychlorinated Biphenyl Mixture Aroclor 1260 by Naturally Occurring Microorganisms i
Keywords
Alternating anaerobic-aerobic treatment, Aroclor 1260, Biodegradation, Bioremediation,
Biosurfactants, Dechlorination, Exoproteome, Facultative anaerobic microorganisms,
Oxidation, Metaproteomics, Polychlorinated biphenyls, Secretome, Two stage
anaerobic-aerobic treatment.

ii Bioremediation of Commercial Polychlorinated Biphenyl Mixture Aroclor 1260 by Naturally Occurring Microorganisms
Abstract
Polychlorinated biphenyls (PCBs) are a group of synthetic organic chemical
compounds that consist of chlorinated aromatic hydrocarbons. PCBs had been
extensively used in numerous industrial applications. Commercial production of PCBs
ceased in 1993 with the wide recognition to their toxicity, bioaccumulation and
persistence characteristics. However, PCBs are still causing harmful effects due to
their presence in environmental systems such as in soil and sediment. Use of
microorganisms for remediation of PCB-contaminated soil has been widely
investigated due to the potential of microorganisms for breaking down complex
contaminants into less harmful by-products. However, the application of microbial
based bioremediation is not yet commonly practiced as the effectiveness is
influenced by a complexity of factors related to the microorganisms involved, the
nature of the PCB mixture and the contaminated environment.
This study was based primarily on the hypothesis that the complete degradation of
complex commercial PCB mixtures can be achieved through a combination of
anaerobic-aerobic treatment when appropriate microbial mixtures capable of
enhancing the aqueous solubility and degradation of PCBs under both anaerobic and
aerobic conditions were incorporated. To test this hypothesis, a detailed
experimental program was undertaken for isolating and identifying suitable PCB
degrading microorganisms from the natural environment, screening the identified
microorganisms for biosurfactant production, determining the PCB degradation
potential of facultative bacterial cultures under separate and combined anaerobic
and aerobic conditions, individually, and as a consortium. Lastly, the detection and
identification of the extracellular proteins released by microorganisms into the
external environment and analysis of their potential roles in PCB hydrolysis were
performed.
Through a selective enrichment process, two obligate aerobic and four facultative
anaerobic bacterial strains capable of utilizing a commercial PCB mixture, Aroclor
1260, as sole source of carbon were isolated. They were identified as

Bioremediation of Commercial Polychlorinated Biphenyl Mixture Aroclor 1260 by Naturally Occurring Microorganisms iii
Chryseobacterium sp. NP01, Delftia sp. NP02, Achromobacter sp. NP03,
Ochrobactrum sp. NP04, Lysinibacillus sp. NP05 and Pseudomonas sp. NP06 using full
length 16S rRNA gene sequence based molecular identification. Based on the results
and after an exhaustive review of the published literature, this study can be
considered as the first evidence of reporting the presence of Chryseobacterium and
Delftia species and their involvement in the remediation of soil contaminated with
PCBs.
The extreme hydrophobicity of PCBs makes them insoluble in aqueous medium and
is one of the main rate limiting factors in PCB bioremediation. Biosurfactants are
known to aid in solubility of hydrophobic chemicals, before certain microbes are able
to use them for energy. The selected bacterial cultures discovered here were further
tested for their ability to produce biosurfactants. Chryseobacterium sp. NP01 and
Lysinibacillus sp. NP05 demonstrated the highest biosurfactant production, PCB
solubility and degradation. The study created new knowledge on the ability of PCB
degrading bacteria to also produce biosurfactants that correlated well with increasing
PCB solubility and subsequently enhanced bioavailability and degradation.
PCB degradation efficiency of four facultative anaerobic bacterial strains
Achromobacter sp. NP03, Ochrobactrum sp. NP04, Lysinibacillus sp. NP05 and
Pseudomonas sp. NP06 were compared in parallel under aerobic, anaerobic and
combined anaerobic-aerobic conditions. This research can be considered as the first
comparative study assessing facultative anaerobic bacteria in degrading PCBs under
anaerobic, aerobic and two-stage anaerobic-aerobic cultivation conditions. The study
found that the four bacterial strains are capable of degrading commercial PCB
mixture as their sole source of carbon under all three tested conditions, with the
highest degradation under two stage anaerobic-aerobic conditions. Lysinibacillus sp.
NP05 performed the best with 9.16±0.8 mg/L maximum chloride yield under two stage
anaerobic-aerobic treatment, resulting in the removal of about one third of the total
chlorines present in the commercial PCB product Aroclor 1260. This positive finding
proves that in soil remediation applications, facultative microorganisms are better
candidates to survive and achieve higher PCB degradation rates under both anaerobic
and aerobic conditions.

iv Bioremediation of Commercial Polychlorinated Biphenyl Mixture Aroclor 1260 by Naturally Occurring Microorganisms
The study outcomes confirmed that a whilst a single bacterium may initially seem to
possess positive characteristics, it is highly probable that one microbe does not possess
the enzymatic capability to degrade all or even most of the PCB congeners present in the
contaminated environments. A consortium of carefully selected suitable microbial
species was expected to perform better than an individual microbe. Therefore, based on
the individual culture study results, three facultative anaerobic strains Achromobacter
sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05 were selected and used as
a consortium. A conventional two stage (TS) anaerobic-aerobic treatment was compared
to alternating (AN) anaerobic-aerobic treatment. The two stage process was set up to
provide an extended anaerobic phase of four weeks followed by a short aerobic phase
of two weeks. In contrast, weekly intervals of anaerobic and aerobic conditions were
applied in the alternating treatment. It was found that the alternating approach was
more efficient compared to the two stage treatment with yields of nearly 50% reduction
in total PCBs reached within the first two weeks compared to 24% reduction obtained in
two stage treatment. This finding is significant as one of the limiting factors in
bioremediation applications is the normal extended time span required and applied in
order to achieve satisfactory degradation of PCBs.
During this study, extracellular proteins released and/or secreted by the consortium
members under alternating and two stage anaerobic-aerobic-aerobic experimental
conditions were investigated. Proteins with diverse functional roles consisting of 319
non-secreted, 212 classically secreted and 87 non-classically secreted proteins were
identified. Out of 212 classically secreted proteins, 58% were identified as transport
related proteins. The identification of proteins involved in the intermediate steps of
PCB degradation pathways and in the detoxification of toxic chemicals provided key
evidences of the occurrence of PCB degradation, by the bacterial consortium. The
outcomes from the metaproteomics study will further advance the knowledge
background on the types and potential role of extracellular proteins and enzymes
detected in the culture supernatant, and their involvement in PCB degradation.

Bioremediation of Commercial Polychlorinated Biphenyl Mixture Aroclor 1260 by Naturally Occurring Microorganisms v
Table of Contents
Keywords .................................................................................................................. i
Abstract ................................................................................................................... ii
Table of Contents .....................................................................................................v
List of Figures .......................................................................................................... xii
List of Tables ......................................................................................................... xvii
List of Abbreviations ............................................................................................ xviii
Statement of Original Authorship .......................................................................... xx
Acknowledgements ............................................................................................... xxi
List of thesis associated publications ................................................................... xxii
Chapter 1: Introduction .................................................................................. 1
1.1 Background .................................................................................................. 1
1.2 Research Problem ........................................................................................ 2
1.3 Research Hypothesis ................................................................................... 3
1.4 Aims and Objectives .................................................................................... 4
1.5 Research Scope ............................................................................................ 4
1.6 Innovation and Contribution to Knowledge ................................................ 5
1.7 Research Design and Methodology ............................................................. 6
1.7.1 Critical review of research literature ...................................................... 9
1.7.2 Isolation, screening and identification of potential PCB degrading microorganisms .................................................................................... 9
1.7.3 Screening of bacterial isolates for their ability to produce biosurfactants to make hydrophobic PCBs soluble in aqueous media ...................... 10
1.7.4 Comparison of the individual facultative anaerobic bacterial isolates during PCB hydrolysis under aerobic, anaerobic and two stage anaerobic-aerobic conditions ............................................................. 11
1.7.5 Comparison of the bacterial consortium during PCB hydrolysis under two modes of combined anaerobic-aerobic treatments ................... 12
1.7.6 Analysis and characterization of proteins detected in the culture supernatants during PCB degradation. .............................................. 12
1.8 Thesis Outline ............................................................................................ 13
Chapter 2: Bioremediation of Polychlorinated biphenyls ............................... 15
2.1 Introduction ............................................................................................... 15

vi Bioremediation of Commercial Polychlorinated Biphenyl Mixture Aroclor 1260 by Naturally Occurring Microorganisms
2.2 Overview of polychlorinated biphenyls ..................................................... 16
2.2.1 Structure, properties and applications ................................................. 16
2.2.2 Influence of PCBs on human and ecosystem health ............................ 19
2.2.3 Regulations and monitoring .................................................................. 20
2.3 Removal of PCBs from contaminated soil ................................................. 21
2.3.1 PCBs as a soil contaminant ................................................................... 21
2.3.2 Remediation of PCB contaminated soil ................................................ 24
2.3.3 In-situ bioremediation .......................................................................... 24
2.3.3.1 Phytoremediation .................................................................... 24
2.3.3.2 Fungal bioremediation ............................................................. 28
2.3.3.3 Bacterial bioremediation ......................................................... 29
2.4 Biodegradation pathways .......................................................................... 29
2.4.1 Anaerobic reductive dechlorination ..................................................... 30
2.4.1.1 Anaerobic reductive dechlorination pathways ........................ 31
2.4.2 Aerobic oxidative degradation .............................................................. 33
2.4.2.1 The upper biphenyl degradation pathway .............................. 34
2.4.2.2 The lower biphenyl degradation pathway ............................... 37
2.4.3 Sequential anaerobic-aerobic degradation .......................................... 39
2.5 Factors affecting microbial remediation ................................................... 40
2.5.1 Soil properties ....................................................................................... 40
2.5.2 Environmental factors ........................................................................... 41
2.5.2.1 pH ............................................................................................. 41
2.5.2.2 Temperature ............................................................................ 42
2.5.2.3 Soil moisture ............................................................................ 42
2.5.3 PCB related properties .......................................................................... 42
2.5.4 Natural microbial diversity .................................................................... 43
2.6 Enhancement of bioremediation ............................................................... 46
2.6.1 Biostimulation ....................................................................................... 46
2.6.2 Bioaugmentation .................................................................................. 47
2.6.3 Mixed microbial consortia .................................................................... 48
2.6.4 Surfactants ............................................................................................ 48
2.6.5 Secondary carbon sources .................................................................... 50
2.6.6 Biocarriers ............................................................................................. 51
2.7 Monitoring of PCB degradation ................................................................. 51
2.8 Summary .................................................................................................... 55

Bioremediation of Commercial Polychlorinated Biphenyl Mixture Aroclor 1260 by Naturally Occurring Microorganisms vii
Chapter 3: Materials and Methods ................................................................ 57
3.1 Chemicals, media and reagents ................................................................. 57
3.1.1 PCB source ............................................................................................ 57
3.1.2 General Media and reagents ................................................................ 58
3.1.2.1 Media used for cultivating PCB degrading microorganisms .... 58
3.1.2.2 Media used in 16S rRNA based bacteria identification ........... 60
3.1.2.3 Media used in biosurfactant screening tests ........................... 61
3.1.2.4 Reducing sugar analysis in microbial growth studies .............. 62
3.1.2.5 Chemicals used in PCB extraction and analysis ....................... 63
3.1.2.6 Chemicals, buffers and materials used in protein extraction .. 64
3.2 Methods ..................................................................................................... 66
3.2.1 Selective enrichment of potential PCB utilizing bacteria ..................... 66
3.2.2 Genetic characterization based on 16S rRNA sequences ..................... 66
3.2.3 Bacterial growth profiles ...................................................................... 66
3.2.4 Bacteria cell density measurement ...................................................... 66
3.2.5 pH 68
3.2.6 Glucose concentration .......................................................................... 68
3.2.7 Chloride ion concentration ................................................................... 68
3.2.8 PCB extraction ....................................................................................... 69
3.2.9 PCB analysis .......................................................................................... 71
3.2.10 Screening tests for biosurfactant production ....................................... 73
3.2.11 Extracellular protein visualization, quantification, extraction and analysis ............................................................................................... 73
3.2.12 Storage of culture supernatant samples .............................................. 73
3.2.13 Bacterial culture maintenance .............................................................. 73
3.3 PCB degradation studies ............................................................................ 73
3.4 Cleaning of glassware used in PCB extraction ........................................... 74
3.5 Quality assurance ...................................................................................... 75
Chapter 4: Selective Enrichment and Identification of Potential PCB Degrading Microorganisms ........................................................................... 77
4.1 Background ................................................................................................ 77
4.2 Materials and Methods ............................................................................. 78
4.2.1 Soil and sediment sample collection and preparation ......................... 78
4.2.2 Selective enrichment of potential PCB utilizing bacteria ..................... 79
4.2.3 Characterization of potential PCB degrading bacteria ......................... 80
4.2.3.1 Morphological characteristics .................................................. 80

viii Bioremediation of Commercial Polychlorinated Biphenyl Mixture Aroclor 1260 by Naturally Occurring Microorganisms
4.2.3.2 Oxygen requirements............................................................... 81
4.2.3.3 Identification of bacterial isolates using 16S rRNA full length gene sequencing ...................................................................... 81
4.2.3.4 Confirmation of the ability to grow on PCBs as sole carbon source ....................................................................................... 84
4.2.3.5 Growth profiles of bacteria ...................................................... 85
4.3 Results and Discussion ............................................................................... 86
4.3.1 Screening and Identification of PCB utilizing culture members ........... 86
4.3.1.1 16S rRNA gene based identification ........................................ 86
4.3.1.2 Morphological characteristics of identified bacteria ............... 93
4.3.1.3 Screening of microorganisms based on tolerance to atmospheric oxygen ................................................................. 96
4.3.2 Basic growth profiles using glucose as the carbon source ................... 98
4.4 Conclusions .............................................................................................. 102
Chapter 5: Screening of bacterial isolates for biosurfactant production ....... 103
5.1 Background .............................................................................................. 103
5.2 Materials and Methods ........................................................................... 104
5.2.1 Experimental setup ............................................................................. 104
5.2.2 Evaluation of PCB solubility and degradation ..................................... 105
5.2.3 Screening for biosurfactant production .............................................. 105
5.2.3.1 Drop collapse test .................................................................. 105
5.2.3.2 Emulsification index (EI24) ...................................................... 106
5.2.3.3 Haemolysis ............................................................................. 106
5.3 Results and Discussion ............................................................................. 107
5.3.1 PCB solubility ....................................................................................... 107
5.3.2 Bacterial growth, chloride ion accumulation and pH ......................... 108
5.3.2.1 Bacterial growth ..................................................................... 108
5.3.2.2 Chloride ion concentration .................................................... 110
5.3.2.3 pH ........................................................................................... 111
5.3.3 Biosurfactant production .................................................................... 112
5.4 Conclusions .............................................................................................. 116
Chapter 6: PCB degradation potential of facultative anaerobic bacterial isolates .................................................................................................. 119
6.1 Background .............................................................................................. 119
6.2 Materials and Methods ........................................................................... 120
6.2.1 Experimental setup ............................................................................. 120

Bioremediation of Commercial Polychlorinated Biphenyl Mixture Aroclor 1260 by Naturally Occurring Microorganisms ix
6.2.2 Bacteria seed culture preparation ...................................................... 121
6.2.3 Controls ............................................................................................... 122
6.2.4 Sample collection, analysis and preservation ..................................... 122
6.3 Results and discussion ............................................................................. 123
6.3.1 Growth profiles - aerobic vs anaerobic vs two stage anaerobic-aerobic 123
6.3.2 PCB degradation and solubilisation .................................................... 126
6.3.3 Chloride ion accumulation .................................................................. 129
6.4 Conclusions .............................................................................................. 133
Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium ........................ 135
7.1 Background .............................................................................................. 135
7.2 Materials and Methods ........................................................................... 136
7.2.1 Laboratory microcosm batch experiments ......................................... 137
7.2.2 Frequency, collection and preservation of samples ........................... 137
7.2.3 Total PCB extraction and analysis ....................................................... 139
7.2.4 Carbon and nitrogen source utilization profiling of bacterial cultures 139
7.2.4.1 Procedure for Gram positive bacteria ................................... 140
7.2.4.2 Procedure for Gram negative bacteria .................................. 141
7.3 Results and Discussion ............................................................................. 142
7.3.1 Test for competition ........................................................................... 142
7.3.2 PCB degradation by the bacterial consortium under AN and TS treatments ........................................................................................ 143
7.3.2.1 Total PCB degradation ........................................................... 143
7.3.2.2 PCB Homolog analysis ............................................................ 146
7.3.3 Chloride ion accumulation and pH variation ...................................... 151
7.3.3.1 Chloride ion analysis .............................................................. 151
7.3.3.2 pH analysis ............................................................................. 154
7.3.4 Carbon and nitrogen wide substrate utilization tests ........................ 156
7.4 Conclusions .............................................................................................. 160
Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05 ................................................................ 163
8.1 Background .............................................................................................. 163

x Bioremediation of Commercial Polychlorinated Biphenyl Mixture Aroclor 1260 by Naturally Occurring Microorganisms
8.2 Materials and Methods ........................................................................... 165
8.2.1 Protein visualization ............................................................................ 166
8.2.1.1 Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) ............................................................................. 166
8.2.1.2 Coomassie blue staining......................................................... 166
8.2.2 Protein quantification using Bicinchoninic acid assay (BCA) .............. 166
8.2.3 Trypsin digestion of extracellular proteins ......................................... 167
8.2.4 Desalting of samples prior to mass spectroscopy analysis ................. 168
8.2.5 Secretome analysis ............................................................................. 169
8.2.5.1 Liquid chromatography – mass spectroscopy ....................... 169
8.2.5.2 Protein identification using ProteinPilot v5 ........................... 171
8.2.5.3 Protein quantitation using PeakView .................................... 171
8.2.5.4 Bioinformatics analysis of peptide sequences ....................... 172
8.3 Results and Discussion ............................................................................. 173
8.3.1 SDS-PAGE analysis: Extracellular protein detection and visualization 173
8.3.1.1 Individual cultures .................................................................. 173
8.3.1.2 Consortium study under AN and TS treatment conditions ... 176
8.3.2 Proteomics analysis ............................................................................. 178
8.3.2.1 Non-secretory proteins .......................................................... 179
8.3.2.2 Secretory proteins: Secretome .............................................. 182
8.4 Conclusions .............................................................................................. 193
Chapter 9: Conclusions, practical applications and recommendations for future research..................................................................................... 197
9.1 Conclusions .............................................................................................. 197
9.1.1 Isolation, screening and identification of potential PCB degrading microorganisms ................................................................................ 201
9.1.2 Screening of bacterial isolates for their ability to produce biosurfactants to make hydrophobic PCBs soluble in aqueous media .................... 201
9.1.3 Comparison of the individual facultative anaerobic bacterial isolates during PCB hydrolysis under aerobic, anaerobic and two stage anaerobic-aerobic conditions ........................................................... 202
9.1.4 Comparison of the bacterial consortium during PCB hydrolysis under two modes of combined anaerobic-aerobic treatments ................. 203
9.1.5 Analysing and characterization of proteins detected in the culture supernatants during PCB degradation ............................................. 204
9.2 Practical applications of research outcomes ........................................... 206
9.3 Recommendations for future research ................................................... 207

Bioremediation of Commercial Polychlorinated Biphenyl Mixture Aroclor 1260 by Naturally Occurring Microorganisms xi
References…….. ................................................................................................ 211
Appendices….. .................................................................................................. 245
Appendix A: PCB analysis ........................................................................... 246
Appendix B: PCB data related to bacterial consortium study ....................... 251
Appendix C: Extracellular protein analysis .................................................. 255
Supplimentary Material .................................................................................... 291
Supplementary Material 1: Abstracts of conference papers relevant to the thesis .......................................................................................... 293
Supplementary Material 2: Publications relevant to the thesis ................... 295

xii Bioremediation of Commercial Polychlorinated Biphenyl Mixture Aroclor 1260 by Naturally Occurring Microorganisms
List of Figures
Figure 1.1 Schematic representation of the research methodology ........................... 8
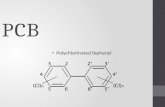
Figure 2.1 Structural form of PCB. Clx and Cly are number of chlorines attached to each benzene ring and x + y = 1 to 10. Adapted from Wiegel and Wu (2000). .......................................................................................................... 17
Figure 2.2 Concentrations of total PCBs in surface soils at global background sites (Li et al., 2010). .................................................................................... 22
Figure 2.3 Potential pathway for anaerobic dechlorination of highly chlorinated congeners. Adapted from Borja et al. (2005). ............................................. 31
Figure 2.4 The upper biphenyl degradation pathway. Modified from Field and Sierra-Alvarez (2008). ................................................................................... 35
Figure 2.5 The lower biphenyl degradation pathways (a) Mineralization of 2-hydroxypenta -2,4-dienoate. Modified from Field and Sierra-Alvarez (2008). (b) Mineralization of chlorobenzoic acid. Modified from ATSDR (2000). .......................................................................................................... 38
Figure 2.6 Overview of factors affecting microbial remediation ............................... 40
Figure 2.7 Variation of the bacterial community at genus level in the bulk, top and rhizosphere soils. The phylum of each genus is reported in brackets (Acid-Acidobacteria; Act-Actinobacteria; Alph-Alphaproteobacteria; Beta-Betaproteobacteria; Chlo-Chloroflexi; Firm-Firmicutes; Gamm-Gammaproteobacteria; Gemm-Gemmatimonatedes). Adapted from Stella et al. (2015). ........................... 45
Figure 2.8 The omics pyramid. Modified from NASEM (2016). ................................. 52
Figure 2.9 Organism based and community based “omic” approaches for assessing bioremediation approaches. Modified from Chovanec et al. (2011). .......................................................................................................... 54
Figure 3.1 Standard plate count technique ............................................................... 67
Figure 3.2 Recovery of Aroclor 1260 soluble in the aqueous minimal salt medium as a percentage of total PCBs added to the mixture, using diethyl ether (DEE) and hexane as the extraction solvents. Error bars represent the standard deviation of mean values (n = 3). .......................... 70
Figure 3.3 The Coy anaerobic chamber with the connecting airlock to the right, used in this research. ................................................................................... 74
Figure 4.1 Selective enrichment of potential PCB degrading bacteria, under aerobic and anaerobic conditions at 28 °C. ................................................. 80
Figure 4.2 Agarose gel electrophoresis of genomic DNA isolated from bacterial isolates using Isolate II genomic DNA kit (Bioline). A1 to A5 were from

Bioremediation of Commercial Polychlorinated Biphenyl Mixture Aroclor 1260 by Naturally Occurring Microorganisms xiii
aerobic selective enrichments and AN1 to AN6 were from anaerobic selective enrichments. ................................................................................. 87
Figure 4.3 Blue-white screening on LB/ampicillin/IPTG/X-Gal plate after the transformation into high efficiency E. coli JM109 competent cells. White colour colonies representing the positive transformations. ............ 88
Figure 4.4 Agarose gel electrophoresis of purified recombinant plasmids. 5 µL from each sample was loaded into the corresponding well. A1 to A5 were from aerobic selective enrichments and AN1 to AN6 were from anaerobic selective enrichments. Instead of plasmids, sterile MilliQ water was used in the negative control. ..................................................... 88
Figure 4.5 Pairwise alignment of forward and reverse DNA sequences of bacterial isolate AN2 using Emboss Needle software. Total length after the alignment was 1459 and number of similarities between two sequences were 738/1459 (50.6%). ............................................................ 89
Figure 4.6 Colony morphology of the bacterial cultures on nutrient agar after 48 h incubation at 28 °C. Culture A to F were under aerobic conditions and culture G was under anaerobic conditions. .......................................... 94
Figure 4.7 Gram stained bacterial cultures under the light microscope (100x magnification). ............................................................................................. 95
Figure 4.8 Bacterial colonies on minimal salt agar with 50 mg/L Aroclor 1260 as sole source of carbon after 48 hrs at 28 °C (A) under aerobic conditions, (B) facultative anaerobic cultures under anaerobic conditions, (C) negative controls under aerobic conditions (D) negative controls under anaerobic conditions. ........................................................................ 97
Figure 4.9 Growth of obligate anaerobic Novosphingobium sp. NP07 on 25 mg/L and 50 mg/L Aroclor 1260 containing minimal salt agar after 48 h incubation at 28 °C under anaerobic conditions. ........................................ 98
Figure 4.10 Basic bacterial growth profiles of the six bacterial isolates grown in minimal salt medium over a nine hour period at 28 °C and 150 rpm using glucose as the carbon source. Error bars represent the standard deviation of mean values (n = 3). ................................................................ 99
Figure 4.11 Variation of pH in the culture medium during the bacterial growth profile studies. Error bars represent the standard deviation of mean values (n = 3). ............................................................................................. 101
Figure 5.1 Variation of the total solubility of Aroclor 1260 in the aqueous minimal salt media inoculated with bacterial cultures. Error bars represent the standard deviation of mean values (n=3 for bacterial cultures and n=2 for abiotic controls). ...................................................... 107
Figure 5.2 Bacterial growth as optical density (OD600) in the batch mesocosms at 28 °C and 150 rpm. Error bars represent the standard deviation of mean values (n=3). ..................................................................................... 109

xiv Bioremediation of Commercial Polychlorinated Biphenyl Mixture Aroclor 1260 by Naturally Occurring Microorganisms
Figure 5.3 Chloride ion accumulation in the batch mesocosms after six weeks of incubation at 28 °C and 150 rpm. The background values from the controls of (1) minimal salt medium only and (2) seed cultures only were subtracted first. Error bars represent the standard deviation of mean values (n=3). ..................................................................................... 110
Figure 5.4 pH variation in the batch mesocosms at 28 °C and 150 rpm. Error bars represent the standard deviation of mean values (n=3). .................. 112
Figure 5.5 Drop collapse test. 1% (w/v) sodium dodecyl sulphate (SDS) solution was used as the positive control. The phosphate buffered saline (PBS) solution and abiotic control (minimal salt medium only) were used as negative controls. ....................................................................................... 114
Figure 5.6 Haemolysis of sheep blood in Tryptone soya agar after incubation at 28 °C for 48 hours (A) Chryseobacterium sp. NP01, (B) Delftia sp. NP02, (C) Achromobacter sp. NP03, (D) Ochrobactrum sp. NP04, (E) Lysinibacillus sp. NP05, (F) Pseudomonas sp. NP06, (G) 1% SDS as positive control, and (H) abiotic control. ................................................... 115
Figure 6.1 Growth of the four facultative anaerobic bacterial strains under (a) aerobic, (b) anaerobic, and (c) two stage anaerobic-aerobic conditions. Error bars represent the standard deviation of mean values (n = 3). ....... 125
Figure 6.2 PCB solubility under (a) aerobic, (b) anaerobic, and (c) two stage anaerobic-aerobic conditions. Prior to the addition of microbes, samples were removed and analysed for Initial soluble PCBs measurement and then after adding microbes, samples were removed immediately and represent week 0. Error bars represent the standard deviation of mean values from triplicates. ................................................ 128
Figure 6.3 Chloride ion accumulation in the culture media after six weeks. The background values from the controls of (1) minimal salt medium only and (2) seed cultures only were subtracted first. Error bars represent the standard deviation of mean values from triplicates. .......................... 130
Figure 6.4 Variation of pH and chloride ion concentrations after six weeks under aerobic, anaerobic and two stage anaerobic-aerobic conditions (Initial pH was adjusted to 7.0). Error bars represent the standard deviation of mean values from triplicates. ................................................................ 132
Figure 6.5 Growth profile, PCB hydrolysis and pH variation of Lysinibacillus sp. NP05 under two stage anaerobic-aerobic conditions. Error bars represent the standard deviation of mean values from triplicates. .......... 133
Figure 7.1 Inoculation of batch mesocosms with bacterial seed cultures inside the anaerobic chamber. ............................................................................. 137
Figure 7.2 Growth of Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05 on minimal salt-Aroclor 1260 agar at 28 °C after 72 h (a) under aerobic conditions, (b) under anaerobic conditions. ......... 143
Figure 7.3 Total PCB degradation as a percentage and bacterial growth as OD600 under (a) alternating and (b) two stage anaerobic-aerobic treatments

Bioremediation of Commercial Polychlorinated Biphenyl Mixture Aroclor 1260 by Naturally Occurring Microorganisms xv
by the bacterial consortium. Error bars represent the standard deviation of mean values (n = 3). .............................................................. 145
Figure 7.4 Variation of PCB homolog groups following AN treatment; (a) lower chlorinated congener groups (mono to tetra), and medium to highly chlorinated congener groups, (b) penta to hepta, (c) octa and nona. Error bars represent the standard deviation of mean values (n = 3). ....... 148
Figure 7.5 Variation of PCB homolog groups following TS conditions; (a) lower chlorinated congener groups (mono to tetra), and highly chlorinated congener groups, (b) penta to hepta, (c) octa and nona. Error bars represent the standard deviation of mean values (n = 3). ........................ 150
Figure 7.6 Chloride ion accumulation under alternating (AN) and two stage (TS) anaerobic-aerobic conditions. The background chloride values from the minimal salt medium were first subtracted from experimental values and media controls. Error bars represent the standard deviation of mean values (n=3 for experimental values and n=2 for media controls). .................................................................................................... 151
Figure 7.7 Measured chloride ion buildup in the culture medium and calculated chloride ion removal from the PCB mixture based on homolog group reductions under; (a) alternating (AN), and (b) two stage (TS) anaerobic-aerobic conditions. Error bars represent the standard deviation of mean values (n = 3). .............................................................. 153
Figure 7.8 pH trends relative to chloride ion concentration. (a) AN and (b) TS anaerobic-aerobic treatments. Error bars represent the standard deviation of mean values (n = 3). .............................................................. 155
Figure 8.1 The secretome and exoproteome of a Gram negative bacterial cell. (Armengaud et al., 2012) ........................................................................... 164
Figure 8.2 Role of extracellular enzymes in insoluble compound metabolism. ...... 165
Figure 8.3 SDS-PAGE analysis of Lysinibacillus sp. NP05 containing controls (minimal salt medium with no added PCBs) under anaerobic conditions at 28 °C. Lane 1, SeeBlue Protein standard as the protein molecular weight markers; lanes 2, time 0 immediately after addition of seed culture; lane 3 to 8, week 1 to week 6....................................................... 173
Figure 8.4 SDS-PAGE analysis of extracellular proteins of Achromobacter sp. NP03 under (A) aerobic and (B) anaerobic conditions at 28 °C. Lane 1, SeeBlue Protein standard as the protein molecular weight markers; lanes 2, time 0 immediately after addition of seed culture; lane 3 to 8, week 1 to week 6. ...................................................................................... 174
Figure 8.5 SDS-PAGE analysis of extracellular proteins of Ochrobactrum sp. NP04 under (A) aerobic and (B) anaerobic conditions at 28 °C. Lane 1, SeeBlue Protein standard as the protein molecular weight markers; lanes 2, time 0 immediately after addition of seed culture; lane 3 to 8, week 1 to week 6. ...................................................................................... 175

xvi Bioremediation of Commercial Polychlorinated Biphenyl Mixture Aroclor 1260 by Naturally Occurring Microorganisms
Figure 8.6 SDS-PAGE analysis of extracellular proteins of Lysinibacillus sp. NP05 under (A) aerobic and (B) anaerobic conditions at 28 °C. Lane 1, SeeBlue Protein standard as the protein molecular weight markers; lanes 2, time 0 immediately after addition of seed culture; lane 3 to 8, week 1 to week 6. ...................................................................................... 176
Figure 8.7 SDS-PAGE analysis of the extracellular proteins of the bacterial consortium consisting of Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05 under (A) AN, and (B) TS anaerobic-aerobic conditions at 28 °C. Lane 1, SeeBlue Protein standard as the protein molecular weight markers; lanes 2, time 0 immediately after addition of seed culture; lane 2 to 5, at fortnightly intervals up to week 6. ................................................................................................................. 177
Figure 8.8 Proportions of classically and non-classically secreted proteins in the culture supernatant of the bacterial consortium Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05. ..................... 183
Figure 8.9 Functional groupings of proteins identified as classically secreted proteins. ..................................................................................................... 184
Figure 8.10 The concentration of the sulfate transporter protein detected in the culture supernatant over time, under the alternating anaerobic-aerobic (AN) conditions. ............................................................................ 188
Figure 8.11 Venn diagram of the distribution of proteins identified as classically secreted proteins among (A) alternating (AN) anaerobic-aerobic, and (B) two stage (TS) anaerobic-aerobic conditions. ...................................... 190
Figure 8.12 Functional groupings of proteins identified as non-classically secreted proteins. ...................................................................................... 191
Figure 8.13 Variation of glutamine synthetase concentration and pH level in the culture supernatant under alternating anaerobic-aerobic conditions. ..... 192
Figure 8.14 Distribution of non-classically secreted proteins under (A) AN anaerobic-aerobic treatment, and (B) TS anaerobic-aerobic treatment. .................................................................................................................... 193
Figure 9.1 A schematic representation of the summary of major findings of the research study. ........................................................................................... 200

Bioremediation of Commercial Polychlorinated Biphenyl Mixture Aroclor 1260 by Naturally Occurring Microorganisms xvii
List of Tables
Table 2.1 PCB homologs and their chlorine substitutions ......................................... 18
Table 2.2 Comparison of PCB levels (µg/kg dry weight) in soils based on selected international studies. ................................................................................... 23
Table 2.3 Plant–microbial relationships associated with PCB degradation .............. 27
Table 2.4 Microbial dechlorination pathways (Wiegel & Wu, 2000). ....................... 32
Table 2.5 Types and microbial origin of biosurfactants. ............................................ 49
Table 3.1 Summary of physicochemical properties of Aroclor 1260 ........................ 57
Table 3.2 Average weight percent of PCB homolog groups and chlorines in Aroclor 1260. ................................................................................................ 72
Table 4.1 Comparison of closest relatives of isolated bacteria based on NCBI and RDP databases ...................................................................................... 91
Table 4.2 Final nomenclature and identification of the pure bacterial isolates ....... 92
Table 4.3 Specific growth rate of bacterial cultures during the growth profile studies using 2 g/L glucose as the carbon source. ..................................... 100
Table 5.1 Summary of biosurfactant screening tests .............................................. 113
Table 6.1 Bacteria cell count in overnight Luria–Bertani liquid medium at 28 °C..................................................................................................................... 123
Table 7.1 Ingredients for the nutrient additive solution, 12x (PM additive) ........... 140
Table 7.2 Preparation of final inoculation fluid to inoculate the Biolog plates....... 141
Table 7.3 Carbon source utilization by consortium members Achromobacter sp. NP03, Ochrobactrum sp. NP04, and Lysinibacillus sp. NP05. .................... 158
Table 7.4 Nitrogen source utilization by consortium members Achromobacter sp. NP03, Ochrobactrum sp. NP04, and Lysinibacillus sp. NP05. ............... 159
Table 8.1 Standard dilutions preparation for BCA assay ......................................... 167
Table 8.2 Analysis of extracellular proteins identified in culture supernatants of consortium under AN and TS conditions. .................................................. 178
Table 8.3 Functional classification of 319 identified proteins that were predicted to be non-secretory proteins by the SignalP 3.0 and SecretomeP 2.0 servers. ............................................................................ 179
Table 8.4 Heat map showing the relative abundance of ten highly secreted extracellular proteins detected in the culture supernatant of the bacterial consortium Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05. .............................................................. 186

xviiiBioremediation of Commercial Polychlorinated Biphenyl Mixture Aroclor 1260 by Naturally Occurring Microorganisms
List of Abbreviations
Abbreviations
AN Alternating anaerobic-aerobic treatment
CFU Colony forming units
DEE Di ethyl ether
DNS Dinitrosalicylic acid
GCMS Gas chromatography-mas spectroscopy
gDNA Genomic DNA
IPTG Isopropyl β-D-1-thiogalactopyranoside
LB Luria–Bertani medium
LCMS Liquid chromatography–mass spectrometry
NA Nutrient Agar
NCBI National Centre for Biotechnology Information
MSM Minimal salt medium
OD600 Optical density at 600 nm
PBS phosphate buffer saline
PCBs Poly chlorinated biphenyls
PCR Polymerase chain reaction
RDP Ribosomal database project
RSDV Relative standard deviation
SDS Sodium dodecyl sulphate
SOC Super optimal broth with catabolite repression
SRM Selective reaction monitoring
SWATH Sequential window acquisition of all theoretical mass spectra
TSA Tryptone soya agar
TS Two stage anaerobic-aerobic treatment

Bioremediation of Commercial Polychlorinated Biphenyl Mixture Aroclor 1260 by Naturally Occurring Microorganisms xix
Units
bp Base pair(s)
g Gram(s)
g Relative centrifugal force in units of gravity
h Hour(s)
kb Kilobase
kDa Kilodalton
min Minute(s)
mol Mole(s)
rpm Revolutions per minute
s Second(s)
v/v Volume per volume
w/v Weight per volume

xx Bioremediation of Commercial Polychlorinated Biphenyl Mixture Aroclor 1260 by Naturally Occurring Microorganisms
Statement of Original Authorship
The work contained in this thesis has not been previously submitted to meet
requirements for an award at this or any other higher education institution. To the
best of my knowledge and belief, the thesis contains no material previously published
or written by another person except where due reference is made.
Signature:
Date: November 2018
QUT Verified Signature

Bioremediation of Commercial Polychlorinated Biphenyl Mixture Aroclor 1260 by Naturally Occurring Microorganisms xxi
Acknowledgements
The successful completion of my thesis would not have been possible without the
guidance, support, encouragement and help from my supervisory committee,
technical staff, scholarship from the Australian Government, my family and friends.
I am extremely thankful to my principal supervisor, Associate Professor V. S. Junior
Te'o, for his invaluable guidance and support throughout my PhD research. I would
also like to express my sincere thanks to my associate supervisor, Professor Ashantha
Goonetilleke for his guidance, support and encouragement to complete my research
study successfully. My sincere thanks is extended to my associate supervisor, Dr.
Prasanna Egodawatta for giving me the opportunity to undertake my PhD study at
QUT and for his continuous support and guidance.
I sincerely acknowledge Queensland University of Technology (QUT) for providing me
financial support through a RTPSD scholarship to conduct this doctoral research
study. I would also wish to acknowledge the staff of Central Analytical Research
Facility (CARF) of QUT, especially Mr. Vincent Chand and Mr. Shane Russell for their
technical support, Ms. Silvia Gemme for supporting me with the GCMS analysis, Dr.
Pawel Sadowski for helping me with proteomics. I am very thankful to Mr. Tony Tuong
Ngo, Powerlink Queensland Oil testing services, for supplying me transformer oil
samples without any hesitation to initiate my experiments.
I would like to extend my thanks to my fellow HDR colleagues for their support,
friendship, and encouragement. I am very grateful to my husband Samanola and kids
Vinuri, Mindulie and Biman for their love, support, patience, and encouragement to
complete my PhD study. Finally, my parents are remembered with love and gratitude
for their guidance and blessings throughout my life.

xxii Bioremediation of Commercial Polychlorinated Biphenyl Mixture Aroclor 1260 by Naturally Occurring Microorganisms
List of thesis associated publications
Conference Papers
• Pathiraja P.M.G., Egodawatta P., Goonetilleke A., Te'o V.S. J. Degradation of
commercial polychlorinated biphenyl mixture by naturally occurring facultative
microorganisms.
Nineteenth International Conference on Environmental Biodegradation
Rates, Sydney, Australia, 2017.
Journal Papers (published)
• Pathiraja, G., Egodawatta, P., Goonetilleke, A., & Te'o, V. S. J. (2019). Solubilization
and degradation of polychlorinated biphenyls (PCBs) by naturally occurring
facultative anaerobic bacteria. Science of The Total Environment, 651, 2197-2207.
Journal Impact Factor: 4.9, SJR Rank Q1.
Journal papers (Under review)
• Effective degradation of polychlorinated biphenyls by three facultative anaerobic
bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium.
Submitted to Science of the Total Environment, Journal Impact Factor: 4.9, SJR Rank
Q1.

Chapter 1: Introduction 1
Chapter 1: Introduction
1.1 Background
The diversity and magnitude of man-made toxic chemicals released into the
environment are creating long-term ecological impacts. Polychlorinated biphenyls
(PCBs) are one such toxic chemical group, consisting of 209 different chlorinated
organic compounds. Due to their unique chemical and physical properties, PCBs had
been used widely in many industrial applications, especially as insulating fluids in
transformers, capacitors, hydraulic systems, and as flame retardants. As a result,
since 1930, PCBs have been manufactured commercially in large scale as complex
mixtures.
The low reactivity, high chemical stability and complex nature of commercial PCB
mixtures have made them highly persistent and less environmentally desirable than
many other organic chemicals (Beyer & Biziuk, 2009). Their affinity to build up in living
organisms though bioaccumulation over time, biomagnification along the food chains
and resistance to biotransformation have led to numerous health implications in
humans and animals. As a result, PCBs were categorized as one of the original twelve
worldwide priority persistent organic pollutants (POPs) covered by the Stockholm
Convention (Bedard et al., 2007). Even though commercial production and use of
PCBs were banned or restricted decades ago, there is still a substantial amount of
PCBs present in the environment due to the continuing use and disposal of
equipment containing PCBs, recycling of PCB-contaminated products, emissions from
combustion of PCB contaminated waste, contaminated sites and disposal areas
(Breivik et al., 2007).
Treating environmental media such as soil contaminated with PCBs is of vital
importance to safeguard human and ecosystem health. In this regard, a range of
physical, chemical and biological treatment technologies have been investigated to
identify new remediation possibilities (Gomes et al., 2013). Bioremediation, the use
of microorganisms or microbial processes to degrade environmental contaminants,

2 Chapter 1: Introduction
is one of the methods intensively investigated for treating PCB-contaminated soils
(Boopathy, 2000).
1.2 Research Problem
Developing an environmentally sustainable and economically viable biological
treatment method as an alternative to existing physical and chemical treatment
methods for treating soil contaminated with PCBs is of critical importance. Over the
last decades, some aerobic and anaerobic microorganisms capable of degrading a
broad range of PCBs have been identified (Dercova et al., 2008; Sowers & May, 2013).
However, the application of microbial based bioremediation to treat PCB
contaminated soil is not yet widely practiced. The effectiveness of bioremediation is
determined by a number of factors, such as the lack of suitable microorganisms in
the contaminated environment, difficulties in maintaining the PCB degrading
bacterial communities in real-world environmental conditions, the nature of the PCB
mixture and the severity of contamination, low bioavailability of PCBs and the
characteristics of the contaminated environment.
As noted by Passatore et al. (2014), complete degradation of commercial PCB
mixtures that consist of compounds with varying degree of chlorination by
microorganisms can be achieved through a combination of anaerobic and aerobic
processes. It was recognized that the anaerobic dechlorination of more highly
chlorinated congeners followed by the aerobic degradation of those dechlorinated
products are the most likely degradation pathways occurring in the environment
(Payne et al., 2013). However, due to the complexity of commercial PCB mixtures
with the varying degree of chlorination, a single bacterium is not capable of degrading
all or even most of the PCB congeners present in contaminated environments (Pieper,
2005). Therefore, the search for appropriate microorganisms with the ability to
survive and degrade complex PCB mixtures under both anaerobic and aerobic
conditions would be a potential solution to achieve an efficient and effective process
for the biodegradation of PCBs.

Chapter 1: Introduction 3
Stella et al. (2015) noted that the extremely hydrophobic nature of PCBs makes them
poorly soluble in aqueous media, and this attribute can lead to PCBs being less
bioavailable for microbial degradation. Therefore, an increase in solubilisation would
enhance the bioavailability and subsequent biodegradation of PCBs (Ohtsubo et al.,
2004). However, the application of chemical and biological surfactants to increase
PCB solubility is limited due to various factors, such as chemical toxicity and high cost.
Therefore, the identification of suitable microorganisms that are capable of surviving
under a PCB contaminated environment, while producing biosurfactants would
accelerate PCB solubility and subsequent degradation.
Moreover, both anaerobic and aerobic PCB degradation pathways so far identified in
microorganisms were found to have occurred intracellularly (Wiegel & Wu, 2000;
Pieper, 2005; Agullo et al., 2017). However, the mechanism of transportation of PCB
molecules across the cytoplasmic membrane of microorganisms is not clear (Parales
& Ditty, 2017). The proteins or enzymes released and/or secreted by the
microorganisms to the extracellular environment may provide novel insights into the
mechanisms involved in modifying and transporting hydrophobic PCB molecules into
the cell (Basak & Dey, 2015). Therefore, identification of proteins released by
microorganisms into their surrounding extracellular environment and the functional
relationship with the PCB uptake into the bacterial cells would be important in order
to accelerate the degradation process.
Accordingly, this study aimed to discover microorganisms that can survive and
effectively degrade PCBs under both anaerobic and aerobic conditions, while
facilitating aqueous solubility and cellular uptake.
1.3 Research Hypothesis
The research study was based on the following hypotheses:
• Complete degradation of complex PCB mixtures can be achieved through a
combination of anaerobic-aerobic treatments.

4 Chapter 1: Introduction
• Microbial mixtures with varied capabilities for increasing the aqueous
solubility of PCBs, dechlorinating highly chlorinated congeners and complete
degradation of lower chlorinated congeners are required for comprehensive
degradation of PCBs.
• Extracellular proteins or enzymes released and/or secreted by the
microorganisms cultivated in minimal salts based medium with PCBs as a
carbon source, facilitate the uptake of PCB molecules into the bacterial cells
for degradation.
1.4 Aims and Objectives
The primary objective of this research was to identify suitable microorganisms from
the natural environment, which are capable of solubilizing and degrading complex
PCB mixtures under varying anaerobic and aerobic conditions in order to effectively
treat soil contaminated with PCBs.
To achieve this objective, the study aimed to:
• Isolate, screen, identify and characterize naturally occurring microorganisms
and select the best ones for further work, based on their ability to effectively
degrade PCBs under aerobic and anaerobic conditions.
• Screen the identified microorganisms for biosurfactant production in order to
increase the water solubility of PCBs.
• Determine the PCB degradation potential of facultative bacterial cultures
under anaerobic and aerobic conditions both individually and as a consortium.
• Analyse the extracellular proteins released by microorganisms into the
external environment.
1.5 Research Scope
The scope of this research study was as follows:

Chapter 1: Introduction 5
• The PCB source used in the present study was limited to Aroclor 1260, one of
the most commonly used and highly chlorinated commercial PCB mixtures
(USEPA, 2013). Its abundant usage has resulted in contamination of many
sites worldwide and has proven to be difficult to biodegrade due to its high
levels of chlorination (Bedard et al., 2007). Aroclor 1260 contains 60 to 90
different PCB congeners out of 209 possibilities (ATSDR, 2000; Breivik et al.,
2007). The outcomes of the study may not be applicable to PCB congeners
outside of the congeners available in Aroclor 1260.
• The research study was confined to treating soil contaminated with Aroclor
1260. However, the knowledge created in relation to the PCB solubility,
uptake and degradation by microorganisms is applicable to other
contaminated media such as sediments.
1.6 Innovation and Contribution to Knowledge
Investigating the performance of facultative anaerobic bacteria on PCB degradation
is expected to provide new insights to the knowledge base and research field. The
use of facultative anaerobic bacteria under anaerobic and aerobic conditions has led
to opportunities for further research based on their ability to degrade PCBs under
both aerobic and anaerobic conditions. Though a range of studies have so far
discussed PCB degradation potential of aerobic and anaerobic bacteria, their practical
use in the real-world environment is limited. Therefore, the innovative outcomes
from this study can be effectively utilized to treat contaminated soil under varying
anaerobic aerobic conditions in the field without losing the degradability and viability
potential.
The study also created new knowledge on the ability of PCB degrading bacteria to
produce biosurfactants. This finding is essential for designing an effective process to
enhance the bioavailability of PCBs in remediation applications, without addition of
toxic chemical surfactants or costly biological surfactants. Furthermore, the new
knowledge created relating to the identification of the alternating anaerobic-aerobic
treatment method as a rapid and efficient treatment option over the conventional

6 Chapter 1: Introduction
long-term two stage anaerobic-aerobic treatment can be successfully applied in
order to increase the effectiveness and reduce the cost of lengthy treatments. These
discoveries will contribute towards enhancing current microorganism based
bioremediation approaches to treat soils contaminated with PCBs and other similar
toxic chemical contaminants, in order to reduce and eventually eliminate their
harmful impacts on ecosystems and human health.
1.7 Research Design and Methodology
Effective biodegradation of PCBs is a complex process that involves a combination of
factors such as having suitable microbial cultures, complexity, concentration and
bioavailability of PCB congener mixtures, and environmental conditions. Therefore, the
research methodology was formulated in order to first, isolate, screen and identify
appropriate microorganisms with PCB degradation potential, second, react selected
microorganisms with PCBs, and third, analyse the results and characterize the key
contributing factors influencing the extent of PCB degradation by the microorganisms.
This section outlines the research methodology adopted.
The research methodology consisted of the following phases:
• Critical review of research literature.
• Isolation, screening and identification of potential PCB degrading
microorganisms.
• Screening of bacterial isolates for their ability to produce biosurfactants to make
hydrophobic PCBs soluble in aqueous media.
• Comparison of PCB degradation rates of facultative anaerobic bacterial isolates
individually under aerobic, anaerobic and two stage anaerobic-aerobic
conditions.
• Comparison of PCB degradation efficiency of selected bacterial isolates as a
consortium under two modes of combined anaerobic-aerobic treatments.
• Detection, identification and bioinformatics analysis of peptides produced from
proteins found in the culture supernatants during PCB hydrolysis indicative of
protein or enzyme secretion.

Chapter 1: Introduction 7
• Assessment of the types of proteins and a discussion of their possible roles in PCB
hydrolysis, and potential mechanisms of protein externalization by the
microorganisms.
The critical review of the research literature was first undertaken to identify the
knowledge gaps and research questions in PCB bioremediation, and thereby to
formulate the approaches to investigate the research problem. Figure 1.1 shows the
schematic of the research methodology describing the development of key study steps,
which are discussed in Chapters 4 to 8.

8 Chapter 1: Introduction
Figure 1.1 Schematic representation of the research methodology
Identification and bioinformatics analysis
Selective enrichment and identification of
potential PCB degrading microorganisms (Chapter 4)
Sediment and soil samples
Contamination with Aroclor 1260
Selective enrichment
Isolation of potential PCB degrading microorganisms
Identification using 16S rDNA and morphology
Long-term storage
PCB degradation potential of facultative anaerobic bacterial isolates under aerobic, anaerobic and two stage anaerobic– aerobic conditions (Chapter 6)
MSM medium + 50 mg/L Aroclor 1260
Analyze for PCB degradation
Develop microbial consortium
PCB degrading microorganism
s
Effective degradation PCBs by selected bacterial isolates as a consortium under combined anaerobic– aerobic conditions (Chapter 7)
Metaproteomics analysis of proteins detected in the culture supernatants (Chapter 8)
Analyse for PCB solubilisation, biosurfactants
MSM medium + 50 mg/L Aroclor 1260
PCB degrading microorganisms
Screening of bacterial isolates for biosurfactant production (Chapter 5)
MSM medium + 50 mg/L Aroclor 1260
Microbial consortium
Comparison of PCB degradation under AN and TS conditions
Visualization Quantification

Chapter 1: Introduction 9
1.7.1 Critical review of research literature
A critical literature review was conducted to identify the current status and
subsequent knowledge gaps in the area of environmental pollution on the use of
polychlorinated biphenyls. Of particular interest was the state of play on known types
of biodegradation pathways, factors affecting PCB degradation, and importantly, the
potential opportunities to improve PCB biodegradation processes using
microorganisms. The main areas of focus for the literature review were:
• Overview of polychlorinated biphenyls
• Different methods for removal of PCBs from contaminated soil
• Biodegradation pathways
• Factors affecting microbial remediation
• Enhancement of bioremediation
• Monitoring of PCB degradation
1.7.2 Isolation, screening and identification of potential PCB degrading
microorganisms
Due to the complexity of the study, it was essential to initially undertake the research
under laboratory conditions in order to limit the number of environmental variables.
This approach would then provide a robust platform to assess the applicability of the
outcomes under field conditions and to undertake appropriate modifications as
needed. The potential for field application of the laboratory study outcomes have
been further discussed in Section 9.3 of Chapter 9, Recommendations for future
research.
Accordingly, soil and sediment samples collected from different sites (locations
around the Brisbane City Botanical Gardens (27.4745° S, 153.0293° E), Brisbane River
(27.4745° S, 153.0293° E) and Coombabah Lake, Gold Coast (27.54° S, 153.22° E )
were artificially mixed with Aroclor 1260 and used to isolate PCB degrading
microorganisms. PCB concentration in soil varies widely depending on number of
factors such as distance to the point of contamination, source of contamination, and

10 Chapter 1: Introduction
soil depth. However, if the concentration of PCBs is ≥ 50 mg/kg in any soil type, then
such type of soil is regarded as PCB remediation waste and under the regulation of
the Toxic Substances Control Act. The PCB concentration used in this study was
limited to 50 mg/L, in order to limit the usage, minimize the waste generated and to
reduce the number of variables.
According to the current literature, soils contaminated with PCBs are most commonly
associated with transformer oil. Therefore, instead of direct contamination with the
commercial PCB mixture, Aroclor 1260, transformer oil samples contaminated with
Aroclor 1260 (supplied by the Powerlink oil testing laboratory) were used to
contaminate the soil and sediment samples. Although the focus of the study was to
remediate the contaminated soil, both soil and sediment samples were used during
the screening process with the aim of obtaining a combination of aerobic and
anaerobic bacteria as complete degradation of complex PCB mixtures favours the use
of both anaerobic degradation and aerobic oxidation.
For bacterial identification, the isolation and sequencing of good quality full length
16S rRNA gene DNAs (1.5kb) were performed through PCR and cloning using plasmids
and the bacterium Escherichia coli. The resulting full length 1.5kb 16S gene DNA
sequences on recombinant plasmids were submitted to the commonly used GenBank
National Center for Biotechnology Information (NCBI)
(https://www.ncbi.nlm.nih.gov/) as a non-curated database (Wang et al., 2007), and
also to the curated database Ribosomal Database Project (RDP)
(http://rdp.cme.msu.edu/). The details of methodologies used during the molecular
identification process are provided in Chapter 4.
1.7.3 Screening of bacterial isolates for their ability to produce biosurfactants to
make hydrophobic PCBs soluble in aqueous media
PCBs are inherently hydrophobic chemicals. As a consequence, insoluble PCBs are
considered one of the limiting factors in microbial bioremediation. Therefore, an
increase in the rate of solubilisation would potentially aid in the internalization
process of PCBs into the cells and subsequent degradation (Ohtsubo et al., 2004). The

Chapter 1: Introduction 11
identified microorganisms with PCB degradation potential were tested for
biosurfactant production. However, the extent of the study was limited to initial
screening for the potential biosurfactant production and PCB solubilisation using
emulsification index and drop collapse tests as quantitative tests and a haemolytic
assay as a qualitative test. The details of the experimental setup and analysis
methods used are discussed in Chapters 3 and 5, respectively.
1.7.4 Comparison of the individual facultative anaerobic bacterial isolates during
PCB hydrolysis under aerobic, anaerobic and two stage anaerobic-aerobic
conditions
Complete degradation of complex PCB mixtures needs a combination of anaerobic
dechlorination and aerobic oxidation bioconversion processes. Therefore, during this
study, the performance of four selected facultative anaerobic bacterial strains were
compared in parallel under anaerobic, aerobic and combined anaerobic-aerobic
conditions to evaluate their capability for PCB degradation. A total of 462 samples
were produced here as a result of replicates, number of strains, variables tested and
the low water solubility of Aroclor 1260. Therefore, it was not possible to maintain
and sacrifice a high number of whole flasks at weekly intervals to extract and measure
the total and congener specific PCB reductions.
Instead, removal of aliquots from each flask at weekly intervals and extraction of
PCBs were performed according to methods that measure the variation of total
soluble PCB levels in the aqueous culture medium (Adrian et al., 2009; Wang & He,
2013b). In addition, PCB hydrolysis was indirectly measured as cell growth and
chloride ion accumulation. All the experiments were conducted at 28 °C as the
average temperature due to the practical difficulties for maintaining the varied real
field conditions in the laboratory environment. The details of the calibration curve
preparation for PCB analysis are shown in the Appendix A while the details of testing
procedures and experimental setup are given in Chapters 3 and 6.

12 Chapter 1: Introduction
1.7.5 Comparison of the bacterial consortium during PCB hydrolysis under two
modes of combined anaerobic-aerobic treatments
Based on the results obtained for PCB solubility, cell growth and chloride
accumulation, the three facultative anaerobic bacteria, Achromobacter sp. NP03,
Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05 were chosen to form the
consortium. Two combined anaerobic-aerobic treatment modes namely alternating
anaerobic-aerobic treatment (AN) and two stage anaerobic-aerobic treatment (TS)
were selected to compare the rates and efficiencies of PCB degradation by the
consortium. Usually, anaerobic degradation is a long-term process and is one of the
limiting factors in the use of bioremediation as a treatment option.
Therefore, the aim of the two stage (TS) process was to mimic the conventional
combined anaerobic-aerobic treatment with an initial long term anaerobic phase
(four weeks) followed by a short term (two weeks) aerobic phase (Master et al., 2002;
Payne et al., 2013). In contrast, the aim of the alternating (AN) mode was to apply
equally short durations (weekly) of anaerobic and aerobic phases alternatively
throughout the six weeks period to allow the facultative anaerobic microorganisms
to convert the highly chlorinated congeners to lower chlorinated congeners under
anaerobic conditions and further break down the resulting lower chlorinated
congeners under aerobic conditions. However, the PCB degradation study was
limited to the total and homolog group based PCB analysis, as the purpose of the
research project was to study the overall PCB degradation potential. Therefore, the
congener specific degradation was not analysed. The data related to PCB analysis are
given in the Appendix B and the details of the total PCB extractions and the
experimental setups are given in Chapters 3 and 7, respectively.
1.7.6 Analysis and characterization of proteins detected in the culture supernatants
during PCB degradation.
PCB degradation by bacteria is believed to be an intracellular process. However, there
is paucity of knowledge available about the mechanism of how the hydrophobic PCB
molecule is transported into the cell across the cytoplasmic membrane. Therefore,

Chapter 1: Introduction 13
the extracellular proteins and/or enzymes either released or secreted by the
microorganisms into their surrounding environment were analysed to identify any
relationships between the rate of PCB degradation in the presence of extracellular
proteins and their concentrations and the potential mechanisms for the uptake of
PCBs. However, detailed analysis of extracellular proteins was limited to the bacterial
consortium as it is an exhaustive and time consuming process. Therefore, it was not
practical to focus on each individual bacterium under variable environmental
conditions. The data related to the relative abundance and categories of proteins and
enzymes is presented in Appendix C. The details of the analytical procedures used are
provided in Chapter 8.
1.8 Thesis Outline
This thesis consists of nine chapters. Chapter 1 introduces the research study providing
details of the research problem, hypotheses, aims and objective, scope, innovation and
contribution to knowledge, and research design and methodology.
The introduction to the research study is followed by the critical review of research
literature in Chapter 2. It provides a critical review of the research literature in the area
of environmental pollution due to polychlorinated biphenyls. It also identified the
knowledge gaps and provided research directions into advancing knowledge on
polychlorinated biphenyls, their biodegradation pathways, and factors affecting the
degradation and potential opportunities to improve the PCB biodegradation processes.
Chapter 3 outlines the general materials and methods used in the research study. It
further includes details on sample preservation and storage, bacterial culture
maintenance and quality assurance.
Chapters 4 to 8 are research chapters that describe the laboratory based experiments
and discuss the results.
Chapter 4 discusses the methodology used for the isolation, screening and identification
of potential PCB degrading microorganisms from soil and sediment samples artificially
contaminated with Aroclor 1260.

14 Chapter 1: Introduction
Chapter 5 presents the relationship between the biosurfactant production ability of
bacterial isolates with PCB solubility in aqueous media.
Chapter 6 compares PCB degradation rates of four facultative anaerobic bacterial isolates
individually under aerobic, anaerobic and two stage anaerobic-aerobic conditions.
Chapter 7 analyses and compares the PCB degradation efficiency of the selected bacterial
isolates as a consortium under alternating (AN) and two stage (TS) anaerobic-aerobic
treatment conditions.
Chapter 8 is the final research chapter and it analyses the extracellular proteins released
by the bacterial consortium members into the culture supernatant. It was important to
identify the types of proteins and establish any relationships between the proteins and
PCB degradation by the consortium members.
Chapter 9 is the final chapter. It provides the overall discussion, important conclusions
derived from the research and recommendations for future research.

Chapter 2: Bioremediation of Polychlorinated biphenyls 15
Chapter 2: Bioremediation of Polychlorinated biphenyls
2.1 Introduction
Contamination of the environment by hazardous and toxic synthetic chemicals is one
of the major problems facing the world today. Polychlorinated biphenyls (PCBs) are
one such synthetic chemical group, which consist of chlorinated aromatic
hydrocarbons. Due to their stable chemical and physical properties, PCBs were widely
used in many industrial applications since the 1930s. Low reactivity and high chemical
stability of PCBs have made them highly persistent (Beyer & Biziuk, 2009). High
lipophilicity makes PCBs soluble in fats and concentrates in body tissues. Resistance
to biodegradation results in increasingly higher concentrations as these compounds
move upwards through the food chain (ATSDR, 2000). Bioaccumulation,
bioconcentration and biomagnification along the food chain contribute to multiple
health effects in humans and animals. PCBs elicit a range of toxic responses including
acute lethality, carcinogenesis, dermal toxicity, genotoxicity, neurotoxicity and
immunosuppressive effects (ASTDR, 2014). As a result, the use of PCBs has been
restricted worldwide and they are categorised as a priority persistent organic
pollutant (POP) under the Stockholm Convention (Bedard et al., 2007; UNEP, 2009).
Among the approximate 1.3 million tons of cumulative global production until 1993,
about one third of PCBs are estimated to have been emitted to the environment prior
to restrictions being imposed. Emissions were due to releases from equipment still in
use and emissions from contaminated waste, contaminated sites and disposal areas
(Breivik et al., 2007; Li et al., 2010). This problem has led to extensive contamination
of all environmental media including soil, sediment and water through accidental
releases and inappropriate disposal.
Remediating soils contaminated with PCBs is a challenging task. The most widely used
method for treatment of PCB contaminated soil is high temperature incineration.
Additionally, various physical, chemical and biological treatment technologies have

16 Chapter 2: Bioremediation of Polychlorinated biphenyls
been investigated (Gomes et al., 2013). Microwave generated steam technology,
dechlorination using metals, base catalyzed decomposition, thermal desorption,
mechanochemical destruction and electrolysis are some of the novel physical and
chemical remediation technologies investigated (Liu et al., 2014; Qi et al., 2014;
Cagnetta et al., 2016). However, these technologies adopt harsh reaction conditions
such as high temperature, pressure, radiation and extreme pH, which have led to loss
of soil fertility (Sharma et al., 2014). PCBs in soils are relatively low in concentration,
being generally less than 1% of the contaminated mass, even at severely
contaminated sites (Passatore et al., 2014). While chemical and physical methods are
economically viable for the destruction of concentrated PCBs in oils and equipment,
the ability of these technologies to effectively treat large volumes of soil with low
concentrations of PCBs is questionable (Passatore et al., 2014).
Use of microorganisms for remediation of PCB-contaminated soil has been widely
investigated (Chuanmin et al., 2015). Microorganisms have the potential to break
down complex contaminants without transferring the pollution to another
environmental medium such as water, sediment or air. However, field scale
bioremediation approaches are not yet widely used as the effectiveness of using
microorganisms to treat PCB contaminated environments is determined by a number
of factors: (1) the nature of PCB mixture; (2) microorganisms involved; and (3) the
properties of the contaminated environment. Therefore, this critical review of
research literature specifically focuses on bringing together the current state of
knowledge on the properties of PCBs, known metabolic pathways, contributing
factors, their limitations as well as the enhancement opportunities in bioremediation
to identify knowledge gaps that hinder bioremediation.
2.2 Overview of polychlorinated biphenyls
2.2.1 Structure, properties and applications
Polychlorinated biphenyls comprise of two benzene rings joined by a carbon-carbon
bond, and on any or all of the remaining 10 carbon atoms, hydrogen atoms are
substituted with chlorine atoms (see Figure 2.1). Depending on the positions where

Chapter 2: Bioremediation of Polychlorinated biphenyls 17
the substituted chlorine is located, chemical characteristics of biphenyls can be
altered. Hence, it is important to use a standard naming convention to distinguish
different chlorinated biphenyls. In the standard convention, the positions of carbons
are numbered 1 to 6 on one ring, and 1′ to 6′ on the other. 2, 2′, 6, and 6′ positions
are called as “ortho,” 3, 3′, 5 and 5′ positions as “meta” while 4 and 4′ positions are
called as “para” (WHO, 2016). Depending on the number of chlorine substituents and
their ortho, meta and/or para positioning in the biphenyl structure, 209 different
chlorinated compounds are possible as outlined in Table 2.1. These compounds are
commonly referred to as “congeners” (ATSDR, 2000). Based on the degree of
chlorination, PCBs with equal number of chlorines are called “homolog” (Robertson
& Hansen, 2015). Homologs that have different substitution patterns are called
“isomers” (Srirangam, 2007).
Figure 2.1 Structural form of PCB. Clx and Cly are number of chlorines attached to
each benzene ring and x + y = 1 to 10. Adapted from Wiegel and Wu (2000).
Physical and chemical characteristics such as low water solubility, low vapor pressure,
low electrical conductivity, non-explosive nature, high solubilisation in nonpolar
solvents, great thermal conductivity, high thermal and chemical stability and high
ignition temperature have made PCBs suitable for many industrial applications (Beyer
& Biziuk, 2009). As a result, from 1930 to 1993, PCBs were mass-produced worldwide
as complex mixtures comprising 60 to 90 different congeners (ATSDR, 2000; Breivik
et al., 2007). They were sold in different trade names, including Aroclor, Pyranol,
Pyroclor (USA), Clophen, Elaol (Germany), Fenchlor, Apirolio (Italy), Fenochlor

18 Chapter 2: Bioremediation of Polychlorinated biphenyls
(Spain), Kanechlor, Santotherm (Japan) Phenoclor, Pyralene (France) and Sovol
(USSR) (IOMC, 2003). Among them, most commonly known and used PCB mixtures
were manufactured under the trade name Aroclor (USEPA, 2013). Approximately
48% of these commercial PCB mixtures were used as transformer oil, 21% for small
capacitors and 10% for other closed systems such as heat transfer systems and
hydraulic systems. Approximately 21% were used for open applications as
caulks/sealants, paints, plasticizers, anti-corrosion coatings, copy paper and flame
retardants (Breivik et al., 2007).
Table 2.1 PCB homologs and their chlorine substitutions
As there are 209 congeners with differing chemical structures, having a standard
nomenclature to identify and label PCBs is important. Currently, International Union
of Pure and Applied Chemistry (IUPAC) and Ballschmiter & Zell (1980) systems are in
use (IOMC, 2003). The IUPAC nomenclature is primarily based on identifying the
positioning of chlorine atoms in the aromatic carbon structure and lists the sequence
Chlorine
Substitutions
Homolog
group
Molecular
weight
Chlorine
(%)
Number of
Possible
Isomers
PCB
number
Mono C12H9Cl 154.1 19 3 1 - 3
Di C12H8Cl2 188 32 12 4 -15
Tri C12H7Cl3 222 41 24 16 -39
Tetra C12H6Cl4 256 49 42 40 -81
Penta C12H5Cl5 289.9 54 46 82 -127
Hexa C12H4Cl6 323.9 59 42 128 -169
Hepta C12H3Cl7 357.8 63 24 170 -193
Octa C12H2Cl8 391.8 66 12 194 -205
Nona C12HCl9 425.8 69 3 206 -208
Deca C12Cl10 459.7 71 1 209
Total 209

Chapter 2: Bioremediation of Polychlorinated biphenyls 19
in name convention. For example, the PCB congener with chlorine atoms in 3, 4, 5,
and 3’, 4’ carbon positions is identified as 33’44’5 pentachlorobiphenyl (see Figure
2.1 for positioning).
In the Ballschmiter & Zell (1980) naming system, congeners are numbered from PCB
1 through PCB 209 depending on the structural arrangement of the PCB congeners in
an ascending order of the number of chlorine substitutions within each sequential
homologue. The commercial PCB mixture Aroclor is identified by a four digit number.
The first two digits represent the 12 carbon atoms in the biphenyl rings and the last
two digits represent the percentage of chlorines in the PCB mixture (Borja et al.,
2005). As an example, in the commercial PCB mixture Aroclor 1260, 12 indicates the
12 carbon atoms and 60 indicates the presence of 60% chlorine by weight.
2.2.2 Influence of PCBs on human and ecosystem health
Low solubility in water and high adsorption capability to soil make PCBs stable in the
environment (Aken et al., 2009). Once contaminated, it is extremely difficult to
remove PCBs from sediment and soil matrices. They are highly soluble in non-polar
organic solvents and biological lipids (US EPA, 1980). Para and meta saturated
congeners are the most non-polar and hence most soluble in lipids (IOMC, 2003). This
extreme solubility of PCBs in oils and fats leads them to bioaccumulate in animal cells
and pass through the food chains (Passatore et al., 2014). Because of this, PCBs are
considered as one of the most extensively distributed chlorinated chemicals in the
food chains (Borja et al., 2005). Among all, ortho chlorinated congeners have the
highest solubility in water, which is attributed to the hydrogen bonding associated
with the more polar character of the molecules (IOMC, 2003). Solubility of PCBs in
aqueous media decreases with increase in the degree of chlorination. Mono
chlorobiphenyl with one chlorine atom has solubility of 6 ppm while the octa
chlorobiphenyl with eight chlorine atoms is 0.007 ppm (Borja et al., 2005).
After entering the body, PCBs are reported to be transported through the blood
stream and accumulate in liver, muscles and adipose tissues (Borja et al., 2005). It
was revealed through various research studies that there is a possibility of having

20 Chapter 2: Bioremediation of Polychlorinated biphenyls
multiple health impacts in exposed animals and humans including immune system
suppression, decrements in cognitive and neurobehavioral function, disruption of sex
steroid and thyroid function (Kjellerup et al., 2012; Yang et al., 2015). The extent of
accumulation and their health effects can vary based on the extent of exposure,
variances in the diet and metabolic processes, age, gender, size, season, physiological
condition and the area of the body where PCBs are concentrated (Brazova et al.,
2012). Worldwide monitoring programs have shown that PCBs are present in breast
milk, where illnesses and symptoms related to PCB poisoning can be shown from a
very young age (Lauby-Secretan et al., 2013). The International Agency for Research
on Cancer (IARC) has categorized PCBs as group I human carcinogens in 2013, based
on the evidence of carcinogenicity in humans and experimental animals (Chen et al.,
2015b).
2.2.3 Regulations and monitoring
In 2001, PCBs were listed as one of the twelve persistent organic pollutants by the
Stockholm Convention (UNEP, 2009). Parties to the Convention are obliged to
eliminate equipment and oils containing PCBs from use by the year 2025 and treat
them under environmentally sound waste management by 2028 (UNEP, 2009). In
Australia, material or waste containing PCBs at a concentration of more than 2 mg/kg
are subjected to regulation (VEPA, 2017). According to Canadian soil quality
guidelines for total PCBs, 0.5 mg/kg for agricultural soil, 1.3 mg/kg for residential soil
and 33 mg/kg for commercial and industrial soil are the recommended acceptable
background concentrations (CCME, 1999). PCBs are also listed as priority organic
pollutants by the United States Environmental Protection Agency (USEPA) (Sowers &
May, 2013). Clean up and disposal of soil and sediments contaminated with PCBs are
regulated under Toxic Substances Control Act (TSCA). The date of contamination, the
concentration in the source of PCB as well as the current PCB concentration in the
contaminated mass are taken into consideration during this process. If PCB level is ≥
50 mg/kg in any soil or sediments, they are regarded as PCB remediation waste and
regulated by the TSCA. Moreover, if the PCB level in soil or sediments is between 2
to 50 mg/kg from a spill after 1978 from a source that contains ≥ 50 mg/ kg or from

Chapter 2: Bioremediation of Polychlorinated biphenyls 21
a source that is unauthorized for use, such contaminated soil or sediment are
regulated as PCB remediation waste (Davila et al., 1993).
For the determination of PCBs in soil, reliable sample collection, storage, extraction,
clean-up, detection and quality assurance techniques are needed (Muir & Sverko,
2006). Ultrasonic extraction and microwave-assisted extraction are superior to the
traditional Soxhlet extraction due to better recovery, and precision (Camel, 2000;
Arulpriya & Lalitha, 2013). For qualitative and quantitative analyses, gas
chromatographic methods based on single column and single electron capture
detector (ECD) were developed in early stages and later upgraded to double column
- double ECD and mass spectrometry detection capabilities (Muir & Sverko, 2006;
USEPA, 2007; Chuanmin et al., 2015).
2.3 Removal of PCBs from contaminated soil
2.3.1 PCBs as a soil contaminant
Soil is considered as a significant environmental sink for PCBs due to their strong
sorption potential with soil colloids and resistance to physicochemical degradation
and biodegradation. Sorption of PCBs to soil particles primarily depends on the
degree of chlorination of the PCB congeners and the properties of soil such as, type
of soil, organic matter content, soil moisture content and pH (CCME, 1999). These
factors are discussed in detail in section 2.5.
Atmospheric transport and deposition of PCBs is regarded as one of the major
sources of surface soil contamination (ATSDR, 2000). This was further confirmed by a
study based on 191 global background surface soils (up to 5 cm depth) collected from
sites, far away from human activities. (Meijer et al., 2003). The study further revealed
that the estimated total global PCB level only in background soils is 21,000 tons. As
summarized in Figure 2.2, total PCBs in background soil are highly variable (ranging
from 0.04 to 100 ng/g dry weight; mean, 4.9 ng/g dry weight) with highest
concentrations in Europe and North America (Li et al., 2010). Although PCB data
coverage is relatively good for Europe, East Asia, and the Great Lakes region of North
America, in many parts of the world such as Africa, Australasia, central and northern

22 Chapter 2: Bioremediation of Polychlorinated biphenyls
Asia, South America, and the Caribbean region, data is relatively scarce (Li et al.,
2010). However, as given in the Table 2.2, the highest concentrations of PCBs in soils
are in the localized regions close to their production, application and disposal
(Vasilyeva & Strijakova, 2007).
Figure 2.2 Concentrations of total PCBs in surface soils at global background sites (Li
et al., 2010).
A recent study conducted by Desborough et al. (2016) found that the total PCB levels
in UK soils (average 4.7 ng/g, range 0.39 - 21 ng/g) have not significantly reduced
when compared to the levels reported in the mid-1980s. They suggests that the rate
of decline of concentrations of PCBs in soils are slower than expected due to the
continuing emissions from the remaining stocks in use in the built environment
(Desborough et al., 2016).

