APOC_Progress Report
-
Upload
lisa-schwarb -
Category
Documents
-
view
216 -
download
2
description
Transcript of APOC_Progress Report
AFRICAN PROGRAMME FOR ONCHOCERCIASIS CONTROL
Progress report 2011 THE YEAR IN REVIEW
www.who.int/apoc
JAF17.5
© Copyright African Programme for Onchocerciasis Control (WHO/APOC), 2011. All rights reserved.
Publications of the WHO/APOC enjoy copyright protection in accordance with the Universal copyright Convention. Any use of information in the WHO/APOC Progress Report should be accompanied by acknowledgement of WHO/APOC as the source.
For rights of reproduction or translation in part or in total, application should be made to: Office of the APOC Director, WHO/APOC, BP 549 Ouagadougou, Burkina Faso, [email protected].
WHO/APOC welcomes such applications.
The WHO African Programme for Onchocerciasis Control
Progress Report 2011
Joint Action Forum (JAF)Office of the Chairman
SEVENTEENTH SESSIONKuwait, 12-14 December, 2011
AFRICAN PROGRAMME FOR ONCHOCERCIASIS CONTROL
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
4
ww
w.w
ho.in
t/ap
oc
List of tables, figures and annexes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6
List of Abbreviations/acronyms . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
Executive summary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 9
THE YEAR IN REVIEW . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
1 Community-Directed Treatment with Ivermectin (CDTI) and Health impact . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 131.1 DiseaseMapping. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 13
1.1.1 Mapping of Onchocerciasis and Loa loa . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 131.1.2 Integrated mapping of NTDs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 15
1.2 GeographicandTherapeuticCoverage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 161.2.1 Status of Geographic coverage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 161.2.2 Status of therapeutic coverage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 17
1.3 Ivermectin(Mectizan®)tabletssuppliedbytheMectizanDonationProgramme(MDP) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 19
1.4Monitoring,Evaluationandsurveillance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 191.4.1 Independent Participatory Monitoring of CDTI Projects . . . . . . . . . . . . . . 191.4.2 Community Self Monitoring (CSM) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 201.4.3 Evaluation of Sustainability of CDTI Projects . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 221.4.4 Surveillance activities . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 221.4.5 Health Impact of CDTI . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 25
2 Moving from Control to Elimination of Onchocerciasis where feasible . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 26
3 Co-implemetation of CDTI with other health interventions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27
Table of contents
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
5
ww
w.w
ho.in
t/ap
oc
4 Strengthening Health Systems . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28
4.1HumanresourcedevelopmentforthecontrolofOnchocerciasisandotherHealthinterventions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 284.1.1 Training of health staff by NOTFs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 284.1.2 Training of health staff by APOC Management . . . . . . . . . . . . . . . . . . . . . . . . . . . . 284.1.3 Building capacity of countries in communication . . . . . . . . . . . . . . . . . . . . . . . . 294.1.4 Gender Mainstreaming . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 29
4.2Logisticssupporttocountries . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 31
5 Partnerships and Government Contributions . . . . . . . . . . . . . . . . . . . . . . . . . . . . 325.1GovernmentfinancialcontributionstoCDTIactivities . . . . . . . . . . . . . . . . . . 325.2Directfinancialsupporttocountriesandmanagement
oftheAPOCTrustFund . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 32
5.3Governance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 355.3.1 The Joint Action Forum . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 355.3.2 Committee of Sponsoring Agencies . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 365.3.3 Technical Consultative Committee and decentralisation
of functions to countrie . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 36
5.4DevelopmentandimplementationofaCDIcurriculumandtrainingmodule . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 37
5.5TechnicalSupporttoCountries . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 38
5.6Partnershipsandcollaborationv385.6.1 CDTI Projects and NGDO support for Onchocerciasis Control . . . . . 385.6.2 Collaboration with other programmes and institutions . . . . . . . . . . . . . 39
6 Macrofil and other research . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 41
7 Transition to National Ownership and Sustainability . . . . . . . . . . 42
Annexes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 43
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
6
ww
w.w
ho.in
t/ap
oc
List of tables, figures and annexes
TablesTable 1: Results of REMO refinement in 512 villages in Ethiopia, 2011
Table 2: Summary of ivermectin treatment in APOC countries in 2010
Table 3: Summary of independent monitoring results in Bengo, Angola
Table 4: Conclusions of epidemiological evaluation results in 23 sites
Table 5: Number of health workers and CDDs trained/retrained in 2010 by NOTFs
Table 6: Capacity building and number of persons trained
Table 7: Number of female CDDs in 12 APOC countries, 2009-2010
Table 8: Logistics and equipment provided by APOC to countries during the reporting period
Table 9: Government funds disbursed for CDTI activities in 2010
Table 10: Number of projects benefiting from APOC Trust Fund in 2010–2011
Table 11: Number of financial returns submitted and analyzed 2010–2011
Table 12: Achievements and challenges of development and implementation of CDI curriculum and training module
Figures Figure 1: Map of the estimated prevalence of eye worm history in Africa
Figure 2: Prevalence of Lymphatic filariasis (ICT) overlapped with estimated prevalence of eye worm history in the southern part of Chad
Figure 3: Trend of geographical coverage (%) in 16 APOC countries between 2006 and 2010
Figure 4: Trend of therapeutic coverage (%) in 16 APOC countries between 2006 and 2010
Figure 5: Reported number of persons treated in APOC countries between 1997 and 2010
Figure 6: Results of sustainability evaluation in 9 CDTI projects and one Onchocerciasis task force Secretariat
Figure 7: Results of Onchocerciasis surveillance in ex-OCP countries and Nigeria in 2010
Figure 8: Delineation of possible transmission zones in Malawi
Figure 9: Sites and results of epidemiological evaluations between 2009 and 2011
Figure 10: Major health interventions co-implemented with ivermectin distribution using CDI in 10 countries in 2010
Figure 11: Proportion of the budget used for administration and logistic cost compared to technical/operational costs, 2010-2011
Figure 12: Allocation of APOC Trust Fund to major activities in 2010 and 2011
AnnexesAnnex 1: Number of Mectizan tablets provided and shipped to APOC member country
programmes from 1997 to 2010 and ex-OCP countries (2010)
Annex 2: Governments’ contributions for CDTI activities, 2010
Annex 3: CDTI Project supported by NGDOs in APOC countries (September 2011)
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
7
ww
w.w
ho.in
t/ap
oc
AfDB African Development Bank
AFRO WHO Regional Office for Africa
APOC African Programme for Onchocerciasis Control
BELACD Bureau d’études, de liaison des actions caritatives et de développement.
CAR Central African Republic
CBM Christoffel-Blindenmission (German NGO)
CCHP Comprehensive Council Health Plan
CDD Community-Directed Distributor
CDI Community-Directed Intervention
CDTI Community-Directed Treatment with Ivermectin
CHAL Christian Health Association of Liberia
CIDA Canadian International Development Agency
CRS Catholic Relief Services
CSA Committee of Sponsoring Agencies
DALYs Disability Adjusted Life Years
DFC Direct Financial Cooperation
DOTs Directly Observed Treatment Short-course
DRC Democratic Republic of Congo
FLHF Front Line Health Facilities
GIS Geographical Information System
GSM Global Management System
HIA Health Impact Assessment
HKI Helen Keller International
HSAM Health Education /Sensitization /Advocacy /Mobilisation
IEC Information, Education, Communication
IEF International Eye Foundation
IFESH International Foundation for Education and Self-Help
IMA Inter-Church Medical Assistance
IPM Independent Participatory Monitoring
IRC International Rescue Committee
ICT Immunochromatographic Card Test (for filariasis)
JAF Joint Action Forum (APOC governing body)
LA Letter of Agreement
LGA Local Government Area
MDGs Millennium Development Goals
MDP Mectizan® Donation Program
MDSC Multi-Disease Surveillance Centre
MITOSATH Mission To Save The Helpless
NGDO Non-Governmental Development Organisation
NGO Non-Governmental Organisation
NOCP National Onchocerciasis Control Programme
NOTF National Onchocerciasis Task Force
NTD Neglected Tropical Disease
OCP Onchocerciasis Control Programme in West Africa
OPC Organisation pour la Prévention de la Cécité
OTD Other Tropical Diseases
RAPLOA Rapid Assessment Procedure for Loa loa
REMO Rapid Epidemiological Mapping of Onchocerciasis
SAE Severe Adverse Event
SSOTF Southern Sudan Onchocerciasis Task Force
SS SightSavers
TCC Technical Consultative Committee (APOC scientific advisory group)
TDR WHO-based Special Programme for Research and Training in Tropical Diseases
TSA Technical Service Agreement
UFAR United Front Against River-blindness
UNICEF United Nations Children’s Fund
USAID United State Agency for International Development
WAHO West African Health Organisation
WHO World Health Organisation
Abbreviations
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
8
ww
w.w
ho.in
t/ap
oc
Executive summary
Following the external mid-term evaluation of APOC in 2010, JAF 16 decided in Abuja that APOC management should assess and cost possible scenarios for APOC after 2015, when the Programme is due to close. CSA established three sub-groups to develop plans for these scenarios. JAF17’s deliberations on the recommendations from the CSA will be of great significance in determining APOC’s future direction.
In the current reporting period (1 September 2010–31 August 2011), APOC management has focussed on intensification of CDTI implementation with special emphasis on post conflict countries and on assessment of progress towards onchocerciasis elimination. Particular attention was given also to development of training materials and introduction of CDI in curriculum of faculties of medicine, health sciences and schools of nursing. Significant resources and efforts were devoted to the work of CSA advisory groups, research and integrated mapping of NTDs.
CDTI implementationIn 2010, over 75.9 million people where treated in 138 448 communities out of 144 837 in 16 countries. Compared to year 2009 (68 419 817 people treated), this represents an increase of 11%. Countries and APOC management have identified where ivermectin treatment needs to expand to reach the Programme’s annual ultimate treatment objective of 90 million people by 2015. This target will be reached with new CDTI areas identified in Ethiopia, Democratic Republic of Congo (DRC) and Uganda as well as the expansion of geographical coverage in Angola, DRC, and South Sudan. Despite poor health infrastructures, shortage of health professionals, instability and co-endemicity of Onchocerciasis and Loiasis, post conflict countries reached an average therapeutic coverage of 71%. Stable countries continue to sustain high therapeutic coverage with an average of 79% in 2010.
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
9
ww
w.w
ho.in
t/ap
oc
Evaluation of sustainability and independent participatory monitoringNine CDTI Projects were evaluated for sustainability in 2011, in DRC, Liberia and South Sudan. Two of the three projects evaluated in Liberia and one out of three in DRC were judged to be making progress towards sustainability. All the three projects evaluated in South Sudan were not showing any progress. This has led APOC management to initiate remedial measures.
One Project, Bengo, in Angola, underwent Independent Participatory monitoring during the reporting period. This Project was found to be performing satisfactorily.
Disease mappingRapid epidemiological mapping of Onchocerciasis refinement was conducted in 580 communities in Angola, DRC and Ethiopia.
APOC undertook to complete Loa loa mapping in areas outside the Onchocerciasis endemic zones not yet covered by RAPLOA surveys. Loa loa mapping was completed in 11 countries and the data has been published. In 2011, APOC provided technical and financial support to Angola, Cameroon, Chad, DRC, and Republic of Congo for the mapping of Lymphatic filariasis, Schistosomiasis and Soil transmitted helminths.
