Biochemistry Lecture 1 October 24 2011
Transcript of Biochemistry Lecture 1 October 24 2011
-
8/3/2019 Biochemistry Lecture 1 October 24 2011
1/28
BIOCHEMISTRYand
MICROBIAL PHYSIOLOGY
MQT 1173
-
8/3/2019 Biochemistry Lecture 1 October 24 2011
2/28
BIOCHEMISTRY
Biochemists discuss chemistry with
biologists, and biology with
chemists, thereby confusing bothgroups. Among themselves, they
talk about baseball.
Credit: Anonymous
-
8/3/2019 Biochemistry Lecture 1 October 24 2011
3/28
Biochemistry is a hybrid science:
Biology is the science of living organisms and chemistry is the
science of atoms and molecules, so biochemistry is the
science of the atoms and molecules in living organisms.
Its domain encompass all the living world with the unifying
interest in the chemical structures and reactions that occur in
living systems.
Where can you find biochemistry? All through science, medicine,
and agriculture.
-
8/3/2019 Biochemistry Lecture 1 October 24 2011
4/28
Biochemistry underlies ordinary life in unseen ways:
For example, take a middle-aged man who:
Takes a drug to lower his serum cholesterol. That drug was
developed by a pharmaceutical company's biochemists to inhibit a
key enzyme involved in cholesterol biosynthesis.
Shaves with a cream containing compounds that soften his
beard. These active agents were developed after studies of thephysical properties of keratin, the protein in hair.
Eats a breakfast cereal fortified with vitamins identified
through nutritional biochemistry.
Wears a shirt made from pest-resistant cotton. The cotton
plants were bioengineered by biochemists through the transfer ofgenes from a bacterium into plants.
Drinks milk before bedtime. His sleep is helped by the amino
acids in the milk, which are converted by his brain into molecular
signals that lead to a resting state in other parts of his brain.
-
8/3/2019 Biochemistry Lecture 1 October 24 2011
5/28
Biochemistry has three main branches
Metabolism study of the conversion of biological molecules,
especially small molecules, from one to another
forexample, the conversion of sugar into carbon dioxide andwater, or the conversion of fats into cholesterol. Metabolicbiochemists are particularly interested in the individualenzyme-catalyzed steps of an overall sequence ofreactions (called a pathway) that leads from onesubstance to another.
Structural Biochemistry study of how molecules in livingcells work chemically. For example, structural biochemiststry to determine how the three-dimensional structure of
an enzyme contributes to its ability to catalyze a singlemetabolic reaction.
Molecular Genetics is concerned with the expression ofgenetic information and the way in which this informationcontributes to the regulation of cellular functions.
-
8/3/2019 Biochemistry Lecture 1 October 24 2011
6/28
Chemical elements and biomolecules
Around two dozen of the 94 naturally-occuring chemical
elements are essential to various kinds of biological life.Most rare elements on Earth are not needed by life (exceptions
being selenium and iodine), while a few common ones(aluminum and titanium) are not used.
Most organisms share element needs, but there are a fewdifferences between plants and animals.
For example ocean algae use bromine but land plants andanimals seem to need none.
All animals require sodium, but some plants do not.
Plants need boron and silicon, but animals may not (or mayneed ultra-small amounts).
Just six elementscarbon, hydrogen, nitrogen, oxygen,calcium, and phosphorusmake up almost 99% of the massof a human body and require smaller amounts of at least 16
more.
-
8/3/2019 Biochemistry Lecture 1 October 24 2011
7/28
The four main classes of molecules in biochemistryare carbohydrates, lipids (Fatty acids), amino
acids (proteins), and nucleic acids.Many biological molecules are polymers: in thisterminology, monomers are relatively smallmicromolecules that are linked together tocreate large macromolecules, which are knownaspolymers.
When monomers are linked together to synthesizea biological polymer, they undergo a processcalled dehydration synthesis.