Chapter 2: Bioremediation of Polychlorinated biphenyls 23
Table 2.2 Comparison of PCB levels (µg/kg dry weight) in soils based on selected
international studies.
Location PCBs, as mean value
and (range)
Number of
samples and
(depths)
Reference
Central Region, Ghana
7.99 ± 3.8 (1.32 -12.94)a
9.15±0.52
7.55±0.56
7.82±0.55
n=78
(0-10 cm)
(10-20 cm)
(20-30 cm)
(Bentum et al., 2016)
Dump site, Italy 920 x103 NM (Di Toro et al., 2006)
Ex-landfill site, Brighton, UK
(1.7 - 13.2) b n=48, (20 cm) (Zhou et al., 2014)
UK 4.7 (0.39-21) n=24, (5 cm) (Desborough et al., 2016)
Cambridgeshire, UK
5.03(0.27 - 80.5)c n=201, (NM) (Heywood et al., 2006)
England, Wales, Scotland, UK
31.8 (1.7 – 1199) n=100, (5 cm) (Creaser et al., 1989)
Dump site, Czech Republic
705.95±22.85 x103, d
375.81±19.45 x103, e
n=10, (2 m)
n-10, (10 cm)
(Stella et al., 2015)
Switzerland (0.86 – 12)f n=23, (10cm) (Schmid et al., 2005)
South Sweden (2.3 - 986) n=66, (5 cm) (Backe et al., 2004)
Dalian, Liaoning Province, China
2.8 (1.3 - 4.8)g n=14, (5 cm) (Wang et al., 2008)
KwaZulu-Natal, South Africa
109.64±116.07h
19.22±33.23i
n=15, (0.5 cm)
n=15, (1–2 cm)
(Batterman et al., 2009)
Notes:
n – number of samples, NM – Not mentioned a 31.89% hexa, 23.98% penta, 18.47% tri, 13.67% tetra & 11.99% hepta PCBs b All PCB congeners were present except PCB 18 c Sum of 33 PCB congeners with 9% PCB 153, 8.6% PCB18, 8.8% PCB28 & 7% PCB138. d 16.6% PCB 56, 9.8% PCB 52, 9.6% PCB 44 e 20.9% PCB 56 f Sum of 7 PCBs g Sum of 57 PCBs h, i As wet weight of 38 congeners, most prevalent PCBs - 41+ 71, 153+132, 138 +163

24 Chapter 2: Bioremediation of Polychlorinated biphenyls
2.3.2 Remediation of PCB contaminated soil
Bioremediation is defined as a process that uses microorganisms, green plants or
their enzymes to treat the polluted sites for regaining their original condition (Glazer
and Nikaido, 1995). It is considered very promising as natural biological activities are
utilized either to partially degrade the contaminants to less harmful products or to
completely mineralize them (Tomei & Daugulis, 2013; Sharma et al., 2014). Generally,
biological treatments are long-term processes as they take a long time to show
satisfactory performance. However, in comparison to the other physical and chemical
treatment methods, it is less expensive and environmentally friendly while having
higher public acceptance (Busset et al., 2012; Tomei & Daugulis, 2013; Sharma et al.,
2014). In bioremediation approaches, in-situ (on site) bioremediation is preferred
over ex-situ (off site) as it involves the treatment of polluted material at the site
without removing the contaminated soil for ex-situ treatment.
2.3.3 In-situ bioremediation
In-situ bioremediation consists of the use of green plants (phytoremediation),
microorganisms (fungi and bacteria), or their enzymes to treat the polluted materials
at the site and is discussed in section 2.3.3.1 to 2.3.3.3.
2.3.3.1 Phytoremediation
In phytoremediation, plants and their associated microorganisms are used for the
treatment of contaminated soil (Aken et al., 2009). Extensive laboratory and
greenhouse studies have been undertaken to remediate PCB contaminated soils
through different phytoremediation approaches that are summarized below.
Phytoextraction is to grow suitable plant varieties capable of accumulating significant
amounts of the contaminant from the soil and storing it in the plant shoots. These
plants with high PCB levels can then be harvested and treated using suitable method
such as high temperature incineration (Aslund et al., 2007; Anyasi & Atagana, 2014;
Luo et al., 2015). This method cannot be regarded as a direct treatment option as it
use plants as a vector to physically transfer the contaminants from one place to other.

Chapter 2: Bioremediation of Polychlorinated biphenyls 25
In phytotransformation, once the pollutants are absorbed by the plant through the
root system, enzymatic breakdown of pollutants takes place inside the plant tissues
(Aken et al., 2009). However, plants generally lack the enzymes that are necessary to
achieve complete degradation of recalcitrant organic compounds and therefore,
results in slow and incomplete degradation (Eapen et al., 2007).
In contrast, rhizoremediation is more effective as some secondary plant metabolites,
which are structurally similar to contaminants are released by plant roots. They
stimulate the growth of PCB degrading microbial community associated with the root
zone (Meggo et al., 2013; Liang et al., 2014; Jha et al., 2015; Liang et al., 2015).
Microorganisms utilize these secondary plant metabolites such as naringin, myricetin
and flavonone as their growth substrate while inducing PCB cometabolism (Leigh et
al., 2006; Musilova et al., 2016). In cometabolism, contaminants which cannot serve
as the primary substrate to provide energy for the microorganisms are degraded
cometabolically while utilizing some other substrate as their primary substrate
(Musilova et al., 2016). Fully developed roots and rhizospheres in switchgrass
(Panicum virgatum) and poplar (Populus deltoids x nigra) planted microcosms have
shown better biotransformation potential than the unplanted reactors (Meggo &
Schnoor, 2013). Table 2.3 summarises recent studies based on plant-microbe
combinations in PCB degradation. Plant growth-promoting rhizobacteria can survive
and multiply under extreme weather and climatic conditions while improving the
plant biota against stress imposed by contaminants and increase their
biomass/efficiency to take up contaminants (Asad, 2017).
Higher degradation rates in the mesocosms of switchgrass rhizospheres
bioaugmented with PCB degrading bacteria suggest that the use of phytoremediation
and bioaugmentation in combination could be an efficient and sustainable strategy
for the treatment of PCB contaminated soil (Liang et al., 2015). However, success in
remediation relies on several factors such as selection of suitable plant - microbial
combinations based on soil conditions, level of contamination and bioavailability of
PCBs as well as maintenance of appropriate conditions such as pH, moisture, and
other growth requirements. Most of the available information are based on small-
scale research studies conducted in confined environments such as hydroponics and

26 Chapter 2: Bioremediation of Polychlorinated biphenyls
greenhouses. Performance under full scale is not yet known as full scale studies are
limited to a few trials and are not yet completed due to the long time span required
for plant based remediation approaches (Passatore et al., 2014). Furthermore, as a
contaminant, PCBs are frequently associated with other environmental contaminants
like dioxins and heavy metals. Therefore, an in-depth knowledge is needed to
understand the effects exerted by these co-contaminants on microorganisms and
plants associated with PCB remediation (Wang & He, 2013a).

Chapter 2: Bioremediation of Polychlorinated biphenyls 27
Table 2.3 Plant–microbial relationships associated with PCB degradation
Microorganism/s Plant PCB reduction % Type of treatment Reference
Pseudomonas putida
Stenotrophomonas maltophilia
Mustard (B. juncea) 7.2–30.3% after 2
months
Greenhouse pots with
biochar as bio-carrier
(Pino et al., 2016)
Actinobacteria sp.
Chloroflexi sp.
Alfalfa 31.4% in 1 yr.
78.4% in 2 yrs.
Contaminated site (Tu et al., 2011)
Rhodococcus sp.
Burkholderia sp.
Austrian pine (P. nigra)
Goat Willow (S. caprea)
- Contaminated site (Leigh et al., 2006)
Geobacter sp. Switchgrass (Panicum
virgatum)
30–40 % after 6 months Soil microcosms (Liang et al., 2015)
Bioaugmentation with Burkholderia
xenovorans LB400
Switchgrass (P. virgatum) 47.3 ± 1.22% after 6
months
Soil microcosms (Liang et al., 2014)
Pseudomonas sp.
Agrobacterium sp.
Ochrobactrum sp.
Horseradish (A. rusticana)
Goat willow (S. caprea)
28.0 %
31.8 % after 6 months
Open air pots
(Ionescu et al., 2009)
Mixed microbial consortium Poplar > 90% after 14 months Contaminated site (Ancona et al., 2016)

28 Chapter 2: Bioremediation of Polychlorinated biphenyls
2.3.3.2 Fungal bioremediation
Knowledge on the potential of applying fungi for PCB breakdown and their
occurrence in contaminated soils is limited. Penicillium chrysogenum, Penicillium
digitatum, Scedosporium apiospermum, and Fusarium solani have been found to
display rapid growth in mineral media containing PCB and glucose while degrading
PCBs (Tigini et al., 2009). However, none of the taxa was able to grow on PCBs as their
sole source of carbon. In microbial community analysis of soil historically
contaminated with high concentrations of PCBs, the microbial community was found
to consist of both bacteria and fungi although their individual contribution towards
PCB degradation was not known (Stella et al., 2015). Fate of some selected mono (2-
chlorobiphenyl and 4-chlorobiphenyl), tri (3,4,5-trichlorobiphenyl) and penta
(3,3`,4,4`,5-pentachlorobiphenyl) chloro biphenyls in a pure culture of Aspergillus
niger were determined. Only 2-chlorobiphenyl and 4-chlorobiphenyl were
transformed to hydrophilic metabolites with 38 and 52 % reductions, respectively,
while there was very low reduction of trichlorobiphenyl (2%) and no observable
reduction of pentachlorobiphenyl concentration (Kim et al., 2016). This result
suggests that the degradation ability of A. niger is limited to simple monochlorinated
PCB congeners.
Federici el al (2012) reported 33.8% overall PCB removal in soils contaminated with
Aroclor 1260 after bioaugmentation with maize stalk-immobilized Lentinus tigrinus.
However, the incubation control which only consisted of residential microflora also
demonstrated 28% overall PCB degradation (Federici et al., 2012). It is not clear that
the 5.8% difference between the L. tigrinus bioaugmented and control microcosms
was due to the degradation ability of L. tigrinus itself, its indirect contribution to
proliferate the residential soil microflora or direct absorption of PCBs into the fungal
mycelium. Other than the degradation, translocation of PCBs into fruiting bodies
were observed in white rot fungus Pleurotus ostreatus (oyster mushroom) growing
on straw spiked with a Delor 103 commercial PCB mixture (Moeder et al., 2005).
Pandey et al (2016) suggest that the metabolism of PCB compounds and their
metabolites by the ligninolytic enzymes secreted by white rot fungi is due to the

Chapter 2: Bioremediation of Polychlorinated biphenyls 29
structural similarities between PCB molecules and lignin molecules (Pandey et al.,
2016).
It can be concluded that the fungal action takes place primarily in the extracellular
environment due to the secretion of enzymes to the external environment (Passatore
et al., 2014). Moreover, fungal based degradation seems to be most effective against
the lower chlorinated PCBs. Assessing the applicability of some fungal strains, which
have the ability to live in symbiosis with plant roots, for remediating PCB
contaminated soils will be beneficial, as they can survive under extreme
environmental conditions which would be an added advantage in remediation
applications.
2.3.3.3 Bacterial bioremediation
When compared to plants (Section 2.3.3.1) and fungi (Section 2.3.3.2), bacteria are
the most widely studied microorganisms in PCB bioremediation due to their
ubiquitous distribution in the environment and the ability to degrade lower and
highly chlorinated congeners by numerous bacterial strains through different
anaerobic metabolism, aerobic co-metabolism and aerobic metabolism pathways
(Passatore et al., 2014). Though the findings of many research studies are available,
bacteria based bioremediation of PCBs is not yet widely practiced as a field scale
treatment technique. Therefore, bacterial bioremediation of PCBs was chosen in the
current study as a way forward to minimize the gaps in available knowledge.
In the following sections, different types of biodegradation pathways, known types
of bacteria with PCB degradation potential, and their applications are discussed.
2.4 Biodegradation pathways
In PCB degradation, two distinctive microbial processes have been identified:
anaerobic reductive dechlorination; and aerobic oxidative degradation. The higher
chlorinated biphenyls are often subjected to anaerobic dechlorination while the
lower chlorinated biphenyls are often subjected to aerobic oxidation (Demirtepe et
al., 2015).

30 Chapter 2: Bioremediation of Polychlorinated biphenyls
2.4.1 Anaerobic reductive dechlorination
The conversion of highly chlorinated congeners (congeners with five or more chlorine
atoms) to less chlorinated congeners (congeners with four or less chlorine atoms) by
replacing a single chlorine atom by a hydrogen atom is termed as dechlorination
(Hughes et al., 2009). This process reduces the potential toxicity and bioaccumulation
of highly chlorinated congeners. As illustrated in Equation 2.1, in the reductive
dechlorination process, PCBs are used as electron acceptors and the chlorine is
replaced with hydrogen (Borja et al., 2005).
R⎼Cl + 2e¯+ H+→ R⎼H + Cl¯ Equation 2.1
Anaerobic dechlorination of PCBs was detected in the sediments of the Hudson River,
Massachusetts (Furukawa & Fujihara, 2008). An escalation in the lower chlorinated
congeners and a reduction in the highly chlorinated congeners were observed when
analyzing the PCBs inanaerobic sediments (Borja et al., 2005). a similar trend was
exhibited during a laboratory study based on electronic waste contaminated soil
using dissimilatory Fe(III) reducing and arylhalorespiring bacteria (Song et al., 2015).
Specialized populations of microorganisms with distinct dehalogenating enzymes
have the potential to carry out reductive dechlorination. So far, several anaerobic
dechlorinating bacteria have been isolated. The microorganisms frequently belong to
the Dehalorespiring Chloroflexi group, such as Dehalococcoides spp. (Adrian et al.,
2009; Praveckova et al., 2015) and Dehalobium spp. (Payne et al., 2011). In addition,
Bacteroides and Betaproteobacterium, Pseudomonas (Bedard et al., 2006),
Desulfomonile, Desulfitobacterium, Dehalobacter, Dehalospirillum, Desulforomonas
(Borja et al., 2005) have also been identified as being able to undertake
dechlorination of PCBs. Furthermore, dissimilatory iron-reducing bacteria (DIRB)
belonging to Geobacteraceae are strictly anaerobic and capable of utilizing
chlorinated hydrocarbons directly as terminal electron acceptors (Song et al., 2015).

Chapter 2: Bioremediation of Polychlorinated biphenyls 31
2.4.1.1 Anaerobic reductive dechlorination pathways
Studies conducted to investigate anaerobic dechlorination suggest that different
microbial populations are responsible for different dechlorination patterns based on
the number and position of chlorine substitutions in the biphenyl structure (Beyer &
Biziuk, 2009). These patterns confirm that dechlorination occurs mainly to meta and
para substituted chlorines producing less chlorinated, mostly ortho-substituted
products as illustrated in Figure 2.3. The rate of dechlorination of meta or para
positions increases when chlorine ions are available in the neighbouring positions.
When there are two chlorines in adjacent positions, it is called as doubly flanked
chlorine and when a single chlorine ion is present in one neighbouring position, it is
referred to as flanked chlorine. The descending order of preference for
dechlorination is reported in the form of doubly flanked chlorine > singly flanked
chlorine > unflanked chlorine (Wiegel & Wu, 2000).
Figure 2.3 Potential pathway for anaerobic dechlorination of highly chlorinated
congeners. Adapted from Borja et al. (2005).
However, the dechlorination order could vary depending on the types of the
microbial population present (Bedard et al., 2007). Dehalogenation is found to be
microbial species specific in terms of the substitution positions attacked and the
numbers of chlorine atoms removed. This conclusion is based on the observations of
different microbial populations generating different dehalogenation patterns under

32 Chapter 2: Bioremediation of Polychlorinated biphenyls
different environmental conditions (Gomes et al., 2013; Song et al., 2015). Based on
congener loss and product accumulation, eight distinct reductive dechlorination
pathways have been recognized so far as M, Q, H’, H, P, N, LP and T (Wiegel & Wu,
2000) (see Table 2.4). It was observed that the removal of chlorines is highly selective
in each dechlorination pathway. As an example, in the pathway P, para chlorines
flanked by at least one meta chlorine were selectively removed from the biphenyl
molecule while the removal of meta chlorines flanked by at least one chlorine in
either the para or the ortho position happened in Pathway N (Bedard & May, 1995).
Table 2.4 Microbial dechlorination pathways (Wiegel & Wu, 2000).
Dechlorination Pathway Chlorines Removed
M Flanked and unflanked meta
Q Flanked and unflanked para, meta of 23-group
H’ Flanked para, meta of 23- and 234-groups
H Flanked para, doubly flanked meta
P Flanked para
N Flanked meta
LP Flanked and unflanked para
T Flanked meta of 2345-group, in hepta and octa
chlorobiphenyls
Imamoglu, et al., (2002) introduced an anaerobic dechlorination model (ADM). ADM
(Imamoglu et al., 2002) helps to identify the possible anaerobic dechlorination
patterns and to quantify the relevant dechlorination pathways in sediment
mesocosms. The model was further improved by Demirtepe et al. (2015), to identify
the possible anaerobic dechlorination patterns and to quantify the relevant
dechlorination pathways. In the modified ADM (Demirtepe et al., 2015),
dechlorination pathways are defined by the targeted chlorines (i.e. meta and para
positions, either as singly or doubly flanked) unlike the dechlorination pathways
based on congener loss and product accumulation (Wiegel & Wu, 2000).
Improvements to the previously modified ADM model have enabled a better

Chapter 2: Bioremediation of Polychlorinated biphenyls 33
understanding of multiple dechlorination activities and co-elution of congeners with
increased accuracy, speed and flexibility by incorporating constraints and a new
goodness of fit evaluation, as reported by Karakas & Imamoglu (2017).
2.4.2 Aerobic oxidative degradation
Aerobic degradation typically occurs in topsoil and sediment layers. As noted by Field
& Sierra-Alvarez (2008), lower chlorinated congeners and the products of anaerobic
reductive dechlorination are subjected to aerobic degradation leading to complete
mineralization (Field & Sierra-Alvarez, 2008). Microbial oxidation of low (mono to
tetra) chlorinated biphenyls is catalysed by aerobic bacteria, which are capable of
degrading biphenyls as their sole source of carbon and energy (Pieper & Seeger,
2008). Various gram negative bacteria such as Acinetobacter, Alcaligenes,
Achromobacter, Burkholderia, Comamonas, Pseudomonas, Ralstonia, Sphingomonas
and gram positive bacteria such as Bacillus, Corynebacterium and Rhodococcus are
responsible for aerobic oxidative degradation of PCBs (Furukawa & Fujihara, 2008;
Hassan, 2014). Complete genomes of Burkholderia xenovorans LB400 and
Rhodococcus jostii RHA1, two of the most widely studied and characterised PCB-
metabolizing strains contributed to improve knowledge on their overall metabolism,
physiology, and evolution as well as their defence mechanisms against PCB toxicity
(Atago et al., 2016; Agullo et al., 2017).
Some intermediate products such as dihydrodiols and dihydroxybiphenyls that are
produced during the PCB degradation by diverse bacteria, including Burkholderia sp.
are referred to as dead-end intermediates as they are highly toxic to bacteria (Camara
et al., 2004). The reason for the toxicity is associated with the increased polarity of
these dihydroxylated metabolic intermediates as it make them more soluble in
aqueous medium (Seeger & Pieper, 2010). Only a few microorganisms have been
reported so far with the capacity to completely mineralize PCBs without
accumulating dead-end intermediates. A novel bacterium, Sphingobium fuliginis was
found to degrade biphenyl, lower chlorinated (mono, di and tri) PCBs and mono
chlorobenzoates without accumulation of dead-end intermediates (Hu et al., 2015).
More recently, Qui et al. (2016), demonstrated that after a 40% inoculation volume,

34 Chapter 2: Bioremediation of Polychlorinated biphenyls
Comamonas testosteroni was capable of completely degrading 100–500 mg/L
decachlorobiphenyl PCB209 as the sole carbon source in 140 hr at low temperature
(10 °C ±0.5 °C) and pH 7–8. The strain was also effective in practical applications of
PCB209 biodegradation in contaminated soil (Qiu et al., 2016).
The aerobic oxidative degradation of PCBs occurs through two distinctive pathways.
Firstly, less chlorinated congeners are converted to the corresponding chlorinated
benzoic acid through the upper biphenyl pathway (Field & Sierra-Alvarez, 2008) as
illustrated in Figure 2.4. Chlorobenzoate is further mineralized to carbon dioxide and
inorganic chlorides through lower biphenyl pathways (ATSDR, 2000; Field & Sierra-
Alvarez, 2008) as illustrated in Figure 2.5 a and b. Utilization of biphenyl, an
unchlorinated analogue of PCBs and benzoate, an intermediate of biphenyl
degradation pathways are mainly performed by distinct bacterial groups with
different ecological growth strategies (Leewis et al., 2016). However, only a small
proportion of the microbial community have demonstrated their ability to assimilate
carbon from both biphenyls and benzoates. This may be due to the possessing of
enzymes that catalyses biochemical reactions in both upper and lower biphenyl
pathways.
2.4.2.1 The upper biphenyl degradation pathway
Four key enzymes are involved and play major roles in the upper biphenyl
degradation pathway (see Figure 2.4).

Chapter 2: Bioremediation of Polychlorinated biphenyls 35
Figure 2.4 The upper biphenyl degradation pathway. Modified from Field and Sierra-Alvarez (2008).

36 Chapter 2: Bioremediation of Polychlorinated biphenyls
(A) Biphenyl-2,3-Dioxygenases (BphA)
Biphenyl dioxygenases initiate the oxidation of polychlorinated biphenyls. The
enzyme belongs to the toluene / biphenyl branch of Rieske non-heme iron
oxygenases (Pieper & Seeger, 2008). It catalyses the formation of chlorinated dihydro
biphenyl-2,3-diol. It was revealed that there are considerable differences in various
biphenyl 2,3-dioxygenases for their congener selectivity patterns, as well as their
preference for the attacked ring structure (Seeger & Pieper, 2010). The order of
preference for the deoxygenation on the biphenyl pathway has been observed based
on Burkholderia xenovorans strain LB400 as; unsubstituted > 2-chloro > 2,5-dichloro
> 2,4-dichloro > 3-chloro > 4-chloro > 2,3-dichloro (Pieper & Seeger, 2008). The bphA1
gene encodes the large subunit of biphenyl 2, 3-dioxygenase enzyme. Major amino
acid differences are found in this subunit in different PCB degrading bacterial species.
This feature may explain the reason for the affinity of different bacteria for different
PCB congeners (Furukawa & Fujihara, 2008; Hoostal & Bouzat, 2016). The presence
of bphA1 gene has been used as a measure of genetic potential for PCB
biodegradation (Dercova et al., 2008).
(B) Cis-2,3-dihydro-2,3-dihydroxybiphenyl dehydrogenase (Cis-dihydrodiol
dehydrogenase) (BphB)
BphB is responsible for the dehydrogenation of cis-2,3-dihydro-2,3-
dihydroxychlorobiphenyls to 2,3 –dihydroxy chlorobiphenyl in the second step of the
upper biphenyl pathway (Furukawa & Fujihara, 2008). They are short chain alcohol
dehydrogenases and show common features such as an absolute requirement for the
coenzyme nicotinamide adenine dinucleotide (NAD+). This group of dehydrogenases
show broad substrate specificity and oxidized the cis-di-hydrodiols derived from
biphenyl and other polycyclic hyrocarbons to catecols through various aromatic
degradation pathways (Jouanneau & Meyer, 2006).
(C) 2,3-dihydroxybiphenyl-1,2-dioxygenase (BphC)
This is the critical step in upper biphenyl degradation pathway as 2, 3-
dihydroxybiphenyl-1,2-dioxygenase catalyses aromatic ring cleavage (Dai et al.,

Chapter 2: Bioremediation of Polychlorinated biphenyls 37
2002). 2,3 –dihydroxy chlorobiphenyl is converted into a yellow meta-cleavage
product called chlorinated 2-hydroxy-6-oxo-6-phenylhexa-2,4-dienoic acid (HOPDA)
by the enzyme 2,3-dihydroxybiphenyl-1,2-dioxygenase (Field & Sierra-Alvarez, 2008).
This enzyme belongs to type I extradiol dioxygenases (Pieper & Seeger, 2008). The
development of yellow coloration from the meta cleavage product was used to
screen the bacteria capable of producing dioxygenases as they are potential
candidates for PCB degradation (Dercova et al., 2008). Though they differ in substrate
specificity, BphC enzymes have the ability to transform various chloro substituted
derivatives into meta cleavage products (Dai et al., 2002). However, it was found that
the presence of 3,4-dihydroxybiphenyl and ortho chlorinated PCB metabolites
strongly inhibited the BphC enzymes responsible for aromatic ring cleavage by
promoting their suicide inactivation (Furukawa, 2006).
(D) 2-hydroxy-6-phenyl-6-oxohexa-2,4-dieneoate (HOPDA) hydrolase (BphD)
The enzyme catalysing the final conversion step for the upper biphenyl pathway is
BphD belonging to α / β hydrolase enzyme superfamily. It hydrolyses HOPDA down
to chlorinated 2-hydroxy-penta-2,4-dienoate and chlorobenzoate (Pieper & Seeger,
2008).
2.4.2.2 The lower biphenyl degradation pathway
Chlorobenzoates and 2-hydroxypenta-2,4-dienoates generated from the upper
biphenyl degradation pathway are further degraded through the lower biphenyl
pathway as shown in Figure 2.5 (a) and (b). Three enzymes called 2-hydroxypenta-
2,4-dienoate hydratase (BphH), 4-hydroxy-2-oxovalerate aldolase (BphI) and an
acylating acetaldehyde dehydrogenase (BphJ) are responsible for the conversion of
2-Hydroxypenta-2,4-dienoate to pyruvate and acetyl-CoA (Seeger & Pieper, 2010).
The resulting acetyl-CoA then enters the tricarboxylic acid (TCA) cycle, which is used
by all aerobic organisms to release their stored energy. Benzoate, the other end
product generated in the upper biphenyl degradation pathway is a growth substrate
for a broad range of bacteria. It can be mineralized to CO2 and H2O either via
chlorocatechol or 3-oxoadipate pathways under aerobic conditions (Pieper, 2005).

38 Chapter 2: Bioremediation of Polychlorinated biphenyls
Figure 2.5 The lower biphenyl degradation pathways (a) Mineralization of 2-hydroxypenta -2,4-dienoate. Modified from Field and Sierra-Alvarez
(2008). (b) Mineralization of chlorobenzoic acid. Modified from ATSDR (2000).

Chapter 2: Bioremediation of Polychlorinated biphenyls 39
2.4.3 Sequential anaerobic-aerobic degradation
To achieve a complete degradation of PCBs, a combination of anaerobic reductive
dechlorination and aerobic oxidation is essential. Recent reviews and studies discuss the
possibility of promising results in biological removal of highly chlorinated PCB congeners
in sediments when anaerobic and aerobic processes are combined (Pieper, 2005; Bedard
et al., 2006; Wang & He, 2013b). The study by Master et al. (2002) observed no PCB
degradation without prior anaerobic treatment in soil slurry mesocosm containing
weathered Aroclor 1260 (Master et al., 2002). During their initial anaerobic treatment,
highly chlorinated congeners of Aroclor 1260 were completely or partially transformed
to less chlorinated congeners. Sequential anaerobic-aerobic composting of 2:3 soil to
waste ratio led to the mineralization of highly chlorinated PCBs in soil, with 25%
reduction of total PCBs and 61% reduction of PCBs containing two to five chlorine atoms
(Long et al., 2015). However, except for some soil–slurry based studies (Tartakovsky et
al., 2001; Master et al., 2002; Rodrigues et al., 2006), limited knowledge is available to-
date in relation to soil based sequential anaerobic-aerobic degradation of PCBs (Long et
al., 2015).
A sediment based study conducted in Guanica Bay, Puerto Rico, contaminated with
relatively high concentrations of PCBs, showed a high relative abundance of Chloroflexi
group, which included known anaerobic PCB-degrading bacteria in bottom sediments
and the presence of prominent aerobic PCB degraders in the intertidal sediments (Klaus
et al., 2016). This is a good example of the occurrence of combination of aerobic and
anaerobic biodegradation processes simultaneously in nature to decontaminate PCB
mixtures. Promising results have been obtained in concurrent anaerobic-aerobic
degradation of weathered Aroclor 1260 contaminated sediment and soil in laboratory
mesocosms (Kjellerup et al., 2012; Payne et al., 2013). Weathered Aroclor-contaminated
sediment mesocosms bioaugmented with anaerobic halorespiring Dehalobium
chlorocoercia and aerobic Burkholderia xenovorans showed 80% reduction in total PCBs
(by mass) after 120 days (Payne et al., 2013). Concurrent anaerobic dechlorination by D.
chlorocoercia and aerobic degradation by B. xenovorans was evident by no substantial
increase in lower chlorinated congeners. Therefore, concurrent degradation would be a
preferred alternative to the sequential anaerobic-aerobic degradation as it more closely

40 Chapter 2: Bioremediation of Polychlorinated biphenyls
mimics natural attenuation processes. In two stage and concurrent degradation
processes, use of facultative bacteria with the ability of both anaerobic dechlorination
and aerobic degradation of PCBs would be a potential area for future research as they
have the ability to survive under aerobic and anaerobic conditions.
2.5 Factors affecting microbial remediation
The rate, extent, and route of PCB degradation in soil are determined by a variety of
biological, chemical and physical factors as they have a direct impact on the specific
bacterial communities involved in the degradation process and the bioavailability
fraction of PCBs (Camara et al., 2004; Vasilyeva & Strijakova, 2007; Chen et al.,
2015a). The major contributing factors are summarised in Figure 2.6 as presented
below.
Figure 2.6 Overview of factors affecting microbial remediation
2.5.1 Soil properties
Soil is an extremely complex system when compared to other environmental media.
Soil has variable physical, chemical and biological characteristics and such variation
influences the rate of PCB biodegradation in soil. As hydrophobic compounds, PCBs

Chapter 2: Bioremediation of Polychlorinated biphenyls 41
are strongly sorbed to soil particles. The sorption capacity is determined primarily by
the quantity and quality of organic matter present in soil (Haluska et al., 1995).
Consequently, organic matter content is an important soil parameter that influences
the effectiveness of soil bioremediation as it has a direct impact on the bioavailability
of PCBs. Therefore, organic matter content is one of the rate limiting factors for the
persistence of inoculated microbial strains in soil and their ability to degrade PCBs
(Mrozik & Piotrowska-Seget, 2010). The presence of aromatic carbon in humic acids
also plays an important role in the survival and activity of inoculants (Long et al.,
2015). A recent study on sediments from the Hudson and Grasse river in the USA
revealed that the degree of dechlorination was mainly determined by the sediment
itself, not by the composition of PCBs (Xu et al., 2016). However, very little is known
about the activity of PCB degrading microorganisms in different soil types, though it
is an important requirement in in-situ bioremediation applications.
2.5.2 Environmental factors
Environmental factors such as pH, temperature and moisture levels play a major role
in PCB degradation. Their influences on PCB degradation are discussed below.
2.5.2.1 pH
Concentration of hydrogen cations (H+) plays an important role in the growth and
metabolism of microorganisms. Sediment samples contaminated with Aroclor
mixtures revealed that the optimal pH for the removal of chlorine under anaerobic
conditions was 7.0–7.5 (Borja et al., 2005). Degradation of PCBs in liquid cultures by
Pseudomonas sp. under aerobic conditions also indicated that near neutral pH
conditions were favourable, whereas a lower pH severely inhibited PCB
biodegradation (Chen et al., 2015a). In their study, the degradation efficiencies of
total PCBs were 4.2 %, 57 %, 63.6 %, and 44.3 % at pH 4, 6, 7, and 8 respectively.
Similarly, a 100 % degradation rate of PCB 209 was achieved with the cold resistant
Comamonas testosteroni strain between pH 7 to 8, whereas the degradation rates
were reduced to 80.6 % and 66.6 %, respectively, at pH 9 and 10 (Qiu et al., 2016).

42 Chapter 2: Bioremediation of Polychlorinated biphenyls
2.5.2.2 Temperature
Temperature can significantly influence the bioavailability of PCBs in the environment
by creating an imbalance between the ratio of PCBs that are in dissolved form and
those adsorbed to organic matter (Asad, 2017). This imbalance can hinder the growth
of microorganisms by having an impact on physiological activities such as uptake and
enzymatic breakdown (Wiegel & Wu, 2000). Investigation of sediment contaminated
with Aroclor 1260 revealed that different optimal temperature ranges were
responsible for different dechlorination patterns (ATSDR, 2000; Borja et al., 2005).
However, it is difficult to draw conclusions solely based on laboratory experiments
where samples are incubated at constant temperature, whereas in nature, soil is
subjected to temperature fluctuations between day and night and seasonally.
Therefore, field scale studies and laboratory scale studies, which mimic real field
conditions, are important to assess the variation of the degradation efficiencies due
to diurnal effects and seasonal climatic changes.
2.5.2.3 Soil moisture
Soil moisture is critical, and a 43% degradation rate of highly chlorinated biphenyls
was obtained at 60% moisture content in anaerobic composting of PCB-contaminated
soil using pig manure (Zhang et al., 2013). Activity of bacteria is reduced when the
soil water content is low as it limits the supply of substrates through diffusion and
causes cell dehydration (Mrozik & Piotrowska-Seget, 2010).
2.5.3 PCB related properties
As a whole, PCBs are relatively insoluble in water, and the solubility decreases with
increased chlorination. As an example, solubility of Aroclor 1216 with 16% chlorines
is 0.42 mg/L in water while the solubility of Aroclor 1260 with 60% chlorine is 0.003
to 0.08 mg/L (ATSDR, 2000). The number and position (ortho, meta and para) of
chlorine atoms in a PCB molecule is directly related to the type, extent and pathway
for bioremediation. PCB congeners having chlorines in one biphenyl ring breakdown
more easily than those having chlorines in both rings, while the congeners with

Chapter 2: Bioremediation of Polychlorinated biphenyls 43
double ortho substituted chlorines (chlorine at position 2, 6- or 2, 2′) are hard to
degrade (Furukawa & Fujihara, 2008). The preferred positions for overall
dechlorination were found to be, meta > para > ortho, while the ortho and double
flanked meta chlorines were primarily targeted followed by single and double flanked
para chlorines (Xu et al., 2016).
Effective rates for PCB dechlorination have been found to happen in the range of
100–1,000 mg/kg (wet weight) concentration in contaminated sediments (ATSDR,
2000). Concentrations above 1000 mg/kg may cause toxicity in bacterial communities
(Passatore et al., 2014). Conversely, at concentrations lower than 50 mg/kg, the
dechlorination process significantly reduced due to the insufficient induction of the
degradative enzymes and difficulty in sustaining the growth of competent organisms
(Passatore et al., 2014). However, it has been observed in some laboratory based
experiments that the reductive dechlorination of many PCB congeners in commercial
PCB mixtures take place even when their individual concentrations are less than 1
mg/kg (ATSDR, 2000). This finding is significant as it demonstrates the ability of PCB
dechlorinating microorganisms to remove the traces of PCBs from contaminated
environments.
2.5.4 Natural microbial diversity
The bioremediation of PCBs is possible due to the activities and interactions of
aerobic and/or anaerobic heterotrophic microorganisms present in soil. Natural
bioremediation of soils contaminated with PCBs is frequently affected by low
bioavailability and the inadequacy of PCB degrading autochthonous microorganisms
(Di Toro et al., 2006). Higher microbial density and diversity can be expected in
surface soil than in the sub surface soil due to the high organic matter content created
by plants (Boopathy, 2000). Organic matter serves as a reserve of carbon, energy and
macronutrients for microorganisms. With depth, microorganisms such as fungi and
actinomycetes decrease in numbers and bacteria become more dominant in the
microbial community (Boopathy, 2000). This is attributed to the ability of bacteria to
use alternative electron acceptors other than oxygen, under anaerobic conditions.

44 Chapter 2: Bioremediation of Polychlorinated biphenyls
Microbial community composition analysis of bulk soil (depth of 2m), top soil (depth
up to 10 cm) and rhizosphere soil samples collected from a historically PCB
contaminated dump site in Czech Republic using pyrosequencing revealed that
proteobacteria were the most dominant group consisting nearly 25% of the total
population (see Figure 2.7) (Stella et al., 2015). Previously isolated bacteria with high
degradation potential to a wide range of PCBs and their halogenated metabolites
belonged to Sphingomonas, Burkholderia and Arthrobacter, which were among the
most abundant genera. Abundance of Burkholderia in the rhizosphere soil was
considerably high when compared to the other two soils, indicating their association
with plants.

Chapter 2: Bioremediation of Polychlorinated biphenyls 45
Figure 2.7 Variation of the bacterial community at genus level in the bulk, top and
rhizosphere soils. The phylum of each genus is reported in brackets (Acid-
Acidobacteria; Act-Actinobacteria; Alph-Alphaproteobacteria; Beta-
Betaproteobacteria; Chlo-Chloroflexi; Firm-Firmicutes; Gamm-
Gammaproteobacteria; Gemm-Gemmatimonatedes). Adapted from Stella et al.
(2015).
One of the factors that hinders the complete degradation of PCB mixtures by diverse
bacteria is accumulation of different metabolic intermediates including
dihydroxylated derivatives (Camara et al., 2004). They act as dead-end metabolites

46 Chapter 2: Bioremediation of Polychlorinated biphenyls
and the increased concentration of dihydroxylated intermediates causes cell lysis and
reduction of viable cell count.
2.6 Enhancement of bioremediation
Biological treatments offer environmentally friendly remediation options. However,
prolonged time frames before being able to observe results is one of the most limiting
factors in bioremediation of PCBs when compared to physical and chemical treatment
technologies. This section summarizes the various techniques proposed to overcome
the limitations due to factors such as low water solubility and complexity of PCBs,
inability of natural microbial flora to carry out PCB degradation and the lack of
additional carbon sources as discussed in section 2.5.
2.6.1 Biostimulation
Biostimulation of native PCB degrading microorganisms has been achieved by
introducing PCBs or other halogenated aromatic compounds to the contaminated soil
as alternate halogenated electron acceptors/co-substrates (haloprimers) (Krumins et
al., 2009). This approach can result in increased biomass of dehalogenating microbial
population, induce genes required for dechlorination and support either
dehalorespiration or cometabolism of PCB compounds (Sowers & May, 2013).
However, to introduce toxic compounds to soil as a treatment option is not an
environmentally acceptable approach as it may worsen the situation. Addition of
carvone to induce the biphenyl pathway (PetricHrsak et al., 2011) and the addition of
elemental ion to supply hydrogen as electron donor through the anaerobic corrosion
of iron (Varadhan et al., 2011) were observed to stimulate the indigenous population
of dechlorinators significantly in contaminated surface sediments. It was observed
that the oxidation of Fe stimulated microbial activity, while the resulting Fe2+
sequestered potentially inhibitory sulfides (Rysavy et al., 2005). However, it is
obvious that biostimulation will not be successful if the soil is deficient in viable
indigenous bacterial population with the PCB degradation potential.

Chapter 2: Bioremediation of Polychlorinated biphenyls 47
2.6.2 Bioaugmentation
Bioaugmentation is the application of native or allochthonous wild type or genetically
modified microorganisms to sites contaminated with hazardous waste to speed up the
removal of undesirable compounds (Mrozik & Piotrowska-Seget, 2010). Successful
bioaugmentation of soil requires knowledge on the nature and level of contaminants and
the suitable microbial strains. Limited data is available on in-situ bioaugmentation as
most experiments are conducted under controlled laboratory environments (Adrian et
al., 2009; Krumins et al., 2009; Payne et al., 2011; Praveckova et al., 2015). However, 56%
reduction of higher chlorinated PCBs was observed after 120 days in Dehalobium
chlorocoercia DF1 bioaugmented sediment mesocosms contaminated with weathered
Aroclor 1260 (Payne et al., 2011). According to Winchell and Novak, (2008), addition of
iron failed to stimulate dechlorination of PCBs in sediment based microcosms without
bioaugmentation of suitable microbial culture (Winchell & Novak, 2008). Appearance of
congeners that were not products of Dehalobium chlorocoercia DF1, indicated the
possibility of stimulating of native PCB halorespiring populations after the
bioaugmentation (Payne et al., 2013).
Competition with indigenous microorganisms for limited resources, their antagonistic
interactions and predation play essential roles in bioaugmentation (Mrozik &
Piotrowska-Seget, 2010; Joutey et al., 2013). Repeated inoculation of the soil with an
active bacterial culture has been suggested to improve the efficiency of bioaugmentation
(Singer et al., 2000; Megharaj et al., 2011). A total of 57% PCB removal was observed
after repeated applications of a co-inoculation consisting of Arthrobacter sp. and
Ralstonia eutrophus, two PCB degrading bacteria with different congener specificities, 17
times during nine weeks duration to the Aroclor 1242 contaminated soil (Singer et al.,
2000). Pre-adaptation of catabolic bacteria to the target environment prior to inoculation
enhanced the rate of remediation (Megharaj et al., 2011), through improved survival,
persistence and degradative activities of microorganisms.

48 Chapter 2: Bioremediation of Polychlorinated biphenyls
2.6.3 Mixed microbial consortia
Bioaugmentation with bacterial consortia having PCB degradation potential and the
ability to respond to different environmental stimuli are more desirable than single
strains (Bedard et al., 2006; Di Toro et al., 2006; Wang & He, 2013b; Sharma et al.,
2014). PCBs are present as complex congener mixtures in the environment and
different catabolic enzymes have been identified as bottlenecks for each congener
(Seeger & Pieper, 2010). Each microbial strain used in the consortium can exhibit a
particular activity range on the type and the extent of PCB metabolized, when
individually tested (Liz et al., 2009). PCB degradation potential of two bacterial
cultures individually and as a consortium were assessed for 15 days and the GC MS
analysis indicated that the 97% of PCB-44 (tetrachlorobiphenyl) reduction in
treatment containing consortium, whereas the individual organisms showed much
lower degradation activity yields at 67% and 82% (Sharma et al., 2014).
2.6.4 Surfactants
Lack of bioaccessibility due to the hydrophobic nature of PCBs is one of the most
limiting factors (Stella et al., 2015). This limitation can be overcome by the addition
of mobilizing agents such as chemical or biological surfactants to the polluted soil
(Banat et al., 2000; Singer et al., 2000; Fava & Di Gioia, 2001; Occulti et al., 2008).
However, use of chemical surfactants requires proper selection, planning and dosing.
Furthermore, prior information is needed about the type and fate of the surfactant
as well as PCBs to avoid possible leaching, ground and surface water pollution.
Biosurfactants are preferred over chemical surfactants due to their high interfacial
tension reduction activities, no or low toxicity and being readily biodegradable
(Mulligan, 2005). So far, various biosurfactants produced by microorganisms have
been identified (see Table 2.5).

Chapter 2: Bioremediation of Polychlorinated biphenyls 49
Table 2.5 Types and microbial origin of biosurfactants.
Type of
biosurfactant
Microorganism Reference
Alasan Acinetobacter
radioresistens
(Navon-Venezia et al., 1995)
Arthrofactin Arthrobacter sp. (Morikawa et al., 2000)
Biosur PM Pseudomonas maltophilla (Phale et al., 1995)
Glycolipid Alcanivorax borkumensis
Serratia rubidea
Serratia marcescens
(Abraham et al., 1998)
(Matsuyama et al., 1990)
(Pruthi & Cameotra, 1997)
Lichenysin Bacillus licheniformis (Lin et al., 1994)
Particulate
surfactant
Pseudomonas marginalis (Burd & Ward, 1996)
Rhamnolipid Pseudomonas aeruginosa
Pseudoxanthomonas sp.
(Abdel-Mawgoud et al., 2010)
(Nayak et al., 2009)
Sophorose lipid Candida bombicola
Candida apicola
(Van Bogaert et al., 2007)
Streptofactin Streptomyces tendae (Richter et al., 1998)
Surfactin Bacillus pumilus
Bacillus subtilis
(Morikawa et al., 1992)
(Kakinuma et al., 1969)
Trehalose lipid Norcardia sp.
Rhodococcus sp.
(Kosaric & Choi, 1990)
(Singer et al., 1990)
Trehalose
tetraester
Arthrobacter sp.
Rhodococcus
wratislaviensis
(Passeri et al., 1991)
(Tuleva et al., 2008)
Viscosin Pseudomonas fluorescens (Laycock et al., 1991)
Application of microbial strains that are equally capable of biosurfactant production
and PCB degradation would be an attractive alternative to addition of surfactants.
Selection of an appropriate host strain/s for the expression of PCB-degrading
enzymes can include biosurfactant producing strains as they are ubiquitously
distributed in the environment (Bodour et al., 2003; Ohtsubo et al., 2004). Bodour et
al. (2003) screened soil samples collected from contaminated and uncontaminated
soils for biosurfactant-producing isolates. Out of the 20 soils tested, 13 including all
undisturbed soils contained a total of 45 putative biosurfactant producers. However,
the authors concluded that further research on the interactions among the possible
microbial candidates, biosurfactants and PCBs is needed as most research studies so

50 Chapter 2: Bioremediation of Polychlorinated biphenyls
far conducted were based on the addition of either chemical or biological surfactants
alone, but not the direct application of surfactant producing microorganisms.
2.6.5 Secondary carbon sources
In anaerobic PCB dechlorination, PCBs are used as electron acceptors, but the rings are
not cleaved. Consequently, the PCB dechlorinating microorganisms require additional
sources of carbon and electrons for their growth. However, the presence of other carbon
sources could either stimulate the use of substances that hinder the PCB dechlorination
or inhibit PCB dechlorination by facilitating to out-compete the non-PCB dechlorinating
microorganisms or providing more preferred electron acceptors than PCBs to the
dechlorinating population (Wiegel & Wu, 2000; Yang et al., 2008; Parnell et al., 2010).
Indeed, the addition of glucose, tryptone, yeast extract (Hu et al., 2015), fumarate,
lactate (Song et al., 2015), malate (Bedard et al., 2006), fatty acids like acetate (Adrian et
al., 2009), propionate, butyrate, and hexanoic acid has been found to stimulate the
dechlorination of PCBs in carbon limited soils and sediments (ATSDR, 2000; Wu et al.,
2000; Sowers & May, 2013; Passatore et al., 2014; Wang et al., 2014).
In addition, enhanced dechlorination of low concentrations of weathered PCBs was
observed in Dehalococcoides ethenogenes bioaugmented sediment microcosms
amended with pentachloronitrobenzene (Krumins et al., 2009). Biphenyl addition was
also shown to enhance the mineralization of PCBs in bioaugmented and non-
bioaugmented treatments (Di Toro et al., 2006; Field & Sierra-Alvarez, 2008; Parnell et
al., 2010; Luo & Hu, 2013). However, the addition of harmful chemicals like
pentachloronitrobenzene and biphenyls to the environment as inducers may not be
appropriate (Ohtsubo et al., 2004) as it might lead to increased pollution levels. The
applicability of inexpensive and non toxic alternatives like agricultural residues, mulch
and compost as carbon sources is a preferred approach as these will also contribute to
upgrading the quality of contaminated soil through increasing water holding capacity,
porosity and microbial diversity.

Chapter 2: Bioremediation of Polychlorinated biphenyls 51
2.6.6 Biocarriers
The introduction and ongoing maintenance of microbial communities in
contaminated sites are important to achieve and sustain successful bioremediation
efforts (Mrozik & Piotrowska-Seget, 2010). In most PCB-related degradation studies,
bacteria are directly introduced as liquid cultures to the contaminated soil though
PCB molecules are hydrophobic and poorly soluble in water (Di Toro et al., 2006; Liz
et al., 2009; Liang et al., 2014). Immobilizing microorganisms to suitable carrier
material helps to disperse the cells in the field while providing a protective niche for
the microorganisms against harsh environmental conditions and competitive
indigenous microflora (Power et al., 2011).
Clay particles have been reported to facilitate biofilm formation by acting as a
nutrient shuttle for the transfer of hydrophobic contaminants to biodegrading
microorganisms (Payne et al., 2013). In addition, bioaugmentation of PCB
contaminated soil by free, alginate and biochar immobilized microbial consortium
have shown that the survival rate is equally high in alginate and biochar than the free
microorganisms, while the highest PCB removal was noted in biochar immobilized
mesocosms (Pino et al., 2016). Furthermore, properly selected granulated activated
carbon (GAC) was tested and found to facilitate immobilization of microorganisms for
efficient PCB degradation by effectively sequestering PCBs from contaminated soil
and sediment (Mercier et al., 2014). The selection of carriers that can play a dual role,
as an absorbent for PCB molecules and an immobilizing agent for microorganisms
would be beneficial in order to concentrate low levels of PCBs while facilitating
microbial survival and PCB breakdown.
2.7 Monitoring of PCB degradation
The effectiveness of bioremediation efforts to remove the PCBs from the environment is
traditionally monitored through the assessment of loss of chlorines from the biphenyl
molecule, disappearance of PCB congeners of interest, total PCB reduction or by
analysing the intermediates generated and metabolic end products (Ganey & Boyd,
2005). However, for the successful development and implementation of bioremediation

52 Chapter 2: Bioremediation of Polychlorinated biphenyls
strategies, understanding and monitoring of indigenous microbial communities, their
metabolic capabilities, and the way they control the major degradation pathways under
in-situ conditions are important.
Introduction of culture independent molecular approaches for the assessment of
diversity, structure, dynamics and functions of the microbial communities within
contaminated environments have revolutionized the concept of bioremediation (Sar &
Islam, 2012). Invention and advancement of molecular tools as well as a wide range of
“omics” technologies now permit analysis of microbial community composition and
activity, while preserving the fingerprints of biotic and abiotic factors, opening up new
perspectives in pollution control (Bell et al., 2014). Genomics, proteomics and
metabolomics are some of the important omics technologies useful in studying the
microorganisms involved in bioremediation by means of their genomes, the protein
products synthesized based on the genetic instructions, and the type of molecules they
metabolize, respectively (see Figure 2. 8). All the small molecules involved in the chemical
reactions in an organism is included in the metabolome. Therefore, metabolomics can
be used to detect the changes occur in the genomes and proteomes of organisms in
response to exposure of the chemical contaminants present in the environment.
Figure 2.8 The omics pyramid. Modified from NASEM (2016).

Chapter 2: Bioremediation of Polychlorinated biphenyls 53
Genomics aims to understand the structure of the genome of single microorganism
through mapping genes and sequencing the DNA while metagenomics gives the
complete picture of microbial communities (microbiome) directly in their natural
environments without isolation and culturing of individual microorganisms (Thomas
et al., 2012). Similarly, proteomics can provide information on the composition of
proteins or proteome of the individual microbial species or the metaproteome of the
microbial community under specific environmental conditions (Figure 2.9) (Chovanec
et al., 2011).
In metagenomics, identification of microbial communities with PCB degradation
potential in contaminated environments is done using culture independent
molecular methods based on direct soil DNA extraction and analyses (PetricHrsak et
al., 2011). In oxidative PCB degradation, the degradation and transformation of PCBs
are generally taking place through the upper and lower biphenyl (bph) pathways as
described in detail under in Section 2.4.2. Two dioxygenases known as BphA and BphC
are the key enzymes responsible for catalyzing the initial steps in the upper biphenyl
pathway. Cell free polymerase chain reaction (PCR) based screening assay was
developed by Hoostal et al. (2002) to determine the PCB degradation potential of
resident microbial populations in PCB contaminated sediments through amplification
of bphA1 gene that encodes the biphenyl dioxygenase enzyme (Hoostal et al., 2002).
Abundance of the PCB degrading functional community in soil was successfully
assessed through the amplification of bphA and bphC sequences by quantitative PCR
assays (PetricHrsak et al., 2011).

54 Chapter 2: Bioremediation of Polychlorinated biphenyls
Figure 2.9 Organism based, and community based “omic” approaches for assessing
bioremediation approaches. Modified from Chovanec et al. (2011).
Environmental proteomics applied to on-site microbial mineralization applications
can provide important information about the different proteins or enzymes and their
potential roles during bioremediation. Such information can be used to develop
biomarker proteins relevant to bioremediation and to build up a proteomic databank
to enable better understanding of the catalytic roles of microorganisms (Ahmad &
Ahmad, 2014). Recent advances in mass spectrometry, with increased resolution,
sensitivity, and throughput has led to an important development in analysing total
proteins (proteomics) (Chovanec et al., 2011). Development of more efficient, simple
and cost-effective methods for protein extraction from microbial communities and

Chapter 2: Bioremediation of Polychlorinated biphenyls 55
improvement in bioinformatics tools to simplify the massive amount of data and
analyses would be helpful for reliable decision making in bioremediation applications.
Metabolomics is the scientific study of small molecules and biochemical
intermediates produced from cellular metabolism. It is a comprehensive extension of
traditional metabolite analysis. Further, the extremely diverse chemical structures of
metabolites can be complex, but there is progress with identification and
quantification being enabled using various technology platforms. In particular, GCMS
based metabolite profiling of microcosms and culture media is rapidly becoming one
of the cornerstones of functional metabolomics (Ahmad & Ahmad, 2014). These
advanced technologies enables a better understanding of the physiological state of
the microorganism and its response to different types of stimuli, including pollutants
(NASEM, 2016).
2.8 Summary
Although commercial production of PCBs was terminated in 1993, recent data have
revealed that there is no significant decline in historic levels of contamination
(Bentum et al., 2016; Desborough et al., 2016). Most of the commonly used physical
and chemical treatment methods to remediate PCB contaminated soil have serious
shortcomings of being unsustainable mainly due to the practical impossibility of
treating large quantities of soil containing low concentration of pollutants and
destruction of soil ecosystem by harsh treatment conditions. Thus, there is a vital
need to search for sustainable alternative approaches. Efficient bioremediation
techniques using sustainable microorganisms have the potential to fulfil this need.
This critical review of research literature has outlined the properties of
polychlorinated biphenyls, their impacts on human and ecosystem health while
critically evaluating the current state of knowledge on bioremediation of PCB
impacted environments with emphasis on potential areas for further improvement.
Factors that limit the efficacy of microbial remediation of PCBs are numerous. As soil
is a heterogeneous medium, the degradation rate is dependent on environmental
conditions as well as the other stressors faced by microorganisms. On the other hand,
PCBs are present in the environment as complex congener mixtures, and

56 Chapter 2: Bioremediation of Polychlorinated biphenyls
bioremediation approaches generally need a longer time span due to the slow
degradation rates. Therefore, improvement and optimization of existing
bioremediation technologies are essential in order to produce viable treatment
options. Based on the promising results of some recent studies, potential areas for
further investigations are: (1). implementation of microbial based treatments which
mimic the real field situations; (2). introduction of microbial communities capable of
degrading multiple PCB congeners and their intermediates instead of using single
microbial strains; and (3). finding strains which are able to tolerate a wide range of
environmental variations, concomitant biosurfactant production and PCB
degradation.
Parallel to bioremediation studies, similar attention needs to be given to developing
reliable and standard monitoring technologies focused on microbial community
structure and their metabolic pathways. Application and development of biomarkers
or bio indicators using novel “omics” based approaches would be an enhanced
alternative to existing techniques that identify and quantify large numbers of
different congeners and their intermediate products. These new technologies require
highly sophisticated instruments, technical knowledge, upskilling, and bioinformatics
tools for data analyses, which are not yet freely available worldwide.

Chapter 3: Materials and Methods 57
Chapter 3: Materials and Methods
This chapter describes the general materials and methods used in the project.
Further details and specific materials and methods are provided in the Chapters 4 - 8
where appropriate.
3.1 Chemicals, media and reagents
All the chemical compounds, reagents and solvents used in the study were of high
purity analytical grade. The water was of MilliQ grade (Millipore), sterilized at 121 °C
for 30 min before use. Solvents used in PCB extraction were GCMS grade and the
chemicals and water used in protein extraction were of LCMS grade. During media
preparation, either HCl or NaOH was used to adjust the pH up to the required level.
3.1.1 PCB source
The PCB source, Aroclor 1260 was obtained as a gas chromatography/ flame
ionization detector (GC/FID) grade technical mixture from AccuStandard Inc. (New
Haven, CT, USA). The complex mixture consisted of about 75 penta to nona chloro
biphenyls with an average of 6.3 chlorines per biphenyl molecule (Bedard et al., 2007)
(Table 3.1).
Table 3.1 Summary of physicochemical properties of Aroclor 1260
(CAS No.110-968-25)
Property
Molecular weight (g/mole) 357.7
Colour Light yellow
Physical state Sticky resin
Boiling point, (°C) 385–420
Density, (g/cm3) at 25 °C 1.62
Water Solubility, (g/m3) 0.003- 0.08

58 Chapter 3: Materials and Methods
3.1.2 General Media and reagents
3.1.2.1 Media used for cultivating PCB degrading microorganisms
(A) Modified mineral salt medium (DSMZ medium 465a)
The mineral salt medium (DSMZ medium 465a) for the cultivation of hydroxybiphenyl
utilizing bacteria (Atlas, 2005) was used in all the culture enrichments and batch
experiments with the following modifications. Aroclor 1260 stock solution was
prepared in GCMS grade acetone (50 mg Aroclor 1260/mL acetone) instead of
ethanol, and used as the sole carbon and energy source to give 50 mg/L final PCB
concentration in the mineral salt medium.
Preparation of PCB stock solution:
50 mg of Aroclor 1260 was dissolved in 1 mL of analytical grade acetone in order to
make 50 mg/mL PCB stock solution. 1 µL of stock solution contained 0.05 mg of
Aroclor 1260. As the intention was to use PCBs as the sole source of carbon, when
dissolving PCBs, a small quantity of acetone (100 µL acetone for every 100 mL of
minimal salt medium) was used to prevent the solvent from being a significant supply
of substrate for dechlorinating microorganisms.
Composition of the mineral salt medium (MSM) per litre:
Na2HPO4·2H2O 3.5 g
KH2PO4 1.0 g
(NH4)2SO4 0.5 g
MgCl2·6H2O 0.1 g
Ca(NO3)2·4H2O 0.05 g
Trace elements solution SL-4 1.0 mL
pH 7.25 ± 0.2 at 25 °C
Components of the minimal salt medium were added and brought to 1.0 L volume
and pH was adjusted to 7. The medium was autoclaved for 30 min at 121 °C. Aroclor
1260 stock solution was aseptically added to the sterile MS medium to give 50 mg/L

Chapter 3: Materials and Methods 59
concentration. To prepare mineral salt agar medium, bacteriological agar was added
to minimal salt medium before autoclaving to give a final concentration of 1.5% (w/v).
Typical working agar volumes were made up to 500 mL total.
Composition of the trace Elements Solution SL-4 (per 250 mL):
EDTA 125 mg
FeSO4·7H2O 50 mg
Trace elements solution SL-6 100 mL
Volume was brought to 250 mL using deionized water after all the components were
added and thoroughly mixed.
Composition of the trace Elements Solution SL-6 (per 250 mL):
H3BO3 75 mg
CoCl2·6H2O 50 mg
ZnSO4·7H2O 25 mg
MnCl2·4H2O 7.5 mg
Na2MoO4·H2O 7.5 mg
NiCl2·6H2O 5 mg
CuCl2·2H2O 2.5 mg
pH was adjusted to 3-4.
Volume was brought to 250 mL using deionized water after all the components were
added. Solution was mixed thoroughly and the pH was adjusted to 3-4.
(B) Nutrient agar (NA)
Nutrient agar was used in bacterial working culture preparation and in bacterial cell
counts. NA was prepared by suspending 28 g of nutrient agar (CM003, Oxoid) powder
in 1 L of distilled water and autoclaving for 30 min at 121 °C. When the mixture cooled
down to about 50 °C, 25-30 mL aliquots were poured into sterile petri dishes and
allowed to solidify.

60 Chapter 3: Materials and Methods
(C) Luria- Bertani (LB) liquid medium
LB medium was used for bacterial seed culture preparations (Dercova et al., 2008).
Composition (per litre)
Bacto tryptone 10 g
BactoR-yeast extract 5 g
NaCl 5 g
Components were added and brought to 1.0 L volume and pH was adjusted to 7.0
with NaOH. The medium was sterilized by autoclaving for 30 min at 121 °C.
3.1.2.2 Media used in 16S rRNA based bacteria identification
(A) 100 mM Isopropyl β-D-1-thiogalactopyranoside (IPTG) stock solution
For 1.2 g IPTG (Promega), sterile deionised water was added to a final volume of 50
mL, vortexed until the powder was fully dissolved, filter-sterilized using sterile 0.2 µm
filter discs and stored at 4 °C until use.
(B) 50 mg/mL X-Gal solution (2 mL)
100 mg 5-bromo-4-chloro-3-indolyl-β-d-galactoside (Thermo Scientific) was dissolved
in 2 mL of N,N´-dimethyl-formamide (DMF, Ambion), covered with aluminium foil and
stored at –20 °C until use.
(C) LB plates with ampicillin/IPTG/X-Gal
15 g bacteriological agar (Oxoid) was added to 1 L LB medium and autoclaved. Once
the medium was cooled to 50 °C, filter sterile ampicillin (AppliChem) was added to
get the 100 μg/mL final concentration. 30–35 mL of medium was poured into 85 mm
petri dishes. When the agar was hardened, 100 μL of 100 mM IPTG and 20 μL of 50
mg/mL X-Gal were spread on the agar surface and allowed to absorb for 30 minutes
at 37 °C prior to use.

Chapter 3: Materials and Methods 61
(D) Super optimal broth with catabolite repression (SOC) medium (per 100 mL)
Tryptone 2 g
Yeast extract 0.5g
1 M NaCl 1 mL
1 M KCl 0.25 mL
2 M Mg2+ stock, filter-sterilized 1mL
2 M glucose, filter-sterilized 1mL
Tryptone, Yeast extract, NaCl and KCl were added to 97 mL distilled water, stirred to
dissolve, and autoclaved and cooled to room temperature. 2M Mg2+ stock solution
and 2M glucose solutions were added to get a final concentration of 20 mM. The
volume was adjusted to 100 mL with sterile, distilled water. The final pH was adjusted
to 7.0. The final solution was sterilized by passing through a 0.2 µm filter. Stored at
4 °C until use.
(E) 2M Mg2+ stock (per 100 mL)
MgCl2.6H2O 20.33 g
MgSO4.7H2O 24.65 g
Powders were weighed and added into 50 mL of distilled water and the final volume
was brought to 100 mL using distilled water. The resulting solution was sterilized by
passing through a 0.2 µm filter.
3.1.2.3 Media used in biosurfactant screening tests
(A) Tryptone soya agar (TSA) + sheep blood (per litre)
Tryptone 15 g
Soya Peptone 5 g
Sodium Chloride 5 g
Bacteriological agar 15 g
Sheep Blood - Defibrinated 50 mL
pH 7.3 ± 0.2

62 Chapter 3: Materials and Methods
Readymade agar plates were purchased from Thermo Fisher Scientific and used in
the haemolytic assay test.
(B) Phosphate buffered saline (PBS) solution (per 250 mL)
NaCl 2 g
KCl 0.05 g
Na2HPO4 0.36 g
KH2PO4 0.06 g
Distilled water 200 mL
pH was adjusted to 7.4 with HCl.
All powders were weighed and added into 200 mL distilled water and the final volume
was adjusted to 250 mL with distilled water. PBS solution was filter sterilized by
passing through a 0.2 µm filter, stored at room temperature and used as the negative
control in the biosurfactant screening tests.
(C) 1 % Sodium dodecyl sulphate (SDS) solution
1 g SDS (Merk) was dissolved in 80 ml of distilled water and the volume was adjusted
up to 100 mL with distilled water. The SDS solution was filter sterilized by passing
through a 0.2 µm filter, stored at room temperature and used as the positive control
in biosurfactant screening tests.
3.1.2.4 Reducing sugar analysis in microbial growth studies
The following chemicals were made and used for the analysis of glucose utilization
during microbial cultivations grown on glucose as a carbon source, and was based on
the method by Miller (1959).

Chapter 3: Materials and Methods 63
(A) Dinitrosalicylic acid (DNS) reagent, 1% (per litre)
10 g Dinitrosalicylic acid, 0.5 g sodium sulfite and 10 g sodium hydroxide were
dissolved in distilled water and the volume was brought to 1 L.
(B) Potassium sodium tartrate solution, 40%
40 g of Potassium sodium tartrate (Sigma Aldrich) was added into 80 mL of distilled
water and brought the volume up to 100 mL using distilled water.
3.1.2.5 Chemicals used in PCB extraction and analysis
(A) Sodium Sulfate, anhydrous granular reagent grade
200 g Sodium sulfate (Merk) was heated at 400 °C for 4 h, cooled in a desiccator, and
stored in a glass Schott bottle until use.
(B) Potassium Carbonate, anhydrous granular reagent grade
200 gm of Potassium carbonate (Merk) was heated at 400 °C for 4 h, cooled in a
desiccator, and stored in a glass Schott bottle until use.
(C) PCB Surrogate standard solutions
2,4,5,6-Tetrachloro-m-xylene (TCMX), 500 µg/mL stock solution in acetone was
purchased as GC/FID grade solution from AccuStandard Inc. (New Haven, CT,
USA). 10 µg/mL surrogate working solutions were prepared in 1 mL aliquots by
adding 980 µL of hexane to 20 µL of TCMX stock solution.
(D) PCB Internal standard stock solution
2,2',4,4',5,5'-Hexabromobiphenyl, 100 µg/mL in Hexane was purchased as GC/FID
grade solution from AccuStandard Inc. (New Haven, CT, USA). PCB internal standard
working solutions were prepared in 1 mL aliquots at a concentration of 10 µg/mL in
hexane.

64 Chapter 3: Materials and Methods
3.1.2.6 Chemicals, buffers and materials used in protein extraction
(A) SDS-Tris lysis buffer (per 10 mL)
10% (w/v) SDS 4 mL
1.5 M Tris (pH 8.5) 667 µL
Dithiothreitol (Agilent) 0.154 g
Dithiothreitol was freshly added.
(B) Urea-Tris buffer (50 mL)
24 g urea was topped up to 50 mL with 100 mM Tris pH 8.5. Buffer was prepared
fresh.
(C) Dithiothreitol-Urea buffer (per 10 mL)
0.038 g Dithiothreitol was dissolved in 10 mL Urea-Tris buffer.
(D) Iodoacetamide-Urea buffer (per 10 mL)
0.092 g Iodoacetamide (Sigma) was dissolved in 10 mL Urea-Tris buffer. Buffer was
freshly prepared and light protected.
(E) 200 mM Ammonium bicarbonate
0.78 g Ammonium bicarbonate was dissolved in 50 mL LC-MS grade water.
(F) Preparation of Trypsin stocks
100 μg trypsin (Promega, catalogue number: V5280) reconstituted in 1 mL 50 mM
ammonium bicarbonate and aliquot 10 μL volumes into 98 tubes. The preparation
was done on ice in a keratin-free environment. The aliquots were stored at -80 °C
until use.

Chapter 3: Materials and Methods 65
(G) iRT (indexed Retention Time) buffer
1 mL of 100 x concentrated iRT peptides (Biognosis) dissolved in 40% (v/v) acetonitrile
(ACN) was aliquot into 10 μL volumes. Aliquots were stored at -80 °C until use. Before
use, vials were transferred on to ice and each 10 µL aliquot was diluted 100 times by
mixing with 990 μL 2% (v/v) ACN/0.1% (v/v) formic acid.
(H) DW
0.2% (v/v) Trifluoro acetic acid (TFA) was prepared using LC-MS grade water.
(I) DE
5% (v/v) Ammonium hydroxide / 80% (v/v) ACN was prepared in a fume hood using
LC-MS grade water.
(J) Preparation of Stage Tips-SCX for desalting of protein samples
An SCX (Empore 3M) single membrane was placed in a clean petri dish. The blunt-end
needle was gently pressed into the membrane to cut out the disks. Needle was
attached to a 1 mL syringe and the membrane disk attached into the needle was
released into a regular 200 µL yellow (Eppendorf, catalogue number: 0030 014.464)
pipette tip using the plunger.