Moving from control to elimination where feasible By August 2011, epidemiological evaluations were undertaken in 23 sites with 43 528 persons examined in 275 villages. In comparison with ONCHOSIM predictions, the results were encouraging in most of the evaluated areas: out of the 23 sites evaluated, 20 showed satisfactory progress towards elimination and nine of these have probably achieved elimination already whilst five are close to elimination. Six of them are on track but still need to treat for the coming years to reach the breakpoint. In three sites the results were unsatisfactory due to inadequate treatment coverage.
Strengthening health systems During the period under review, 535,156 Community-directed distributors (CDDs) and 53 135 health workers from all levels were trained/retrained by CDTI projects. CDI training workshops were held for 407 participants from Burundi,
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
10
ww
w.w
ho.in
t/ap
oc
DRC, Ethiopia, Guinea Bissau, Liberia and Tanzania. Training on epidemiological and entomological evaluations was also provided to 72 nationals from eigth countries. Fourteen health professionals from 10 countries, who were enrolled with financial support from APOC in Master of Science programs in Entomology, Parasitology and Public Health graduated in 2011.
Logistics and equipment were provided to 14 APOC and six ex-OCP countries, including 29 vehicles, 2338 bicycles, 140 motorcycles. Details of other equipment provided to countries are described in this report.
Strengthening of APOC management to support countriesAPOC continued its efforts to strengthen its management capacity as well as technical support to post-conflict countries during the period under review. In this regard, fixed term positions of Health Systems Specialist, Research, Administrative and Finance Officers have been filled. In addition, a short term Technical Advisor has been appointed in Guinea Bissau to assist the country in achieving elimination of the disease within a two to three year period. Furthermore, in partnership with CBM, APOC co-financed Technical Advisor positions in two countries (Angola and DRC) where CDTI activities need to be reinforced to reach the recommended therapeutic and geographic coverage threshold. APOC financially supported two NPO/NTD positions in Angola and Burundi within the WHO country offices’ work plans.
Co-implementation of multiple health interventions Expanded co-implementation of NTDs in the Democratic Republic of Congo and Tanzania, supported by funds from USAID and the APOC Trust Fund continued during the reporting period. The other countries involved in co-implementation activities in 2010 are Burundi, Cameroon, CAR, Ethiopia, Liberia, Malawi, Nigeria, and Uganda.
During the reporting period, a total of 48 million treatments/health interventions were provided alongside ivermectin distribution.
Financial support from the APOC trust fundDirect financial support was provided to 15 APOC countries and limited support to ten ex-OCP countries. In 2010/2011, a total amount of 47.59 million represents the programme budget implemented as of 31st October 2011. For the given period, 86% of this amount was allocated to technical/operational activities and 14% to administrative/management cost.
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
11
ww
w.w
ho.in
t/ap
oc
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
12
ww
w.w
ho.in
t/ap
oc
1.1 Disease mapping1.1.1 Mapping of Onchocerciasis and Loa loa
Mapping of OnchocerciasisDuring the period under review, APOC supported National Onchocerciasis Task Forces (NOTFs) in conducting refinement of REMO in Angola, Democratic Republic of Congo (DRC) and Ethiopia. An integrated mapping of Onchocerciasis and Loiasis was conducted in DRC. It covered 16 villages in Ituri district in Province Orientale. The results showed a median prevalence of Onchocerciasis nodule carriers of 41.7%. Based on these results the country submitted a project proposal to extend CDTI from the present northern area of
Ituri district to cover the entire district. In Angola, 52 villages were surveyed in the provinces of Bie, Huambo, Kuando Kubango and Moxico and the refinement results confirmed the data available.
In Ethiopia, REMO refinement was conducted in 512 villages in eight zones (North Gondar, Assosa, Kamashi, East Wollega, Illubabor, Jimma, West Shewa and West Wollega). The results for the zones surveyed for REMO are presented in Table 1. Based on these results, 34 woredas have been identified for CDTI.
In addition to allowing NOTFs identify new areas targeted for CDTI, the results of these REMO refinement exercises
Table 1: Results of REMO refinement in 512 villages in Ethiopia, 2011
Onchocercal nodule prevalence (%)
Region Zone Median Minimum Maximum N*
Amhara North Gondar 6.4 0 45.8 17
Benishangul-Gumuz Mao Komo Special Woreda
5.7 0 21.2 7
Assosa 4.7 0 34.3 14
Kamashi 16.7 3 26.7 9
Oromiya East Wollega 30.0 0 81.5 74
Illubabor 30.3 0 50.0 95
Jimma 20.0 0 58.5 89
West Shewa 5.0 0 35.0 32
West Wollega 13.9 0 74.3 175
Overall 14.7 0 81.5 512
N* = number of CDTI projects
THE YEAR IN REVIEW 1. Community-Directed Treatment with Ivermectin (CDTI) and health impact
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
13
ww
w.w
ho.in
t/ap
oc
in the three countries have provided APOC with additional data to better delineate the transmission pattern of Onchocerciasis. Mapping of Loiasis
Mapping of LoiasisWith the increasing expansion of NTD programmes that included the distribution of ivermectin for the elimination of Lymphatic filariasis, there was an urgent need for country programmes and partners to have better knowledge of the distribution of Loiasis throughout the African region, including areas that were not targeted for Onchocerciasis control. APOC undertook to complete Loa loa mapping in areas outside the Onchocerciasis endemic zones not yet covered by RAPLOA surveys. From October 2010 to
February 2011, RAPLOA surveys were conducted in 849 villages in Angola, Central African Republic (CAR), Chad, Congo, DRC and Gabon.
In February 2011, APOC carried out an in-depth analysis of all RAPLOA data available for 11 sub-Saharan African countries. The analysis was done in collaboration with the University of Lancaster, United Kingdom. A geo-statistical analysis method called kriging applied to the data collected in 4798 sampled villages generated a contour map of Loiasis prevalence, providing the first global map of Loiasis based on actual survey data (Figure 1). This map shows prevalence of Loiasis in 10 countries. It is an estimated that 14.4 million people live in high risk areas.
Figure 1: Map of the estimated prevalence of eye worm history in AfricaSource: Zouré et al. PLoS Negl Trop Dis 5(6): e1210. doi:10.1371/journal.pntd.0001210
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
14
ww
w.w
ho.in
t/ap
oc
Figure 2: Prevalence of Lymphatic filariasis overlapped with estimated prevalence of eye worm history in the southern part of Chad
Following this analysis a paper entitled “The geographic distribution of Loa loa in Africa: results of large-scale implementation of the Rapid Assessment Procedure for Loiasis (RAPLOA)” has been published in PLoS NTDs (PLoSNeglTropDis5(6):e1210.doi:10.1371/journal.pntd.0001210).
1.1.2 Integrated mapping of NTDs
In 2011, APOC provided technical and financial support for the mapping of other NTDs to Chad (Lymphatic filariasis), Angola (Lymphatic filariasis), Republic of Congo (Schistosomiasis and soil-transmitted helminths), DRC (Lymphatic filariasis, Schistosomiasis and soil-transmitted helminths) and Cameroon (Schistosomiasis and soil-
transmitted helminths). The data of Angola and Congo is being analysed. The surveys are on-going in DRC and are at a planning stage in Cameroon.
In Chad, the surveys were conducted in an integrated manner for Loiasis using the RAPLOA method and for Lymphatic filariasis using ICT cards in 42 villages in 25 districts endemic for Onchocerciasis. Figure 2, shows Lymphatic filariasis prevalence overlapped with the estimated prevalence of history of eye worm. It appears that in the districts of Moundou, Beinamar, Bebebdja, Benoye, Goré and Baiboloum, which are endemic for Lymphatic filariasis, the prevalence of history of eye worm is also high (>= 40%).
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
15
ww
w.w
ho.in
t/ap
oc
1.2 Geographic and Therapeutic CoverageIn 2010, ivermectin mass distribution was implemented by 107 CDTI projects in 16 countries. Thirty-four projects are in post conflict countries (Burundi, Central African Republic, Chad, Democratic Republic of Congo, Liberia, South Sudan and Sudan). The remaining 73 projects are in stable countries. Overall, 75,947,196 people were treated in 138,448 communities (Table 2).
1.2.1 Status of Geographic coverage
In 2010, 138,448 communities out of 144,837 were treated. Forty percent
(55,359) of the communities treated were in post conflict countries and the remaining 83,089 communities in stable countries. The average geographical coverage was 96%.
Post-conflict countriesA total of 55,359 communities out of 59,756 were treated in seven post conflict countries with an average geographical coverage of 93%. The geographic coverage ranged from 86% (South Sudan) to 100% (Burundi, Chad and Sudan). Thus, the geographical coverage has significantly improved compared to 2009, increasing from 83% to an average of 93%.
Post-conflict countries
Burundi 3 368 368 100.0 1,456,186 1,146,033 78.7
conflict 1 5,572 4,835 86.8 1,543,972 1,264,508 81.9
Chad 1 3,250 3,250 100.0 1,905,121 1,542,377 81.0
DRC 20 40,407 37,669 93.2 27,902,550 20,290,244 72.7
Liberia 3 3,413 3,387 99.2 2,475,647 2,003,343 80.9
South Sudan 1 6,511 5,615 86.2 5,707,127 2,981,506 52.2
Sudan 5 235 235 100.0 391,810 329,702 84.1
Total 34 59,756 55,359 92.6 41,382,413 29,557,713 71.4
Stable countries
Angola 6 2,486 1,997 80.3 1,046,563 711,276 68.0
Cameroon 15 10,296 10,132 98.4 6,722,905 5,300,025 78.8
Congo 2 770 770 100.0 803,026 651,922 81.2
Eq. Guinea 1 129 129 100.0 81,318 57,735 71.0
Ethiopia 9 22,484 22,484 100.0 5,967,179 4,809,869 80.6
Malawi 2 2,186 2,186 100.0 2,017,712 1,666,048 82.6
Nigeria 27 36,117 35,773 99.0 36,396,877 29,116,332 80.0
Tanzania 7 5,825 5,825 100.0 2,373,351 1,901,542 80.1
Uganda 4 4,788 3,793 79.2 3,135,530 2,174,734 69.4
Total 73 85,081 83,089 97.7 58,544,461 46,389,483 79.2
Grand Total 107 144,837 138,448 95.6 99,926,874 75,947,196 76.0
Com
mun
itie
sTr
eate
d
Com
mun
itie
sTo
tal
Tota
l CD
TIpr
ojec
t
Geo
grap
hic
cove
rage
%
Popu
lati
on
Trea
ted
Popu
lati
on
Tota
l
Ther
apeu
tic
cove
rage
%Country
Table 2: Summary of ivermectin treatment in APOC countries in 2010
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
16
ww
w.w
ho.in
t/ap
oc
Figure 3 reveals that Burundi and Chad sustained full geographic coverage during five consecutive years (2006-2010). Continuous progress had been recorded in South Sudan where geographical coverage improved from 25% in 2006 to 86% in 2010. Progress towards high geographical coverage observed in Liberia, Central African Republic and Democratic Republic of Congo is commendable and should be maintained.
Stable countriesA total of 83,089 communities out of 85,081 were treated in stable countries; this represents a geographic coverage of 98%. Geographical coverage remained above 90% in all the stable countries (Table 2 and Figure 3) except for Angola and Uganda. In Angola, shortage of human resources may explain the low coverage observed. The drop in geographical coverage in Uganda resulted from the fact that three districts in the Phase III project
of Uganda were not treated because Albendazole was not available. These districts had planned to administer the drugs as a package under NTD Programme. It was subsequently decided that in future, ivermectin treatment would not be withheld due to the lack of availability of other NTD drugs.