Most of these are optically active, that is, they arefound in only one of the possible stereoisomers.
(Stereoisomers are compounds that have the same kinds andnumbers of atoms but have different molecular arrangements)
-
8/3/2019 Biochemistry Lecture 1 October 24 2011
8/28
-
8/3/2019 Biochemistry Lecture 1 October 24 2011
9/28
Amino acids
Generally, amino acids found in nature are the L-stereoisomers.
Amino acids are the building blocks of proteins, and have an
important role in energy metabolism and in cellular signaling.
They are also a small but important part ofcell membranes.
are molecules containing an amine group, a carboxylic acid groupand a side-chain that varies between different amino acids.
The key elements of an amino acid are carbon, hydrogen, oxygen,and nitrogen.
They are particularly important in biochemistry, where the termusually refers to alpha-amino acids.
http://en.wikipedia.org/wiki/Moleculehttp://en.wikipedia.org/wiki/Aminehttp://en.wikipedia.org/wiki/Carboxylic_acidhttp://en.wikipedia.org/wiki/Side_chainhttp://en.wikipedia.org/wiki/Carbonhttp://en.wikipedia.org/wiki/Hydrogenhttp://en.wikipedia.org/wiki/Oxygenhttp://en.wikipedia.org/wiki/Nitrogenhttp://upload.wikimedia.org/wikipedia/commons/c/ce/AminoAcidball.svghttp://en.wikipedia.org/wiki/Nitrogenhttp://en.wikipedia.org/wiki/Oxygenhttp://en.wikipedia.org/wiki/Hydrogenhttp://en.wikipedia.org/wiki/Carbonhttp://en.wikipedia.org/wiki/Side_chainhttp://en.wikipedia.org/wiki/Side_chainhttp://en.wikipedia.org/wiki/Side_chainhttp://en.wikipedia.org/wiki/Carboxylic_acidhttp://en.wikipedia.org/wiki/Aminehttp://en.wikipedia.org/wiki/Molecule -
8/3/2019 Biochemistry Lecture 1 October 24 2011
10/28
Carbohydrates
are molecules of the empirical formula Cm
(H2O)
nwhere n usually
ranges from 3
7 (where m could be different from n).
They are found in sugars and starches and make up parts ofnucleotides (the energy currency of a cell, and the building blocksfor genetic information).
They are also present in some components of all cell membranes.
They are the central components of energy-producing pathways inbiology.
Carbohydrates are made from monomers called monosaccharides.
Monosaccharides include glucose , fructose, and deoxyribose
When two monosaccharides undergo dehydration synthesis,water is produced, as two hydrogen atoms and one oxygen atomare lost from the two monosaccharides' hydroxyl group.
http://en.wikipedia.org/wiki/Monosaccharideshttp://en.wikipedia.org/wiki/Glucosehttp://en.wikipedia.org/wiki/Fructosehttp://en.wikipedia.org/wiki/Deoxyribosehttp://en.wikipedia.org/wiki/Hydrogenhttp://en.wikipedia.org/wiki/Oxygenhttp://en.wikipedia.org/wiki/Hydrogenhttp://en.wikipedia.org/wiki/Hydroxyl_grouphttp://en.wikipedia.org/wiki/Hydroxyl_grouphttp://en.wikipedia.org/wiki/Oxygenhttp://en.wikipedia.org/wiki/Hydroxyl_grouphttp://en.wikipedia.org/wiki/Hydroxyl_grouphttp://en.wikipedia.org/wiki/Hydroxyl_grouphttp://en.wikipedia.org/wiki/Hydroxyl_grouphttp://en.wikipedia.org/wiki/Oxygenhttp://en.wikipedia.org/wiki/Hydrogenhttp://en.wikipedia.org/wiki/Deoxyribosehttp://en.wikipedia.org/wiki/Fructosehttp://en.wikipedia.org/wiki/Glucosehttp://en.wikipedia.org/wiki/Monosaccharides -