66 Chapter 3: Materials and Methods
3.2 Methods
3.2.1 Selective enrichment of potential PCB utilizing bacteria
Refer to Chapter 4.
3.2.2 Genetic characterization based on 16S rRNA sequences
Refer to Chapter 4.
3.2.3 Bacterial growth profiles
Refer to Chapter 4.
3.2.4 Bacteria cell density measurement
Bacterial growth was measured as biomass concentration using two standard
techniques; optical density (OD600) measurement and standard plate count (SPC).
Although the standard plate count can detect viable cells, it is time consuming as it
requires preparation of serial dilutions, NA plates in duplicates for each dilution
incubation for 24 - 48 h for microbial growth as described in Section 3.3.1 B. On the
other hand, optical density measurement is simple and effective as the background
minimal medium used in all the growth studies was colorless. Therefore, optical
density was used as the main bacterial growth measurement method and SPC was
mainly used to countercheck the optical density measurements and to calculate the
number of colony forming units (CFU) used as the seed cultures.
(A) Optical density (OD600)
During the PCB degradation studies, the cell growth of microorganisms grown in
MSM spiked with Aroclor 1260 was measured as optical density at 600 nm using the
DU730 Beckman Coulter UV/VIS spectrophotometer. Cell free minimal salt medium
spiked with Aroclor 1260 was used as the media blank. Samples were diluted
appropriately to get the absorbance readings ≤ 0.3, to take into account the

Chapter 3: Materials and Methods 67
spectrophotometer detection limit. The final optical density readings were then
produced by multiplying the OD600 readings by the dilution factor.
(B) Standard Plate count
Bacterial cell counts were determined for each bacterial strain. A serial dilution was
carried out using 0.85% (w/v) sterile saline solution as shown in Figure 3.1. Bacterial
cell counts in the original samples were calculated as colony forming units (CFU)/mL
using the following equation.
cfu/mL = (number of colonies x dilution factor) / volume plated on NA plate
Equation 3.1
Figure 3.1 Standard plate count technique

68 Chapter 3: Materials and Methods
3.2.5 pH
Hanna HI 2221 pH meter was used to measure the pH according to the APHA method
4500-H (APHA, 2012). The pH meter was calibrated using pH 4.0, 7.0 and 10.0
calibration buffers each time before use.
3.2.6 Glucose concentration
The Dinitrosalicylic colorimetric method (Miller, 1959) was used to measure the
glucose concentration during the bacterial growth profile studies. The process
involves the oxidation of the aldehyde functional group in glucose. It reduces 3,5-
dinitrosalicylic acid (DNS) to 3- amino-5-nitrosalicylic acid which under alkaline
conditions is converted to a reddish brown coloured complex. DNS reagent and
potassium sodium tartrate solution were prepared as per the Section 3.1.2.4 A and
Section 3.1.2.4 B respectively. A Glucose standard curve was prepared using 0.2 g/L,
0.4 g/L, 0.6 g/L, 0.8 g/L and 1.0 g/L glucose solutions. 3 mL of DNS reagent was added
to 3 mL of glucose sample in a lightly capped test tube and the mixture was heated
in a water bath maintained at 90 °C for 5-15 min to develop the red-brown colour. 1
mL of 40% potassium sodium tartrate solution was added to stabilize the colour and
cooled to room temperature in a cold water bath. Absorbance was measured at 575
nm using the DU730 Beckman Coulter UV/VIS spectrophotometer, and glucose
concentration was calculated using the standard curve of glucose.
3.2.7 Chloride ion concentration
Chloride ion concentration in the culture medium was measured in order to
determine the amount of chlorines released from the PCB mixture, by the direct
action of the microbes. If bacteria were able to dechlorinate the PCB molecules, the
released chloride ions need to be accumulated in the culture medium. Therefore, the
chloride ions accumulated in the culture medium is directly proportional to the
degree of dechlorination. Samples were first centrifuged at 5000 x g for 10 min and
the resultant supernatant was filtered through 0.2 µm sterile filter discs to remove
the bacterial cells. Cell free culture supernatant was used to measure the chloride ion

Chapter 3: Materials and Methods 69
concentration using the Dionex ICS-2100 ion chromatography according to USEPA
method 300.0 (USEPA, 1993). For the calibration curve preparation, 0.1 ppm, 1 ppm,
5 ppm, 10 ppm, 20 ppm and 100 ppm standard sodium chloride solutions were used.
The following controls were used in order to subtract the background chloride levels
coming from the minimal salt medium, lysis of bacterial cells and leaking of any
chloride occurred, and from any traces from the LB medium used to cultivate the
bacterial seed cultures.
(A) Abiotic controls: Minimal salt medium spiked with 50 mg/L Aroclor 1260.
(B) Media controls: Minimal salt medium inoculated with similar amount of
bacterial seed cultures used in the experiment. Chloride measurements were
done before and after the sonication to see the contribution from cell lysis.
(C) Minimal salt medium only.
3.2.8 PCB extraction
During the experiments, PCB concentrations were measured in two ways as; (1) total
soluble PCBs (used in Chapter 5 and Chapter 6), and (2) total PCBs (used in Chapter
7). PCBs are poorly soluble in water and the solubility of Aroclor 1260 is reported to
be in the range of 0.003-0.08 g/m3 (ATSDR, 2000). Therefore, before starting the PCB
extractions, the extraction efficiencies of two solvents (1), hexane and (2), diethyl
ether (DEE) were tested in order to determine the best solvent to use. 1 mL aliquots
of minimal salt media containing PCBs (50 mg/L, 25 mg/L and 10 mg/L in triplicates)
were transferred to duplicate 8 mL glass vials fitted with Teflon-lined screw caps
(THC15151083 and THC15091703, Thermo Fisher Scientific). PCBs that were soluble
in the aquous medium were extracted with 2.5 mL of GC grade n-hexane (USEPA,
2007) and GC grade DEE ((Bedard et al., 2006; Adrian et al., 2009) by vigorous
horizontal shaking on a platform shaker at 250 rpm for 4 h under room temperature,
followed by centrifugation at 5000 rpm for 10 min (Adrian et al., 2009).
The solvent phase was removed and used for subsequent analysis using the GCMS as
described under Section 3.3.4.2. 50 ppm/mL Aroclor 1260 in hexane was used to
compare the recovery efficiencies of PCBs dissolved in the aqueous medium as given
in Figure 3.2. All the DEE extracts were evaporated to dryness and redissolved in 1

70 Chapter 3: Materials and Methods
mL hexane before analysis. Although recovery rates of PCBs were comparatively low
in hexane, the variation of the results in triplicate samples were minimal. Extraction
using DEE also needed additional step as once the PCBs are extracted, DEE need to
be removed by evaporation and resuspension of PCBs in hexane need to be done
before injecting samples to the gas chromatograph. Accordingly, hexane was chosen
as the extraction solvent.
Figure 3.2 Recovery of Aroclor 1260 soluble in the aqueous minimal salt medium as
a percentage of total PCBs added to the mixture, using diethyl ether (DEE) and hexane
as the extraction solvents. Error bars represent the standard deviation of mean values
(n = 3).
(A) Total soluble PCB extraction
Liquid aliquots (1 mL) withdrawn from batch culture flasks were transferred into 8
mL glass vials fitted with Teflon-lined screw caps. PCBs were extracted with 2.5 mL of
GC grade n-hexane (USEPA, 2007) by vigorous horizontal shaking at 250 rpm on a
platform shaker for 4 h at room temperature, followed by centrifugation at 5000 rpm
for 10 min (Adrian et al., 2009). The solvent phase was separated and used for
subsequent total soluble PCB analysis. Before extraction, 25 µL of 2, 4, 5, 6-

Chapter 3: Materials and Methods 71
tetrachloro-m-xylene (10 µg/mL in hexane) was added to each sample as the
surrogate standard to determine the extraction efficiency (USEPA, 2007). The
surrogate recovery was 98.98% ± 19.11% (n=462).
(B) Total PCB extraction
Total PCB extraction was done by sacrificing the content of the whole flask based on
the method described by Murinova et al. (2014) with some modifications. 5 mL of n-
hexane was added to each flask and the flasks were sonicated in an ultrasonic bath
at medium speed for 10 min to disrupt the cells and release the bound PCBs. The
content of the flask was transferred into a 250 mL separatory funnel and vigorously
shaken for 2 min. Anhydrous K2CO3 (1 g) was added to the mixture to reduce the foam
generated from biomass and shaken for 15 s. The n-hexane layer was collected into
a flask containing anhydrous Na2SO4. The extraction was repeated twice with 5 mL of
n-hexane at each time. The content was centrifuged at 4500 rpm for 10 min to
separate the emulsion layer. The content was added to 25 mL volumetric flask and
the final volume was adjusted to a 25 mL with n-hexane and stored in amber colour
glass bottles at 4 °C until subsequent analysis by gas chromatography (GC). To
determine the extraction efficiency, 250 µL of 100 ppm of GC grade 2,4,5,6-
tetrachloro-m-xylene was used as the surrogate standard and added to flasks
immediately before starting the extraction.
3.2.9 PCB analysis
PCB extracts and standards were spiked with 2,2',4,4',5,5'-hexabromobiphenyl stock
solution as an internal standard before GCMS analysis (USEPA, 2007) to give 100 ppb
final concentration. PCB levels were determined as per the USEPA method 8082A
(USEPA, 2007). Thermo Scientific Trace 1310 (in splitless mode, at 260 °C inlet
temperature, 80 mL/min split flow and 1.2 min splitless time) equipped with SSL
injector with splitless liner with glass wool, Thermo TG-5SilMS analytical column (30
m × 0.25 mm ID×0.25 µm), TriPlus RSH auto sampler was used for PCB analysis.
Helium was used as the carrier gas at 1.2 mL/min constant flow. The column was kept
at 40 °C for 2 min, and then the temperature was raised to 300 °C at 15 °C/min and

72 Chapter 3: Materials and Methods
kept for 5 min. The Thermo Scientific TraceFinder EFS software was used for the
screening and quantitation of total PCBs and PCB homolog groups using the Thermo
Scientific triple stage quadrupole Mass spectrometer (TSQ8000 EVO). Retention
times were taken from the existing literature (Walker & Feyerherm, 2013) and full
scan acquisition and timed selective reaction monitoring (SRM) modes were used to
distinguish the PCB homolog groups. 5 ppb, 10 ppb, 20 ppb, 50 ppb, 250 ppb, 500
ppb, 1000 ppb, 2000 ppb and 10 000 ppb Aroclor 1260 standard solutions were used
for calibration curve preparation. Average weight percent of PCB homolog groups
and chlorines in Aroclor 1260 used in the study were calculated based on the
calibration curve and given in Table 3.2. Relative amounts of PCB levels and homolog
groups in the samples were determined by nine-point calibration using Aroclor 1260
standard solutions and area integration. Details of calibration and surrogate standard
preparations were given in Appendix A.
Table 3.2 Average weight percent of PCB homolog groups and chlorines in Aroclor
1260.
Chlorine
Substitutions
Homolog group Homolog group (%)
(by weight)
Cl %
(by weight)
mono C12H9Cl 0.24 ± 0.03 0.05 ± 0.00
Di C12H8Cl2 0.82 ± 0.07 0.26 ± 0.02
Tri C12H7Cl3 1.97 ± 0.20 0.81 ± 0.08
Tetra C12H6Cl4 1.59 ± 0.06 0.78 ± 0.03
Penta C12H5Cl5 20.06 ± 0.33 10.83 ± 0.18
Hexa C12H4Cl6 50.69 ± 0.47 29.91 ± 0.28
Hepta C12H3Cl7 21.22 ± 0.61 13.37 ± 0.38
Octa C12H2Cl8 2.72 ± 0.23 1.80 ± 0.15
Nona C12HCl9 0.63 ± 0.03 0.43 ± 0.02
Deca C12Cl10 0.05 ± 0.00 0.04 ± 0.00
Total 100.00 ± 2.03 58.27 ± 1.15

Chapter 3: Materials and Methods 73
3.2.10 Screening tests for biosurfactant production
Refer to Chapter 5.
3.2.11 Extracellular protein visualization, quantification, extraction and analysis
Refer to Chapter 8.
3.2.12 Storage of culture supernatant samples
Samples withdrawn during batch cultivations for protein extraction (1 mL aliquots),
chloride analysis (5 mL aliquots) and extraction of total soluble PCBs (1 mL aliquots)
were stored at -80 °C until analysis.
3.2.13 Bacterial culture maintenance
(A) Long term storage at -80 °C using glycerol stocks
For long term storage of bacterial cultures, 1 mL of samples containing bacterial cells
from 20 mL cultures grown overnight (at 28 °C and 150 rpm) in LB were removed, and
centrifuged at 5000 rpm for 10 min. For each bacterium, the supernatant was
discarded, and the cells were taken up in 1.5 mL sterile 50% glycerol solution and
aliquot to 3 x 500 µL samples, and stored at -80 °C.
(B) Bacteria working cultures
Bacterial cultures were streaked onto nutrient agar (NA) plates in duplicate and
stored at 4 °C. Sub culturing was performed at monthly intervals by streaking onto
fresh NA plates.
3.3 PCB degradation studies
All the PCB degradation studies were conducted at 28 °C. The experiments conducted
under aerobic conditions were done at 150 rpm in a platform shaker incubator. The
experiments conducted under anaerobic conditions were performed inside an
anaerobic chamber (COY laboratory products, Inc.) under strict anaerobic conditions.

74 Chapter 3: Materials and Methods
Palladium catalysts located inside the chamber were used to scrub any residual
oxygen present in the chamber. Shake flasks and agar plates were transferred under
anaerobic conditions without changes to the internal atmosphere through a heavy
duty vacuum airlock compartment. The anaerobic environment inside the chamber
was maintained constant under 4.9% H2, 10.7% CO2 and 84.4% N2 (BOC Australia).
Once minimal salt medium containing flasks were prepared, they were kept
equilibrated for one week inside the anaerobic chamber with loosed caps before they
were inoculated with the bacterial cultures. Anaerobic experiments carried out at 28
°C were kept in a temperature controlled incubator inside the anaerobic chamber
(see Figure 3.3).
Figure 3.3 The Coy anaerobic chamber with the connecting airlock to the right, used
in this research.
3.4 Cleaning of glassware used in PCB extraction
All the glassware used in the PCB extractions were first soaked in detergent and
thoroughly washed with hot water. They were then rinsed with tap and distilled
water respectively followed by a thorough rinse with hexane. After drying at 50 °C,
all the cleaned glassware to be used in PCB extraction were sealed and stored in a
clean environment to prevent any accumulation of dust or other contaminants.

Chapter 3: Materials and Methods 75
3.5 Quality assurance
To obtain accurate data is critical in scientific research and therefore, quality control
and quality assurance are very important. As such, the following measures were
taken to minimize errors. All the experiments were run in triplicate with identical
conditions to test the repeatability and the results were reported as means ±
standard deviations. In all the measurements and analyses, the Relative Standard
Deviation (RVSD) was calculated as defined by Equation 3.2.
RVSD (%)= (σr
μr
) ×100 Equation 3.2
Where:
µr – mean concentration of replicates
σr – standard deviation of the concentrations of replicates
With every set of experiments, controls (as described under each chapter) were run
and used in sample and data analysis. In PCB analysis, a series of blanks and standard
solutions were used as specified in the method 8082A (USEPA, 2007). This approach
included method blanks, calibration blanks, calibration standards, intermediate
checks, internal standards and surrogate standards. The surrogate standard 2,4,5,6-
Tetrachloro-m-xylene was added to all samples, controls, method blanks and
calibration standards as the surrogate standard, prior to extraction at the
concentrations described in the Sections 3.1.2.5 C. The recoveries of the surrogate
standards were calculated using the standard curve prepared for surrogate standard
in order to find out the extraction efficiencies of PCBs. Similarly, 5 µL of a 10 µg/mL
internal standard in hexane (see Section 3.1.2.5 D and Appendix A) was added to all
standard solutions used in the calibration curve, intermediate checks, and the
samples including the method blanks and controls before GCMS analysis. A nine point
calibration curve was prepared using a range of Aroclor 1260 concentrations from 5
ppb to 10,000 ppb and used in quantification of PCBs. In addition to that, method
blanks were run using similar volumes of deionized water only through the entire
analytical procedure for each batch of extractions. During PCB analysis, 5 ppm, 10

76 Chapter 3: Materials and Methods
ppm, 20 ppm and 100 ppm Aroclor 1260 standard solutions were prepared and run
in between each of the 25 samples as intermediate checks.
In chloride analysis, six calibration standards 0.1 ppm, 1 ppm, 5 ppm, 10 ppm, 20 ppm
and 100 ppm sodium chloride solutions were used to prepare the calibration curve.
Additionally, in between each of the 20 samples, 5 ppm and 20 ppm sodium chloride
standards were run as intermediate checks.

Chapter 4: Selective Enrichment and Identification of Potential PCB Degrading Microorganisms 77
Chapter 4: Selective Enrichment and Identification of Potential PCB Degrading Microorganisms
4.1 Background
Bioremediation, the use of microorganisms capable of degrading toxic compounds to
decontaminate the polluted environments, has become an attractive alternative to
existing physical and chemical treatments. However, commercial application of
bioremediation to remediate PCB contaminated environments is not yet widely
practiced mainly due to long reaction times and lower degradation rates. Therefore,
further research is needed to improve and optimize the existing bioremediation
technologies in order to be cost effective as a viable treatment option for onsite
applications.
The aim of this chapter is to identify aerobic and anaerobic PCB degrading
microorganisms from the natural environment for use in subsequent PCB
degradation related studies discussed in Chapter 5 to Chapter 8. Soil and sediment
samples collected from the natural environment were contaminated with Aroclor
1260 as the PCB source and subjected to selective enrichment to isolate
microorganisms. During the selective enrichment, samples went through four serial
transfers using a fresh minimal salt PCB liquid medium at weekly intervals in order to
obtain soil and sediment free enrichment medium as a source of microorganisms that
are capable of utilizing PCBs as their sole source of carbon. Once the initial screening
was done, bacterial isolates were tested for their oxygen tolerance while searching
for any facultative anaerobic organisms, as the ability to survive under both anaerobic
and aerobic conditions would be an added advantage in field scale remediation
applications.
Full length 16S rRNA gene (1.5kb) isolation and sequencing was used for identification
of the bacterial isolates at the genus level as a minimum. The 16S rRNA gene is the
most commonly used genetic marker in bacterial identification due to a number of

78 Chapter 4: Selective Enrichment and Identification of Potential PCB Degrading Microorganisms
reasons, namely; (i) 16S rRNA is a component of the small subunit (30S) of ribosomal
RNA genes and is found in all bacteria (Janda & Abbott, 2007), and (ii) it is
comparatively short (~1.5 kb) and comprise a particular combination of conserved,
variable and hypervariable regions that have evolved at different rates (Srinivasan et
al., 2015). These characteristics allow the use of 16S rRNA to identify bacteria to the
genus or the species level by comparing against the existing databases that include
near full length sequences of a large number of bacterial strains.
4.2 Materials and Methods
4.2.1 Soil and sediment sample collection and preparation
In order to isolate possible aerobic PCB degrading bacteria, soil samples were
collected from locations around the Brisbane City Botanical Gardens and Queensland
University of Technology, Gardens Point Campus (27.4745° S, 153.0293° E). Samples
were collected into 500 mL sterile glass Schott bottles and transported in ice to the
laboratory. Samples were homogenised, and 50 g aliquots of the composite was
added to two 250 mL sterile Erlenmeyer flasks. Each sample was contaminated using
transformer oil mixed with Aroclor 1260 to obtain 50 mg/kg PCB concentration.
Flasks containing soil were mixed thoroughly and incubated stationary under aerobic
conditions at room temperature (23 °C ± 1 °C) for one month period to obtain PCB
tolerant microorganisms for selective enrichment screening.
In order to isolate potential anaerobic PCB degrading bacteria, three sites were
chosen for soil and sediment sampling. The sites were the Brisbane City Botanical
Gardens, Brisbane River (27.4745° S, 153.0293° E) and Coombabah Lake, Gold Coast
(27.54° S, 153.22° E). Samples were collected into 250 mL sterile glass Schott bottles
and transported under anaerobic conditions in an anaerobic jar. Similar to the
process adopted for aerobic soil samples, 50 g of composite samples were added to
two 50 mL polypropylene vials with screw caps under anaerobic conditions and
contaminated with Aroclor 1260 containing transformer oil to obtain 50 mg/kg PCB
concentration. The vials were sealed and incubated stationary inside the anaerobic
chamber (COY laboratory products) main compartment at room temperature (23 °C
± 1 °C) for one month. The atmosphere inside the anaerobic chamber was maintained

Chapter 4: Selective Enrichment and Identification of Potential PCB Degrading Microorganisms 79
constant using a special mixed gas made up of 4.9% H2, 10.7% CO2 and 84.4 % N2
(BOC, Australia).
4.2.2 Selective enrichment of potential PCB utilizing bacteria
Isolation of microorganisms capable of utilising PCBs as carbon and energy source
under aerobic conditions was carried out through a series of selective enrichments
as illustrated in Figure 4.1a. Modified DSMZ medium 465a (see Section 3.1.2.1 A in
Chapter 3) was used as the base minimal salt medium with 50 mg/L Aroclor 1260 as
the sole source of carbon. During the study, two 250 mL Erlenmeyer flasks each
containing 100 mL of sterile minimal salt medium were prepared and inoculated with
10 g of composite soil previously contaminated with transformer oil containing
Aroclor 1260. In order to enrich the possible aerobic PCB degrading bacteria, flasks
were incubated aerobically in a platform shaker at 28 °C and 150 rpm for one week.
Three further serial transfers (10% of the enrichment medium) were carried out every
7 days into fresh sterile minimal salt medium. From the final flask, 1 mL of the
supernatant was serially diluted with sterile 0.85% saline water and plated in
duplicate on nutrient agar (CM003, Oxoid) and incubated for 24 to 48 h at 28 °C.
Morphologically different colonies obtained from the selective enrichment were
isolated and streaked on fresh nutrient agar plates.
The same procedure was followed for the enrichment of possible anaerobic PCB
degrading bacteria with the following modifications as illustrated in Figure 4.1b. After
inoculation with 10 g of composite soil and sediment samples previously
contaminated with Aroclor 1260, flasks were incubated anaerobically under static
conditions in an incubator kept inside the anaerobic chamber with the temperature
maintained at 28 °C (see Figure 3.3 in Chapter 3).

80 Chapter 4: Selective Enrichment and Identification of Potential PCB Degrading Microorganisms
Figure 4.1 Selective enrichment of potential PCB degrading bacteria, under aerobic
and anaerobic conditions at 28 °C.
4.2.3 Characterization of potential PCB degrading bacteria
Bacterial isolates obtained from the selective enrichment were further characterized
based on their colony morphology on nutrient agar and cell morphology using Gram
staining. Genomic DNAs were first isolated from pure isolates, before full-length 16S
rRNA gene sequences were PCR amplified for sequencing, in order to ascertain their
identification.
4.2.3.1 Morphological characteristics
Smears of bacterial isolates were prepared on microscopic slides and stained using
the standard Gram staining technique to differentiate the Gram positive and negative
bacteria (Barthomolew & Mittwer, 1952). Bacterial cells were examined under oil
immersion (100x magnification) using Nikon Eclipse Ni light microscope.

Chapter 4: Selective Enrichment and Identification of Potential PCB Degrading Microorganisms 81
4.2.3.2 Oxygen requirements
Bacterial isolates obtained from selective enrichment were inoculated in duplicate
on nutrient agar plates and incubated at 28 °C in parallel under anaerobic and aerobic
conditions to determine their growth tolerance in the presence and absence of
oxygen.
4.2.3.3 Identification of bacterial isolates using 16S rRNA full length gene
sequencing
The pair of 27F and 1492R universal primers were used for the amplification of the
full-length 1.5 kb 16S RNA gene sequences (Fredriksson et al., 2013). The resulting
1.5 kb polymerase chain reaction (PCR) products for the different bacterial isolates
were cloned into the pGEM-T vector plasmid (Promega) and the 1.5 kb RNA gene
inserts on the recombinant plasmids were sequenced using the dideoxy Sanger
sequencing method of the same plasmid in two separate reactions (Sanger &
Coulson, 1975). In a typical run, up to 900 bp of good quality readable nucleotide
sequences are obtained in both, forward and reverse directions.
(A) Genomic DNA (gDNA) extraction
Bacterial cultures initially isolated from selective enrichment were separately
inoculated into 5 mL of sterile Luria-Bertani broth and incubated overnight at 28 °C
and 150 rpm in a rotary incubator. Isolate II genomic DNA kit (Bioline) was used to
extract the bacterial DNA following the manufacturer’s protocol. The gDNA isolation
kit was successful in the isolation of good quality gDNAs from all the isolates except
two isolates. In order to extract the gDNA of these two isolates, the same procedure
was repeated, but with following modification. After addition of the Lysis buffer and
proteinase K solution, the tubes were incubated overnight at 37 °C to help with cell
wall Lysis, instead of the standard 3 hour incubation at 56 °C. The quality of the
extracted gDNA was determined by electrophoresis at 100 V for 1 h using 1% agarose
gel using 1KB plus hyper ladder (Bioline) as the marker.

82 Chapter 4: Selective Enrichment and Identification of Potential PCB Degrading Microorganisms
(B) Polymerase chain reactions (PCR)
The extracted gDNA was diluted 1:10 with sterile ultra-pure water, and 1 µL aliquot
was used as a template for PCR amplification in a 50 µL reaction mixture. Universal
16S rRNA primers (Integrated DNA Technologies, Inc.), 27F (5'-
AGAGTTTGATCCTGGCTCAG-3') and 1492R (5'-GGTTA CCTTGTTACGACTT-3') were
used to amplify the 16S rRNA genes of isolated bacteria according to the MyTaq HS
Red DNA polymerase protocol (Bioline). One µL of ultra-pure water without DNA was
used as the negative control. The Eppendorf Master cycler machine was used for the
PCR reactions with the following PCR cycling conditions: initial denaturation at 95 °C
for 10 min, 30 cycles of 30 s at 95 °C, 30 s at 50 °C, and 2 min at 72 °C followed by
final extension at 72 °C for 7 min and a final hold at 4 °C. The quality of the PCR
products was checked by electrophoresis by running 5 µL on a 1% agarose gel at 100
V for one hour next to a 1KB plus hyper ladder as the marker.
The remainder of the PCR samples were run on a new 1% agarose gel and the DNA
fragments were excised and purified according to the Isolate II PCR and gel kit
protocol (Bioline). Following gel extractions, the concentrations of the DNA was
determined using the NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific)
set at 260/280nm, expressed as ng/µL, as well as checking 5 µL on a 1% agarose gel
for 1 h at 100 V.
(C) Ligation, transformation and cloning in Escherichia coli (E. coli)
pGEM-T Easy Vector system (Promega) was used to clone the 16S rRNA gene PCR
product into E. coli. The gel purified ~1.5 kb 16S gene fragments were ligated into
the pGEM-T Easy Vector plasmids (Promega) according to the ligation protocol of
pGEM-T Easy Vector system (Promega) using 1:3 insert:vector ratio. The 16S rRNA
gene ligated vector plasmids were transformed into high efficiency E. coli JM 109
competent cells. The transformed cells were screened for blue and white colonies by
plating on Luria-Bertani (LB) agar plates containing ampicillin (100 µg/mL), Isopropyl
β-D-1-thiogalactopyranoside (IPTG, 0.5mM) and 5-bromo-4-chloro-3-indolyl-β-D-
galactopyranoside (X-Gal, 80 µg/mL). Three white colonies indicative of being
positive for recombinant clones were picked from plates representing each bacterial

Chapter 4: Selective Enrichment and Identification of Potential PCB Degrading Microorganisms 83
isolate and patched onto fresh LB plates containing ampicillin, IPTG and X-Gal.
Potential recombinant bacterial clones were checked for the presence of 16S gene
inserts by colony PCR with M13 forward and reverse primers (Integrated DNA
Technologies, Inc.) that anneal to outside of the pGEM-T vector multiple cloning site
(Promega) as follows: PCR mixtures were prepared with no template using 5µL 5X
MyTaq reaction buffer, 1 µL of M13 forward and reverse primers, 0.25 µL MyTaq DNA
polymerase and 17.75 µL ultra-pure water to make 20 µL. Then a sterile inoculating
needle was used to gently touch a white colony and a few cells were transferred to
the reaction mixture. The following amplification program was used: 1 cycle at 94 °C
for 15 min, and then 35 cycles of 30 s at 94 °C, 30 s at 50 °C, and 2 min at 72 °C
followed by final hold at 4 °C. Resulting PCR products (5 µL) were checked on a 1%
agarose gel at 100 V for 1 h.
(D) Plasmid isolation and Sanger sequencing
After checking for the presence of 1.5 kb 16S gene fragments on the pGEM-T vector
by PCR, a single positive recombinant clone for each bacterial isolate was grown in 5
mL LB broth containing ampicillin (100 µg/mL) and incubated for 12 to 16 h at 37 °C
with vigorous shaking at 250 rpm. The plasmid isolation and purification was
performed using the QIAprep Spin Miniprep Kit (QIAGEN) according to the
manufacturer’s protocol. The purified plasmids were checked using gel
electrophoresis.
For Sanger sequencing, two separate sequence reactions using the M13F and M13R
primers were carried out for each recombinant plasmid, according to the standard
BigDye Terminator (BDT) v3.1 cycle sequencing kit protocol (Thermo Fisher
Scientific). The following cycling conditions were used: 1 cycle at 96 °C for 1 min, and
then 25 cycles of 10 s at 96 °C, 5 s at 50 °C, and 4 min at 60 °C followed by a final hold
at 4 °C.
Ethanol/EDTA precipitations were carried out to purify and precipitate the sequence
reactions. DNA sequencing was performed using 3500 Genetic Analyzer (Applied
Biosystems, Hitachi). FinchTV 1.4.0 (Geospiza Inc.) was used for editing raw
nucleotide DNA sequence data. The raw DNA sequences were further polished by

84 Chapter 4: Selective Enrichment and Identification of Potential PCB Degrading Microorganisms
the removal of the plasmid vector sequences. Edited forward and reverse sequences
were aligned to obtain full length 1.5 kb contiguous sequences using the EMBOSS
Needle tool of the European Bioinformatics Institute (EMBL-EBI). The full length 16S
rRNA gene sequences were then submitted to determine the closest matching
sequences by comparing with the bacterial and archaeal 16S RNA databases using
the Sequence Match tool of the Ribosomal Database Project (RDP) and Basic Local
Alignment Search Tool (BLAST) of NCBI, USA. The searches were limited to curated
‘Type’ strains.
There is no universal definition existing for species level identification using 16S rRNA
gene sequencing and use of acceptable criteria for establishing a species match varies
widely in different studies (Janda & Abbott, 2007). However, a study based on 571
distinct pairs of strains, a threshold between 98.2 - 99.0% 16S rRNA gene sequence
identity has been suggested as a reasonable species boundary (Meier-Kolthoff et al.,
2013). Yarza et al. (2014) used a 98.7% 16S rRNA gene threshold in estimating global
species richness based on the 98.7 – 99% similarity threshold recommended by
Stackebrandt and Eber (2006). Therefore, if similarity was not 100%, identification
was limited up to the generic level as there is no guarantee of having 1% divergence
to obtain an accurate identification.
4.2.3.4 Confirmation of the ability to grow on PCBs as sole carbon source
All the bacterial strains identified in Section 4.2.3.2 found to be able to grow in the
presence of oxygen were cultivated in triplicate on minimal salt agar containing 50
mg/L of Aroclor 1260 as the sole source of carbon and incubated at 28 °C under
aerobic conditions for 24 to 72 h. This was to further confirm the ability of identified
bacteria strains to grow under aerobic conditions using PCBs as the sole source of
carbon. Similarly, all the facultative anaerobic cultures were streaked onto minimal
salt agar containing 50 mg/L of Aroclor 1260 as sole source of carbon and incubated
at 28 °C under anaerobic conditions for 24 to 72 h. Minimal salt agar with no added
Aroclor 1260, but an equal volume of acetone that was used to add Aroclor 1260 into
the minimal salt agar in the growth experiment, was used as the negative control to
determine whether there was any contribution from leftover acetone on bacterial

Chapter 4: Selective Enrichment and Identification of Potential PCB Degrading Microorganisms 85
growth. In comparison, the minimal salt agar with 0.1% (w/v) glucose added and solid
rich nutrient agar medium alone were used as the positive controls.
4.2.3.5 Growth profiles of bacteria
To understand the growth of isolated bacteria under controlled conditions, bacterial
cultures were grown in a closed system as pure cultures and the rate of cell
population increase, rate of glucose consumption and the variation of pH over time
were measured over a nine hour period.
For each bacterial culture, triplicate 500 mL Erlenmeyer flasks containing 250 mL
sterile MSM and 2 g/L glucose were used to develop their growth curves. Flasks were
inoculated with 10% (v/v) overnight grown bacterial cultures in liquid LB broth as the
seed and incubated at 28 °C and 150 rpm in a shaker incubator. Cell density of the
bacterial cultures were calculated as colony forming units (CFU) using standards plate
count as described in Section 3.3.1.B to obtain the growth curves. The specific growth
rate (µ) of each bacteria were calculated for the exponential phase using Equation
4.1 (Maier, 2009).
𝑋 = Xₒeµᵗ Equation. 4.1
Where:
X – number of cells after time t (as CFU)
Xₒ- initial number of cells (as CFU)
µ- specific growth rate
Negative controls were also adopted and conducted parallel to each bacterial isolate.
In this regard, minimal salt medium containing 2 g/L glucose without inoculation with
bacterial seed culture, and minimal salt medium without glucose inoculated with
10% (v/v) seed culture were used as negative controls. Immediately after the
inoculation at time 0 and at hourly intervals up to nine hours, 6 mL aliquots were
withdrawn from each flask and analysed for optical density at 600 nm, pH and glucose

86 Chapter 4: Selective Enrichment and Identification of Potential PCB Degrading Microorganisms
as reducing sugars using dinitrosalicylic colorimetric method (see Section 3.2.4 A,
Section 3.2.5 and Section 3.2.6 in Chapter 3 respectively for methods adopted).
4.3 Results and Discussion
4.3.1 Screening and Identification of PCB utilizing culture members
After the selective enrichment screening, five microbial isolates from aerobic
enrichments (labelled as A1 to A5) and six bacterial isolates from anaerobic
enrichments (labelled as AN1 to AN6) that grew on the minimal salt medium with
Aroclor 1260 as the sole carbon source were isolated, as pure cultures. The 11 isolates
were subjected to further analyses and their identities determined using 16S rRNA
gene sequencing and morphological characterization.
4.3.1.1 16S rRNA gene based identification
Full length (1.5kb) 16S rRNA gene isolation and sequencing was performed as
described in Section 4.2.3.3 in order to identify the eleven purified bacterial isolates.
Genomic DNAs were first isolated from overnight grown cells using Isolate II genomic
DNA kit (Bioline) and run on a 1% agarose gel as shown in Figure 4.2. Although equal
volume (5 mL) from each genomic DNA sample was loaded on to the gel, the intensity
of the DNA bands on the gel was different. The possible reasons for the differences
in extraction efficiency is attributed to the variations of the cell densities of each
bacterial culture and the differences of the bacterial strains, constitution of the cell
wall and the physiological state of the cells (Van Tongeren et al., 2011).

Chapter 4: Selective Enrichment and Identification of Potential PCB Degrading Microorganisms 87
Figure 4.2 Agarose gel electrophoresis of genomic DNA isolated from bacterial
isolates using Isolate II genomic DNA kit (Bioline). A1 to A5 were from aerobic
selective enrichments and AN1 to AN6 were from anaerobic selective enrichments.
The genomic DNA of two bacterial isolates AN3 and AN4 (as shown in Figure 4.2) were
unable to be extracted using the standard Isolate II genomic DNA kit (Bioline)
protocol. The standard lysis protocol is not adequate to lyse some bacterial cell walls
and subsequent release of DNA (Van Tongeren et al., 2011). If they are Gram positive
bacteria, the thick peptidoglycan layer in the cell walls usually made them difficult to
break using the normal cell lysis method when compared to Gram negative bacteria
(Vingataramin & Frost, 2015).
Once the PCR amplifications of extracted genomic DNA was extracted from agarose
gel and purified, the resulting 1.5 kb DNA fragments were ligated into pGEMR-T Easy
Vector to obtain recombinant DNA. The ligated products were then transformed into
high efficiency E. coli JM109 competent cells to produce multiple copies of
recombinant DNA molecules as described in Section 4.2.3.3 C. After transformation,
positive white colonies were observed on the LB/ampicillin/IPTG/X-Gal agar plates
representing all the different bacterial isolates (Figure 4.3). Recombinant plasmid
DNAs were then isolated from E. coli, purified and the checked on a 1 % agarose gel
to assess the yield and quality (Figure 4.4), before subjecting them for Sanger
sequencing.

88 Chapter 4: Selective Enrichment and Identification of Potential PCB Degrading Microorganisms
Figure 4.3 Blue-white screening on LB/ampicillin/IPTG/X-Gal plate after the
transformation into high efficiency E. coli JM109 competent cells. White colour
colonies representing the positive transformations.
Figure 4.4 Agarose gel electrophoresis of purified recombinant plasmids. 5 µL from
each sample was loaded into the corresponding well. A1 to A5 were from aerobic
selective enrichments and AN1 to AN6 were from anaerobic selective enrichments.
Instead of plasmids, sterile MilliQ water was used in the negative control.
Raw DNA sequences in the forward and reverse directions were first edited using
bioinformatics tool FinchTV 1.4.0 (Geospiza Inc.). The two sequences were merged
using EMBOSS Needle tool (EMBL-EBI) as described in 4.2.3.3 D and as shown in
Figure 4.5, before the 1.5kb 16S rRNA contiguous sequences were submitted to NCBI
and RDP databases for matches.

Chapter 4: Selective Enrichment and Identification of Potential PCB Degrading Microorganisms 89
Figure 4.5 Pairwise alignment of forward and reverse DNA sequences of bacterial
isolate AN2 using Emboss Needle software. Total length after the alignment was 1459
and number of similarities between two sequences were 738/1459 (50.6%).

90 Chapter 4: Selective Enrichment and Identification of Potential PCB Degrading Microorganisms
The closest matching sequences were obtained from NCBI and RDP databases and
are summarized in Table 4.1. The Ribosomal Database Project (RDP) is a curated
database as it provides the aligned and annotated rRNA gene sequence data (Cole et
al., 2014; Wang et al., 2007) while NCBI is not a curated database. There were no
differences between the two databases for culture number NP02, NP04, NP05 and
NP07 as they obtained the same closest relatives from both databases. However,
similarity of culture number NP01, NP03 and NP06 were limited to the generic level
between the two databases. None of the cultures showed 100% similarity to the
existing bacterial cultures in the databases. Therefore, during this study, the different
isolates were identified and labelled according to the genus name followed by strain
number (see Table 4.2). 16S rRNA sequences of the six viable cultures (NP01 to NP06)
were deposited in GenBank (National Centre for Biotechnology Information-NCBI)
database under the accession numbers KY427122, KY427123, KY711179, KY711180,
KY711181 and KY711182 respectively.

Chapter 4: Selective Enrichment and Identification of Potential PCB Degrading Microorganisms 91
Table 4.1 Comparison of closest relatives of isolated bacteria based on NCBI and RDP databases
Notes:
a Similarity score - percent sequence identity over all pairwise comparable positions when ran with aligned myRDP sequences.
b S_ab score (Seqmatch score) - number of (unique) 7-base oligomers shared between the tested sequence and a given RDP sequence divided by the lowest
number of unique oligos in either of the two sequences (Wang et al., 2007)
Culture No.
RDP NCBI
Similarity score a
S_ab score b
Unique common oligomers
Name Maximum Score
Identities Gaps Name
NP 01 0.990 0.956 1344 Chryseobacterium rhizoplanae; JM-534; KP033261
2564 1422/1439(99%) 0 (0%) Chryseobacterium lactis KC1864
NP 02 0.995 0.947 1435 Delftia lacustris; 332; EU888308 2671 1479/1495(99%) 4 (0%) Delftia lacustris 332
NP 03 0.996 0.943 1364 Achromobacter insolitus; LMG 6003; AY170847
2660 1476/1493(99%) 5(0%) Achromobacter denitrificans DSM 30026
NP 04 0.994 0.979 1394 Ochrobactrum lupini; LUP21; AY457038
2625 1437/1445(99%) 0(0%) Ochrobactrum lupini LUP21
NP 05 0.997 0.967 1413 Lysinibacillus macroides; LMG 18474; AJ628749
2717 1486/1494(99%) 0(0%) Lysinibacillus macroides LMG 18474
NP 06 0.995 0.948 1416 Pseudomonas citronellolis; DSM 50332T; Z76659
2686 1486/1501(99%) 4(0%) Pseudomonas knackmussii B13
NP 07 0.985 0.926 1390 Novosphingobium naphthalenivorans; TUT562; AB177883
2564 1430/1451(99%) 0(0%) Novosphingobium naphthalenivorans TUT562

92 Chapter 4: Selective Enrichment and Identification of Potential PCB Degrading Microorganisms
In summary, the eleven bacterial isolates initially identified were reduced to seven
distinct strains based on the outcomes of comparison with existing databases (Table
4.1) and labelled as NP01 to NP07 as shown in Table 4.2.
Table 4.2 Final nomenclature and identification of the pure bacterial isolates
Bacterial isolate New culture no. Proposed name
A1, A4 NP01 Chryseobacterium sp. NP01
A2 NP02 Delftia sp. NP02
A3, A5, AN5 NP03 Achromobacter sp. NP03
AN1 NP04 Ochrobactrum sp. NP04
AN3, AN4 NP05 Lysinibacillus sp. NP05
AN6 NP06 Pseudomonas sp. NP06
AN2 NP07 Novosphingobium sp. NP07
Various microorganisms associated with either anaerobic dechlorination (Adrian et
al., 2009; Payne et al., 2011; Praveckova et al., 2015) or aerobic oxidative
degradations (Borja et al., 2005; (Furukawa & Fujihara, 2008; Dudasova et al., 2014;
Hassan, 2014; Murinova & Dercova, 2014) of PCBs have previously been isolated from
different sediment and soil samples. However, there is no information available
about the bacterial species capable of utilizing PCBs as their sole carbon source
simultaneously under both, anaerobic and aerobic conditions.
Furthermore, a comprehensive search of published literature failed to identify any
studies reporting on the ability of Chryseobacterium sp. and Delftia sp. to grow on
PCBs. Therefore, the two obligate aerobic organisms identified as Chryseobacterium
sp. NP01 and Delftia sp. NP02 in the present study can be considered as new additions
to the existing list of PCB degrading bacteria.

Chapter 4: Selective Enrichment and Identification of Potential PCB Degrading Microorganisms 93
4.3.1.2 Morphological characteristics of identified bacteria
The seven strains identified have shown different colony morphology on nutrient
agar as shown in Figure 4.6. Chryseobacterium sp. NP01 and Ochrobactrum sp. NP04
had mucilaginous colonies while Chryseobacterium sp. NP01 colonies were yellow
pigmented. Novosphingobium sp. NP07 colonies were orange pigmented on nutrient
agar. After the Gram staining, Lysinibacillus sp. NP05 was identified as a Gram
positive, rod shape, spore forming bacterium while all the other six bacterial strains
were identified as Gram negative, rod shape bacteria (see Figure 4.7 for cell
morphology).
When the Gram staining results were compared with the genomic DNA extraction
results as stated in Section 4.3.1.1, the two bacterial isolates AN3 and AN4 that was
not able to lyse using standard cell lysis protocol were identified as Gram positive,
spore forming Lysinibacillus sp. NP05. This further confirmed their resistance to cell
lysis due to the thick peptidoglycan layer (Vingataramin & Frost, 2015).

94 Chapter 4: Selective Enrichment and Identification of Potential PCB Degrading Microorganisms
Figure 4.6 Colony morphology of the bacterial cultures on nutrient agar after 48 h incubation at 28 °C. Culture A to F were under aerobic
conditions and culture G was under anaerobic conditions.

Chapter 4: Selective Enrichment and Identification of Potential PCB Degrading Microorganisms 95
Figure 4.7 Gram stained bacterial cultures under the light microscope (100x magnification).

96 Chapter 4: Selective Enrichment and Identification of Potential PCB Degrading Microorganisms
4.3.1.3 Screening of microorganisms based on tolerance to atmospheric oxygen
The seven pure isolates were also tested for growth tolerance to atmospheric oxygen.
After growing them parallel under both, anaerobic and aerobic conditions, NP01 and
NP02 were identified as obligate aerobic bacteria as they have shown growth only
under aerobic conditions. NP03, NP04, NP05 and NP06 were able to grow under both,
aerobic and anaerobic conditions and therefore identified as facultative anaerobic
bacteria. NP07 was found to be obligate anaerobic as it was able to grow only under
anaerobic conditions.
Importantly, all the bacterial cultures confirmed their ability to grow and utilize PCBs
as their sole source of carbon by showing growth on minimal salt agar containing
Aroclor 1260 (Figure 4.8A and Figure 4.8B). The four facultative anaerobic bacterial
strains Achromobacter sp. NP03, Ochrobactrum sp. NP04, Lysinibacillus sp. NP05 and
Pseudomonas sp. NP06 were able to grow on minimal salt Aroclor 1260 agar under
both, aerobic (Figure 4.8A) and anaerobic (Figure 4.8B) conditions indicating their
ability to utilize PCBs as their sole source of carbon under both environmental
conditions. There was no visible growth in negative controls that contained solid
MSM with equal volume of acetone used to dissolved Aroclor 1260 (Figure 4.8C and
Figure 4.8D) This confirmed that the bacteria were not able to utilize acetone as their
carbon source even if there was residual acetone left in the agar medium after
evaporation. The characteristic yellow colour shown by Chryseobacterium sp. NP01
on nutrient agar (Figure 4.6A) was not present when growing on minimal salt agar as
shown in Figure 4.8A.

Chapter 4: Selective Enrichment and Identification of Potential PCB Degrading Microorganisms 97
Figure 4.8 Bacterial colonies on minimal salt agar with 50 mg/L Aroclor 1260 as sole
source of carbon after 48 hrs at 28 °C (A) under aerobic conditions, (B) facultative
anaerobic cultures under anaerobic conditions, (C) negative controls under aerobic
conditions (D) negative controls under anaerobic conditions.
Out of the five bacterial cultures identified through anaerobic enrichment (NP03 to
NP07), only one culture, identified as Novosphingobium sp. NP07, was found to be
an obligate anaerobe. As shown in Figure 4.9, Novosphingobium sp. NP07
demonstrated the fastest growth on 25 mg/L and 50 mg/L Aroclor 1260 containing
MSA after 48 hrs. However, after few transfers to fresh minimal salt agar, the culture
lost its viability and was not able to recover. According to the literature, most PCB
dechlorinating obligate anaerobic cultures require the presence of sediment or

98 Chapter 4: Selective Enrichment and Identification of Potential PCB Degrading Microorganisms
sediment substitutes to maintain their dechlorination ability. Therefore, it is difficult
to conduct subsequent bacterial enrichment and isolation processes involving such
bacteria (Wiegel & Wu, 2000). One of the main functions of added sediments to PCB
dechlorinating cultures is to enhance the bioavailability of PCB congener mixtures
(Adrian et al., 2009). There are only a few studies on maintaining sediment free
cultures by adding lactate (Wang & He, 2013b) and acetate (Bedard et al., 2006) as
carbon sources to the medium other than PCBs. This may explain why the viability of
obligate anaerobe Novosphingobium sp. NP07 was lost, and it was not included in
subsequent studies.
Figure 4.9 Growth of obligate anaerobic Novosphingobium sp. NP07 on 25 mg/L and
50 mg/L Aroclor 1260 containing minimal salt agar after 48 h incubation at 28 °C
under anaerobic conditions.
4.3.2 Basic growth profiles using glucose as the carbon source
Typical growth profiles of different and newly isolated microorganisms have generally
been performed using glucose as the carbon source, as it is readily used by most
microorganisms. During this study, cultures were set up as described in Section
4.2.3.5 and incubated over a nine hour period at 28 °C and 150 rpm in a shaker
incubator. Bacterial growth curves were developed based on the samples obtained
and analyzed for bacterial growth (optical density measurements) and glucose
consumption (Figure 4.10). The glucose test was based on the DNS reducing sugar
assay described in Section 3.2.6 in Chapter 3.

Chapter 4: Selective Enrichment and Identification of Potential PCB Degrading Microorganisms 99
Figure 4.10 Basic bacterial growth profiles of the six bacterial isolates grown in minimal salt medium over a nine hour period at 28 °C and 150
rpm using glucose as the carbon source. Error bars represent the standard deviation of mean values (n = 3).

100 Selective Enrichment and Identification of Potential PCB Degrading Microorganisms
It was observed that only Chryseobacterium sp. NP01, Delftia sp. NP02,
Achromobacter sp. NP03 and Ochrobactrum sp. NP04 were able to utilize glucose as
their carbon source. The specific growth rate (µ) of these bacterial cultures were
calculated using the equation 4.1 as described in Section 4.2.3.5 and Table 4.3.
Samples removed over time demonstrated no significant change in the pH in all the
cultures throughout the study period (see Figure 4.11 for pH variation). However,
parallel to the glucose consumption, initial 7.2 pH in the Chryseobacterium sp. NP01,
Delftia sp. NP02 and Achromobacter sp. NP03 containing culture media indicated
slight reduction of pH over time until six to seven hours and started to increase again
once the glucose utilization was stopped. The possible reason for the pH reduction at
the beginning is attributed to the breakdown of glucose into acidic metabolites. Once
the glucose utilization was over, further combustion of acidic metabolites would have
again contributed to increase the pH.
Table 4.3 Specific growth rate of bacterial cultures during the growth profile studies
using 2 g/L glucose as the carbon source.
Bacterial culture Specific growth rate (µ) (h-1)
Chryseobacterium sp. NP01 0.47
Delftia sp. NP02 0.45
Achromobacter sp. NP03 0.34
Ochrobactrum sp. NP04 0.3
Lysinibacillus sp. NP05 Slight growth
Pseudomonas sp. NP06 No growth
There was no glucose reduction or biomass increase observed in the flasks containing
Pseudomonas sp. NP06 indicating the inability of this strain to consume glucose as its
carbon source. Interestingly, the glucose concentration measured as reducing sugars
in the Lysinibacillus sp. NP05 containing flasks started to increase slowly with time.
At the end of the nine hours, it increased up to ~ 2.27 g/L from the initially added
glucose level of 2 g/L. There was no noticeable increase in the glucose level in the
negative control as indicated in the Figure 4.10. This results suggested that under

Chapter 4: Selective Enrichment and Identification of Potential PCB Degrading Microorganisms 101
nutrient limiting conditions, microbes such as Lysinibacillus sp. NP05 may have the
ability to synthesize some extracellular polymeric substances (EPS) such as
exopolysaccharides to protect the cells through cellular adhesion (Characklis &
Cooksey, 1983). The common sugars such as D-glucose, D-galactose and D-mannose
are frequently found in bacterial exopolysaccharides (Flemming et al., 2016). Some
Lysinibacillus species were previously identified with the ability to secrete some EPS
with high protein content (Francois et al., 2012). Parallel to the increase in glucose
level in the culture medium, Lysinibacillus sp. NP05 cell density as the OD600 also
indicated a gradual increase. The research literature suggests the ability of some
bacteria to utilize the secreted exopolysaccharides for protein production under
nutrient limiting conditions (Flemming et al., 2016).
Figure 4.11 Variation of pH in the culture medium during the bacterial growth profile
studies. Error bars represent the standard deviation of mean values (n = 3).

102 Selective Enrichment and Identification of Potential PCB Degrading Microorganisms
4.4 Conclusions
The overall aim of the selective enrichment screening in the present study was to
isolate, screen and identify potential PCB degrading microorganisms from the natural
environment for use in PCB degradation. Two obligate aerobic, four facultative
anaerobic and one obligate anaerobic bacterial strains were isolated by selective
enrichment screening. Based on the 16S rRNA sequence based molecular
identification, they were identified as Chryseobacterium sp. NP01, Delftia sp. NP02,
Achromobacter sp. NP03, Ochrobactrum sp. NP04, Lysinibacillus sp. NP05,
Pseudomonas sp. NP06 and Novosphingobium sp. NP07. Their survival on PCB was
confirmed by the growth on minimal salt agar containing commercial PCB mixture,
Aroclor 1260. However, the obligate anaerobe Sphingomonas sp. NP07 lost its
viability to grow and therefore, was not able to be used in further degradation
studies.
Importantly, this is also the first instance of reporting of the presence of
Chryseobacterium and Delftia species involved in PCB contaminated and hydrolysis
conditions. Furthermore, to date, information is not available on the isolation of
facultative anaerobic microorganisms capable of growing under both, anaerobic and
aerobic conditions while utilizing PCBs as their sole source of carbon. Therefore,
based on the results obtained in this study, the isolated facultative anaerobic strains
Achromobacter sp. NP03, Ochrobactrum sp. NP04, Lysinibacillus sp. NP05 and
Pseudomonas sp. NP06 were chosen and individually compared for their PCB
degradation potential under parallel; aerobic, anaerobic and combined anaerobic-
aerobic conditions, as described in Chapter 6.

Chapter 5: Screening of bacterial isolates for biosurfactant production 103
Chapter 5: Screening of bacterial isolates for biosurfactant production
5.1 Background
PCBs are hydrophobic chemicals with poor water solubility. This feature and the low
abundance of suitable microbial communities in the contaminated environments can
prevent natural biodegradation occurring at faster rates (Edwards & Kjellerup, 2013;
Stella et al., 2015). Past research literature on biochemical pathways and intracellular
localization of enzymes responsible for PCB degradation suggest that PCBs have to
be solubilized for easier passage through the cell membrane and into the cytoplasm
prior to being metabolized. Therefore, an increase in the rate of solubilisation may
accelerate the entrance of PCBs into cells and their subsequent degradation (Ohtsubo
et al., 2004). One way of increasing the rate of solubilisation of hydrophobic PCBs is
the addition of mobilizing agents such as chemical and biological surfactants (Singer
et al., 2000; Fava & Di Gioia, 2001; Occulti et al., 2008). Chemical surfactants have the
advantage of being economical, but are often toxic to biological systems (Abraham
et al., 2002). In comparison, biosurfactants generally exhibit higher interfacial tension
reduction activities compared to chemical surfactants, and are less toxic and readily
biodegradable (Viisimaa et al., 2013). However, the main disadvantage of the use of
commercially available biosurfactants is the high cost (Aparna et al., 2012).
The use of suitable biosurfactant producing microbial strains, which are also capable
of degrading PCBs, would be an attractive alternative to the use of chemical and
biological surfactants. Indeed, the application of biosurfactant-producing and
pollutant-degrading microorganisms offers a dual advantage of a continuous supply
of biodegradable surfactant and the ability to degrade pollutants (Megharaj et al.,
2011). However, limited information is available on the fundamentals regarding the
regulation and mechanisms on why and how microbial cultures produce
biosurfactants, and the eventual hydrolysis PCBs. Most studies on PCB solubility have
been based on the addition of chemical or biological surfactants with no investigation

104 Chapter 5: Screening of bacterial isolates for biosurfactant production
into the actual application of microorganisms producing surfactants (Singer et al.,
2000; Fava & Di Gioia, 2001; Occulti et al., 2008; Viisimaa et al., 2013).
In this context, this chapter focuses on investigating the microorganisms with PCB
degradation potential described in Chapter 4, and whether they can also produce
biosurfactants during the hydrolysis of PCBs. The main outcome from this part of the
study was used in the selection of suitable bacterial strains to form the consortium
for further tests, as described in Chapter 7.
5.2 Materials and Methods
In this study, the six bacterial cultures isolated through selective enrichment
described in Chapter 4, namely, Chryseobacterium sp. NP01, Delftia sp. NP02,
Achromobacter sp. NP03, Ochrobactrum sp. NP04, Lysinibacillus sp. NP05 and
Pseudomonas sp. NP06 were individually tested for their PCB solubilization and
biosurfactant production ability.
5.2.1 Experimental setup
Batch mesocosm experiments were conducted for each bacterial strain under aerobic
conditions for six weeks. Erlenmeyer flasks containing 75 mL of sterile minimal salt
medium spiked with 50 mg/L Aroclor 1260 were prepared as described in Section
3.1.2.1 A. Triplicate flasks were tested for each microorganism. Two flasks containing
75 mL of sterile minimal salt medium spiked with 50 mg/L Aroclor 1260 (without
adding bacteria) were used as abiotic controls. Additionally, media controls were
prepared by spiking 75 mL of sterile minimal salt medium with an equal volume of
acetone used to dissolve Aroclor 1260. Parallel to each bacterial batch culture
experiment, two media controls were conducted after inoculation with bacterial seed
culture in a similar way to the experiments.
Each bacterial culture was inoculated into 40 mL of sterile LB medium and incubated
overnight at 150 rpm and 28 °C in a platform shaker. Contents were transferred to 50
mL falcon tubes and centrifuged at 5000 x g for 10 minutes to separate the cell
pellets. Cell pellets were washed twice in minimal salt medium and re-suspended in

Chapter 5: Screening of bacterial isolates for biosurfactant production 105
5 mL of fresh minimal salt medium. 1 mL aliquots of the seed culture suspension were
used to inoculate the batch mesocosms and media controls. All the mesocosms,
abiotic controls and media controls were incubated at 28 °C and 150 rpm in a rotary
shaker for six weeks.
5.2.2 Evaluation of PCB solubility and degradation
PCB solubilisation was measured in the form of total PCBs in solution as hydrophobic
portion of PCBs remained at the bottom of the flask and on the surface of the
aqueous medium. PCB degradation was measured indirectly using cell density, pH
variation and chloride ion accumulation in the batch mesocosms. Initially and at
weekly intervals, 1 mL aliquots of culture supernatants were removed, extracted (as
per Section 3.2.8 A) and analysed (as per Section 3.2.9) for total soluble PCBs. In order
to have homogeneous samples, aliquots were withdrawn from the middle part of the
liquid in the flasks. Parallel to the sampling for PCB analysis, 1 mL aliquots were
removed to measure the cell growth as optical density as per Section 3.2.4 A and 2
mL aliquots were removed to measure the pH as per Section 3.2.5. At the end of week
six, 10 mL from the remaining culture medium was removed, centrifuged at 5000 x g
for 10 min and passed through 0.2 µm filters to separate the bacterial cells and the
filtrate was analysed for chlorine ion accumulation as described in Section 3.2.7.
5.2.3 Screening for biosurfactant production
After six weeks of cultivation, 10 mL culture supernatant from each flask of the batch
experiment were centrifuged at 5000 x g for 10 min and filtered through 0.2 µm filters
to separate the bacterial cells. The cell free supernatants were used for the
subsequent biosurfactant screening tests.
5.2.3.1 Drop collapse test
The drop collapse test was used as a primary screening to determine the ability of
bacterial strains for their biosurfactant production capacity based on past literature
(Bodour et al., 2003; Alvarez et al., 2015; Panjiar et al., 2015; Joy et al., 2017). 20 μL

106 Chapter 5: Screening of bacterial isolates for biosurfactant production
cell free culture supernatant was mixed with 5 μL of 0.1% methylene blue solution
and placed on a parafilm as a drop using a 100 μL micropipette (Panjiar et al., 2015).
The purpose of adding methylene blue was for easy visualization of the droplet.
Diameter of the drop was measured after one minute using 1 mm grit paper placed
underneath the parafilm paper. 1% (w/v) sodium dodecyl sulphate (SDS) solution was
used as the positive control while phosphate buffered saline (PBS) solution and
abiotic controls were used as negative controls. The results where the diameter of
the droplet was at least one millimetre larger than the one made by the negative
control were considered as positive for biosurfactant production.
5.2.3.2 Emulsification index (EI24)
3 mL of cell free culture supernatant and 3 mL of mineral oil were taken into a
graduated test tube and vigorously shaken for 2 min to form an emulsion. The
mixture was allowed to stand still for 24 h and the height of emulsion layer was
measured (Nayak et al., 2009). The emulsification index was calculated using
Equation 5.1 (Panjiar et al., 2015).
EI₂₄(%) = Height of emulsion formed after 24 hrs / Total height of solution x 100
Equation 5.1
Emulsions formed following the reactions with different culture supernatants were
compared to the controls. The positive control used was a 1% (w/v) solution of
synthetic surfactant sodium dodecyl sulphate in deionised water, whereas the abiotic
and medium only control samples were used as the negative controls.
5.2.3.3 Haemolysis
To detect the haemolytic activity indicative of biosurfactant production, 50 µL of the
cell free culture supernatant was spotted on the middle of Tryptone soya agar plates
containing 5% sheep blood (Thermo Fisher Scientific) and the plates were incubated
at 28 °C for 48 h (Alvarez et al., 2015). Plates were visually examined for haemolysis.
The level of clearance of red blood cells was considered proportional to the
concentration of biosurfactant. A yellow transparent zone indicated complete lysis of

Chapter 5: Screening of bacterial isolates for biosurfactant production 107
red blood cells and was regarded as beta () or complete haemolysis. The appearance
of dark green zones beneath the place where the supernatant was spotted was
considered as alpha () or partial haemolysis of blood cells. Alpha and beta
haemolysis were considered as positive for biosurfactant production. No change in
the blood agar plates indicates gamma () or no haemolysis (Joy et al., 2017).
5.3 Results and Discussion
5.3.1 PCB solubility
At the beginning of the experiment, Aroclor 1260 was added to the minimal salt
medium to give 50 mg/L total PCB concentration. Due to the low water solubility of
this highly chlorinated PCB mixture, the initial soluble PCB concentration in the
aqueous minimal salt medium was very low. The results are shown in Figure 5.1.
Figure 5.1 Variation of the total solubility of Aroclor 1260 in the aqueous minimal salt
media inoculated with bacterial cultures. Error bars represent the standard deviation
of mean values (n=3 for bacterial cultures and n=2 for abiotic controls).
As shown in Figure 5.1, the results for the abiotic controls demonstrated that the
total soluble PCB concentration remained stable throughout the six weeks study

108 Chapter 5: Screening of bacterial isolates for biosurfactant production
period and it was measured and found to be 0.15±0.03 mg/L (Figure 5.1). The results
from the obligate aerobic Chryseobacterium sp. NP01 and facultative anaerobic
Lysinibacillus sp. NP05 demonstrated increased concentrations of PCBs in solution
over time. In comparison, there was no significant increase in the PCB concentrations
in solution for the other four culture members (Figure 5.1).
For Lysinibacillus sp. NP05, the PCB concentration in the solution gradually increased
over time and reached a maximum solubility of 14.9±0.55 mg/L at the end of week 5.
In comparison, nearly half of the initially added 50 mg/L Aroclor 1260 became soluble
at the end of the week 6 for Chryseobacterium sp. NP01 and it was measured as
23.8±1.03 mg/L. However, increase in PCB concentration in solution does not
necessarily indicate the net solubility of PCBs. This is due to the potential of bacteria
for increasing solubility and breaking down of PCBs concomitantly under the provided
conditions. When the rate of degradation is similar to the rate of solubility, it would
not indicate any increase of PCB concentration in the solution.
5.3.2 Bacterial growth, chloride ion accumulation and pH
A holistic investigation into the performance of the bacterial strains was carried out
based on one experimental approach while obtaining results for; (1) PCB solubility,
(2) bacterial growth, (3) pH, and (4) chloride ion accumulation in the culture medium.
This is primarily due to the potential of the bacterial strains to increase PCB solubility,
while removing chlorines from biphenyl rings and breaking of the biphenyl rings
concomitantly when PCBs is the only carbon source available. Therefore, this
approach warranted a detailed assessment of bacterial growth, chloride ion build up
and pH variation, in addition to the testing for biosurfactant production during the
bacterial cultivations with PCBs, as an energy source.
5.3.2.1 Bacterial growth
During the six weeks experimental period, growth of all six cultures were very low
and optical density values were less than one (Figure 5.2). Chryseobacterium sp. NP01
reached its maximum growth within the first week of incubation with an OD600 value
of 0.65±0.03 despite the increasing PCB solubility over time (Figure 5.2 vs 5.1). In

Chapter 5: Screening of bacterial isolates for biosurfactant production 109
comparison, the OD600 readings of Achromobacter sp. NP03, Ochrobactrum sp. NP04,
Lysinibacillus sp. NP05 and Pseudomonas sp. NP06 showed minor increase over time.
Figure 5.2 Bacterial growth as optical density (OD600) in the batch mesocosms at 28
°C and 150 rpm. Error bars represent the standard deviation of mean values (n=3).
When cell growth results (Figure 5.2) were compared to PCB solubility results (Figure
5.1), the total PCB solubility capacity of Chryseobacterium sp. NP01 and Lysinibacillus
sp. NP05 were significantly high (see Figure 5.1), but there was no significant increase
in growth. In particular, Chryseobacterium sp. NP01 reached high cell growth by the
end of week 1, followed by a steep decline between weeks 2 and 3, and then followed
a similar trend like most of the other bacteria in weeks, 4, 5 and 6 (Figure 5.2). At the
other extreme, Delftia sp. NP02 seemed to have little growth.
These results suggested that although Chryseobacterium sp. NP01 and Lysinibacillus
sp. NP05 were capable of transforming an extremely hydrophobic PCB mixture
soluble in aqueous medium, their PCB degradation ability under aerobic conditions
was limited to lower chlorinated congeners as suggested by Borja et al. (2005).

110 Chapter 5: Screening of bacterial isolates for biosurfactant production
5.3.2.2 Chloride ion concentration
At the end of week six, the chloride ion concentrations were measured and are shown
in Figure 5.3. The contribution of chloride ion ions from controls (from minimal salt
medium and seed cultures) for all six cultures (NP01 to NP06) were 33.88±0.13,
34.78±0.08, 34.70±0.10, 35.33±0.03, 34.29±0.08 and 34.24±0.09 mg/L respectively.
The background chloride values were first subtracted from the experimental values
before the final values were plotted in order to clearly demonstrate the contribution
of chlorides due to the activity of each bacterial culture.
Figure 5.3 Chloride ion accumulation in the batch mesocosms after six weeks of
incubation at 28 °C and 150 rpm. The background values from the controls of (1)
minimal salt medium only and (2) seed cultures only were subtracted first. Error bars
represent the standard deviation of mean values (n=3).
As shown in Figure 5.3, the highest chloride ion concentrations were from
Chryseobacterium sp. NP01 and Achromobacter sp. NP03 both indicating similar
values of 2.4±0.3 mg/L and 2.4±0.5 mg/L respectively (Figure 5.3). These chloride
concentrations were followed by Lysinibacillus sp. NP05 (2.0±0.3 mg/L) and
Ochrobactrum sp. NP04 (1.5±0.3 mg/L). According to past research literature, under

Chapter 5: Screening of bacterial isolates for biosurfactant production 111
aerobic conditions, bacteria are usually capable of degrading lower chlorinated
congeners through biphenyl ring cleavage (Borja et al., 2005; Field & Sierra-Alvarez,
2008). As previously analysed, the percentage of total chlorine in the Aroclor 1260
mixture used in this study was 58.27±1.15 (see Table 3.2 in Chapter 3). Based on the
homolog group composition in Table 3.2, out of 58.27±1.15% total chlorine, only
1.9±0.13% of chlorine was from the lower chlorinated congeners (congeners with 4
or less chlorines). Therefore, the theoretical maximum chloride concentration
expected to be released from a complete degradation of lower chlorinated congeners
in a 50 mg/L Aroclor 1260 solution is 0.95±0.06 mg/L. As shown in Figure 5.3, except
for Pseudomonas sp. NP06, the rest of the bacterial strains all released different, but
higher amounts of chlorides compared to the expected theoretical maximum yield.
These results suggested that under aerobic conditions, the six bacteria tested have
different mechanisms to attack PCBs, with the concomitant release of different
concentration of chloride ions, depending on their ability to degrade different lower
and moderately chlorinated homolog groups.
5.3.2.3 pH
There was no significant change in pH in all the batch mesocosms and they were in
the range of 7.02±0.01 to 7.78±0.02 as shown in Figure 5.4. However, throughout the
six weeks period, pH variation of flasks containing Chryseobacterium sp. NP01 and
Lysinibacillus sp. NP05 were similar and always slightly higher than the flasks
containing rest of the cultures. As PCB solubility levels were also high in the
mesocosms inoculated with Chryseobacterium sp. NP01 and Lysinibacillus sp. NP05,
any potential relationship between the pH of the medium and the agents responsible
for increased PCB solubility needs to be further investigated. One possible
explanation for all the pH to move towards the alkaline range is the potential build-
up of CO2 in the form of HCO3- in the medium, during cellular respiration under
aerobic cultivation with agitation set at 150 rpm.

112 Chapter 5: Screening of bacterial isolates for biosurfactant production
Figure 5.4 pH variation in the batch mesocosms at 28 °C and 150 rpm. Error bars
represent the standard deviation of mean values (n=3).
5.3.3 Biosurfactant production
The bacterium Chryseobacterium sp. (formerly known as Flavobacterium) was
previously reported with the ability to produce biosurfactants named flavolipids. The
mixture was found to consist of at least 37 strong and stable different flavolipids even
at low concentrations (Bodour et al., 2004). When tested with the hydrocarbon
hexadecane, the apparent solubility of hexadecane increased by several orders of
magnitude. An extracellular bioemulsifier producing bacterium Lysinibacillus sp. was
also isolated from soil contaminated with petroleum oil and found to be positive with
different aliphatic and aromatic hydrocarbons like hexane, benzene, toluene, diesel
and kerosene for its biosurfactant production ability (Panjiar et al., 2015). In addition,
a bacterium isolated from refinery wastewater was identified as Ochrobactrum sp.
with the potential to produce exopolysaccharide bioemulsifier and was able to
degrade diesel oil (Ramasamy et al., 2014), n-octane, mineral light and heavy oils,
crude oil (Calvo et al., 2008). However, there is no research literature available that
confirms the ability of the same bacterial species to produce biosurfactants, which

Chapter 5: Screening of bacterial isolates for biosurfactant production 113
makes extreme hydrophobic PCBs soluble in aqueous medium while concomitantly
degrading PCBs. Therefore, this study can be considered a first to report the potential
of Chryseobacterium, Lysinibacillus and Ochrobactrum species to produce
biosurfactants that ultimately increased the solubility of PCBs.
In this study, the drop collapse and emulsification index tests were used as
quantitative methods (Ramasamy et al., 2014), while the haemolytic assay was used
as the qualitative method (Thavasi et al., 2011) to test for biosurfactant production.
Results of these three tests are summarised in Table 5.1.
Table 5.1 Summary of biosurfactant screening tests
Notes:
* The zones of clearing were scored as follows: ‘+’ incomplete haemolysis; ‘++’ incomplete
haemolysis with semitransparent zones surrounded by green colour areas; ‘+++’ complete
haemolysis.
**1% (w/v) sodium dodecyl sulphate (SDS) solution
***Phosphate buffered saline (PBS) solution
ND - not detected.
Among the six bacterial cultures, four cultures showed positive results for the drop
collapse test. A maximum drop diameter of 6 mm was observed with the culture
Description Drop collapse test
(Diameter in mm)
Emulsification
index (%)
Haemolysis*
Positive Control** 5.3±0.3 50.0 +++
Negative Control*** 3.3±0.3 ND ND
Abiotic control 3.2±0.3 ND ND
Chryseobacterium sp. NP01 6.0±0.0 50.0 +++
Delftia sp. NP02 4.0±0.0 16.7 +
Achromobacter sp. NP03 5.3±0.3 33.3 ++
Ochrobactrum sp. NP04 5.0±0.0 16.7 ++
Lysinibacillus sp. NP05 5.0±0.0 50.0 +++
Pseudomonas sp. NP06 3.5±0.0 ND +

114 Chapter 5: Screening of bacterial isolates for biosurfactant production
supernatant of Chryseobacterium sp. NP01. Achromobacter sp. NP03, Ochrobactrum
sp. NP04 and Lysinibacillus sp. NP05 were also demonstrated to have relatively high
diameters of 5.3±0.3 mm, 5.0±0.0 mm and 5.0±0.0 mm, respectively, when compared
to 3.3±0.3 mm diameter in the negative control (see Table 5.1 and Figure 5.5).
Figure 5.5 Drop collapse test. 1% (w/v) sodium dodecyl sulphate (SDS) solution was
used as the positive control. The phosphate buffered saline (PBS) solution and abiotic
control (minimal salt medium only) were used as negative controls.
As summarised in Table 5.1, the highest emulsification index of 50% was observed in
culture supernatants of both Chryseobacterium sp. NP01 and Lysinibacillus sp. NP05.
This high result was followed by Achromobacter sp. NP03 (33.3%), Ochrobactrum sp.
NP04 (16.7%) and Delftia sp. NP02 (16.7%). Emulsion formation was not observed in
the culture supernatant of Pseudomonas sp. NP06.
Similar to positive results from the emulsification index test, Chryseobacterium sp.
NP01 and Lysinibacillus sp. NP05 performed well during the haemolytic assay. Both
microbes showed strong yellow transparent zones in Tryptone soya agar containing
sheep blood after incubation at 28 °C for 48 hours indicative of complete haemolysis
of red blood cells (Figure 5.6). In contrast, Achromobacter sp. NP03 and
Ochrobactrum sp. NP04 showed partial haemolysis having semi-transparent zones
surrounded by dark green areas. Delftia sp. NP02 and Pseudomonas sp. NP06 showed

Chapter 5: Screening of bacterial isolates for biosurfactant production 115
negative to minor haemolysis, when compared to other positive results and this also
coincides with the low chloride accumulation observed.
Figure 5.6 Haemolysis of sheep blood in Tryptone soya agar after incubation at 28 °C
for 48 hours (A) Chryseobacterium sp. NP01, (B) Delftia sp. NP02, (C) Achromobacter
sp. NP03, (D) Ochrobactrum sp. NP04, (E) Lysinibacillus sp. NP05, (F) Pseudomonas
sp. NP06, (G) 1% SDS as positive control, and (H) abiotic control.
When all three biosurfactant tests conducted were considered, the descending order
of preference for the potential of biosurfactant production is Chryseobacterium sp.
NP01 > Lysinibacillus sp. NP05 > Achromobacter sp. NP03 > Ochrobactrum sp. NP04
> Delftia sp. NP02 > Pseudomonas sp. NP06. According to Ohtsubo et al. (2004), the
following events need to take place for PCBs to be degraded by microorganisms: (1)
solubilisation of PCBs and their entry into cells; (2) expression of PCB degrading
enzymes in the cells; and (3) catalytic breakdown of the PCBs. When the results of
biosurfactant screening tests were compared with PCB solubility and chloride ion
accumulation, Chryseobacterium sp. NP01 and Lysinibacillus sp. NP05 that exhibited
the highest biosurfactant production potential also demonstrated the highest total
PCB solubility and chloride ion accumulation. These results provide a clear and
positive correlation between biosurfactant production and PCB solubilization and
subsequent degradation. Although the solubility of PCBs by the other four cultures

116 Chapter 5: Screening of bacterial isolates for biosurfactant production
were comparatively low, they all displayed different levels of chloride ion
accumulation suggesting they had different mechanisms for attacking PCBs.
Not surprisingly, the two cultures, Delftia sp. NP02 and Pseudomonas sp. NP06 that
demonstrated the lowest biosurfactant production capability also had the lowest
chloride ion levels in the culture supernatant. When the growth profiles of bacterial
cultures were considered, the low growth rate would suggest it is due to the
availability of limited lower chlorinated congeners in the medium. Under aerobic
conditions, these bacteria are expected to only attack and consume lower
chlorinated congeners as their carbon source. Therefore, it is important to study the
performance of these culture members with secondary carbon sources, for cell
growth. The outcomes of such an experiment can potentially help enhance the
biosurfactant production and PCB degradation.
All these bacterial cultures were initially screened as discussed in Chapter 4 for their
ability to survive by utilizing PCBs as their sole source of carbon. According to the
results presented in this Chapter, it can be argued that the rate of microbial
degradation of PCBs is decided not only by their ability for breaking down the PCB
molecules, but also by their ability for making hydrophobic PCBs soluble in the
aqueous medium. Therefore, it is important to include suitable and different culture
members with the ability to produce biosurfactants in order to facilitate the
bioavailability of PCBs. Such a strategy may assist in producing effective on field and
scale up bioremediation applications.
5.4 Conclusions
Six bacterial isolates identified during an initial selective screening for PCB
degradation potential were also individually tested for their ability to produce
biosurfactants. Emulsification index and drop collapse tests were used as quantitative
tests and a haemolytic assay was used as a qualitative test to measure the
biosurfactant production ability of the selected microorganisms. Concomitantly, the
total PCB solubility, bacterial growth, and chloride ion accumulation were also
analysed to measure the PCB solubilisation and degradation potential.

