BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap...
-
Upload
alban-hood -
Category
Documents
-
view
216 -
download
2
Transcript of BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap...
BIOL 200 (Section 921)Lecture # 11 [July 4, 2006]
UNIT 8: Cytoskeleton
• Reading:
• ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.
• ECB, 1st ed. Chap 16. pp 513-542; Questions 16-1, 16-2, 16-10 to 16-21.
UNIT 8: Cytoskeleton - Objectives1. Two major roles for cytoskeleton - skeletal support and motility 2. Distinguish between three major cytoskeletal systems - intermediate filaments,
microtubules and actin filaments (microfilaments). 3. Be able to describe how intermediate filaments are assembled from polypeptides
to form a microscopically visible fibre. 4. Know major cell function of intermediate filaments 5. Know structure of microtubules, their process of assembly, the meaning of plus
and minus ends, and the role of MTOCs. 6. Understand dynamic instability and how it may be applied to microtubules and
microtubule containing structures. 7. Understand the role of GTP in the generation and control of dynamic instability of
microtubules. 8. Understand how motor proteins work and how their movement relates to the
polarity of their molecular substrates 9. Be able to describe the structure of flagella and the molecular basis of flagellar
bending 10. assembly of actin filaments, 11. dynamic instability of actin filaments; comparison with that of microtubules. 12. role of actin filaments in formation of the cell cortex, and regulation of cell
structure and movement, 13. myosins and the myosin activity cycle as it relates to muscle.
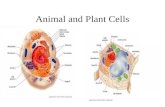
The cytoskeleton is a network of filaments that regulatesa cell’s shape, strength and movement [Fig. 1-27]
Actin filaments Microtubules Intermediatefilaments
An overview of the cytoskeleton [Fig. 17-2]
Intermediate Filaments-tough ropes
Microtubules-big hollow tubes support cell structures
Actin Microfilaments- helical polymers involved in movement/shape
17_03_Interm_filaments.jpgIntermediate filaments form a strong, durable network in
the cytoplasm of the cell [Fig. 17-3]
Intermediate keratin filaments (green, fluorescent) from different cells are
connected through the desmosomes [Immunofluorescence micrograph]
A drawing from the electronmicrograph showing the bundlesof intermediate filaments throughthe desmosomes
Structure Long, hollow cylinders made of 13 protofilaments
Diameter 25 nm
Protein Subunit Alpha & Beta Tubulin = globular Proteins
Location in Cell -end: attached to centrosome (or Microtubule organization Center)
+ end is free
Function 1. Chromosome movement during cell division
2. Maintaining cell shape
3. Movement of vesicles
4. Movement of cilia and flagella
5. Positioning organelles within cell
Drug Sensitivity A) Colchicine: binds free tubulin and inhibits formation of microtubulues
B) Taxol: stabilizes microtubules by preventing loss of subunits
MICROTUBULES
-The growing end of the microtubule (MT), at the top, has subunits arranged with the beta-tubulin on the outside. The subunits in the microtubule all show a uniform polarity
tubulin dimer
-Microtubules, like poly-peptides and nucleic acids, grow by addition of subunits at only one end:Growth = plus endNo Growth = minus end.
[Fig. 17-10]
Growingend
Non-growingor fixed end
Fig. 16-11 Alberts MBOC-
GTP-bound tubulin packs efficiently into protofilament
GDP-bound tubulin bind less strongly to each other-depolymerize MT
GTP hydrolyzes to GDP in MT
17_11_centrosome.jpg
The centrosome is the major MT-organizing Center. It contains nucleating sites (rings of of γ-tubulin) which serve
as starting point for growth of MTs [Fig. 17-11]
Centrioles are arrays of short MTs and are identical to basal bodies.
-Tubulin (a G-protein) dimers carrying GTP (red) bind more tightly to one another than tubulin dimers carrying GDP (dark green). -microtubules with freshly added tubulin dimers and GTP keep growing. -when microtubule growth is slow, the subunits in this "GTP cap" will hydrolyze their GTP to GDP before fresh subunits loaded with GTP have time to bind. The GTP cap is then lost-the GDP-carrying subunits are less tightly bound in the polymer and are readily released from the free end, so that the microtubule begins to shrink continuously.
