Balance each of the following chemical reactions AND explain what type of reaction it is: a. F 2(g)...
-
Upload
allyson-jenkins -
Category
Documents
-
view
220 -
download
4
Transcript of Balance each of the following chemical reactions AND explain what type of reaction it is: a. F 2(g)...

Balance each of the following chemical reactions AND explain what type of reaction it is:
a. F2(g) + Na(s) NaF(s)
b. Al(s) + CuCl2(aq) AlCl3(aq) + Cu(s)
Q Of The Day Day 5 5-9
2 2 synthesis
3 322
single replacement

First finish and hand in yesterday’s assignment: Review section 11.2 AND complete #s 13 on page 359 and 20-22 (skip 21 a and d) and 24 on page 367
Second complete and hand in Assignment # 2 Unit 9 – you must hand this in before you leave!
Day 5 5-9

Balance the following chemical reaction AND answer the question below:
F2(g) + AlCl3 AlF3(s) + Cl2(g)
What is produced (BOTH!)?
Q Of The DayDay 6
5-10

H2(g) + F2(g) 2HF(g)
What type of reaction is:
Notes page # 6

What type of reaction is:
NaBr + Cl2 NaCl + Br22 2

2KI + Pb(NO3)2 PbI2 + 2KNO3
What type of reaction is:

What type of reaction is:
2H2O(l) 2H2(g) + O2(g)electricity

What type of reaction requires aqueous reactants?
What type of reaction requires addition of heat and/or electricity?

What do most combustion reaction have in common?
Q Of The Day Day 1 5-13

usatestprep.com – account ID = stroudsburg94:
Keystone Test Prep Day 1 5-13
Partners Login Password
Tony & Brianna harrisondavis davisharrison
Angelo & Matt angelomatt psp123
Tyler & Ciarra tylerc 142650
Desiree & Kendra desiree desiree
Tim & Chris ChrisDavonn dragonbutterfly212
Kevin marvinstrickland ilovemarvin1
Jacob & Keith TylerH longboarding1
Complete Practice Test 3 and Practice Test 4

Keystone Test Prep Day 1 5-13
Kashawn = study hall
Davonn = Balancing websites
Johnny = make up book work

How does the Law of Conservation of Matter apply to chemical reactions?
How do substances react to form products?
Day 2 5-8

1. How do you know if a synthesis reaction has taken place?
Q Of The Day Day 3 5-9
2. How do you know if a decomposition reaction has taken place?



1. How do you know if a single replacement reaction has taken place?
Q Of The Day Day 4 5-10
2. How do you know if a double replacement reaction has taken place?

What type of reaction:
can produce a precipitate?
requires an energy source?
produces only one product?
Q Of The Day Day 5 5-11


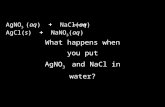



![Aula #23 · AgCl (s) Ag+ (aq) + Cl-(aq) Ksp = [Ag +][Cl K sp is the solubility product constant MgF 2 (s) Mg2+ (aq) + 2F-(aq) Ksp = [Mg 2+][F]2 Ag 2 CO 3 (s) 2Ag+ (aq) + CO3 2-(aq)](https://static.fdocuments.in/doc/165x107/5f08237a7e708231d42087a7/aula-23-agcl-s-ag-aq-cl-aq-ksp-ag-cl-k-sp-is-the-solubility-product.jpg)