Chapter 5: Screening of bacterial isolates for biosurfactant production 117
Biosurfactants produced by the microorganisms were found to increase the solubility
of hydrophobic PCB mixture in the aqueous medium and consequently enhanced the
bioavailability and degradation of PCBs. The rate of PCB solubility was positively
correlated with the rate of biosurfactant production. Chryseobacterium sp. NP01 and
Lysinibacillus sp. NP05 demonstrated the highest biosurfactant production, PCB
solubilisation and chloride ion accumulation when compared to the other four
bacterial cultures, Delftia sp. NP02, Achromobacter sp. NP03, Ochrobactrum sp. NP04
and Pseudomonas sp. NP06.
In conclusion, the results suggested that microorganisms capable of degrading PCBs
also have a potential to produce some surface active substances to facilitate
hydrophobic PCBs soluble in aqueous media to make them available for utilization.
However, as this study was an initial screening to see the potential of isolated PCB
degrading microorganisms for biosurfactant production, further research is needed
to identify the types and physicochemical properties of the biosurfactants through
extraction, quantification and characterization and to assess their performance in
PCB contaminated soil remediation applications. This latter part was outside the
scope of this study.

118 Chapter 5: Screening of bacterial isolates for biosurfactant production

Chapter 6: PCB degradation potential of facultative anaerobic bacterial isolates 119
Chapter 6: PCB degradation potential of facultative anaerobic bacterial isolates
6.1 Background
Biological conversion of highly and moderately chlorinated congeners into less
chlorinated congeners has been reported to take place under anaerobic conditions
(Praveckova et al., 2015; Agullo et al., 2017). In comparison to highly chlorinated
congeners, lower and moderately chlorinated congeners can be degraded by
oxidative bacteria under aerobic conditions through the upper and lower biphenyl
degradation pathways (Field & Sierra-Alvarez, 2008). This highlights the need of a
bioremediation strategy involving a combined approach of anaerobic dechlorination
and aerobic oxidation steps, in order to obtain the complete degradation of PCBs
(Passatore et al., 2014). Studies undertaken to understand PCB degradation by
combined anaerobic-aerobic conditions have used two separate groups of bacteria,
one capable of reductive dechlorination and the other capable of aerobic oxidation
(Evans et al., 1996; Master et al., 2002). However, there has been no comprehensive
study undertaken to understand PCB degradation using facultative anaerobic
microorganisms under a two-stage anaerobic-aerobic (dechlorination and oxidation)
bioprocess approach. Use of facultative anaerobic microorganisms can potentially
break down PCBs using the two stage anaerobic-aerobic processes while being able
to be used in field scale bioremediation applications as they have the potential to
survive and carry out degradation under varying environmental conditions.
The main aim of Chapter 6 is to investigate the capability of four facultative anaerobic
bacterial cultures Achromobacter sp. NP03, Ochrobactrum sp. NP04, Lysinibacillus sp.
NP05 and Pseudomonas sp. NP06 isolated through selective enrichment as described
in Chapter 4, for degrading Aroclor 1260. Experiments were conducted in parallel for
each facultative anaerobic culture member under (1) anaerobic, (2) aerobic and (3)
two stage (combined) anaerobic-aerobic conditions. The outcomes from this part of

120 Chapter 6: PCB degradation potential of facultative anaerobic bacterial isolates
the study are expected to contribute towards the development of more efficient and
effective bacterial mediated bioremediation treatment discussed in Chapter 7.
6.2 Materials and Methods
The batch mesocosm experiments were conducted for four facultative anaerobic
bacterial cultures Achromobacter sp. NP03, Ochrobactrum sp. NP04, Lysinibacillus sp.
NP05 and Pseudomonas sp. NP06 isolated and identified in Chapter 4 with the
potential to degrade PCBs under both aerobic and anaerobic conditions. For each
facultative anaerobic culture, three separate sets of experiments were conducted in
parallel under aerobic, anaerobic and two-stage anaerobic aerobic conditions,
respectively. All the experiments were conducted in triplicate for each
microorganism under each tested condition.
According to past research literature, the cleavage of the biphenyl ring usually does
not occur during PCB dechlorination, but uses PCBs as electron acceptors (Wiegel &
Wu, 2000). Therefore, it is expected that suitable secondary or additional carbon
sources would be needed and be accessible by the microorganisms for growth.
However, based on the results obtained during the initial selective screening
described in Chapter 4, all the facultative anaerobic microorganisms demonstrated
growth on minimal salt agar containing 50 mg/L of Aroclor 1260 as the only carbon
source available, under anaerobic conditions (see Figure 4.8B in Chapter 4).
Therefore, no additional carbon sources were added to the culture medium other
than PCBs, during the study.
6.2.1 Experimental setup
Erlenmeyer flasks containing 75 mL of sterile minimal salt medium (see Section
3.1.2.1A for mesocosm preparations) were prepared and Aroclor 1260 stock solution
in GCMS grade acetone was added to each flask as the sole source of carbon so that
the final Aroclor 1260 concentration was 50 mg/L. Contents of the flasks were
vigorously shaken for few minutes to evaporate acetone. In all anaerobic and two
stage experiments, flasks were prepared, inoculated and incubated in an anaerobic

Chapter 6: PCB degradation potential of facultative anaerobic bacterial isolates 121
chamber (COY laboratory products, Inc.) to maintain strict anaerobic conditions. The
flasks with minimal salt medium used for anaerobic and two stage experiments were
kept equilibrated for one week inside the anaerobic chamber main compartment
before they were inoculated with the seed cultures. The environment inside the
anaerobic chamber was maintained at a condition with 4.9 % H2, 10.7 % CO2 and 84.4
% N2 (BOC Australia).
Anaerobic dechlorination is a reductive process. PCBs act as electron acceptors and
the chlorine substituents are replaced with hydrogen (Borja et al., 2005). Therefore,
suitable electron donors are needed for the dechlorination process by
microorganisms, which may ultimately lead to the conversion of highly chlorinated
congeners into the lower chlorinated compounds. Hydrogen is assumed to act
directly or indirectly as the electron donor in the dechlorination process (Wiegel &
Wu, 2000). Therefore, the hydrogen gas present inside the anaerobic chamber and
controlled at 4.9% (mol/mol) concentration was the potential electron donor in the
present study.
In aerobic experiments, flasks were incubated at 28 °C and 150 rpm in a rotary shaker
for six weeks. Flasks used in the anaerobic experiments were retained at 28 °C in an
incubator kept inside the anaerobic chamber, with occasional gentle shaking by hand
over the 6 weeks period. The two-stage experiment started at 28 °C under anaerobic
conditions (with occasional shaking by hand) for the first four weeks, and then
removed from the anaerobic chamber and transferred to aerobic conditions at 28 °C
and 150 rpm for the last two weeks.
6.2.2 Bacteria seed culture preparation
Bacteria cultures were inoculated into sterile LB medium in 50 mL falcon tubes and
incubated overnight at 150 rpm and 28 °C in a platform shaker. 7.5 mL of culture
suspension was aseptically transferred to a sterile vial and centrifuged at 5000 x g for
10 min. The supernatant was discarded, and the resulting cell pellet was washed
twice in minimal salt medium. The cell pellet was collected again by centrifugation
and resuspended in 1 mL of minimal salt medium to be used as the inoculum.

122 Chapter 6: PCB degradation potential of facultative anaerobic bacterial isolates
Bacterial cell densities of the seed cultures were calculated as colony forming units
(CFU) using standard plate count as described in Section 3.2.4B.
6.2.3 Controls
The following controls were tested in parallel under each condition.
1. Abiotic controls: 75 mL of minimal salt medium spiked with 50 mg/L Aroclor
1260 without the addition of bacteria seed culture.
2. Media controls: 75 mL minimal salt medium spiked with an equal volume of
acetone used to dissolve 50 mg/L Aroclor 1260 and inoculated with an equal
amount of seed culture used in the experiment.
6.2.4 Sample collection, analysis and preservation
Samples were withdrawn from each flask at time zero and at weekly intervals for the
analyses of pH (2 mL), cell growth (1 mL), extracellular proteins (1 mL) and PCB
solubility (1 mL). In order to have representative samples, liquid aliquots were
removed from the middle of the culture medium while flasks were kept under
agitation. The cell growth was measured as the optical density as per Section 3.2.4A.
The pH and chloride ion concentration were measured as per Section 3.2.5 and
Section 3.2.7 respectively. The total soluble PCBs were extracted as per Section 3.2.8
A and analysed as per Section 3.2.9. Samples withdrawn for extracellular protein
analysis were stored in sterile low protein binding Eppendorf tubes at -80 °C until
subsequent analysis. Analytical processes and outcomes of extracellular protein
analysis are discussed in Chapter 8.

Chapter 6: PCB degradation potential of facultative anaerobic bacterial isolates 123
6.3 Results and discussion
Cell densities of the overnight grown four bacterial seed cultures are summarized in
Table 6.1.
Table 6.1 Bacteria cell count in overnight Luria–Bertani liquid medium at 28 °C.
Bacterial culture No. of colonies in 10-5 dilution
using 100 µL seed culture
No. of CFU/mL
seed culture
1 2 3
Achromobacter sp. NP03 296 288 282 2.9x108 ± 0.07x108
Ochrobactrum sp. NP04 151 144 155 1.5x108 ± 0.09x108
Lysinibacillus sp. NP05 162 143 152 1.5x108 ± 0.09x108
Pseudomonas sp. NP06 180 168 173 1.7x108 ± 0.12x108
6.3.1 Growth profiles - aerobic vs anaerobic vs two stage anaerobic-aerobic
As described in Section 6.2.1, the four facultative strains were tested for growth on
Aroclor 1260 as a carbon source in liquid batch cultures. All tests were monitored at
weekly intervals under three different conditions; aerobic, anaerobic and two stage
anaerobic-aerobic. The results are shown in Figure 6.1.
During aerobic conditions as shown in Figure 6.1a, all four strains reached saturation
by week one with optical density (OD600) readings of 0.51, 0.7, 0.62 and 0.46 for NP03,
NP04, NP05 and NP06 respectively. These optical densities are relatively similar and
demonstrated less variations over time. According to the literature, these results
suggest the possibility of NP03, NP04, NP05 and NP06 consuming lower chlorinated
PCB congeners within the first week and were unable to degrade highly chlorinated
congeners under aerobic conditions (Field & Sierra-Alvarez, 2008). This concept can
be supported by the fact that Aroclor 1260, the PCB source used in this study consists
of less than 10% (by weight) of lower chlorinated homolog groups (containing four or
fewer chlorines per biphenyl molecule) compared to the highly chlorinated homologs
(Mayes et al., 1998; ATSDR, 2000) (see Table 3.2 in Chapter 3). To provide further
support for these observations, Pieper and Seeger (2008) reported that aerobic

124 Chapter 6: PCB degradation potential of facultative anaerobic bacterial isolates
bacteria were capable of degrading biphenyl as the sole source of carbon and energy,
and usually involves the biodegradation of PCBs with less than four chlorine atoms.
Based on these results, under aerobic conditions, the four facultative anaerobic
strains NP03, NP04, NP05 and NP06 may have hydrolysed the lower chlorinated PCBs
homolog groups present in Aroclor 1260. The reactions appeared to occur within the
first one to two weeks of incubation (see Figure 6.1a).
In comparison, during anaerobic conditions, all four strains of NP03, NP04, NP05 and
NP06, showed higher cell densities with OD600 of 1.22, 1.31, 1.04 and 0.82,
respectively. Variations of cell densities indicate that the maximum growth was
reached by week 4 (see Figure 6.1b). Significantly high growth rates under anaerobic
conditions without the presence of carbon sources other than PCBs is an indication
of biphenyl ring cleavage in addition to dechlorination. According to the current
literature, anaerobic dechlorination is a reductive process that uses PCBs as electron
acceptors, but the carbon rings are usually not cleaved (Wiegel & Wu, 2000; Hughes
et al., 2009). In cases where bacteria are not capable of breaking down the carbon
ring structure, they will require additional carbon and electron sources in order to
maintain their growth during dechlorination of PCBs (Wu et al., 2000; Bedard et al.,
2006; Adrian et al., 2009; Wang & He, 2013b). In this case, the only carbon source
available is PCBs. During this study, 8.7x106, 5.0x106, 4.9x106 and 4.5x106 cells/mL
initial cell densities at week zero were increased by 200 fold to 2.0x108, 1.3x108,
1.3x108 and 1.0x108 cells/mL at week four for NP03, NP04, NP05 and NP06,
respectively. This fact confirms the capability of these microorganisms to utilize PCBs
as their carbon source under anaerobic conditions without the requirement for
additional carbon sources.

Chapter 6: PCB degradation potential of facultative anaerobic bacterial isolates 125
Figure 6.1 Growth of the four facultative anaerobic bacterial strains under (a) aerobic,
(b) anaerobic, and (c) two stage anaerobic-aerobic conditions. Error bars represent
the standard deviation of mean values (n = 3).
In the two-stage anaerobic-aerobic cultivations as shown in Figure 6.1c, all four
strains showed similar growth patterns to the anaerobic conditions (Figure 6.1b) up
to week four. However, after switching from anaerobic to aerobic conditions

126 Chapter 6: PCB degradation potential of facultative anaerobic bacterial isolates
(between weeks four to six), Lysinibacillus sp. NP05 started increasing in growth from
OD600 of 1.04±0.07 to 2.63±0.22, which is close to three times its original cell density.
Similarly, Ochrobactrum sp. NP03, Achromobacter sp. NP04 and Pseudomonas sp.
NP06 also showed a slight increase in optical densities of 1.39±0.08, 1.48±0.23 and
0.99±0.06, respectively, when conditions changed from anaerobic to aerobic as
indicated in Figure 6.1c. Based on these results, it can be postulated that all four
organisms are capable of performing dechlorination under anaerobic conditions in a
similar way at different rates and Lysinibacillus sp. NP05 subsequently hydrolyses the
carbon ring structure extensively under aerobic conditions compared to the other
three organisms. However, these earlier observations based on cell growth need
further confirmation through chemical analyses of PCB degradation and release of
chlorides into the culture supernatant.
6.3.2 PCB degradation and solubilisation
PCB concentrations were measured as total soluble PCBs in order to investigate the
change of solubility levels in the medium resulting from potential bacterial activity
under aerobic, anaerobic and two stage conditions. Solubility of PCBs in the medium
was an indirect measure of the microorganisms attacking the PCBs and converting
them from insoluble to soluble forms. The results are shown in Figure 6.2.
At the initial concentration of 50 mg/L, it was observed that the PCBs added to the
flasks appeared not completely soluble, but remained at the bottom as small clumps,
prior to the addition of the bacterial cultures. The total soluble PCB levels in the
abiotic controls with no bacteria added was measured and found to remain very low
throughout the cultivation period, with values found to be 0.15±0.02 mg/L, 0.57±0.07
mg/L and 0.5±0.23 mg/L under aerobic, anaerobic and two stage anaerobic-aerobic
conditions, respectively. According to Figure 6.2a and under aerobic conditions,
Lysinibacillus sp. NP05 showed the highest capacity to solubilize PCBs compared to
Ochrobactrum sp. NP03, Achromobacter sp. NP04 and Pseudomonas sp. NP06. This
increase of activity by Lysinibacillus sp. NP05 started after week one and reached
optimal activity by week five before it started to decrease. However, Lysinibacillus sp.
NP05 did not increase in cell growth, but showed similar cell densities to the rest of

Chapter 6: PCB degradation potential of facultative anaerobic bacterial isolates 127
the three strains as shown in Figure 6.1a. The increased solubility over the aerobic
incubation period with no corresponding increase in cell growth shown by
Lysinibacillus sp. NP05 could possibly be due to two reasons. First, Lysinibacillus sp.
NP05 is capable of creating some surface-active compounds or biosurfactants that
can ultimately lead to increased solubility of hydrophobic PCBs as discussed Chapter
5. Second, the bacteria is unable to carry out the dechorination of highly chlorinated
congeners under aerobic conditions even though it may have the capacity to
solubilize them as reported by Furukawa (2000). Camara et al. (2004) reported that
the accumulation of different metabolic intermediates of PCBs such as dihydrodiols
and dihydroxybiphenyls by aerobic oxidation are highly toxic to bacteria. Therefore,
while increased polarity of these dihydroxylated metabolites can increase their
aqueous solubility, toxic effects can ultimately lead to the inhibition of microbial cell
growth (Seeger & Pieper, 2010). Although the presence of these metabolites was not
analyzed, it was unlikely that cell growth was inhibited by such metabolites, indicated
by steady cell growth during the aerobic period.
In contrast to the aerobic conditions and as shown in Figure 6.2b, PCB solubility of all
four cultures increased significantly under anaerobic conditions. A similar trend was
also observed in the first four weeks during the anaerobic stage of the two stage
anaerobic-aerobic conditions (see Figure 6.2c). It was noted that soon after the
addition of bacterial cultures, the insoluble PCB pellets started to disappear in the
flasks, presumably as the PCBs became soluble due to the action of the
microorganisms. There are two possible explanations for the mode of action by the
microorganisms resulted in the increase in solubility of PCBs. First reason is that
dechlorination of PCBs could transform low water soluble highly chlorinated
congeners into more water soluble lower chlorinated congeners as reported by Yin
et al. (2011). If highly chlorinated congeners were dechlorinated into lower
chlorinated congeners, there should be more chlorides released to the medium.
Therefore, measurement of chloride ions accumulated in the medium is a direct
indication of dechlorination, which was discussed in Section 6.3.3. The second reason
is that the facilitation of the solubility of hydrophobic PCBs due to the production of

128 Chapter 6: PCB degradation potential of facultative anaerobic bacterial isolates
bioemulsifiers or surface-active molecules (biosurfactants) by microorganisms
(FedericiGiubileiCovino et al., 2012) as discussed in details in Chapter 5.
Figure 6.2 PCB solubility under (a) aerobic, (b) anaerobic, and (c) two stage anaerobic-
aerobic conditions. Prior to the addition of microbes, samples were removed and
analysed for Initial soluble PCBs measurement and then after adding microbes,
samples were removed immediately and represent week 0. Error bars represent the
standard deviation of mean values from triplicates.

Chapter 6: PCB degradation potential of facultative anaerobic bacterial isolates 129
Findings of the two stage experiments are shown in Figure 6.2c. As shown in Figure
6.2c, first four weeks were dedicated to anaerobic conditions before switching over
to aerobic conditions during the last two weeks. All four strains demonstrated similar
trends in the first four weeks under anaerobic conditions (Figure 6.2b vs Figure 6.2c).
However, in the second stage, when the conditions shifted from anaerobic to aerobic,
the solubility of PCBs reduced in Achromobacter sp NP03, Ochrobactrum sp. NP04
and Pseudomonas sp. NP06, whereas in Lysinibacillus sp. NP05, there was a
significant increase in the PCB solubility. Parallel to the increased PCB solubility,
growth of Lysinibacillus sp. NP05 also increased significantly during this period as
shown in Figure 6.1c aerobic phase.
6.3.3 Chloride ion accumulation
The release of chlorides and their concentration in the liquid medium were measured
as explained in Section 3.2.7. These measurements were taken as an indication of the
dechlorination process that took place following the addition of the four bacteria
strains. The chloride ion concentrations in the abiotic controls were also measured
and found to be relatively constant throughout the experiment and there was no
considerable difference between the initial and final chloride levels under aerobic,
anaerobic and two stage anaerobic-aerobic conditions. The chloride ion
concentrations in the controls were in the range of 37.0±0.9 mg/L. These background
levels were concluded to come from chloride containing compounds in the basal
minimal salt medium comprised of MgCl2·6H2O, CoCl2·6H2O, MnCl2·4H2O, NiCl2·6H2O
and CuCl2·2H2O. The background chloride values were first subtracted from the
experimental values obtained from samples of the medium containing cells. Similarly,
background values from the controls of seed cultures only were also subtracted from
the experimental values, before the final values were plotted as shown in Figure 6.3.

130 Chapter 6: PCB degradation potential of facultative anaerobic bacterial isolates
Figure 6.3 Chloride ion accumulation in the culture media after six weeks. The
background values from the controls of (1) minimal salt medium only and (2) seed
cultures only were subtracted first. Error bars represent the standard deviation of
mean values from triplicates.
According to Figure 6.3, accumulation of chloride ions in aerobic and anaerobic
conditions provided clear evidence that the four facultative microbes discovered
have the ability to dechlorinate the Aroclor 1260 mixture under both, aerobic and
anaerobic conditions. Significantly high chloride ion concentrations in the combined
anaerobic-aerobic treatments of all four cultures when compared to aerobic and
anaerobic treatments further confirms the effectiveness of combining anaerobic and
aerobic degradation rather than isolated aerobic or anaerobic applications
(Tartakovsky et al., 2001; Long et al., 2015). Increasing levels of chloride ions in the
culture medium were also reported as a direct indication of dechlorination of PCB
molecules by Yin et al. (2011). Under anaerobic and two stage conditions,
Lysinibacillus sp. NP05 demonstrated the highest chloride ion levels when compared
to the other three cultures and they were 9.16±0.8 mg/L and 5.2±0.7 mg/L,
respectively. Pseudomonas sp. NP06 has the lowest chloride levels under all three
conditions. As Aroclor 1260 theoretically contains 60% chlorine by weight, maximum
chlorine level expected in 50 mg/L Aroclor 1260 concentration is 30 mg/L. Therefore,

Chapter 6: PCB degradation potential of facultative anaerobic bacterial isolates 131
maximum chloride yield of 9.16±0.8 mg/L observed in Lysinibacillus sp. NP05 under
two stage anaerobic-aerobic treatment is an indication of the removal of one third of
total chlorine present in the Aroclor 1260 mixture.
The pH of the samples was also monitored, and the results from week 6 are shown
together with chloride ions accumulated in Figure 6.4. Before inoculation of the
cultures, flasks were maintained at pH 7. The pH values of the abiotic controls
remained relatively constant throughout the experiment (7.07±0.14) under
anaerobic, aerobic and two stage conditions. At the end of the aerobic and anaerobic
experiments, the pH did not significantly change and were 7.49±0.07 and 6.95±0.03,
respectively. However, significant pH reduction was observed at the end of all the
two stage anaerobic-aerobic experiments with values of 5.15±0.03, 4.98±0.06,
4.97±0.01 and 6.14±0.03 obtained for Achromobacter sp. NP03, Ochrobactrum sp.
NP04, Lysinibacillus sp. NP05, and Pseudomonas sp. NP06, respectively, as shown in
Figure 6.4. When variations of the pH values were compared with the accumulation
of the chloride ion levels in the medium, the negative correlation is clearly visible.
Based on these results, the lowering of pH under two stage anaerobic-aerobic
conditions appeared to have correlated well with the increase in chloride ions. The
high levels of chlorides under combined anaerobic-aerobic conditions is attributed to
the dechlorination of highly chlorinated congeners under anaerobic conditions first,
followed by oxidation of resulting lower chlorinated congeners into the
corresponding chlorinated intermediates through the upper biphenyl pathway as
reported by Field and Sierra-Alvarez (2008). Finally, it can be postulated that further
mineralization of the intermediates into carbon dioxide and inorganic chlorides
through the lower biphenyl pathways (ATSDR, 2000; FedericiGiubileiCovino et al.,
2012) has occurred after switching to aerobic conditions.

132 Chapter 6: PCB degradation potential of facultative anaerobic bacterial isolates
Figure 6.4 Variation of pH and chloride ion concentrations after six weeks under
aerobic, anaerobic and two stage anaerobic-aerobic conditions (Initial pH was
adjusted to 7.0). Error bars represent the standard deviation of mean values from
triplicates.
Overall, the data from the growth characteristics (Figure 6.1), PCB solubility (Figure
6.2), chloride accumulation levels (Figure 6.3), and pH (Figure 6.4) support the
identification of Lysinibacillus sp. NP05 as the best performer out of the four
facultative anaerobic strains tested followed by Achromobacter sp. NP03 and
Ochrobactrum sp. NP04 for PCB degradation. As shown in Figure 6.5, increase in
growth rate parallel to the increased PCB solubility under combined anaerobic-
aerobic treatment by Lysinibacillus sp. NP05 is an indication of the consumption of
Aroclor 1260 as their carbon and energy source other than solubilisation. Moreover,
decrease in pH over the two weeks of aerobic phase as per Figure 6.5 would coincide
with the occurrence of advanced PCB degradation steps to further breakdown the
chlorinated intermediates.

Chapter 6: PCB degradation potential of facultative anaerobic bacterial isolates 133
Figure 6.5 Growth profile, PCB hydrolysis and pH variation of Lysinibacillus sp. NP05
under two stage anaerobic-aerobic conditions. Error bars represent the standard
deviation of mean values from triplicates.
6.4 Conclusions
Based on an exhaustive review of past research literature, this can be considered as
the first comparative study assessing the capability and growth characteristics of
facultative anaerobic bacteria in degrading PCBs under anaerobic, aerobic and two-
stage anaerobic-aerobic cultivation conditions. The study found four bacterial strains
identified as Achromobacter sp. NP03, Ochrobactrum sp. NP04, Lysinibacillus sp.
NP05 and Pseudomonas sp. NP06, to have the capability for degrading the
commercial PCB mixture, Aroclor 1260 as the sole source of carbon under both,
anaerobic and aerobic conditions. Among the four strains tested, Lysinibacillus sp.
NP05 performed best based on the results from the comparative experiments on cell
growth, PCB solubility and chloride release and accumulation, under the three
process conditions tested.

134 Chapter 6: PCB degradation potential of facultative anaerobic bacterial isolates
All four strains performed better under anaerobic conditions compared to aerobic
conditions. However, the two stage anaerobic-aerobic conditions produced the best
overall results when assessed on cell growth, PCB solubility and chloride build up.
Importantly, it was found that these microorganisms can carry out these reactions
relatively rapidly within the first one to two weeks. This is despite the fact that a
number of research studies carried out so far have shown comparatively long
durations ranging from three to six months for the reactions to be completed (Master
et al., 2002; Chen et al., 2014).
The presence of chloride ions under all the three conditions tested found that the
highest chloride ion concentrations was achieved under two stage anaerobic-aerobic
conditions which suggest that the combined anaerobic-aerobic conditions could
further enhance the chlorine removal from the biphenyl structure. Therefore, in field
scale soil remediation applications, facultative microorganisms have the potential to
be better candidates as they can survive and degrade PCBs under both anaerobic and
aerobic conditions, while achieving relatively higher degradation rates.
The limitations of biological breakdown due to the characteristic hydrophobic
properties of PCBs can be overcome by the use of suitable bacterial strains, which
can simultaneously solubilize and breakdown PCBs. During this study, Lysinibacillus
sp. NP05 was found to have high potential as a candidate to effectively
decontaminate environmental pollutants such as highly chlorinated complex PCB
mixture, Aroclor 1260. Therefore, based on these results there is an opportunity to
produce and apply tailored-made consortia for future process designs and
applications resulting in shorter time frames, while effectively hydrolyzing PCBs.

Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium 135
Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium
7.1 Background
Reductive dechlorination and oxidative degradation are the two main processes
involved in the biodegradation of PCBs. Therefore, a combination of anaerobic
dechlorination and aerobic oxidation is essential to effectively degrade these
complex polychlorinated biphenyl mixtures to less toxic products. Due to the complex
metabolic network responsible for PCB degradation, a single bacterium does not
possess the enzymatic capability to degrade all or even most of the PCB congeners
present in polluted environments (Fritsche & Hofrichter, 2005; Pieper, 2005). Based
on the availability of genes encoding enzymes that degrade PCBs (Seeger & Pieper,
2010; Hassan, 2014; Wang et al., 2014), types of degradation products (Petric et al.,
2007; PetricBru et al., 2011) and information on how well individual microorganisms
degrade PCBs, a consortium of carefully selected microbial species is expected to
perform better than the application of individual microbes (Liz et al., 2009; Chen et
al., 2015a).
Currently, two modes of combined anaerobic dechlorination and aerobic oxidation
applications have been reported in PCB bioremediation studies, and have been
labelled as: (1) two stage (or sequential) anaerobic-aerobic degradation; and (2)
concurrent (or alternating) anaerobic-aerobic degradation (Master et al., 2002;
Payne et al., 2013; Chen et al., 2014; Long et al., 2015). Based on the results discussed
in Chapter 6, three individual strains Achromobacter sp. NP03, Ochrobactrum sp.
NP04 and Lysinibacillus sp. NP05 provided positive indicators as potential candidates
to undergo further tests as a consortium.

136 Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium
Accordingly, Chapter 7 primarily focuses on testing a consortium made up of
facultative anaerobic Achromobacter sp. NP03, Ochrobactrum sp. NP04 and
Lysinibacillus sp. NP05 based on their ability as individuals to degrade the PCB mixture
Aroclor 1260 under both, anaerobic and aerobic conditions. The rates and efficiencies
of PCB biodegradation by the selected three strains as a consortium were compared
under; (1) alternating (AN), and (2) two stage (TS) anaerobic-aerobic modes.
7.2 Materials and Methods
Three facultative anaerobic bacterial strains Achromobacter sp. NP03, Ochrobactrum
sp. NP04 and Lysinibacillus sp. NP05 capable of degrading PCBs under both, anaerobic
and aerobic conditions were used as a consortium in the present study. Initially they
were streaked on solid minimal salt medium containing 50 mg/L Aroclor 1260 and
duplicate plates were incubated anaerobically and aerobically at 28 °C to see whether
there was any visible competition among them for the growth substrate.
The two modes of treatments were tested at 28 °C for six weeks in order to compare
PCB degradation rates under; (1) two stage anaerobic-aerobic (TS), and (2)
alternating anaerobic-aerobic (AN) conditions. The TS experiment continued for four
weeks under anaerobic static conditions (as described in Chapter 6.2.1) and then
switched to aerobic mixing conditions at 150 rpm for the last two weeks. Under AN
conditions, the experiment was kept under anaerobic static conditions in the first
week and switched to aerobic mixing conditions in the second week. Switching from
one week of anaerobic to one week of aerobic incubations was continued throughout
the six-week study. In anaerobic stages, flasks were kept at 28 °C in an incubator kept
inside the anaerobic chamber with occasional gentle shaking by hand. Hydrogen gas
(H2) at 4.92% (BOC Australia) was provided as the electron donor during the
anaerobic phases.
Additionally, three culture members used in the consortium were checked for their
carbon and nitrogen source preferences. Understanding their preferences is
important so that cost, availability and applicability of carbon and nitrogen sources

Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium 137
during inoculation preparation, scale up and on-site remediation applications can be
determined.
7.2.1 Laboratory microcosm batch experiments
The liquid microcosms were prepared using 250 mL Erlenmeyer flasks containing 20
mL of sterile minimal salt medium (see Section 3.1.2.1A). 50 mg/mL Aroclor 1260
stock solution in GCMS grade acetone was added to each flask as sole source of
carbon to give 50 mg/L Aroclor 1260 final concentration.
For seed culture preparations, single bacterial colonies were picked and inoculated
into 250 mL of sterile Luria-Bertani (LB) broth and grown overnight at 28 °C, 150 rpm.
The seed cultures were collected by centrifuging at 5000 x g for 10 min, washed twice
in minimal salt medium, and the resulting cell pellets were resuspended in 1 mL of
sterile minimal salt medium. Previously prepared batch mesocosms were inoculated
with seed cultures under anaerobic conditions (Figure 7.1).
Figure 7.1 Inoculation of batch mesocosms with bacterial seed cultures inside the
anaerobic chamber.
7.2.2 Frequency, collection and preservation of samples
In order to obtain the total PCBs concentrations, the entire content in each flask was
extracted and tested. This was done as PCBs are poorly soluble in water and taking

138 Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium
aliquots may result in unrepresentative samples. Due to this, the experiment was set
up to sacrifice six whole flasks at each sampling from each TS and AN experiment.
These six flasks included one set of triplicate flasks for total PCB extraction and
another set of triplicate flasks for the analyses of bacterial growth, pH, chloride ion
concentration and extracellular proteins. Sampling was done initially (at time zero)
and at fortnightly intervals (at week 2, 4 and 6) for both, AN and TS treatments.
Two controls were also prepared to test parallel to the samples. First control was an
abiotic control without bacterial seed culture additions (i.e. Minimal salt medium
spiked with 50 mg/L Aroclor 1260) and the second control was a growth medium
control (i.e. minimal salt medium with bacterial seed culture addition, but no Aroclor
1260 was added). These controls were conducted in parallel to the experiments
under each TS and AN treatments, and duplicate flasks from each abiotic and growth
medium controls were sacrificed at each sampling time for analyses.
The following is the summary of the test set up of the mesocosms at each sampling
under AN and TS conditions:
• Three flasks for extractions for total PCBs and PCB homolog groups;
• Three flasks for bacterial growth, pH, chlorides and proteomic analyses;
• Two flasks as abiotic controls (minimal salt medium spiked with 50 mg/L
Aroclor 1260, but no seed culture added) ; and
• Two flasks as media controls (minimal salt medium spiked with an equal
volume of acetone used to dissolve Aroclor 1260 and inoculated with seed
culture, but no 50 mg/L Aroclor 1260 added).
Additionally, to see the difference when switching from one week of anaerobic to
one week of aerobic under AN treatment conditions, additional three flasks from AN
treatment and two flasks from each control were used for each consecutive week (at
week 1, 3 and 5) to take samples for cell growth, chloride, pH and extracellular
proteins.
Bacterial growth, pH and chloride ion concentrations were measured as per Section
3.2.4A, Section 3.2.5 and Section 3.2.7, respectively. Final chloride ion concentrations

Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium 139
were determined by subtracting the chloride concentrations of the controls. To see
whether there was any contribution of chloride from the cell lysis was also tested by
comparing the chloride levels of samples before and after being subjected to 10 min
sonication for cell disruption. Samples collected for protein analysis were stored at -
80 °C freezer until subsequent analysis. Analytical processes and outcomes of
extracellular protein analysis are discussed in Chapter 8.
7.2.3 Total PCB extraction and analysis
PCB extraction was performed according to the method described by Murinova et al.
(2014) using GC grade n-hexane as the extraction solvent. Further details of the
extraction method is as described in Section 3.2.8B. Recovery of the surrogate
standard was obtained at 77.0% ± 4.3% (n = 61) and found to be within the USEPA
recommended recovery limits of 70-130% (Eichelberger et al., 1995). A method blank
was also carried out in parallel to each batch of PCB extractions through the entire
procedure using sterile distilled water to evaluate any presence of contamination
from the complete preparation and analytical procedure.
PCB extracts were diluted four fold using n-hexane and analyzed using gas
chromatography (GC) as per the method described in Section 3.2.9 to determine the
total and homolog group specific PCB levels. PCB degradation rates were calculated
as a percentage using Equation 7.1.
PCB degradation (%)
=(Initial PCB concentration - PCB concentration in culture suspension)
Initial PCB concentrationx 100
Equation 7.1
7.2.4 Carbon and nitrogen source utilization profiling of bacterial cultures
Phenotype microarray plates (PM1 and PM3B, Biolog) containing 95 separate sole
carbon and nitrogen sources were used to identify carbon and nitrogen utilization
preferences of the three facultative bacterial consortium members. Depending on
the ability of bacteria to oxidize carbon and nitrogen sources in the wells, initially

140 Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium
colourless tetrazolium dye was reduced to form a purple colour (Miller & Rhoden,
1991). The colour intensity ranged from dark purple to colourless depending on the
intensity of bacterial growth. Biolog phenotype microarray (PM) procedures for Gram
negative and positive bacteria were followed during the cell suspension preparation
and inoculation.
7.2.4.1 Procedure for Gram positive bacteria
(A) Bacterial cell suspension preparation
Gram positive Lysinibacillus sp. NP05 was grown overnight at 30 °C on nutrient agar
to produce pure and isolated colonies. A cell suspension was prepared by transferring
bacterial colonies from the nutrient agar plate into 5 mL 1x inoculating fluid (Biolog
IF-0a GN/GP base) using a sterile cotton swab until the transmittance of 81% (0.0915
absorbance at 600 nm) was reached.
(B) Nutrient additive solution preparation
Nutrient solutions as indicated in Table 7.1 were prepared to use in the final
inoculation fluid, filter sterilized through 0.2 µm filters and stored at 4 °C until use.
Table 7.1 Ingredients for the nutrient additive solution, 12x (PM additive)
Ingredient Gm/50 mL PM1 (mL) PM3 (mL)
Sodium succinate 4.0515 - 3
MgCl2.6H2O
CaCl2.2H2O
2.44
0.88 1 1
L-arginine, HCl
L-glutamate, Na
0.0315
0.0505 1 -
L-cystine, pH 8.5 0.006 3 -
Yeast extract 0.3 1 1
Tween 80 0.3 1 1
Sterile water 3 4
Total 10 10

Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium 141
(C) Final inoculation fluid preparation
The recipe as indicated in Table 7.2 was used to prepare the final inoculating fluids.
Duplicate PM1 and PM3 Biolog plates were inoculated with respective final
inoculation fluid (100 µL/well). Plates were incubated at 30 °C and colour
development was monitored for 24 h in 30 min intervals and measured at 595 nm
wavelength using a plate reader (Synergy HTX). Equivalent volumes of bacterial cell
suspensions with no added carbon and nitrogen sources were used as negative
controls.
Table 7.2 Preparation of final inoculation fluid to inoculate the Biolog plates
Description mL/PM1 Biolog plate
(For carbon sources)
mL/PM3 Biolog plate
(For nitrogen sources)
1.2X stock inoculation fluid
(Biolog IF-0a GN/GP base)
10.0 10.0
Redox dye mix D, 100x (Biolog,
for Gram positive bacteria)
0.12 0.12
Nutrient additive solution, 12x
(PM additive)
1.0* 1.0**
Cell suspension (with 81% T) 0.88 0.88
Total 12.0 12.0
* From PM1 nutrient solution from Table 7.1.
**From PM3 nutrient solution from Table 7.1.
7.2.4.2 Procedure for Gram negative bacteria
(A) Bacterial cell suspension preparation
Gram negative bacteria, Achromobacter sp. NP03 and Ochrobactrum sp. NP04 were
separately grown overnight at 30 °C on nutrient agar to obtain pure colonies. Cell
suspensions were prepared by transferring the bacterial colonies from the nutrient
agar plates into 5 mL 1x inoculating fluid (Biolog IF-0a GN/GP base) using sterile
cotton swabs until the transmittance of 42% (0.3768 absorbance at 600 nm) was
reached.

142 Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium
(B) Final inoculation fluid preparation and inoculation of PM1 and PM3 plates
0.6 mL redox dye H (Biolog, for Gram negative bacteria) and 7.734 mL sterile water
were added to a sterile 50 mL falcon tube containing 41.666 mL of 1.2X stock
inoculation fluid (Biolog IF-0a GN/GP base) and thoroughly mixed to form a uniform
solution. To prepare the final inoculation fluid, 40 mL of the mixture was transferred
into a sterile 50 mL falcon tube and mixed gently with 8 mL of previously prepared
cell suspension having 42% Transmittance. 24 mL from the final inoculation fluid was
transferred to a sterile reservoir and PM1 plates were inoculated in duplicate for each
bacterial culture (100 µL/well). Then, 240 µL of the previously prepared 2M sodium
succinate solution was added to the remaining 24 mL of the final inoculation fluid.
The mixture was gently mixed to make a uniform suspension and used to inoculate
in duplicate PM3 Biolog plates (100 µL/well). Similar to the plates for the Gram
positive bacterium, plates for the two Gram negative bacteria were incubated at 30
°C and the colour development was monitored for 24 h in 30 min intervals and
measured as absorbance at 595 nm wavelength using a plate reader (Synergy HTX).
Bacterial cell suspensions with no added carbon or nitrogen sources were used as
negative controls.
7.3 Results and Discussion
7.3.1 Test for competition
At the start of the experiment, the three chosen bacterial cultures Achromobacter sp.
NP03, Ochrobactrum sp. NP 04 and Lysinibacillus sp. NP05 were streaked on to minimal
salt agar plates spiked with 50 mg/L of Aroclor 1260 as the sole source of carbon and
grown under both, aerobic and anaerobic conditions to test for growth inhibition. All
three strains grew well without any visible growth inhibition from the neighbouring
organism after 72 hours, and in particular, no obvious zones of inhibition were observed
at the areas where each strain merged with each other (Figure 7.2).

Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium 143
Figure 7.2 Growth of Achromobacter sp. NP03, Ochrobactrum sp. NP04 and
Lysinibacillus sp. NP05 on minimal salt-Aroclor 1260 agar at 28 °C after 72 h (a) under
aerobic conditions, (b) under anaerobic conditions.
7.3.2 PCB degradation by the bacterial consortium under AN and TS treatments
7.3.2.1 Total PCB degradation
Typically, anaerobic processes have been shown to be slow and require long
durations in order to achieve effective and higher degradation of PCBs. Adrian et al.
(2009) reported that 64% total PCB reduction was observed after four months of
incubation under anaerobic conditions using anaerobic Dehalococcoides sp. CBDB1,
whereas 36% reduction of total PCBs by a consortium of anaerobic microorganisms
after 10 months of anaerobic incubation was reported by Praveckova et al. (2015) .
Differences in rate of PCB degradation could be due to the differences in types of
microorganisms used and the application or treatment conditions. However, as
discussed in Chapter 6, when facultative anaerobic bacterial strains were tested
during this work as individuals for their PCB degradation potential under aerobic,
anaerobic and combined anaerobic-aerobic conditions, the highest results were
obtained under the combined anaerobic-aerobic conditions.
There are only limited research studies on conventional combined anaerobic-aerobic
treatments (Master et al., 2002; Chen et al., 2014; Long et al., 2015). Most of these

144 Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium
studies consist of lengthy (70 to 180 days) anaerobic phase followed by short aerobic
phase (28 to 60 days). Based on these earlier studies, a two stage (TS) treatment
process was designed for this study, which included a four week anaerobic stage
followed by a two week aerobic stage. In the alternating (AN) treatment mode,
cultures were placed in anaerobic conditions for one week and then switched to one
week long aerobic conditions. Alternation of anaerobic and aerobic conditions were
repeated throughout the six week testing period.
The results of PCB degradation in relation to cell growth under the AN vs TS
conditions are shown in Figure 7.3. According to Figure 7.3, the rate of PCB
degradation occurred faster under AN compared to TS conditions, with 49.2±2.5%
total PCB reduction reached during the first two weeks under AN (Figure 7.3a). In
comparison, only about 25% of PCB degradation was obtained in the first two weeks
of just anaerobic conditions under TS (Figure 7.3b). The results from the AN
conditions showed better degradation efficiency within a short period than that of
previous combined anaerobic-aerobic studies. In the study conducted by Master et
al. (2002), it took four months of anaerobic treatment followed by 28 days of aerobic
treatment to achieve 66% PCB reduction. Total PCB reduction reported by Long et al.
(2015) after 70 days of anaerobic treatment and 28 days of aerobic treatment was
only 25%. Therefore, the results of this study are superior in terms of treatment
efficiency compared to past studies.
According to Figure 7.3, though 49.2±2.5% total PCB reduction occurred after the first
two weeks under AN, only a 4.9% further reduction occurred during the last four
weeks reaching up to 54.1±0.49% degradation by week 6. However, despite the fact
that there was no considerable increase in PCB reduction after the second week,
bacterial growth continued to rise from week 2, demonstrated by the optical density
increase from 0.59±0.01 to 2.49±0.16 (Figure 7.3a). This suggests the possibility of a
consortium utilizing the intermediate products produced during the degradation of
Aroclor 1260 as their growth substrates. This hypothesis was confirmed by the fact
that there was no other carbon source added to the medium other than Aroclor 1260
and the increase in growth cannot be expected while there is no substantial PCB
reduction. However, the types of intermediate products produced and their fate

Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium 145
during the degradation process needed to be confirmed through further
investigation.
Figure 7.3 Total PCB degradation as a percentage and bacterial growth as OD600 under
(a) alternating and (b) two stage anaerobic-aerobic treatments by the bacterial
consortium. Error bars represent the standard deviation of mean values (n = 3).
The results from the two stage (TS) anaerobic-aerobic approach are shown in Figure
7.3b. Under TS conditions, the total PCB degradation rate after two weeks of
anaerobic treatment was 24.44±2.46% compared to 49.2±2.5% achieved during the
AN conditions (Figure 7.3b vs Figure 7.3a). From week 2 to 4 under continuing
anaerobic conditions, PCBs were further degraded up to 34.89±2.26%. Anaerobic PCB
dechlorination is a reductive process that uses PCBs as electron acceptors, but the
rings are not usually cleaved as reported by Borja et al. (2005). Therefore, PCB
dechlorinating only microorganisms require additional carbon and electrons sources
for their growth. During the first four weeks of anaerobic growth, the gradual
increase in the growth based on values of OD600 0.18±0.01 to 0.66±0.14,
corresponded well with total PCB concentration reduction (Figure 7.3b). These
results indicate that the consortium members were able to utilize PCBs as their
carbon source under anaerobic conditions. Furthermore, these results were
comparable to the findings from the comparative growth studies including the same

146 Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium
three microorganisms when tested individually under similar conditions as described
in Chapter 6.
Under TS conditions, when the cultures were switched from anaerobic to aerobic
conditions during the last two weeks, the PCB degradation rate increased from
34.89±2.26% (in week 4) to 47.99±1.55% (in week 6, Figure 7.3b). Meanwhile the
optical density value increased nearly three times from 0.66±0.14 in week 4 to
1.56±0.03 in week 6. Microbial growth thrived once it changed from anaerobic to
aerobic conditions. The increased PCB degradation and growth rates were highly
likely due to ring cleavage and hydrolysis of lower chlorinated PCB congeners
generated during the initial anaerobic phase.
7.3.2.2 PCB Homolog analysis
PCB congeners are categorized into ten homolog groups and labelled from
monochlorobiphenyls to decachlorobiphenyls based on the degree of chlorination in
the biphenyl molecule (ATSDR, 2000). However, decachlorobiphenyls were not
included in the analysis as they were present in trace quantities (Less than 0.05% of
the total PCB mixture). According to the existing literature, highly chlorinated
congeners (with 5 or more chlorines) are converted into lower chlorinated (four or
less) congeners under anaerobic conditions. Under aerobic oxidations, lower
chlorinated congeners breakdown into intermediate products such as
chlorobenzoates and benzoates based on upper biphenyl pathway (Borja et al.,
2005). Benzoate is a growth substrate for a broad range of bacteria and it can be
further mineralized to CO2 and H2O via lower biphenyl pathways such as the
chlorocatechol or 3-oxoadipate pathways (Pieper, 2005).
To determine the capability of the consortium to degrade the different PCB homolog
groups under alternating (AN) and two stage (TS) anaerobic-aerobic conditions,
samples were removed, extracted and analysed as described in Sections 7.2.2, 7.2.3
and 7.2.4, respectively. The results are shown in Figure 7.4 for the AN treatment and
in Figure 7.5 for the TS treatment. Since the range of homolog concentrations were
too broad to illustrate in a single graph, three graphs are plotted for each of the AN

Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium 147
and TS treatments in three different scales as: (a) homologs containing lower
chlorinated congeners (from mono to tetra); and (b) penta, hexa, hepta groups; and
(c) octa, nona groups of highly chlorinated congeners.
As shown in Figure 7.4, the concentrations of all the PCB homolog groups decrease
significantly during the first two weeks under AN conditions. Similar to total PCBs,
there was no considerable reduction in the concentrations of homolog groups after
the second week indicating the ability of the consortium to reach their optimum
degradation within the first two weeks under AN conditions. However, when
individual homolog groups were considered, the rate of degradation was negatively
correlated with the increasing number of chlorine atoms in the PCB molecules.
Congeners with single chlorine atoms (monochlorobiphenyl) displayed the highest
reduction rate reaching a 98.69±0.05% overall reduction at the end of week 6 (Figure
7.4a), whereas the nonachlorobiphenyls showed the lowest reduction rate of
47.12±1.64% at the end of week 6 (Figure 7.4c). Based on these results, it can be
concluded that the higher the number of chlorines attached to the biphenyl rings, the
more resistant the PCBs are to microbial biodegradation. A similar observation was
noted by Furukuwa et al (2006). When compared to other homolog groups,
hexachloribiphenyls represent 50.69±0.47% (by weight) of Aroclor 1260 mixture.
Therefore, reduction of initial 19.73±1.12 mg/L hexachlorobiphenyl level to
10.46±0.05 mg/L by week 2 is nearly 47% reduction of the original
hexachlorobiphenyl concentration and this is the main contribution to 49.2±2.5%
overall total PCB reduction (Figure 7.4b).

148 Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium
Figure 7.4 Variation of PCB homolog groups following AN treatment; (a) lower
chlorinated congener groups (mono to tetra), and medium to highly chlorinated
congener groups, (b) penta to hepta, (c) octa and nona. Error bars represent the
standard deviation of mean values (n = 3).
In the TS treatment, the first four weeks were kept under anaerobic conditions and
switched to aerobic conditions in the last two weeks. When lower chlorinated
congener groups were analyzed, the concentration of each group slightly increased
during the anaerobic phase with the highest increase observed in dichlorobiphenyl
from 0.21±0.02 mg/L in week 0 to 1.55±0.11 mg/L by the end of week 4 (Figure 7.5a).

Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium 149
However, once conditions changed from anaerobic to aerobic after week 4,
concentrations of all the lower chlorinated congener groups reduced far below the
initial concentrations (Figure 7.5a). These results indicate that the microorganisms
utilized the lower chlorinated PCBs as an energy source under the aerobic conditions.
The subsequent increase in cell density during the last two weeks under aerobic
conditions as demonstrated in Figure 7.3b would support this conclusion.
Furthermore, these findings are in agreement with the study outcomes by Borja et
al. (2005).
In contrast, the concentrations of highly chlorinated congeners gradually decreased
during the four week anaerobic phase as shown in Figure 7.5. The highest reductions
were observed for penta, hexa and octa homolog groups at the end of anaerobic
phase with 48.2±3.85%, 42.09±1.8% and 39.28±1.62% reductions, respectively (see
Figure 7.5b and Figure 7.5c). These reductions in the highly chlorinated congener
groups suggested the possibility of dechlorination during the anaerobic conditions. It
can also be pointed out that if these highly chlorinated congener groups were
dechlorinated into lower chlorinated congeners, increase in the lower chlorinated
groups need to be higher than what is shown in Figure 7.5a. As there is no significant
increase in lower chlorinated congeners during the anaerobic phase as expected, this
was due to the ability of consortium members to utilize the lower chlorinated
congeners as their carbon and energy source under anaerobic conditions. This can be
further confirmed by the increase in cell density during the anaerobic phase as shown
in Figure7.3b. After the conditions changed from anaerobic to aerobic, there was no
substantial reduction of any highly chlorinated congener groups. However, the
overall reduction in homolog groups was higher in the AN treatment with nearly 90%
of the total reductions achieved within the first two weeks, when compared to the
TS treatment.

150 Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium
Figure 7.5 Variation of PCB homolog groups following TS conditions; (a) lower
chlorinated congener groups (mono to tetra), and highly chlorinated congener
groups, (b) penta to hepta, (c) octa and nona. Error bars represent the standard
deviation of mean values (n = 3).

Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium 151
7.3.3 Chloride ion accumulation and pH variation
7.3.3.1 Chloride ion analysis
Monitoring of chloride ion accumulation in the culture medium was performed to
further confirm the removal of chlorines from the PCB mixture. It was expected that
the chloride ion build up in the culture medium would increase proportionately to
the decrease in PCB homolog groups over time. Accordingly, the presence and
buildup of chloride ion concentrations in both AN and TS treatments were measured
as described in Section 7.2.2, and the results are shown in Figure 7.6.
Figure 7.6 Chloride ion accumulation under alternating (AN) and two stage (TS)
anaerobic-aerobic conditions. The background chloride values from the minimal salt
medium were first subtracted from experimental values and media controls. Error
bars represent the standard deviation of mean values (n=3 for experimental values
and n=2 for media controls).

152 Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium
Primary contributors of chloride levels in the experimental setup are minimal salt
medium used for the experiments and dechlorination of PCBs. In order to eliminate
the contributions of chlorides from minimal salt medium, chloride concentrations of
controls were first examined. The chloride ions in the abiotic controls were expected
to come from chloride containing compounds in the basal minimal salt medium and
the average chloride level in the abiotic control was 37.36±0.47 mg/L. To remove the
contribution from the minimal salt medium, chloride values of the abiotic controls
were first subtracted from the experimental values and media controls before final
values were plotted as shown in Figure 7.6. There was no significant difference of the
chloride levels before and after sonication of the samples and the difference is less
than 1 mg/L. This is indicative of no significant contribution of chlorides to the final
chloride concentration from cell lysis.
As shown in Figure 7.6, increasing levels of chloride concentrations occurred over the
six weeks under both AN and TS treatments. However, higher yields of chlorides were
detected in AN compared to TS at the end of week 4. By the end of week 6, a yield of
17.63±0.91 mg/L chlorides were measured under AN conditions, compared to 11.79±
1.28 mg/L measured under TS conditions.
In addition to the measurement of chloride ion build up in the culture medium, the
theoretical chloride ion removal from Aroclor 1260 present in the medium was also
calculated based on the PCB homolog group reduction rates as discussed in Section
7.3.2.2. Calculated and measured chloride ion levels under each treatment condition
are shown in Figure 7.7.

Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium 153
Figure 7.7 Measured chloride ion buildup in the culture medium and calculated
chloride ion removal from the PCB mixture based on homolog group reductions
under; (a) alternating (AN), and (b) two stage (TS) anaerobic-aerobic conditions. Error
bars represent the standard deviation of mean values (n = 3).
It can be postulated that the release of chlorines is proportional to the homolog
group reduction rates and released chlorides are accumulating in the culture
medium. This leads to an ideal scenario where calculated chloride ions based on
homolog group reductions is equal to the measured chloride levels in the culture
medium. As shown in Figure 7.3a, Figure 7.4b and Figure 7.4c, nearly 50% reduction
in total PCBs and highly chlorinated penta to nona homolog groups were recorded in
week 2 under AN conditions and this would result in high chloride ion concentration
in the medium. Based on the PCB homolog group reduction rates, the calculated
chloride ions released at week 2 was 14.09±0.41 mg/L. However, measured chloride
ion concentration in the culture medium was only 3.9±0.8 mg/L in week 2 (Figure
7.7a) and it was significantly lower than that of week 4 and the calculated chloride
level. This suggests the possibility of transformation of PCBs into chlorinated
intermediate products such as chlorobenzoates and chlorocatechols rather than
releasing chlorine from biphenyl molecules as suggested by Petric et al. (2007).
However, by week 4, measured chloride level increased significantly up to 16.03±0.7
mg/L, a similar value in comparison to calculated chloride level of 14.19±0.65 mg/L.
This indicates the breakdown of intermediate products and release of bound
chlorides. This was further confirmed by the continued growth demonstrated by the

154 Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium
bacterial consortium from week 2 to week 4 with increased optical density from
0.59±0.01 at week 2 to 1.11±0.21 at week 4 as demonstrated in Figure 7.3a, even
when there was no significant increase in the PCB reduction after the second week.
When compared to AN treatment, there was no significant difference between the
calculated and measured chloride concentrations in TS treatment. As both values
were lower than that of AN treatment, it indicates that AN treatment is more
effective than TS treatment.
7.3.3.2 pH analysis
The pH of the culture medium was also measured as described in Section 7.2.2 and
the results are shown in Figure 7.8. As expected, the controls remained almost
constant throughout the study period (7.09±0.1) and were not included in Figure 7.8.
Overall, the pH in both AN and TS treatments under anaerobic conditions
demonstrated negative trends, while the pH trend was positive under aerobic
condition (Figure 7.8). In the AN treatment, the pH dropped from 7.0±0.04 to
6.67±0.09 at the end of each anaerobic phase. However, when switching from
anaerobic to aerobic, the pH appeared to have self-recovered and adjusted back to
its original neutral condition irrespective of the increasing chloride ion concentration
(Figure 7.8a). According to White (1986), some bacteria can undergo metabolic
processes that can lead to the secretion of compounds with the ability to alter the pH
of the medium . Under the aerobic conditions, it is possible that the consortium
members have metabolized the salts in the minimal salt medium such as ammonium
sulfate as their nitrogen source that resulted in the generation of ammonia (Park &
Lee, 1998). Moreover, under aerobic conditions, the flasks are shaken at 150 rpm, in
the presence of oxygen. It is common that microbes under respiration produce CO2,
which can be converted to HCO3 that will also contribute to the alkalinity of the
medium (Hem & Survey, 1989).

Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium 155
Figure 7.8 pH trends relative to chloride ion concentration. (a) AN and (b) TS
anaerobic-aerobic treatments. Error bars represent the standard deviation of mean
values (n = 3).
In the TS treatment, the initial pH dropped from 7.0±0.04 to about 5.51±0.27 by the
end of week 4 (Figure 7.8b). In contrast, the chloride ions from PCB degradation
increased linearly reaching 20.76±0.46 mg/L by the end of week 4. When compared
between the two conditions, it was of interest to see that during the first four weeks
under AN conditions, the buildup in chloride concentration was more gradual
compared to the linear and exponential increase under TS conditions (see Figure 7.8a
vs Figure 7.8b). However, once the conditions switched from anaerobic to aerobic
after week 4, pH level gradually recovered back to its original neutral value of 7.05.
Although, under TS conditions three consortium members individually were not able
to change the pH back to its original neutral position as shown in Figure 6.4 in Chapter
6. When used as a consortium, the ability to self-recover pH from acidic to neutral
condition is an added advantage in soil remediation applications as low pH severely
inhibit PCB biodegradation while.prolonged acidic pH can lead to leaching of acid
soluble toxic compounds present in the soil (Chen et al., 2015a).

156 Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium
7.3.4 Carbon and nitrogen wide substrate utilization tests
Availability of different carbon and nitrogen sources can be a major growth
requirement for microorganisms and play a significant role in the metabolism and
hydrolysis of environmental pollutants such as PCBs. Anaerobic dechlorination is a
reductive process and biphenyl rings are not cleaved unless the microorganisms are
capable of utilizing PCBs as a carbon source as reported by Wiegel and Wu (2000).
Therefore, supplementing a suitable additional carbon source would be essential for
the microorganisms for their survival while dechlorinating PCBs, under anaerobic
conditions. It was apparent that during the first four weeks, the growth rate under TS
conditions was much slower than the AN treatment (see Figure 7.3). Therefore,
providing an additional carbon source, which can be utilized by all the consortium
members, could enhance the PCB dechlorination rate. Addition of a desirable carbon
source was also shown to enhance the mineralization of PCBs under aerobic
conditions through co-metabolism of biphenyl as reported by Beyer and Biziuk
(2009). In the present study, (NH4)2SO4 in the minimal salt medium was provided as
the inorganic nitrogen source for the microorganisms. However, addition of an
economical organic nitrogen source which can be utilized by all the consortium
members would be important to maintain bacterial growth, either under laboratory
conditions, or in on-site applications.
Therefore, as part of further strain characterization and potential future applications,
the three strains Achromobacter sp. NP03, Ochrobactrum sp. NP04, and Lysinibacillus
sp. NP05 were subjected to a carbon and nitrogen-wide substrate utilization screen.
The Biolog system PM1 for carbon and PM3B for nitrogen substrate were used as
described in Section 7.2.4.
The results for different carbon substrate tests are shown in Table 7.3. Likewise,
results for the different nitrogen substrate tests are summarized in Table 7.4. If
bacteria were able to utilize particular carbon or nitrogen source, the initially
colourless tetrazolium dye is reduced to purple colour compound. The intensity of
colour change is proportional to the the degree of substrate utilization. Based on the
colour intensities, L-proline was identified as the best substrate that all three

Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium 157
consortium members, Achromobacter sp. NP03, Ochrobactrum sp. NP04, and
Lysinibacillus sp. NP05, could utilize as their sole source of carbon and nitrogen. L-
Proline is one of the twenty amino acids used by living organisms as the building
blocks of proteins (Al-Mailem et al., 2018). L-proline is produced at industrial scale
and some of the raw materials rich with L-proline are algae Chlorella (Leavitt, 1983)
and keratin separated from waste biomass (Sharma & Gupta, 2016). In addition to L-
proline, all three consortium culture members were able to utilize L-lactic acid and
methyl pyruvate as carbon sources (Table 7.3) and L-Glutamic acid, Ala-His, Ala-Leu
and Gly-Gln as nitrogen sources (Table 7.4) at high rates.
However, it should be further confirmed by additional research as the presence of
other carbon sources could either stimulate or inhibit PCB dechlorination by
facilitating out-competing the non-PCB dechlorinators or providing more preferred
electron acceptors other than PCBs to the dechlorinators in the natural environment
(Wiegel & Wu, 2000; Yang et al., 2008; Parnell et al., 2010). Additionally, potential for
the use of these chemical compounds as nutritional supplements to the soils in on-
site applications need to be carefully decided based on the associated cost and their
impact on the soil ecosystem.

158 Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium
Table 7.3 Carbon source utilization by consortium members Achromobacter sp. NP03, Ochrobactrum sp. NP04, and Lysinibacillus sp. NP05.
A - Achromobacter sp. NP03, O – Ochrobactrum sp. NP04, L – Lysinibacillus sp. NP05
High Moderate Low Negative
Carbon Source A O L Carbon Source A O L Carbon Source A O L Carbon Source A O L
L-Arabinose Citric Acid D,L-α-Glycerol- Phosphate Tricarballylic Acid
Adonitol D,L-Malic Acid D-Glucose-1-Phosphate L-Serine
D-saccharic Acid D-Ribose D-Fructose-6-Phosphate L-Threonine
Succinic Acid Tween 20 D-Glucose-6-Phosphate L-Alanine
D-galactose L-Rhamnose α-hydroxy glutaric acid-γ-lactone L-Alanyl-Glycine
L-aspartic Acid D-Fructose α-Hydroxy Butyric Acid Acetoacetic Acid
L-proline Acetic Acid β-Methyl-D-Glucoside N-Acetyl-β-D-Mannosamine
D-alanine α-D-Glucose N-acetyl-D-glucosamine Mono Methyl Succinate
D-Trehalose Maltose Maltotriose Methyl Pyruvate
D-Mannose D-Melibiose 2-Deoxy Adenosine D-Malic Acid
Dulcitol Thymidine Adenosine L-Malic Acid
D-Serine L-Asparagine Glycyl-L-Aspartic Acid Glycyl-L-Proline
D-Sorbitol D-Aspartic Acid D-galactonic acid-γ-lactone p-hydroxy phenyl acetic acid
Glycerol D-Glucosaminic Acid m-Inositol m-hydroxy phenyl acetic acid
L-Fucose 1,2-Propanediol D-Threonine Tyramine
D-Glucuronic Acid Tween 40 Fumaric Acid D-Psicose
D-Gluconic Acid α-Keto-Glutaric Acid Bromo Succinic Acid L-Lyxose
m-Tartaric Acid α-Keto-Butyric Acid Propionic Acid Glucuronamide
D-Xylose α-Methyl-D-Galactoside Mucic Acid Pyruvic Acid
L-Lactic Acid α-D-Lactose Glycolic Acid L-Galactonic Acid-γ-Lactone
Formic Acid Lactulose Glyoxylic Acid D-Galacturonic Acid
D-Mannitol Sucrose D-Cellobiose Phenylethyl-amine
L-Glutamic Acid Uridine Inosine 2-Aminoethanol
Tween 80 L-Glutamine Glycyl-L-Glutamic Acid

Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium 159
Table 7.4 Nitrogen source utilization by consortium members Achromobacter sp. NP03, Ochrobactrum sp. NP04, and Lysinibacillus sp. NP05.
Nitrogen Source A O L Nitrogen Source A O L Nitrogen Source A O L Nitrogen Source A O L
Ammonia L-Valine -Phenylethyl- amine Xanthosine
Nitrite D-Alanine Tyramine Uric Acid
Nitrate D-Asparagine Acetamide Alloxan
Urea D-Aspartic Acid Formamide Allantoin
Biuret D-Glutamic Acid Glucuronamide Parabanic Acid
L-Alanine D-Lysine D,L-Lactamide D,L--Amino-N-Butyric Acid
L-Arginine D-Serine D-Glucosamine -Amino-N-Butyric Acid
L-Asparagine D-Valine D-Galactosamine -Amino-N-Caproic Acid
L-Aspartic Acid L-Citrulline D-Mannosamine D,L--Amino- Caprylic Acid
L-Cysteine L-Homoserine N-Acetyl-D-Glucosamine -Amino-N-Valeric Acid
L-Glutamic Acid L-Ornithine N-Acetyl-D-Galactosamine -Amino-N-Valeric Acid
L-Glutamine N-Acetyl-L-Glutamic Acid N-Acetyl-D-Mannosamine Ala-Asp
Glycine N-Phthaloyl-L-Glutamic Acid Adenine Ala-Gln
L-Histidine L-Pyroglutamic Acid Adenosine Ala-Glu
L-Isoleucine Hydroxylamine Cytidine Ala-Gly
L-Leucine Methylamine Cytosine Ala-His
L-Lysine N-Amylamine Guanine Ala-Leu
L-Methionine N-Butylamine Guanosine Ala-Thr
L-Phenylalanine Ethylamine Thymine Gly-Asn
L-Proline Ethanolamine Thymidine Gly-Gln
L-Serine Ethylenediamine Uracil Gly-Glu
L-Threonine Putrescine Uridine Gly-Met
L-Tryptophan Agmatine Inosine Met-Ala
L-Tyrosine Histamine Xanthine
A - Achromobacter sp. NP03, O – Ochrobactrum sp. NP04, L – Lysinibacillus sp. NP05
High Moderate Low Negative

160 Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium
7.4 Conclusions
Based on an extensive search of published literature, this appears to be the first
comparative study assessing PCB degradation efficiency of three facultative
anaerobic bacteria as a consortium under two different combined anaerobic-aerobic
modes; alternating (AN) and two stage (TS). Based on the literature, microbial
degradation of PCBs under anaerobic conditions has been shown to be a long-term
process ranging from 70 to 180 days. Therefore, the two stage (TS) process was set
up to have an extended anaerobic phase of four weeks followed by a short aerobic
phase of two weeks. In contrast, weekly intervals of anaerobic and aerobic
conditions, and vice versa was applied in the alternating (AN) study, over a six week
period. The study found that the alternating approach was more efficient compared
to the two stage treatment conditions with nearly 50% reduction in total PCBs
achieved within the first two weeks compared to only 24% from the two stage
treatment. This is significant because one of the limiting factors in bioremediation
applications is the long time span required to achieve a satisfactory degradation rate.
During both treatments, the consortium exhibited a greater ability to degrade the
lower chlorinated PCB homolog groups under aerobic conditions compared to highly
chlorinated homolog groups. However, the total PCB reductions were always higher
under alternating conditions, suggesting the weekly switching between anaerobic
and aerobic conditions favored the consortium to change between dechlorination
and oxidation activities. Even after the PCB degradation appeared to have reached a
plateau at week two, the bacterial cell density and chloride ion concentration in the
culture medium under alternating conditions kept increasing, suggesting the ability
of the consortium to further break down the intermediate products as their sole
carbon source and release the bound chlorides.
L-proline was found to be the best substrate to be utilized by all three consortium
members as their carbon and nitrogen source. Additionally, all three bacterial strains
were able to utilize L-lactic acid and methyl pyruvate as their carbon source and L-
Glutamic acid, Ala-His, Ala-Leu and Gly-Gln as their nitrogen source. However,
applicability of the addition of such chemical compounds as nutritional supplements

Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium 161
to soil in bioremediation applications needs to be considered only after additional
research on their suitability for the soil ecosystem.
Finally, it can be concluded that the alternating (AN) anaerobic-aerobic treatment
would be a preferred approach compared to the two stage (TS) anaerobic-aerobic
process in PCB remediation applications when compared to degradation efficiencies
and time. Importantly, the use of facultative anaerobic bacteria such as
Achromobacter sp. NP03, Ochrobactrum sp. NP04, and Lysinibacillus sp. NP05 as a
consortium would be advantageous as they have the ability to survive and degrade
PCBs under both, aerobic and anaerobic conditions more efficiently than the
individual organisms.

162 Chapter 7: Effective degradation of polychlorinated biphenyls by three facultative anaerobic bacterial species Achromobacter, Ochrobactrum and Lysinibacillus as a consortium

Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05 163
Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05
8.1 Background
Recent research literature provide confirming evidences to suggest that the
intracellular degradation of PCBs occurs via a series of enzymes through anaerobic
dechlorination and aerobic oxidation (Wiegel & Wu, 2000; Pieper, 2005; Agullo et al.,
2017). However, there is no real knowledgeabout specific proteins involved in active
transport of hydrocarbons across the biological membranes (Parales & Ditty, 2017).
As PCBs are insoluble in aqueous media, it is a prerequisite to transform PCBs into
soluble and / or smaller molecules to diffuse more easily into the cells across the cell
membranes (Hearn et al., 2008). Extracellular enzymes released by microorganisms
into their surrounding environment may play a major role in transformation of
hydrophobic pollutants (Basak & Dey, 2015).
According to Desvaux et al. (2009), actively secreted and non-secreted proteins by
an organism are collectively called as exoproteome (see illustration in Figure 8.1).
However, the total proteins or enzymes exported from inside the cells to the external
environment by an organism is defined as the secretome. The secretome consists of
proteins that are actively transported to outside the cell through the cytoplasmic
membrane via classical or non-classical secretion mechanisms, proteins shedding
from the cell membrane, proteins released from the membrane vesicles, as well as
from the secretion machinery itself (Caccia et al., 2013).