1.2.2 Status of therapeutic coverage
In 2010, 75,947,196 people were treated in 16 countries of whom 61% (46,389,483 people) were in stable countries and the remaining 29,557,713 were in post-conflict countries.
Post-conflict countriesIn the post-conflict countries, the average therapeutic coverage was 71%. It ranged from 52% in South Sudan to 84% in Sudan (see Table 2). As of 2010, all the post conflict countries except South Sudan have reached the threshold of 65% for control
0
10
20
30
40
50
60
70
80
90
100
Burundi
Chad
Sudan
Liberia
DRC CAR
South Sudan
Uganda
Angola
Cameroon
Nigeria
Congo
Equatoria
l Guinea
Ethiopia
Malawi
Tanzania
Post conflict countries Stable countries
2006 2007 2008 2009 2010
Figure 3: Trend of the geographical coverage (%) in 16 APOC countries between 2006 and 2010
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
17
ww
w.w
ho.in
t/ap
oc
(Figure 4). Central African Republic (82%), Chad (81%), Liberia (81%) and Sudan (84%) reached in 2010 a therapeutic coverage above the 80% recommended for moving from Onchocerciasis control to elimination of infection and interruption of transmission. Burundi, with an average therapeutic coverage of 79%, was moving towards the targeted threshold for elimination (80%). Its project of Cibitoke-Bubanza reached 80.1% therapeutic coverage and Rutana and Bururi achieved respectively 77.5% and 76.4%. The average therapeutic coverage of the 20 CDTI projects of DRC was 73%. Six projects reached a therapeutic coverage ranging between 80% (Ueles) and 82% (Bandundu). The therapeutic coverage ranged from 69% (Kasongo) to 79% (Katanga Sud) in nine projects. While the therapeutic coverage remained low in the remaining five projects, namely Ubangi Sud (39.5%), Lubutu (44.1%), Ubangi Nord (49.8%), Butembo-Beni (62.1%) and Tshopo (62.4%). Armed
conflicts and Onchocerciasis-Loiasis co-endemicity,have hampered ivermectin mass distribution in these projects.
Stable countriesThe average therapeutic coverage in stable countries was 79%. It ranged from 68% in Angola to 83% in Malawi. Excluding Equatorial Guinea, where ivermectin treatment was not conducted in 2006, all the stable countries have maintained a therapeutic coverage above the control threshold of 65%.
Figure 4 summarises the treatment figures in APOC Programme countries from 2006 to 2010. Malawi has consistently maintained a therapeutic coverage of 80% and above during the last five years followed by Congo in the last two years.
Figure 5 reveals the number of persons treated through CDTI strategy between 1997 and 2010.
Compared to 2009, the number of people treated in 2010 (75,947,196 persons) has increased by 11%.
Figure 4: Trend of therapeutic coverage (%) in 16 APOC countries between 2006 and 2010
0
10
20
30
40
50
60
70
80
Burundi
Chad
Sudan
Liberia
DRC CAR
South Sudan
Uganda
Angola
Cameroon
Nigeria
Congo
Equatoria
l Guinea
Ethiopia
Malawi
Tanzania
Post conflict countries Stable countries
2006 2007 2008 2009 2010
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
18
ww
w.w
ho.in
t/ap
oc
The remaining 14 million people to be covered to enable the programme to reach its ultimate treatment objective of 90 million annually are identified. In this context, 34 new CDTI worreda will initiate distribution in 2012 in Ethiopia as well as Kitgum and Pader districts in Uganda. In DRC, one new project will be launched in South Ituri and treatment coverage that was restricted by Onchocerciasis and Loiasis co-endemicity will be expanded. Angola and South Sudan also will expand their treatment coverage.
1.3 Ivermectin (Mectizan®) tablets supplied by the Mectizan Donation Programme (MDP) Between 1997 and 2010, Merck & Co. Inc. through its Mectizan Donation Programme has donated 1,816,138,200 tablets to APOC countries. In 2010 alone, 345,552,000 tablets were distributed to 15 APOC countries and 24,493,500
tablets to three ex-OCP countries (Ghana, Côte d’Ivoire and Sierra Leone). Details of the numbers of tablets distributed to APOC countries (1997-2010) and ex-OCP countries (2010) are shown in Annex 1.
1.4 Monitoring, Evaluation and surveillance 1.4.1 Independent participatory monitoring of CDTI projects
Independent Participatory Monitoring exercises of CDTI projects verify that they are on schedule to achieving sustainability. According to existing guidelines, these exercises are initiated in the 2nd year of launching a CDTI project. In a participatory manner, teams composed of external monitors, programme managers from other project areas and community members undertake these exercises. Currently, 15 years after the inception of APOC, very few projects are still in their 2nd or 3rd year. These are mainly located in
Figure 5: Reported number of persons treated in APOC countries between 1997 and 2010
0
10
20
30
40
50
60
70
80
1997
0.08
75.95
68.45
57.0954.47
48.67
40.3637.82
32.4627.90
24.1920.39
14.09
1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010
Num
ber o
f peo
ple
trea
ted
(mili
ons)
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
19
ww
w.w
ho.in
t/ap
oc
post-conflict countries or in areas of co-endemicity in which projects were launched late because of fear of the occurrence of severe adverse events (SAEs). During the reporting period, only Bengo CDTI project in Angola has undergone Independent Participatory Monitoring. The exercise covered three health districts Bula Atumba (1 heath area), Pango Alequm (2) and Dembos Quibaxe (3). In each health district, 10 communities were sampled totalizing 30 communities in the project area. Table 3 shows the findings of the monitors during the exercise. It is hoped that this progress will continue over the next years as the country continues to enjoy stability. To strengthen the country capacity, APOC Management is in the process of recruiting a full time Technical Advisor in addition to the Administrative and Finance Assistant already stationed in Angola.
1.4.2 Community Self Monitoring (CSM)
Over the years communities have demonstrated that they are not only capable of distributing simple drugs and commodities to their members but are also capable of monitoring themselves
and finding realistic solutions for improving their performance as shown by reported numbers of communities which implemented CSM in 2007 (24,807), 2008 (23,697), 2009 (28,892) and 2010 (15,545).
The outcome of the CSM is reported annually at a Stakeholders meeting, a forum at which the communities and other health workers have the opportunity to discuss the aggregate results from CSM exercises. In 2008, NOTFs requested that APOC management financially assist countries to initiate and scale up CSM activities to empower all communities to carry out this very important activity. Since then, to better understand the barriers to the upscale of CSM, APOC management held a series of in-country consultations, using a bottom-up approach with Community Leaders/Members and health staff facilitated by social anthropologists. In 2010, two such consultations took place in Burundi and Congo in both communities practicing CSM and those that have not been implementing. This was followed by consultations in Ouagadougou in 2011, which regrouped a small group of experts on CDTI/CDI,
Indicator monitored Performance
1Treatment coverage – geographic 87.7% (67.4 – 100.0) relatively good
– therapeutic 72.8 (65.7 – 76.4) relatively good
2 Community participation and programme ownership fair
3 Health Education/Mobilisation/Sensitisation fair
4 Mectizan supply, collection and distribution fair
5 CDD performance poor
6 Gender Issues and Minority groups/non-indigenous poor
7 Training, monitoring and supervision poor
8 Integration poor
9 Partnership poor
10 Resources poor
Table 3: Summary of the independent monitoring results in Bengo, Angola
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
20
ww
w.w
ho.in
t/ap
oc
social anthropologists, NGDO partner representatives and community members from Burundi, Congo, and DRC, during which the reports of in-country consultations were analyzed. The meeting made 13 recommendations below to upscale the CSM by 40% in 2012, 60% in 2013 and in all communities by 2015:
To APOC Management:
1. Standardise CDTI training approach, revise training materials and develop training modules and job aids for the practical application of the CDTI including CSM at all levels especially at frontline and community levels.
2. Expand on the pool of trainers (TOT) for CDTI including CSM, to reinforce knowledge and skills transfer for CSM at country level.
3. Develop and/or strengthen IEC activities and materials, in order to reinforce community understanding of the CDTI philosophy, and ownership of CDTI including CSM.
4. Facilitate capacity building of community leaders including women to acquire skills in the use of practical and innovative strategies for resource mobilisation, so as to improve community level support for CDTI including CSM activities.
5. Continue the CSM consultation in selected countries. In this regards, strengthen the methodology and existing data collection instrument for country consultations on CSM, to build upon the evidence base for the scale up of CSM. And provide feedback to stakeholders including communities.
6. Gender mainstreaming should remain a priority in the implementation of all these recommendations.
7. Facilitate dialogue between different health intervention programmes to integrate monitoring and other activities especially at community level.
To countries and field partners:
8. Allocate adequate funding for core CDTI activities including CSM so as to promote community ownership of CDTI and empower communities to track and resolve their own health problems.
9. Fund-raising efforts directed at potential local donors and local NGOs should be made by NOTFs and communities to up scale CSM as essential activity of CDTI
10. Communities should be encouraged to provide incentives to community
Focus group discussions in collines Miremera in Cibitoke, Burundi.
Focus group discussions in the village urban CDTI area in Congo (Health District Mfilou).
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
21
ww
w.w
ho.in
t/ap
oc
monitors in addition to CDDs so as to provide motivation for carrying out CSM
11. Improve capacity for planning at all levels through trainings especially at FLHF level for the timely implementation of CDTI activities including CSM.
12. Because of the variation in providing incentives to community volunteers (CDDs and monitors) and in order to integrate monitoring by different health programmes the policies for providing incentives by community and health programmes should be harmonized.
13. Intensify HSAM so as to sustain community interest for ivermectin uptake and the value of implementing CSM to improve their health status
1.4.3 Evaluation of sustainability of CDTI projects
Evaluation of sustainability of CDTI projects using the tools/instruments developed for this purpose (APOC documents WHO/APOC/MG/04.1)1 , is a means of ensuring that CDTI projects will continue to perform sustainably after the closure of APOC in 2015. The evaluations provide a quantitative and qualitative assessment of key elements determining the sustainability of a project in their third or fifth year. They give an opportunity to project staff and managers at all levels from the front line health facilities (FLHF) up to the senior policy and decision making officials of the Ministries of Health, NGDOs and the National coordinating office to review progress, identify weaknesses and prepare plans for consolidating sustainability based on the outcomes of the reviews.
During the reporting period, evaluations of sustainability were conducted for nine CDTI projects (plus the Secretariat of South Sudan Onchocerciasis task force) in three countries (DRC, Liberia and South Sudan). Sustainability plans were produced for all evaluated projects in accordance with evaluation guidelines. Projects are judged to be making progress towards sustainability if they achieve a score of 2.5 or above out of a total possible score of four.
In Liberia, the evaluation reports judged two projects, South Eastern (2.95) and South Western (2.70), sustainable or making progress towards sustainability whilst North West which scored 2.20 out of four is still below making progress. In DRC, only the CDTI project of Lualaba that scored 2.95 was making progress towards sustainability, the projects of Bas-Congo (1.58) and Equateur Kiri (1.17) were judged not to be making progress. The Secretariat of South Sudan Onchocerciasis task force (SSOTF) and the three CDTI projects of West Equatoria, East Equatoria and East Bahr el Ghazal were not showing progress towards sustainability (Figure 6).