GTP cap leads to stability and growth of MTs
GTP cap
Dynamic instability (loss of GTP cap) leads to MT shrinking
Fig. 17-13Inhibitor: COLCHICINE Inhibitor: TAXOL
Three classes of MTs make up the mitotic spindle at metaphase [Fig. 19-13]
Aster MTs Kinetochore MTs Interpolar MTs
17_12_grows_shrinks.jpg
Each microtubule filament grows and shrinks
independent of its neighbors [Fig.17-12]
17_14_polarize_cell.jpgThe selective stabilization of MTs can
polarize a cell [Fig. 17-14]
•A MT can be stabilized by attaching its plus end to a capping protein or cell structure that prevents tubulin depolymerization
•This is how organelles are positioned in cells
Motor proteins [Dynein and Kinesin] transport vesicles along MTs in a nerve cell [Fig. 17-15]
cell body
MT
axon terminal
+-
dynein kinesin
Nerve cell polarity maintained by microtubules
Motor proteins [Dynein and Kinesin] move along MTs using their globular heads [Fig. 17-17]
dynein
dynein
kinesin
kinesin
Kinesins move ER outward and Dyneins move Golgi inward to maintain cell structure [Fig. 17-23]
ER
MTs
kinesins
Golgi
MTs
dynein
ER
Golgi
MT
Nucleus
17_22_kinesin_moves.jpg
Kinesin walks along a MT [Fig. 17-22]
Moves in aSeries of 8 nm stepsHeads
Kinesin-GFPmoves alonga MT
Motor proteins• Two families of motor proteins are involved in
moving vesicles and other membrane-bound organelles along MT tracks
• Both binding sites for tubulin (head) and for their cargo (tail)
• Both use ATP hydrolysis to change conformation and move along MT
• Kinesins move vesicles to plus end of MT away from centrosome [e.g. Kinesins pull ER ouward along MTs]
• Dyneins move vesicles towards minus end of MT, towards the centrosome [e.g. Dyneins pull the Golgi apparatus towards the centre of the cell]
Cilia and Flagella• An array of stabilized
MTs and MT-associated proteins (MAPS)
• Same structure throughout all kingdoms.
• Cilia are short and many. Flagella are long, single or paired.
• Air pollution and cigarette smoking can cause loss of cilia on epithelium of the respiratory tract.
Ciliated epithelium in airway [Fig. 17-24]
Flagella propel a sperm cell [Fig. 17-26]
17_27_9_+_2_array.jpgMTs in a cilium or flagellum are arranged
In a “9 + 2” array [Fig. 17-27]
•9 Doublet MTs and 2 central singlets•Many different MAPs including radial spokes, central sheath element, nexin links, dynein arms•Dynein hydrolyzes ATP and generates a sliding force between MT doublets
17_28_dynein_flagell.jpg
The movement of dynein causes bending of flagellum
[Fig. 17-28]Linkers removed
17_29_Actin_filaments.jpgDistribution of actin filaments in different cells
Determines their shape and function [Fig. 17-29]
Microvilli in Intestine (increase surface area)
Contractile bundlesin cytoplasm
Sheetlike (lamellipodia)and fingerlike (filipodia)protrusionsof a moving cell [importantin cell crawling, endo- andexo-cytosis
Contractile ring during cell divn.
17_30_protein threads.jpg
Two F-actin strands wind around each other to form an actin filament [Fig. 17-30]
Twist-repeatingdistance
Actin with bound ADP
minus end
Actin with bound ATP
plus end
ATP hydrolysis induce dynamic instability of actin filaments [Fig.17-31]
Cytochalasin D: A fungal metabolite, Inhibits the polymerization of actin microfilaments
Phalloidin: A cyclic peptide from the deathcap fungus, Amanita phalloides, inhibits the depolymerization of actin, thereby stabilizing actin microfilaments
Microfilaments or Actin Filaments
• Distribution: in bundles lying parallel to plasma membrane
• Diameter: 7 mm• Structure: made of a small globular protein known
as G-actin• Polymerizes into filaments known as F-actin• Two F-actin molecules wind around each other to
form a microfilament• Show structural polarity• Show dynamic instability• Associate with actin-binding proteins
17_32_Actin_binding.jpg
Actin-binding proteins regulate the behavior of actin filaments [Fig. 17-32]
(e.g. thymosin and profilin)
(e.g. gelsolin)
Filopodium grows by nucleation of actin microfilaments [Fig. 16-29, ECB 1st ed.]
nucleation complex at PM
Growing filopodium
Growing microfilament
monomers added
17_36_actin_meshwork.jpg
Association of actin and actin related proteins pushes forward lamellipodium
17_38_myosin_I.jpg
Roles of actin-dependent motor protein, myosin I [Fig. 17-38]
Myosin I: Move a vesicle relative to an actin filament.
Myosin I: Move an actin filament.
The head group of myosin I walks towards the plus end of the actin filament.
17_40_Myosin_II.jpgMyosin-II molecules can associate with one another to
form myosin filaments [Fig. 17-40]
[Coiled-coil]
Bipolar myosin filament
Tails
17_41_slide_actin.jpgRoles of actin-dependent motor protein, myosin II [Fig. 17-38
Myosin II: Regulate contraction – move actin filaments relative to each other.
The head group of myosin II walks towards the plus end of the actin filament.