8/3/2019 Biochemistry Lecture 1 October 24 2011
11/28
Properties of GlucoseMolecular formula C6H12O6
Molar mass 180.16 g/mol
Melting point -D-glucose: 46 C,
-D-glucose: 150 C
Solubility in water Very soluble
Properties of FructoseMolecular formula C6H12O6
Molar mass 180.16 g/mol
Melting point 103 C
Solubility in water Very soluble
http://en.wikipedia.org/wiki/Molar_masshttp://en.wikipedia.org/wiki/Melting_pointhttp://en.wikipedia.org/wiki/Solubilityhttp://en.wikipedia.org/wiki/Waterhttp://en.wikipedia.org/wiki/Molar_masshttp://en.wikipedia.org/wiki/Melting_pointhttp://en.wikipedia.org/wiki/Solubilityhttp://en.wikipedia.org/wiki/Waterhttp://upload.wikimedia.org/wikipedia/commons/e/e5/D-Fructose.svghttp://en.wikipedia.org/wiki/Waterhttp://en.wikipedia.org/wiki/Solubilityhttp://en.wikipedia.org/wiki/Melting_pointhttp://en.wikipedia.org/wiki/Molar_masshttp://upload.wikimedia.org/wikipedia/commons/1/14/DGlucose_Fischer.svghttp://en.wikipedia.org/wiki/Waterhttp://en.wikipedia.org/wiki/Solubilityhttp://en.wikipedia.org/wiki/Melting_pointhttp://en.wikipedia.org/wiki/Molar_mass -
8/3/2019 Biochemistry Lecture 1 October 24 2011
12/28
Properties of Deoxyribose
Molecular formula C5H10O4
Molar mass 134.13 g mol1
Melting point 91 C
Solubility in water Very soluble
A molecule of sucrose (glucose + fructose), a disaccharide
http://en.wikipedia.org/wiki/Molar_masshttp://en.wikipedia.org/wiki/Melting_pointhttp://en.wikipedia.org/wiki/Solubilityhttp://en.wikipedia.org/wiki/Waterhttp://en.wikipedia.org/wiki/Sucrosehttp://en.wikipedia.org/wiki/Disaccharidehttp://en.wikipedia.org/wiki/Disaccharidehttp://en.wikipedia.org/wiki/Sucrosehttp://upload.wikimedia.org/wikipedia/commons/b/b3/Sucrose-inkscape.svghttp://en.wikipedia.org/wiki/Waterhttp://en.wikipedia.org/wiki/Solubilityhttp://en.wikipedia.org/wiki/Melting_pointhttp://en.wikipedia.org/wiki/Molar_mass -
8/3/2019 Biochemistry Lecture 1 October 24 2011
13/28
LipidsClosely related to hydrocarbons (compounds containing
hydrogen and carbon atoms exclusively), although they usually haveother atoms beside C and H.
Constitute a broad group of naturally occurring molecules
which include fats, waxes, sterols, fat-soluble vitamins (such as
vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids,and others.
Characterized by limited solubility in water, essential
components of membranes, important energy stores in plants and
animals and signaling molecules
May be broadly defined as hydrophobic or amphiphilic small
molecules; the amphiphilic nature of some lipids allows them to form
structures such as vesicles, liposomes, or membranes in an aqueous
environment.
http://en.wikipedia.org/wiki/Vesicle_%28biology%29http://en.wikipedia.org/wiki/Liposomehttp://en.wikipedia.org/wiki/Liposomehttp://en.wikipedia.org/wiki/Vesicle_%28biology%29 -
8/3/2019 Biochemistry Lecture 1 October 24 2011
14/28
Biological lipids originate entirely or in part from two
distinct types of biochemical subunits or "building blocks":
ketoacyl and isoprene groups.