1.4.4 Surveillance activities
During the reporting period, APOC management put emphasis on establishing systems, capacity and structures to assess the level of transmission in five countries (Cameroon, Chad, Nigeria, Tanzania and Uganda) in which Onchocerciasis infection has been virtually eliminated in 11 foci. In addition, APOC finalised in Malawi, as a first test, the delineation of transmission zones using cyto-
1 Guidelines for conducting an evaluation of the sustainability of CDTI projects, APOC management, September 2004, revised version
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
22
ww
w.w
ho.in
t/ap
oc
taxonomy and chromosomal inversions techniques. Also, following the setting up structures and capacities, APOC launched entomological evaluations in the above-mentioned five countries. APOC also continued to monitor the status of Onchocerciasis transmission in ex-OCP countries in order to assist them with timely detection of any recrudescence of transmission.
As far as the former OCP countries are concerned, fifty-four catching points in seven countries (Benin, Burkina Faso, Côte d’Ivoire, Ghana, Guinea Bissau, Niger and Togo) were monitored by the countries with financial and technical support of APOC and with assistance of the Onchocerciasis entomological surveillance unit of MDSC.
A total of 385,178 blackflies captured during these surveillance activities as well as through studies of vector movement between Benin and Nigeria and Onchocerciasis elimination feasibility studies were screened, providing a preliminary status of the transmission in most of the areas. Figure 7 gives an overall picture of the results of this entomological surveillance
0.0
0.5
1.0
1.5
2.0
2.5
3.0
3.5
4.0
West E
quatoria
East Bahr e
l Ghazal
SSTOF
West E
quatoria
South Eate
rn
South W
estern
North W
est
Lualaba
Bas-Congo
Equateur K
iri
South Sudan
Liberia
D.R. Congo
Figure 6: Results of sustainability evaluation in 9 CDTI projects and one Onchocerciasis task force secretariat
in the former OCP countries as summarized below:
i. Only few of the Simulium flies caught in Benin, Côte d’Ivoire, Guinea Bissau, Niger and Togo were carriers of the human Onchocerciasis parasite. The infectivity rate was lower than the threshold of 0.5 ‰, indicating that transmission has been interrupted and therefore, there is no or insignificant risk of recrudescence of Onchocerciasis;
ii. In Burkina Faso and Ghana, the infectivity rates at some of the assessment sites located towards the border with Côte d’Ivoire are higher than the acceptable threshold of 0.5‰. Meetings between Ministry of Health officials and technicians from Burkina Faso and Côte d’Ivoire were organised with technical and financial support of APOC and SightSavers. This provided an opportunity to review the Onchocerciasis situation and prepare a plan of action and budget to support the two countries in assessing the epidemiological situation thoroughly and in taking appropriate corrective measures.
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
23
ww
w.w
ho.in
t/ap
oc
Although the fly movement study is not completed and some other assessments need to be performed, data available allow for the following observations:
i. The Simulium flies sampled did not show any infection in Benin while three out of the five catching points in Nigeria presented infected females with infectivity rates higher than the threshold of 0.5 ‰. An epidemiological evaluation will be undertaken in the same areas in Benin and Nigeria before the end of 2011 to verify if there is any correlation between the indicators of two domains (entomology and epidemiology). This might indicate the presence of a natural barrier (the wide uninhabited forest area for instance) that prevent the infected females from Nigeria from crossing the border and infecting people in Benin;
ii. The predominant species of blackfly in Nigeria were of the forest type, with a few savanna flies caught in January at the catching points closest to Benin;
iii. Most of the flies in Benin were of the savanna form. Hetero-duplex analysis characterized the savanna flies as S.damnosum s.s. and the forest flies as S.soubrense Beffa form.
Once the epidemiological evaluation is concluded and all available entomological samples are processed, a conclusion would be drawn on eventual fly movement and its impact on transmission at the border of the two countries.
Within the framework of Onchocerciasis transmission assessments, an entomo-logical working group was set up by APOC in March 2011 to review the draft protocol, agree on the steps to follow for the assessments, agree on the chrono-gram of activities and share tasks among the members. Following a situational analysis and sensitisation of authorities, training of technicians was undertaken in July/August 2011 in Chad, Nigeria and Uganda for 36 technicians. Fly catch-ing sites were then selected and the assessments started in the second half of August 2011 in most of the catching points in Nigeria and Chad. Preliminary results after two weeks of sampling of female flies show that there is almost no transmission in Kaduna focus while the number of flies caught in the other sam-pling sites both in Nigeria and Chad were almost nil. The evaluation will continue during the rainy season and the forth-coming dry season for a better apprecia-tion of the transmission in the foci.
Figure 7: Results of Onchocerciasis surveillance in ex-OCP countries and Nigeria in 2010
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
24
ww
w.w
ho.in
t/ap
oc
As far as the delineation of transmission zones is concerned, the vector cytospecies identification and the determination of the frequency of polymorphic inversions were undertaken in Malawi from May/June 2010 to June 2011 by nationals trained by APOC, with the technical support of consultants. The data shows the existence of two distinct Simulium populations distributed as indicated in Figure 8, representing two transmission zones. It is important to indicate that these transmission zones are all covered by ivermectin treatment and therefore, elimination of infection will be easy if high therapeutic coverage is achieved. The same exercise is being undertaken in Nigeria where 10 cyto-taxonomy technicians were trained in August 2011. The first comprehensive results will be available in 2012 to assist in a better delineation of the treatment areas.
1.4.5 Health Impact of CDTI
APOC is collaborating with Erasmus University of Rotterdam, the Netherlands, on a comprehensive health impact assessment of APOC operations. The plan of work includes (i) continued analysis of disease prevalence data; (ii) prediction of trends of Onchocerciasis infection in relation with ivermectin treatment; (iii) finalising the APOC Onchocerciasis simulation model (ONCHOSIM) which will include in addition to infection, skin and eye disease. In this framework, capacity at national level and APOC HQ is being built to facilitate operational use of the APOC ONCHOSIM model by countries and APOC Secretariat staff. Thus, capacity will be enhanced to assess health impact by project/country, to assess the trends of the disease infection for decision making on where and when to stop ivermectin treatment, and to determine the DALYs gained through the programme’s operations.
Figure 8: Delineation of possible transmission zones in Malawi
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
25
ww
w.w
ho.in
t/ap
oc
2. Moving from control to elimination of Onchocerciasis where feasible
Epidemiological evaluationAPOC started conducting epidemio-logical evaluations in 2008. The major objective of these evaluations was to col-lect enough empirical evidence to help countries to determine when and where to stop ivermectin treatment. Since then, epidemiological evaluations using a skin-snip parasitological survey have been conducted or are on-going in 33 Onchocerciasis foci in 32 CDTI projects in 10 countries. The countries are Cam-eroon, CAR, Chad, Congo, DRC, Ethiopia, Malawi, Nigeria, Tanzania and Uganda (Figure 9). At the time of writing the report, evaluations had been completed and results analysed for 23 Onchocercia-sis foci where 43,528 persons were ex-amined in 275 villages. The results were very encouraging in most of the evalu-ated areas. Compared to ONCHOSIM predictions the results are encouraging in 20 out of the 23 sites evaluated. In 9 sites the infection had probably been eliminated; five are close to elimination; six are on track whilst in the 3 remain-ing sites the results are not satisfactory
(Table 4). At the time of writing the report, epidemiological evaluations were still ongoing in nine sites. In sites where the trend towards elimination seems con-firmed, entomological and epidemiologi-cal evaluations will be intensified in 2012 to gather information required for deci-sion making regarding the cessation of ivermectin treatment. Other sites should continue the treatment in order to reach the break point. Also, evaluations for the feasibility of elimination will continue in projects that are eligible, i.e2,3,4 at least 10 years of ivermectin treatment, pre-control endemicity higher than 40% (nodule palpation)/60% (bloodless skin snip), a therapeutic coverage higher than 70%.
Conclusion of evaluation Nb of sites
Elimination probably already achieved 9
Close to elimination 5
On track 6
Unsatisfactory progress 3
Total evaluated 23
Table 4: Conclusions of epidemiological evaluation results in 23 sites
Figure 9: Sites and results of epidemiological evaluations between 2009 and 2011
2 Evaluation of the impact of community treatment with ivermectin using skin snip method3 Guidelines for the Evaluation of Therapeutic Coverage4 Conceptual and Operational Framework of Onchocerciasis Elimination with Ivermectin Treatment
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
26
ww
w.w
ho.in
t/ap
oc
3. Co-implementation of CDTI with other health interventions
Control of NTDs building on CDTIExpanded co-implementation of NTDs in the Democratic Republic of Congo and Tanzania, supported by funds from USAID and the APOC Trust Fund continued during the reporting period. The other countries involved in co-implementation activities in 2010 were Burundi, Cameroon, CAR, Ethiopia, Liberia, Malawi, Nigeria, and Uganda. 48 million treatments/health interventions were provided alongside ivermectin distribution. Figure 10 shows that STH treatments
were administered to more than 15 million persons; ITN to 8.2 million. Vit A distribution, home management of malaria and mass treatment for LF benefitted each to more than six million persons.
0
2
4
6
8
10
12
14
16
STH
Malarie IT
M
VAS (pre
vention of
Vitamin A de�ency)
Malaria (H
MM)
SchistoLF
Trachoma
HIV/A
IDS (h
ealth educatio
n)
Epi (EPI ta
rgete
d diseases)
Hygiène (pre
vention of C
holera)
Catara
ct
15.17
8.226.73 6.60 6.47
2.481.12 0.72 0.44 0.11 0
Num
ber o
f peo
ple
trea
ted
(mili
ons)
Figure 10: Major health interventions co-implemented with ivermectin distribution using CDI in 10 countries in 2010
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
27
ww
w.w
ho.in
t/ap
oc
4. Co-implementation of CDTI with other health interventions
4.1 Human resource development for the control of Onchocerciasis and other Health interventions 4.1.1 Training of health staff by NOTFsA total of 53,135 health workers from all levels were trained/re-trained by CDTI projects with technical assistance from NGDO partners and coordination from the NOTFs (Table 5). The high percentage of new trainees resulted from the need to increase the number of
CDDs, particularly in Cameroon, Chad, Uganda, Congo and South Sudan, and for scaling-up implementation in new CDTI projects in Angola, Liberia and Southern Sudan.