164 Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05
Figure 8.1 The secretome and exoproteome of a Gram negative bacterial cell.
(Armengaud et al., 2012)
As illustrated in Figure 8.2, oxidoreductases and hydrolases are some of the secreted
proteins from microorganisms with the ability to convert polymeric chemical
substances such as poly aromatic hydrocarbons to partially degraded or oxidized
products that can be easily absorbed by microorganisms (Gianfreda & Rao, 2004;
Basak & Dey, 2015). Hydrolases or hydrolytic enzymes facilitate the cleavage of major
chemical bonds such as C–C, C–O, C–N in toxic molecules and help to reduce their
toxicity (Karigar & Rao, 2011). However, there is limited knowledge about the role of
extracellular proteins or enzymes secreted by that can attacking and hydrolysing PCB
molecules to produce more easily diffusible intermediates. Proteins that facilitate
uptake of those intermediates into cells for further degradation, and subsequent
conversion to energy are also poorly known.

Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05 165
Figure 8.2 Role of extracellular enzymes in insoluble compound metabolism.
Chapter 8 focuses on extracellular proteins detected in the culture supernatants of
Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05 both
individually as described in Chapter 6 and as a consortium reacting with PCBs under
AN and TS conditions, as described in Chapter 7. This chapter also discusses; (1) mass
spectrometry based proteomics approaches for the identification of proteins present
in bacterial culture meda (2) bioinformatics analyses of these protein sequences, and
(3) their possible roles during the degradation of PCBs by the three facultative
anaerobic bacteria acting as a consortium.
8.2 Materials and Methods
Supernatant samples (1 mL) were collected from individual culture experiments of
facultative anaerobic members Achromobacter sp. NP03, Ochrobactrum sp. NP04
and Lysinibacillus sp. NP05 (as described in Chapter 6), and from the bacterial
consortium based experiments (as described in Chapter 7). These were used for the

166 Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05
analysis of extracellular proteins found in the culture supernatants. The samples were
centrifuged at 10,000 × g for 15 min at 4 °C to remove the bacterial cells, and the
resulting cell free culture supernatants containing proteins were used for protein
visualization, quantification and extraction prior to mass spectrometry analyses.
8.2.1 Protein visualization
8.2.1.1 Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)
SDS-PAGE was used to visualise the proteins in the culture supernatants. Protein
sample (32.5 µL) was mixed with 12.5 µL of 4x Laemmli loading buffer (Thermo Fisher
Scientific) and 5 µL of 10x Bolt reducing agent (Thermo Fisher Scientific). The mixture
was then denatured by boiling at 95 ̊ C for 5 min. Samples were resolved by SDS-PAGE
using Bolt 4-12% Bis-Tris Plus SDS-PAGE gels (Invitrogen, Thermo Fisher scientific).
SeeBlue Protein standard (Thermo Fisher scientific) was used as the protein
molecular weight (MW) marker. A 40 µL aliquot was loaded to each well and
electrophoresis was performed under constant voltage of 200 V for 30 min in mini
gel tanks (Life Technologies) using 1x Bolt MES SDS running buffer (Novex).
8.2.1.2 Coomassie blue staining
Following electrophoresis, gels were removed from tank and fixed by adding 50 mL
fixing solution (10% (v/v) acetic acid and 40% (v/v) ethanol) and agitating gently for
five minutes in a shallow tray. Gels were then rinsed with deionised water and stained
in QC Coomassie stain (Biorad) overnight at room temperature with gentle agitation.
The next day, gels were destained in deionized water for 1 to 3 hr with gentle
agitation at room temperature until the background cleared, and the protein bands
became clearly visible.
8.2.2 Protein quantification using Bicinchoninic acid assay (BCA)
The total protein concentrations in the culture supernatants were quantified using a
microplate bicinchoninic acid assay as per the Pierce BCA Protein Assay Kit protocol
(Catalog number 23227, Thermo Scientific). The protein concentrations were

Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05 167
determined relative to the triplicates of standard dilutions (0-2000 ng/μL) of bovine
serum albumin (BSA, stock solution of 2 mg/mL, Sigma-Aldrich (Table 8.1). 25 μL of
each sample and 200 μL working reagent (50 parts of BCA reagent A with 1 part of
BCA reagent B) was mixed to give 1:8 ratio in a 96-well microtiter plate and incubated
at 37 ˚C for 30 min. The plate was cooled to room temperature and the absorbance
was measured at 562 nm using a microplate reader (FLUOStar Optima, BMG Labtech).
Table 8.1 Standard dilutions preparation for BCA assay
Vial Volume of
Diluent* (μL)
Volume and Source of BSA
(μL)
Final BSA concentration
(μg/mL)
A 0 300 of Stock 2000
B 125 375 of Stock 1500
C 325 325 of Stock 1000
D 175 175 of vial B 750
E 325 325 of vial C 500
F 325 325 of vial E 250
G 325 325 of vial F 125
H 400 0 0 = Blank
*Sterile MilliQ water was used as the diluent.
8.2.3 Trypsin digestion of extracellular proteins
A modified in-filter digestion (FASP) protocol based on the work of Wisniewski et al.
(2009) was used to digest the proteins in the culture supernatant into peptides prior
to mass spectroscopy. Based on the protein concentration measurements in Section
8.2.2, supernatants containing 20 µg of proteins were used for the trypsin digestion.
Parallel to each batch of samples, 10 µg of BSA was also analyzed as a quality control
measure. Buffers and chemicals needed for the protein extractions were prepared as
per Section 3.1.2.6 of Chapter 3.
During sample preparation, protein samples were combined with 200 μL of
Dithiothreitol -Urea buffer and loaded onto 30 kDa Microcon YM-30 centrifugal filter

168 Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05
tubes (catalogue number MRCF0R030, Millipore) and incubated at room
temperature for 60 min in an agitator. Filters were centrifuged at 14,000 x g, 21 °C
for 15 min and the filtrate was discarded. Filters were washed with 200 μL Urea-Tris
buffer and centrifuged at 14,000 x g, 21 °C for 15 min, and the filtrate was discarded.
100 μL Iodoacetamide-Urea buffer was then added to each of the filters and
incubated at room temperature for 20 min in an agitator. Filters were centrifuged at
14,000 x g, 21 °C for 10 min and the filtrate was discarded. The filters were washed
twice with 100 μL of Urea-Tris buffer and centrifuged at 14,000 x g, 21 °C for 15 min
each, with the filtrate discarded after each time. The filters were then equilibrated
by washing twice in 100 μL of 100 mM ammonium bicarbonate buffer and
centrifuged at 14,000 x g, 21 °C for 10 min. The filtrate was removed after both
centrifugation steps.
Trypsin stock (10 µL of 1 µg/µL in 100 mM ammonium bicarbonate buffer) was
defrosted on ice and the working trypsin solution was prepared by adding 90 μL of
100 mM ammonium bicarbonate buffer to the trypsin stock. The working trypsin
solution was added to the tubes containing filters to achieve an enzyme : protein
ratio of 1:50 and the tubes were placed in a humidified chamber and incubated
overnight at 37 °C with gentle agitation.
Following protein tryptic digestions, the filters were transferred to clean 1.5 mL
Eppendorf tubes and the resultant peptides were eluted by centrifugation at 14,000
x g, 21 °C for 15 min. An additional elution step was performed with one wash of the
filters with 20 μL of 100 mM ammonium bicarbonate buffer and collected at 14,000
x g, 21 °C for 15 min. Samples containing tryptic peptides were vacuum dried using a
rotational vacuum concentrator (RVC 2-33IR, John Morris Scientific) for 30 min at 40
°C and reconstituted in 10 μL of 2% (v/v) Acetonitrile (ACN) / 0.1% (v/v) trifluoroacetic
acid (TFA) solution, before proceeding to desalting.
8.2.4 Desalting of samples prior to mass spectroscopy analysis
Following elution, the peptide samples were further purified using solid phase
extraction as described by Rappsilber et al (2003). In particular, 200 µL pipette tips

Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05 169
were filled with single SCX membrane disc (Empore 3M) as described in Section
3.1.2.6 J in Chapter 3 and activated by passing 30 μL of 100% ACN through using a
centrifugal force (2 min spin at 2,000 rpm). Next, 30 μL of 5% (v/v) ammonium
hydroxide/80% (v/v) ACN was added to the tips just before the last remainder of ACN
left the tip and collected for 2 min at 2,000 rpm. Finally, 30 μL of 0.2% TFA (v/v) was
added and collected for 2 min at 2,000 rpm. Peptide samples were then loaded onto
the tips and centrifuged for 2 min at 2,000 rpm. A 30 μL 0.2% TFA solution was added
and the tips were centrifuged for 2 min at 2,000 rpm and this step was repeated.
Peptide samples were eluted to clean 1.5 mL Eppendorf tubes by adding 30 μL of 5%
ammonium hydroxide/80% ACN to the tips followed by 2 min spin at 2,000 rpm. The
eluted peptide samples were vacuum dried as before using a rotational vacuum
concentrator and the pellets were reconstituted in 10 μL iRT buffer (see Section
3.1.2.6 G for preparation) ready for liquid chromatography - mass spectroscopy (LC-
MS) analyses.
8.2.5 Secretome analysis
8.2.5.1 Liquid chromatography – mass spectroscopy
(A) Chromatography
The peptide samples were analysed using the TripleTOF® 5600+ mass spectrometer
(SCIEX) in either DDA or DIA mode as described below. Approximately 400 ng – 1 µg
of peptide material was injected to the instrument. Peptides were separated by
performing reversed-phase chromatography using an Eksigent ekspert™ nanoLC 400
System directly interfaced to the MS/MS instrument. The LC platform was set up in a
trap and elute configuration with a 10 mm × 0.3 mm trap cartridge packed with
ChromXP C18CL 5 µm 120 Å material and a 150 mm × 75 µm analytical column packed
with ChromXP C18 3 µm 120 Å (Eksigent Technologies, Dublin, CA). The mobile phase
solvents were composed of mobile phase A: water/0.1% formic acid (FA); mobile
phase B: ACN/0.1% FA; and mobile phase C: water/2% ACN/0.1% FA. Trapping was
performed in mobile phase C for 5 min at 5 µL/min followed by an elution
configuration across either 9.5 min or 40 min gradient (depending whether 25 min or

170 Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05
65 min method was used) using mobile phases A and B at a conserved flowrate of
300 nL/min. The proportions of both solvents (A and B) were adjusted at specified
time-points of:
a) 0, 30, 35, 40, 49, 50 and 60 min corresponding to 98, 60, 35, 10, 10, 98 and 98 % of
solvent A in the case of the 65 min method.
b) 0, 5, 7, 9.5, 10.2 and 20 min corresponding to 95, 60, 10, 10, 95 and 95 % of solvent
A in the case of the 25 min method.
To minimise retention time drift, the analytical column was maintained at 40 °C.
(B) Data-dependent acquisition (DDA)
For spectral library generation, peptides were analysed by data-dependent
acquisition (DDA) using 25 min (for BSA QC samples) and 65 min method (for culture
supernatant samples). The DDA mode of the instrument was set to obtain high
resolution (resolving power: 30,000) TOF-MS scans over a range of 350-1350 m/z,
followed by up to 15 (in the case of 25 min method) or 40 (in the case of 65 min
method) high sensitivity MS/MS scans of the most abundant peptide ions per cycle
over the range of 100-2000 m/z. The selection criteria for the peptide ions included
intensity greater than 150 cps and charge state of 2-5. The dynamic exclusion
duration was set at either 3 s or 9 s to account for the difference in chromatographic
peak width between the two methods used. Each survey (TOF-MS) scan lasted 250
ms and the product ion (MS/MS) scan was acquired for 50 ms, resulting in a total
cycle time of either 1 s or 2.3 s depending on the method. The ions were fragmented
in the collision cell using rolling collision energy, and collision energy spread (CES) was
set to 5. The collected peptide ion fragmentation spectra were stored in two files per
sample with extensions format of .wiff and .wiff.scan (SCIEX).
(C) Data-independent acquisition (DIA)
For quantitation, eluted peptides were subjected to a cyclic data-independent
acquisition (DIA) using even isolation windows SWATH-MS™ acquisition 65 min
method (SCIEX) based on its earlier description (Gillet et al., 2012). In particular, a

Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05 171
survey scan data (MS) was acquired for 80 ms, followed by MS/MS on all precursors
within a particular isolation window in a cyclic manner using an accumulation time of
80 ms per individual SWATH-MS window. 36 overlapping windows, each 26 m/z units
wide, were used to cover the peptide ions in a range of 350 – 1250 m/z which resulted
in the cycle time of 3 s. Fragment ions were recorded in a high sensitivity mode and
in a range of 100 – 1800 m/z. Given the peptide chromatographic peaks were
approximately 18 s wide, the above parameters allowed the collection of at least 6
data points for each chromatographic peak to ensure accurate quantitation. The
collected data were stored in two files per sample with extensions format of .wiff and
.wiff.scan (SCIEX).
8.2.5.2 Protein identification using ProteinPilot v5
All DDA data files of individual culture samples of Achromobacter sp. NP03,
Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05 (as discussed in Chapter 6) were
submitted together for peak picking and database search through ProteinPilot (V5.0,
ABSciex) software using Paragon algorithm (Shilov et al, 2007). The database was
created using the existing protein sequences of the three microorganisms used in the
consortium study at genus level and protein sequences of the microorganisms
identified with PCB degradation potential in the present and previously published
studies downloaded (on 09.08.2017) from the NCBI protein database. The resulting
protein list was stored as a single .group file.
8.2.5.3 Protein quantitation using PeakView
The resulting .group file generated from ProteinPilot software via SWATH microapp
to create a spectral library with false discovery rate set to 1% for protein identification
and requirement of only unmodified peptides to be used. Next, SWATH™-MS data
files were imported into PeakView and SWATH microapp was used for targeted
extraction of fragment ion signals specific to peptides represented in the library from
each individual SWATH™-MS run. This peak detection step was conducted under
stringent criteria (99% confidence and 0% false discovery rate). SWATH™-MS runs
were performed in triplicate for each sample analyzed. At least four different

172 Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05
peptides were required per protein and their signals were averaged at a protein level
inside SWATH microapp. A protein was considered valid when it was detected in all
three replicates, and absent in the media controls. Protein level data were normalized
using the Most Likely Ratio (MLR) method using MarkerView software (V1.3.1, SCIEX)
which was also used to statistically identify the significantly changed proteins through
principal component analysis (PCA) before any further analysis using bioinformatics.
Finally, using Excel, relative abundance of significantly changed proteins were
determined as heat maps.
8.2.5.4 Bioinformatics analysis of peptide sequences
Once the identity of significantly changed proteins was established, the
corresponding peptide sequences were analysed whether that protein would fall
under a ‘secreted’ or ‘non-secreted’ protein using bioinformatics tools. The proteins
that would fall under classically secreted extracellular proteins were identified using
SignalP 4.1 (http://www.cbs.dtu.dk/services/SignalP) (Armengaud et al., 2012).
Default cut-off values were used during the search for both Gram positive and Gram
negative bacteria. In contrast, the proteins classified as non-classical secreted
proteins were predicted with SecretomeP 2.0
(http://www.cbs.dtu.dk/services/SecretomeP) (Vazquez-Gutierrez et al., 2017). A
protein without a signal peptide was considered positive as a non-classically secreted
protein when the SecP score was above 0.5. Analysis using SignalP first, followed by
SecretomeP, was considered as the preferential order when the same protein was
found positive under both prediction tools. All protein sequences were further
scrutinized through the redundancy check using Geneious (R10.2.3, Biomatters Ltd.).
Proteins that were grouped under secreted and non-secreted proteins were also
analysed according to their potential cellular and biological functions, following NCBI,
UniProtKB (UniProt Consortium, 2016), SwissProt (Bairoch & Apweiler, 2000)
databases and close homolog searches.

Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05 173
8.3 Results and Discussion
8.3.1 SDS-PAGE analysis: Extracellular protein detection and visualization
8.3.1.1 Individual cultures
As an initial screening step, 1 mL samples were collected at weekly intervals from the
supernatants of individual batch culture experiments of the three facultative
anaerobic culture members Achromobacter sp. NP03, Ochrobactrum sp. NP04 and
Lysinibacillus sp. NP05 as described in Chapter 6. Following separation of supernatant
from cells by centrifugation at 10,000 × g for 15 min at 4 °C, a total of 32.5 µL of clear
culture supernatants was checked from each sample, in order to visualize the
presence or absence of proteins in the supernatant over time (see SDS-PAGE protein
analysis in Section 8.2.1). Parallel to each set of samples, separate SDS-PAGE gels
were run for control samples collected at weekly intervals from flasks containing
minimal salt medium inoculated with bacterial seed cultures, containing no PCBs and
there were no visible proteins detected in any of the control SDS-PAGE gels (see
Figure 8.3 for Lysinibacillus sp. NP05 controls under anaerobic conditions).
Figure 8.3 SDS-PAGE analysis of Lysinibacillus sp. NP05 containing controls (minimal
salt medium with no added PCBs) under anaerobic conditions at 28 °C. Lane 1,
SeeBlue Protein standard as the protein molecular weight markers; lanes 2, time 0
immediately after addition of seed culture; lane 3 to 8, week 1 to week 6.

174 Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05
The SDS-PAGE results are shown in Figure 8.4 for Achromobacter sp. NP03, Figure 8.5
for Ochrobactrum sp. NP04 and Figure 8.6 for Lysinibacillus sp. NP05, respectively.
Notably, none of the three strains had any visible protein bands present in week 0,
directly after the addition of the seed cultures.
According to Figure 8.4, the main proteins detected in the supernatants of
Achromobacter sp. NP03 cultures are shown running at ~ 40 kDa that first appeared
in week 1 and were present up to week 6, during anaerobic conditions (Figure 8.4 B).
In contrast, lower yield proteins running at about 50 kDa appeared at weeks 1-6
under aerobic conditions. However, more proteins around the 90 and 40 kDa were
present during week 5 (Figure 8.4 A).
Figure 8.4 SDS-PAGE analysis of extracellular proteins of Achromobacter sp. NP03
under (A) aerobic and (B) anaerobic conditions at 28 °C. Lane 1, SeeBlue Protein
standard as the protein molecular weight markers; lanes 2, time 0 immediately after
addition of seed culture; lane 3 to 8, week 1 to week 6.
Similar to Achromobacter sp. NP03, low amounts of proteins were detected for
Ochrobactrum sp. NP04 under aerobic conditions during weeks 1 to 4 and 6, with
even low to no proteins appearing in week 5 (Figure 8.5 A). More proteins were
present under anaerobic conditions in weeks 2 to 6, with no obvious proteins
detected in week 1 (Figure 8.5 B).

Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05 175
Figure 8.5 SDS-PAGE analysis of extracellular proteins of Ochrobactrum sp. NP04
under (A) aerobic and (B) anaerobic conditions at 28 °C. Lane 1, SeeBlue Protein
standard as the protein molecular weight markers; lanes 2, time 0 immediately after
addition of seed culture; lane 3 to 8, week 1 to week 6.
The proteins detected in the Lysinibacillus sp. NP05 culture supernatants are shown
in Figure 8.6. Under aerobic conditions, a dominant high molecular weight proteins
running at about 100 kDa appeared during week 1 and was show continuing presence
from weeks 2 to 6 (Figure 8.6 A). In contrast, more proteins were detected under
anaerobic conditions, with the yield and pattern of proteins appearing similar in
weeks 1 and 3, and, weeks 4 and 6 (Figure 8.6 B). It was of interest that high amounts
of proteins running approximately at 100 kDa appeared in weeks 1 and 3, while lesser
amounts in weeks 2, 4 and 6. When compared to other weeks, relatively low amounts
of proteins were present in the supernatant in week 5.

176 Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05
Figure 8.6 SDS-PAGE analysis of extracellular proteins of Lysinibacillus sp. NP05 under
(A) aerobic and (B) anaerobic conditions at 28 °C. Lane 1, SeeBlue Protein standard
as the protein molecular weight markers; lanes 2, time 0 immediately after addition
of seed culture; lane 3 to 8, week 1 to week 6.
Despite the fact that the samples were normalized to the volume and not protein
amount, and therefore, change in the protein signal likely correlated with bacterial
density/growth as well as increased/decreased secretion. In summary, Lysinibacillus
sp. NP05 under anaerobic conditions showed the highest number of protein bands
present in the culture supernatants among all the samples (compare Figures 8.4, 8.5
and 8.6). In addition, protein levels in the extracellular environment under aerobic
conditions seemed lower than under anaerobic conditions. This may be due to the
low concentration of lower chlorinated PCB congeners present in the PCB mixture
(see Table 3.2 in Chapter 3) as the only available carbon source for the
microorganisms to utilize under aerobic conditions.
8.3.1.2 Consortium study under AN and TS treatment conditions
In the consortium experiments as described in Chapter 7, supernatant samples
collected at fortnightly intervals from the alternating (AN) and the two stage (TS)
anaerobic-aerobic experiments were also analysed using SDS-PAGE protein analysis.
The results are shown in Figure 8.7. Similar to the individual cultures (see Figures 8.4,

Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05 177
8.5 and 8.6), no obvious proteins can be seen in week 0, straight after the addition of
the seed comprising of Achromobacter sp. NP03, Ochrobactrum sp. NP04 and
Lysinibacillus sp. NP05, as a consortium. However, obvious proteins at around 38-39
kDa are present in week 2, and lesser amounts of the similar sized proteins in weeks
4 and 6 during the AN conditions (Figure 8.7 A). Another protein at about 29 kDa is
present in higher amounts in week 2, under the AN conditions compared to the week
2 results under the TS conditions (Figure 8.7 B). Incidentally, the high levels of
extracellular proteins in week 2 under the AN conditions coincides with the high PCB
degradation rate of 49.2±2.5% observed in week 2 under AN treatment conditions as
discussed in Section 7.3.2.1 in Chapter 7.
Figure 8.7 SDS-PAGE analysis of the extracellular proteins of the bacterial consortium
consisting of Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp.
NP05 under (A) AN, and (B) TS anaerobic-aerobic conditions at 28 °C. Lane 1, SeeBlue
Protein standard as the protein molecular weight markers; lanes 2, time 0
immediately after addition of seed culture; lane 2 to 5, at fortnightly intervals up to
week 6.
In comparison, similar sized proteins (~38-39 and 29 kDa) are also present in weeks
2 and 4 under anaerobic conditions in TS treatment (Fig. 8.7 B). However, it appeared
that the same proteins are not present in the culture medium, during the last 2 weeks
of cultivation when the condition was changed from anaerobic to aerobic in TS
conditions (Figure 8.7 B).

178 Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05
8.3.2 Proteomics analysis
The proteins in the culture supernatants from the consortium study were further
analysed using mass spectrometry in order to enable their identification. Proteins
isolated from the culture supernatants were digested into peptides, purified using
solid phase extraction and resultant peptides were submitted for mass spectrometric
analysis. After quantification using SWATH, a total of 618 proteins representing the
extracellular protein fractions in both AN and TS conditions were identified for
further analyses, following quality control measures as described in 8.2.3.4.
All the 618 total proteins were then subjected to bioinformatics analyses in order to
determine: (1) the presence of signal peptides characteristic of classically secreted
proteins using the SignalP 3.0 server (Petersen et al., 2011); and (2) proteins
characterized as non-classically secreted proteins using the SecretomeP 2.0 server, a
prediction method for identifying the proteins using the signal peptide independent
secretion pathways (Bendtsen et al., 2005). The remaining proteins were grouped as
non-secretory proteins. Analysis of 618 total proteins from AN and TS conditions over
the six weeks period is shown in Table 8.2. The highest number of total proteins (n-
542) detected in week 6 under AN conditions coincides with the maximum PCB
degradation rate (54.1±0.49%), optical density (2.49±0.16 (see Figure 7.3a in Chapter
7) and chloride ion accumulation (17.63±0.91 mg/L) (see Figure 7.6 in Chapter 7).
Table 8.2 Analysis of extracellular proteins identified in culture supernatants of
consortium under AN and TS conditions.
Category
Number of proteins
AN Treatment (weeks) TS Treatment (weeks)
2 4 6 2 4 6
Non secretory 265 220 273 261 249 263
Secretory 260 254 269 266 278 254
Total 525 474 542 527 527 517

Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05 179
8.3.2.1 Non-secretory proteins
Out of the 618 total proteins, 319 (52%) of the proteins found in the culture
supernatant were predicted to be non-secretory by the SignalP 3.0 and SecretomeP
2.0 servers. Both the highest (n=273) and lowest (n=220) number of non-secretory
proteins were detected in week 6 and week 4 under AN conditions as summarized in
Table 8.2. The predicted 319 non-secretory proteins were grouped according to their
potential functional activities (Table 8.3) and the detailed functional classification of
predicted non-secretory proteins in the culture supernatant is given in Table C.3 in
Annex C.
Table 8.3 Functional classification of 319 identified proteins that were predicted to
be non-secretory proteins by the SignalP 3.0 and SecretomeP 2.0 servers.
Function Number of proteins
Amino acid metabolism 43
Aromatics & Xenobiotic degradation 16
Carbohydrate metabolism 32
Carboxylic acid metabolism 15
Elongation factors and chaperones 11
Genetic Information Processing 41
Hypothetical 20
Lipid metabolism 22
Membrane related 6
Metabolism of cofactors and vitamins 6
Nucleotide metabolism 21
Signal transduction and chemotaxis 11
Stress and redox signalling 26
Transport and secretion 8
Other (uncategorised) 41
Total 319
The functional classification of these predicted non-secretory proteins revealed that
the large proportion matched to proteins involved in major metabolic processes
essential for the survival of cells such as amino acid metabolism (n=43), genetic
information processing (n=41) and carbohydrate metabolism (n=32) as shown in

180 Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05
Table 8.3. There were also 16 proteins related to aromatics and xenobiotic
degradation including 4-oxalocrotonate tautomerase, phenyl phosphate carboxylase,
3-oxoadipate CoA transferase, carboxymuconolactone decarboxylase, 2-
aminobenzoate-CoA ligase, and glutathione S-transferase.
4-Oxalocrotonate tautomerase enzyme is part of the bacterial plasmid-encoded
pathway, which allows the microorganisms harbouring the plasmid to use various
aromatic hydrocarbons as their sole sources of carbon and energy (Whitman, 2002).
Phenylphosphate carboxylase has been found to be responsible for catalysing the
para carboxylation of phenylphosphate to 4-hydroxybenzoate in anaerobic
metabolism of phenol (Schuhle & Fuchs, 2004). During aerobic degradation, aromatic
compounds are transformed to intermediates like catechol and protocatechuate
(Altenschmidt & Fuchs, 1992). However, there is a limited number of bacterial species
which are able to both, convert the chloroaromatics into catechol and
protocatechuate intermediates and further mineralize them, due to the lack of
chlorocatechol pathway enzymes (Pieper & Reineke, 2004). Both 3-oxoadipate CoA-
transferase and carboxymuconolactone decarboxylase detected here, are two such
enzymes involved in the catechol and protocatechuate branches of the 3-oxoadipate
pathway, which are important for the degradation of aromatic compounds (Eulberg
et al., 1998). 2-aminobenzoate-CoA ligase was found to be able to catalyse the first
step of aerobic metabolism of 2-aminobenzoate, an intermediate of PCB degradation
into the coenzyme A thioester of 2-aminobenzoate (Altenschmidt & Fuchs, 1992).
Bacterial glutathione transferases (GSTs) are one of the superfamily of enzymes that
play an important role in cellular detoxification against toxic xenobiotics via
conjugation of reduced glutathione and protection against chemical and oxidative
stresses (Nebert & Vasiliou, 2004; Allocati et al., 2009). Glutathione S-transferase is
also known as BphK and catalyzes the dechlorination of 3-chloro-2-hydroxy-6-oxo-6-
phenyl-2,4-dienoates (HOPDAs) compounds that are produced during the
degradation of PCBs in some bacterial biphenyl catabolic (bph) pathways. The
enzyme also catalyzed the dechlorination of 5 chloro HOPDA and 3,9,11-trichloro
HOPDA (Fortin et al., 2006). Additionally, GSTs are also involved in the reductive
dechlorination of pentachlorophenol (Van Agteren et al., 2013).

Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05 181
Oxidoreductases were present in culture supernatant throughout the study period
under both AN and TS conditions with the maximum concentration observed at week
2 under AN conditions. These enzymes are responsible for bacterial mediation of
detoxification of toxic organic compounds through oxidative coupling.
Microorganisms extract energy via energy-yielding biochemical reactions mediated
by oxidoreductases to cleave chemical bonds and to assist in the transfer of electrons
from a reduced organic substrate (donor) to another chemical compound (acceptor)
(Karigar & Rao, 2011). As a result of such oxidation-reduction reactions, the toxic
contaminants are oxidized to harmless compounds.
In addition to these results, the release of certain proteins without known peptide
secretion signals have also been reported in some comparative proteomics studies of
extracellular proteins of Escherichia coli. (Li et al., 2004; Xia et al., 2008). Li et al.
(2004) reported the presence of some enzymes that are involved in metabolic
processes such as nucleotide metabolism, glycolytic pathway, amino acid metabolism
as well as some outer membrane, periplasmic, and cytosolic proteins in the
extracellular proteome of some virulent Escherichia coli strains. Enzymes related to
metabolism and cellular processes such as isocitrate lyase, malate synthase without
signal peptide sequences were also detected in the extracellular proteome of some
other Escherichia coli (9BL21 and W3110) strains (Xia et al., 2008).
Importantly, the potential for cell lysis could not be excluded as a cause for the
release of these proteins, since increased concentrations of some major cytoplasmic
proteins such as chaperonin GroEL, DnaK suppressor protein and thioredoxin TrxA
were detected in the culture medium over time under both AN and TS treatments.
However, occurrence of cell lysis leading to cell death is less likely since there is a
strong trend in increasing cell density and subsequent PCB degradation rates in the
culture medium over time (see Figure 7.3 in Chapter 7).
Selective leakage of some cytoplasmic proteins by unidentified mechanisms was
previously reported (Xia et al., 2008). Therefore, alterations to the cell membrane
and cell wall structures is likely to occur rather than complete cell lysis due to various
stresses such as osmotic shock-like response (Kaakoush et al., 2010). Vazquez-Laslop
et al. (2001) reported that the increased permeability of the outer membrane due to

182 Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05
sudden drop in osmolarity in the medium leads to selective release of some small
cytoplasmic proteins. Thioredoxin and DnaK, which were present in high
concentrations in the exoproteome of the present study, were also among the highly
released proteins due to the osmolarity shock (Vazquez-Laslop et al., 2001).
Furthermore, Cl- ions build up during PCB dechlorination (see Figures 7.6 in Chapter
7) may have contributed to changing the osmolality of the culture medium and may
have some impact on the release of non-secretory proteins. However, this needs to
be further investigated.
Overall, the presence of enzymes that are responsible for the intermediate steps of
PCB degradation pathways and detoxification of toxic chemicals in the extracellular
environment by cell lysis or by selective straining provides strong support for the
occurrence of PCB degradation by the bacterial consortium.
8.3.2.2 Secretory proteins: Secretome
Out of 618 total proteins found in the exoproteome, 299 (48%) were
bioinformatically predicted to be secreted. Of the 299 total proteins identified to be
secreted in the culture supernatant, 71% (212 proteins) were predicted to have signal
peptides and grouped to be classically secreted proteins, while only 29% (87) proteins
were predicted to be secreted by non-classical secretion pathways (Figure 8.8).
Detailed information related to secretory proteins under AN and TS conditions are
given in Table C.1 in Appendix C.

Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05 183
Figure 8.8 Proportions of classically and non-classically secreted proteins in the
culture supernatant of the bacterial consortium Achromobacter sp. NP03,
Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05.
4.3.2.2.1 Classically secreted proteins
Classically secreted proteins were further categorized based on their functional
groups in Figure 8.9. Some proteins such as superoxide dismutase that were found to
have a specific function in the cytoplasm were also recognized to be actively involved
in some biological processes in the extracellular environment (Bendtsen et al., 2005;
Henderson & Martin, 2011). The functions they perform in the cytoplasmic and
extracellular environments are not always identical. Such proteins involved in
multiple functions are known as moonlighting proteins (Jeffery, 2003).

184 Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05
Figure 8.9 Functional groupings of proteins identified as classically secreted proteins.
As shown in Figure 8.9, most abundantly detected classically secreted proteins were
transporters and they represented 58% (122 proteins) of the total classically secreted
proteins. Transporter proteins are one of organism’s primary interfaces with the
environment. Bacterial transport proteins comprise a heterogeneous group
representative of their diverse biological functions (Giuliani et al., 2011). The ability
of bacteria to occupy specific environments mainly depends on their capacity to
obtain sufficient supplies of nutrients that are essential for their growth (Vazquez-
Gutierrez et al., 2017). Bacterial transport proteins play an important role in
facilitating the uptake of essential nutrients, regulate the cytoplasmic concentrations
of metabolites, export large molecules to the outer surface of the cell and prevent
toxic effects of toxins by catalyzing their active efflux (Yen et al., 2009). These proteins
comprise a heterogeneous group representative of their diverse functional and
cellular roles.
Among the ten extracellular proteins identified with the highest protein
concentrations, eight were also transport related classically secreted proteins (see
Table 8.4). The majority of the transporters such as sulfate transporter, leucine ABC
transporter subunit substrate-binding protein, glutamine ABC transporter substrate-
binding protein, amino acid ABC transporter substrate-binding protein, branched
chain amino acid ABC transporter substrate-binding protein, iron ABC transporter

Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05 185
substrate-binding protein, molybdate ABC transporter substrate-binding protein,
were present in the extracellular environment and are involved in the transport of
nutrients. The synthesis of a wide range such transporters in the cell is essential for
the microorganisms to uptake the nutrients from the environment (Christie-Oleza et
al., 2012).
Bacterial biodegradation of hydrocarbons, an important process for environmental
remediation, requires the passage of hydrophobic substrates across the cell
membrane. Membrane proteins are believed to be required to facilitate the
transportation of hydrophobic compounds across the outer membrane of Gram-
negative bacteria surrounded by a hydrophilic lipopolysaccharide layer (Hearn et al.,
2008). ATP-binding cassette (ABC) transporters couple ATP hydrolysis to aid in the
transport of various molecules across cellular membranes (Michalska et al., 2012).
The involvement of ABC transporters, via their associated substrate binding protein
specificity in the uptake and metabolism of benzoic acid and other aromatics was
revealed by Giuliani et al., (2011).

186 Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05
Table 8.4 Heat map showing the relative abundance of ten highly secreted extracellular proteins detected in the culture supernatant of the
bacterial consortium Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05.
No. Accession number
Name AN
(Weeks) TS
(Weeks)
Secreted*
2 4 6 2 4 6
1 OLU07503.1 Sulfate transporter subunit Yes (27-28) 2 EHK64178.1 Histone-like DNA-binding protein Yes# 3 WP_088138276.1 ABC transporter substrate-binding protein Yes (23-24) 4 WP_062683598.1 Amino acid ABC transporter substrate-binding protein Yes (22-23) 5 WP_062682236.1 Leucine ABC transporter subunit substrate-binding protein LivK Yes (25-26) 6 KOQ40547.1 Membrane protein Yes (22-23) 7 OLU06668.1 Iron ABC transporter substrate-binding protein Yes (28-29) 8 KGD98287.1 Glutamine ABC transporter substrate-binding protein Yes (26-27) 9 OLU08696.1 ABC transporter substrate-binding protein Yes (28-29)
10 WP_088595533.1 Glutamine ABC transporter substrate-binding protein GlnH Yes (26-27) Notes:
AN – Alternating anaerobic-aerobic treatment, TS – Two stage anaerobic-aerobic treatment
* Numbers given within brackets correspond to the signal peptide cleavage site location of classically secreted proteins.
# Non-classically secreted proteins.
Protein yield
Low <----------> high

Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05 187
There were three classically secreted proteins identified here that matched to
glutathione transporter proteins. They are; glutathione ABC transporter substrate-
binding protein, Glutathione-binding protein precursor and glutathione-binding
protein (Table C.1 in Appendix C). One of the main roles of glutathione is protection
of cells through the regulation of the concentrations of xenobiotics within the cell by
conjugating xenobiotics and exporting them from the cells (Cole & Deeley, 2006).
PCBs are highly hydrophobic and lipophilic molecules, and therefore they are
believed to be capable of unregulated entry into the cells through the cytoplasmic
membrane which can ultimately cause intracellular toxicity (Parales & Ditty, 2017).
Therefore, bacteria capable of utilizing PCBs as their growth substrate must have
some mechanism to control the intracellular PCB concentration at sufficient level to
permit cell growth while maintaining below the intracellular toxic levels. Therefore,
the presence of glutathione related transporter proteins in the culture supernatant
might have a role in maintaining the internal PCB concentration at sub-toxic levels.
The protein with the highest concentration found in the secretome was identified as
a sulfate transporter protein (Table 8.4). Sulfur plays several key roles in bacterial
cells as it is a part of some amino acids such as cysteine, methionine and some cellular
cofactors such as biotin, coenzyme A, S-adenosylmethionine, thiamine, glutathione,
and iron-sulfur clusters (Scott et al., 2007). Sulfate is the preferred sulphur source for
most of the organisms (Aguilar-Barajas et al., 2011). The concentration of the sulfate
transporter was analyzed at different time intervals and found that increased
amounts were detected during the aerobic stages compared to lower yields in the
anaerobic stages of both AN and TS conditions (see Table C.2 in Appendix C, and
Figure 8.10). However, role of sulphate transporters in PCB hydrolysis was not clear.

188 Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05
Figure 8.10 The concentration of the sulfate transporter protein detected in the
culture supernatant over time, under the alternating anaerobic-aerobic (AN)
conditions.
Lykidis et al. (2010) reported that the Cupriavidus necator, a Gram negative
bacterium that utilizes a variety of chloroaromatic compounds as its sole carbon and
energy source contains several ABC transporters that are able to transport aromatic
compounds. Aroclor 1260 is a synthetic chlorinated aromatic hydrocarbon mixture
used as the sole source of carbon, but there were no specific classically secreted
aromatic and xenobiotic degradation related proteins identified in the culture
supernatant. However, about 10% of uncategorized proteins were grouped together
and labelled as unclassified (Figure 8.9). In addition, about 10% of the classically
secreted proteins were grouped as ‘hypothetical’ proteins with unknown functions.
It is possible that these unclassified and unknown proteins identified here may form
new and novel secretion systems used by bacteria for growth depending on the
medium and substrates (Maffei et al., 2017).
About 10% of membrane type proteins were predicted as classically secreted
proteins (Table C.1 in Appendix C). However, it was not clear whether they are
actually secreted proteins or false positives, as they are usually not considered as
secreted proteins, but remain within the membranes of the bacterium. It has been

Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05 189
suggested that the SecretomeP server would identify them as secreted proteins due
to the presence of cleavable signal peptide sequences at the N-terminus (Song et al.,
2009). However, Maffei et al. (2017) recently revealed that the lipoproteins that are
fully or partially exposed to the surface occur either through known secretion
systems or by novel mechanisms. Additionally, some lipoproteins were identified as
surface-active amphiphilic metabolites or biosurfactants that play a major role in
bioremediation of hydrophobic chemical pollutants such as PCBs (Basak & Dey,
2015). The biosurfactants found during the initial biosufactant screening in Chapter
5 may have some link to the lipoproteins identified as part of the classically secreted
proteins.
The distribution of the 212 identified as classically secreted proteins at fortnightly
intervals were compared under AN and TS anaerobic-aerobic treatment conditions
(Figure 8.11). Out of 211 proteins identified in the AN conditions, 160 proteins were
found common in weeks 2, 4 and 6, with the highest number of proteins (193)
identified at Week 6 (see Figure 8.11A). 209 proteins were identified in the TS
conditions of which 161 proteins were found common in weeks 2, 4 and 6 (see Figure
8.11B). The highest number of proteins (199) with identifications were present at
week 4 under TS conditions. Even though most (140) of the proteins were found
common in all three fortnightly intervals under both AN and TS conditions, they were
present in different concentrations under each condition as indicated in the heat map
in Table C.1, Appendix C. Sulfate ABC transporter substrate-binding protein and
ethanolamine utilization protein were present only in week 6, under AN conditions.
Proteins involved in ethanolamine utilization (EUTs) have been studied and found to
be part of bacterial micro compartments (BMC) whereby BMC functions to control
metabolic reactions by confining volatile/toxic metabolite intermediates such as
alcohols and acids (Axen et al., 2014; Sturms et al., 2015). In the TS treatment,
ferrichrome ABC transporter substrate-binding protein was absent during the first
four weeks of anaerobic treatment and only present under aerobic condition at week
6. Sixteen out of twenty one common proteins found in week 2 and week 4
(anaerobic phase) of TS treatment were found to be transport related proteins.

190 Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05
Figure 8.11 Venn diagram of the distribution of proteins identified as classically
secreted proteins among (A) alternating (AN) anaerobic-aerobic, and (B) two stage
(TS) anaerobic-aerobic conditions.
8.3.2.2.2 Non-classically secreted proteins
Cytoplasmic proteins lacking typical signal peptides or secretion motifs, but found in
the extracellular environment of bacteria are called non-classically secreted proteins
(Zhao et al., 2017). A total of 87 proteins quantified using SWATH were predicted to
be non-classically secreted proteins by the SecretomeP server (Figure 8.8). However,
the mechanisms and cellular membrane locations and features responsible for non-
classical protein secretion are not well understood (Bendtsen et al., 2005).
Similar to the analysis undertaken for classically secreted proteins, non-classically
secreted proteins were further analyzed based on their potential functions and
outcomes are presented in Figure 8.12. Out of the 87 proteins, 17% (15 proteins)
were grouped as hypothetical proteins, indicative of novel or new proteins. Six non-
classically secreted proteins were identified to be involved in motility while five
proteins were related to transporter proteins.
Only one protein matched to an enzyme called Dienelactone hydrolase in relation to
aromatic and xenobiotic degradation. This enzyme plays a crucial role in microbial
degradation of chloroaromatics via the modified chlorocatechol ortho cleavage
pathway (Schlomann et al., 1993) by catalysing the hydrolysis of dienelactone to
maleylacetate (Park et al., 2010).

Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05 191
Figure 8.12 Functional groupings of proteins identified as non-classically secreted
proteins.
The first published study on non-classical protein secretion in bacteria reported the
secretion of an enzyme called glutamine synthetase (GlnA) (Bendtsen et al., 2005).
The enzymes glutamine synthetase and transglutaminase were detected in the
culture supernatants. These two enzymes may have had a direct impact on the
variation of pH in the culture medium. In the AN anaerobic-aerobic conditions, weeks
1, 3 and 5 were maintained under anaerobic conditions while weeks 2, 4 and 6 were
maintained under aerobic conditions. The starting pH of the medium was set at 7.0.
At each time, when the conditions changed to anaerobic, the pH slightly dropped
(Figure 8.13). However, once conditions were shifted from anaerobic to aerobic, the
pH was found to be closer to its original neutral value of 7, especially in weeks 2, 4
and 6 (Figure 8.13). Glutamine synthetase uses ammonia during the metabolism of
nitrogen by catalysing the condensation of glutamate and ammonia to form
glutamine (Takeo et al., 2013). At each anaerobic phase, the concentration of
glutamine synthetase was relatively high when compared to aerobic phase, indicating
more ammonium ion removal from the medium. The increased glutamine synthetase
concentrations may have coincided with the pH drop under anaerobic conditions as
shown in Figure 8.13. In contrast to glutamine synthetase, the enzyme
transglutaminase concentration was relatively high under each aerobic phase when

192 Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05
compared to anaerobic phases (see heat map in Table C.2, Appendix C). The enzyme
transglutaminase is responsible for catalysing the formation of a crosslink between a
free amine group and the γ-carboxamide group of a protein bound glutamine. The
reaction also produces ammonia (Zhang et al., 2009). Therefore, it can be assumed
that the variation of glutamine synthetase and transglutaminase concentrations
under aerobic and anaerobic conditions may have contributed to the variation of pH
levels in the medium (Figure 8.13).
Figure 8.13 Variation of glutamine synthetase concentration and pH level in the
culture supernatant under alternating anaerobic-aerobic conditions.
The distribution of the 87 non-classically secreted proteins at fortnightly intervals
were also compared under AN and TS anaerobic-aerobic treatment conditions (Figure
8.14). Similar to the classically secreted proteins, a high amount of the released
proteins under both AN and TS conditions were shared among all the fortnightly
collected samples (see Figure 8.14A and Figure 8.14B). However, and perhaps not
surprisingly, the concentrations of each protein in the culture supernatant varied at
different time intervals as shown in the Table C.1, Appendix C. When AN and TS
treatments were compared, the number of proteins shared during weeks 2, 4 and 6

Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05 193
was high in the culture supernatant under the TS treatment (63 proteins) compared
to the AN treatment (51 proteins). Under TS conditions, transglutaminase and
glutamine synthase were absent in anaerobic phase (in week 2 and week 4) and only
present in aerobic phase (week 6).
Figure 8.14 Distribution of non-classically secreted proteins under (A) AN anaerobic-
aerobic treatment, and (B) TS anaerobic-aerobic treatment.
Under both AN and TS conditions, a phospholipid-binding protein, dienelactone
hydrolase family protein, histone-like DNA-binding protein and flagella hook-
associated protein FlgK demonstrated relatively high concentrations throughout the
six weeks study period. An elongation factor protein concentration was relatively
high only during the initial two weeks in both, AN and TS conditions. In the TS
treatment, the only protein detected in week 2 was an efflux RND transporter
periplasmic adaptor subunit. Resistance nodulation division (RND) family
transporters belong to the bacterial efflux pumps and are widespread among Gram
negative bacteria (Nikaido & Takatsuka, 2009). They are known to catalyse and
regulate the active transport of substrates such as antibiotics and chemotherapeutic
agents and therefore may have a similar role during cell growth on PCBs.
8.4 Conclusions
So far, various studies have been conducted to identify the PCB degradation
pathways and the relevant enzymes responsible by analysing the bacterial

194 Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05
proteomes. However, no information is available on the types and potential role of
the enzymes secreted to the extracellular environment during microbial
bioremediation of pollutants such as PCBs. The proteomic approach to investigate
the exoproteome of the bacterial consortium grown on PCBs allowed a detailed study
on the composition and concentration of the proteins extracted from the culture
supernatant, under the AN and TS anaerobic-aerobic cultivation conditions.
During protein visualization using SDS-PAGE, it was revealed that proteins were
detected externally under both anaerobic and aerobic conditions, by the individual
facultative anaerobic bacterial culture members Achromobacter sp. NP03,
Ochrobactrum sp NP04 and Lysinibacillus sp. NP05 while growing on Aroclor 1260 as
the sole source of carbon and energy. These protein results suggested that these
bacteria were capable of secreting proteins or enzymes to the external environment
containing PCBs. In general, protein levels in the extracellular environment under
aerobic conditions were found to be lower than under anaerobic conditions.
Lysinibacillus sp. NP05 under anaerobic conditions demonstrated the highest number
of protein bands among all the samples, although the concentrations were different
for each week. In contrast and in the experiments using the consortium, the second
week of the alternating mode showed relatively high number of protein bands when
compared to the other samples, and this result correlated with the high PCB
degradation rate (49.2±2.5%) observed at week two under the alternating treatment
conditions (Figure 7.3 in Chapter 7).
Analysis of the exoproteome from the bacterial consortium reaction with Aroclor
1260 as the sole source of carbon resulted in the identification of 319 non-secreted,
212 classically secreted and 87 non-classically secreted proteins in the culture
supernatant. These proteins consisted of a heterogeneous group representative of
their diverse functional and cellular roles. Although the contribution of a high number
of cytoplasmic proteins in the extracellular environment is not clear, potentially
resulting from cell lysis or from selective straining, the presence of some proteins
involved in the intermediate steps of the PCB degradation pathways and
detoxification of toxic chemicals is a direct indication for the occurrence of PCB

Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05 195
degradation by the bacterial consortium. Transporters were the largest protein group
that represented 58% of the total classically secreted proteins. However, the
mechanisms for protein or enzyme release, adaptations for cell protection from toxic
pollutants and their specific functions in PCB degradation as an extracellular protein
or enzyme remains to be further investigated.

196 Chapter 8: Metaproteomics analysis of extracellular proteins detected in the culture supernatant during PCB hydrolysis by the bacterial consortium: Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05

Chapter 9: Conclusions, practical applications and recommendations for future research 197
Chapter 9: Conclusions, practical applications and recommendations for future research
9.1 Conclusions
Polychlorinated biphenyls are regarded as legacy pollutants. Even though commercial
production and use ceased in 1993, extremely stable properties have made PCBs
persistent in the environment, leading to a range of problems with ecosystem and
human health. There is a vital need to search for sustainable treatment approaches
to remediate PCB contaminated soil due to the serious shortcomings of current
physical and chemical treatment methods. Development of sustainable and effective
bioremediation techniques using microorganisms have the potential to fulfil this
need.
The effectiveness of microbial remediation of PCB contaminated soil is determined
by number of factors. The degradation rate is mainly dependent on the
environmental conditions, soil characteristics, complexity and severity of PCB
contamination, ability of native or bioaugmented microorganisms to survive and to
degrade the PCB congeners and their metabolic intermediates. As PCBs are usually
manufactured and applied as complex congener mixtures, treatment of
contaminated environments is also a complex process. As a result, bioremediation
approaches generally need a longer time span due to the slow degradation rates.
Therefore, improvement and optimization of existing bioremediation technologies
are essential in order to produce viable and cost effective treatment approaches.
The present research study focused on addressing the knowledge gaps in PCB
bioremediation that have been identified in the research literature. The study was
based on the primary hypotheses that complete degradation of complex
commercially available PCB mixtures can be achieved through a combination of
anaerobic-aerobic treatment when appropriate microbial mixtures which are capable

198 Chapter 9: Conclusions, practical applications and recommendations for future research
of increasing the aqueous solubility of PCBs and degrading PCB congeners under both
anaerobic and aerobic conditions are incorporated.
Accordingly, potential PCB degrading microorganisms were isolated from the natural
environment and their PCB degradation potentials were determined as individuals
and as a consortium under different environmental conditions. The ability of bacteria
to make hydrophobic PCBs soluble in aqueous media and to facilitate the uptake of
PCBs into the cells by releasing extracellular proteins and/or secreted enzymes were
also tested. The findings presented in this thesis details the new knowledge created
to bridge a number of current knowledge gaps in the microbial based bioremediation
of PCBs.
The schematic representation of the summary of major research findings described
in Chapters 4-8, is given in Figure 9.1 and the main conclusions derived from the study
are discussed below in Sections 9.1.1 to 9.1.5.
According to Figure 9.1, PCBs were added into the minimal salts based bacterial
growth medium as the sole source of carbon. Biosurfactants that were released and
detected in the extracellular environment showed a direct impact on increasing the
solubility of hydrophobic PCBs in the aqueous medium by the PCB degrading
microorganisms, as discussed in detailed in Chapter 5. The major proteins that were
found to be related to PCB degradation and detoxification and discussed in Chapter
8 are only shown in the Figure 9.1. The lipoproteins detected in the culture
supernatant may have increased PCB solubilization together with the biosurfactants.
According to the existing literature (see chapters 8.1), PCB degradation is believed to
be an intracellular process and therefore the enzymes responsible for anaerobic
degradation and aerobic oxidation pathways shown inside the cell in the Figure 9.1
would not be expected in the extracellular environment. However, some of the
previously identified enzymes such as dienelactone hydrolase and 3-oxoadipate co A
transferase that are responsible for some of the intermediate steps in PCB
degradation pathways were found outside in the culture supernatant as highlighted
in Figure 9.1 (also see Sections 8.3.2.1 and 8.3.2.2). Relatively high levels of different
oxidoreductases were also found in the culture supernatant as both secretory and

Chapter 9: Conclusions, practical applications and recommendations for future research 199
non-secretory proteins, which are usually engaged in detoxification of toxic
hydrocarbons.
It is postulated that bacteria capable of utilizing PCBs as their growth substrate need
some mechanism to control the intracellular PCB concentration at sufficient level to
permit cell growth while maintaining and controlling the intracellular toxic levels.
Therefore, as shown in Figure 9.1, the presence of some classically secreted
glutathione related transporter proteins in the culture supernatant might have a key
role in maintaining the internal PCB concentration at sub-toxic levels as one of the
main functions of glutathione is protection of cells through the regulation of internal
xenobiotic concentrations by conjugating and exporting them from the cells. Nearly
half of the secretory proteins were related to cellular transport (see Figures 8.9 and
9.1) and they were found to be involved in various cellular functions such as uptake
of nutrients, regulation of cytoplasmic metabolites concentration, and prevention of
the effects of toxins by catalyzing their active efflux.

200 Chapter 9: Conclusions, practical applications and recommendations for future research
Figure 9.1 A schematic representation of the summary of major findings of the research study.

Chapter 9: Conclusions, practical applications and recommendations for future research 201
9.1.1 Isolation, screening and identification of potential PCB degrading
microorganisms
As discussed in Chapter 4, the selective enrichment screening was done to isolate and
identify the potential PCB degrading microorganisms from the natural environment.
The main conclusions derived are as follows:
• From 11 microorganisms initially tested, two obligate aerobic and four
facultative anaerobic bacterial strains that can utilize commercial PCB mixture,
Aroclor 1260 as their sole source of carbon were isolated from the natural
environment.
• They were identified as Chryseobacterium sp. NP01, Delftia sp. NP02,
Achromobacter sp. NP03, Ochrobactrum sp. NP04, Lysinibacillus sp. NP05 and
Pseudomonas sp. NP06 using 16S rRNA gene sequence based molecular
identification.
• This finding is also the first evidence of reporting the PCB degradation ability of
Chryseobacterium and Delftia species.
9.1.2 Screening of bacterial isolates for their ability to produce biosurfactants to
make hydrophobic PCBs soluble in aqueous media
As discussed in Chapter 5, six bacterial cultures isolated during Chapter 4 were further
tested for their ability to produce biosurfactants as one of the limitations in biological
breakdown is poor bioavailability due to the extreme hydrophobic nature of PCBs. The
main conclusions from chapter 5 are as follows:
• Microorganisms capable of degrading PCBs discovered in this study, were also
found to have the potential to produce some surface active substances to facilitate
the enhancement of solubility of the hydrophobic PCBs.
• The PCB solubility results was positively correlated with the biosurfactant
production.

202 Chapter 9: Conclusions, practical applications and recommendations for future research
• Chryseobacterium sp. NP01 and Lysinibacillus sp. NP05 demonstrated the highest
biosurfactant production, PCB solubility and chloride ion accumulation when
compared to Delftia sp. NP02, Achromobacter sp. NP03, Ochrobactrum sp. NP04
and Pseudomonas sp. NP06
9.1.3 Comparison of the individual facultative anaerobic bacterial isolates during
PCB hydrolysis under aerobic, anaerobic and two stage anaerobic-aerobic
conditions
PCB degradation efficiency of four facultative anaerobic culture members, namely,
Achromobacter sp. NP03, Ochrobactrum sp. NP04, Lysinibacillus sp. NP05 and
Pseudomonas sp. NP06 were compared under aerobic, anaerobic and combined
anaerobic-aerobic conditions for their PCB degradation potential as discussed in Chapter
6. The significant conclusions of the Chapter 6 are as follows:
• Based on the review of publicly available literature, this is the first comparative
study assessing the capability of facultative anaerobic bacteria for degrading PCBs
under anaerobic, aerobic and two-stage anaerobic-aerobic cultivation conditions.
• All four facultative anaerobic bacterial strains were found to be capable of
degrading the commercial PCB mixture, Aroclor 1260 as a sole source of carbon
under both, anaerobic and aerobic conditions at varying degrees.
• All four stains showed limitations in growth under aerobic conditions. This is
attributed to the low number of lower chlorinated congeners available in the PCB
mixture. In contrast, the growth increased under anaerobic conditions, suggesting
the microbes were capable in attacking the biphenyl ring and used PCBs as their
carbon source.
• The highest chloride ion accumulation from all the four facultative anaerobic
cultures was observed under two stage anaerobic-aerobic conditions, suggesting
the dechlorination of highly chlorinated congeners under anaerobic conditions
occurred first, followed by the degradation of the resulting lower chlorinated
congeners under aerobic conditions.

Chapter 9: Conclusions, practical applications and recommendations for future research 203
• A maximum chloride yield of 9.16±0.8 mg/L was observed for Lysinibacillus sp.
NP05 under the two stage anaerobic-aerobic treatment. This value corresponded
to one third of the total chlorine present in the Aroclor 1260 mixture indicating
33% total chlorine removal.
• In field scale soil remediation applications, facultative microorganisms have the
potential as better candidates as they can survive and degrade PCBs under both,
anaerobic and aerobic conditions while achieving high PCB degradation rates.
• Out of the four facultative anaerobic microorganisms, Lysinibacillus sp. NP05 was
found to have high potential as a single candidate to effectively dechlorinate the
highly chlorinated complex PCB mixture, Aroclor 1260.
9.1.4 Comparison of the bacterial consortium during PCB hydrolysis under two
modes of combined anaerobic-aerobic treatments
According to previous studies, when the two stage (or combined) anaerobic-aerobic
treatment was employed to treat the PCBs, a relatively long anaerobic phase (from 70 -
180 days) followed by a short aerobic phase (from 28-60 days) is the usual approach used
as anaerobic degradation is believed to be a slow process. In addition to that, anaerobic
PCB dechlorinating bacteria have been used under the anaerobic phase while only
aerobic PCB degrading bacteria were used under the aerobic phase. In Chapter 7, a
conventional two stage anaerobic-aerobic treatment (TS) with lengthy anaerobic phase
(of four weeks) followed by short aerobic phase (of two weeks) was compared to the
alternating anaerobic-aerobic-aerobic treatment (AN) having equally short anaerobic
and aerobic phases (one week each). Based on the results obtained in Chapters 5 and 6,
the bacterial consortium was developed consisting of Achromobacter sp. NP03,
Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05 and used in the comparison of TS and
AN anaerobic-aerobic treatments. The major conclusions derived from Chapter 7 are
summarized below:
• Based on the review of publicly available literature, this is the first comparative
study assessing the PCB degradation efficiency under two different combined
anaerobic-aerobic modes; alternating (AN) and two stage (TS), using a consortium

204 Chapter 9: Conclusions, practical applications and recommendations for future research
of three facultative anaerobic bacteria Achromobacter sp. NP03, Ochrobactrum sp.
NP04 and Lysinibacillus sp. NP05 that were found capable of degrading PCBs under
both, anaerobic and aerobic conditions.
• In both treatments, lower chlorinated PCB homolog groups exhibited greater
degradation capability under aerobic conditions when compared to highly
chlorinated homolog groups, although the degradation rates were always high
under alternating conditions.
• Even after the PCB degradation reached a plateau at week two, continuous
increases in bacterial cell density and chloride ion concentration in the culture
medium under alternating conditions highlighted the ability of the consortium
member/s to further breaking down the intermediate products.
• The alternating treatment (AN) was found to be the preferred method over the
two stage (TS) treatment with nearly 50% reduction in total PCBs within the first
two weeks when compared to 24% reduction achieved under the two stage
treatment.
• The switching between short anaerobic and aerobic phases at weekly intervals
under alterating conditions favoured the consortium to change in between
dechlorination and oxidation. This alternating switching between two phases
facilitated the overall PCB degradation rate due to increased PCB degradation
under aerobic phases through ring cleavage and hydrolysis of lower chlorinated
PCB congeners that were generated during the initial anaerobic phases.
9.1.5 Analysing and characterization of proteins detected in the culture
supernatants during PCB degradation
Various studies have been conducted to-date to identify the PCB degradation
pathways and the relevant enzymes responsible by analysing the bacterial
proteomes. However, knowledge is limited about the types and roles of proteins
and/or enzymes secreted into the extracellular environment during PCB degradation.
Therefore, research discussed in Chapter 8 aimed to investigate the composition,

Chapter 9: Conclusions, practical applications and recommendations for future research 205
concentration and characteristics of extracellular proteins released by three PCB
degrading facultative anaerobic bacteria as a consortium under alternating and two
stage anaerobic-aerobic-aerobic experimental conditions and to relate them to PCB
degradation data. The important conclusions derived from the extracellular protein
analysis are given below:
• During protein visualization, it was found that under both anaerobic and
aerobic conditions, three facultative anaerobic bacterial culture members
Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05
were individually capable of secreting proteins into the liquid medium at
varying levels while Lysinibacillus sp. NP05 under anaerobic conditions
demonstrated the highest number of protein bands.
• From a metaproteomics approach, a total of 618 proteins consisted of a
heterogeneous group, representative of their diverse functional and cellular
roles including 319 non-secreted, 212 classically secreted and 87 non-
classically secreted proteins were identified in the culture supernatant of the
bacterial consortium.
• Transport related proteins represented 58% of the total classically secreted
proteins. This is a significant finding because bacterial transport proteins play
an important role in facilitating the uptake of essential nutrients, regulate the
cytoplasmic concentrations of metabolites, export large molecules to the
outer surface of the cell and prevent toxic effects of toxins by catalyzing their
active efflux.
• The presence of large number of non-secretory proteins (n=319) in the
extracellular environment was not clear whether it was from cell lysis or from
any other cellular mechanism such as selective straining.
• The occurrence and identification of some proteins involved in the
intermediate steps of the known PCB degradation pathways, regulation of
internal xenobiotic concentration by conjugating and exporting excess
xenobiotic from the cells, detoxification of toxic chemicals and allows
microorganisms to use aromatic hydrocarbons as their sole source of carbon

206 Chapter 9: Conclusions, practical applications and recommendations for future research
and energy was a direct indication of the occurrence of PCB degradation by
the bacterial consortium.
9.2 Practical applications of research outcomes
The objective of the research study was to identify suitable microorganisms from the
natural environment that are capable of solubilizing and degrading complex PCB
mixtures under varying anaerobic and aerobic conditions to effectively treat the
contaminated soil. Although many studies have been conducted to assess the possibility
of using bioremediation as a biological treatment option to treat the soils contaminated
with PCBs, bioremediation is still not widely practiced as a field scale remediation option
due to various challenges.
In biological degradation of PCBs, two main processes are fundamentally involved,
namely, anaerobic dechlorination and aerobic oxidation. Highly chlorinated PCB
congeners first need to be dechlorinated into lower chlorinated congeners. Under
anaerobic conditions, dechlorinating bacteria are usually involved in this step. The next
step is partial or complete degradation of lower chlorinated congeners, which is an
oxidative process and aerobic PCB degrading bacteria are responsible for the conversion.
The studies undertaken in the past have mainly reported on the use of two separate
groups of microorganisms when both anaerobic dechlorination and aerobic oxidation
steps are involved in combination. These microorganisms and process conditions are
referred to as, anaerobic dechlorinating microorganisms under anaerobic conditions and
aerobic degradative microorganisms under aerobic conditions. However, both,
anaerobic and aerobic conditions are present in the natural soil environment and
changes in the environmental conditions can lead to death of PCB degrading
microorganisms introduced to the soil if they cannot survive under varying oxygen levels.
During the present study, it was found that there are some facultative anaerobic
microorganisms in the natural environment that can survive and carryout PCB
degradation successfully under both anaerobic and aerobic conditions simultaneously.
This novel finding can be incorporated into bioremediation based soil decontamination
applications.

Chapter 9: Conclusions, practical applications and recommendations for future research 207
The existing studies on the combined anaerobic and aerobic treatments usually use a
prolonged initial anaerobic phase followed by short aerobic phase assuming that the
anaerobic dechlorination is a slow process, and therefore requires a long period ranging
from 70 to 180 days to achieve the expected outcome. However, in the present study,
application of equally short alternating anaerobic and aerobic phases demonstrated
higher PCB degradation rates within two weeks compared to the conventional two stage
treatments having lengthy anaerobic phase typically in months, followed by a short
aerobic phase. This research outcome will create new opportunities to overcome one of
the major limitations in bioremediation by reducing the extended time required for
conventional bioremediation applications.
Extreme hydrophobic nature of PCB mixtures is another rate limiting factor that hinders
the effective biodegradation as it reduces the bioavailability of PCBs to the potential
degrading bacterial community. Research has been undertaken to improve the aqueous
solubility of PCBs by adding chemical surfactants and biosurfactants to the soil. However,
such applications were limited due to the toxicity of chemical surfactants to the PCB
degrading and other native soil microbial community and to the high cost associated with
commercially available biosurfactants. In the present study, some of the
microorganisms, which were identified as having PCB degradation potential, were also
found to be capable of making the hydrophobic PCBs soluble in aqueous medium by
producing biosurfactants. This is an added advantage in the field of bioremediation as
the appropriate microbial mixture having both degradation and solubilization capabilities
can replace the addition of toxic chemicals or biological surfactants externally.
In summary, the outcomes from this research study can be incorporated in future
bioremediation applications to effectively treat soils contaminated with PCBs and other
similar toxic xenobiotic compounds.
9.3 Recommendations for future research
The outcomes of this research study have contributed new knowledge on
bioremediation of contaminated environments by PCBs. In order to further consolidate
the outcomes of this study, the following additional research studies are recommended.

208 Chapter 9: Conclusions, practical applications and recommendations for future research
Based on the review of publicly available literature, the present study is the first to
identify the facultative anaerobic microorganisms with PCB degradation ability under
both anaerobic and aerobic conditions. However, total PCB degradation was
determined using PCB homolog group concentration and chloride ion accumulation.
Therefore, in future studies, incorporation of the analysis of PCB degradation at
individual congener level is recommended to understand the potential pathways
used by microorganisms during PCB degradation.
In the present study, a commercial PCB mixture, Aroclor 1260 was used as the sole source
of carbon for PCB degrading microorganisms. According to the literature, during the
anaerobic PCB dechlorination, the biphenyl ring is usually not cleaved unless the
microorganisms are capable of utilizing PCBs as their carbon source. Therefore, providing
a suitable carbon source is essential for the microorganisms for their survival while
carrying out PCB dechlorination. Even under aerobic conditions, addition of a desirable
carbon source was shown to enhance the mineralization of PCBs through co-metabolism.
Therefore, providing a suitable secondary carbon source, which can be utilized by all the
members in the bacterial consortium, could enhance the PCB degradation rate. During
the screening for potential secondary carbon sources, L-proline, L-lactic acid and methyl
pyruvate were identified as sources that can be utilized by all three consortium
members. In addition, L-proline was found to be utilized by all the bacterial strains used
in the consortium as their nitrogen source. Therefore, it needs to be further confirmed
by additional research to see whether the addition of secondary carbon sources will
either stimulate or inhibit PCB degradation and their capability in field scale remediation
applications.
The reason for the increased solubility of hydrophobic PCB mixture in the aqueous
medium over time was found to be due to the biosurfactants produced by the PCB
degrading bacterial strains. However, as this study was an initial screening to see the
potential of isolated PCB degrading microorganisms for biosurfactant production, further
research is needed to identify the types and physico-chemical properties of the
biosurfactants through extraction, quantification and characterization and to assess their
performance in PCB contaminated soil.

Chapter 9: Conclusions, practical applications and recommendations for future research 209
The study found that the alternating approach was more efficient compared to the two
stage treatment suggesting the switching between short anaerobic and aerobic phases
of weekly intervals favoured the consortium to changing between dechlorination and
oxidation. However, the sampling frequency in this study under both treatment
conditions was limited to fortnightly intervals and PCB degradation analysis were limited
to total PCB degradation based on the PCB homolog concentration. Therefore, it is
recommended to closely monitor the behaviour of PCB degradation and product build
up under each anaerobic and aerobic phase at daily intervals to get detailed information
to further confirm the scientific principles behind the alternating approach.
Generally, a project of this nature has to be undertaken in the laboratory first and
then transferred to the field. As the present study was limited to liquid based
experiments, it is highly recommended to study the performance of bacterial strains on
PCB degradation and variations in composition profile of the bacterial consortium in PCB
contaminated soil under alternating anaerobic-aerobic conditions.

210 Chapter 9: Conclusions, practical applications and recommendations for future research

References 211
References
Abdel-Mawgoud, A. M., Lepine, F., & Deziel, E. (2010). Rhamnolipids: Diversity of
structures, microbial origins and roles. Applied microbiology and
biotechnology, 86(5), 1323-1336. doi:http://dx.doi.org/10.1007/s00253-010-
2498-2
Abraham, W. R., Meyer, H., & Yakimov, M. (1998). Novel glycine containing
glucolipids from the alkane using bacterium alcanivorax borkumensis.
Biochimica et Biophysica Acta (BBA)-Lipids and Lipid Metabolism, 1393(1), 57-
62.
Abraham, W. R., Nogales, B., Golyshin, P. N., Pieper, D. H., & Timmis, K. N. (2002).
Polychlorinated biphenyl-degrading microbial communities in soils and
sediments. Current Opinion in Microbiology, 5(3), 246-253.
doi:http://dx.doi.org/10.1016/S1369-5274(02)00323-5
Adrian, L., Dudkova, V., Demnerova, K., & Bedard, D. L. (2009). “Dehalococcoides” sp.
Strain CBDB1 extensively dechlorinates the commercial polychlorinated
biphenyl mixture aroclor 1260. Applied and environmental microbiology,
75(13), 4516-4524. doi:https://doi.org/10.1128/AEM.00102-09
Aguilar-Barajas, E., Diaz-Perez, C., Ramirez-Diaz, M. I., Riveros-Rosas, H., & Cervantes,
C. (2011). Bacterial transport of sulfate, molybdate, and related oxyanions.
Biometals, 24(4), 687-707. doi:http://dx.doi.org/10.1007/s10534-011-9421-x
Agullo, L., Pieper, D. H., & Seeger, M. (2017). Genetics and biochemistry of biphenyl
and PCB biodegradation. In R. Fernando (Ed.), Aerobic utilization of
hydrocarbons, oils and lipids (pp. 1-28): Springer International Publishing.
Ahmad, M., & Ahmad, I. (2014). Recent advances in the field of bioremediation. In
Biodegradation and bioremediation (Vol. 11, pp. 1-42): Studium Press, LLC.
Aken, B. V., Correa, P. A., & Schnoor, J. L. (2009). Phytoremediation of polychlorinated
biphenyls: New trends and promises. Environmental science & technology,
44(8), 2767-2776. doi:http://dx.doi.org/10.1021/es902514d

212 References
Al-Mailem, D. M., Eliyas, M., & Radwan, S. S. (2018). Ferric sulfate and proline
enhance heavy-metal tolerance of halophilic/halotolerant soil
microorganisms and their bioremediation potential for spilled-oil under
multiple stresses. Frontiers in microbiology, 9, 394.
doi:http://dx.doi.org/10.3389/fmicb.2018.00394
Allocati, N., Federici, L., Masulli, M., & Di Ilio, C. (2009). Glutathione transferases in
bacteria. The FEBS journal, 276(1), 58-75.
doi:http://dx.doi.org/10.1111/j.1742-4658.2008.06743.x
Altenschmidt, U., & Fuchs, G. (1992). Novel aerobic 2-aminobenzoate metabolism:
Purification and characterization of 2-aminobenzoate-coA ligase, localisation
of the gene on a 8-kbp plasmid, and cloning and sequencing of the gene from
a denitrifying Pseudomonas sp. . European. Journal of Biochemistry, 205, 721-
727.
Alvarez, V. M., Jurelevicius, D., Marques, J. M., de Souza, P. M., de Araujo, L. V.,
Barros, T. G., de Souza, R. O. M. A., Freire, D. M. G., & Seldin, L. (2015). Bacillus
amyloliquefaciens TSBSO 3.8, a biosurfactant-producing strain with
biotechnological potential for microbial enhanced oil recovery. Colloids and
Surfaces B: Biointerfaces, 136, 14-21.
doi:http://dx.doi.org/10.1016/j.colsurfb.2015.08.046
Ancona, V., Barra Caracciolo, A., Grenni, P., Di Lenola, M., Campanale, C., Calabrese,
A., Uricchio, V. F., Mascolo, G., & Massacci, A. (2016). Plant-assisted
bioremediation of a historically PCB and heavy metal-contaminated area in
Southern Italy. New Biotechnology.
doi:https://doi.org/10.1016/j.nbt.2016.09.006
Anyasi, R. O., & Atagana, H. I. (2014). Phytotreatment of polychlorinated biphenyls
contaminated soil by Chromolaena odorata (L) king and robinson.
International Journal of Environmental Pollution & Remediation, 1929, 2732.
doi:http://dx.doi.org/10.11159/ijepr.2014.011
Aparna, A., Srinikethan, G., & Smitha, H. (2012). Production and characterization of
biosurfactant produced by a novel Pseudomonas sp. 2B. Colloids and Surfaces

References 213
B: Biointerfaces, 95 (Supplement C), 23-29.
doi:https://doi.org/10.1016/j.colsurfb.2012.01.043
APHA. (2012). Standard methods for the examination of water and wastewater, 22nd
edn. : American Public Health Association, Washington, DC.
Armengaud, J., Christie-Oleza, J. A., Clair, G., Malard, V., & Duport, C. (2012).
Exoproteomics: Exploring the world around biological systems. Expert review
of proteomics, 9(5), 561-575. doi:http://dx.doi.org/10.1586/EPR.12.52
Arulpriya, P., & Lalitha, P. (2013). Evaluation of different extraction methods for
optimization of extraction of aerial roots of Rhaphidophora aurea entwined
over two diverse host trees. International Journal of ChemTech Research, 5(5),
2173-2176.
Asad, S. A. (2017). Soil–PCB–PGPR interactions in changing climate scenarios. In
Xenobiotics in the soil environment (pp. 281-298): Springer.
Aslund, M. L. W., Zeeb, B. A., Rutter, A., & Reimer, K. J. (2007). In situ phytoextraction
of polychlorinated biphenyl - (PCB) contaminated soil. Sci Total Environ,
374(1), 1-12. doi:http://dx.doi.org/10.1016/j.scitotenv.2006.11.052
ASTDR. (2014). ATSDR case studies in environmental medicine polychlorinated
biphenyls (PCBs) toxicity. Agency for Toxic Substances and Disease Registry,
U.S. Department of Health and Human Services.
Atago, Y., Shimodaira, J., Araki, N., Bin Othman, N. a., Zakaria, Z., Fukuda, M., Futami,
J., & Hara, H. (2016). Identification of novel extracellular protein for
PCB/biphenyl metabolism in Rhodococcus jostii RHA1. Bioscience,
biotechnology, and biochemistry, 80(5), 1012-1019.
doi:http://dx.doi.org/10.1080/09168451.2015.1127134
Atlas, R. M. (2005). Handbook of media for environmental microbiology (2 ed.): CRC
press.
ATSDR. (2000). Toxicological profile for polychlorinated biphenyls (PCBs). Division of
Toxicology/Toxicology Information Branch, U.S. Department of Health and
human Services.

