Atomic Structure. All waves have a characteristic wavelength,, and amplitude, A. Frequency,, of a...
-
Upload
tabitha-gilbert -
Category
Documents
-
view
221 -
download
0
Transcript of Atomic Structure. All waves have a characteristic wavelength,, and amplitude, A. Frequency,, of a...

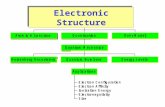
Atom ic H Spectrum
Heisenberg Uncerta inty
Electron ConfigurationElectron AffinityIonization EnergyElectronegativityS ize
Applications
Quantum Num bers Energy Levels
Quantum M echanics
Quantization Bohr M odel
W ave/Partic le Concept
Atomic Structure

• All waves have a characteristic wavelength, , and amplitude, A.
• Frequency, , of a wave is the number of cycles which pass a point in one second.
• Speed of a wave, c, is given by its frequency multiplied by its wavelength:
• For light, speed = c = 3.00x108 m s-1 . • A Brief History of Time
The Wave Nature of LightThe Wave Nature of Light
c

The Wave Nature of LightThe Wave Nature of Light

The Wave Nature of LightThe Wave Nature of Light

The Wave Nature of LightThe Wave Nature of Light

• Planck: energy can only be absorbed or released from atoms in certain amounts called quanta.
• The relationship between energy and frequency is
where h is Planck’s constant ( 6.626 10-34 J s ) .
Quantized Energy and PhotonsQuantized Energy and Photons
hE

The Photoelectric Effect and Photons• Einstein assumed that light traveled in energy packets
called photons.• The energy of one photon is:
Quantized Energy and PhotonsQuantized Energy and Photons
hE

Nature of Waves: Quantized Energy and PhotonsNature of Waves: Quantized Energy and Photons

Line Spectra• Radiation composed of only one wavelength is called
monochromatic.• Radiation that spans a whole array of different
wavelengths is called continuous.• White light can be separated into a continuous spectrum
of colors.• Note that there are no dark spots on the continuous
spectrum that would correspond to different lines.
Line Spectra and the Bohr ModelLine Spectra and the Bohr Model


Bohr Model• Colors from excited gases arise because electrons move between energy states
in the atom. (Electronic Transition)
Line Spectra and the Bohr ModelLine Spectra and the Bohr Model

Bohr Model• Since the energy states are quantized, the light emitted
from excited atoms must be quantized and appear as line spectra.
• After lots of math, Bohr showed that
where n is the principal quantum number (i.e., n = 1, 2, 3, … and nothing else).
Line Spectra and the Bohr ModelLine Spectra and the Bohr Model
218 1J 10178.2
nEn

Bohr Model
• We can show that
• When ni > nf, energy is emitted.
• When nf > ni, energy is absorbed
Line Spectra and the Bohr ModelLine Spectra and the Bohr Model
2218 11J 10178.2
iffi nn
hchE
hEEE if
218 1J 10178.2
nEn

Bohr Model
Line Spectra and Line Spectra and the Bohr Modelthe Bohr Model
CyberChem (Fireworks) video
Mathcad (Balmer Series)

Line Spectra and the Bohr Model: Balmer Series CalculationsLine Spectra and the Bohr Model: Balmer Series Calculations

4to2 486.8nm4to26.62610 34
J s 3.00108 m s 1
E4to2
h cE
E4to2 4.084 10 19 JE4to2 2.178 10 18 J1
22
1
42
nf 2ni 4Blue Line:
E3to2 3.025 10 19 JNote that:
3to2 657.1nm3to26.62610 34 J s 3.00108 m s 1
E3to2
h c
E
E3to2 3.025 10 19 JE3to2 2.178 10 18 J1
22
1
32
Ei.to.f 2.178 10 18 J1
nf2
1
ni2
nf 2ni 3Red Line:
nm 10 9 mc 3.00108 m s 1h 6.62610 34 J s
Balmer Series: Electronic transitions from higher E-levels (ni>2) down to the second E-level (nf=2).
Calculation of the first two lines in the Balmer Series for the H-atom

Limitations of the Bohr Model• Can only explain the line spectrum of hydrogen
adequately.• Can only work for (at least) one electron atoms.• Cannot explain multi-lines with each color.
• Electrons are not completely described as small particles.• Electrons can have both wave and particle properties.
Line Spectra and the Bohr ModelLine Spectra and the Bohr Model

• Knowing that light has a particle nature, it seems reasonable to ask if matter has a wave nature.
• Using Einstein’s and Planck’s equations, de Broglie showed:
• The momentum, mv, is a particle property, whereas is a wave property.
• de Broglie summarized the concepts of waves and particles, with noticeable effects if the objects are small.
The Wave Behavior of MatterThe Wave Behavior of Matter
mvh

The Uncertainty Principle• Heisenberg’s Uncertainty Principle: on the mass scale
of atomic particles, we cannot determine exactly the position, direction of motion, and speed simultaneously.
• For electrons: we cannot determine their momentum and position simultaneously.
• If x is the uncertainty in position and mv is the uncertainty in momentum, then
The Wave Behavior of MatterThe Wave Behavior of Matter
4· hmvx

Energy and Matter
Size of Matter Particle Property Wave Property
Large – macroscopic Mainly Unobservable
Intermediate – electron Some Some
Small – photon Few Mainly
E = m c2

• Schrödinger proposed an equation that contains both wave and particle terms.
• Solving the equation leads to wave functions. • The wave function gives the shape of the electronic
orbital. [“Shape” really refers to density of electronic charges.]
• The square of the wave function, gives the probability of finding the electron ( electron density ).
Quantum Mechanics and Atomic OrbitalsQuantum Mechanics and Atomic Orbitals
EH^

Quantum Mechanics and Atomic OrbitalsQuantum Mechanics and Atomic Orbitals
Solving Schrodinger’s Equation gives rise to ‘Orbitals.’
These orbitals provide the electron density distributed about the nucleus.
Orbitals are described by quantum numbers.

