Atomic Structure Test Review 166 point total. 1.The atomic number is the number of protons. 2. The...
-
Upload
neal-dennis -
Category
Documents
-
view
217 -
download
0
Transcript of Atomic Structure Test Review 166 point total. 1.The atomic number is the number of protons. 2. The...

Atomic Structure Test Review
166 point total

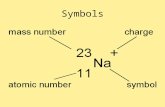
1. The atomic number is the number of protons.
2. The mass number is the number of protons and neutrons.
3. Subatomic particles with an atomic mass of 1 amu are the protons and neutrons.

1. The atomic number is the number of protons.
2. The mass number is the number of protons and neutrons.
3. Subatomic particles with an atomic mass of 1 amu are the protons and neutrons.

1. The atomic number is the number of protons.
2. The mass number is the number of protons and neutrons.
3. Subatomic particles with an atomic mass of 1 amu are the protons and neutrons.

1. The atomic number is the number of protons.
2. The mass number is the number of protons and neutrons.
3. Subatomic particles with an atomic mass of 1 amu are the protons and neutrons.

4. A neutral atom will have equal numbers of protons and electrons.
5. Isotopes have different numbers of neutrons. 6. Most mass in the atom is located in the
nucleus.

4. A neutral atom will have equal numbers of protons and electrons.
5. Isotopes have different numbers of neutrons. 6. Most mass in the atom is located in the
nucleus.

4. A neutral atom will have equal numbers of protons and electrons.
5. Isotopes have different numbers of neutrons. 6. Most mass in the atom is located in the
nucleus.

4. A neutral atom will have equal numbers of protons and electrons.
5. Isotopes have different numbers of neutrons. 6. Most mass in the atom is located in the
nucleus.

7. Subatomic particles with a positive charge are called protons.
8. Subatomic particles with a negative charge are
called electrons. 9. Subatomic particles with a neutral charge are
called neutrons.

7. Subatomic particles with a positive charge are called protons.
8. Subatomic particles with a negative charge are
called electrons. 9. Subatomic particles with a neutral charge are
called neutrons.

7. Subatomic particles with a positive charge are called protons.
8. Subatomic particles with a negative charge are
called electrons. 9. Subatomic particles with a neutral charge are
called neutrons.

7. Subatomic particles with a positive charge are called protons.
8. Subatomic particles with a negative charge are
called electrons. 9. Subatomic particles with a neutral charge are
called neutrons.

10. The subatomic particles found in the nucleus are the protons and the neutrons.
11. The subatomic particles found in energy
levels are the electrons.

10. The subatomic particles found in the nucleus are the protons and the neutrons.
11. The subatomic particles found in energy
levels are the electrons.

10. The subatomic particles found in the nucleus are the protons and the neutrons.
11. The subatomic particles found in energy
levels are the electrons.

12. The energy level closest to the nucleus can contain up to 2 electrons.
13. The second energy level in an atom can hold
up to 8 electrons.

12. The energy level closest to the nucleus can contain up to 2 electrons.
13. The second energy level in an atom can hold
up to 8 electrons.

12. The energy level closest to the nucleus can contain up to 2 electrons.
13. The second energy level in an atom can hold
up to 8 electrons.

Element Atomic Number
Mass Number
Number of
Protons
Number of
Neutrons
Number of
Electrons
Electron Arrangement
14. C 6 12 6 6 6 2-415. Ca 20 40 20 20 20 2-8-8-216. Al 13 27 13 14 13 2-8-317. S 16 32 16 16 16 2-8-618. O 8 16 8 8 8 2-619. Cl 17 35 17 18 17 2-8-7

Element Atomic Number
Mass Number
Number of
Protons
Number of
Neutrons
Number of
Electrons
Electron Arrangement
14. C 6 12 6 6 6 2-415. Ca 20 40 20 20 20 2-8-8-216. Al 13 27 13 14 13 2-8-317. S 16 32 16 16 16 2-8-618. O 8 16 8 8 8 2-619. Cl 17 35 17 18 17 2-8-7

Element Atomic Number
Mass Number
Number of
Protons
Number of
Neutrons
Number of
Electrons
Electron Arrangement
14. C 6 12 6 6 6 2-415. Ca 20 40 20 20 20 2-8-8-216. Al 13 27 13 14 13 2-8-317. S 16 32 16 16 16 2-8-618. O 8 16 8 8 8 2-619. Cl 17 35 17 18 17 2-8-7

Element Atomic Number
Mass Number
Number of
Protons
Number of
Neutrons
Number of
Electrons
Electron Arrangement
14. C 6 12 6 6 6 2-415. Ca 20 40 20 20 20 2-8-8-216. Al 13 27 13 14 13 2-8-317. S 16 32 16 16 16 2-8-618. O 8 16 8 8 8 2-619. Cl 17 35 17 18 17 2-8-7

Element Atomic Number
Mass Number
Number of
Protons
Number of
Neutrons
Number of
Electrons
Electron Arrangement
14. C 6 12 6 6 6 2-415. Ca 20 40 20 20 20 2-8-8-216. Al 13 27 13 14 13 2-8-317. S 16 32 16 16 16 2-8-618. O 8 16 8 8 8 2-619. Cl 17 35 17 18 17 2-8-7

Element Atomic Number
Mass Number
Number of
Protons
Number of
Neutrons
Number of
Electrons
Electron Arrangement
14. C 6 12 6 6 6 2-415. Ca 20 40 20 20 20 2-8-8-216. Al 13 27 13 14 13 2-8-317. S 16 32 16 16 16 2-8-618. O 8 16 8 8 8 2-619. Cl 17 35 17 18 17 2-8-7