214 References
Axen, S. D., Erbilgin, O., & Kerfeld, C. A. (2014). A taxonomy of bacterial
microcompartment loci constructed by a novel scoring method. PLoS
computational biology, 10(10), e1003898.
doi:http://dx.doi.org/10.1371/journal.pcbi.1003898
Backe, C., Cousins, I. T., & Larsson, P. (2004). PCB in soils and estimated soil–air
exchange fluxes of selected PCB congeners in the south of Sweden.
Environmental Pollution, 128(1–2), 59-72.
doi:http://dx.doi.org/10.1016/j.envpol.2003.08.038
Bairoch, A., & Apweiler, R. (2000). The SWISS-PROT protein sequence database and
its supplement TrEMBL in 2000. Nucleic acids research, 28(1), 45-48.
doi: https://doi.org/10.1093/nar/28.1.45
Banat, I. M., Makkar, R. S., & Cameotra, S. (2000). Potential commercial applications
of microbial surfactants. Applied microbiology and biotechnology, 53(5), 495-
508.
Barthomolew, J. W., & Mittwer, T. (1952). The Gram stain. Bacteriological Review 16,
1-29.
Basak, B., & Dey, A. (2015). Bioremediation approaches for recalcitrant pollutants:
Potentiality, success and limitation. In A. K. Rathoure (Ed.), Toxicity and waste
management using bioremediation (pp. 178-197): IGI Global.
Batterman, S., Chernyak, S., Gouden, Y., Hayes, J., Robins, T., & Chetty, S. (2009). PCBs
in air, soil and milk in industrialized and urban areas of Kwazulu-natal, South
Africa. Environmental Pollution, 157(2), 654-663.
doi:http://dx.doi.org/10.1016/j.envpol.2008.08.015
Bedard, D. L., Bailey, J. J., Reiss, B. L., & Jerzak, G. V. S. (2006). Development and
characterization of stable sediment-free anaerobic bacterial enrichment
cultures that dechlorinate Aroclor 1260. Applied & Environmental
Microbiology, 72(4), 2460-2470.
doi:http://dx.doi.org/10.1128/AEM.72.4.2460-2470.2006

References 215
Bedard, D. L., & May, R. J. (1995). Characterization of the polychlorinated biphenyls
in the sediments of woods pond: Evidence for microbial dechlorination of
Aroclor 1260 in situ. Environmental science & technology, 30(1), 237-245.
Bedard, D. L., Ritalahti, K. M., & Loffler, F. E. (2007). The dehalococcoides population
in sediment-free mixed cultures metabolically dechlorinates the commercial
polychlorinated biphenyl mixture Aroclor 1260. Applied and environmental
microbiology, 73(8), 2513-2521. doi:http://dx.doi.org/10.1128/AEM.02909-
06
Bell, T. H., Joly, S., Pitre, F. E., & Yergeau, E. (2014). Increasing phytoremediation
efficiency and reliability using novel omics approaches. Trends in
Biotechnology, 32(5), 271-280.
doi:http://dx.doi.org/10.1016/j.tibtech.2014.02.008
Bendtsen, J. D., Kiemer, L., Fausboll, A., & Brunak, S. (2005). Non-classical protein
secretion in bacteria. BMC Microbiology, 5, 58-58. Retrieved from
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1266369/.
doi:10.1186/1471-2180-5-58
Bentum, J. K., Dodoo, D. K., Kwakye, P. K., Essumang, D. K., & Adjei, G. A. (2016).
Spatial and temporal distribution of polychlorinated biphenyl residues in
tropical soils. Open Journal of Applied Sciences, 6(04), 234.
doi:http://dx.doi.org/10.4236/ojapps.2016.64024
Beyer, A., & Biziuk, M. (2009). Environmental fate and global distribution of
polychlorinated biphenyls. In D. M. Whitacre (Ed.), Reviews of environmental
contamination and toxicology (Vol. 201, pp. 137-158): Springer US.
Bodour, A. A., Drees, K. P., & Maier, R. M. (2003). Distribution of biosurfactant-
producing bacteria in undisturbed and contaminated arid southwestern soils.
Applied & Environmental Microbiology, 69(6), 3280-3287.
doi:http://dx.doi.org/10.1128/AEM.69.6.3280–3287.2003
Bodour, A. A., Guerrero-Barajas, C., Jiorle, B. V., Malcomson, M. E., Paull, A. K.,
Somogyi, A., Trinh, L. N., Bates, R. B., & Maier, R. M. (2004). Structure and
characterization of flavolipids, a novel class of biosurfactants produced by

216 References
Flavobacterium sp. strain MTN11. Applied and environmental microbiology,
70(1), 114-120. doi:http://dx.doi.org/10.1128/AEM.70.1.114–120.2004
Boopathy, R. (2000). Factors limiting bioremediation technologies. Bioresource
Technology, 74(1), 63-67.
Borja, J., Taleon, D. M., Auresenia, J., & Gallardo, S. (2005). Polychlorinated biphenyls
and their biodegradation. Process Biochemistry, 40(6), 1999-2013.
doi:http://dx.doi.org/10.1016/j.procbio.2004.08.006
Brazova, T., Hanzelova, V., Miklisova, D., Salgovicova, D., & Turcekova, L. (2012).
Biomonitoring of polychlorinated biphenyls (PCBs) in heavily polluted aquatic
environment in different fish species. Environmental monitoring and
assessment, 184(11), 6553-6561. doi:http://dx.doi.org/10.1007/s10661-011-
2440-9
Breivik, K., Sweetman, A., Pacyna, J. M., & Jones, K. C. (2007). Towards a global
historical emission inventory for selected PCB congeners — a mass balance
approach: 3. An update. Science of The Total Environment, 377(2–3), 296-307.
doi:http://dx.doi.org/10.1016/j.scitotenv.2007.02.026
Burd, G., & Ward, O. (1996). Physicochemical properties of pm-factor, a surface-
active agent produced by Pseudomonas marginalis. Canadian journal of
microbiology, 42(3), 243-251.
Busset, G., Sangely, M., Montrejaud-Vignoles, M., Thannberger, L., & Sablayrolles, C.
(2012). Life cycle assessment of polychlorinated biphenyl contaminated soil
remediation processes. The International Journal of Life Cycle Assessment,
17(3), 325-336. doi:http://dx.doi.org/10.1007/s11367-011-0366-7
Caccia, D., Dugo, M., Callari, M., & Bongarzone, I. (2013). Bioinformatics tools for
secretome analysis. Biochimica et Biophysica Acta (BBA) - Proteins and
Proteomics, 1834(11), 2442-2453.
doi:http://dx.doi.org/10.1016/j.bbapap.2013.01.039
Cagnetta, G., Robertson, J., Huang, J., Zhang, K., & Yu, G. (2016). Mechanochemical
destruction of halogenated organic pollutants: A critical review. Journal of

References 217
Hazardous Materials, 313, 85-102.
doi:http://dx.doi.org/10.1016/j.jhazmat.2016.03.076
Calvo, C., Silva-Castro, G., Uad, I., Fandiño, C. G., Laguna, J., & Gonzalez-Lopez, J.
(2008). Efficiency of the eps emulsifier produced by Ochrobactrum anthropi
in different hydrocarbon bioremediation assays. Journal of industrial
microbiology & biotechnology, 35(11), 1493-1501.
doi:https://doi.org/10.1007/s10295-008-0451-5
Camara, B., Herrera, C., Gonzalez, M., Couve, E., Hofer, B., & Seeger, M. (2004). From
PCBs to highly toxic metabolites by the biphenyl pathway. Environmental
Microbiology, 6(8), 842-850. doi:http://dx.doi.org/10.1111/j.1462-
2920.2004.00630.x
Camel, V. (2000). Microwave-assisted solvent extraction of environmental samples.
TrAC Trends in Analytical Chemistry, 19(4), 229-248.
doi:http://dx.doi.org/10.1016/S0165-9936(99)00185-5
CCME. (1999). Canadian soil quality guidelines for the protection of environmental
and human health: Polychlorinated biphenyls (total). Canadian Council of
Ministers of the Environment, Winnipeg.
Characklis, W. G., & Cooksey, K. E. (1983). Biofilms and microbial fouling. In A. I. Laskin
(Ed.), Advances in applied microbiology (Vol. 29, pp. 93-138): Academic Press.
Chen, C., Yu, C., Shen, C., Tang, X., Qin, Z., Yang, K., Hashmi, M. Z., Huang, R., & Shi,
H. (2014). Paddy field – a natural sequential anaerobic–aerobic bioreactor for
polychlorinated biphenyls transformation. Environmental Pollution, 190(0),
43-50. doi:http://dx.doi.org/10.1016/j.envpol.2014.03.018
Chen, F., Hao, S., Qu, J., Ma, J., & Zhang, S. (2015a). Enhanced biodegradation of
polychlorinated biphenyls by defined bacteria-yeast consortium. Annals of
Microbiology, 65(4), 1847-1854. doi:http://dx.doi.org/10.1007/s13213-014-
1023-8
Chen, Y., Huang, Q., Chen, Q., Lin, Y., Sun, X., Zhang, H., Zhu, M., & Dong, S. (2015b).
The inflammation and estrogen metabolism impacts of polychlorinated

218 References
biphenyls on endometrial cancer cells. Toxicology in Vitro, 29(2), 308-313.
doi:http://dx.doi.org/10.1016/j.tiv.2014.11.008
Chovanec, P., Basu, P., & Stolz, J. F. (2011). Application of proteomics in
bioremediation. In Microbial metal and metalloid metabolism (pp. 247-259):
American Society of Microbiology.
Christie-Oleza, J. A., Pina-Villalonga, J. M., Bosch, R., Nogales, B., & Armengaud, J.
(2012). Comparative proteogenomics of twelve roseobacter exoproteomes
reveals different adaptive strategies among these marine bacteria. Molecular
& Cellular Proteomics, 11(2), M111. 013110.
doi:http://dx.doi.org/10.1074/mcp.M111.013110–1
Chuanmin, C., Xuan, L., Tao, C., & Songtao, L. (2015). The detection and remediation
technologies of PCB-contaminated soils. Applied Mechanics & Materials, 768.
doi:https://doi.org/10.4028/www.scientific.net/AMM.768.212
Cole, S. P. C., & Deeley, R. G. (2006). Transport of glutathione and glutathione
conjugates by MRP1. Trends in Pharmacological Sciences, 27(8), 438-446.
doi:https://doi.org/10.1016/j.tips.2006.06.008
Creaser, C. S., Fernandes, A. R., Harrad, S. J., Hurst, T., & Cox, E. A. (1989). Background
levels of polychlorinated biphenyls in British soils - ii. Chemosphere, 19(8),
1457-1466. doi:http://dx.doi.org/10.1016/0045-6535(89)90094-5
Dai, S., Vaillancourt, F. H., Maaroufi, H., Drouin, N. M., Neau, D. B., Snieckus, V., Bolin,
J. T., & Eltis, L. D. (2002). Identification and analysis of a bottleneck in PCB
biodegradation. Nature Structural and Molecular Biology, 9(12), 934.
doi:http://dx.doi.org/10.1038/nsb866
Davila, B., Whitford, K., & Saylor, E. (1993). Technology alternatives for the
remediation of PCB-contaminated soil and sediment: US Environmental
Protection Agency, Center for Environmental Research Information.
Demirtepe, H., Kjellerup, B., Sowers, K. R., & Imamoglu, I. (2015). Evaluation of PCB
dechlorination pathways in anaerobic sediment microcosms using an
anaerobic dechlorination model. Journal of Hazardous Materials, 296(0), 120-
127. doi:http://dx.doi.org/10.1016/j.jhazmat.2015.04.033

References 219
Dercova, K., Cicmanova, J., Lovecka, P., Demnerova, K., Mackova, M., Hucko, P., &
Kusnir, P. (2008). Isolation and identification of PCB-degrading
microorganisms from contaminated sediments. International
Biodeterioration & Biodegradation, 62(3), 219-225.
doi:http://dx.doi.org/10.1016/j.ibiod.2008.01.016
Desborough, J., Evans, T., Muller, J., & Harrad, S. (2016). Polychlorinated biphenyls
(PCBs), hexabromocyclododecanes (HBCDDs) and degradation products in
topsoil from Australia and the United Kingdom. Emerging Contaminants, 2(1),
37-41. doi:http://dx.doi.org/10.1016/j.emcon.2016.03.003
Desvaux, M., Hebraud, M., Talon, R., & Henderson, I. R. (2009). Secretion and
subcellular localizations of bacterial proteins: A semantic awareness issue.
Trends in microbiology, 17(4), 139-145.
doi:https://doi.org/10.1016/j.tim.2009.01.004
Di Toro, S., Zanaroli, G., & Fava, F. (2006). Intensification of the aerobic
bioremediation of an actual site soil historically contaminated by
polychlorinated biphenyls (PCBs) through bioaugmentation with a non
acclimated, complex source of microorganisms. Microbial cell factories, 5(1),
11. doi:http://dx.doi.org/10.1186/1475-2859-5-11
Dudasova, H., Lukacova, L., Murinova, S., Puskarova, A., Pangallo, D., & Dercova, K.
(2014). Bacterial strains isolated from PCB-contaminated sediments and their
use for bioaugmentation strategy in microcosms. Journal of basic
microbiology, 54(4), 253-260. doi:https://doi.org/10.1002/jobm.201200369
Eapen, S., Singh, S., & D'Souza, S. F. (2007). Advances in development of transgenic
plants for remediation of xenobiotic pollutants. Biotechnology Advances,
25(5), 442-451. doi:https://doi.org/10.1016/j.biotechadv.2007.05.001
Edwards, S. J., & Kjellerup, B. V. (2013). Applications of biofilms in bioremediation and
biotransformation of persistent organic pollutants, pharmaceuticals/personal
care products, and heavy metals. Applied microbiology and biotechnology,
97(23), 9909-9921. doi:http://dx.doi.org/10.1007/s00253-013-5216-z

220 References
Eichelberger, J. W., Behymer, T. D., Budde, W. L., & Munch, J. W. (1995). Method
525.2, determination of organic compounds in drinking water by liquid-solid
extraction and capillary column gas chromatography Mass spectrometry.
National Exposure Research Laboratory Office of Research and Development
USEPA.
Eulberg, D., Lakner, S., Golovleva, L. A., & Schlömann, M. (1998). Characterization of
a protocatechuate catabolic gene cluster from Rhodococcus opacus 1CP:
Evidence for a merged enzyme with 4-carboxymuconolactone-
decarboxylating and 3-oxoadipate enol-lactone-hydrolyzing activity. Journal
of bacteriology, 180(5), 1072-1081.
Evans, B. S., Dudley, C. A., & Klasson, K. T. (1996). Sequential anaerobic-aerobic
biodegradation of PCBs in soil slurry microcosms. Applied biochemistry and
biotechnology, 57(1), 885-894.
Fava, F., & Di Gioia, D. (2001). Soya lecithin effects on the aerobic biodegradation of
polychlorinated biphenyls in an artificially contaminated soil. Biotechnology
and bioengineering, 72(2), 177-184.
Federici, E., Giubilei, M., Santi, G., Zanaroli, G., Negroni, A., Fava, F., Petruccioli, M.,
& D'Annibale, A. (2012). Bioaugmentation of a historically contaminated soil
by polychlorinated biphenyls with Lentinus tigrinus. Microbial cell factories,
11(1), 35.
Federici, E., Giubilei, M. A., Covino, S., Zanaroli, G., Fava, F., D’Annibale, A., &
Petruccioli, M. (2012). Addition of maize stalks and soybean oil to a historically
PCB-contaminated soil: Effect on degradation performance and indigenous
microbiota. New biotechnology, 30(1), 69-79.
doi:http://dx.doi.org/10.1016/j.nbt.2012.07.007
Field, J. A., & Sierra-Alvarez, R. (2008). Microbial transformation and degradation of
polychlorinated biphenyls. Environmental pollution, 155(1), 1-12.
doi:https://doi.org/10.1016/j.envpol.2007.10.016
Flemming, H.-C., Neu, T. R., & Wingender, J. (2016). The perfect slime: Microbial
extracellular polymeric substances (EPS): IWA publishing.

References 221
Fortin, P. D., Horsman, G. P., Yang, H. M., & Eltis, L. D. (2006). A glutathione s-
transferase catalyzes the dehalogenation of inhibitory metabolites of
polychlorinated biphenyls. Journal of bacteriology, 188(12), 4424-4430.
doi:http://dx.doi.org/10.1128/JB.01849-05
Francois, F., Lombard, C., Guigner, J.-M., Soreau, P., Brian-Jaisson, F., Martino, G.,
Vandervennet, M., Garcia, D., Molinier, A.-L., & Pignol, D. (2012). Isolation and
characterization of environmental bacteria capable of extracellular
biosorption of mercury. Applied and environmental microbiology, 78(4), 1097-
1106. doi:http://dx.doi.org/10.1128/AEM.06522-11
Fredriksson, N. J., Hermansson, M., & Wilen, B.-M. (2013). The choice of PCR primers
has great impact on assessments of bacterial community diversity and
dynamics in a wastewater treatment plant. PLoS ONE, 8(10), e76431.
doi:http://dx.doi.org/10.1371/journal.pone.0076431
Fritsche, W., & Hofrichter, M. (2005). Aerobic degradation of recalcitrant organic
compounds by microorganisms. In H.-J. Jordening & J. Winter (Eds.),
Environmental biotechnology concepts and applications: Wiley-VCH Verlag
GmbH & Co.
Furukawa, K. (2000). Biochemical and genetic bases of microbial degradation of
polychlorinated biphenyls (PCBs). The Journal of general and applied
microbiology, 46(6), 283-296.
Furukawa, K. (2006). Oxygenases and dehalogenases: Molecular approaches to
efficient degradation of chlorinated environmental pollutants. Bioscience,
Biotechnology, and Biochemistry, 70(10), 2335-2348.
doi:http://dx.doi.org/10.1271/bbb.60218
Furukawa, K., & Fujihara, H. (2008). Microbial degradation of polychlorinated
biphenyls: Biochemical and molecular features. Journal of Bioscience and
Bioengineering, 105(5), 433-449. doi:http://dx.doi.org/10.1263/jbb.105.433
Ganey, P. E., & Boyd, S. A. (2005). An approach to evaluation of the effect of
bioremediation on biological activity of environmental contaminants:

222 References
Dechlorination of polychlorinated biphenyls. Environmental Health
Perspectives, 113(2), 180-185. doi: https://doi.org/10.1289/ehp.6935
Gianfreda, L., & Rao, M. A. (2004). Potential of extra cellular enzymes in remediation
of polluted soils: A review. Enzyme and Microbial Technology, 35(4), 339-354.
doi:https://doi.org/10.1016/j.enzmictec.2004.05.006
Gillet, L. C., Navarro, P., Tate, S., Rost, H., Selevsek, N., Reiter, L., Bonner, R., &
Aebersold, R. (2012). Targeted data extraction of the MS/MS spectra
generated by Data-Independent Acquisition: A new concept for consistent
and accurate proteome analysis. Molecular & Cellular Proteomics, 11(6),
doi:http://dx.doi.org/10.1074/mcp.O111.016717
Giuliani, S. E., Frank, A. M., Corgliano, D. M., Seifert, C., Hauser, L., & Collart, F. R.
(2011). Environment sensing and response mediated by ABC transporters.
BMC Genomics, 12(1), S8. doi:http://dx.doi.org/10.1186/1471-2164-12-s1-s8
Gomes, H. I., Dias-Ferreira, C., & Ribeiro, A. B. (2013). Overview of in situ and ex situ
remediation technologies for PCB-contaminated soils and sediments and
obstacles for full-scale application. Science of The Total Environment, 445–
446(0), 237-260. doi:http://dx.doi.org/10.1016/j.scitotenv.2012.11.098
Haluska, L. u., Baranciková, G., Balaz, S., Dercova, K., Vrana, B., Paz-Weisshaar, M.,
Furciova, E., & Bielek, P. (1995). Degradation of PCB in different soils by
inoculated Alcaligenes xylosoxidans. Science of The Total Environment, 175(3),
275-285. doi:http://dx.doi.org/10.1016/0048-9697(95)04927-4
Hassan, H. A. (2014). Detection, cloning, and expression of catechol 2, 3 dioxygenase
genes from novel polychlorinated biphenyl (PCB) degraders. Life Science
Journal, 11(1).
Hearn, E. M., Patel, D. R., & van den Berg, B. (2008). Outer-membrane transport of
aromatic hydrocarbons as a first step in biodegradation. Proceedings of the
National Academy of Sciences, 105(25), 8601-8606.
doi:http://dx.doi.org/10.1073/pnas.0801264105

References 223
Hem, J. D., & Survey, G. (1989). Study and interpretation of the chemical
characteristics of natural water. US Geological survey water supply paper, 3
ed.
Henderson, B., & Martin, A. (2011). Bacterial virulence in the moonlight: Multitasking
bacterial moonlighting proteins are virulence determinants in infectious
disease. Infection and immunity, 79(9), 3476-3491.
doi:https://doi.org/10.1128/IAI.00179-11
Heywood, E., Wright, J., Wienburg, C. L., Black, H. I. J., Long, S. M., Osborn, D., &
Spurgeon, D. J. (2006). Factors influencing the national distribution of
polycyclic aromatic hydrocarbons and polychlorinated biphenyls in British
soils. Environmental Science & Technology, 40(24), 7629-7635.
doi:http://dx.doi.org/10.1021/es061296x
Hoostal, M. J., & Bouzat, J. L. (2016). Spatial patterns of bpha gene diversity reveal
local adaptation of microbial communities to PCB and PAH contaminants.
Microbial Ecology, 72(3), 559-570. doi: http://dx.doi.org/10.1007/s00248-
016-0812-y
Hoostal, M. J., Bullerjahn, G. S., & McKay, R. M. L. (2002). Molecular assessment of
the potential for in situ bioremediation of PCBs from aquatic sediments.
Hydrobiologia, 469(1-3), 59-65.
Hu, J., Qian, M., Zhang, Q., Cui, J., Yu, C., Su, X., Shen, C., Hashmi, M. Z., & Shi, J. (2015).
Sphingobium fuliginis HC3: A novel and robust isolated biphenyl-and
polychlorinated biphenyls-degrading bacterium without dead-end
intermediates accumulation. PloS one, 10(4), e0122740.
doi:http://dx.doi.org/10.1371/journal.pone.0122740
Hughes, A. S., VanBriesen, J. M., & Small, M. J. (2009). Identification of structural
properties associated with polychlorinated biphenyl dechlorination
processes. Environmental science & technology, 44(8), 2842-2848.
doi:http://dx.doi.org/10.1021/es902109w

224 References
Imamoglu, I., Li, K., & Christensen, E. R. (2002). Modeling polychlorinated biphenyl
congener patterns and dechlorination in dated sediments from the Ashtabula
river, Ohio, USA. Environmental Toxicology and Chemistry, 21(11), 2283-2291.
IOMC. (2003). Polychlorinated biphenyls : Human health aspects.: World Health
Organization, Geneva
Ionescu, M., Beranova, K., Dudkova, V., Kochankova, L., Demnerova, K., Macek, T., &
Mackova, M. (2009). Isolation and characterization of different plant
associated bacteria and their potential to degrade polychlorinated biphenyls.
International Biodeterioration & Biodegradation, 63(6), 667-672.
doi:http://dx.doi.org/10.1016/j.ibiod.2009.03.009
Janda, J. M., & Abbott, S. L. (2007). 16s rRNA gene sequencing for bacterial
identification in the diagnostic laboratory: Pluses, perils, and pitfalls. Journal
of Clinical Microbiology, 45(9), 2761-2764.
doi:https://doi.org/10.1128/JCM.01228-07
Jeffery, C. J. (2003). Moonlighting proteins: Old proteins learning new tricks. Trends
in Genetics, 19(8), 415-417. doi:https://doi.org/10.1016/S0168-
9525(03)00167-7
Jha, P., Panwar, J., & Jha, P. N. (2015). Secondary plant metabolites and root
exudates: Guiding tools for polychlorinated biphenyl biodegradation.
International Journal of Environmental Science and Technology, 12(2), 789-
802. doi:http://dx.doi.org/10.1007/s13762-014-0515-1
Jouanneau, Y., & Meyer, C. (2006). Purification and characterization of an arene cis-
dihydrodiol dehydrogenase endowed with broad substrate specificity toward
polycyclic aromatic hydrocarbon dihydrodiols. Applied and environmental
microbiology, 72(7), 4726-4734. doi:http://dx.doi.org/10.1128/AEM.00395-
06
Joutey, N. T., Bahafid, W., Sayel, H., & El Ghachtouli, N. (2013). Biodegradation:
Involved microorganisms and genetically engineered microorganisms.
Biodegradation-life of science. InTech, Rijeka, 289-320.
doi:http://dx.doi.org/10.5772/56194

References 225
Joy, S., Rahman, P. K. S. M., & Sharma, S. (2017). Biosurfactant production and
concomitant hydrocarbon degradation potentials of bacteria isolated from
extreme and hydrocarbon contaminated environments. Chemical
Engineering Journal, 317, 232-241.
doi:https://doi.org/10.1016/j.cej.2017.02.054
Kaakoush, N. O., Man, S. M., Lamb, S., Raftery, M. J., Wilkins, M. R., Kovach, Z., &
Mitchell, H. (2010). The secretome of Campylobacter concisus. The FEBS
journal, 277(7), 1606-1617. doi:https://doi.org/10.1111/j.1742-
4658.2010.07587.x
Kakinuma, A., Hori, M., Isono, M., Tamura, G., & Arima, K. (1969). Determination of
amino acid sequence in surfactin, a crystalline peptidelipid surfactant
produced by Bacillus subtilis. Agricultural and Biological Chemistry, 33(6),
971-972.
Karakas, F., & Imamoglu, I. (2017). Estimation of rate constants of PCB dechlorination
reactions using an anaerobic dehalogenation model. Journal of Hazardous
Materials, 324, Part B, 554-563.
doi:https://doi.org/10.1016/j.jhazmat.2016.11.026
Karigar, C. S., & Rao, S. S. (2011). Role of microbial enzymes in the bioremediation of
pollutants: A review. Enzyme research, 2011.
doi:http://dx.doi.org/10.4061/2011/805187
Kim, C.-S., Lim, D.-H., & Keum, Y.-S. (2016). Biodegradation pathways of
polychlorinated biphenyls by soil fungus Aspergillus niger. The Korean Journal
of Pesticide Science, 20(1), 7-13.
doi:http://dx.doi.org/10.7585/kjps.2016.20.1.7
Kjellerup, B. V., Paul, P., Ghosh, U., May, H. D., & Sowers, K. R. (2012). Spatial
distribution of PCB dechlorinating bacteria and activities in contaminated soil.
Applied and Environmental Soil Science, 2012.
doi:http://dx.doi.org/10.1155/2012/584970
Klaus, J. S., Kourafalou, V. H., Piggot, A. M., Reniers, A., Kang, H., Kumar, N., Zahran,
E. M., Bachas, L. G., Fernandez, A., & Gardinali, P. (2016). Potential impacts of

226 References
PCBs on sediment microbiomes in a tropical marine environment. Journal of
Marine Science and Engineering, 4(1), 13.
doi:http://dx.doi.org/10.3390/jmse4010013
Kosaric, N., & Choi, H. (1990). Biosurfactant production from Nocardia SFC-D:
Biosurfactant production in a nitrogen limited hydrocarbon medium from
Nocardia SFC-D. Tenside, surfactants, detergents, 27(5), 294-297.
Krumins, V., Park, J.W., Son, E.K., Rodenburg, L. A., Kerkhof, L. J., Haggblom, M. M., &
Fennell, D. E. (2009). Pcb dechlorination enhancement in anacostia river
sediment microcosms. Water Research, 43(18), 4549-4558.
doi:http://dx.doi.org/10.1016/j.watres.2009.08.003
Lauby-Secretan, B., Loomis, D., Grosse, Y., El Ghissassi, F., Bouvard, V., Benbrahim-
Tallaa, L., Guha, N., Baan, R., Mattock, H., & Straif, K. (2013). Carcinogenicity
of polychlorinated biphenyls and polybrominated biphenyls. The Lancet.
Oncology, 14(4), 287. doi:http://dx.doi.org/10.1016/S1470-2045(13)70104-9
Laycock, M. V., Hildebrand, P. D., Thibault, P., Walter, J. A., & Wright, J. L. (1991).
Viscosin, a potent peptidolipid biosurfactant and phytopathogenic mediator
produced by a pectolytic strain of Pseudomonas fluorescens. Journal of
agricultural and food chemistry, 39(3), 483-489.
Leavitt, R. I. (1983). [Process for the preparation of l-proline by cultivating algae].
Leewis, M.C., Uhlik, O., & Leigh, M. B. (2016). Synergistic processing of biphenyl and
benzoate: Carbon flow through the bacterial community in polychlorinated-
biphenyl-contaminated soil. Scientific reports, 6.
doi:https://doi.org/10.1038/srep22145
Leigh, M. B., Prouzova, P., Macková, M., Macek, T., Nagle, D. P., & Fletcher, J. S.
(2006). Polychlorinated biphenyl (PCB)-degrading bacteria associated with
trees in a PCBb-contaminated site. Applied and Environmental Microbiology,
72(4), 2331-2342. doi:http://dx.doi.org/10.1128/AEM.72.4.2331–2342.2006
Li, M., Rosenshine, I., Tung, S., Wang, X., Friedberg, D., Hew, C., & Leung, K. (2004).
Comparative proteomic analysis of extracellular proteins of
enterohemorrhagic and enteropathogenic Escherichia coli strains and their ihf

References 227
and ler mutants. Applied and environmental microbiology, 70(9), 5274-5282.
doi:http://dx.doi.org/10.1128/AEM.70.9.5274–5282.2004
Li, Y.F., Harner, T., Liu, L., Zhang, Z., Ren, N.-Q., Jia, H., Ma, J., & Sverko, E. (2010).
Polychlorinated biphenyls in global air and surface soil: Distributions, air−soil
exchange, and fractionation effect. Environmental Science & Technology,
44(8), 2784-2790. doi:http://dx.doi.org/10.1021/es901871e
Liang, Y., Meggo, R., Hu, D., Schnoor, J., & Mattes, T. (2015). Microbial community
analysis of switchgrass planted and unplanted soil microcosms displaying PCB
dechlorination. Applied Microbiology and Biotechnology, 1-12.
doi:http://dx.doi.org/10.1007/s00253-015-6545-x
Liang, Y., Meggo, R., Hu, D., Schnoor, J. L., & Mattes, T. E. (2014). Enhanced
polychlorinated biphenyl removal in a switchgrass rhizosphere by
bioaugmentation with Burkholderia xenovorans LB400. Ecological
engineering, 71, 215-222.
doi:http://dx.doi.org/10.1016/j.ecoleng.2014.07.046
Lin, S.-C., Minton, M. A., Sharma, M. M., & Georgiou, G. (1994). Structural and
immunological characterization of a biosurfactant produced by Bacillus
licheniformis IF-2. Applied and environmental microbiology, 60(1), 31-38.
Liu, J., Chen, T., Qi, Z., Yan, J., Buekens, A., & Li, X. (2014). Thermal desorption of PCBs
from contaminated soil using nano zerovalent iron. Environmental Science
and Pollution Research, 21(22), 12739-12746.
doi:http://dx.doi.org/10.1007/s11356-014-3226-8
Liz, J. A. Z.E., Jan-Roblero, J., de la Serna, J. Z.D., de Leon, A. V.P., & Hernandez-
Rodriguez, C. (2009). Degradation of polychlorinated biphenyl (PCB) by a
consortium obtained from a contaminated soil composed of Brevibacterium,
Pandoraea and Ochrobactrum. World Journal of Microbiology and
Biotechnology, 25(1), 165-170. doi:http://dx.doi.org/10.1007/s11274-008-
9875-3
Long, Y.Y., Fang, Y., Zhang, C., Du, Y., Shentu, J., & Shen, D.S. (2015). Degradation of
polychlorinated biphenyls by sequential anaerobic–aerobic composting.

228 References
Water, Air, & Soil Pollution, 226(3), 1-12.
doi:http://dx.doi.org/10.1007/s11270-015-2333-6
Luo, C., Wang, S., Wang, Y., Yang, R., Zhang, G., & Shen, Z. (2015). Effects of EDDS and
plant-growth-promoting bacteria on plant uptake of trace metals and PCBs
from e-waste–contaminated soil. Journal of Hazardous Materials.
doi:http://dx.doi.org/10.1016/j.jhazmat.2015.01.010
Luo, W., & Hu, C. (2013). Interaction of plant secondary metabolites and organic
carbon substrates affected on biodegradation of polychlorinated biphenyl.
Journal of Environmental Biology, 34(2 suppl), 337.
Lykidis, A., Perez-Pantoja, D., Ledger, T., Mavromatis, K., Anderson, I. J., Ivanova, N.
N., Hooper, S. D., Lapidus, A., Lucas, S., & Gonzalez, B. (2010). The complete
multipartite genome sequence of Cupriavidus necator JMP134, a versatile
pollutant degrader. PloS one, 5(3), e9729.
doi:http://dx.doi.org/10.1371/journal.pone.0009729
Maffei, B., Francetic, O., & Subtil, A. (2017). Tracking proteins secreted by bacteria:
What's in the toolbox? Frontiers in cellular and infection microbiology, 7, 221.
doi:http://dx.doi.org/10.3389/fcimb.2017.00221
Maier, R. M. (2009). Bacterial growth. In R. M. Maier, I. L. Pepper & C. P. Gerba (Eds.),
Environmental microbiology (2 ed., Vol. 397, pp. 37-54): Academic press.
Master, E. R., Lai, V. W.-M., Kuipers, B., Cullen, W. R., & Mohn, W. W. (2002).
Sequential anaerobic-aerobic treatment of soil contaminated with weathered
Aroclor 1260. Environmental science & technology, 36(1), 100-103.
Matsuyama, T., Kaneda, K., Ishizuka, I., Toida, T., & Yano, I. (1990). Surface-active
novel glycolipid and linked 3-hydroxy fatty acids produced by Serratia
rubidaea. Journal of bacteriology, 172(6), 3015-3022.
Mayes, B. A., Mc Connell, E. E., Neal, B. H., Brunner, M. J., Hamilton, S. B., Peters, A.
C., Ryan, M. J., Toft, J. D., Singer, A. W., & Brown Jr, J. F. (1998). Comparative
carcinogenicity in sprague-dawley rats of the polychlorinated biphenyl
mixtures Aroclors 1016, 1242, 1254, and 1260. Toxicological Sciences, 41(1),
62-76.

References 229
Meggo, R. E., & Schnoor, J. L. (2013). Rhizospere redox cycling and implications for
rhizosphere biotransformation of selected polychlorinated biphenyl (PCB)
congeners. Ecological Engineering, 57, 285-292.
doi:http://dx.doi.org/10.1016/j.ecoleng.2013.04.052
Meggo, R. E., Schnoor, J. L., & Hu, D. (2013). Dechlorination of PCBs in the rhizosphere
of switchgrass and poplar. Environmental Pollution, 178, 312-321.
doi:http://dx.doi.org/10.1016/j.envpol.2013.02.035
Megharaj, M., Ramakrishnan, B., Venkateswarlu, K., Sethunathan, N., & Naidu, R.
(2011). Bioremediation approaches for organic pollutants: A critical
perspective. Environment International, 37(8), 1362-1375.
doi:http://dx.doi.org/10.1016/j.envint.2011.06.003
Meier-Kolthoff, J. P., Goker, M., Sproer, C., & Klenk, H.P. (2013). When should a DDH
experiment be mandatory in microbial taxonomy? Archives of Microbiology,
195(6), 413-418. doi:http://dx.doi.org/10.1007/s00203-013-0888-4
Mercier, A., Joulian, C., Michel, C., Auger, P., Coulon, S., Amalric, L., Morlay, C., &
Battaglia-Brunet, F. (2014). Evaluation of three activated carbons for
combined adsorption and biodegradation of PCBs in aquatic sediment. Water
Research, 59, 304-315. doi:http://dx.doi.org/10.1016/j.watres.2014.04.021
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing
sugar. Analytical chemistry, 31(3), 426-428.
Miller, J. M., & Rhoden, D. L. (1991). Preliminary evaluation of biolog, a carbon source
utilization method for bacterial identification. Journal of clinical microbiology,
29(6), 1143-1147.
Moeder, M., Cajthaml, T., Koeller, G., Erbanova, P., & Sasek, V. (2005). Structure
selectivity in degradation and translocation of polychlorinated biphenyls
(Delor 103) with a Pleurotus ostreatus (oyster mushroom) culture.
Chemosphere, 61(9), 1370-1378.
doi:http://dx.doi.org/10.1016/j.chemosphere.2005.02.098
Morikawa, M., Hirata, Y., & Imanaka, T. (2000). A study on the structure–function
relationship of lipopeptide biosurfactants. Biochimica et Biophysica Acta

230 References
(BBA) - Molecular and Cell Biology of Lipids, 1488(3), 211-218.
doi:http://dx.doi.org/10.1016/S1388-1981(00)00124-4
Morikawa, M., Ito, M., & Imanaka, T. (1992). Isolation of a new surfactin producer
Bacillus pumilus A-1, and cloning and nucleotide sequence of the regulator
gene, PSF-1. Journal of fermentation and bioengineering, 74(5), 255-261.
Mrozik, A., & Piotrowska-Seget, Z. (2010). Bioaugmentation as a strategy for cleaning
up of soils contaminated with aromatic compounds. Microbiological
Research, 165(5), 363-375.
doi:http://dx.doi.org/10.1016/j.micres.2009.08.001
Muir, D., & Sverko, E. (2006). Analytical methods for PCBs and organochlorine
pesticides in environmental monitoring and surveillance: A critical appraisal.
Analytical and Bioanalytical Chemistry, 386(4), 769-789.
doi:http://dx.doi.org/10.1007/s00216-006-0765-y
Mulligan, C. N. (2005). Environmental applications for biosurfactants. Environmental
Pollution, 133(2), 183-198.
doi:http://dx.doi.org/10.1016/j.envpol.2004.06.009
Murinova, S., & Dercova, K. (2014). Potential use of newly isolated bacterial strain
Ochrobactrum anthropi in bioremediation of polychlorinated biphenyls.
Water, Air, & Soil Pollution, 225(6), 1-16.
doi:http://dx.doi.org/10.1007/s11270-014-1980-3
Murinova, S., Dercova, K., & Dudasova, H. (2014). Degradation of polychlorinated
biphenyls (pcbs) by four bacterial isolates obtained from the PCB-
contaminated soil and PCB-contaminated sediment. International
Biodeterioration & Biodegradation, 91(0), 52-59.
doi:http://dx.doi.org/10.1016/j.ibiod.2014.03.011
Musilova, L., Ridl, J., Polivkova, M., Macek, T., & Uhlik, O. (2016). Effects of secondary
plant metabolites on microbial populations: Changes in community structure
and metabolic activity in contaminated environments. International Journal
of Molecular Sciences, 17(8), 1205.
doi:http://dx.doi.org/10.3390/ijms17081205

References 231
NASEM. (2016). Use of metabolomics to advance research on environmental
exposures and the human exposome: Workshop in brief. : The National
Academies Press, Washington, DC.
Navon-Venezia, S., Zosim, Z., Gottlieb, A., Legmann, R., Carmeli, S., Ron, E., &
Rosenberg, E. (1995). Alasan, a new bioemulsifier from Acinetobacter
radioresistens. Applied and environmental microbiology, 61(9), 3240-3244.
Nayak, A. S., Vijaykumar, M. H., & Karegoudar, T. B. (2009). Characterization of
biosurfactant produced by Pseudoxanthomonas sp. PNK-04 and its
application in bioremediation. International Biodeterioration &
Biodegradation, 63(1), 73-79.
doi:http://dx.doi.org/10.1016/j.ibiod.2008.07.003
Nebert, D. W., & Vasiliou, V. (2004). Analysis of the glutathione s-transferase (GST)
gene family. Human Genomics, 1(6), 460-464..
doi:http://dx.doi.org/10.1186/1479-7364-1-6-460
Nikaido, H., & Takatsuka, Y. (2009). Mechanisms of RND multidrug efflux pumps.
Biochimica et biophysica acta, 1794(5), 769-781.
doi:http://dx.doi.org/10.1016/j.bbapap.2008.10.004
Occulti, F., Roda, G. C., Berselli, S., & Fava, F. (2008). Sustainable decontamination of
an actual-site aged PCB-polluted soil through a biosurfactant-based washing
followed by a photocatalytic treatment. Biotechnology and bioengineering,
99(6), 1525-1534. doi:http://dx.doi.org/10.1002/bit.21703
Ohtsubo, Y., Kudo, T., Tsuda, M., & Nagata, Y. (2004). Strategies for bioremediation
of polychlorinated biphenyls. Applied microbiology and biotechnology, 65(3),
250-258. doi:http://dx.doi.org/10.1007/s00253-004-1654-y
Pandey, P., Pathak, H., & Dave, S. (2016). Microbial ecology of hydrocarbon
degradation in the soil: A review. Research Journal of Environmental
Toxicology, 10(1), 1. doi:http://dx.doi.org/10.3923/rjet.2016.1.15
Panjiar, N., Sachan, S. G., & Sachan, A. (2015). Screening of bioemulsifier-producing
micro-organisms isolated from oil-contaminated sites. Annals of

232 References
Microbiology, 65(2), 753-764. doi:http://dx.doi.org/10.1007/s13213-014-
0915-y
Parales, R. E., & Ditty, J. L. (2017). Substrate transport. In T. Krell (Ed.), Cellular
ecophysiology of microbe (pp. 1-16): Springer International.
Park, C. B., & Lee, S. B. (1998). Ammonia production from yeast extract and its effect
on growth of the hyperthermophilic Archaeonsulfolobus solfataricus.
Biotechnology and Bioprocess Engineering, 3(2), 115-118.
doi:https://doi.org/10.1007/BF02932514
Parnell, J. J., Denef, V. J., Park, J., Tsoi, T., & Tiedje, J. M. (2010). Environmentally
relevant parameters affecting PCB degradation: Carbon source- and growth
phase-mitigated effects of the expression of the biphenyl pathway and
associated genes in Burkholderia xenovorans LB400. Biodegradation, 21(1),
147-156. doi:http://dx.doi.org/10.1007/s10532-009-9289-4
Passatore, L., Rossetti, S., Juwarkar, A. A., & Massacci, A. (2014). Phytoremediation
and bioremediation of polychlorinated biphenyls (PCBs): State of knowledge
and research perspectives. Journal of hazardous materials, 278, 189-202.
doi:http://dx.doi.org/10.1016/j.jhazmat.2014.05.051
Passeri, A., Lang, S., Wagner, F., & Wray, V. (1991). Marine biosurfactants, ii.
Production and characterization of an anionic trehalose tetraester from the
marine bacterium Arthrobacter sp. EK 1. Zeitschrift fur Naturforschung C,
46(3-4), 204-209.
Payne, R. B., Fagervold, S. K., May, H. D., & Sowers, K. R. (2013). Remediation of
polychlorinated biphenyl impacted sediment by concurrent bioaugmentation
with anaerobic halorespiring and aerobic degrading bacteria. Environmental
science & technology, 47(8), 3807-3815.
doi:http://dx.doi.org/10.1021/es304372t
Payne, R. B., May, H. D., & Sowers, K. R. (2011). Enhanced reductive dechlorination
of polychlorinated biphenyl impacted sediment by bioaugmentation with a
dehalorespiring bacterium. Environmental science & technology, 45(20),
8772-8779. doi:http://dx.doi.org/10.1021/es201553c

References 233
Petersen, T. N., Brunak, S., von Heijne, G., & Nielsen, H. (2011). Signalp 4.0:
Discriminating signal peptides from transmembrane regions. Nature methods,
8(10), 785. doi:https://doi.org/10.1038/nmeth.1701
Petric, I., Bru, D., Udikovic-Kolic, N., Hrsak, D., Philippot, L., & Martin-Laurent, F.
(2011). Evidence for shifts in the structure and abundance of the microbial
community in a long-term PCB-contaminated soil under bioremediation.
Journal of Hazardous Materials, 195(0), 254-260.
doi:http://dx.doi.org/10.1016/j.jhazmat.2011.08.036
Petric, I., Hrsak, D., Fingler, S., Udikovic-Kolic, N., Bru, D., & Martin-Laurent, F. (2011).
Insight in the PCB-degrading functional community in long-term
contaminated soil under bioremediation. Journal of soils and sediments,
11(2), 290-300. doi:http://dx.doi.org/10.1007/s11368-010-0299-y
Petric, I., Hrsak, D., Fingler, S., Voncina, E., Cetkovic, H., Begonja Kolar, A., & Udikovic
Kolic, N. (2007). Enrichment and characterization of PCB-degrading bacteria
as potential seed cultures for bioremediation of contaminated soil. Food
Technology and Biotechnology, 45(1), 11-20.
Phale, P. S., Savithri, H. S., Rao, N. A., & Vaidyanathan, C. S. (1995). Production of
biosurfactant “biosur-PM” by Pseudomonas maltophila csv89:
Characterization and role in hydrocarbon uptake. Archives of microbiology,
163(6), 424-431.
Pieper, D. (2005). Aerobic degradation of polychlorinated biphenyls. Applied
Microbiology and Biotechnology, 67(2), 170-191.
doi:http://dx.doi.org/10.1007/s00253-004-1810-4
Pieper, D. H., & Reineke, W. (2004). Degradation of chloroaromatics by Pseudomona
(d) s. In Pseudomonas (pp. 509-574): Springer.
Pieper, D. H., & Seeger, M. (2008). Bacterial metabolism of polychlorinated biphenyls.
Journal of Molecular Microbiology and Biotechnology, 15(2-3), 121-138.
doi:http://dx.doi.org/10.1159/000121325
Pino, N. J., Munera, L. M., & Penuela, G. A. (2016). Bioaugmentation with immobilized
microorganisms to enhance phytoremediation of PCB-contaminated soil. Soil

234 References
and Sediment Contamination: An International Journal.
doi:http://dx.doi.org/10.1080/15320383.2016.1148010
Power, B., Liu, X., Germaine, K. J., Ryan, D., Brazil, D., & Dowling, D. N. (2011). Alginate
beads as a storage, delivery and containment system for genetically modified
PCB degrader and PCB biosensor derivatives of Pseudomonas fluorescens
F113. Journal of Applied Microbiology, 110(5), 1351-1358.
doi:http://dx.doi.org/10.1111/j.1365-2672.2011.04993.x
Praveckova, M., Brennerova, M. V., Cvancarova, M., De Alencastro, L. F., Holliger, C.,
& Rossi, P. (2015). Divergent PCB organohalide-respiring consortia enriched
from the efflux channel of a former delor manufacturer in eastern europe.
Ecotoxicology and Environmental Safety, 120(0), 223-234.
doi:http://dx.doi.org/10.1016/j.ecoenv.2015.05.038
Pruthi, V., & Cameotra, S. S. (1997). Production of a biosurfactant exhibiting excellent
emulsification and surface active properties by Serratia marcescens. World
journal of microbiology and biotechnology, 13(1), 133-135.
Qi, Z., Chen, T., Bai, S., Yan, M., Lu, S., Buekens, A., Yan, J., Bulmau, C., & Li, X. (2014).
Effect of temperature and particle size on the thermal desorption of PCBs
from contaminated soil. Environmental Science and Pollution Research, 21(6),
4697-4704. doi:http://dx.doi.org/10.1007/s11356-013-2392-4
Qiu, L., Wang, H., & Wang, X. (2016). Isolation and characterization of a cold-resistant
PCB209-degrading bacterial strain from river sediment and its application in
bioremediation of contaminated soil. Journal of Environmental Science and
Health, Part A, 51(3), 204-212.
doi:http://dx.doi.org/10.1080/10934529.2015.1094324
Ramasamy, S., Mathiyalagan, P., & Chandran, P. (2014). Characterization and
optimization of EPS-producing and diesel oil-degrading Ochrobactrum
anthropi MP3 isolated from refinery wastewater. Petroleum Science, 11(3),
439-445. doi:http://dx.doi.org/10.1007/s12182-014-0359-9
Rappsilber, J., Ishihama, Y., & Mann, M. (2003). Stop and go extraction tips for matrix-
assisted laser desorption/ionization, nanoelectrospray, and LC/MS sample

References 235
pretreatment in proteomics. Analytical chemistry, 75(3), 663-670. doi:
http://dx.doi.org/10.1021/ac026117i
Richter, M., Willey, J. M., Submuth, R., Jung, G., & Fiedler, H.-P. (1998). Streptofactin,
a novel biosurfactant with aerial mycelium inducing activity from
Streptomyces tendae TU 901/8C. FEMS microbiology letters, 163(2), 165-171.
Robertson, L. W., & Hansen, L. G. (2015). PCBs: Recent advances in environmental
toxicology and health effects: University Press of Kentucky.
Rodrigues, J. L., Kachel, C. A., Aiello, M. R., Quensen, J. F., Maltseva, O. V., Tsoi, T. V.,
& Tiedje, J. M. (2006). Degradation of Aroclor 1242 dechlorination products
in sediments by Burkholderia xenovorans LB400 (OHB) and Rhodococcus sp.
strain RHA1 (FCB). Applied and environmental microbiology, 72(4), 2476-
2482. doi:http://dx.doi.org/10.1128/AEM.72.4.2476–2482.2006
Rysavy, J. P., Yan, T., & Novak, P. J. (2005). Enrichment of anaerobic polychlorinated
biphenyl dechlorinators from sediment with iron as a hydrogen source. Water
Research, 39(4), 569-578.
doi:http://dx.doi.org/10.1016/j.watres.2004.11.009
Sanger, F., & Coulson, A. R. (1975). A rapid method for determining sequences in DNA
by primed synthesis with DNA polymerase. Journal of molecular biology,
94(3), 441-448.
Sar, P., & Islam, E. (2012). Metagenomic approaches in microbial bioremediation of
metals and radionuclides. In Microorganisms in environmental management
(pp. 525-546): Springer.
Schlomann, M., Ngai, K. L., Ornston, L. N., & Knackmuss, H. J. (1993). Dienelactone
hydrolase from Pseudomonas cepacia. Journal of bacteriology, 175(10), 2994-
3001. doi:http://dx.doi.org/10.1128/jb.175.10.2994-3001.1993
Schmid, P., Gujer, E., Zennegg, M., Bucheli, T. D., & Desaules, A. (2005). Correlation
of PCDD/F and PCB concentrations in soil samples from the swiss soil
monitoring network (NABO) to specific parameters of the observation sites.
Chemosphere, 58(3), 227-234.
doi:http://dx.doi.org/10.1016/j.chemosphere.2004.08.045

236 References
Schuhle, K., & Fuchs, G. (2004). Phenylphosphate carboxylase: A new CC lyase
involved in anaerobic phenol metabolism in Thauera aromatica. Journal of
bacteriology, 186(14), 4556-4567.
doi:http://dx.doi.org/10.1128/JB.186.14.4556–4567.2004
Scott, C., Hilton, M. E., Coppin, C. W., Russell, R. J., Oakeshott, J. G., & Sutherland, T.
D. (2007). A global response to sulfur starvation in Pseudomonas putida and
its relationship to the expression of low-sulfur-content proteins. FEMS
microbiology letters, 267(2), 184-193.
Seeger, M., & Pieper, D. (2010). Genetics of biphenyl biodegradation and co-
metabolism of PCBs. In Handbook of hydrocarbon and lipid microbiology (pp.
1179-1199): Springer.
Sharma, J., Gautam, R., Misra, R., Kashyap, S., Singh, S., & Juwarkar, A. (2014).
Degradation of di- through hepta-chlorobiphenyls in clophen oil using
microorganisms isolated from long term PCBs contaminated soil. Indian
Journal of Microbiology, 54(3), 337-342.
doi:http://dx.doi.org/10.1007/s12088-014-0459-7
Sharma, S., & Gupta, A. (2016). Sustainable management of keratin waste biomass:
Applications and future perspectives. Brazilian Archives of Biology and
Technology, 59. doi:http://dx.doi.org/10.1590/1678-4324-2016150684
Shilov, I. V., Seymour, S. L., Patel, A. A., Loboda, A., Tang, W. H., Keating, S. P., ... &
Schaeffer, D. A. (2007). The Paragon Algorithm, a next generation search
engine that uses sequence temperature values and feature probabilities to
identify peptides from tandem mass spectra. Molecular & Cellular
Proteomics, 6(9), 1638-1655. doi: http://dx.doi.org/10.1074/mcp.T600050-
MCP200
Singer, A., Gilbert, E., Luepromchai, E., & Crowley, D. (2000). Bioremediation of
polychlorinated biphenyl-contaminated soil using carvone and surfactant-
grown bacteria. Applied Microbiology and Biotechnology, 54(6), 838-843.

References 237
Singer, M. V., Finnerty, W., & Tunelid, A. (1990). Physical and chemical properties of
a biosurfactant synthesized by Rhodococcus species H13-A. Canadian journal
of microbiology, 36(11), 746-750.
Song, C., Kumar, A., & Saleh, M. (2009). Bioinformatic comparison of bacterial
secretomes. Genomics, proteomics & bioinformatics, 7(1), 37-46.
doi:http://dx.doi.org/10.1016/S1672-0229(08)60031-5
Song, M., Luo, C., Li, F., Jiang, L., Wang, Y., Zhang, D., & Zhang, G. (2015). Anaerobic
degradation of polychlorinated biphenyls (PCBs) and polychlorinated
biphenyls ethers (PBDEs), and microbial community dynamics of electronic
waste-contaminated soil. Science of The Total Environment, 502, 426-433.
doi:http://dx.doi.org/10.1016/j.scitotenv.2014.09.045
Sowers, K. R., & May, H. D. (2013). In situ treatment of PCBs by anaerobic microbial
dechlorination in aquatic sediment: Are we there yet? Current Opinion in
Biotechnology, 24(3), 482-488.
doi:http://dx.doi.org/10.1016/j.copbio.2012.10.004
Srinivasan, R., Karaoz, U., Volegova, M., MacKichan, J., Kato-Maeda, M., Miller, S.,
Nadarajan, R., Brodie, E. L., & Lynch, S. V. (2015). Use of 16s rRNA gene for
identification of a broad range of clinically relevant bacterial pathogens. PloS
one, 10(2), e0117617. doi:http://dx.doi.org/10.1371/journal.pone.0117617
Srirangam, R. S. (2007). Enhanced anaerobic biodegradation of PCBs in contaminated
sediments using hydrogen. University of Illinois, Chicago.
Stackebrandt, E., & Eber, J. (2006). Taxonomic parameters revisited: Tarnished gold
standards. Microbiology Today, 6, 152-155.
Stella, T., Covino, S., Burianova, E., Filipova, A., Kresinova, Z., Voriskova, J., Vetrovsky,
T., Baldrian, P., & Cajthaml, T. (2015). Chemical and microbiological
characterization of an aged PCB-contaminated soil. Science of The Total
Environment, 533, 177-186.
doi:http://dx.doi.org/10.1016/j.scitotenv.2015.06.019
Sturms, R., Streauslin, N. A., Cheng, S., & Bobik, T. A. (2015). In Salmonella enterica,
ethanolamine utilization is repressed by 1, 2-propanediol to prevent

238 References
detrimental mixing of components of two different bacterial
microcompartments. Journal of bacteriology, 197(14), 2412-2421.
doi:http://dx.doi.org/10.1128/JB.00215-15
Takeo, M., Ohara, A., Sakae, S., Okamoto, Y., Kitamura, C., Kato, D.i., & Negoro, S.
(2013). Function of a glutamine synthetase-like protein in bacterial aniline
oxidation via γ-glutamylanilide. Journal of Bacteriology, 195(19), 4406-4414.
Retrieved from PMC. doi:http://dx.doi.org/10.1128/JB.00397-13
Tartakovsky, B., Michotte, A., Cadieux, J. C. A., Lau, P. C. K., Hawari, J., & Guiot, S. R.
(2001). Degradation of Aroclor 1242 in a single - stage coupled
anaerobic/aerobic bioreactor. Water Research, 35(18), 4323-4330.
doi:http://dx.doi.org/10.1016/S0043-1354(01)00175-0
Thavasi, R., Sharma, S., & Jayalakshmi, S. (2011). Evaluation of screening methods for
the isolation of biosurfactant producing marine bacteria. Journal of Petroleum
& Environmental Biotechnology. doi:http://dx.doi.org/10.4172/2157-
7463.S1-001
Thomas, T., Gilbert, J., & Meyer, F. (2012). Metagenomics-A guide from sampling to
data analysis. Microbial informatics and experimentation, 2(1), 3.
Tigini, V., Prigione, V., Di Toro, S., Fava, F., & Varese, G. C. (2009). Isolation and
characterisation of polychlorinated biphenyl (PCB) degrading fungi from a
historically contaminated soil. Microbial Cell Factories, 8(5).
doi:http://dx.doi.org/10.1186/1475-2859-8-5
Tomei, M. C., & Daugulis, A. J. (2013). Ex situ bioremediation of contaminated soils:
An overview of conventional and innovative technologies. Critical Reviews in
Environmental Science and Technology, 43(20), 2107-2139.
doi:http://dx.doi.org/10.1080/10643389.2012.672056
Tu, C., Teng, Y., Luo, Y., Sun, X., Deng, S., Li, Z., Liu, W., & Xu, Z. (2011). PCB removal,
soil enzyme activities, and microbial community structures during the
phytoremediation by alfalfa in field soils. Journal of Soils and Sediments, 11(4),
649-656. doi:http://dx.doi.org/10.1007/s11368-011-0344-5

References 239
Tuleva, B., Christova, N., Cohen, R., Stoev, G., & Stoineva, I. (2008). Production and
structural elucidation of trehalose tetraesters (biosurfactants) from a novel
alkanothrophic Rhodococcus wratislaviensis strain. Journal of applied
microbiology, 104(6), 1703-1710. doi:https://doi.org/10.1111/j.1365-
2672.2007.03680.x
UNEP. (2009). Stockholm convention on persistent organic pollutants (POPs) as
amended in 2009. The Secretariat of the Stockholm Convention, United
Nations, Geneva.
UniProt Consortium. (2016). UniProt: the universal protein knowledgebase. Nucleic
acids research, 45(D1), D158-D169. doi: https://doi.org/10.1093-
/nar/gkw1099
USEPA. (1993). Method 300.0 determination of inorganic ions by ion
chromatography. Environmental monitoring systems laboratory, Office of
research and development, USEPA, Cincinnati, Ohio 45268
USEPA. (2007). Method 8082a. Polychlorinated biphenyls (PCBs) by gas
chromatography. United States Environmental Protection Agency.
USEPA. (2013). Polychlorinated biphenyls (PCBs). Unites States Environmental
Protection Agency.
Van Agteren, M. H., Keuning, S., & Janssen, D. (2013). Aromatic compounds. In
Handbook on biodegradation and biological treatment of hazardous organic
compounds (Vol. 2): Springer Science & Business Media.
Van Bogaert, I. N., Saerens, K., De Muynck, C., Develter, D., Soetaert, W., &
Vandamme, E. J. (2007). Microbial production and application of
sophorolipids. Applied microbiology and biotechnology, 76(1), 23-34.
doi:https://doi.org/10.1007/s00253-007-0988-7
Van Tongeren, S. P., Degener, J. E., & Harmsen, H. J. M. (2011). Comparison of three
rapid and easy bacterial DNA extraction methods for use with quantitative
real-time PCR. European Journal of Clinical Microbiology & Infectious
Diseases, 30(9), 1053-1061. Retrieved from PMC.
doi:https://doi.org/10.1007/s10096-011-1191-4

240 References
Varadhan, A. S., Khodadoust, A. P., & Brenner, R. C. (2011). Effect of biostimulation
on the microbial community in PCB-contaminated sediments through
periodic amendment of sediment with iron. Journal of industrial microbiology
& biotechnology, 38(10), 1691-1707. doi:http://dx.doi.org/10.1007/s10295-
011-0959-y
Vasilyeva, G., & Strijakova, E. (2007). Bioremediation of soils and sediments
contaminated by polychlorinated biphenyls. Microbiology, 76(6), 639-653.
Vazquez-Gutierrez, P., Stevens, M. J., Gehrig, P., Barkow-Oesterreicher, S., Lacroix, C.,
& Chassard, C. (2017). The extracellular proteome of two Bifidobacterium
species reveals different adaptation strategies to low iron conditions. BMC
genomics, 18(1), 41. doi:http://dx.doi.org/10.1186/s12864-016-3472-x
Vazquez-Laslop, N., Lee, H., Hu, R., & Neyfakh, A. A. (2001). Molecular sieve
mechanism of selective release of cytoplasmic proteins by osmotically
shocked Escherichia coli. Journal of bacteriology, 183(8), 2399-2404.
VEPA. (2017). Polychlorinated biphenyls (PCB) management, industrial waste
resource guidelines. Environmental Protection Authority, Victoria [Publication
IWRG643.2. December] Retrieved from
http://www.epa.vic.gov.au/~/media/Publications/IWRG643%202.pdf.
Viisimaa, M., Karpenko, O., Novikov, V., Trapido, M., & Goi, A. (2013). Influence of
biosurfactant on combined chemical–biological treatment of PCB-
contaminated soil. Chemical Engineering Journal, 220, 352-359.
doi:http://dx.doi.org/10.1016/j.cej.2013.01.041
Vingataramin, L., & Frost, E. (2015). A single protocol for extraction of gDNA from
bacteria and yeast. BioTechniques, 58(3), 120.
Walker, D. R., & Feyerherm, F. (2013). Identification and quantitation of. PCB Aroclor
mixtures in a single run using the Agilent 7000B triple quadrupole GC/MS.
Retrieved from https://www.agilent.com/cs/library/applications/5991-
3537EN.pdf
Wang, D.G., Yang, M., Jia, H.L., Zhou, L., & Li, Y.F. (2008). Levels, distributions and
profiles of polychlorinated biphenyls in surface soils of Dalian, China.

References 241
Chemosphere, 73(1), 38-42.
doi:http://dx.doi.org/10.1016/j.chemosphere.2008.05.055
Wang, Q., Garrity, G. M., Tiedje, J. M., & Cole, J. R. (2007). Naive bayesian classifier
for rapid assignment of rrna sequences into the new bacterial taxonomy.
Applied and Environmental Microbiology , 73 (16), 5261-5267.
doi:http://dx.doi.org/10.1128/AEM.00062-07
Wang, S., Chng, K. R., Wilm, A., Zhao, S., Yang, K.-L., Nagarajan, N., & He, J. (2014).
Genomic characterization of three unique Dehalococcoides that respire on
persistent polychlorinated biphenyls. Proceedings of the National Academy of
Sciences, 111(33), 12103-12108.
doi:http://dx.doi.org/10.1073/pnas.1404845111
Wang, S., & He, J. (2013a). Dechlorination of commercial PCBs and other multiple
halogenated compounds by a sediment-free culture containing
Dehalococcoides and Dehalobacter. Environmental science & technology,
47(18), 10526-10534.
Wang, S., & He, J. (2013b). Phylogenetically distinct bacteria involve extensive
dechlorination of Aroclor 1260 in sediment-free cultures. PloS one, 8(3),
e59178. doi:http://dx.doi.org/10.1371/journal.pone.0059178
White, D. C. (1986). Quantitative physicochemical characterization of bacterial
habitats. Bacteria in nature, 2, 177-203.
Whitman, C. P. (2002). The 4-oxalocrotonate tautomerase family of enzymes: How
nature makes new enzymes using a beta-alpha-beta structural motif. Arch
Biochem Biophys, 402(1), 1-13. doi:http://dx.doi.org/10.1016/s0003-
9861(02)00052-8
WHO. (2016). Iarc working group on the evaluation of carcinogenic risk to humans.
Polychlorinated biphenyls and polybrominated biphenyls. International
Agency for Research on Cancer, France.
Wiegel, J., & Wu, Q. (2000). Microbial reductive dehalogenation of polychlorinated
biphenyls. FEMS microbiology ecology, 32(1), 1-15.