Orbitals and Quantum Numbers• Schrödinger’s equation requires 3 quantum numbers:
1. Principal Quantum Number, n. This is the same as Bohr’s n. As n becomes larger, the atom becomes larger and the electron is further from the nucleus. ( n = 1 , 2 , 3 , 4 , …. )
2. Azimuthal Quantum Number, . This quantum number depends on the value of n. The values of begin at 0 and increase to (n - 1). We usually use letters for (s, p, d and f for = 0, 1, 2, and 3). Usually we refer to the s, p, d and f-orbitals.
3. Magnetic Quantum Number, m. This quantum number depends on . The magnetic quantum number has integral values between - and + . Magnetic quantum numbers give the 3D orientation of each orbital.
Quantum Mechanics and Atomic OrbitalsQuantum Mechanics and Atomic Orbitals

Quantum Numbers of Wavefuntions
Quantum # Symbol Values Description
Principle n 1,2,3,4,… Size & Energy of orbital
Azimuthal 0,1,2,…(n-1) for each n
Shape of orbital
Magnetic m…,0,…+ for each
Relative orientation of orbitals within same
Spin ms +1/2 or –1/2 Spin up or Spin down
Azimuthal Quantum # Name of Orbital
0 s (sharp)
1 p (principal)
2 d (diffuse) 3 f (fundamental) 4 g

Quantum Mechanics and Atomic OrbitalsQuantum Mechanics and Atomic Orbitals
n ℓ Orbital Name mℓ (“sub-orbitals) Comment

Orbitals and Quantum Numbers
Quantum Mechanics and Atomic OrbitalsQuantum Mechanics and Atomic Orbitals

The s-Orbitals
Representations of OrbitalsRepresentations of Orbitals

The p-Orbitals
Representations of OrbitalsRepresentations of Orbitals

d-orbitals

Many-Electron Atoms Many-Electron Atoms
Orbitals and Their Energies
Orbitals CD

Electron Spin and the Pauli Exclusion Principle
Many-Electron Atoms Many-Electron Atoms

Electron Spin and the Pauli Exclusion Principle
• Since electron spin is quantized, we define ms = spin quantum number = ½.
• Pauli’s Exclusions Principle:: no two electrons can have the same set of 4 quantum numbers.• Therefore, two electrons in the same orbital must have
opposite spins.
Many-Electron Atoms Many-Electron Atoms

Figure 6.27
Figure 6.27 Orbitals CD

Figure 6.28 Orbitals CD

Many-Electron Atoms Many-Electron Atoms
Orbitals and Their Energies
Orbitals CD

Electron ConfigurationsSpecies Electron Configuration Box Orbital Comment

Metals, Nonmetals, and MetalloidsMetals, Nonmetals, and Metalloids
Metals
Figure 7.14

Two Major Factors:•principal quantum number, n, and
•the effective nuclear charge, Zeff.
Periodic Trends

Figure 7.5: Radius video Clip

Figure 7.6

Figure 7.10 IE clip

Figure 7.9

Electron AffinitiesElectron Affinities
• Electron affinity is the opposite of ionization energy.• Electron affinity: the energy change when a gaseous atom
gains an electron to form a gaseous ion:Cl(g) + e- Cl-(g)
• Electron affinity can either be exothermic (as the above example) or endothermic:
Ar(g) + e- Ar-(g)

Figure 7.11: Electron AffinitiesFigure 7.11: Electron Affinities

Group Trends for the Active MetalsGroup Trends for the Active Metals
Group 1A: The Alkali Metals

Group Trends for the Active MetalsGroup Trends for the Active Metals
Group 2A: The Alkaline Earth Metals

Group Trends for Selected NonmetalsGroup Trends for Selected Nonmetals
Group 6A: The Oxygen Group

Group Trends for Selected NonmetalsGroup Trends for Selected Nonmetals
Group 7A: The Halogens

Group Trends for the Active MetalsGroup Trends for the Active Metals
Group 1A: The Alkali Metals• Alkali metals are all soft.• Chemistry dominated by the loss of their single s
electron:M M+ + e-
• Reactivity increases as we move down the group.• Alkali metals react with water to form MOH and
hydrogen gas:2M(s) + 2H2O(l) 2MOH(aq) + H2(g)

Group Trends for the Active MetalsGroup Trends for the Active Metals
Group 2A: The Alkaline Earth Metals• Alkaline earth metals are harder and more dense than the
alkali metals.• The chemistry is dominated by the loss of two s
electrons:M M2+ + 2e-.
Mg(s) + Cl2(g) MgCl2(s)2Mg(s) + O2(g) 2MgO(s)
• Be does not react with water. Mg will only react with steam. Ca onwards:
Ca(s) + 2H2O(l) Ca(OH)2(aq) + H2(g)

Atom ic H Spectrum
Heisenberg Uncerta inty
Electron ConfigurationElectron AffinityIonization EnergyElectronegativityS ize
Applications
Quantum Num bers Energy Levels
Quantum M echanics
Quantization Bohr M odel
W ave/Partic le Concept
Atomic Structure
c
)( photonperchE
2218 1110178.2
iffi nn
JE],,,[ smmn
![General Certi 2016 Double Award Science: Physics Higher ... · 10042.03 MV18 3 [Turn over (i) State the amplitude of the waves. [1 mark] Amplitude = mm (ii) State the wavelength of](https://static.fdocuments.in/doc/165x107/5f028cea7e708231d404d254/general-certi-2016-double-award-science-physics-higher-1004203-mv18-3-turn.jpg)

