4.1.2 Training of health staff by APOC ManagementWithin the reporting period, APOC management, with the assistance of country experts and MDSC, also built the capacity of health personnel of Ministries of Health and NGDO partners in different areas. Table 6 summarises
Health workers Community-directed distributors
Country N* Newly trained
Re-trained Total % newly
trainedNewly
trainedRe-
trained Total % newly trained
Angola 6 237 200 437 54.2% 2,329 1,752 4,081 57.1%
Burundi 3 35 179 214 16.4% 80 8,792 8,872 0.9%
Cameroon 15 853 1,984 2,837 30.1% 17,962 22,716 40,678 44.2%
CAR 1 9 438 447 2.0% 552 5,060 5,612 9.8%
Chad 1 70 151 221 31.7% 2,096 2,851 4,947 42.4%
Congo 2 17 181 198 8.6% 582 1,064 1,646 35.4%
Ethiopia 9 354 4,327 4,681 7.6% 6,414 60,209 66,623 9.6%
Liberia 3 233 474 707 33% 3,527 6,046 9,573 36.8%
Malawi 2 153 2,743 2,896 5.3% 2,265 12,413 14,678 15.4%
Nigeria 27 9,632 19,432 29,064 33.1% 57,659 125,817 183,476 31.4%
DRC 20 1,226 5,831 7,057 17.4% 20,999 74,762 102,537 20.5%
Southern Sudan 5 527 455 982 53.7% 6,959 5,245 12,204 57%
Sudan 1 0 0 0 0 1,375 1,826 3,201 43%
Tanzania 7 330 1,346 1,676 19.7% 1,830 11,390 13,220 13.8%
Uganda 4 941 777 1,718 54.8% 22,897 40,911 63,808 35.9%
Total 106 14,617 38,518 53,135 27.5 147,526 380,854 535,156 27.6
N* = number of CDTI projects
Table 5: Number of health workers and CDDs trained/retrained in 2010 by NOTFs
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
28
ww
w.w
ho.in
t/ap
oc
Area of capacity building Number of trainees Countries
Strategy on CDTI / CDI 407 Burundi, DRC, Ethiopia, Guinea Bissau, Liberia, Tanzania
Management of SAEs 26 DRC
Disease mapping 246 Angola, CAR, Chad, DRC, Gabon, Ethiopia
Epidemiological evaluation 36 CAR, Congo, Ethiopia, Malawi, Tanzania
Entomological evaluation 36 Chad, Nigeria, Uganda
Data management 41 Angola, Cameroon, South Sudan
GIS 56 Angola, Burundi, CAR, Chad, Cameroon, Congo, DRC, Equatorial Guinea
ONCHOSIM modelling 33
Burkina Faso, Cameroon, Ethiopia, Liberia, Malawi, Nigeria, South Sudan, Tanzania, Uganda, Burundi, Congo, Equatorial Guinea, CAR, Chad, DRC and Côte d’Ivoire
MSc training 25 Burundi, Cameroon, CAR, Chad, Congo, DRC, Kenya, Malawi, Mozambique and Uganda
Financial management 113 Angola, Chad, DRC, Ethiopia, Ghana, Nigeria, Sierra Leone, Tanzania, Uganda
Grand Total 1004
Table 6: Number of health workers and CDDs trained/retrained in 2010 by NOTFs
the areas in which capacity building was achieved and the number of persons trained.
Out of a total of 15 candidates from 10 countries who benefitted from APOC-funded scholarships in 2009, 14 have completed their Masters degrees. For the academic year starting 2011, ten candidates from six countries were selected for scholarships (CAR: 2, Equatorial Guinea: 3, Côte d’Ivoire: 1, DRC: 2, Cameroon: 1, Chad: 1). The target areas of training are: Entomology (2), Parasitology (1) and Public Health (7). Fifty six percent of the selected candidates were females.
4.1.3 Building capacity of countries in communicationAPOC management provided regular technical assistance to countries (CAR, Chad and Nigeria) in the area of health education, sensitisation advocacy and communication throughout the
period. A mission was undertaken to Taraba state in Nigeria in June 2011 to provide assistance to the Taraba project and assist project staff to develop and implement effective health education, sensitisation, advocacy and mobilization (HSAM) for the Onchocerciasis project in the state. Technical assistance is being provided to CAR and Chad for the development of a communication strategy. These countries are also being assisted in planning IEC activities and development of appropriate IEC materials.
4.1.4 Gender MainstreamingCurrent status in the countries The policy of gender mainstreaming was initiated in APOC Programme in 2007. Since then countries have been reporting the number of CDDs involved in CDTI activities by gender.Table 7 shows, the total number of CDDs and the proportion of female CDDs
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
29
ww
w.w
ho.in
t/ap
oc
in 2009 and 2010 for 12 countries that provided disaggregated data,. In the overall, the number of CDDs increased by 15% between 2009 and 2010 and the number of female CDDs by 17%, from 121 976 in 2009 to 143 167 in 2010. The increase in number of female CDDs was higher in post conflict countries (31.0%) than in stable countries (13%).During the reporting period the following activities were carried out: (i) training on Gender Leadership and integrated participatory management of communities’ health (Cameroon, CAR, Chad, Congo and DRC); (ii) Plans of action for gender mainstreaming in CDTI activities are under development in CAR, Cameroon, Chad and Congo.APOC, in accordance with its policy on gender mainstreaming, continues to encourage the participation of women in different training sessions and workshops. During the reporting period,
Female CDDs Total CDDs % female CDDs
2009 2010 2009 2010 2009 2010
Post-conflict countries
Burundi 4,089 4,045 8,828 8,872 46.3 45.6
CAR 85 260 4,431 5,612 1.9 4.6
Chad 2,251 4,348 13,602 14,201 16.5 30.6
DRC 18,913 24,608 112,254 115,194 16.8 21.4
Liberia 1,316 1,693 6,124 7,343 21.5 23.1
Sudan 1,182 1,499 2,911 3,201 40.6 46.8
Total 27,836 36,453 148,150 154,423 18.8 23.6
Stable countries
Angola 138 531 2,179 4,421 6.3 12.0
Cameroon 11,635 18,559 38,983 65,764 29.8 28.2
Congo 332 393 1,668 1,646 19.9 23.9
Ethiopia 8,058 7,158 64,919 66,623 12.4 10.7
Malawi 8,237 9,326 14,147 15,559 58.2 59.9
Nigeria 65,740 70,747 200,770 232,790 32.7 30.4
Total 94,140 106,714 322,666 386,803 29.2 27.6
Grand Total 121,976 143,167 470,816 541,226 25.9 26.5
Table 7: Number of female CDDs in 12 APOC countries, 2009-2010
female participation was as described below:
• Training in cytotaxonomic identification of members of the S.damnosum complex: 4 females/11 people trained in Nigeria.
• Training in entomological surveillance of onchocerciasis: 6 females /16 technicians trained in Nigeria and 13 health personnel in Chad (training session included some women).
• A total of 14 women benefited from scholarships for completion of Masters Degree in Epidemiology and Public Health between 2010 and 2011.
Out of a total of 135 participants who underwent Training on Gender, Leadership in GPISC (Integrated Participatory Management of Communities Health) 68 were female community leaders and CDDs.
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
30
ww
w.w
ho.in
t/ap
oc
Current status in APOC HeadquartersAt APOC HQ, the proportion of women represents 40% of the entire staff. However, for new recruitment in 2011, APOC has achieved parity and the Management is determined to maintain this trend. Despite the existing gap between male and female staff at the APOC/HQ, the Management values female contributions and encouraged their involvement in implementing and evaluating field activities by including 7 HQ female staff and female temporary advisers on missions to several countries (Liberia, Ghana, Nigeria, DRC, etc.) between September 2010 and August 2011.
4.2 Logistics support to countriesDuring the period under review, APOC supported 14 of its member countries (Angola, Burundi, Cameroon, Chad, Congo, Ethiopia, Liberia, Malawi, Nigeria, Uganda, Central African Republic, Democratic Republic of Congo, South Sudan and Tanzania) and six ex-OCP countries (Benin, Côte d’Ivoire, Ghana, Guinea-Bissau, Guinea and Sierra Leone) with logistics and equipment. Table 8 shows the equipment/logistics provided to countries.
Logistics support APOC Ex-OCP
Transport
Vehicle (4x4) 22 7
Bicycle 2,038 300
Motorcycle 140
Computers and accessories
Desktop computer 41 1
Laptop computer 28 4
Laser printer 39
Scanner 30
UPS 7 1
Communication & other
TV 8
LCD projector 7
Photo 5
Photocopier 4
Generator 4
Table 8: Logistics and equipment provided by APOC to countries during the reporting period PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
31
ww
w.w
ho.in
t/ap
oc
5. Partnerships and government contributions
5.1 Government financial contributions to CDTI activitiesDuring the reporting period, Governments of 15 countries disbursed US$ 16 937 214 for core CDTI activities, equipment and salaries of health personnel of various CDTI implementation units.
Governments disbursed US$ 3 012 750 to core CDTI activities. 12% of the contributions were made by post-conflict countries and the remaining US$ 2 657 771 by stable countries. Table 9and Annex 2 show that countries paid more attention to health education, sensitization, advocacy and mobilization (34.0%) followed by ivermectin delivery and management of severe adverse events (30.1%).
5.2 Direct financial support to countries and management of the APOC Trust FundIn addition to the logistic and technical support to countries over the period 2010–2011, direct financial support was provided to 15 APOC countries and limited support to ten ex-OCP countries. The details of countries/projects that benefited from the APOC Trust Fund in years 2010–2011are shown in Table 10.
In 2010 and 2011, a total amount of US$ 47.59 million (US$ 24.06 million for year 2010 and US$ 23.53 million for year 2011), represents the total implemented Programme budget as of 31st October 2011. For the given period, 86.41% of this amount was allocated to technical/operational activities and 13.59% to
Angola, Burundi, Cameroon, CAR, Chad, Congo, DRC, Equatorial Guinea, Ethiopia, Liberia, Malawi, Nigeria, South Sudan, Tanzania and Uganda disbursed for core CDTI.
Equipment and salaries in 2010: US$ 16,937,214
Core CDTI activitiesAmount
disbursed US$
Percentage
Health education, sen-sitization, advocacy and mobilization
1,025,751 34.0%
Ivermectin delivery and management of severe adverse events
905,417 30.1%
Training/retraining of Community-directed distributors and Health workers
546,705 18.1%
Supervision, monitoring and evaluation 534,877 17.8%
Total for core CDTI 3,012,750 100%
Table 9: Government funds disbursed for CDTI activities in 2010
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
32
ww
w.w
ho.in
t/ap
oc
Country Nb projected funded/ total project Nb DFC prepared by country (specific activities included)
Year/Period 2010 2011* 2010 2011*
Support to APOC countries
Angola 7/8 9/9 9 11
Burundi 3/3 3/3 9 6
Cameroon 16/16 16/16 23 24
CAR 1/1 1/1 6 2
Chad 1/1 1/1 6 6
Congo 2/2 2/2 5 3
DRC 21/22 21/22 26 33
Equatorial Guinea 1/1 1/1 N/A 2
Ethiopia 9/10 10/10 10 13
Liberia 3/3 3/3 6 7
Malawi 2/2 2/2 2 5
Nigeria 28/28 28/28 45 45
South Sudan 5/5 5/5 11 10
Sudan 1/1 1/1 N/A N/A
Tanzania 8/8 7/7 9 8
Uganda 6/7 7/7 3 4
Sub total 114/118 115/117 170 181
Limited support to ex OCP country programmes
Benin 1/1 1/1 3 2
Bissau Guinea 1/1 1/1 3 4
Burkina Faso 1/1 1/1 3 2
Ghana 1/1 1/1 3 3
Guinea 1/1 1/1 1 2
Mali 1/1 1/1 1 1
Niger 1/1 1/1 1 1
RCI 1/1 1/1 2 4
Sierra Leone 1/1 1/1 2 2
Togo 1/1 1/1 N/A N/A
Sub total 10/10 10/10 19 21
Grand Total 124/128 125/127 189 202
* Data as of October 2011
Table 10: Number of female CDDs in 12 APOC countries, 2009-2010
administrative/management cost (Figure 11).
Overall, 107 CDTI projects and 7 NOTF Secretariat support projects were funded for all the countries during the reporting period. About 35.86 % of the approved/obligated Plan budget was allocated and released directly to national projects.
Figure 12 shows that CDTI activities represented 62.14 % expenditures in year 2010 and 66.16% in the 2011 (as of 31st October 2011).
