1 CH1. Atomic Structure orbitals orbitals periodicity periodicity.
(B) Periodicity. After completing this topic you should be able to : ATOMIC STRUCTURE Bonding in the...
-
Upload
beverly-cole -
Category
Documents
-
view
215 -
download
0
Transcript of (B) Periodicity. After completing this topic you should be able to : ATOMIC STRUCTURE Bonding in the...

(B) Periodicity(B) Periodicity

After completing this topic you should be able to :
ATOMIC STRUCTUREATOMIC STRUCTURE
Bonding in the first 20 elements Bonding in the first 20 elements
• Learners should be familiar with the Periodic Table in terms of elements being arranged in order of increasing atomic number.
• The first 20 elements in the Periodic Table are categorised according to bonding and structure: - metallic (Li, Be, Na, Mg, Al, K, Ca) - covalent molecular (H2, N2, O2, F2, Cl2, P4- S8 and fullerenes (eg C60))- covalent network (B, C (diamond, graphite), Si) - monatomic (noble gases)
• Learn about these weak intermolecular forces of attraction which exist between all atoms and molecules (London dispersion forces.
• Learn how to explain differences in physical properties such as viscosity, melting point and boiling point in terms of differences in strength of intermolecular forces.

The Chemical Bond
Chemical Bond
Intramolecular(Within)
Intermolecular(between)
Van der Waals’
INTERMOLECULAR FORCES OF ATTRACTION
This animation describes and explains the key intermolecular forces of attraction (Van der Waals forces of attraction) including London dispersion forces, permanent dipole-permanent dipole attractions and Hydrogen Bonding.

Types of bonding in elements
• Metallic• Covalent• Londons forces

The Metals Metallic bonding
Groups I, II, III

Metallic elements
+ + + +
+ + + +
The outer shell in metals is not full and so the outer electrons in metal atoms can move randomly between these partially filled outer shells. The electrons are delocalised (sometimes called a ‘sea’ or ‘cloud’ ofElectrons) i.e. they are held in common by all the atoms.
Metallic bonding is the strong electrostatic force of attraction, between the positive charged ions, formed by the loss of the outer shell electrons of a metal atom and these delocalised electrons.
Positive nucleus (core)
Electron shells
Delocalisedelectron

The positive metal ions are held together by this electron “Glue”.
The outer electrons are delocalised and free to move throughout the lattice.
The greater the number of electrons in the outer shell the stronger the metallic bond.
So the melting point of Al>Mg>Na

Metallic Character The strength of the metallic bond depends on
1) The elements tendency to lose electrons (ionise)2)The packing arrangement of the metal atoms. 3)The size of the atom. 4)The number of valence electrons in the outer most shell.5) The number of shells.

Physical properties of metals
A. Metals are malleable and ductile
Metal atoms can ‘slip’ past each other because the metallicbond is not fixed and it acts in all directions.

Physical properties of metals
B. Conduction of electricity and thermal energy.
Solid and liquid metals conduct heat and electricity.
The delocalised electrons are free to move in the solid lattice. These mobile electrons can act as charge carriers in the conduction of electricity or as energy conductors in the conduction of heat.

Physical properties of metals
In general, metals have high melting and boiling points because of the strength of the metallic bond.
When a metal is molten the metallic bonds are still present.
B.p.’s are much higher as you need to break the metallic bonds throughout the metal lattice.
C. Change of state

Metal boiling point trendsBoiling point of alkali metals
0
200
400
600
800
1000
1200
1400
1600
Lithium Sodium Potassium
Metal
Bo
ilin
g p
oin
t /o
C
Series1
The strength of metallic bonding decreases the forces of attraction get weaker.
The covalent radius decreases as the positive core is increasing in charge, this has the effect of pulling the outer closer to the nucleus the forces of attraction increase.
Down a group
Across a periodBoiling point across a period
0
500
1000
1500
2000
2500
3000
Potassium Calcium Gallium
Metal
Bo
ilin
g p
oin
t /o
C
Series1

