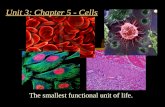
Cell – the basic unit of life. Cells Smallest living unit Most are microscopic.
Atom = smallest unit of an element Molecule = smallest unit of compound
4
• Atom = smallest unit of an element • Molecule = smallest unit of compound
-
Upload
ahmed-benjamin -
Category
Documents
-
view
21 -
download
2
description
Atom = smallest unit of an element Molecule = smallest unit of compound. Valence electrons. The electrons in one atom that are able to form bonds with another atom. The closer the atom is to having 8 valence electrons , the more stable it is. Where are the valence electrons?. Co valent Bond. - PowerPoint PPT Presentation
Transcript of Atom = smallest unit of an element Molecule = smallest unit of compound

• Atom = smallest unit of an element• Molecule = smallest unit of compound

Valence electrons• The electrons in one atom that are able to form bonds with
another atom. • The closer the atom is to having 8 valence electrons , the more
stable it is.
Where are the valence electrons?

Covalent Bond• Electrons are shared between atoms. • 1 bond is when atoms share 2 electrons• Nonmetal with nonmetal• Neither atom is strong enough to pull an
electron away from the other so they share.

Bonds
• Two atoms can form single, double or triple bonds between them.
• Two atoms can form no more than 3 bonds between them.