242 References
Winchell, L. J., & Novak, P. J. (2008). Enhancing polychlorinated biphenyl
dechlorination in fresh water sediment with biostimulation and
bioaugmentation. Chemosphere, 71(1), 176-182.
doi:http://dx.doi.org/10.1016/j.chemosphere.2007.10.021
Wisniewski, J. R., Zougman, A., Nagaraj, N., & Mann, M. (2009). Universal sample
preparation method for proteome analysis. Nature Methods, 6(5), 359-362.
doi:http://dx.doi.org/10.1038/nmeth.1322
Wu, Q., Sowers, K. R., & May, H. D. (2000). Establishment of a polychlorinated
biphenyl-dechlorinating microbial consortium, specific for doubly flanked
chlorines, in a defined, sediment-free medium. Applied and environmental
microbiology, 66(1), 49-53.
Xia, X. X., Han, M. J., Lee, S. Y., & Yoo, J. S. (2008). Comparison of the extracellular
proteomes of Escherichia coli B and K-12 strains during high cell density
cultivation. Proteomics, 8(10), 2089-2103.
doi:https://doi.org/10.1002/pmic.200700826
Xu, Y., Gregory, K. B., & VanBriesen, J. M. (2016). Microbial-catalyzed reductive
dechlorination of polychlorinated biphenyls in Hudson and Grasse river
sediment microcosms: Determination of dechlorination preferences and
identification of rare ortho removal pathways. Environmental Science &
Technology, 50(23), 12767-12778.
doi:http://dx.doi.org/10.1021/acs.est.6b03892
Yang, C.Y., Chiou, S.L., Wang, J.D., & Guo, Y. L. (2015). Health related quality of life
and polychlorinated biphenyls and dibenzofurans exposure: 30 years follow-
up of Yucheng cohort. Environmental Research, 137(0), 59-64.
doi:http://dx.doi.org/10.1016/j.envres.2014.11.020
Yang, S., Yoshida, N., Baba, D., & Katayama, A. (2008). Anaerobic biodegradation of
biphenyl in various paddy soils and river sediment. Chemosphere, 71(2), 328-
336. doi:http://dx.doi.org/10.1016/j.chemosphere.2007.09.002
Yarza, P., Yilmaz, P., Pruesse, E., Glockner, F. O., Ludwig, W., Schleifer, K.H., Whitman,
W. B., Euzeby, J., Amann, R., & Rossello-Mora, R. (2014). Uniting the

References 243
classification of cultured and uncultured bacteria and archaea using 16s rrna
gene sequences. Nature Reviews Microbiology, 12(9), 635.
doi:http://dx.doi.org/10.1038/nrmicro3330
Yen, M. R., Choi, J., & Saier Jr, M. H. (2009). Bioinformatic analyses of transmembrane
transport: Novel software for deducing protein phylogeny, topology, and
evolution. Journal of molecular microbiology and biotechnology, 17(4), 163-
176. doi:https://doi.org/10.1159/000239667
Yin, Y., Guo, J., Zheng, L., Tian, L., & Wang, X. (2011). Capability of polychlorinated
biophenyl (PCBs) degrading fungi segregated from sediments. World Journal
of Microbiology and Biotechnology, 27(11), 2567.
doi:https://doi.org/10.1007/s11274-011-0728-0
Zhang, C., Du, Y., Tao, X.Q., Zhang, K., Shen, D.S., & Long, Y.Y. (2013). Dechlorination
of polychlorinated biphenyl-contaminated soil via anaerobic composting with
pig manure. Journal of Hazardous Materials, 261, 826-832.
doi:http://dx.doi.org/10.1016/j.jhazmat.2013.05.060
Zhang, D., Zhu, Y., & Chen, J. (2009). Microbial transglutaminase production:
Understanding the mechanism. Biotechnology and Genetic Engineering
Reviews, 26(1), 205-222. doi:https://doi.org/10.5661/bger-26-205
Zhao, L., Chen, J., Sun, J., & Zhang, D. (2017). Multimer recognition and secretion by
the non-classical secretion pathway in Bacillus subtilis. Scientific reports, 7,
44023. doi:http://dx.doi.org/10.1038/srep44023
Zhou, J. L., Siddiqui, E., Ngo, H. H., & Guo, W. (2014). Estimation of uncertainty in the
sampling and analysis of polychlorinated biphenyls and polycyclic aromatic
hydrocarbons from contaminated soil in Brighton, Uk. Science of The Total
Environment, 497–498(0), 163-171.
doi:http://dx.doi.org/10.1016/j.scitotenv.2014.07.097

244 References

Appendices 245
Appendices

246 Appendix A: PCB analysis
Appendix A: PCB analysis

Appendix A: PCB analysis 247
Table A. 1 Selective reaction monitoring (SRM) transitions
Name Retention
Time Ion
Polarity Window
Pre-width
Post-width
Mass Product
Mass Collision Energy
mono 10 Positive 1 7 13 188 152 22
mono 10 Positive 1 7 13 190 152 22
di 10 Positive 1 7 13 222 152 22
di 10 Positive 1 7 13 224 152 22
tri 10 Positive 1 7 13 256 186 22
tri 10 Positive 1 7 13 258 186 22
tetra 10 Positive 1 7 13 289.9 219.9 22
tetra 10 Positive 1 7 13 291.9 222 22
penta 10 Positive 1 7 13 323.9 253.9 22
penta 10 Positive 1 7 13 325.9 255.9 22
hexa 10 Positive 1 7 13 357.9 287.9 22
hexa 10 Positive 1 7 13 359.8 289.9 22
hepta 10 Positive 1 7 13 391.9 321.9 22
hepta 10 Positive 1 7 13 393.8 323.9 22
octa 10 Positive 1 7 13 427.8 355.8 22
octa 10 Positive 1 7 13 429.8 357.8 22
nona 10 Positive 1 7 13 461.7 391.8 22
nona 10 Positive 1 7 13 463.8 393.8 22
deca 10 Positive 1 7 13 495.7 425.7 22
deca 10 Positive 1 7 13 497.7 427.7 22
2456 tetrachloro-m-xylene 12.53 Positive 2 1 1 242 207 15
2456 tetrachloro-m-xylene 12.53 Positive 2 1 1 244 209 15
2456 tetrachloro-m-xylene 12.53 Positive 2 1 1 246 211 15
224455-hexabromobiphenyl 20.74 Positive 2 1 1 468 306 30
224455-hexabromobiphenyl 20.74 Positive 2 1 1 468 308 30
224455-hexabromobiphenyl 20.74 Positive 2 1 1 547 467 20

248 Appendix A: PCB analysis
Table A. 2 Retention times used for homolog groups and standards
Retention Time (min) Compound
11.50-12.70 Mono
12.53 2,4,5,6-tetrachloro-m-xylene
12.50-13.50 Di
13.40-14.95 Tri
14.60-15.81 Tetra
15.00-17.00 Penta
16.45-18.20 Hexa
17.20-18.75 Hepta
17.90-19.55 Octa
18.70-20.00 Nona
20.20 Deca
20.74 2,2′,4,4′,5,5′-hexabromo biphenyl

Appendix B: PCB data related to bacterial consortium study 249
Table A. 3 Aroclor 1260 calibration curve preparation from 50 mg/L stock solution
Notes: * Internal standard 2,2′,4,4′,5,5′-hexabromo biphenyl
**Surrogate – 2,4,5,6-tetrachloro-m-xylene
Aroclor 1260 V2 (µL) Final concentration of surrogate in
calibration levels (ppb)
Ratio M1/M2
M1 (ppb)
V1 (µL)
M2 (ppb) V2 (µL) Solvent
(Hexane)
Internal standard*
(10000 ppb)
Surrogate**
(1000 ppb)
1.0 50000 200 10000 500 395 5 250 250
0.5 10000 500 5000 500 245 5 0 125
0.25 5000 400 2000 500 295 5 0 50
0.125 2000 250 1000 500 245 5 0 25
0.05 2000 125 500 500 340 5 0 12.5
0.5 2000 62.5 250 500 432.5 5 0 6.25
0.2 2000 25 100 500 470 5 0 2.5
0.1 100 250 50 500 245 5 0 1.25
0.05 100 100 20 500 395 5 0 0.5
100 50 10 500 445 5 0 0.25

250 Appendix A: PCB analysis
Table A. 4 Peak area values obtained for each homolog group at different Aroclor 1260 standard concentrations
Homolog
group
Peak area
5 ppb 10 ppb 20 ppb 50 ppb 250 ppb 500 ppb 1000 ppb 2000 ppb 10000 ppb
mono 1889.348 1707.92589 2468.0219 3465.0801 15574.9853 30401.777 76422.231 129119.086 750636.22
di 550.9803 1188.7858 5080.5965 8521.2593 56355.3414 117940.77 270306.73 498293.522 2950682.9
tri 4497.273 4884.74261 11812.728 24380.686 122937.635 251359.76 594197.13 1087982.18 6107066.8
tetra 2845.426 3695.28486 7324.9622 17024.727 124256.795 257673.05 627861.52 1121852.91 6265611.3
penta 30426.95 45411.7234 105299.96 240614.42 1319259.75 2751272.7 6568636.5 11776736.5 67231764
hexa 67817.83 109574.906 259315.13 595372.54 3350325.52 6928845.8 16565257 30207732.6 173350129
hepta 30819.58 49218.3871 115007.26 268515.69 1546399.88 3202036.2 7659288.8 13826669.3 80016143
octa 4640.051 8502.89597 15613.646 26168.11 176444.942 354261.01 852198.37 1538777.25 8835023.5
nona 730.4066 1319.00774 2175.074 5331.9759 41249.352 79538.784 192950.88 350151.504 2011603.3
deca 12.63421 385.909366 250.08227 437.05678 3016.55984 5866.5805 13860.713 28517.889 160508.93
Total 144230.5 225889.569 524347.46 1189831.6 6755820.76 13979196 33420980 60565832.8 347679170

Appendix B: PCB data related to bacterial consortium study 251
Appendix B: PCB data related to bacterial
consortium study

252 Appendix B: PCB data related to bacterial consortium study
Table B. 1 Homolog and total PCB composition in the Two stage anaerobic-aerobic treatment
Concentration
(µg/L)
Uninoculated
control STDV Week 0 STDV Week 2 STDV Week 4 STDV Week 6 STDV
mono 25.75 1.74 22.09 1.27 83.04 5.83 354.37 30.29 0.90 0.50
di 217.32 19.06 209.73 20.69 314.97 7.99 1550.56 110.93 33.35 3.60
tri 593.56 26.00 591.56 12.17 596.27 38.47 942.59 83.67 164.46 16.30
tetra 790.20 30.73 728.15 103.91 725.93 25.73 831.86 42.64 236.22 86.16
penta 7974.02 238.11 8285.12 675.16 5621.28 406.15 4130.70 307.46 3658.28 384.09
hexa 19728.43 1125.69 18060.61 338.91 14691.42 313.85 11424.79 356.11 10576.86 910.62
hepta 7777.20 743.16 7283.13 292.32 6035.75 144.05 4958.36 157.50 4545.18 221.73
octa 926.88 86.36 798.70 69.01 689.67 10.16 562.77 15.06 536.66 48.25
nona 202.99 21.01 189.99 9.78 153.26 4.36 127.29 1.59 124.55 9.46
deca 18.51 1.78 15.91 0.75 13.05 0.83 10.57 0.75 9.29 0.74
Total PCB 38232.24 1934.50 36184.98 85.69 28924.63 944.11 24893.86 863.58 19885.74 1452.83

Appendix B: PCB data related to bacterial consortium study 253
Table B. 2 Homolog and total PCB concentrations in the alternating anaerobic-aerobic treatment
Concentration
(µg/L)
uninoculated
control STDV Week 0 STDV Week 2 STDV Week 4 STDV Week 6 STDV
mono 25.755 1.735 22.085 1.268 1.380 0.718 0.725 0.066 0.336 0.013
di 217.322 19.063 209.733 20.693 15.404 1.676 10.060 0.299 9.542 1.197
tri 593.557 25.997 591.555 12.168 125.624 19.200 120.412 10.854 95.087 2.713
tetra 790.205 30.734 728.152 103.905 280.507 37.452 288.415 7.861 278.337 4.132
penta 7974.023 238.110 8285.116 675.162 3485.743 181.287 3653.442 50.885 3233.831 226.179
hexa 19728.433 1125.692 18060.611 338.912 10462.199 549.223 10309.500 358.778 9527.195 372.236
hepta 7777.203 743.160 7283.129 292.323 4450.881 218.022 4312.760 158.724 3875.390 24.000
octa 926.876 86.362 798.696 69.013 481.680 27.793 500.159 16.609 418.755 16.982
nona 202.990 21.005 189.989 9.779 108.509 5.285 109.904 0.671 107.336 3.326
deca 18.509 1.784 15.914 0.751 8.925 1.391 8.542 1.284 9.568 0.881
Total PCB 38232.240 1934.498 36184.980 85.689 19420.851 957.890 19313.918 564.092 17555.378 188.643

254 Appendix B: PCB data related to bacterial consortium study

Appendix C: Extracellular protein analysis 255
Appendix C: Extracellular protein analysis

256 Appendix C: Extracellular protein analysis
Table C.1 Relative abundance of extracellular proteins of bacterial consortium Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05 found in the culture supernatant under alternating (AN) and two stage (TS) anaerobic-aerobic conditions as a heat map.
No. Accession number Protein
AN (Weeks)
TS (Weeks) Secreted*
2 4 6 2 4 6
Transport related
1 WP_054452161.1 ABC transporter Yes (28-29)
2 WP_043545028.1 ABC transporter Yes (27-28)
3 WP_088148777.1 ABC transporter permease Yes (22-23)
4 WP_088587964.1 ABC transporter permease Yes (24-25)
5 WP_082601631.1 ABC transporter permease Yes (30-31)
6 WP_050448859.1 ABC transporter permease Yes (29-30)
7 WP_088154810.1 ABC transporter permease Yes (24-25)
8 WP_024899300.1 ABC transporter permease Yes (20-21)
9 KNY13962.1 ABC transporter permease Yes (20-21)
10 WP_088447256.1 ABC transporter substrate-binding protein Yes (28-29)
11 WP_088447395.1 ABC transporter substrate-binding protein Yes (36-37)
12 WP_088146376.1 ABC transporter substrate-binding protein Yes (33-34)
13 WP_088147153.1 ABC transporter substrate-binding protein Yes (22-23)
14 ASC66145.1 ABC transporter substrate-binding protein Yes (22-23)
15 WP_088147688.1 ABC transporter substrate-binding protein Yes (22-23)
16 ASC63496.1 ABC transporter substrate-binding protein Yes (22-23)
17 ASC64180.1 ABC transporter substrate-binding protein Yes (20-21)
18 ASC67639.1 ABC transporter substrate-binding protein Yes (24-25)
19 OWT57704.1 ABC transporter substrate-binding protein Yes (35-36)
20 WP_054454039.1 ABC transporter substrate-binding protein Yes (23-24)
21 OAD17456.1 ABC transporter substrate-binding protein Yes (26-27)

Appendix C: Extracellular protein analysis 257
No. Accession number Protein
AN
(Weeks)
TS
(Weeks) Secreted*
2 4 6 2 4 6
22 WP_025139398.1 ABC transporter substrate-binding protein Yes (23-24)
23 OCZ60996.1 ABC transporter substrate-binding protein Yes (26-27)
24 OAE68865.1 ABC transporter substrate-binding protein Yes (26-27)
25 WP_076521077.1 ABC transporter substrate-binding protein Yes (23-24)
26 WP_043547493.1 ABC transporter substrate-binding protein Yes (26-27)
27 KGD96204.1 ABC transporter substrate-binding protein Yes (25-26)
28 WP_054456606.1 ABC transporter substrate-binding protein Yes (22-23)
29 WP_088155937.1 ABC transporter substrate-binding protein Yes (23-24)
30 OLT99158.1 ABC transporter substrate-binding protein Yes (21-22)
31 WP_088154240.1 ABC transporter substrate-binding protein Yes (23-24)
32 OLU02054.1 ABC transporter substrate-binding protein Yes (24-25)
33 WP_088138972.1 ABC transporter substrate-binding protein Yes (21-22)
34 WP_088138276.1 ABC transporter substrate-binding protein Yes (23-24)
35 OLU08696.1 ABC transporter substrate-binding protein Yes (28-29)
36 OWG18014.1 ABC transporter substrate-binding protein Yes (25-26)
37 WP_085982324.1 ABC transporter substrate-binding protein Yes (21-22)
38 WP_061347109.1 ABC transporter substrate-binding protein Yes (22-23)
39 KXO76717.1 ABC transporter substrate-binding protein Yes (27-28)
40 OOC59559.1 ABC transporter substrate-binding protein Yes (37-38)
41 KXO75178.1 amino acid ABC transporter Yes (21-22)
42 WP_088589881.1 amino acid ABC transporter substrate-binding protein Yes (22-23)
43 WP_061306665.1 amino acid ABC transporter substrate-binding protein Yes (22-23)
44 WP_054420698.1 amino acid ABC transporter substrate-binding protein Yes (22-23)
45 KGD90086.1 amino acid ABC transporter substrate-binding protein Yes (22-23)
46 KGE00355.1 amino acid ABC transporter substrate-binding protein Yes (26-27)

258 Appendix C: Extracellular protein analysis
No. Accession number Protein
AN
(Weeks)
TS
(Weeks) Secreted*
2
4
6
2
4
6
47 KGD94165.1 amino acid ABC transporter substrate-binding protein Yes (23-24)
48 KNE29344.1 amino acid ABC transporter substrate-binding protein Yes (31-32)
49 OLU07976.1 amino acid ABC transporter substrate-binding protein Yes (22-23)
50 WP_016992241.1 amino acid ABC transporter substrate-binding protein Yes (28-29)
51 WP_025141122.1 amino acid ABC transporter substrate-binding protein Yes (37-38)
52 WP_043541220.1 amino acid ABC transporter substrate-binding protein Yes (35-36)
53 WP_088146425.1 branched-chain amino acid ABC transporter substrate-binding protein Yes (23-24)
54 WP_088141350.1 branched-chain amino acid ABC transporter substrate-binding protein Yes (25-26)
55 AKP90359.1 Branched-chain amino acid ABC transporter, amino acid-binding protein Yes (28-29)
56 WP_054450546.1 branched-chain amino acid ABC transporter substrate-binding protein Yes (24-25)
57 KGY25861.1 C4-dicarboxylate ABC transporter Yes (24-25)
58 WP_056567163.1 C4-dicarboxylate ABC transporter substrate-binding protein Yes (23-24)
59 WP_088156564.1 C4-dicarboxylate ABC transporter substrate-binding protein Yes (34-35)
60 WP_085947802.1 C4-dicarboxylate ABC transporter substrate-binding protein Yes (39-40)
61 WP_088148288.1 carbohydrate ABC transporter substrate-binding protein Yes (22-23)
62 EEQ96006.1 extracellular solute-binding protein Yes (27-28)
63 EHK65400.1 extracellular solute-binding family protein Yes (22-23)
64 ABS16514.1 extracellular solute-binding protein family 3 Yes (23-24)
65 WP_048394203.1 ferrichrome ABC transporter substrate-binding protein Yes (27-28)
66 KGD98287.1 glutamine ABC transporter substrate-bindnig protein Yes (26-27)
67 OLU03919.1 glutamine ABC transporter substrate-binding protein Yes (25-26)
68 KNE26968.1 glutathione ABC transporter substrate-binding protein Yes (28-29)
69 CUJ34547.1 Glutathione-binding protein gsiB precursor Yes (28-29)
70 ADP14939.1 glutathione-binding protein GsiB Yes (28-29)

Appendix C: Extracellular protein analysis 259
No. Accession number Protein
AN
(Weeks)
TS
(Weeks) Secreted*
2 4 6 2 4 6
71 WP_057284748.1 glycine/betaine ABC transporter substrate-binding protein Yes (27-28)
72 WP_063952087.1 hemin ABC transporter substrate-binding protein Yes (18-19)
73 WP_088146328.1 iron ABC transporter substrate-binding protein Yes (29-30)
74 WP_043542652.1 iron ABC transporter substrate-binding protein Yes (24-25)
75 KGD93619.1 iron ABC transporter substrate-binding protein Yes (24-25)
76 OLU06668.1 iron ABC transporter substrate-binding protein Yes (28-29)
77 KMN36448.1 iron ABC transporter substrate-binding protein Yes (33-34)
78 KXO74055.1 iron ABC transporter substrate-binding protein Yes (23-24)
79 KXO75074.1 iron ABC transporter substrate-binding protein Yes (23-24)
80 OCX15298.1 iron ABC transporter substrate-binding protein Yes (22-23)
81 CUJ13421.1 leucine ABC transporter subunit substrate-binding protein Yes (24-25)
82 KGD96135.1 membrane protein Yes (22-23)
83 KOQ40547.1 membrane protein Yes (22-23)
84 WP_008160825.1 metal ABC transporter substrate-binding protein Yes (29-30)
85 WP_088153545.1 molybdate ABC transporter substrate-binding protein Yes (24-25)
86 WP_043212569.1 MULTISPECIES: ABC transporter permease Yes (24-25)
87 WP_062685045.1 MULTISPECIES: ABC transporter substrate-binding protein Yes (30-31)
88 WP_054549123.1 MULTISPECIES: ABC transporter substrate-binding protein Yes (32-33)
89 WP_062683233.1 MULTISPECIES: amino acid ABC transporter substrate-binding protein Yes (23-24)
90 WP_088140661.1 MULTISPECIES: amino acid ABC transporter substrate-binding protein Yes (25-26)
91 WP_063961606.1 MULTISPECIES: amino acid ABC transporter substrate-binding protein Yes (22-23)
92 WP_062683598.1 MULTISPECIES: amino acid ABC transporter substrate-binding protein Yes (22-23)
93 WP_056317574.1 MULTISPECIES: branched chain amino acid ABC transporter substrate-binding protein Yes (25-26)
94 WP_088595533.1 MULTISPECIES: glutamine ABC transporter substrate-binding protein GlnH Yes (26-27)
95 WP_062684476.1 MULTISPECIES: hemin ABC transporter substrate-binding protein Yes (19-20)

260 Appendix C: Extracellular protein analysis
No. Accession number Protein
AN
(Weeks)
TS
(Weeks) Secreted*
2
4
6
2
4
6
96 WP_062682236.1 MULTISPECIES: leucine ABC transporter subunit substrate-binding protein LivK Yes (25-26)
97 WP_034397436.1 MULTISPECIES: outer membrane protein assembly factor BamE Yes (22-23)
98 WP_082601759.1 peptide ABC transporter Yes (31-32)
99 ASC64693.1 peptide ABC transporter substrate-binding protein Yes (28-29)
100 WP_056569658.1 peptide ABC transporter substrate-binding protein Yes (27-28)
101 KGD99111.1 peptide ABC transporter substrate-binding protein Yes (28-29)
102 WP_054550856.1 peptide ABC transporter substrate-binding protein Yes (24-25)
103 WP_088145960.1 phosphate ABC transporter substrate-binding protein PstS Yes (24-25)
104 WP_054423127.1 phosphate/phosphite/phosphonate ABC transporter substrate-binding protein Yes (24-25)
105 WP_043544234.1 polyamine ABC transporter substrate-binding protein Yes (25-26)
106 WP_049054386.1 sugar ABC transporter substrate-binding protein Yes (30-31)
107 KMN40622.1 sugar ABC transporter substrate-binding protein Yes (28-29)
108 WP_061346078.1 sulfate ABC transporter substrate-binding protein Yes (21-22)
109 OLU07503.1 sulfate transporter subunit Yes (27-28)
110 WP_078339521.1 thiamine ABC transporter substrate binding subunit Yes (23-24)
111 WP_088147934.1 transporter Yes (21-22)
112 KGD94171.1 transporter Yes (24-25)
113 AOU92447.1 tripartite tricarboxylate transporter Yes (31-32)
114 WP_008160088.1 tripartite tricarboxylate transporter substrate binding protein Yes (29-30)
115 WP_088146063.1 tripartite tricarboxylate transporter substrate binding protein Yes (30-31)
116 WP_006224550.1 tripartite tricarboxylate transporter substrate binding protein Yes (23-24)
117 WP_088158369.1 tripartite tricarboxylate transporter substrate binding protein Yes (23-24)
118 WP_085947886.1 tripartite tricarboxylate transporter substrate binding protein Yes (23-24)
119 WP_088147839.1 tripartite tricarboxylate transporter substrate binding protein Yes (25-26)

Appendix C: Extracellular protein analysis 261
No. Accession number Protein
AN
(Weeks)
TS
(Weeks) Secreted*
2 4 6 2 4 6
120 WP_088447842.1 tripartite tricarboxylate transporter substrate binding protein Yes (26-27)
121 WP_076518986.1 tripartite tricarboxylate transporter substrate binding protein Yes (28-29)
122 OMG92355.1 urea ABC transporter substrate-binding protein Yes (27-28)
123 OLU07484.1 ABC transporter substrate-binding protein Yes#
124 WP_043547903.1 C4-dicarboxylate ABC transporter Yes#
125 WP_076815687.1 electron transporter Yes#
126 CUJ75845.1 Membrane protein involved in colicin uptake Yes#
127 WP_063959745.1 MULTISPECIES: efflux RND transporter periplasmic adaptor subunit Yes#
Membrane related
128 KGD89451.1 lipoprotein Yes (17-18)
129 WP_043542536.1 lipoprotein, YaeC family Yes (25-26)
130 WP_043546947.1 lipoprotein, YaeC family Yes (24-25)
131 WP_088164242.1 lipoprotein, YaeC family Yes (32-33)
132 KGD96135.1 membrane protein Yes (22-23)
133 WP_043542943.1 membrane protein Yes (20-21)
134 KOQ40547.1 membrane protein Yes (22-23)
135 WP_062681332.1 MULTISPECIES: outer membrane protein assembly factor BamC Yes (18-19)
136 WP_062681256.1 MULTISPECIES: peptidoglycan-associated lipoprotein Yes (22-23)
137 ADP14169.1 NLPA lipoprotein family protein 1 Yes (25-26)
138 WP_088146536.1 peptidoglycan-binding protein Yes (24-25)
139 SIT23972.1 periplasmic chaperone for outer membrane proteins SurA Yes (29-30)
140 CUJ96470.1 Probable phospholipid-binding protein mlaC precursor Yes (28-29)
141 EZP50560.1 putative lipoprotein Yes(23-24)
142 WP_043547462.1 TonB-dependent siderophore receptor Yes (21-22)
143 WP_013395985.1 TonB-dependent siderophore receptor Yes (37-38)

262 Appendix C: Extracellular protein analysis
No. Accession number Protein
AN
(Weeks)
TS
(Weeks) Secreted*
2
4
6
2
4
6
144 WP_088141626.1 TonB-dependent receptor Yes (22-23)
145 WP_082306154.1 TonB-dependent siderophore receptor Yes (22-23)
146 WP_057283984.1 TonB-dependent siderophore receptor Yes (33-34)
147 WP_043548035.1 carboxypeptidase regulatory-like domain-containing protein [ Yes (22-23)
148 WP_079242778.1 SusC/RagA family TonB-linked outer membrane protein Yes (22-23)
149 CUJ75845.1 Membrane protein involved in colicin uptake Yes#
150 WP_054481236.1 MULTISPECIES: phospholipid-binding protein Yes#
151 OWT75564.1 phospholipid-binding protein Yes#
152 KGD93668.1 phospholipid-binding protein Yes#
153 WP_043544462.1 TonB-dependent receptor Yes#
154 WP_043547031.1 TonB-dependent siderophore receptor Yes#
Amino acids and protein
155 KGD95192.1 amino acid-binding protein Yes (24-25)
156 WP_085946870.1 argininosuccinate lyase Yes (16-17)
157 OLU10332.1 argininosuccinate lyase Yes (23-24)
158 CUI77092.1 Leucine-isoleucine-valine-threonine-alanine-binding protein precursor Yes (25-26)
159 KGD89270.1 peptidase Yes (21-22)
160 CUI97220.1 Peptidyl-prolyl cis-trans isomerase cyp18 Yes (21-22)
161 KGD95891.1 peptidylprolyl isomerase Yes (19-20)
162 KGD90035.1 porin Yes (32-33)
163 WP_009370175.1 protein sphX precursor Yes (26-27)
164 WP_088154790.1 serine peptidase Yes (34-35)
165 WP_057286328.1 transglutaminase Yes (26-27)
166 WP_043548331.1 twin-arginine translocation pathway signal protein Yes (34-35)

Appendix C: Extracellular protein analysis 263
No. Accession number Protein
AN
(Weeks)
TS
(Weeks) Secreted*
2 4 6 2 4 6
167 WP_054453210.1 peptidase Yes (21-22)
168 OLU07391.1 S9 family peptidase Yes (23-24)
169 WP_043541316.1 thiol:disulfide interchange protein Yes (27-28)
170 EZP63473.1 Glutamine synthetase Yes#
171 KGD87014.1 glutamine synthetase Yes#
172 KGE00438.1 leucine--tRNA ligase Yes#
173 WP_043547732.1 peptidase M20 Yes#
174 WP_025139792.1 transglutaminase Yes#
175 WP_061346249.1 type I glutamate-ammonia ligase Yes#
176 OLU09649.1 serine peptidase Yes#
177 WP_062682562.1 MULTISPECIES: peptidase Yes#
178 KGD95842.1 sulfoxide reductase catalytic subunit YedY Yes#
179 WP_036567469.1 sarcosine oxidase subunit alpha family protein Yes#
Energy Metabolism
180 ASC63703.1 cytochrome Yes (22-23)
181 WP_068983280.1 cytochrome B6 Yes#
182 WP_088147109.1 cytochrome C biogenesis protein Yes#
183 WP_058665008.1 NAD(P)H-dependent oxidoreductase Yes#
184 WP_088139396.1 energy transducer TonB Yes#
185 WP_008164054.1 electron transfer flavoprotein subunit alpha/FixB family protein Yes#
186 WP_042792534.1 MULTISPECIES: electron transfer flavoprotein subunit alpha/FixB family protein Yes#
Carbohydrate metabolism
187 ADP18914.1 VI polysaccharide biosynthesis protein VipB/TviC Yes#
188 SEJ19686.1 glyceraldehyde-3-phosphate dehydrogenase (NAD+) Yes#
189 WP_078339602.1 alcohol dehydrogenase AdhP
Yes#

264 Appendix C: Extracellular protein analysis
No. Accession number Protein
AN
(Weeks)
TS
(Weeks) Secreted*
2 4 6 2 4 6
190 OPD83684.1 aldehyde dehydrogenase, partial Yes#
Aromatics & Xenobiotic degradation
191 WP_043544412.1 dienelactone hydrolase family protein Yes#
Genetic Information Processing
192 WP_088146602.1 LacI family transcriptional regulator Yes (27-28)
193 OOC51395.1 LacI family transcriptional regulator yes (23-24)
194 WP_016992165.1 MULTISPECIES: ethanolamine utilization protein EutJ Yes (30-31)
195 SDY72801.1 nucleoside-binding protein Yes (24-25)
196 WP_088159385.1 transcription initiation protein Yes#
197 WP_043541446.1 MULTISPECIES: 30S ribosomal protein S16 Yes#
198 KGD95836.1 50S ribosomal protein L32 Yes#
199 KGD92132.1 50S ribosomal protein L35 Yes#
200 OBY85802.1 50S ribosomal protein L6 Yes#
201 KEH14151.1 50S ribosomal protein L27 Yes#
202 WP_088587769.1 nucleotide exchange factor GrpE Yes#
203 KGD93761.1 translocation protein TolB Yes#
204 EHK64371.1 endoribonuclease L-PSP family protein Yes#
205 EHK64178.1 histone-like DNA-binding protein Yes#
206 OLU00781.1 guanylate kinase Yes#
Hypothetical
207 WP_013394864.1 hypothetical protein Yes (22-23)
208 WP_053245268.1 hypothetical protein Yes (27-28)
209 OWT64198.1 hypothetical protein Yes (21-22)
210 WP_088148216.1 hypothetical protein Yes (22-23)

Appendix C: Extracellular protein analysis 265
No. Accession number Protein
AN
(Weeks)
TS
(Weeks) Secreted*
2 4 6 2 4 6
211 KXO76461.1 hypothetical protein Yes (19-20)
212 WP_043541445.1 hypothetical protein Yes (32-33)
213 WP_054500944.1 hypothetical protein yes (23-24)
214 WP_048390832.1 hypothetical protein Yes (26-27)
215 WP_016451823.1 hypothetical protein Yes (25-26)
216 WP_047422111.1 hypothetical protein Yes (34-35)
217 WP_054548726.1 hypothetical protein Yes (26-27)
218 WP_088155737.1 hypothetical protein Yes (26-27)
219 WP_054550744.1 hypothetical protein Yes (26-27)
220 WP_012092824.1 hypothetical protein Yes (22-23)
221 WP_061347242.1 hypothetical protein Yes (26-27)
222 ASC65605.1 hypothetical protein Yes (50-51)
223 KGD93886.1 hypothetical protein Yes (25-26)
224 KMN36246.1 hypothetical protein Yes (23-24)
225 WP_088146808.1 hypothetical protein Yes (29-30)
226 WP_053075708.1 hypothetical protein Yes (26-27)
227 KXO76479.1 hypothetical protein Yes (25-26)
228 WP_053075736.1 hypothetical protein Yes (30-31)
229 WP_088447542.1 hypothetical protein Yes#
230 WP_061344534.1 hypothetical protein Yes#
231 WP_008165350.1 hypothetical protein Yes#
232 WP_048395293.1 hypothetical protein Yes#
233 WP_063584394.1 hypothetical protein Yes#
234 KGE00062.1 hypothetical protein
Yes#

266 Appendix C: Extracellular protein analysis
No. Accession number Protein
AN
(Weeks)
TS
(Weeks) Secreted*
2
4
6
2
4
6
235 SEJ51186.1 hypothetical protein Yes#
236 WP_062681187.1 hypothetical protein Yes#
237 WP_043544879.1 hypothetical protein Yes#
238 WP_088148921.1 hypothetical protein Yes#
239 KOF53565.1 hypothetical protein Yes#
240 WP_052097204.1 hypothetical protein Yes#
241 WP_088448398.1 hypothetical protein Yes#
242 KGD93750.1 hypothetical protein Yes#
243 WP_077418331.1 hypothetical protein Yes#
Mineral absorption
244 WP_061347015.1 iron uptake system protein EfeO Yes (25-26)
245 KGD96157.1 zinc protease Yes (27-28)
246 KGD88097.1 superoxide dismutase Yes (22-23)
247 WP_063643475.1 MULTISPECIES: copper chaperone PCu(A)C Yes (20-21) 248 WP_008167322.1 copper chaperone PCu(A)C Yes (26-27)
249 WP_043541183.1 copper chaperone PCu(A)C Yes (22-23)
250 WP_050446790.1 Co2+/Mg2+ efflux protein ApaG Yes#
251 WP_088598926.1 MULTISPECIES: iron-sulfur cluster insertion protein ErpA Yes#
252 WP_056559067.1 superoxide dismutase Yes#
253 ASC66955.1 superoxide dismutase [Fe] Yes#
Fatty acid metabolism
254 WP_056567753.1 beta-ketoacyl-ACP reductase Yes#
Motility
255 OWT67573.1 elongation factor P Yes#

Appendix C: Extracellular protein analysis 267
No. Accession number Protein
AN
(Weeks)
TS
(Weeks) Secreted*
2 4 6 2 4 6
256 WP_057284408.1 flagellar biosynthesis protein FlgE Yes#
257 WP_076521318.1 flagellar hook protein FliD Yes#
258 OLT99557.1 flagellar hook protein FliD Yes#
259 ASC68125.1 flagellar hook-associated protein 3 Yes#
260 WP_043545126.1 flagellar hook-associated protein FlgK Yes#
Other
261 WP_043547752.1 dehydratase Yes (24-25)
262 WP_013391069.1 DUF3300 domain-containing protein Yes (24-25)
263 WP_088148638.1 DUF4198 domain-containing protein Yes (21-22)
264 WP_088589970.1 DUF4198 domain-containing protein Yes (21-22)
265 WP_082308508.1 DUF4198 domain-containing protein Yes (21-22)
266 WP_062683029.1 MULTISPECIES: BON domain-containing protein Yes (24-25)
267 WP_010658359.1 MULTISPECIES: DUF1344 domain-containing protein Yes (21-22)
268 WP_062685071.1 MULTISPECIES: DUF4136 domain-containing protein Yes (20-21)
269 EJO30942.1 extra-cytoplasmic solute receptor family protein 174 Yes (23-24)
270 WP_065345553.1 Ig family protein Yes (20-21)
271 WP_061345471.1 immunogenic protein Yes (28-29)
272 WP_062681529.1 MULTISPECIES: immunogenic protein Yes (25-26)
273 WP_061347347.1 ligand-binding protein SH3 Yes (22-23)
274 WP_050447996.1 nitrate reductase Yes (24-25)
275 WP_047422444.1 OmpA family protein Yes (21-22)

268 Appendix C: Extracellular protein analysis
No. Accession number Protein
AN
(Weeks)
TS
(Weeks) Secreted*
2 4 6 2 4 6
276 EHK66933.1 periplasmic protein Yes (20-21)
277 WP_088159604.1 peroxiredoxin Yes (21-22)
278 WP_052097643.1 polyisoprenoid-binding protein Yes (23-24)
279 WP_076408159.1 polyketide cyclase Yes (24-25)
280 WP_088148447.1 receptor Yes (28-29)
281 CUI89627.1 Uncharacterised protein Yes (22-23)
282 SFB23670.1 2-oxoglutarate dehydrogenase E2 component Yes#
283 WP_076519821.1 arsenate reductase Yes#
284 ADP18479.1 carboxymethylenebutenolidase 4 Yes#
285 WP_062676177.1 catalase/peroxidase HPI Yes#
286 WP_071633427.1 ferredoxin family protein Yes#
287 WP_061347851.1 hemolysin expression modulating protein Yes#
288 WP_088138258.1 histone Yes#
289 WP_076519581.1 MULTISPECIES: peroxidase Yes#
290 WP_088141995.1 NAD(P)H-dependent oxidoreductase Yes#
291 WP_043547559.1 peroxiredoxin OsmC Yes#
292 WP_088141124.1 OsmC family peroxiredoxin Yes#
293 KGD97163.1 peroxidase Yes#
294 WP_043543107.1 nitronate monooxygenase Yes#
295 KGY26538.1 nucleoid-associated protein Yes#
296 OLU09883.1 Phasin (PHA-granule associated protein) Yes#
297 WP_054550133.1 SRPBCC domain-containing protein Yes#

Appendix C: Extracellular protein analysis 269
No. Accession number Protein
AN
(Weeks)
TS
(Weeks) Secreted*
2 4 6 2 4 6
298 OLU07233.1 TIGR01244 family protein Yes#
299 WP_088153633.1 DUF2950 domain-containing protein Yes#
Notes:
AN – Alternating anaerobic-aerobic treatment, TS – Two stage anaerobic-aerobic treatment
* Numbers given within brackets correspond to the cleavage site location within the amino acid sequence of classically secreted proteins.
# Non-classically secreted proteins.
Protein yield
Negative Low <----------> high

270 Appendix C: Extracellular protein analysis
Table C.2 Relative abundance of extracellular proteins of bacterial consortium Achromobacter sp. NP03, Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05
found in the culture supernatant under alternating (AN) anaerobic-aerobic conditions as a heat map. Classically secreted proteins were predicted using the
SignalP 3.0 Server and the non-classically secreted proteins were predicted using the SecretomeP 2.0 Server. Week 1, 3, 5 were kept under anaerobic and
week 2, 4, 6 were kept under aerobic conditions.
No. Accession number Protein Weeks
Secreted* 1 2 3 4 5 6
Transport related
1 WP_054452161.1 ABC transporter Yes (28-29)
2 WP_043545028.1 ABC transporter Yes (27-28)
3 WP_088148777.1 ABC transporter permease Yes (22-23)
4 WP_088587964.1 ABC transporter permease Yes (24-25)
5 WP_082601631.1 ABC transporter permease Yes (30-31)
6 WP_050448859.1 ABC transporter permease Yes (29-30)
7 WP_088154810.1 ABC transporter permease Yes (24-25)
8 WP_024899300.1 ABC transporter permease Yes (20-21)
9 KNY13962.1 ABC transporter permease Yes (20-21)
10 WP_088447256.1 ABC transporter substrate-binding protein Yes (28-29)
11 WP_088447395.1 ABC transporter substrate-binding protein Yes (36-37)
12 WP_088146376.1 ABC transporter substrate-binding protein Yes (33-34)
13 WP_088147153.1 ABC transporter substrate-binding protein Yes (22-23)
14 ASC66145.1 ABC transporter substrate-binding protein Yes (22-23)
15 WP_088147688.1 ABC transporter substrate-binding protein Yes (22-23)
16 ASC63496.1 ABC transporter substrate-binding protein Yes (22-23)
17 ASC64180.1 ABC transporter substrate-binding protein Yes (20-21)
18 ASC67639.1 ABC transporter substrate-binding protein Yes (24-25)

Appendix C: Extracellular protein analysis 271
No. Accession number Protein Weeks
Secreted* 1 2 3 4 5 6
19 OWT57704.1 ABC transporter substrate-binding protein Yes (35-36)
20 WP_054454039.1 ABC transporter substrate-binding protein Yes (23-24)
21 OAD17456.1 ABC transporter substrate-binding protein Yes (26-27)
22 WP_025139398.1 ABC transporter substrate-binding protein Yes (23-24)
23 OCZ60996.1 ABC transporter substrate-binding protein Yes (26-27)
24 OAE68865.1 ABC transporter substrate-binding protein Yes (26-27)
25 WP_076521077.1 ABC transporter substrate-binding protein Yes (23-24)
26 WP_043547493.1 ABC transporter substrate-binding protein Yes (26-27)
27 KGD96204.1 ABC transporter substrate-binding protein Yes (25-26)
28 WP_054456606.1 ABC transporter substrate-binding protein Yes (22-23)
29 WP_088155937.1 ABC transporter substrate-binding protein Yes (23-24)
30 OLT99158.1 ABC transporter substrate-binding protein Yes (21-22)
31 WP_088154240.1 ABC transporter substrate-binding protein Yes (23-24)
32 OLU02054.1 ABC transporter substrate-binding protein Yes (24-25)
33 WP_088138972.1 ABC transporter substrate-binding protein Yes (21-22)
34 WP_088138276.1 ABC transporter substrate-binding protein Yes (23-24)
35 OLU08696.1 ABC transporter substrate-binding protein Yes (28-29)
36 OWG18014.1 ABC transporter substrate-binding protein Yes (25-26)
37 WP_085982324.1 ABC transporter substrate-binding protein Yes (21-22)
38 WP_061347109.1 ABC transporter substrate-binding protein Yes (22-23)
39 KXO76717.1 ABC transporter substrate-binding protein Yes (27-28)
40 OOC59559.1 ABC transporter substrate-binding protein Yes (37-38)
41 KXO75178.1 amino acid ABC transporter Yes (21-22)
42 WP_088589881.1 amino acid ABC transporter substrate-binding protein Yes (22-23)
43 WP_061306665.1 amino acid ABC transporter substrate-binding protein Yes (22-23)
44 WP_054420698.1 amino acid ABC transporter substrate-binding protein Yes (22-23)

272 Appendix C: Extracellular protein analysis
No. Accession number Protein
Weeks
Secreted* 1
2
3
4
5
6
45 KGD90086.1 amino acid ABC transporter substrate-binding protein Yes (22-23)
46 KGE00355.1 amino acid ABC transporter substrate-binding protein Yes (26-27)
47 KGD94165.1 amino acid ABC transporter substrate-binding protein Yes (23-24)
48 KNE29344.1 amino acid ABC transporter substrate-binding protein Yes (31-32)
49 OLU07976.1 amino acid ABC transporter substrate-binding protein Yes (22-23)
50 WP_016992241.1 amino acid ABC transporter substrate-binding protein Yes (28-29)
51 WP_025141122.1 amino acid ABC transporter substrate-binding protein Yes (37-38)
52 WP_043541220.1 amino acid ABC transporter substrate-binding protein Yes (35-36)
53 WP_088146425.1 branched-chain amino acid ABC transporter substrate-binding protein Yes (23-24)
54 WP_088141350.1 branched-chain amino acid ABC transporter substrate-binding protein Yes (25-26)
55 AKP90359.1 Branched-chain amino acid ABC transporter, amino acid-binding protein Yes (28-29)
56 WP_054450546.1 branched-chain amino acid ABC transporter substrate-binding protein Yes (24-25)
57 KGY25861.1 C4-dicarboxylate ABC transporter Yes (24-25)
58 WP_056567163.1 C4-dicarboxylate ABC transporter substrate-binding protein Yes (23-24)
59 WP_088156564.1 C4-dicarboxylate ABC transporter substrate-binding protein Yes (34-35)
60 WP_085947802.1 C4-dicarboxylate ABC transporter substrate-binding protein Yes (39-40)
61 WP_088148288.1 carbohydrate ABC transporter substrate-binding protein Yes (22-23)
62 EEQ96006.1 extracellular solute-binding protein Yes (27-28)
63 EHK65400.1 extracellular solute-binding family protein Yes (22-23)
64 ABS16514.1 extracellular solute-binding protein family 3 Yes (23-24)
65 WP_048394203.1 ferrichrome ABC transporter substrate-binding protein Yes (27-28)
66 KGD98287.1 glutamine ABC transporter substrate-bindnig protein Yes (26-27)
67 OLU03919.1 glutamine ABC transporter substrate-binding protein Yes (25-26)
68 KNE26968.1 glutathione ABC transporter substrate-binding protein Yes (28-29)

Appendix C: Extracellular protein analysis 273
No. Accession number Protein Weeks
Secreted* 1 2 3 4 5 6
69 CUJ34547.1 Glutathione-binding protein gsiB precursor Yes (28-29)
70 ADP14939.1 glutathione-binding protein GsiB Yes (28-29)
71 WP_057284748.1 glycine/betaine ABC transporter substrate-binding protein Yes (27-28)
72 WP_063952087.1 hemin ABC transporter substrate-binding protein Yes (18-19)
73 WP_088146328.1 iron ABC transporter substrate-binding protein Yes (29-30)
74 WP_043542652.1 iron ABC transporter substrate-binding protein Yes (24-25)
75 KGD93619.1 iron ABC transporter substrate-binding protein Yes (24-25)
76 OLU06668.1 iron ABC transporter substrate-binding protein Yes (28-29)
77 KMN36448.1 iron ABC transporter substrate-binding protein Yes (33-34)
78 KXO74055.1 iron ABC transporter substrate-binding protein Yes (23-24)
79 KXO75074.1 iron ABC transporter substrate-binding protein Yes (23-24)
80 OCX15298.1 iron ABC transporter substrate-binding protein Yes (22-23)
81 CUJ13421.1 leucine ABC transporter subunit substrate-binding protein Yes (24-25)
82 KGD96135.1 membrane protein Yes (22-23)
83 KOQ40547.1 membrane protein Yes (22-23)
84 WP_008160825.1 metal ABC transporter substrate-binding protein Yes (29-30)
85 WP_088153545.1 molybdate ABC transporter substrate-binding protein Yes (24-25)
86 WP_043212569.1 MULTISPECIES: ABC transporter permease Yes (24-25)
87 WP_062685045.1 MULTISPECIES: ABC transporter substrate-binding protein Yes (30-31)
88 WP_054549123.1 MULTISPECIES: ABC transporter substrate-binding protein Yes (32-33)
89 WP_062683233.1 MULTISPECIES: amino acid ABC transporter substrate-binding protein Yes (23-24)
90 WP_088140661.1 MULTISPECIES: amino acid ABC transporter substrate-binding protein Yes (25-26)
91 WP_063961606.1 MULTISPECIES: amino acid ABC transporter substrate-binding protein Yes (22-23)
92 WP_062683598.1 MULTISPECIES: amino acid ABC transporter substrate-binding protein Yes (22-23)
93 WP_056317574.1 MULTISPECIES: branched chain amino acid ABC transporter substrate-binding protein Yes (25-26)
94 WP_088595533.1 MULTISPECIES: glutamine ABC transporter substrate-binding protein GlnH Yes (26-27)

274 Appendix C: Extracellular protein analysis
No.
Accession number
Protein
Weeks
Secreted*
1 2 3 4 5 6
95 WP_062684476.1 MULTISPECIES: hemin ABC transporter substrate-binding protein Yes (19-20)
96 WP_062682236.1 MULTISPECIES: leucine ABC transporter subunit substrate-binding protein LivK Yes (25-26)
97 WP_034397436.1 MULTISPECIES: outer membrane protein assembly factor BamE Yes (22-23)
98 WP_082601759.1 peptide ABC transporter Yes (31-32)
99 ASC64693.1 peptide ABC transporter substrate-binding protein Yes (28-29)
100 WP_056569658.1 peptide ABC transporter substrate-binding protein Yes (27-28)
101 KGD99111.1 peptide ABC transporter substrate-binding protein Yes (28-29)
102 WP_054550856.1 peptide ABC transporter substrate-binding protein Yes (24-25)
103 WP_088145960.1 phosphate ABC transporter substrate-binding protein PstS Yes (24-25)
104 WP_054423127.1 phosphate/phosphite/phosphonate ABC transporter substrate-binding protein Yes (24-25)
105 WP_043544234.1 polyamine ABC transporter substrate-binding protein Yes (25-26)
106 WP_049054386.1 sugar ABC transporter substrate-binding protein Yes (30-31)
107 KMN40622.1 sugar ABC transporter substrate-binding protein Yes (28-29)
108 WP_061346078.1 sulfate ABC transporter substrate-binding protein Yes (21-22)
109 OLU07503.1 sulfate transporter subunit Yes (27-28)
110 WP_078339521.1 thiamine ABC transporter substrate binding subunit Yes (23-24)
111 WP_088147934.1 transporter Yes (21-22)
112 KGD94171.1 transporter Yes (24-25)
113 AOU92447.1 tripartite tricarboxylate transporter Yes (31-32)
114 WP_008160088.1 tripartite tricarboxylate transporter substrate binding protein Yes (29-30)
115 WP_088146063.1 tripartite tricarboxylate transporter substrate binding protein Yes (30-31)
116 WP_006224550.1 tripartite tricarboxylate transporter substrate binding protein Yes (23-24)
117 WP_088158369.1 tripartite tricarboxylate transporter substrate binding protein Yes (23-24)
118 WP_085947886.1 tripartite tricarboxylate transporter substrate binding protein Yes (23-24)

Appendix C: Extracellular protein analysis 275
No. Accession number Protein Weeks
Secreted* 1 2 3 4 5 6
119 WP_088147839.1 tripartite tricarboxylate transporter substrate binding protein Yes (25-26)
120 WP_088447842.1 tripartite tricarboxylate transporter substrate binding protein Yes (26-27)
121 WP_076518986.1 tripartite tricarboxylate transporter substrate binding protein Yes (28-29)
122 OMG92355.1 urea ABC transporter substrate-binding protein Yes (27-28)
123 OLU07484.1 ABC transporter substrate-binding protein Yes#
124 WP_043547903.1 C4-dicarboxylate ABC transporter Yes#
125 WP_076815687.1 electron transporter Yes#
126 CUJ75845.1 Membrane protein involved in colicin uptake Yes#
127 WP_063959745.1 MULTISPECIES: efflux RND transporter periplasmic adaptor subunit Yes#
Membrane related
128 KGD89451.1 lipoprotein Yes (17-18)
129 WP_043542536.1 lipoprotein, YaeC family Yes (25-26)
130 WP_043546947.1 lipoprotein, YaeC family Yes (24-25)
131 WP_088164242.1 lipoprotein, YaeC family Yes (32-33)
132 KGD96135.1 membrane protein Yes (22-23)
133 WP_043542943.1 membrane protein Yes (20-21)
134 KOQ40547.1 membrane protein Yes (22-23)
135 WP_062681332.1 MULTISPECIES: outer membrane protein assembly factor BamC Yes (18-19)
136 WP_062681256.1 MULTISPECIES: peptidoglycan-associated lipoprotein Yes (22-23)
137 ADP14169.1 NLPA lipoprotein family protein 1 Yes (25-26)
138 WP_088146536.1 peptidoglycan-binding protein Yes (24-25)
139 SIT23972.1 periplasmic chaperone for outer membrane proteins SurA Yes (29-30)
140 CUJ96470.1 Probable phospholipid-binding protein mlaC precursor Yes (28-29)
141 EZP50560.1 putative lipoprotein Yes(23-24)
142 WP_043547462.1 TonB-dependent siderophore receptor Yes (21-22)

276 Appendix C: Extracellular protein analysis
No. Accession number Protein
Weeks
Secreted* 1
2
3
4
5
6
Amino acids and protein
143 WP_013395985.1 TonB-dependent siderophore receptor Yes (37-38)
144 WP_088141626.1 TonB-dependent receptor Yes (22-23)
145 WP_082306154.1 TonB-dependent siderophore receptor Yes (22-23)
146 WP_057283984.1 TonB-dependent siderophore receptor Yes (33-34)
147 WP_043548035.1 carboxypeptidase regulatory-like domain-containing protein [ Yes (22-23)
148 WP_079242778.1 SusC/RagA family TonB-linked outer membrane protein Yes (22-23)
149 CUJ75845.1 Membrane protein involved in colicin uptake Yes#
150 WP_054481236.1 MULTISPECIES: phospholipid-binding protein Yes#
151 OWT75564.1 phospholipid-binding protein Yes#
152 KGD93668.1 phospholipid-binding protein Yes#
153 WP_043544462.1 TonB-dependent receptor Yes#
154 WP_043547031.1 TonB-dependent siderophore receptor Yes#
155 KGD95192.1 amino acid-binding protein Yes (24-25)
156 WP_085946870.1 argininosuccinate lyase Yes (16-17)
157 OLU10332.1 argininosuccinate lyase Yes (23-24)
158 CUI77092.1 Leucine-isoleucine-valine-threonine-alanine-binding protein precursor Yes (25-26)
159 KGD89270.1 peptidase Yes (21-22)
160 CUI97220.1 Peptidyl-prolyl cis-trans isomerase cyp18 Yes (21-22)
161 KGD95891.1 peptidylprolyl isomerase Yes (19-20)
162 KGD90035.1 porin Yes (32-33)
163 WP_009370175.1 protein sphX precursor Yes (26-27)
164 WP_088154790.1 serine peptidase Yes (34-35)
165 WP_057286328.1 transglutaminase Yes (26-27)
166 WP_043548331.1 twin-arginine translocation pathway signal protein Yes (34-35)

Appendix C: Extracellular protein analysis 277
No. Accession number Protein Weeks
Secreted* 1 2 3 4 5 6
167 WP_054453210.1 peptidase Yes (21-22)
168 OLU07391.1 S9 family peptidase Yes (23-24)
169 WP_043541316.1 thiol:disulfide interchange protein Yes (27-28)
170 EZP63473.1 Glutamine synthetase Yes#
171 KGD87014.1 glutamine synthetase Yes#
172 KGE00438.1 leucine--tRNA ligase Yes#
173 WP_043547732.1 peptidase M20 Yes#
174 WP_025139792.1 transglutaminase Yes#
175 WP_061346249.1 type I glutamate-ammonia ligase Yes#
176 OLU09649.1 serine peptidase [Achromobacter xylosoxidans] Yes#
177 WP_062682562.1 MULTISPECIES: peptidase Yes#
178 KGD95842.1 sulfoxide reductase catalytic subunit YedY Yes#
179 WP_036567469.1 sarcosine oxidase subunit alpha family protein Yes#
Energy Metabolism
180 ASC63703.1 cytochrome Yes (22-23)
181 WP_068983280.1 cytochrome B6 Yes#
182 WP_088147109.1 cytochrome C biogenesis protein Yes#
183 WP_058665008.1 NAD(P)H-dependent oxidoreductase Yes#
184 WP_088139396.1 energy transducer TonB Yes#
185 WP_008164054.1 electron transfer flavoprotein subunit alpha/FixB family protein Yes#
186 WP_042792534.1 MULTISPECIES: electron transfer flavoprotein subunit alpha/FixB family protein Yes#
Carbohydrate metabolism
187 ADP18914.1 VI polysaccharide biosynthesis protein VipB/TviC Yes#
188 SEJ19686.1 glyceraldehyde-3-phosphate dehydrogenase (NAD+) Yes#
189 WP_078339602.1 alcohol dehydrogenase AdhP Yes#
190 OPD83684.1 aldehyde dehydrogenase, partial Yes#

278 Appendix C: Extracellular protein analysis
No. Accession number Protein Weeks
Secreted* 1 2 3 4 5 6
Aromatics & Xenobiotic degradation
191 WP_043544412.1 dienelactone hydrolase family protein Yes#
Genetic Information Processing
192 WP_088146602.1 LacI family transcriptional regulator Yes (27-28)
193 OOC51395.1 LacI family transcriptional regulator yes (23-24)
194 WP_016992165.1 MULTISPECIES: ethanolamine utilization protein EutJ Yes (30-31)
195 SDY72801.1 nucleoside-binding protein Yes (24-25)
196 WP_088159385.1 transcription initiation protein Yes#
197 WP_043541446.1 MULTISPECIES: 30S ribosomal protein S16 Yes#
198 KGD95836.1 50S ribosomal protein L32 Yes#
199 KGD92132.1 50S ribosomal protein L35 Yes#
200 OBY85802.1 50S ribosomal protein L6 Yes#
201 KEH14151.1 50S ribosomal protein L27 Yes#
202 WP_088587769.1 nucleotide exchange factor GrpE Yes#
203 KGD93761.1 translocation protein TolB Yes#
204 EHK64371.1 endoribonuclease L-PSP family protein Yes#
205 EHK64178.1 histone-like DNA-binding protein Yes#
206 OLU00781.1 guanylate kinase Yes#
Hypothetical
207 WP_013394864.1 hypothetical protein Yes (22-23)
208 WP_053245268.1 hypothetical protein Yes (27-28)
209 OWT64198.1 hypothetical protein Yes (21-22)
210 WP_088148216.1 hypothetical protein Yes (22-23)

Appendix C: Extracellular protein analysis 279
No. Accession number Protein Weeks
Secreted* 1 2 3 4 5 6
211 KXO76461.1 hypothetical protein Yes (19-20)
212 WP_043541445.1 hypothetical protein Yes (32-33)
213 WP_054500944.1 hypothetical protein yes (23-24)
214 WP_048390832.1 hypothetical protein Yes (26-27)
215 WP_016451823.1 hypothetical protein Yes (25-26)
216 WP_047422111.1 hypothetical protein Yes (34-35)
217 WP_054548726.1 hypothetical protein Yes (26-27)
218 WP_088155737.1 hypothetical protein Yes (26-27)
219 WP_054550744.1 hypothetical protein Yes (26-27)
220 WP_012092824.1 hypothetical protein Yes (22-23)
221 WP_061347242.1 hypothetical protein Yes (26-27)
222 ASC65605.1 hypothetical protein Yes (50-51)
223 KGD93886.1 hypothetical protein Yes (25-26)
224 KMN36246.1 hypothetical protein Yes (23-24)
225 WP_088146808.1 hypothetical protein Yes (29-30)
226 WP_053075708.1 hypothetical protein Yes (26-27)
227 KXO76479.1 hypothetical protein Yes (25-26)
228 WP_053075736.1 hypothetical protein Yes (30-31)
229 WP_088447542.1 hypothetical protein Yes#
230 WP_061344534.1 hypothetical protein Yes#
231 WP_008165350.1 hypothetical protein Yes#
232 WP_048395293.1 hypothetical protein Yes# 233 WP_063584394.1 hypothetical protein Yes#
234 KGE00062.1 hypothetical protein Yes#
235 SEJ51186.1 hypothetical protein Yes#

280 Appendix C: Extracellular protein analysis
No. Accession number Protein
Weeks
Secreted* 1
2
3
4
5
6
236 WP_062681187.1 hypothetical protein Yes#
237 WP_043544879.1 hypothetical protein Yes#
238 WP_088148921.1 hypothetical protein Yes#
239 KOF53565.1 hypothetical protein Yes#
240 WP_052097204.1 hypothetical protein Yes#
241 WP_088448398.1 hypothetical protein Yes#
242 KGD93750.1 hypothetical protein Yes#
243 WP_077418331.1 hypothetical protein Yes#
Mineral absorption
244 WP_061347015.1 iron uptake system protein EfeO Yes (25-26)
245 KGD96157.1 zinc protease Yes (27-28)
246 KGD88097.1 superoxide dismutase Yes (22-23)
247 WP_063643475.1 MULTISPECIES: copper chaperone PCu(A)C Yes (20-21)
248 WP_008167322.1 copper chaperone PCu(A)C Yes (26-27)
249 WP_043541183.1 copper chaperone PCu(A)C Yes (22-23)
250 WP_050446790.1 Co2+/Mg2+ efflux protein ApaG Yes#
251 WP_088598926.1 MULTISPECIES: iron-sulfur cluster insertion protein ErpA Yes#
252 WP_056559067.1 superoxide dismutase Yes#
253 ASC66955.1 superoxide dismutase [Fe] Yes#
Fatty acid metabolism
254 WP_056567753.1 beta-ketoacyl-ACP reductase Yes#
Motility
255 OWT67573.1 elongation factor P Yes#
256 WP_057284408.1 flagellar biosynthesis protein FlgE Yes#
257 WP_076521318.1 flagellar hook protein FliD Yes#

Appendix C: Extracellular protein analysis 281
No. Accession number Protein Weeks
Secreted* 1 2 3 4 5 6
258 OLT99557.1 flagellar hook protein FliD Yes#
259 ASC68125.1 flagellar hook-associated protein 3 Yes#
260 WP_043545126.1 flagellar hook-associated protein FlgK Yes#
Others
261 WP_043547752.1 dehydratase Yes (24-25)
262 WP_013391069.1 DUF3300 domain-containing protein Yes (24-25)
263 WP_088148638.1 DUF4198 domain-containing protein Yes (21-22)
264 WP_088589970.1 DUF4198 domain-containing protein Yes (21-22)
265 WP_082308508.1 DUF4198 domain-containing protein Yes (21-22)
266 WP_062683029.1 MULTISPECIES: BON domain-containing protein Yes (24-25)
267 WP_010658359.1 MULTISPECIES: DUF1344 domain-containing protein Yes (21-22)
268 WP_062685071.1 MULTISPECIES: DUF4136 domain-containing protein Yes (20-21)
269 EJO30942.1 extra-cytoplasmic solute receptor family protein 174 Yes (23-24)
270 WP_065345553.1 Ig family protein Yes (20-21)
271 WP_061345471.1 immunogenic protein Yes (28-29)
272 WP_062681529.1 MULTISPECIES: immunogenic protein Yes (25-26)
273 WP_061347347.1 ligand-binding protein SH3 Yes (22-23)
274 WP_050447996.1 nitrate reductase Yes (24-25)
275 WP_047422444.1 OmpA family protein Yes (21-22)
276 EHK66933.1 periplasmic protein Yes (20-21)
277 WP_088159604.1 peroxiredoxin Yes (21-22)
278 WP_052097643.1 polyisoprenoid-binding protein Yes (23-24)
279 WP_076408159.1 polyketide cyclase Yes (24-25)
280 WP_088148447.1 receptor Yes (28-29)
281 CUI89627.1 Uncharacterised protein Yes (22-23)
282 WP_076519821.1 arsenate reductase Yes#

282 Appendix C: Extracellular protein analysis
No. Accession number Protein Weeks Secreted* 1 2 3 4 5 6
283 ADP18479.1 carboxymethylenebutenolidase 4 Yes#
284 WP_062676177.1 catalase/peroxidase HPI Yes#
285 WP_071633427.1 ferredoxin family protein Yes#
286 WP_061347851.1 hemolysin expression modulating protein Yes#
287 WP_088138258.1 histone Yes#
288 WP_076519581.1 MULTISPECIES: peroxidase Yes#
289 WP_088141995.1 NAD(P)H-dependent oxidoreductase Yes#
290 WP_043547559.1 peroxiredoxin OsmC Yes#
291 WP_088141124.1 OsmC family peroxiredoxin Yes#
292 KGD95842.1 sulfoxide reductase catalytic subunit YedY Yes#
293 KGD97163.1 peroxidase Yes#
294 WP_043543107.1 nitronate monooxygenase Yes#
295 KGY26538.1 nucleoid-associated protein Yes#
296 OLU09883.1 Phasin (PHA-granule associated protein) Yes#
297 WP_054550133.1 SRPBCC domain-containing protein Yes#
298 OLU07233.1 TIGR01244 family protein Yes#
299 WP_088153633.1 DUF2950 domain-containing protein Yes#
Notes:
* Numbers given within brackets correspond to the cleavage site location within the amino acid sequence of classically secreted proteins.
# Non-classically secreted proteins.
Week 1, 3 and 5 were maintained under anaerobic conditions and week 2, 4 and 6 were maintained under aerobic conditions.
Protein yield
Negative Low <----------> high

Appendix C: Extracellular protein analysis 283
Table C.3 Detailed functional classification of predicted non-secretory proteins in the culture
supernatant of the bacterial consortium Achromobacter sp. NP03, Ochrobactrum sp. NP04
and Lysinibacillus sp. NP05 grown in minimal salt medium using 50 mg/L Aroclor 1260 as the
sole source of carbon
Function Accession No. nnumbernumber
Protein
Aromatics & Xenobiotic degradation
OWG17491.1 glutathione S-transferase
ASC68025.1 glutathione peroxidase
WP_088447182.1
carboxymuconolactone decarboxylase family protein WP_043547979.
1 carboxymuconolactone decarboxylase family protein WP_088164555.
1 glutathione-dependent disulfide-bond oxidoreductase WP_088146345.
1 glutathione S-transferase
KGD95298.1 carboxymuconolactone decarboxylase
WP_006391478.1
glutathione S-transferase
WP_043546720.1
glutathione S-transferase
KGD86957.1 glutathione S-transferase
KGD90634.1 3-oxoadipate CoA-transferase
KGD95901.1 carboxymuconolactone decarboxylase
WP_013391342.1
2-aminobenzoate-CoA ligase
WP_088139343.1
carboxymethylenebutenolidase
WP_088146045.1
phenylphosphate carboxylase subunit delta
OAD15100.1 4-oxalocrotonate tautomerase
Carbohydrate metabolism
WP_048395937.1
MULTISPECIES: malate synthase A
OLE09158.1 malate synthase G
KXO78488.1 glutamine--fructose-6-phosphate aminotransferase
CUI32503.1 L-lactate dehydrogenase
A9BQA5.1 Succinate--CoA ligase [ADP-forming] subunit beta
APE47142.1 succinate--CoA ligase subunit alpha
WP_088154574.1
malate synthase G
WP_043544785.1
carbohydrate kinase family protein
KGD89899.1 fructose-1,6-bisphosphate aldolase
WP_062684727.1
acyl-ACP--UDP-N-acetylglucosamine O-acyltransferase KIU67857.1 isocitrate lyase
KGE00280.1 isocitrate lyase
EHK65803.1 fructose-1,6-bisphosphate aldolase
AMG47497.1 fructose-bisphosphatase
WP_088148834.1
malate synthase G
OWT65266.1 class II fumarate hydratase
WP_004233046.1
MULTISPECIES: isocitrate lyase
KGD95789.1 succinyl-CoA:3-ketoacid-CoA transferase
WP_008158821.1
isocitrate dehydrogenase (NADP(+))
OAD13600.1 succinate--CoA ligase subunit beta
CUI44931.1 Malate dehydrogenase
WP_061344542.1
malate dehydrogenase
WP_043542435.1
2-dehydro-3-deoxy-phosphogluconate aldolase
WP_088138267.1
glutamate--tRNA ligase
OWT65337.1 inositol monophosphatase
WP_043542539.1
glycosyl hydrolase

284 Appendix C: Extracellular protein analysis
Function Accession No. Protein
WP_061347956.1
NAD-glutamate dehydrogenase
ASC68281.1 N-formylglutamate deformylase
WP_046807028.1
triose-phosphate isomerase
KGD98528.1 3-isopropylmalate dehydrogenase
OJV49620.1 NAD-dependent epimerase
WP_006389696.1
NAD dependent epimerase/dehydratase
Elongation factors and chaperones
SDY63415.1 chaperonin GroEL
WP_081955120.1
Tir chaperone family protein
KZK25390.1 ATP-dependent chaperone ClpB
KGD90204.1 molecular chaperone GroEL
KRB12805.1 Fe-S protein assembly chaperone HscA
KRC86368.1 chaperonin
WP_054451905.1
chaperonin GroEL
OCZ53579.1 translation elongation factor G
KZK30311.1 elongation factor Tu
WP_088148204.1
elongation factor G
WP_088140169.1
elongation factor Ts
Genetic Information Processing
OLU08504.1 asparaginyl/glutamyl-tRNA amidotransferase subunit C WP_043543299.
1 ribonuclease III
WP_057283073.1
non-canonical purine NTP pyrophosphatase
KOQ46086.1 50S ribosomal protein L29
WP_088446340.1
nicotinate-nucleotide diphosphorylase (carboxylating) WP_062681662.
1 MULTISPECIES: GntR family transcriptional regulator
KFJ12144.1 ribosomal protein L19
KOF53552.1 ATP synthase F0F1 subunit epsilon
KOF53046.1 transcriptional regulator
KNE28388.1 glutamyl-tRNA amidotransferase
KEH14150.1 50S ribosomal protein L21
WP_008164520.1
ribose-5-phosphate isomerase RpiA
KOQ22336.1 DNA-directed RNA polymerase subunit omega
ADP15126.1 translation initiation factor IF-2
KGD94250.1 transcriptional regulator
WP_043211401.1
MULTISPECIES: transcription elongation factor GreB
WP_061344667.1
DNA starvation/stationary phase protection protein Dps APX77825.1 50S ribosomal protein L24
APE50426.1 translation elongation factor Ts
OLU10224.1 DNA polymerase III subunit beta
WP_043549030.1
transcription termination/antitermination protein NusA KGD85622.1 50S ribosomal protein L9
WP_062683079.1
MULTISPECIES: 50S ribosomal protein L25
CUJ77722.1 Transcription antitermination protein nusG
ASC68086.1 30S ribosomal protein S2
WP_043545730.1
MULTISPECIES: DNA-binding response regulator
ASC68083.1 ribosome-recycling factor
OWT65989.1 adenylate kinase
OAE68850.1 DNA starvation/stationary phase protection protein

Appendix C: Extracellular protein analysis 285
Function Accession No. nnumbernumber
Protein
EHK64962.1 acetaldehyde dehydrogenase
CUI94695.1 DnaK suppressor protein
WP_071633166.1
heat-inducible transcriptional repressor HrcA
ASC68256.1 exodeoxyribonuclease III
WP_088165862.1
phosphoribosylaminoimidazolesuccinocarboxamide synthase WP_062682787.
1 MULTISPECIES: transcriptional repressor LexA
WP_056568752.1
ferric iron uptake transcriptional regulator
OFL34502.1 DNA-directed RNA polymerase subunit alpha
BAP44485.1 transaldolase B
WP_088148869.1
molybdenum-dependent transcriptional regulator
WP_057285634.1
GTPase ObgE
WP_076519393.1
MULTISPECIES: GTPase Era
Hypothetical WP_028691874.1
MULTISPECIES: hypothetical protein
AIK41363.1 hypothetical protein DR92_4327
WP_062681152.1
MULTISPECIES: hypothetical protein
KXO79421.1 hypothetical protein AYJ56_01890
WP_062681189.1
MULTISPECIES: hypothetical protein
WP_043548951.1
hypothetical protein
WP_006391526.1
hypothetical protein
WP_043212744.1
MULTISPECIES: hypothetical protein
WP_088141988.1
hypothetical protein
OCX62666.1 hypothetical protein BFM98_01295
EIK53096.1 hypothetical protein YO5_02683
ASC64511.1 hypothetical protein B9P52_09445
KGE01814.1 hypothetical protein JL37_00900
WP_054458252.1
MULTISPECIES: hypothetical protein
WP_036077843.1
hypothetical protein
WP_043544852.1
hypothetical protein
WP_088138363.1
hypothetical protein
WP_082775659.1
hypothetical protein
WP_076521683.1
hypothetical protein
OLU09627.1 hypothetical protein BVK87_03785
Lipid metabolism
WP_010661441.1
acetyl-CoA C-acetyltransferase
OAE44373.1 3-hydroxyacyl-[acyl-carrier-protein] dehydratase FabZ OLU06419.1 acyl dehydratase
WP_016453937.1
acyl-CoA dehydrogenase
WP_062681131.1
MULTISPECIES: acyl carrier protein
ASC68058.1 enoyl-CoA hydratase
WP_076521293.1
3-hydroxyacyl-[acyl-carrier-protein] dehydratase FabZ WP_088587684.
1 enoyl-CoA hydratase
WP_061346094.1
acyl-CoA dehydrogenase
WP_050446775.1
enoyl-CoA hydratase
OWT77500.1 acyl carrier protein
WP_021586121.1
beta-ketoacyl-ACP reductase
WP_088147327.1
beta-ketoacyl-ACP reductase
WP_012203459.1
MULTISPECIES: enoyl-CoA hydratase
OKO45451.1 REVERSED long-chain-acyl-CoA synthetase
CUI26675.1 Uncharacterized acyl-CoA thioester hydrolase HI_0827

286 Appendix C: Extracellular protein analysis
Function Accession No. Protein
WP_034394989.1
MULTISPECIES: 3-hydroxyacyl-CoA dehydrogenase
AEF90758.1 Enoyl-(acyl-carrier-protein) reductase (NADH)
KGD95832.1 3-ketoacyl-ACP reductase
ASC66946.1 3-hydroxyacyl-CoA dehydrogenase
WP_043543263.1
propionate--CoA ligase
WP_061346536.1
3-hydroxyacyl-CoA dehydrogenase
Membrane related
WP_043547278.1
membrane protein
AKP92101.1 putative lipoprotein
WP_034397436.1
MULTISPECIES: outer membrane protein assembly factor BamE WP_043544857.
1 outer protein B
WP_057284478.1
outer membrane protein chaperone
WP_043544088.1
exopolyphosphatase
Nucleotide metabolism
WP_088153872.1
oxidoreductase
OWT72173.1 NADP-dependent oxidoreductase
KGD93926.1 cytochrome C550
WP_058665727.1
redox-regulated ATPase YchF
WP_076518982.1
fumarate hydratase, class II
WP_061071973.1
ribose-5-phosphate isomerase RpiA
WP_076815687.1
electron transporter RnfB
OLU00760.1 phosphoribosylamine--glycine ligase
KGD96735.1 ATP synthase F0F1 subunit delta
WP_006218219.1
electron transfer flavoprotein subunit beta
WP_048395540.1
MULTISPECIES: ATP-dependent Clp protease ATP-binding subunit WP_054452508.
1 ATP-dependent chaperone ClpB
WP_020924632.1
MULTISPECIES: dihydrolipoyl dehydrogenase
WP_062684019.1
MULTISPECIES: NAD(P)(+) transhydrogenase (Re/Si-specific) subunit alpha SEI90423.1 dihydrolipoamide dehydrogenase
WP_088419875.1
orotate phosphoribosyltransferase
WP_046804414.1
phosphoribosylaminoimidazolesuccinocarboxamide synthase WP_043210047.
1 MULTISPECIES: glycosyl transferase
KNE24372.1 inosine-5-monophosphate dehydrogenase
KGY26696.1 nucleotide-binding protein
KGD90752.1 phosphoglyceromutase
Protein and amino acid metabolism
WP_043547631.1
endopeptidase La
WP_068986654.1
type I methionyl aminopeptidase
WP_088147382.1
arginine--tRNA ligase
WP_062682748.1
MULTISPECIES: succinyl-diaminopimelate desuccinylase WP_006390545.
1 MULTISPECIES: isocitrate lyase
WP_012206888.1
MULTISPECIES: 4-hydroxy-tetrahydrodipicolinate synthase WP_062679845.
1 MULTISPECIES: branched-chain amino acid transaminase OCZ66373.1 cysteine synthase B
WP_061345481.1
urease subunit alpha
EFI67045.1 threonine synthase
WP_066036939.1
peptide chain release factor 1
WP_088146963.1
serine--tRNA ligase
KXO79104.1 cysteine synthase