The Non-metals Nobel gases
Group 0

Noble gasesNoble gases have full outer electron shells
They do not need to combine with other atoms.
They are said to be monatomic.
However, the monatomic gases do form weak inter-atomicbonds at very low temperatures.
Group 0 are all gases and exist as individual atoms.
He
++

Monatomic elementsSometimes the electrons can end up on oneside of the atom, i.e. the electron cloud can wobble
This means that one side of the atom is morenegative than the other side.
A temporary dipole is therefore formed.
Londons forces
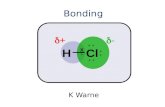
These charges are given the symbol δ ‘delta’
A dipole can induce other atoms to form dipoles, resulting indipole –dipole attraction.
++
++
δ-
δ+
δ-
δ+
δ-
δ+

London dispersion forces are very weak attractive forces
Monatomic elements


Noble gases b.p.’s
b.p / K
0 K = -293o C
B.p.’s increase as the size of the atom increases.
This happens because the London forces increases with increasing number of electrons. The more electrons the bigger the dipole the stronger the London forces.
4 27
87
121
166
0
20
40
60
80
100
120
140
160
180
Helium
NeonArgon
Krypton
Xeon

The Non-metals Covalent molecules

Covalent molecular elements
Most non-metals exist as discrete covalent molecules held together by covalent bonds.
Discrete molecules have a definite formula with a definite number of atoms bonded together

Covalent Bond
A covalent bond is formed when a pair of electrons are shared.
The atoms in a covalent bond is the mutual attraction of two positive nuclei for a shared pair of electrons.
17+
Chlorine atom2,8,7
17+ 17+
Chlorine molecule Cl2
2,8,8
diatomic

Group VII
F F
F F
Strong covalentbond
Weak London forces
Strong intra-molecular bonding and weak inter-molecular bonding exist in this diatomic molecule.F 2 m.p. -220o C
Cl Cl
Cl ClStrong covalentbond
Strong intra-molecular bonding and weak inter-molecular bonding exist in this diatomic molecule.Cl 2 m.p. -101oC
Chlorine Cl2
Fluorine F2
Weak London forces

Halogens b.p.’s
b.p./ K
As the size of the halogen atom increases (more electrons), so does the size of the London forces between the halogen molecule.
0
50
100
150
200
250
300
350
400
450
500
Fluorine
Chlorine
Bromine
Iodine
85
238
332
457

Group VI
Sulphur S8
m.p. 113oC
Higher m.p. because there arestronger Londons’ forcesbetween larger molecules(more electrons).
Weak Londonsforces
O O
O O
Strong double covalent bond Weak Londons force
Strong intra-molecular bonding and weak inter-molecular bonding exist in this diatomic molecule.O 2 m.p. -218o C

Group V
Phosphorus P4
m.p. 44oC
Weak Londonsforces
Strong intra-molecular bonding and weak inter-molecular bonding exist in this diatomic molecule.N 2 m.p. -210o C
Strong covalentbonds
N N
N N
Strong triple covalent bond Weak Londons force

Buckminster fullerene (Bucky Balls) were discovered in the 1980’s.
C60
Due to the large molecules , fullerenes have stronger London forces between their molecules, compared to elements made from smaller molecules.
C70
Group IV
Fullerenes are a family of carbon molecules made up of rings with definite formula.
They are discrete covalent molecules

Covalent Network Elements
In the first 20 elements, only Boron, Carbon and Silicon have covalent network structures.

Diamond forms an infinite 3D network structure.
Each carbon atom forms 4 covalent bonds to 4 other carbon atoms.
Very rigid strong structure.
Diamond is one of the hardest materials known to man.
C sublimes 3642oC
Carbon Diamond

Graphite
London forces between the layers allows layers to slide overeach other.
Carbon bonded to only 3 other Carbons
So the spare electrons are delocalised and so free to move. Graphite is a conductor.
Graphite can be used as a lubricant

Silicon
Silicon has the same infinite 3D network structure as diamond Si mp 1410oC.