Element Atomic Number
Mass Number
Number of
Protons
Number of
Neutrons
Number of
Electrons
Electron Arrangement
14. C 6 12 6 6 6 2-415. Ca 20 40 20 20 20 2-8-8-216. Al 13 27 13 14 13 2-8-317. S 16 32 16 16 16 2-8-618. O 8 16 8 8 8 2-619. Cl 17 35 17 18 17 2-8-7

Element Atomic Number
Mass Number
Number of
Protons
Number of
Neutrons
Number of
Electrons
Electron Arrangement
20. P 15 31 15 16 15 2-8-521. Ne 10 20 10 10 10 2-822. K 19 39 19 20 19 2-8-8-123. N 7 14 7 7 7 2-524. Si 14 28 14 14 14 2-8-425. F 9 19 9 10 9 2-7

Element Atomic Number
Mass Number
Number of
Protons
Number of
Neutrons
Number of
Electrons
Electron Arrangement
20. P 15 31 15 16 15 2-8-521. Ne 10 20 10 10 10 2-822. K 19 39 19 20 19 2-8-8-123. N 7 14 7 7 7 2-524. Si 14 28 14 14 14 2-8-425. F 9 19 9 10 9 2-7

Element Atomic Number
Mass Number
Number of
Protons
Number of
Neutrons
Number of
Electrons
Electron Arrangement
20. P 15 31 15 16 15 2-8-521. Ne 10 20 10 10 10 2-822. K 19 39 19 20 19 2-8-8-123. N 7 14 7 7 7 2-524. Si 14 28 14 14 14 2-8-425. F 9 19 9 10 9 2-7

Element Atomic Number
Mass Number
Number of
Protons
Number of
Neutrons
Number of
Electrons
Electron Arrangement
20. P 15 31 15 16 15 2-8-521. Ne 10 20 10 10 10 2-822. K 19 39 19 20 19 2-8-8-123. N 7 14 7 7 7 2-524. Si 14 28 14 14 14 2-8-425. F 9 19 9 10 9 2-7

Element Atomic Number
Mass Number
Number of
Protons
Number of
Neutrons
Number of
Electrons
Electron Arrangement
20. P 15 31 15 16 15 2-8-521. Ne 10 20 10 10 10 2-822. K 19 39 19 20 19 2-8-8-123. N 7 14 7 7 7 2-524. Si 14 28 14 14 14 2-8-425. F 9 19 9 10 9 2-7

Element Atomic Number
Mass Number
Number of
Protons
Number of
Neutrons
Number of
Electrons
Electron Arrangement
20. P 15 31 15 16 15 2-8-521. Ne 10 20 10 10 10 2-822. K 19 39 19 20 19 2-8-8-123. N 7 14 7 7 7 2-524. Si 14 28 14 14 14 2-8-425. F 9 19 9 10 9 2-7

Element Atomic Number
Mass Number
Number of
Protons
Number of
Neutrons
Number of
Electrons
Electron Arrangement
20. P 15 31 15 16 15 2-8-521. Ne 10 20 10 10 10 2-822. K 19 39 19 20 19 2-8-8-123. N 7 14 7 7 7 2-524. Si 14 28 14 14 14 2-8-425. F 9 19 9 10 9 2-7

Element Atomic Number
Mass Number
Number of
Protons
Number of
Neutrons
Number of
Electrons
Electron Arrangement
26. Ar 18 40 18 22 18 2-8-827. B 5 11 5 6 5 2-328. Mg 12 24 12 12 12 2-8-229. Na 11 23 11 12 11 2-8-130. Be 4 9 4 5 4 2-2

Element Atomic Number
Mass Number
Number of
Protons
Number of
Neutrons
Number of
Electrons
Electron Arrangement
26. Ar 18 40 18 22 18 2-8-827. B 5 11 5 6 5 2-328. Mg 12 24 12 12 12 2-8-229. Na 11 23 11 12 11 2-8-130. Be 4 9 4 5 4 2-2

Element Atomic Number
Mass Number
Number of
Protons
Number of
Neutrons
Number of
Electrons
Electron Arrangement
26. Ar 18 40 18 22 18 2-8-827. B 5 11 5 6 5 2-328. Mg 12 24 12 12 12 2-8-229. Na 11 23 11 12 11 2-8-130. Be 4 9 4 5 4 2-2

Element Atomic Number
Mass Number
Number of
Protons
Number of
Neutrons
Number of
Electrons
Electron Arrangement
26. Ar 18 40 18 22 18 2-8-827. B 5 11 5 6 5 2-328. Mg 12 24 12 12 12 2-8-229. Na 11 23 11 12 11 2-8-130. Be 4 9 4 5 4 2-2

Element Atomic Number
Mass Number
Number of
Protons
Number of
Neutrons
Number of
Electrons
Electron Arrangement
26. Ar 18 40 18 22 18 2-8-827. B 5 11 5 6 5 2-328. Mg 12 24 12 12 12 2-8-229. Na 11 23 11 12 11 2-8-130. Be 4 9 4 5 4 2-2

Element Atomic Number
Mass Number
Number of
Protons
Number of
Neutrons
Number of
Electrons
Electron Arrangement
26. Ar 18 40 18 22 18 2-8-827. B 5 11 5 6 5 2-328. Mg 12 24 12 12 12 2-8-229. Na 11 23 11 12 11 2-8-130. Be 4 9 4 5 4 2-2