For 2010, three hundred and forty-eight (348) Funding Authorization and Certification of Expenditure reports (FACE) were received from the projects, 99% (346) of which were analyzed/
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
33
ww
w.w
ho.in
t/ap
oc
Figure 11: Proportion of the budget used for administration and logistic costs compared to technical and operational costs in 2010 and 2011
Figure 12: Allocation of APOC Trust Fund to major activities in 2010 and 2011
certified by the APOC Finance Team (Table 11). The FACEs of year 2011 (mid-term and the final) were due respectively in July and in December 2011 and will be reported on in 2012.
The noticeable improvement in the rate of submission of financial returns by APOC Country/Projects in 2010 is driven primarily by changes made to the financial and administrative procedures of APOC as dictated by the new WHO
Global Management System (GSM). In addition, efforts were made in training most of the national partners involved in the Trust Fund and Project management. Finally, frequent visits to countries (twice per year at least) by the APOC Finance Team, have also significantly enhanced the financial reporting and monitoring of APOC Country/Projects and is expected to contribute to a smooth transition to national ownership and sustainability.
2010 2011
Technical / Operational related activity cost 86.96%
Administrative & Logistic (APOC Mangement) cost 13.04%
Administrative & Logistic (APOC Mangement) cost 14.13%
Technical / Operational related activity cost 85.87%
2010 2011
Strengthening health systems 15.95%
Administrative & Logistic (APOC Mangement) 14.13%
Partnerships 4.05%
Gender 0.79%
Monitoring/ Evaluation 3.90%
Epidemiological evaluation & surveillance 7.48%
CDTI Implementation 50.76%
Research and Drug development 2.94%
Strengthening health systems 13.06%
Administrative & Logistic (APOC Mangement) 13.04%
Partnerships 3.66%
Gender 1.60%
Monitoring/ Evaluation 2.89%
Epidemiological evaluation & surveillance 11.88%
CDTI Implementation 51.39%
Research and Drug development 2.47%
CDTI=62.14% CDTI=66.16%
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
34
ww
w.w
ho.in
t/ap
oc
Table 11: Number of financial returns submitted and analysed in 2010–2011
Country
Nb. Financial returns sub-mitted by country / Nb. of
returns expected (Inclu-ding previous years)
Nb. FACE submitted by country / Nb. Of FACE
expected (Including previous years and specifics activities)
Nb. FACE certified (Including previous years and specifics activities) / Nb. FACE submitted by country
Year/Period 2010* 2011** 2010 2011** 2010 2011
Support to APOC countries
Angola 159/159 24/63 20/20 5/9 20/20 0/5
Burundi 70/70 20/28 8/15 0/9 8/8 0/0
Cameroon 325/356 61/143 48/81 5/49 48/48 5/5
CAR 15/15 6/7 8/9 0/2 6/8 0/0
Chad 29/29 6/7 7/7 0/1 7/7 0/0
Congo 33/33 8/16 6/10 0/6 6/6 0/0
DRC 508/508 102/147 73/73 1/21 73/73 0/1
Equatorial Guinea N/A N/A N/A N/A N/A N/A
Ethiopia 237/237 0/63 18/21 0/12 18/18 0/0
Liberia 72/72 6/21 12/13 0/4 12/12 4/4
Malawi 30/30 14/14 5/6 4/4 5/5 0/4
Nigeria 595/595 92/196 94/96 7/30 94/94 0/7
South Sudan 101/101 0/35 0/18 0/23 0/0 0/0
Sudan N/A N/A N/A N/A N/A N/A
Tanzania 214/214 38/42 21/29 0/15 21/21 0/0
Uganda 23/32 9/9 5/5 0/0 5/5 0/0
Sub total 2411/2451 386/791 325/403 22/185 323/325 9/38
Limited support to ex OCP country programmes
Benin 0/12 12/12 6/6 0/6 6/6 0/0
Bissau Guinea 0/12 0/12 0/3 0/4 0/0 0/0
Burkina Faso 0/12 0/12 0/5 0/5 0/0 3/3
Ghana 0/12 12/12 7/7 0/0 7/7 0/0
Guinea 0/12 0/12 0/2 0/2 0/0 0/0
Mali 0/12 0/12 0/2 0/2 0/0 0/0
Niger 0/12 0/12 0/2 0/2 0/0 0/0
RCI 0/12 12/12 7/7 0/0 7/7 0/0
Sierra Leone 12/12 6/7 3/3 1/1 3/3 1/1
Togo 0/12 0/12 0/1 0/1 0/0 0/0
Sub total 12/120 42/115 23/39 1/23 23/23 4/4
Grand Total 2423/2571 428/906 348/442 23/208 346/348 13/23
* Monthly returns as per the former APOC admninistrative an financial procedures* * Data as of October 2011. The mid term FACE of year 2011 is due in July 2011 and the final FACE expected in
December 2011
5.3 Governance5.3.1 The Joint Action ForumThe sixteenth Session of the Joint Action Forum (JAF), the Governing body of APOC, was hosted by the Government of the Federal Republic of Nigeria, from 7-9 December 2010 in Abuja, Nigeria, and
was officially opened by Professor C.O. Onyebuchi Chukwu, Federal Minister of Health, on behalf of His Excellency the President, Goodluck Jonathan. The final report of the external mid-term evaluation of APOC was presented to JAF by Prof. Habbema and was discussed. It was agreed that, in principle, APOC
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
35
ww
w.w
ho.in
t/ap
oc
should not close in 2015 and JAF decided that CSA should investigate scenarios for the Programme’s continuation and that these scenarios should be presented to JAF 17 in December 2011 for consideration. CSA set up three sub-advisory groups on future of APOC, elimination of Onchocerciasis and co-implementation. The recommendations of the sub-advisory groups will be submitted to the 17th session of JAF by CSA.
5.3.2 Committee of Sponsoring Agencies The Committee of Sponsoring Agencies (CSA) continued its activities of monitoring the APOC Programme management and operations. Four meetings of the CSA were held during the reporting period. The highlights of these meetings included: (i) Follow-up of the decisions of JAF16 (ii) discussions on strategies to improve the sponsorships of the Programme with additional UN organisations and revamp the contribution/participation of the statutory ones; (iii) the revision of the MemorandumofUnderstandingofAPOC to take into account recent developments with the APOC partnership and the move from control to elimination; (iv) review and recommendations for the appointment of new TCC members; (v) Preparations for JAF17 including the review of the draft reflections of the CSA to JAF; (vi) Criteria and guidelines for certification of elimination of Onchocerciasis; (vii) review of APOC operations and its financing, (viii) determination of criteria for collaboration between APOC and MDSC.
In addition, CSA sessions received updates on APOC operations, reports of TCC 31, 32, and NGDO Group meetings,
provision of technical support to Onchocerciasis control in Yemen and participation of the West African Health Organisation (WAHO) in the Joint Action Forum.
5.3.3 Technical Consultative Committee and decentralisation of functions to countriesThe 31st and 32nd sessions of the Technical Consultative Committee (TCC) of APOC were held in September 2010 and March 2011 respectively in Ouagadougou, Burkina Faso. TCC31 discussed the preliminary findings of the external mid-term evaluation of APOC, which were summarised in a presentation by Dr Habbema. Other issues that were discussed were progress towards elimination of Onchocerciasis infection and interruption of transmission and completion of mapping of areas of Africa co-endemic for Onchocerciasis and Loiasis. TCC members expressed their great pleasure for the completion of this mapping and APOC was urged to publish these data as soon as possible.
TCC32 discussed progress with the CDI curriculum and commented that it was critical for APOC to develop a textbook on CDI to ensure that the training is uniform. The committee reviewed the progress of activities related to the elimination of infection and interruption of transmission, emphasising the need for both entomological and epidemiological evaluations and after a presentation on the OV16 diagnostic test for Onchocerciasis, TCC noted that this test could be useful in the post elimination surveillance phase.
In 2008, TCC recommended that countries set up Technical Review
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
36
ww
w.w
ho.in
t/ap
oc
Committees (TRC) to perform functions (in-country) similar to those performed by TCC. This reflects APOC’s philosophy of empowering countries to take more ownership of the programme, thus enhancing sustainability. TRCs have been set up in Cameroon, Nigeria and Uganda. The committees have responsibility for the in-country review of Annual Technical Reports of projects that are seven years or older and of proposals for operational research. TRC also has the mandate to advise the NOTF on measures to sustain and improve CDTI implementation in the country, as well as strengthening health systems at different levels. The chairs of these committees make presentations to TCC during its twice-yearly sessions at APOC-HQ.
During the period under review APOC funded the 5th and 6th TRC meetings in Nigeria; the 2nd TRC meeting in Uganda; and the 3rd and 4th TRC meetings in Cameroon. The summaries of these meetings are provided in the respective TCC reports.
5.4 Development and implementation of a CDI curriculum and training moduleRecognising the importance of involving communities in the design, planning and implementation of their own health services, APOC through the CDTI strategy has been instrumental in implementing the Alma-Ata Declaration (1978), the Ottawa Charter (1986), the Djakarta Charter (1997), and the Ouagadougou Declaration (2008). Therefore, in 2010, Vice-chancellors, deans of faculties of medicine and health sciences and heads of nursing schools of the Eastern and
Southern regions of Africa held a three-day meeting in Nairobi on the curriculum and training module on the Community-Directed Intervention (CDI) strategy. The meeting, which was co-facilitated by the Kenya WHO Country Office and the WHO African Programme for Onchocerciasis Control (WHO-APOC), deliberated on the use and inclusion of the Community-Directed Intervention (CDI) strategy in the curricula for training of medical professionals from East and Southern African countries. The meeting was attended by 44 participants representing 16 Universities, Medical and Nursing schools from Angola, Burundi, Kenya, Malawi, Namibia, Rwanda, South Africa, Tanzania and Zimbabwe. Also in attendance were representatives of the Ministry of Health of Kenya, WHO/AFRO and the Country Office, Kenya, WHO-APOC, the African Medical Research Foundation (AMREF) and SightSavers in Kenya.
A two-day Seminar was also held at the University of Medical Sciences and Technology, Khartoum, from 24–25 November 2010, on the inclusion of the CDI Strategy Curriculum and Training Module, in Universities in Sudan. At the end of the seminar consensus was reached by the 18 Universities to pre-test the CDI curriculum.
A total of 52 faculties of medicine, health sciences and nursing schools in 19 countries across Africa have accepted to pre-test for incorporation the teaching of the CDI strategy in the curricula of their Institutions. Since 2009, APOC has received plans of actions and budgets from 15 institutions and approved over US$ 183 840.67 for pre-testing activities in 13 of the 15 Institutions.
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
37
ww
w.w
ho.in
t/ap
oc
5.5 Technical support to countriesStrengthening of APOC Management, and support to countriesAPOC continued its efforts to strengthen its management capacity as well as technical support to post-conflict countries during the period under review. In this regard, fixed-term positions of Health Systems Specialist, Research, Administrative and Finance Officers have been filled. In addition, a short-term Technical Advisor has been appointed in Guinea Bissau to assist the country in achieving elimination of Onchocerciasis within a two to three year period. Furthermore, in partnership with CBM, APOC co-financed Technical Advisor positions in two countries (Angola and DRC) in which CDTI activities still need more support to reach the recommended therapeutic and geographic coverage thresholds. The Programme also financially supported two NPO/NTD positions in Angola and Burundi within the WHO country offices’ work plans.