Density change across a period
Densityg/cm3
Cl and is a covalent molecular gas at room temperature.
0
0.5
1
1.5
2
2.5
3
SodiumMagnesiumAluminiumSilicon
PhosphorusSulphurChlorineArgon
NaMgAl Si P S Cl ArNa to Al the atom size decreases leading to greater packing in metal lattice.
Si is a covalent network, tightly packed atoms in covalent lattice.P and S are covalent molecular solids with quite densely packed molecules.
Ar and is a monomolecular gas at room temperature.

Bond Strengths
Bond Type Strength (kJ mol –1)
Metallic 80 to 600
Ionic 100 to 500
Covalent 100 to 500
Hydrogen 40
Dipole-Dipole 30
Londons forces 1 to 20

H
Li Be B C N O F
Na Mg Al Si P S Cl
K Ca
Bonding in the first 20 elements
Ne
Ar
He
Monatomic elements
Ne
Ar
He
The noble gases exist as individual (monatomic) atoms. There are only weak Londons forces between the atoms. Very little energy is needed to break these forces and so the noble gases have very low melting and boiling points.
Covalent molecular gases
H
N O F
Cl
These elements occur as diatomic (two atom) molecules with strong covalent bonds between the atoms (intramolecular bonds) and weak Londons forces between the molecules (intermolecular bonds). The weak Londons forces mean low melting and boiling points.
Covalent networks
B
Si
C
Giant network of atoms with strong covalent bonds between the atoms.
Very high melting and boiling points.
Metallic bonding.
Giant network of positively charged nuclei surrounded by delocalised electrons.
Delocalised electrons make these elements good conductors.
Li Be
Na Mg Al
K CaCovalent molecular solidsPolyatomic (many atom) molecules.
Fullerenes C60 C70
P4 and S8. These molecules have many electrons and this produces larger London forces than the diatomic molecules.
The stronger London forces (temporary dipoles) gives these two elements higher melting and boiling points.
These two elements are solids at room temperature.
P S
C

H
He
Ne
Ar
Li Be B N O F
Na Mg Al Si P S Cl
K Ca
C
He
Ne
Ar
Uneven distribution of the electrons in the electron cloud create temporary dipoles ( + and -) which result in a weak attraction between atoms which come close to each other. These weak attractions are called Londons forces.
S
H
N O F
Cl
Strong covalent bonds between the atoms inside the diatomic molecules.
Weak van der Waal’s forces between molecules which come close to each other.
B
Si
C
Diagram shows part of the covalent network of carbon atoms in diamond.
Each carbon atom is covalently bonded to 4 other carbon atoms.
Li Be
Na Mg Al
K Ca
+ + + + + + +
+ + + + + + +
+ + + + + + +
-
- - - - - -
-
-
--------
Metallic bonding with a network of positively charged nuclei surrounded by a ‘sea’ of delocalised electrons.
P S
Diagram shows S8 molecules in sulphur with the londons forces shown by the dotted lines.
These large molecules have stronger Londons forces than the diatomic molecules.
C

Bonding in the first twenty elements This interactive animation provides a visual representation of the bonding and structure of the first twenty elements in the periodic table, taking into account both the intra- and inter-molecular forces involved.

Questions on elements – bonding and structure
1. Explain why the covalent network elements have high melting and boiling points.
2. Explain why the discrete molecular and monatomic elements have low melting and boiling points.
3. Does diamond conduct electricity? Explain.4. Does graphite conduct electricity? Explain.5. How does the hardness of diamond compare
with graphite? Explain.6. Give a use for both diamond and graphite.7. Complete the following table:

Questions on elements – bonding and structure
7. Complete the following table:Type of bonding
and structureProperties
Metallic solids ……………. of electricity
Covalent network solids
……….. …. melting points
……………. of electricity
exception ……………….
Covalent molecular solids
………….. melting points
…………… of electricity
Covalent molecular (diatomic) gases
and monatomic gases
…………… boiling points