Myofibrils made up of actin and myosin II packed into chains of sarcomeres [Fig. 17-42]
Muscle contraction depends on bundlesof actin and myosin
Sarcomeres (contractile units of muscle) are arrays of actin and myosin [Fig. 17-43]
Z disc: attachment pointsFor actin filaments
17_44_Muscles contract.jpg
Muscles contract by a sliding-filament mechanism [Fig. 17-44]
+ +
The myosin heads walk toward the plus end of the adjacent actin filamentdriving a sliding motion during muscle contraction.
17_45_myosin_walks.jpg
1. The Myosin head tightly locked onto an actin filament.
2. ATP binds to the myosinhead. The Myosin head released from actin.
3. The myosin head displaced by 5 nm. ATP hydrolysis.
4. The myosin head attachesto a new site on actin filament.Pi released. Myosin headregains its originalconformation (power stroke).ADP released.
5. The myosin head is again locked tightly to the actin filament.
Experimental Methodology, Techniques and Approaches for
Studying the Cytoskeleton
1. Modern microscopy techniques
2. Drugs and mutations to disrupt cytoskeletal structures
Modern microscopy techniques to study cytoskeleton
1. Immunofluorescence microscopy: Primary antibodies bind to cytoskeletal proteins. Secondary antibodies labeled with a fluorescent tag bind to the primary antibody. Cytoskeletal proteins glow in the fluorescence microscope. [Fig. A fibroblast stained with fluorescent antibodies against actin filaments].
2. Fluorescence techniques: Fluorescent versions of cytoskeletal proteins are made and introduced into living cells. Flurescence microscopy and video cameras are used to view the proteins as they function in the cell [Fig.Fluorescent tubulin molecules form MTs in fibroblast cells].
3. Computer-enhanced digital videomicroscopy: High resolution images from a video camera attached to a microscope are computer processed to increase contrast and remove background features that obscure the image. [ Several MTs processed to make them visible in detail].
4. Electron microscopy: EM can resolve individual filaments prepared by thin section, quick-freeze deep- etch, or direct-mount techniques. [Bundles of actin filaments in a fibroblast cell prepared by the quick-freeze deep-etch method].
Becker et al. The World of the Cell
Drug Treatments1. Colchicine: An alkaloid from the Autumn
crocus, Colchicum autumnale). Binds to tubulin monomers and prevents polymerization in MTs.
2. Taxol: from the Pacific Yew tree, Taxus brevifolis binds tightly to MTs and stabilizes them. It prevents MTs from dissociating.
3. Cytochalasin D: A fungal metabolite, inhibits the polymerization of actin microfilaments.
4. Phalloidin: A cyclic peptide from the death cap fungus, Amanita phalloides, inhibits the depolymerization of actin, thereby stabilizing actin microfilaments
![Page 1: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/1.jpg)
![Page 2: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/2.jpg)
![Page 3: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/3.jpg)
![Page 4: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/4.jpg)
![Page 5: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/5.jpg)
![Page 6: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/6.jpg)
![Page 7: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/7.jpg)
![Page 8: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/8.jpg)
![Page 9: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/9.jpg)
![Page 10: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/10.jpg)
![Page 11: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/11.jpg)
![Page 12: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/12.jpg)
![Page 13: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/13.jpg)
![Page 14: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/14.jpg)
![Page 15: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/15.jpg)
![Page 16: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/16.jpg)
![Page 17: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/17.jpg)
![Page 18: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/18.jpg)
![Page 19: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/19.jpg)
![Page 20: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/20.jpg)
![Page 21: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/21.jpg)
![Page 22: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/22.jpg)
![Page 23: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/23.jpg)
![Page 24: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/24.jpg)
![Page 25: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/25.jpg)
![Page 26: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/26.jpg)
![Page 27: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/27.jpg)
![Page 28: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/28.jpg)
![Page 29: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/29.jpg)
![Page 30: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/30.jpg)
![Page 31: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/31.jpg)
![Page 32: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/32.jpg)
![Page 33: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/33.jpg)
![Page 34: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/34.jpg)
![Page 35: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/35.jpg)
![Page 36: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/36.jpg)
![Page 37: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/37.jpg)
![Page 38: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/38.jpg)
![Page 39: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/39.jpg)
![Page 40: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/40.jpg)
![Page 41: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/41.jpg)
![Page 42: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/42.jpg)
![Page 43: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/43.jpg)
![Page 44: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/44.jpg)
![Page 45: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/45.jpg)
![Page 46: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/46.jpg)
![Page 47: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/47.jpg)
![Page 48: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/48.jpg)
![Page 49: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/49.jpg)
![Page 50: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/50.jpg)
![Page 51: BIOL 200 (Section 921) Lecture # 11 [July 4, 2006] UNIT 8: Cytoskeleton Reading: ECB, 2nd ed. Chap 17. pp 573-606; Questions 17-1, 17-2, 17-12 to 17-23.](https://reader042.fdocuments.in/reader042/viewer/2022032804/56649e565503460f94b4d645/html5/thumbnails/51.jpg)