Table 12: Achievements and challenges of development and implementation of CDI curriculum and training module
Achievements Challenges
• Curriculum and training module developed and revised during Nairobi meeting
• Faculties of medical and nursing schools to incorporate the teaching of CDI strategy in their curricula in 19 Countries in Eastern and Southern West, Central and Northern
• Ongoing pretesting activities in 15 out of 52 faculties of medicine, health sciences and nursing schools that have agreed to pre-test the CDI curriculum
• A standardized guidelines developed for submitting PABs
• Ongoing activities for finalising teachers hand book of the CDI curriculum
• CDI text book write up (Outline Matrix of topics developed)
• Monitoring, supervision of pretesting
• External evaluation of pretesting
• Long term impact evaluation of strategy in the curriculum on the performance of future generation of health professionals and communities health systems
• Continued APOC support to Faculties of medicine, health sciences and nursing schools (provision resources human, mate-rial and financials for effective implementa-tion of the Curriculum)
• Development of oncho control libraries in Faculties of medicine, health sciences and nursing schools; and WHO country offices.
During the period under review, more than 150 Temporary Advisers and consultants were utilised in the countries in various domains including epidemiological evaluations, hydro-biological data collection and/or analysis, training and elaboration of guides on NTDs mapping, implementation of combined RAPLOA/REMO surveys, entomological assessments, independent participatory monitoring, sustainability evaluations, training of accountants at country level, CDTI data collection and management by projects, among others.
5.6 Partnerships and collaboration5.6.1 CDTI Projects and NGDO support for Onchocerciasis ControlOne of the guiding principles in the unique APOC partnership is to maintain multiple partners so that NGDOs can continue to contribute technical expertise and logistical support to most CDTI projects in the field. The global
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
38
ww
w.w
ho.in
t/ap
oc
financial crisis has hit many of the partners making it difficult to ensure that all CDTI projects have supporting partners.
In the framework of moving from control to elimination APOC Management is putting a lot of efforts to make sure that all CDTI projects benefit from NGDO partner support. In this respect, APOC is partnering with CBM in supporting a Technical Adviser position in Angola and DRC since the begining of 2011. In addition, APOC welcomed SightSavers’ initiative to partner with UFAR in supporting two additional CDTI projects in DRC.
It should be noted that 16 CDTI projects do not have NGDO partner support in the field. They are mainly projects in Angola and DRC as shown in Annex 3. The 37th session of the NGDO Coordination Group for Onchocerciasis Control, held from 2-4 March 2011 in Ouagadougou, Burkina Faso, had representatives of 10 member organisations (Charitable Society for Social Welfare, Christoffel-Blindenmission, Helen Keller International, Mectizan Donation Program, Mission to Save the Helpless, Organisation pour la Prévention de la Cécité, Schistosomiasis Control Initiative, SightSavers, United Front Against Riverblindness and the US Fund for UNICEF) and two Associate members (International Agency for the Prevention of Blindness and Merck & Co., Inc.). Representatives from IMA Global Health, Light for the World, Lions Clubs International Foundation, Malaria Consortium, the Carter Center, and the Liverpool University Centre for Neglected Tropical Diseases were unable to attend the meeting.
As many participants attended that session for the first time, the meeting focused mainly on the APOC philosophy and the Community-Directed Treatment with Ivermectin (CDTI) strategy, which was extensively presented by APOC Director.
The Group addressed issues related to some CDTI projects that lack NGDO partners, the impact of global financial crisis on the implementation of Onchocerciasis control activities and the challenges of moving from Onchocerciasis control to elimination in some selected areas in APOC countries.
5.6.2 Collaboration with other programmes and institutionsTo maintain linkages and keep all partners informed, APOC Management continues to invite different partners to meetings and workshops organised either at APOC/HQ or at country level. Also, to keep abreast of the changing priorities of partners APOC Management attended several meetings and workshops organised by different partners during the reporting period. These included:
• 15th River Blindness Review for Carter Center assisted countries in Atlanta,
• 44th Mectizan Expert Committee meeting in Baltimore;
• Tanzania NTD Stakeholders Meeting
• DRC NTD stakeholders Meeting
• Joint APOC/CBM/USAID mission to monitor co-implementation activities in DRC
• Briefing session for accelerating the finalisation of country NTD plans of action, Harare, Zimbabwe, 1-3 September 2010
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
39
ww
w.w
ho.in
t/ap
oc
• Preventive Chemotherapy (LF, Schisto and STH) Programme Managers and LFE Regional Programme Review Group (RPRG) meetings, Lusaka
The World BankAs the fiscal Agent of the Programme, the World Bank has been instrumental in resource mobilization and management of APOC Trust Fund through their advices to the Progamme and advocacy visits to donors’ countries. It is in this regard that a joint fund mobilization visit was carried out by the Bank and APOC management to Vienna to meet the authorities of the OPEP Funds for International Development. The Bank was also active in all sessions of the Committee of Sponsoring Agencies which took place during the period under review.
West African Health Organization (WAHO)As the sub-regional organization in charge of Health matters in West Africa, WAHO has developed a keen interest in APOC activities. WAHO representative attended all TCC and ex-OCP coordinators’ meetings during the period under review. WAHO co-funded the 3rd Programme National Coordinators meeting of Ex-OCP countries which took place in Ouagadougou in November 2010.
World Health Organization (WHO)WHO/HQ continued to support the Programme in different domains (legal advice, finance,technical guidance, procurement, communication, audit, Global Management System). WHO/AFRO Regional Director continued to advocate for APOC during meetings and working sessions with country leaders and partners. WHO Regional Office for Africa provided support to
APOC in the areas of staff recruitment, transfer of funds to CDTI projects and NOTF Secretariats, technical involvement of Regional staff in APOC’s activities.
WHO Country Offices (WCO) continued to play pivotal roles in country Onchocerciasis control programmes management, local procurement for the Programme, travel arrangements, indirect payments from WHO Global Service Centre (Kuala Lumpur) to partners and suppliers. All APOC missions to the countries were assisted by the WCO.. All APOC Technical Advisors posted in post-conflict countries are given office space within the WCOs.
MDSC, based on a business plan, provided technical service in Onchocerciasis surveillance in ex OCP and in APOC countries to detect recrudescence of the disease. In addition the MDSC participated in APOC research and capacity building activities (details are provided in paragraphs 1.4.4, 4.1.2 and 6)
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
40
ww
w.w
ho.in
t/ap
oc
6. Macrofil and other research
Moxidectin development for Onchocerciasis control: Development of moxidectin was conducted jointly by WHO and Wyeth. In 2009 Pfizer acquired Wyeth and terminated the collaboration agreement with WHO effective 4 July 2011. An External Advisory Committee (EAC) convened by TDR and APOC met on 10 September 2011 to review the data available on moxidectin. The EAC was asked to advise WHO on further development of moxidectin. The EAC’s principal recommendations were:
• WHO should continue development of moxidectin in the most time efficient manner all the way to completion of the community study, provided that the Phase 3 data and the paediatric study data confirm current data on efficacy and safety of moxidectin.
• TDR needs to ensure that the paediatric study is initiated as soon as possible and can be conducted with the drug manufactured for the Phase 3 study to avoid delays and additional costs due to identification and qualification of a new manufacturing site and conduct of a bioequivalence study.
• WHO needs to negotiate with Pfizer to obtain all rights for use of moxidectin for NTDs and all related data, technology, and knowledge, including the methods and costs for manufacturing of the human formulation developed by Wyeth at the scale required for use by control
programmes. This will avoid further delays in moxidectin development and WHO having to spend money for redoing work already done by Wyeth or Pfizer. It will furthermore facilitate finding a license holder and donor, in particular once all data as per development plan are available.
• Evaluation of the safety of moxidectin in Loaloa co-infected subjects should be initiated with testing of moxidectin in animals experimentally infected with Loaloa (not included in current cost estimates).
• TDR should develop a plan to assess the efficacy and safety of moxidectin for Lymphatic filariasis control.
• TDR and APOC should jointly initiate the search for a license holder and donor immediately. Lack of a license holder and donor should, however, not delay studies and other work required for obtaining a recommendation on control programme use.
TCC endorsed the EAC recommendations which are consistent with TCC 32 recommendations. TDR and APOC need to mobilise the funds required, taking into account the financial requirements for continuation of the Ivermectin Response Marker project.
Ivermectin response markers: This project aims to assess whether long term use of ivermectin results in selection of O.volvulus with a reduced sensitivity to the embryostatic effect
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
41
ww
w.w
ho.in
t/ap
oc
of ivermectin by identifying genetic differences between O.volvulus from previously untreated subjects and previously treated subjects with different extent of repopulation of the skin after ivermectin treatment. If such differences are identified and subsequently validated, the project aims to develop a diagnostic tool for surveillance by Onchocerciasis control programmes and build the capacity at MDSC, in Ghana and Cameroon to support use of the tools by control programmes and data analysis. The work was initiated in 1st and 2nd quarters of 2011 in collaboration between researchers in Australia, Cameroun, Canada, France, Ghana and at the MDSC. As a first step, the whole genome of
7. Transition to national ownership and sustainability
There is no doubt that the APOC Programme has established sustainable national drug distribution systems which would continue ivermectin distribution for Onchocerciasis control activities if the Programme closes in 2015. The activity of developing exit plans started a few years back and to date a total of eight APOC countries have developed exit plans. These countries are Cameroon, Chad, Congo, Ethiopia, Malawi, Nigeria, Central African Republic and Tanzania.
Given the move from control to elimination of Onchocerciasis and interruption of transmission, there is need to rethink the value of developing exit plans at this point. During the reporting period APOC Management has put on hold the continuation of exit plans development for the remaining APOC countries until the outcome of JAF17 session. This will give an indication of the nature of exit plans to be developed.
O.volvulus was sequenced and is now publicly available at an FTP site. Bio-informatic analysis of 800 differences in genome sequences between parasites from untreated, ‘good responders’ and ‘poor responders’ from Ghana and Cameroon is ongoing. MDSC has verified that archived parasite samples from areas with different levels of endemicity, forest/savannah, different vectors and different ivermectin treatment history can be used for genetic analysis. Thus, these samples, as well as microfilaria samples collected during ongoing APOC epidemiological evaluation since 2009 will be available for validation of genetic differences resulting from the bio-informatic analysis.