Using this approach, lipids may be divided into eight
categories: fatty acyls, glycerolipids, glycerophospholipids,
sphingolipids, saccharolipids and polyketides (derived from
condensation of ketoacyl subunits); and sterol lipids and prenol
lipids (derived from condensation of isoprene subunits)
Palmitic acid (C16H32O2)
http://en.wikipedia.org/wiki/Fatty_acylshttp://en.wikipedia.org/wiki/Glycerolipidhttp://en.wikipedia.org/wiki/Glycerophospholipidhttp://en.wikipedia.org/wiki/Sphingolipidhttp://en.wikipedia.org/wiki/Saccharolipidhttp://en.wikipedia.org/wiki/Polyketidehttp://en.wikipedia.org/wiki/Polyketidehttp://en.wikipedia.org/wiki/Saccharolipidhttp://en.wikipedia.org/wiki/Sphingolipidhttp://en.wikipedia.org/wiki/Glycerophospholipidhttp://en.wikipedia.org/wiki/Glycerolipidhttp://en.wikipedia.org/wiki/Fatty_acyls -
8/3/2019 Biochemistry Lecture 1 October 24 2011
15/28
A nucleic acid is a complex, high-molecular-weight biochemical
macromolecule composed of nucleotide chains that convey geneticinformation.
The most common nucleic acids are deoxyribonucleic acid (DNA) andribonucleic acid (RNA).
Nucleic acids are found in all living cells and viruses.
Aside from the genetic material of the cell, nucleic acids often play arole as second messengers, as well as forming the base molecule for
adenosine triphosphate, the primary energy-carrier molecule foundin all living organisms.
Nucleic acid, so called because of its prevalence in cellular nuclei, isthe generic name of the family ofbiopolymers.
Nucleosides and nucleotides
http://en.wikipedia.org/wiki/Second_messengerhttp://en.wikipedia.org/wiki/Adenosine_triphosphatehttp://en.wikipedia.org/wiki/Cell_nucleushttp://en.wikipedia.org/wiki/Biopolymerhttp://en.wikipedia.org/wiki/Biopolymerhttp://en.wikipedia.org/wiki/Cell_nucleushttp://en.wikipedia.org/wiki/Adenosine_triphosphatehttp://en.wikipedia.org/wiki/Second_messenger -
8/3/2019 Biochemistry Lecture 1 October 24 2011
16/28
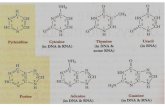
The monomers are called nucleotides, and each consists of threecomponents: a nitrogenous heterocyclic base(either a purine or apyrimidine), a pentosesugar, and a phosphate group.
Different nucleic acid types differ in the specific sugar found in their
chain (e.g. DNA or deoxyribonucleic acid contains 2-deoxyriboses).
Possibly nitrogenous bases are different in the two nucleic acids :adenine, cytosine, and guanine occur in both RNA and DNA,
while thymine occurs only in DNA and uracil occurs in RNA.