Appendix C: Extracellular protein analysis 287
Function Accession No. nnumbernumber
Protein
SEI93094.1 cysteine synthase
KGE00121.1 indole-3-glycerol-phosphate synthase
WP_071632907.1
tryptophan--tRNA ligase
WP_062681333.1
MULTISPECIES: 4-hydroxy-tetrahydrodipicolinate synthase SIT05832.1 protease I
ASC68057.1 3-hydroxyisobutyrate dehydrogenase
KXO76179.1 UDP-N-acetylmuramoyl-L-alanyl-D-glutamate--2,6-diaminopimelate ligase WP_071632036.
1 methylcrotonoyl-CoA carboxylase
WP_081010950.1
MULTISPECIES: aminopeptidase P family protein
WP_062682745.1
MULTISPECIES: chorismate mutase
WP_043543277.1
leucyl aminopeptidase
ASC63154.1 cysteine synthase B
OAD13310.1 aromatic amino acid aminotransferase
ASC64081.1 serine hydroxymethyltransferase
WP_085943758.1
thioredoxin TrxA
EFI68384.1 cysteine synthase
WP_016995150.1
NADP-specific glutamate dehydrogenase
KGD86968.1 pyrroline-5-carboxylate reductase
ADP13864.1 thiazole biosynthesis protein ThiG family protein
WP_063327214.1
3-deoxy-7-phosphoheptulonate synthase
KGD97156.1 aspartate aminotransferase
KRC76684.1 ornithine carbamoyltransferase
WP_071630174.1
3-deoxy-7-phosphoheptulonate synthase class II
WP_025140033.1
acetylornithine transaminase
KGD90458.1 proline iminopeptidase
KGD87877.1 peptide chain release factor 2
OLU09675.1 acetylornithine deacetylase
WP_043547594.1
2,3,4,5-tetrahydropyridine-2,6-dicarboxylate N-succinyltransferase KOF52692.1 acetolactate synthase
WP_043544334.1
threonine synthase [
Stress and redox signalling
WP_050448221.1
universal stress protein
WP_062683504.1
MULTISPECIES: universal stress protein
CUJ55705.1 General stress protein 17o
WP_049075673.1
MULTISPECIES: universal stress protein
ASC63586.1 universal stress protein
KGR86650.1 chemical-damaging agent resistance protein C
OLU07232.1 universal stress protein
WP_043544650.1
universal stress protein
WP_006393278.1
peroxide stress protein YaaA
ASC63654.1 peroxide stress protein YaaA
EZP63695.1 Peroxiredoxin Q/BCP
WP_076521074.1
MULTISPECIES: thioredoxin
WP_062684140.1
MULTISPECIES: peroxiredoxin
OXC90540.1 thioredoxin-disulfide reductase
WP_061072286.1
thioredoxin-disulfide reductase
OMG90645.1 oxidoreductase
OAD13580.1 alkyl hydroperoxide reductase
KRC70798.1 oxidoreductase

288 Appendix C: Extracellular protein analysis
Function Accession No. Protein
WP_063960063.1
MULTISPECIES: NAD(P)H-quinone oxidoreductase
OAE65212.1 alkyl hydroperoxide reductase
KGD92171.1 oxidoreductase
WP_075041485.1
gfo/Idh/MocA family oxidoreductase
SFB54940.1 Nucleotide-binding universal stress protein, UspA family WP_062683028.
1 CsbD family protein
WP_088148284.1
NADP-dependent oxidoreductase
WP_057286974.1
N-acetyl-gamma-glutamyl-phosphate reductase
Signal transduction and chemotaxis
WP_047377097.1
glucokinase
OLU00781.1 guanylate kinase
WP_054441174.1
MULTISPECIES: homoserine kinase
KGD99719.1 adenylate kinase
ASC66688.1 histidine kinase
KGY28956.1 phosphoglycerate kinase
SIT27796.1 nucleoside diphosphate kinase
WP_088446300.1
pyruvate kinase
SEK01422.1 purine-binding chemotaxis protein CheW
WP_047470594.1
methyl-accepting chemotaxis protein
WP_054427381.1
translocation protein TolB
Transport and secretion
WP_063952513.1
electron transporter RnfB
WP_088139428.1
phosphate transport system regulatory protein PhoU KRB12177.1 ABC transporter substrate-binding protein
OCR22505.1 transporter
WP_006218564.1
thiol:disulfide interchange protein DsbD
WP_062684286.1
MULTISPECIES: PTS sugar transporter subunit IIA
WP_088146814.1
diguanylate cyclase
WP_066037445.1
HPr family phosphocarrier protein
Carboxylic acid metabolism
OCA80479.1 aldehyde dehydrogenase
WP_054550108.1
acetate--CoA ligase
KGD97042.1 2-methylisocitrate lyase
WP_076411594.1
O-acetylhomoserine aminocarboxypropyltransferase ASC63275.1 propionate--CoA ligase
OLU08467.1 3-hydroxybutyrate dehydrogenase
WP_088446703.1
2-hydroxyhepta-2,4-diene-1,7-dioate isomerase
WP_062683435.1
MULTISPECIES: lactoylglutathione lyase
OXC92513.1 uroporphyrinogen decarboxylase
KFJ11781.1 acetyl-CoA carboxylase, biotin carboxyl carrier protein WP_013800519.
1 NAD(P)-dependent alcohol dehydrogenase
WP_029926123.1
MULTISPECIES: glyoxylate/hydroxypyruvate reductase A KNY09037.1 2,3,4,5-tetrahydropyridine-2,6-carboxylate N-succinyltransferase WP_054548608.
1 2-oxoglutarate dehydrogenase E1 component
WP_088139068.1
acetate--CoA ligase
Metabolism of cofactors and vitamins
WP_010658361.1
3,4-dihydroxy-2-butanone-4-phosphate synthase
WP_062683274.1
MULTISPECIES: molybdopterin adenylyltransferase
WP_062680922.1
MULTISPECIES: iron donor protein CyaY
WP_056560178.1
bacterioferritin

Appendix C: Extracellular protein analysis 289
Function Accession No. nnumbernumber
Protein
SIT19086.1 bacterioferritin
APE48441.1 3,4-dihydroxy-2-butanone-4-phosphate synthase
Others WP_043543107.1
nitronate monooxygenase
WP_043517811.1
nitronate monooxygenase
WP_047993075.1
DUF779 domain-containing protein
WP_088143023.1
DUF1987 domain-containing protein
CUJ39015.1 Uncharacterized protein conserved in bacteria
KGY24760.1 CBS domain-containing protein
OMG77958.1 gamma carbonic anhydrase family protein
WP_043544356.1
GNAT family N-acetyltransferase
WP_043541508.1
CBS domain-containing protein
WP_008165630.1
diaminopimelate epimerase
WP_043547280.1
threonylcarbamoyl-AMP synthase
WP_025135677.1
DUF1993 domain-containing protein
WP_088159720.1
ubiquinone-binding protein
WP_043545226.1
MULTISPECIES: nitrogen regulatory protein P-II 1
CUK06870.1 Arsenate reductase and related proteins glutaredoxin family WP_082400968.
1 HAD family hydrolase, partial
WP_063959978.1
MULTISPECIES: superoxide dismutase
WP_006218564.1
thiol:disulfide interchange protein DsbD
WP_049667999.1
response regulator
WP_062684908.1
MULTISPECIES: dUTPase
WP_062682845.1
MULTISPECIES: DUF2513 domain-containing protein
KXO75649.1 histidine--tRNA ligase
WP_063587851.1
cell division protein FtsA
WP_088138739.1
hydrolase
OWT64701.1 cupredoxin-domain containing protein
OAE50901.1 deoxycytidine triphosphate deaminase
WP_088148655.1
glycerophosphodiester phosphodiesterase
KMN39865.1 reductase
WP_088147916.1
hydroxyacid dehydrogenase
CUK07244.1 Uncharacterized protein conserved in bacteria
WP_061072417.1
amidohydrolase
WP_041653427.1
MULTISPECIES: nitroreductase
WP_071632572.1
RidA family protein
EEQ95822.1 Nitrogen regulatory protein P-II
WP_076516740.1
MULTISPECIES: MBL fold metallo-hydrolase
EFF77190.1 SWIB/MDM2 domain protein
WP_088148618.1
bifunctional ornithine acetyltransferase/N-acetylglutamate synthase KGE01752.1 acetylornithine aminotransferase
ASC62876.1 quinone oxidoreductase
CUJ75942.1 2-hydroxy-3-oxopropionate reductase
OLU06774.1 aconitate hydratase 1

290 Appendix C: Extracellular protein analysis

Supplimentary Material 291
Supplimentary Material

292 Supplimentary Material

Supplementary Material 1: Abstracts of conference papers relevant to the thesis 293
Supplementary Material 1: Abstracts of
conference papers relevant to the thesis

294 Supplementary Material 1: Abstracts of conference papers relevant to the thesis

Supplementary Material 2: Publications relevant to the thesis 295
Supplementary Material 2: Publications
relevant to the thesis

Science of the Total Environment 651 (2019) 2197–2207
Contents lists available at ScienceDirect
Science of the Total Environment
j ourna l homepage: www.e lsev ie r .com/ locate /sc i totenv
Solubilization and degradation of polychlorinated biphenyls (PCBs) bynaturally occurring facultative anaerobic bacteria
Gathanayana Pathiraja a, Prasanna Egodawatta a, Ashantha Goonetilleke b, Valentino S. Junior Te'o a,⁎a School of Earth, Environmental and Biological Sciences, Queensland University of Technology (QUT), Brisbane 4001, Queensland, Australiab School of Civil Engineering and Built Environment, Queensland University of Technology (QUT), Brisbane 4001, Queensland, Australia
H I G H L I G H T S G R A P H I C A L A B S T R A C T
• The best strains degraded PCBs underboth anaerobic and aerobic conditions.
• Natural bacterial isolates exhibitedconcomitant PCB solubilization anddegradation.
• PCB solubility positively correlated withpotential biosurfactant production.
• Highest chlorine removal was achievedunder two stage anaerobic-aerobicconditions.
• Lysinibacillus sp. demonstrated thehighest PCB solubility and chloridebuild up.
⁎ Corresponding author.E-mail addresses: [email protected] (G
[email protected] (P. Egodawatta), [email protected] (V.S.J. Te'o).
https://doi.org/10.1016/j.scitotenv.2018.10.1270048-9697/© 2018 Published by Elsevier B.V.
a b s t r a c t
a r t i c l e i n f oArticle history:Received 20 August 2018Received in revised form 9 October 2018Accepted 9 October 2018Available online 11 October 2018
Editor: Jay Gan
A combination of solubilization and degradation is essential for the bioremediation of environments contami-natedwith complex polychlorinated biphenyls (PCB)mixtures. However, the application of facultative anaerobicmicroorganisms that can both solubilize and breakdown hydrophobic PCBs in aqueousmedia under both anaer-obic and aerobic conditions, has not been reported widely. In this comprehensive study, four bacteria discoveredfrom soil and sediments and identified as Achromobacter sp. NP03,Ochrobactrum sp. NP04, Lysinibacillus sp. NP05and Pseudomonas sp. NP06, were investigated for their PCB degradation efficiencies. Aroclor 1260 (50 mg/L), acommercial and highly chlorinated PCB mixture was exposed to the different bacterial strains under aerobic,anaerobic and two stage anaerobic–aerobic conditions. The results confirmed that all four facultative anaerobicmicroorganisms were capable of degrading PCBs under both anaerobic and aerobic conditions. The highest chlo-rine removal (9.16± 0.8mg/L), PCB solubility (14.7± 0.93mg/L) and growth rates as OD600 (2.63 ± 0.22)wereobtained for Lysinibacillus sp. NP05 under two stage anaerobic-aerobic conditions. The presence of biosurfactantsin the culturemedium suggested their role in solubility of PCBs. Overall, the positive results obtained suggest thathigh PCB hydrolysis can be achieved using suitable facultative anaerobic microorganisms under two stageanaerobic-aerobic conditions. Such facultative microbial strains capable of solubilization as well as degradationof PCBs under both anaerobic and aerobic conditions provide an efficient and effective alternative to commonlyused bioaugmentation methods utilizing specific obligate aerobic and anaerobic microorganisms, separately.
© 2018 Published by Elsevier B.V.
Keywords:BioremediationBiosurfactantChloride build upTwo stage anaerobic-aerobic treatment
. Pathiraja),@qut.edu.au (A. Goonetilleke),
1. Introduction
The diversity and magnitude of synthetic toxic chemicals releasedinto the environment are creating long-term human and ecosystem

2198 G. Pathiraja et al. / Science of the Total Environment 651 (2019) 2197–2207
health impacts. Polychlorinated biphenyls (PCBs) are one such toxicchemical group consisting of 209 different chlorinated organic com-pounds. PCBs are persistent in the environment due to low reactivity,high chemical stability and extreme hydrophobicity (Beyer and Biziuk,2009). Conversely, high lipophilicity makes PCBs soluble in fats.Such characteristics results in bioaccumulation, bioconcentration andbiomagnification along the food chains leading to numerous healthimplications in humans and animals (ATSDR, 2000). Due to theirpersistence in the environment, sites contaminated with PCBs such aselectricity distribution stations, service areas and dumpsites as well assediments in the nearby waterbodies are still posing significant threatsto human and ecosystem health.
Microorganisms play an important role in the removal of toxicchemical compounds from the environment. Biological conversion ofhighly and moderately chlorinated PCB congeners into less chlorinatedcongeners has been reported to take place through dechlorinationunder anaerobic conditions (Praveckova et al., 2015; Agullo et al.,2017). In comparison, lower and moderately chlorinated congenerscan be degraded by oxidative bacteria under aerobic conditions throughupper and lower biphenyl degradation pathways (Field and Sierra-Alvarez, 2008). Therefore, to achieve complete degradation, one of themost promising bioremediation strategies is to combine the anaerobicdechlorination and aerobic oxidation (Passatore et al., 2014). Althoughnumerous sediment and soil based studies have been conducted eitherunder anaerobic or aerobic conditions separately, using potentialPCB degrading bacteria (Adrian et al., 2009; Payne et al., 2011; Wangand He, 2013; Murinova et al., 2014), studies based on combinedanaerobic-aerobic conditions are limited (Evans et al., 1996; Masteret al., 2002; Long et al., 2015). Studies by Evans et al. (1996) andMaster et al. (2002) used two separate groups of bacteria capable ofreductive dechlorination and aerobic oxidation, respectively, to degradePCBs in contaminated soil slurry. No studies appear to have beenundertaken so far on PCB degradation using facultative anaerobic mi-croorganisms under two-stage anaerobic-aerobic conditions. However,there is a recent study based on facultative anaerobic bacteria mediatedin situ delignification and enhanced gas release under microaerophilicconditions in soil containing lignocellulose (Rashid et al., 2017).
Past research literature on biochemical pathways and intracellularlocalization of enzymes responsible for PCB degradation suggest thatPCBs have to be solubilized first for easier passage through the cellwall and into the cytoplasm prior to being metabolized. Therefore, anincrease in the rate of solubilization could accelerate the entrance ofPCBs into the cells and their subsequent degradation (Ohtsubo et al.,2004). Most of the research studies undertaken so far on PCB solubilityhave been based on the addition of chemical or biological surfactantswith limited investigation into the actual application ofmicroorganismsproducing surfactants (Singer et al., 2000; Fava and Di Gioia, 2001;Occulti et al., 2008; Viisimaa et al., 2013). Chemical surfactants havethe advantage of being economical, but are often toxic to biologicalsystems (Abraham et al., 2002). In comparison, biosurfactants generallyexhibit higher interfacial tension reduction activities compared tochemical surfactants, and are less toxic and readily biodegradable(Viisimaa et al., 2013). However, the main disadvantage in the use ofcommercially available biosurfactants is the high cost (Aparna et al.,2012).
Therefore, the use of suitable biosurfactant producing microbialstrains, which are also capable of degrading PCBs under both, anaerobicand aerobic conditions, would be an attractive alternative to the use ofeither chemical or biological surfactants or PCB degrading aerobicand anaerobic bacterial groups, separately. Indeed, the application ofbiosurfactant-producing and pollutant-degrading microorganismsoffers the dual advantage of a continuous supply of biodegradablesurfactants and the ability to degrade pollutants (Megharaj et al., 2011).
The aim of this study was to isolate potential naturally occurringfacultative anaerobic bacteria from soil and sediment environmentsand to investigate their capability for degrading Aroclor 1260, a complex
and widely used commercial PCB mixture, under comparative anaero-bic, aerobic and two stage anaerobic-aerobic conditions while solubiliz-ing a hydrophobic PCBmixture. The outcomes of the study are expectedto contribute to the development of more efficient and effective bacte-rial mediated bioremediation treatment of PCB contaminated soils andsediments.
2. Materials and methods
2.1. PCB source
Aroclor 1260 was selected as the commercial grade PCB source forthis study and obtained as a GC/FID grade technical mixture fromAccuStandard Inc. (New Haven, CT, USA). Aroclor 1260 represents acomplex PCB mixture consisting of about 75 different penta to nonachloro biphenylswith an average of 6.3 chlorines per biphenylmolecule(Bedard et al., 2007). Aroclor 1260was prepared as a 50mg/mL stock inGCMS grade acetone, before use.
2.2. Screening, enrichment and identification of possible PCB degradingmicroorganisms
2.2.1. Sampling of soil and sedimentsIn order to isolate possible facultative anaerobic PCB degrading bac-
teria, six soil samples around the Brisbane City area and six sedimentsamples from Brisbane River (27.4745° S, 153.0293° E) and CoombabahLake, Gold Coast (27.54° S, 153.22° E), Australia were collected intosterile glass bottles and transported on ice to the laboratory. Soil andsediment samples were separately homogenised and 50 g from eachcomposite soil and sediment mixtures were added to duplicate250mLErlenmeyer flasks. After contaminationwith Aroclor 1260 to ob-tain 50 mg/kg PCB concentration, flasks were incubated stationaryunder aerobic conditions at room temperature (23 °C ± 1 °C) for onemonth. Similarly, 50 g of each composite soil and sediment mixturewere added to duplicate 50 mL polypropylene vials with screw caps,contaminated with Aroclor 1260 to obtain 50 mg/L concentration andincubated stationary inside the anaerobic chamber (COY lab products)main compartment at room temperature (23 °C ± 1 °C) for onemonth. The atmosphere inside the anaerobic chamber was maintainedconstant at 4.9% H2, 10.7% CO2 and 84.4% N2.
2.2.2. Selective enrichment of possible PCB degrading bacteriaIsolation ofmicroorganisms capable of utilizing PCBswas carried out
through a series of selective enrichments using DSMZ medium 465a(Atlas, 2005) as the base minimal salt medium (MSM) with the follow-ing modifications. After autoclaving the medium, instead of 0.5 g/Lhydroxybiphenyl in ethanol, Aroclor 1260 in GCMS grade acetone wasadded as the sole carbon and energy source to give 50 mg/L final PCBconcentration. Four 250 mL Erlenmeyer flasks containing 100 mL ofsterile MSM medium were prepared. Two flasks were inoculated with10 g of composite soil, and the other two were inoculated with 10 gof sediment previously contaminated with Aroclor 1260. Two flasks(onewith soil and the other with sediment) were incubated aerobicallyin a platform shakermaintained at 150 rpmand 28 °C. The other two re-maining flasks were incubated anaerobically under static conditions inan incubator kept inside the anaerobic chamber with the temperaturekept at 28 °C. Four serial transfers (10% of the enrichment medium)were carried out from each flask at weekly intervals into fresh sterileMSM containing 50mg/LAroclor 1260. From the final flask, supernatantwas plated on nutrient agar (CM003, Oxoid) and incubated overnight at28 °C. Morphologically different colonies were isolated and streaked onfresh nutrient agar plates.
2.2.3. Characterization of potential PCB degrading bacteriaBacterial isolates obtained from selective enrichment were inocu-
lated in duplicate on minimal salt agar plates containing 50 mg/L of

2199G. Pathiraja et al. / Science of the Total Environment 651 (2019) 2197–2207
Aroclor 1260 as sole source of carbon and incubated at 28 °C in parallelunder anaerobic and aerobic conditions to determine their growth tol-erance in the presence/absence of atmospheric oxygen. Minimal saltagar with no added Aroclor 1260, but an equal volume of acetone thatwas used to add Aroclor 1260 into the minimal salt agar in the growthexperiment, was used as the negative control to determine whetherthere was any contribution from leftover acetone on bacterial growth.In comparison, nutrient agar medium alone was used as the positivecontrol. Isolates with the ability to grow on PCBs under both, anaerobicand aerobic conditions were selected for further characterization basedon colony morphology on nutrient agar plates and cell morphologyusing Gram staining. Additionally, the bacterial isolates with highPCB solubility and degradation potential were further characterizedbased on their ability to utilize different carbon and nitrogen sourcesusing Biolog PM1 and PM3B plates containing 95 separate sole carbonand nitrogen sources. Full-length 16S rRNA gene sequences were PCRamplified from purified genomic DNA (gDNA) isolated from puremicrobial isolates in order to ascertain their identification as shown inFig. 1.
2.2.3.1. Genomic DNA extraction and polymerase chain reactions (PCR).Bacterial gDNAs were extracted using Isolate II genomic DNA kit(Bioline) and different gDNAs were used as templates for PCRamplification of 16S rRNA genes. Full length universal 16S rRNAprimers, 27F and 1492R (Integrated DNA Technologies, Inc.), wereused and amplifications were performed according to the MyTaq HSRed DNA polymerase protocol (Bioline). The following PCR cyclingconditions were used: 1 × cycle: 95 °C/10 min, 30 × cycles: 95 °C/30 s,50 °C/30 s, 72 °C/2 min, 1 × cycle: 72 °C/7 min, and held at 4 °C. Thequality of the PCR products was checked by electrophoresis at 100 Vfor 1 h using a 1% agarose gel. The ~1.5 kb RNA gene PCR productswere extracted and purified using the Isolate II PCR and gel extractionkit protocol (Bioline).
2.2.3.2. Ligation, transformation and cloning of PCR products for sequencing.The gel purified ~1.5 kb 16S RNA gene fragments were ligated intothe pGEM-T Easy Vector (Promega) and transformed into high efficiencyE. coli JM 109 competent cells. The transformed cells were screened forblue andwhite colonies by plating on Luria-Bertani (LB) agar plates con-taining ampicillin (100 μg/mL), Isopropyl β-D-1-thiogalactopyranoside
Fig. 1. Process overview for full length (1.5 kb) 16S rRNA based genomic D
(IPTG, 0.5mM) and 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside(X-Gal, 80 μg/mL). Recombinant colonies were selected and checked forthe presence of 16S gene inserts by colony PCR using M13 forward andreverse primers (Integrated DNA Technologies, Inc.) that anneal to out-side of the pGEM-T vector (Promega) multiple cloning site.
2.2.3.3. Recombinant plasmid isolation and sequencing. Recombinantclones of each bacterial culture were grown in liquid LB broth contain-ing ampicillin (100 μg/mL), before plasmid isolation and purificationswere performed using the QIAprep (QIAGEN) Spin Miniprep Kit. Twoseparate sequence reactions using M13F and M13R primers werecarried out for each recombinant plasmid, according to the standardBigDye Terminator (BDT) v3.1 cycle sequencing kit (Thermo FisherScientific) protocol. DNA sequencing was performed using 3500Genetic Analyzer (Applied Biosystems, Hitachi) and the programFinchTV 1.4.0 (Geospiza Inc.) was used for editing of raw nucleotideDNA sequence data. The DNA sequences were polished by the removalof the plasmid vector sequences and merging of the two overlappingsequences to obtain a full length 1.5 kb contiguous sequence wasdone using the Emboss Needle tool of the European BioinformaticsInstitute (EMBL-EBI). The resulting full length 16S rRNA genesequences were then submitted to determine the closest matchingsequences by comparing with the bacterial and archaeal 16S RNAdatabases using the Sequence Match tool of the Ribosomal DatabaseProject (RDP) (Cole et al., 2014) and Basic Local Alignment SearchTool (BLAST) of NCBI, USA. The searches were limited to ‘Type’ bacterialstrains.
2.3. PCB degradation by individual bacterial cultures under aerobic, anaer-obic and two-stage anaerobic-aerobic conditions
Erlenmeyer flasks containing 75 mL of sterile MSM (same mediumused for selective enrichment) were prepared and 50 mg/mL Aroclor1260 stock solution in GCMS grade acetone was added to each flask assole source of carbon to give 50 mg/L Aroclor 1260 concentration.Contents of theflaskswere vigorously shaken for 3min to allow acetoneto evaporate. For each bacterial culture, three separate sets of experi-ments were carried out in parallel under aerobic, anaerobic and two-stage anaerobic-aerobic conditions. In all anaerobic and two stageexperiments, flasks containing media were prepared and incubated in
NA isolation, cloning and sequencing based molecular identification.

2200 G. Pathiraja et al. / Science of the Total Environment 651 (2019) 2197–2207
an anaerobic chamber (COY laboratory products, Inc.) under strict an-aerobic conditions. The liquid minimal salt medium in the flasks werekept equilibrated for oneweek inside the anaerobic chambermain com-partment before theywere inoculatedwith the seed cultures. Palladiumcatalysts located inside the chamberwere used to scrub any residual ox-ygen present in the chamber and the samples were transferred underanaerobic conditions without changes to the internal atmosphere inthe chamber through a heavy duty vacuum airlock compartment. Theenvironment inside the anaerobic chamber was maintained constantunder 4.9% H2, 10.7% CO2 and 84.4% N2 (BOC Australia). Under these an-aerobic conditions, hydrogen gas present inside the anaerobic chamberand controlled at 4.9% (mol/mol) concentration as thepotential electrondonor in the present study.
Aliquots of bacterial seed cultures (7.5 mL) grown overnight in LBbroth were centrifuged at 5000 ×g for 10 min. The resulting cell pelletswere washed twice in MSM, resuspended in 1 mL of the samemedium,and then transferred into the anaerobic chamber and used as theinoculum for each flask. Before inoculation of bacterial cultures, flaskswere maintained at pH 7. Flasks used in anaerobic experiments werekept at 28 °C in an incubator kept inside the anaerobic chamber, withoccasional gentle shaking by hand over the six weeks period. In aerobicexperiments, after inoculationwith seed cultures,flaskswere incubatedat 28 °C and 150 rpm under aerobic conditions for six weeks. The two-stage experiment was started and continued at 28 °C under similaranaerobic conditions (with occasional shaking by hand) for the firstfour weeks, and the flasks were removed from the anaerobic chamberand transferred to aerobic conditions at 28 °C and 150 rpm during thelast two weeks. The experiments were conducted in triplicate whilethe controls were conducted in duplicate. Minimal salt media spikedwith 50 mg/L Aroclor 1260 with no added bacteria seed cultures wereused as abiotic controls. Minimal salt media spiked with equal volumesof acetone that were used to dissolve 50 mg/L Aroclor 1260 were inoc-ulated with bacterial seed cultures similar to the experiment and usedas media controls.
2.3.1. Sample collection and analysisSamples (4mL) were withdrawn from each flask at time zero and in
weekly intervals to determine pH, cell growth and PCB solubility. Inorder to have representative samples, liquid aliquots were removedfrom themiddle of the culturemediumwhile flaskswere kept under ag-itation. Bacterial cell densities were calculated as colony forming units(CFU) using standard plate count. The DU730 Beckman Coulter UV/VISspectrophotometer was used to measure the cell growth at 600 nmoptical density and calibrated Hanna HI 2221 pH meter was used tomeasure the pH according to the APHA method 4500-H (APHA, 2012).At the end of six weeks, chloride ion concentration in each flask wasmeasured as described below in order to determine the amount ofchlorines released from the PCB mixture, by the direct action of themicrobes. If bacteria were able to dechlorinate the PCB molecules, thereleased chloride ions were expected to accumulate in the culturemedium. To see whether there is any significant contribution to thechloride level in the culture medium due to the lysis of bacterialcells, the chloride measurements were performed before and afterthe sonication of the samples. Samples were first centrifuged at5000 ×g for 10 min and the resultant supernatant was filteredthrough 0.2 μm sterile filter discs to remove the bacterial cells. Thecell free culture supernatant was used to measure the chloride ionconcentration using the Dionex ICS-2100 ion chromatographyaccording to USEPA method 300.0 (USEPA, 1993). For the calibrationcurve preparation, 0.1 ppm, 1 ppm, 5 ppm, 10 ppm, 20 ppm and100 ppm standard sodium chloride solutions were used. The abioticcontrols andmedia controls (see Section 2.2) were used in parallel inorder to subtract the background chloride levels coming from theminimal salt medium, leaking of any chloride due to bacterial celllysis and any traces left from the LB medium used to cultivate thebacterial seed cultures.
2.3.2. PCB extraction and analysisLiquid aliquots (1mL) sampled in weekly intervals were transferred
to 8 mL glass vials fitted with Teflon-lined screw caps. PCBs were ex-tracted with 2.5 mL of GC grade n-hexane (USEPA, 2007) by vigoroushorizontal shaking on a platform shaker for 4 h at 250 rpm underroom temperature, followed by centrifugation at 5000 rpm for 10 min(Adrian et al., 2009). The solvent phase was used for subsequentPCB analysis. Before extraction, 25 μL of 2, 4, 5, 6-tetrachloro-m-xylene(10 μg/mL in hexane) was added to each sample as the surrogate stan-dard to determine the extraction efficiency (USEPA, 2007). Surrogaterecovery was 98.98% ±19.11% (n = 308).
PCB extracts and standards were spiked with 2,2′,4,4′,5,5′-hexabromobiphenyl as an internal standard (USEPA, 2007). Total solu-ble PCB levels were determined as per the USEPA method 8082A(USEPA, 2007). Thermo Scientific Trace 1310 (in splitless mode, at260 °C inlet temperature, 80 mL/min split flow and 1.2 min splitlesstime) equipped with SSL injector with splitless liner with glass wool,Thermo TG-5SilMS analytical column (30m× 0.25mm ID × 0.25 μm)and, TriPlus RSH auto sampler were used for PCB analysis. Heliumwas used as the carrier gas at 1.2 mL/min constant flowrate. Thecolumn was kept at 40 °C for 2 min, and then the temperature wasraised to 300 °C at 15 °C/min and kept for 5 min. The ThermoScientific Trace Finder EFS software was used for the screening andquantitation of total soluble PCBs and PCB homolog groups usingthe Thermo Scientific triple stage quadrupole Mass spectrometer(TSQ8000 EVO). Retention times for each PCB homolog group wastaken from the existing literature (Walker and Feyerherm, 2013)and full scan acquisition and timed selective reaction monitoring(SRM) modes were used to distinguish the PCB homolog groups.Relative amounts of dissolved PCB levels and homolog groups weredetermined by nine-point calibration using Aroclor 1260 standardsolutions and area integration.
2.4. Screening for biosurfactant production
At the end of the six week experiment, 10 mL culture supernatantfrom each flask of the aerobic batch experiment were centrifuged at5000 ×g for 10 min and filtered through 0.2 μm filters to separate thebacterial cells. The cell free supernatants were used for the subsequentbiosurfactant screening tests.
2.4.1. Drop collapse testThe drop collapse test was used as a primary screening to deter-
mine the ability of bacterial strains for their biosurfactant productioncapacity based on past literature (Bodour et al., 2003; Alvarez et al.,2015; Panjiar et al., 2015; Joy et al., 2017). 20 μL cell free culturesupernatant was mixed with 5 μL of 0.1% methylene blue solutionand placed on parafilm paper as a drop using a pipette. The purposeof adding methylene blue was for easy visualization of the droplet.Diameter of the drop was measured after 1 min using 1 mm gritpaper placed underneath the parafilm paper. 1% (w/v) sodium dode-cyl sulphate (SDS) solution was used as the positive control whilephosphate buffered saline (PBS) solution and abiotic controls wereused as negative controls. The results where the diameter of thedroplet was at least 1 mL larger than the one made by the negativecontrol were considered as positive for biosurfactant production.When there is no biosurfactant present, the droplet remains stableas the polar water molecules are repelled from the hydrophobicsurface. When the interfacial tension between the liquid droplet andthe hydrophobic surface is reduced, the drop spreads or collapses(Alvarez et al., 2015).
2.4.2. Emulsification index (EI24)3 mL of cell free culture supernatant and 3 mL of mineral oil were
taken into a graduated test tube and vigorously shaken for 2 min toform an emulsion. The mixture was allowed to stand still for 24 h and

2201G. Pathiraja et al. / Science of the Total Environment 651 (2019) 2197–2207
the height of emulsion layer was measured (Nayak et al., 2009). Theemulsification index was calculated using Eq. (1) (Panjiar et al., 2015).
EI₂₄ %ð Þ ¼ Height of emulsion formed after 24 hrs=Total height of solution� 100
ð1Þ
Emulsions formed following the reactions with different culturesupernatants were compared to the controls. The positive control usedwas a 1% (w/v) solution of synthetic surfactant sodiumdodecyl sulphatein deionised water, whereas the abiotic and medium only controlsamples were used as the negative controls.
2.4.3. HaemolysisTo detect the haemolytic activity indicative of biosurfactant
production, 50 μL of the cell free culture supernatant was spotted onthe middle of Tryptone soya agar plates containing 5% sheep blood(Thermo Fisher Scientific) and the plates were incubated at 28 °C for48 h (Alvarez et al., 2015). Plates were visually examined forhaemolysis. The level of clearance of red blood cellswas considered pro-portional to the concentration of biosurfactant. A yellow transparentzone indicated complete lysis of red blood cells and was regarded asbeta (β) or complete haemolysis. The appearance of dark green zonesbeneath the place where the supernatant was spotted was consideredas alpha (α) or partial haemolysis of blood cells. Alpha and betahaemolysis were considered as positive for biosurfactant production.No change in the blood agar plates indicated gamma (γ) or nohaemolysis (Joy et al., 2017).
3. Results and discussion
3.1. Identification of PCB utilizing facultative anaerobic culture members
After selective enrichment screening, three Gram negative (NP 03,04 and 06) and one Gram-positive (NP05) rod-like shape bacterialstrains capable of utilizing PCBs as sole source of carbon under bothanaerobic and aerobic conditions were isolated. During 16S rRNA genesequencing based identification, the closest matching sequences wereobtained from National Center for Biotechnology Information (NCBI)and Ribosomal Database Project (RDP) databases and are summarisedin Table 1. The RDP database was used as a curated database as itprovides the aligned and annotated rRNA gene sequence data (Coleet al., 2014; Wang et al., 2007), while NCBI was used as a non-curateddatabase. There were no differences between the two databases forculture numbers NP04 and NP05 as they obtained the same closestrelatives from both databases. However, similarity of culture numbersNP03 and NP06 were limited to the generic level between the twodatabases. There is no universal definition existing for species levelidentification using 16S rRNA gene sequencing and use of acceptablecriteria for establishing a species match varies widely in differentstudies (Janda and Abbott, 2007). Therefore, if similarity was not
Table 1Comparison of closest relatives of isolated bacteria based on NCBI and RDP databases.
Culture no. RDP database NCBI d
Similarityscorea
Unique commonoligomers
Closest match Maximscore
NP 03 0.996 1364 Achromobacter insolitus LMG6003, AY170847
2660
NP 04 0.994 1394 Ochrobactrum lupini LUP21,AY457038
2625
NP 05 0.997 1413 Lysinibacillus macroides LMG18474, AJ628749
2717
NP 06 0.995 1416 Pseudomonas citronellolis DSM50332T, Z76659
2686
a Similarity score - percent sequence identity over all pairwise comparable positions when r
100%, then identification was limited up to the generic level as there isno guarantee of having 1% divergence to obtain accurate identification.As none of the cultures showed 100% similarity to the existing data-bases, the four bacterial cultures were named according to their genusfollowed by the strain number. 16S rRNA sequences of the four cultures(NP03, NP04, NP05 and NP06) were deposited in NCBI GenBank data-base under the accession numbers KY711179, KY711180, KY711181and KY711182, respectively.
There was no visible growth in the media controls that containedequal volume of acetone used to dissolve Aroclor 1260. This findingconfirmed that the bacteria were not able to utilize acetone as their car-bon source even if there was residual acetone left in the medium afterevaporation. Bacteria such as Achromobacter sp. and Ochrobactrum sp.have been found in PCB contaminated sediments, but only under aero-bic conditions (Dudasova et al., 2014; Murinova and Dercova, 2014).However, in this study, it was found that the four facultative anaerobicbacterial strains Achromobacter sp. NP03, Ochrobactrum sp. NP04,Lysinibacillus sp. NP05 and Pseudomonas sp. NP06 were able to growon minimal salt agar containing Aroclor 1260 under aerobic and anaer-obic conditions indicating their ability to utilize PCBs as their sole sourceof carbon under both environmental conditions.
The facultative anaerobic bacterial strains with high PCB solubilityand degradation potential were further characterized based on theirability to utilize different carbon and nitrogen sources using BiologPM1 and PM3B plates. Achromobacter sp. NP03, Ochrobactrum sp.NP04 and Lysinibacillus sp. NP05 were able to utilize L-Proline as solesource of carbon and nitrogen. In addition, L-lactic acid and methyl py-ruvate were utilized as sole source of carbon (Table S1, SupplementaryInformation) and L-Glutamic acid, Ala-His, Ala-Leu and Gly-Gln wereused as nitrogen sources (Table S2, Supplementary Information) athigh rates by Achromobacter sp. NP03, Ochrobactrum sp. NP04 andLysinibacillus sp. NP05.
3.2. PCB degradation under aerobic, anaerobic and two stage anaerobic-aerobic conditions
3.2.1. Growth profiles measured at OD600 while grown on Aroclor 1260 ascarbon source
Fig. 2 shows the growth profiles of four facultative anaerobic strainsunder aerobic, anaerobic and two stage anaerobic-aerobic conditions.As evident in Fig. 2a, all four strains reached saturation by week oneunder aerobic conditions (OD600 of 0.51, 0.7, 0.62 and 0.46 for NP03,04, 05 and 06, respectively) and did not improve much further withtime. According to the literature, these results suggest the possibilityof all four strains consuming lower chlorinated PCB congeners withinthe firstweek andwere unable to degrade highly chlorinated congenersunder aerobic conditions (Field and Sierra-Alvarez, 2008). This conceptcan be supported by the fact that Aroclor 1260, the PCB source used inthis study consists of less than 10% (by weight) of lower chlorinatedhomolog groups (containing four or fewer chlorines per biphenyl
atabase Proposed name
um Identities Gaps Closest match
1476/1493(99%)
5(0%) Achromobacter denitrificansDSM 30026
Achromobacter sp.NP03
1437/1445(99%)
0(0%) Ochrobactrum lupini LUP21 Ochrobactrum sp.NP04
1486/1494(99%)
0(0%) Lysinibacillus macroidesLMG 18474
Lysinibacillus sp.NP05
1486/1501(99%)
4(0%) Pseudomonas knackmussiiB13
Pseudomonas sp.NP06
an with aligned myRDP sequences.

Fig. 2. Growth of four facultative anaerobic bacterial strains under (a) aerobic,(b) anaerobic, and (c) two stage anaerobic-aerobic conditions. Error bars represent thestandard deviation of mean values (n = 3).
2202 G. Pathiraja et al. / Science of the Total Environment 651 (2019) 2197–2207
molecule) compared to the highly chlorinated homologs (Mayes et al.,1998; ATSDR, 2000). To provide further support for these observations,Pieper and Seeger (2008) reported that aerobic bacteria are capable ofdegrading biphenyls as the sole source of carbon and energy, andusually involves the biodegradation of PCBs with less than four chlorineatoms. Therefore, the inability of bacterial cultures to degrade highlychlorinated congeners and the limited availability of lower chlorinatedcongeners in the medium may have negatively contributed to the lim-ited growth rate under aerobic conditions.
In comparison, during anaerobic conditions, all four strains of NP03,NP04, NP05 and NP06, showed higher cell densities with OD600 of 1.22,1.31, 1.04 and 0.82, respectively, reaching maximum growth by week 4(Fig. 2b). Significantly high growth rates under anaerobic conditionswithout the presence of carbon sources other than PCBs is an indicationof biphenyl ring cleavage in addition to dechlorination. According toexisting literature, anaerobic dechlorination is a reductive process thatuses PCBs as electron acceptors, but the carbon rings are usually notcleaved (Wiegel andWu, 2000;Hughes et al., 2009). In caseswhere bac-teria are not capable of breaking down the carbon ring structure, they
will require additional carbon sources in order tomaintain their growthduring dechlorination of PCBs (Wu et al., 2000; Bedard et al., 2006;Adrian et al., 2009; Wang and He, 2013). During this study, 8.7 × 106,5.0 × 106, 4.9 × 106 and 4.5 × 106 cells/mL initial cell densitiesat week zero were increased by 200 folds to 2.0 × 108, 1.3 × 108,1.3 × 108 and 1.0 × 108 cells/mL at week four for NP03, NP04, NP05and NP06, respectively. This fact confirmed the capability of these mi-croorganisms to utilize PCBs as their carbon source other than electronacceptors under anaerobic conditions without the requirement foradditional carbon sources.
In the two-stage anaerobic-aerobic cultivations as shown in Fig. 2c,all four strains showed similar growth patterns to the anaerobic condi-tions (Fig. 2b) up to week four during anaerobic conditions. However,after switching from anaerobic to aerobic conditions between weekfour to six, Lysinibacillus sp. NP05 started increasing in growth fromOD600 of 1.04± 0.07 to 2.63± 0.22, which is nearly three times its orig-inal cell density. Similarly, Ochrobactrum sp. NP03, Achromobacter sp.NP04 and Pseudomonas sp. NP06 also indicated slight increase in celldensities (1.39 ± 0.08, 1.48 ± 0.23 and 0.99 ± 0.06, respectively)when changing conditions from anaerobic to aerobic as indicated inFig. 2c. Based on these results, it can be postulated that all fourorganisms are capable of performing dechlorination under anaerobicconditions in a similar way at different rates and Lysinibacillus sp.NP05 subsequently hydrolyses the carbon ring structure extensivelyunder aerobic conditions compared to the other three organisms.
3.2.2. Variation of PCB solubility in aqueous mediumPCB concentrations were measured as total soluble PCBs in order to
investigate the change of solubility levels in themedium resulting frompotential bacterial activity under aerobic, anaerobic and two stage con-ditions. Solubility of PCBs in themediumwas an indirectmeasure of themicroorganisms attacking the PCBs and converting them from insolubleto soluble forms. Theoutcomes of this experiment are shown in Fig. 3. Atthe initial concentration of 50mg/L, it was observed that the PCBs addedto the flasks appeared to be not completely soluble, but most remainedat the bottom as small clumps prior to the addition of the bacterialcultures. The total soluble PCB levels in the abiotic controls with no bac-teria addedwasmeasured and found to remain very low throughout thecultivation period, with values found to be 0.15 ± 0.02 mg/L, 0.57 ±0.07 mg/L and 0.5 ± 0.23 mg/L under aerobic, anaerobic and twostage anaerobic-aerobic conditions, respectively. Such solubility rangesare consistentwith previous reports (ATSDR, 2000; Bedard et al., 2007).
Under aerobic conditions, Lysinibacillus sp. NP05 showed the highestcapacity to solubilize PCBs compared to Ochrobactrum sp. NP03,Achromobacter sp. NP04 and Pseudomonas sp. NP06 (Fig. 3a). Thisincrease in activity by Lysinibacillus sp. NP05 started after week oneand reached optimal activity by week five before it started to decrease.However, Lysinibacillus sp. NP05 did not increase in cell growth, butshowed similar cell densities to the rest of the three strains as shownin Fig. 2a. The increased solubility over the aerobic incubation periodwith no corresponding increase in cell growth shown by Lysinibacillussp. NP05 could possibly be due to two reasons. Firstly, their ability tosecrete some surface-active compounds that ultimately lead to in-creased solubility of the hydrophobic PCB mixture (Singer et al., 2000;Cameotra and Bollag, 2003). Secondly, the bacterium was unable tocarry out the dechorination of highly chlorinated congeners under aer-obic conditions as reported by Furukawa (2000) even though it mayhave the capacity to solubilize them.
In contrast to the aerobic conditions and as shown in Fig. 3b, PCBsolubility of all four cultures increased significantly under anaerobicconditions. A similar trendwas also observed in thefirst fourweeks dur-ing the anaerobic stage of the two stage anaerobic-aerobic conditions(see Fig. 3c). It was noted that soon after the addition of bacterialcultures, the insoluble PCB pellets started to disappear in the flasks,presumably as the PCBs became soluble due to the action of the micro-organisms. There are two possible explanations for the mode of action

Fig. 3. PCB solubility under (a) aerobic, (b) anaerobic, and (c) two stage anaerobic-aerobicconditions. Error bars represent the standard deviation of mean values from triplicates.Initial is the total soluble PCBs prior to the addition of microbes and week 0 isimmediately after addition of microbes.
2203G. Pathiraja et al. / Science of the Total Environment 651 (2019) 2197–2207
by themicroorganisms that resulted in the increase in solubility of PCBs.Firstly, the dechlorination of PCBs could transform low water solublehighly chlorinated congeners intomorewater soluble lower chlorinatedcongeners as reported by Yin et al. (2011). If highly chlorinated conge-ners were dechlorinated into lower chlorinated congeners, there shouldbe more chlorides released to the medium. Therefore, measurementof chloride ions accumulated in the medium is a direct indicationof dechlorination, which is discussed in Section 3.2.3. Secondly, theenhancement of the solubility of hydrophobic PCBs was due to the pro-duction of bioemulsifiers or surface-active molecules (biosurfactants)by the microorganisms (Federici et al., 2012).
Findings of the two stage experiments are shown in Fig. 3c. Duringthe two stage study, the first four weeks were dedicated to anaerobicconditions before switching over to aerobic conditions in the last twoweeks. All four strains demonstrated similar trends in the first fourweeks under anaerobic conditions (Fig. 3b vs Fig. 3c). However, in thesecond stage, when the conditions shifted from anaerobic to aerobic,
the solubility of PCBs declined in Achromobacter sp. NP03,Ochrobactrumsp. NP04 and Pseudomonas sp. NP06, whereas in Lysinibacillus sp. NP05,there was a significant increase in PCB solubility. Parallel to theincreased PCB solubility, the growth of Lysinibacillus sp. NP05 also in-creased significantly during this period as shown in Fig. 2c aerobicphase. This confirmed the ability of Lysinibacillus to make hydrophobicPCB mixture soluble in the aqueous medium under both, anaerobicand aerobic conditions. Furthermore, it also confirmed the ability to de-chlorinate the highly chlorinated congeners during the anaerobic phaseand to degrade the resulting lower chlorinated congeners during theaerobic phase. This is an added advantage in bioremediation applica-tions as the organism can survive and degrade the PCB compoundsunder varying anaerobic and aerobic conditions.
3.2.3. Chloride ion build up and variation of pH in the aqueous mediumThe release of chlorides and their concentrations in the liquid me-
dium were measured as explained in Section 2.2.1. These measure-ments were taken as an indication of the dechlorination process thatoccurred following the addition of the four bacteria strains. The chlorideion concentrations in the abiotic controlswere alsomeasured and foundto be relatively constant throughout the experiment and there was noconsiderable difference between the initial and final chloride levelsunder aerobic, anaerobic and two stage anaerobic-aerobic conditions.The chloride ion concentrations in the controls were in the range of37.0 ± 0.9 mg/L. These background levels were concluded to comefrom chloride containing compounds in the basal minimal salt mediumcomprising of MgCl2·6H2O, CoCl2·6H2O, MnCl2·4H2O, NiCl2·6H2O andCuCl2·2H2O. The background chloride values coming from the abioticcontrols and media controls were first subtracted from the experimen-tal values, before the final values were plotted as shown in Fig. 4.
According to Fig. 4, accumulation of chloride ions in aerobic andanaerobic conditions provided clear evidence that the four facultativemicrobes discovered have the ability to dechlorinate the Aroclor 1260mixture under both, aerobic and anaerobic conditions. Significantlyhigh chloride ion concentrations in the combined anaerobic-aerobictreatment of all four cultures when compared to separate aerobic andanaerobic treatments further confirmed the effectiveness of combininganaerobic and aerobic degradation rather than isolated aerobic oranaerobic applications (Tartakovsky et al., 2001; Long et al., 2015). In-creasing levels of chloride ions in the culture medium were reportedby Yin et al. (2011) as a direct indication of dechlorination of PCB mol-ecules. Under anaerobic and two stage conditions, Lysinibacillus sp.NP05 demonstrated the highest chloride ion levels when compared tothe other three cultures and they were 5.2 ± 0.7 mg/L and 9.16 ±0.8 mg/L, respectively. Pseudomonas sp. NP06 had the lowest chloridelevels under all three conditions. As Aroclor 1260 theoretically contains60% chlorine by weight, the maximum chlorine level expected in50 mg/L Aroclor 1260 concentration is 30 mg/L. Therefore, the chlorideyield of 9.16 ± 0.8 mg/L observed in Lysinibacillus sp. NP05 under twostage anaerobic-aerobic treatment is an indication of the removal ofone third of total chlorine present in the Aroclor 1260 mixture.
The pH of the culture supernatants were also monitored togetherwith chloride ion build up and are shown in Fig. 5. The pH values ofthe abiotic controls remained relatively constant throughout the exper-iment (7.07 ± 0.14) under anaerobic, aerobic and two stage conditions.At the end of the aerobic and anaerobic experiments, the pHdid not sig-nificantly change and were 7.49 ± 0.07 and 6.95 ± 0.03, respectively.However, significant pH reduction was observed at the end of all thetwo stage anaerobic-aerobic experiments with values of 5.15 ± 0.03,4.98 ± 0.06, 4.97 ± 0.01 and 6.14 ± 0.03 obtained for Achromobactersp. NP03,Ochrobactrum sp. NP04, Lysinibacillus sp. NP05, and Pseudomo-nas sp. NP06, respectively (Fig. 5). When variations of the pH valueswere compared with the accumulation of the chloride ion levels in themedium, the negative correlation is clearly visible. Based on these re-sults, the lowering of pH under two stage anaerobic-aerobic conditionsappeared to have correlated well with the increase in chloride ions. The

Fig. 4. Chloride ion accumulation in the culturemedia after six weeks. The values shown here are after subtracting the background chloride levels coming from abiotic controls andmediacontrols. Error bars represent the standard deviation of mean values from triplicates.
2204 G. Pathiraja et al. / Science of the Total Environment 651 (2019) 2197–2207
high levels of chlorides under combined anaerobic-aerobic conditions isattributed to the dechlorination of highly chlorinated congeners underanaerobic conditions first, followed by further degradation of resultinglower chlorinated congeners after switching to aerobic conditions.
3.3. Biosurfactant production
An extracellular biosurfactant producing Lysinibacillus sp. waspreviously reported with the ability to solubilize different aliphaticand aromatic hydrocarbons such as hexane, benzene, toluene, dieseland kerosene (Panjiar et al., 2015). In addition, a bacterium isolatedfrom refinery wastewater was identified as Ochrobactrum sp. with thepotential to produce exopolysaccharide bioemulsifier and was able todegrade diesel oil (Ramasamy et al., 2014), n-octane, mineral light andheavy oils, crude oil (Calvo et al., 2008). However, there is no research
Fig. 5. Variation of pH and chloride ion concentrations after six weeks under aerobic, anaerobicrepresent the standard deviation of mean values from triplicates.
literature available that confirms the ability of these bacterial speciesto produce biosurfactants, which makes extremely hydrophobic PCBssoluble in aqueous medium while concomitantly degrading PCBs.Therefore, this study can be considered a first to report the potentialof Lysinibacillus, Achromobacter and Ochrobactrum species to producebiosurfactants that ultimately increased the solubility of PCBs.
In this study, the drop collapse and emulsification index tests wereused as quantitative methods (Ramasamy et al., 2014), while thehaemolytic assay was used as the qualitative method (Thavasi et al.,2011) to test for biosurfactant production. Results of these three testsare summarised in Table 2.
Among the four bacterial cultures, three showed positive results forthe drop collapse test. The drop spreads or collapses proportional to theconcentration of biosurfactants in the supernatant, due to the reductionof interfacial tension between the liquid droplet and the hydrophobic
and two stage anaerobic-aerobic conditions the initial pH was adjusted to 7.0. Error bars

Table 2Summary of biosurfactant screening tests.
Description Drop collapse test(diameter in mm)
Emulsificationindex (%)
Haemolysisa
Positive controlb 5.3 ± 0.3 50.0 +++Negative controlc 3.3 ± 0.3 ND NDAbiotic control 3.2 ± 0.3 ND NDAchromobacter sp. NP03 5.3 ± 0.3 33.3 ++Ochrobactrum sp. NP04 5.0 16.7 ++Lysinibacillus sp. NP05 5.0 50.0 +++Pseudomonas sp. NP06 3.5 ND +
ND - not detected.a The zones of clearingwere scored as follows: ‘+’ incomplete haemolysis; ‘++’ partial
haemolysis with semitransparent zones surrounded by green colour areas; ‘+++’ com-plete haemolysis.
b 1% (w/v) sodium dodecyl sulphate (SDS) solution.c Phosphate buffered saline (PBS) solution.
2205G. Pathiraja et al. / Science of the Total Environment 651 (2019) 2197–2207
surface (Alvarez et al., 2015). A maximum drop diameter of 5.3 ±0.3 mmwas observed for the culture supernatant of Achromobacter sp.NP03. Ochrobactrum sp. NP04 and Lysinibacillus sp. NP05 also demon-strated to have relatively high diameters of 5.0 mm, when comparedto 3.3 ± 0.3mmdiameter in the negative control (see Table 2). The rel-atively high droplet diameters of the supernatants confirms the releaseof biosurfactants by these bacterial cultures into the culture medium.
As summarised in Table 2, the highest emulsification index of 50%was observed in culture supernatants of Lysinibacillus sp. NP05. Thishigh result was followed by Achromobacter sp. NP03 (33.3%) andOchrobactrum sp. NP04 (16.7%). Emulsion formation was not observedin the culture supernatant of Pseudomonas sp. NP06. The production ofbiosurfactants was evident by the formation of the emulsion layer byLysinibacillus, Achromobacter and Ochrobactrum strains. The ability ofbiosurfactant production by these potential PCB degradingmicroorgan-isms to increase the solubility of hydrophobic PCBs without addition ofchemical or biological surfactant is an added advantage in bioremedia-tion applications.
Similar to positive results from the emulsification index test,Lysinibacillus sp. NP05 performed well during the haemolytic assay. Itshowed strong yellow transparent zones in Tryptone soya agar contain-ing sheep blood after incubation at 28 °C for 48 h indicative of completehaemolysis of red blood cells. In contrast, Achromobacter sp. NP03and Ochrobactrum sp. NP04 showed partial haemolysis having semi-
Fig. 6. Growth profile, PCB hydrolysis and pH variation of Lysinibacillus sp. NP05 under two stagfrom triplicates.
transparent zones surrounded by dark green areas. Pseudomonas sp.NP06 showed negative to minor haemolysis and this also coincideswith the low chloride accumulation observed.
Based on the three biosurfactant tests, the descending order of pref-erence for the potential of biosurfactant production is Lysinibacillus sp.NP05 N Achromobacter sp. NP03 N Ochrobactrum sp. NP04 N Pseudomo-nas sp. NP06. When the results of biosurfactant screening testswere compared with PCB solubility and chloride ion accumulation,Lysinibacillus sp. NP05 that exhibited the highest biosurfactant produc-tion potential, also demonstrated the highest total PCB solubility andchloride ion accumulation. These results provide a clear and positivecorrelation between biosurfactant production and PCB solubilizationand subsequent degradation. Not surprisingly, Pseudomonas sp. NP06that demonstrated the lowest biosurfactant production capability alsohad the lowest chloride ion levels in the culture supernatant.
According to the results presented in this study, it can be argued thatthe rate of microbial degradation of PCBs is decided not only by theirability of break down the PCB molecules, but also bymaking the hydro-phobic PCBs soluble in the aqueous medium. Therefore, it is importantto include suitable and different culture members with the ability toproduce biosurfactants in order to facilitate the bioavailability of PCBs.Such a strategy will be highly effective in field and scale up bioremedi-ation applications.
Overall, the data from the growth characteristics (Fig. 2), PCBsolubility (Fig. 3), chloride build up (Fig. 4), pH variation (Fig. 5)and biosurfactant production (Table 2) support the identificationof Lysinibacillus sp. NP05 as the best performer out of four facultativeanaerobic strains tested followed by Achromobacter sp. NP03,Ochrobactrum sp. NP04 and Pseudomonas sp. NP06. As shown inFig. 6, increase in growth rate parallel to the increased PCB solubilityunder combined anaerobic-aerobic treatment by Lysinibacillus sp.NP05 is an indication of the consumption of Aroclor 1260 as its carbonand energy source other than solubilization. Moreover, decrease in pHover the two weeks of aerobic phase as per Fig. 6 would coincide withthe occurrence of advanced PCB degradation steps to further break-down the chlorinated intermediates.
4. Conclusions
Based on an exhaustive review of past research literature, this re-search can be considered as the first comparative study which assessed
e anaerobic-aerobic conditions. Error bars represent the standard deviation of mean values

2206 G. Pathiraja et al. / Science of the Total Environment 651 (2019) 2197–2207
the capability and growth characteristics of facultative anaerobicbacteria in degrading PCBs under anaerobic, aerobic and two-stageanaerobic-aerobic cultivation conditions. The study found four bacterialstrains identified as Achromobacter sp. NP03, Ochrobactrum sp. NP04,Lysinibacillus sp. NP05 and Pseudomonas sp. NP06, to have the capabilityfor degrading commercial PCB mixture, Aroclor 1260 as the sole sourceof carbon under both, anaerobic and aerobic conditions. Among the fourstrains tested, Lysinibacillus sp. NP05 performed the best based on theresults from the comparative experiments on cell growth, PCB solubil-ity, chloride build up and biosurfactant production.
The results suggested that microorganisms capable of degradingPCBs also have the potential to produce surface active substances tofacilitate hydrophobic PCBs which are soluble in aqueous media andconsequently enhanced the bioavailability and degradation of PCBs.The two stage anaerobic-aerobic conditions produced the best overallresults when assessed on cell growth, PCB solubility and chloride buildup. In field scale soil remediation applications, facultative microorgan-isms have the potential to be better candidates as they can surviveand degrade PCBs under both anaerobic and aerobic conditions, whileachieving relatively higher degradation rates. Furthermore, based onthese results there is an opportunity to produce and apply tailor-madebacterial consortia for future process designs and applications resultingin shorter time frames, while effectively hydrolyzing PCBs.
Acknowledgement
The authors would like to acknowledge the Queensland University ofTechnology (QUT) for the Australian Government Research TrainingProgram (RTP) scholarship provided to the first author for undertakingthis doctoral study. We also gratefully thank the QUT Central AnalyticalResearch Facility (CARF) operated by the Institute of Future Environments(IFE) where the analytical data reported in this paper were obtained.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.scitotenv.2018.10.127.
References
Abraham, W.R., Nogales, B., Golyshin, P.N., Pieper, D.H., Timmis, K.N., 2002. Polychlorinatedbiphenyl-degrading microbial communities in soils and sediments. Curr. Opin.Microbiol. 5 (3), 246–253. https://doi.org/10.1016/S1369-5274(02)00323-5.
Adrian, L., Dudkova, V., Demnerova, K., Bedard, D.L., 2009. “Dehalococcoides” sp. straincbdb1 extensively dechlorinates the commercial polychlorinated biphenyl mixturearoclor 1260. Appl. Environ. Microbiol. 75 (13), 4516–4524.
Agullo, L., Pieper, D.H., Seeger, M., 2017. Genetics and biochemistry of biphenyl and pcbbiodegradation. In: Fernando, R. (Ed.), Aerobic Utilization of Hydrocarbons, Oils andLipids. Springer International Publishing, pp. 1–28.
Alvarez, V.M., Jurelevicius, D., Marques, J.M., de Souza, P.M., de Araujo, L.V., Barros, T.G., deSouza, R.O.M.A., Freire, D.M.G., Seldin, L., 2015. Bacillus amyloliquefaciens tsbso 3.8, abiosurfactant-producing strain with biotechnological potential for microbial en-hanced oil recovery. Colloids Surf. B: Biointerfaces 136, 14–21. https://doi.org/10.1016/j.colsurfb.2015.08.046.
Aparna, A., Srinikethan, G., Smitha, H., 2012. Production and characterization ofbiosurfactant produced by a novel pseudomonas sp. 2b. Colloids Surf. B: Biointerfaces95 (Supplement C), 23–29. https://doi.org/10.1016/j.colsurfb.2012.01.043.
APHA, 2012. StandardMethods for the Examination of Water andWastewater. 22nd edn.American Public Health Association, Washington, DC.
Atlas, R.M., 2005. Handbook of Media for Environmental Microbiology. 2nd ed. CRC press.ATSDR, 2000. Toxicological profile for polychlorinated biphenyls (pcbs). Division of Toxicol-
ogy/Toxicology Information Branch, U.S. Department of Health and Human Services.Bedard, D.L., Bailey, J.J., Reiss, B.L., Jerzak, G.V.S., 2006. Development and characterization
of stable sediment-free anaerobic bacterial enrichment cultures that dechlorinatearoclor 1260. Appl. Environ. Microbiol. 72 (4), 2460–2470 Retrieved from PMC.https://doi.org/10.1128/AEM.72.4.2460-2470.2006.
Bedard, D.L., Ritalahti, K.M., Loffler, F.E., 2007. The dehalococcoides population insediment-free mixed cultures metabolically dechlorinates the commercialpolychlorinated biphenyl mixture aroclor 1260. Appl. Environ. Microbiol. 73 (8),2513–2521. https://doi.org/10.1128/AEM.02909-06.
Beyer, A., Biziuk, M., 2009. Environmental fate and global distribution of polychlorinatedbiphenyls. In: Whitacre, D.M. (Ed.), Reviews of environmental contamination andtoxicology. Vol. 201. Springer US, pp. 137–158.
Bodour, A.A., Drees, K.P., Maier, R.M., 2003. Distribution of biosurfactant-producing bacte-ria in undisturbed and contaminated arid southwestern soils. Appl. Environ.Microbiol. 69 (6), 3280–3287. https://doi.org/10.1128/AEM.69.6.3280–3287.2003.
Calvo, C., Silva-Castro, G., Uad, I., Fandiño, C.G., Laguna, J., González-López, J., 2008. Effi-ciency of the eps emulsifier produced by ochrobactrum anthropi in different hydro-carbon bioremediation assays. J. Ind. Microbiol. Biotechnol. 35 (11), 1493–1501.https://doi.org/10.1007/s10295-008-0451-5.
Cameotra, S.S., Bollag, J.-M., 2003. Biosurfactant-enhanced bioremediation of polycyclicaromatic hydrocarbons. Crit. Rev. Environ. Sci. Technol. 33 (2), 111–126. https://doi.org/10.1080/10643380390814505.
Cole, J.R., Wang, Q., Fish, J.A., Chai, B.M.D.M., Sun, Y., Brown, C.T., Porras-Alfaro, A., Kuske,C.R., Tiedje, J.M., 2014. Ribosomal database project: data and tools for high through-put rrna analysis. Nucleic Acids Res. 42 (Database issue), D633–D642. https://doi.org/10.1093/nar/gkt1244.
Dudasova, H., Lukacova, L., Murinova, S., Puskarova, A., Pangallo, D., Dercova, K., 2014.Bacterial strains isolated from pcb-contaminated sediments and their use for bioaug-mentation strategy in microcosms. J. Basic Microbiol. 54 (4), 253–260. https://doi.org/10.1002/jobm.201200369.
Evans, B.S., Dudley, C.A., Klasson, K.T., 1996. Sequential anaerobic-aerobic biodegradationof pcbs in soil slurry microcosms. Appl. Biochem. Biotechnol. 57 (1), 885–894.
Fava, F., Di Gioia, D., 2001. Soya lecithin effects on the aerobic biodegradation ofpolychlorinated biphenyls in an artificially contaminated soil. Biotechnol. Bioeng.72 (2), 177–184.
Federici, E., Giubilei, M.A., Covino, S., Zanaroli, G., Fava, F., D'Annibale, A., Petruccioli, M.,2012. Addition of maize stalks and soybean oil to a historically pcb-contaminatedsoil: effect on degradation performance and indigenous microbiota. New Biotechnol.30 (1), 69–79. https://doi.org/10.1016/j.nbt.2012.07.007.
Field, J.A., Sierra-Alvarez, R., 2008. Microbial transformation and degradation ofpolychlorinated biphenyls. Environ. Pollut. 155 (1), 1–12. https://doi.org/10.1016/j.envpol.2007.10.016.
Furukawa, K., 2000. Biochemical and genetic bases of microbial degradation ofpolychlorinated biphenyls (pcbs). J. Gen. Appl. Microbiol. 46 (6), 283–296.
Hughes, A.S., VanBriesen, J.M., Small, M.J., 2009. Identification of structural properties as-sociated with polychlorinated biphenyl dechlorination processes. Environ. Sci.Technol. 44 (8), 2842–2848. https://doi.org/10.1021/es902109w.
Janda, J.M., Abbott, S.L., 2007. 16s rrna gene sequencing for bacterial identification in thediagnostic laboratory: pluses, perils, and pitfalls. J. Clin. Microbiol. 45 (9), 2761–2764Retrieved from PMC. https://doi.org/10.1128/JCM.01228-07.
Joy, S., Rahman, P.K.S.M., Sharma, S., 2017. Biosurfactant production and concomitant hy-drocarbon degradation potentials of bacteria isolated from extreme and hydrocarboncontaminated environments. Chem. Eng. J. 317, 232–241. https://doi.org/10.1016/j.cej.2017.02.054.
Long, Y.-Y., Fang, Y., Zhang, C., Du, Y., Shentu, J., Shen, D.-S., 2015. Degradation ofpolychlorinated biphenyls by sequential anaerobic–aerobic composting. Water AirSoil Pollut. 226 (3), 1–12. https://doi.org/10.1007/s11270-015-2333-6.
Master, E.R., Lai, V.W.-M., Kuipers, B., Cullen, W.R., Mohn, W.W., 2002. Sequentialanaerobic-aerobic treatment of soil contaminated with weathered aroclor 1260. En-viron. Sci. Technol. 36 (1), 100–103.
Mayes, B.A., Mc Connell, E.E., Neal, B.H., Brunner, M.J., Hamilton, S.B., Peters, A.C., Ryan,M.J., Toft, J.D., Singer, A.W., Brown Jr., J.F., 1998. Comparative carcinogenicity inSprague-dawley rats of the polychlorinated biphenyl mixtures aroclors 1016, 1242,1254, and 1260. Toxicol. Sci. 41 (1), 62–76.
Megharaj, M., Ramakrishnan, B., Venkateswarlu, K., Sethunathan, N., Naidu, R., 2011. Bio-remediation approaches for organic pollutants: a critical perspective. Environ. Int. 37(8), 1362–1375. https://doi.org/10.1016/j.envint.2011.06.003.
Murinova, S., Dercova, K., 2014. Potential use of newly isolated bacterial strainochrobactrum anthropi in bioremediation of polychlorinated biphenyls. Water AirSoil Pollut. 225 (6), 1–16. https://doi.org/10.1007/s11270-014-1980-3.
Murinova, S., Dercova, K., Dudasova, H., 2014. Degradation of polychlorinated biphenyls(pcbs) by four bacterial isolates obtained from the pcb-contaminated soil and pcb-contaminated sediment. Int. Biodeterior. Biodegrad. 91 (0), 52–59. https://doi.org/10.1016/j.ibiod.2014.03.011.
Nayak, A.S., Vijaykumar, M.H., Karegoudar, T.B., 2009. Characterization of biosurfactant pro-duced by pseudoxanthomonas sp. Pnk-04 and its application in bioremediation. Int.Biodeterior. Biodegrad. 63 (1), 73–79. https://doi.org/10.1016/j.ibiod.2008.07.003.
Occulti, F., Roda, G.C., Berselli, S., Fava, F., 2008. Sustainable decontamination of an actual-site aged pcb-polluted soil through a biosurfactant-based washing followed by aphotocatalytic treatment. Biotechnol. Bioeng. 99 (6), 1525–1534. https://doi.org/10.1002/bit.21703.
Ohtsubo, Y., Kudo, T., Tsuda, M., Nagata, Y., 2004. Strategies for bioremediation ofpolychlorinated biphenyls. Appl. Microbiol. Biotechnol. 65 (3), 250–258. https://doi.org/10.1007/s00253-004-1654-y.
Panjiar, N., Sachan, S.G., Sachan, A., 2015. Screening of bioemulsifier-producing micro-organisms isolated from oil-contaminated sites. Ann. Microbiol. 65 (2), 753–764.https://doi.org/10.1007/s13213-014-0915-y.
Passatore, L., Rossetti, S., Juwarkar, A.A., Massacci, A., 2014. Phytoremediation and bioreme-diation of polychlorinated biphenyls (pcbs): state of knowledge and research perspec-tives. J. Hazard. Mater. 278, 189–202. https://doi.org/10.1016/j.jhazmat.2014.05.051.
Payne, R.B., May, H.D., Sowers, K.R., 2011. Enhanced reductive dechlorination ofpolychlorinated biphenyl impacted sediment by bioaugmentation with adehalorespiring bacterium. Environ. Sci. Technol. 45 (20), 8772–8779. https://doi.org/10.1021/es201553c.
Pieper, D.H., Seeger, M., 2008. Bacterial metabolism of polychlorinated biphenyls. J. Mol.Microbiol. Biotechnol. 15 (2–3), 121–138. https://doi.org/10.1159/000121325.
Praveckova,M., Brennerova, M.V., Cvancarova, M., De Alencastro, L.F., Holliger, C., Rossi, P.,2015. Divergent pcb organohalide-respiring consortia enriched from the efflux

2207G. Pathiraja et al. / Science of the Total Environment 651 (2019) 2197–2207
channel of a former delor manufacturer in eastern europe. Ecotoxicol. Environ. Saf.120 (0), 223–234. https://doi.org/10.1016/j.ecoenv.2015.05.038.
Ramasamy, S., Mathiyalagan, P., Chandran, P., 2014. Characterization and optimization ofeps-producing and diesel oil-degrading ochrobactrum anthropi mp3 isolated fromrefinery wastewater. Pet. Sci. 11 (3), 439–445. https://doi.org/10.1007/s12182-014-0359-9.
Rashid, G., Durán-Peña, M., Rahmanpour, R., Sapsford, D., Bugg, T., 2017. Delignificationand enhanced gas release from soil containing lignocellulose by treatment with bac-terial lignin degraders. J. Appl. Microbiol. 123 (1), 159–171.
Singer, A., Gilbert, E., Luepromchai, E., Crowley, D., 2000. Bioremediation ofpolychlorinated biphenyl-contaminated soil using carvone and surfactant-grownbacteria. Appl. Microbiol. Biotechnol. 54 (6), 838–843.
Tartakovsky, B., Michotte, A., Cadieux, J.C.A., Lau, P.C.K., Hawari, J., Guiot, S.R., 2001.Degradation of aroclor 1242 in a single - stage coupled anaerobic/aerobicbioreactor. Water Res. 35 (18), 4323–4330. https://doi.org/10.1016/S0043-1354(01)00175-0.
Thavasi, R., Sharma, S., Jayalakshmi, S., 2011. Evaluation of screening methods for theisolation of biosurfactant producing marine bacteria. J. Pet. Environ. Biotechnol.https://doi.org/10.4172/2157-7463.S1-001.
USEPA, 1993. Method 300.0 Determination of Inorganic Ions by Ion Chromatography. En-vironmental Monitoring Systems Laboratory, Office of Research and Development,USEPA, Cincinnati, Ohio 45268.
USEPA, 2007. Method 8082a. Polychlorinated Biphenyls (Pcbs) by Gas Chromatography.Environmental Protection Agency, United States.
Viisimaa, M., Karpenko, O., Novikov, V., Trapido, M., Goi, A., 2013. Influence ofbiosurfactant on combined chemical–biological treatment of pcb-contaminated soil.Chem. Eng. J. 220, 352–359. https://doi.org/10.1016/j.cej.2013.01.041.
Walker, D.R., Feyerherm, F., 2013. Identification and quantitation of. Pcb aroclor mixturesin a single. Run using the agilent 7000b triple. Quadrupole gc/ms. Retrieved from.https://www.agilent.com/cs/library/applications/5991-3537EN.pdf.
Wang, S., He, J., 2013. Phylogenetically distinct bacteria involve extensive dechlorinationof aroclor 1260 in sediment-free cultures. PLoS One 8 (3), e59178. https://doi.org/10.1371/journal.pone.0059178.
Wang, Q., Garrity, G.M., Tiedje, J.M., Cole, J.R., 2007. Naive bayesian classifier for rapid as-signment of rRNA sequences into the new bacterial taxonomy. Appl. Environ.Microbiol. 73 (16), 5261–5267. https://doi.org/10.1128/AEM.00062-07.
Wiegel, J., Wu, Q., 2000. Microbial reductive dehalogenation of polychlorinated biphenyls.FEMS Microbiol. Ecol. 32 (1), 1–15.
Wu, Q., Sowers, K.R., May, H.D., 2000. Establishment of a polychlorinated biphenyl-dechlorinating microbial consortium, specific for doubly flanked chlorines, in a de-fined, sediment-free medium. Appl. Environ. Microbiol. 66 (1), 49–53.
Yin, Y., Guo, J., Zheng, L., Tian, L., Wang, X., 2011. Capability of polychlorinated biophenyl(pcbs) degrading fungi segregated from sediments. World J. Microbiol. Biotechnol. 27(11), 2567. https://doi.org/10.1007/s11274-011-0728-0.