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
42
ww
w.w
ho.in
t/ap
oc
Annexes
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
43
ww
w.w
ho.in
t/ap
oc
Ann
ex 1
: Num
ber o
f Mec
tizan
tabl
ets*
pro
vide
d an
d sh
ippe
d to
APO
C m
embe
r cou
ntry
pro
gram
mes
from
19
97 to
201
0 an
d to
ex-
OCP
cou
ntrie
s (2
010)
APO
C Co
untr
y19
9719
9819
9920
0020
0120
0220
0320
0420
0520
0620
0720
0820
0920
10To
tal
Ang
ola
8,00
00
00
00
01,
199,
000
00
2,88
0,50
00
01,
352,
000
5,43
9,50
0
Buru
ndi
00
00
00
440,
000
056
6,00
02,
249,
000
2,21
5,00
02,
380,
000
2,51
6,00
02,
758,
000
13,1
24,0
00
Cam
eroo
n2,
770,
800
2,64
7,80
05,
434,
900
4,87
4,00
04,
853,
500
9,61
5,50
010
,156
,000
5,27
6,00
010
,388
,500
12,8
34,0
0014
,424
,000
13,0
23,0
0014
,061
,000
22,0
40,0
0013
2,39
9,00
0
CA
R2,
182,
600
3,25
4,00
02,
604,
000
3,01
9,00
04,
350,
000
2,80
0,00
04,
200,
000
00
3,00
0,00
05,
214,
000
2,85
2,00
05,
156,
000
4,97
0,00
043
,601
,600
Chad
1,59
5,80
02,
611,
400
689,
000
1,86
0,00
02,
897,
500
3,05
5,50
03,
487,
000
3,58
0,00
03,
833,
500
4,05
3,00
04,
010,
500
4,25
0,50
04,
250,
500
4,39
0,50
044
,564
,700
Cong
o0
00
876,
500
877,
500
635,
500
774,
000
1,22
0,00
01,
106,
500
1,13
4,50
01,
029,
000
1,36
4,50
01,
137,
000
1,89
8,50
012
,053
,500
DRC
850,
000
370,
000
1,44
9,00
02,
019,
000
7,56
5,50
01,
676,
000
28,2
65,0
0017
,262
,500
12,0
51,5
0016
,243
,500
31,6
26,5
0013
,315
,500
55,8
49,5
0050
,596
,500
239,
140,
000
Eq G
uine
a53
,500
057
,000
74,0
0061
,500
66,5
000
196,
000
020
5,00
00
180,
000
018
0,50
01,
074,
000
Ethi
opia
00
062
9,00
01,
645,
500
6,31
2,00
03,
922,
500
5,17
9,50
018
,440
,000
9,61
7,50
013
,837
,000
13,0
32,5
0013
,032
,500
13,2
80,0
0098
,928
,000
Gab
on0
026
,000
013
0,00
015
,000
25,0
0010
,000
20,5
000
00
00
226,
500
Libe
ria
00
300,
000
916,
000
4,23
2,00
04,
511,
000
3,00
0,00
00
3,00
8,00
00
5,05
3,00
00
08,
625,
500
29,6
45,5
00
Mal
awi
071
2,00
068
6,00
01,
226,
000
2,29
5,50
075
,000
1,69
4,00
04,
217,
500
2,23
9,50
03,
519,
500
4,78
4,50
03,
440,
500
4,25
2,00
032
,734
,500
61,8
76,5
00
Nig
eria
43,3
45,6
0023
,863
,600
54,9
37,0
0057
,746
,000
60,9
84,0
0056
,672
,000
62,6
01,0
0057
,067
,000
90,1
50,0
0043
,483
,500
89,7
72,5
0029
,471
,500
55,1
68,5
0010
9,61
6,50
083
4,87
8,70
0
Suda
n95
5,40
01,
595,
000
1,80
0,00
01,
902,
000
3,11
4,00
02,
250,
000
3,26
9,00
01,
759,
000
1,33
0,00
03,
204,
000
6,15
4,00
07,1
40,0
0010
,688
,000
12,5
57,0
0057
,717
,400
Tanz
ania
720,
600
840,
600
1,01
8,10
01,
474,
000
2,50
7,50
02,
588,
000
2,38
4,50
013
,168
,500
3,18
3,50
02,
121,
500
54,1
70,5
0047
0,00
03,
322,
500
36,3
23,0
0012
4,29
2,80
0
Uga
nda
200,
000
7,34
9,00
03,
900,
000
5,53
6,50
05,
008,
000
5,03
4,00
06,
006,
500
5,82
4,00
06,
554,
000
8,04
5,00
00
8,53
4,00
010
,956
,000
44,2
29,5
0011
7,17
6,50
0
TOTA
L52
,682
,300
43,2
43,4
0072
,901
,000
82,1
52,0
0010
0,52
2,00
095
,306
,000
130,
224,
500
115,
959,
000
152,
871,
500
109,
710,
000
235,
171,
000
99,4
54,0
0018
0,38
9,50
034
5,55
2,00
01,
816,
138,
200
* Th
e ta
blet
s in
199
7 w
ere
excl
usiv
ely
6 m
g ta
blet
s an
d fr
om 1
998
to 1
999
both
6 a
nd 3
mg
tabl
ets.
Fro
m 2
000
to
2008
onl
y 3
mg
tabl
ets
wer
e do
nate
d. T
he 6
mg
tabl
et is
cou
nted
as
twic
e “3
mg
tabl
et e
quiv
alen
t”Ex
-OCP
cou
ntri
es
2010
Cote
D’Iv
oire
5,
572,
500
Gha
na
8,99
9,00
0Si
erra
Leo
ne
9,92
2,00
0TO
TAL
ex-O
CP c
ount
ries
24
,493
,500
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
44
ww
w.w
ho.in
t/ap
oc
Annex 2: Governments’ contributions for CDTI activities, 2010
Funds disbursed in US$ in %
Budget line post conflict Stable Total allocated
Core CDTI activities 1997 1998 1999 2000
Health education, sensitisation, advocacy, mobilisation
Health Education 9,700 128,152 137,852 13.4%
Mobilization 9,252 170,105 179,357 17.5%
Sensitization 16,263 264,658 280,921 27.4%
Advocacy 11,062 416,559 427,621 41.7%
Total 46,277 979,474 1,025,751 100%
Training
Training/retraining of community-directed distributors
23,019 323,484 346,503 63.4%
Training/retraining of health workers 18,017 182,185 200,202 36.6%
Total 41,036 505,669 546,705 100%
Supervision, monitoring and evaluation
Evaluation 144,896 50,392 195,288 36.5%
Monitoring 8,906 70,342 79,248 14.8%
Supervision 25,197 235,144 260,341 48.7%
Total 178,999 355,878 534,877 100%
Ivermectin distribution and management of SAEs
Ivermectin distribution 36,667 696,291 732,958 81%
Management of Severe adverse events 52,000 120,459 172,459 19%
Total 88,667 816,750 905,417 100%
Overall core CDTI activities 354,979 2,657,771 3,012,750
Equipment 21,863 781,689 803,552
Salaries 895,585 12,225,327 13,120,912
Contributions of 15 countries US$
1,272,427 15,664,787 16,937,214
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
45
ww
w.w
ho.in
t/ap
oc
Country Project NGDO
Angola 1 Lunda Norte
2 Lunda Sul
3 Moxico I
4 Bengo CBM
5Kuando Kubango
6 Kwanza Norte
7 Huige CBM
8 Huila
Burundi 9Cibitoke Bubanza
CBM
10 Bururi CBM
11 Rutana CBM
Cameroon 12 Adamaoua I IEF
13 Adamaoua II IEF
14 North Province HKI/Carter Center*
15 South West I Sightsavers
16 South West II Sightsavers
17 Littoral II Perspective/HH
18 Centre 1 HKI
19 Centre 2 HKI
20 Centre 3 HKI
21Western Province
Carter Center
22 North West Sightsavers
23 South Province IEF
24 East Province HKI
25 Far North HKI
26 Littoral I Perspective/HH
CAR 27 CAR CBM
Chad 28 Chad BELAC
Congo 29 Congo I OPC
30Congo Extension
OPC
DRC 31 Kasai CBM
32 Ueles CBM
33 Sankuru CBM
34 Bandundu IMA World Health
35 Tshopo CBM
36 Bas Congo
Country Project NGDO
37 Nord Ubangui CBM
38 Sud Ubangui CBM
39 Mongola CBM
40 Equateur-Kiri CBM
41 Tshuapa CBM
42 Katanga Nord
43 Katanga Sud
44 Lualaba
45 Lubutu UFAR/Sightsavers
46Masisi-Walikale
47Rutshuru-Ngoma
48 Kasongo UFAR
49 Butembo-Beni
50 Ituri Nord UFAR/Sightsavers
51 Ituri Sud
Ethiopia 51 Kaffa Sheka Carter Center
52 Bench Maji Carter Center
53 North Gondar Carter Center
54 East Wellega Light of the World**
55 West Wellega Light of the World**
56 Illubabor Carter Center
57 Jimma Carter Center
58 Metekel Carter Center
59 Gambella Carter Center
Eq. Guinea 60 Equatorial
Guinea
Gabon 61 Gabon
Liberia 62 North West Sightsavers
63 South East Sightsavers
64 South West Sightsavers
Malawi 65 Thyolo & Mwanza Sightsavers
67 Malawi extension Sightsavers
Sudan 68 Northern sector Carter Center
69 West Equatoria CBM
70 East Equatoria CBM
Annex 3: CDTI Project supported by NGDOs in APOC countries (September 2011)
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
46
ww
w.w
ho.in
t/ap
oc
Country Project NGDO
72 West Bahr el Ghazal CBM
73 East Bahr el Ghazal CBM
74 Upper Nile CBM
Nigeria 75 Kaduna Sightsavers
76 Taraba MITOSATH
77 Cross River UNICEF
78 Kogi Sightsavers
79 Adamawa HKI
80 Borno HKI
81 FCT CBM
82 Plateau Nassarawa Carter Centre
83 Osun UNICEF
84Enugu/Anambra/ Ebonyi
Carter Center
85 Kano CBM
86 Imo Abia Carter Center
87 Yobe CBM
88 Zamfara Sightsavers
89 Edo Delta Carter Center
90 Oyo UNICEF
91 Jigawa CBM
92 Benue UNICEF
93 Kebbi Sightsavers
94 Kwara Sightsavers
95 Bauchi UNICEF
96 Ekiti UNICEF
97 Gombe UNICEF
98 Niger UNICEF
99 Ondo UNICEF/MITOSATH
100 Ogun UNICEF
101 Akwa Ibom HKI
Tanzania 102 Mahenge/MOROGORO Sightsavers
103 Ruvuma/RUVUMA Sightsavers
104 Tanga/TANGA Sightsavers
Country Project NGDO
105 Tukuyu/MBEYA Sightsavers
106 Kilosa/MOROGORO Sightsavers
107 Morogoro/MOROGORO Sightsavers
108 Tunduru/RUVUMA Sightsavers
Uganda 109 Phase I SSI, Carter Center
110 Phase II Carter Center
111 Phase III Carter Center
112 Phase IV Carter Center
113 Phase V
* HKI took over from the Carter Center in 2011.** Light for the World ceased being a member of the NGDO group
in August 2011 but will continue to provide financial support to the projects for the next two years.
Green color indicates no NGDO partner support.
PRO
GR
ESS
REP
OR
T 20
11 •
AFR
ICA
N P
RO
GR
AM
ME
FOR
ON
CH
OC
ERC
IASI
S C
ON
TR
OL
47
ww
w.w
ho.in
t/ap
oc
African Programme for Onchocerciasis Control (APOC)World Health OrganizationB.P. 549 – Ouagadougou – BURKINA FASOTel: +226-50 34 29 53 / 50 34 29 59 / 50 34 29 60Fax: +226-50 34 28 75 / 50 34 26 [email protected]
AFRICAN PROGRAMME FOR ONCHOCERCIASIS CONTROL
© A
POC
2011
– G
raph
ic d
esig
n: L
isa
Schw
arb
– Ph
otos
: APO
C –
WH
O/A
POC/
JAF1
7.5




























































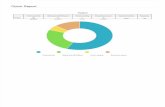





![For The Region: Report, Report, Report [Eng]](https://static.fdocuments.in/doc/165x107/579079761a28ab6874c751c6/for-the-region-report-report-report-eng.jpg)