http://en.wikipedia.org/wiki/Nucleotidehttp://en.wikipedia.org/wiki/Base_%28chemistry%29http://en.wikipedia.org/wiki/Purinehttp://en.wikipedia.org/wiki/Pyrimidinehttp://en.wikipedia.org/wiki/Pentosehttp://en.wikipedia.org/wiki/Sugarhttp://en.wikipedia.org/wiki/Phosphatehttp://en.wikipedia.org/wiki/Deoxyribosehttp://en.wikipedia.org/wiki/Adeninehttp://en.wikipedia.org/wiki/Cytosinehttp://en.wikipedia.org/wiki/Guaninehttp://en.wikipedia.org/wiki/Guaninehttp://en.wikipedia.org/wiki/Cytosinehttp://en.wikipedia.org/wiki/Adeninehttp://en.wikipedia.org/wiki/Deoxyribosehttp://en.wikipedia.org/wiki/Phosphatehttp://en.wikipedia.org/wiki/Sugarhttp://en.wikipedia.org/wiki/Pentosehttp://en.wikipedia.org/wiki/Pyrimidinehttp://en.wikipedia.org/wiki/Purinehttp://en.wikipedia.org/wiki/Base_%28chemistry%29http://en.wikipedia.org/wiki/Nucleotide -
8/3/2019 Biochemistry Lecture 1 October 24 2011
17/28
Adenosine, a nucleoside
http://upload.wikimedia.org/wikipedia/commons/e/e8/Adenosin.svghttp://upload.wikimedia.org/wikipedia/commons/e/e2/Nucleotides_1.svg -
8/3/2019 Biochemistry Lecture 1 October 24 2011
18/28
General formulas of organic compoundsR represents an alkyl group (CH3(CH2)n)
-
8/3/2019 Biochemistry Lecture 1 October 24 2011
19/28
General formulas of functional groups
R represents an alkyl group (CH3(CH2)n)
-
8/3/2019 Biochemistry Lecture 1 October 24 2011
20/28
General formulas of linkages
common in biochemistry
-
8/3/2019 Biochemistry Lecture 1 October 24 2011
21/28
Representations of the structure of ribose.
(a) In the Fischer projection, ribose is drawn as a linear molecule.
(b) In its usual biochemical form, the ribose molecule is in a ring, shown
here as a Fischer projection.
(c) In a Haworth projection, the ring is depicted as lying perpendicular to
the page (as indicated by the thick lines, which represent the bonds closest
to the viewer).
(d) The ring of ribose is not actually planar but can adopt 20 possible
conformations in which certain ring atoms are out-of-plane. In the
conformation shown, C-2 lies above the plane formed by the rest of the
ring atoms.
-
8/3/2019 Biochemistry Lecture 1 October 24 2011
22/28
Types of Biochemical Reactions
Many possible biochemical reactions fall into only a few types to
consider:
Oxidation and reduction:For example, the interconversion of an alcohol and an aldehyde.
Movement of functional groups within/between moleculesFor example, the transfer of phosphate groups from one oxygen
to another.
Addition and removal of water:For example, hydrolysis of an amide linkage to an amine and a
carboxyl group.
Bond-breaking reactions:
For example, carbon-carbon bond breakage.
-
8/3/2019 Biochemistry Lecture 1 October 24 2011
23/28
All Organisms are Related
The classification and grouping of organisms, the science called
taxonomy, regards organisms as similar based on their visible
characteristics. Thus, plants and animals were regarded as the two
main kingdoms of life.
Later, cell biologists divided organisms into prokaryotes and
eukaryotes, that is, organisms without and with a nucleus.
Most recently, a new taxonomy has been developed, largely byCarl Woese and associates, based on the information in the
ribosomal RNA sequences.
-
8/3/2019 Biochemistry Lecture 1 October 24 2011
24/28
Remarkably, the most information-rich classifications of life shows
three main divisions, sometimes called domains, which aremore fundamental than the distinction between plants and
animals, or prokaryotes and eukaryotes. These domains are:
Eukarya: Includes organisms with a nucleus.This division includesplants, animals, and a large number of what are sometimes called
protists, or organisms that can be seen only under a microscope,
such as yeasts or paramecia.
Bacteria: The second domain includes microorganisms without anucleus, including many that are familiar, like Escherichia coli.
Archaea: The third groupis as different from bacteria as they arefrom eukarya on the molecular/biochemical level. These
remarkable microorganisms inhabit niches often thought of as
inhospitable to life for example, locations with high
temperature, low oxygen, or high salt. Their biochemistry is
unique and largely unexplored.
-
8/3/2019 Biochemistry Lecture 1 October 24 2011
25/28
-
8/3/2019 Biochemistry Lecture 1 October 24 2011
26/28
-
8/3/2019 Biochemistry Lecture 1 October 24 2011
27/28
-
8/3/2019 Biochemistry Lecture 1 October 24 2011
28/28