Aortic Abdominal Aneurysm · An Abdominal Aortic Aneurysm (AAA) is defined as a focal dilatation of...
Transcript of Aortic Abdominal Aneurysm · An Abdominal Aortic Aneurysm (AAA) is defined as a focal dilatation of...
-
Aortic Abdominal Aneurysm
Continued surveillance or elective repair.
Stacey Biggs BBiomedSc, s2148529
Course: 7208NSC Physiology Clinical Placement A
Convener: Mrs. Alison White
Due Date: 11 Sept 2015
-
1
Introduction
An Abdominal Aortic Aneurysm (AAA) is defined as a focal dilatation of the
abdominal aorta greater than 3.0cm or 1.5 times its original size [5]. The aortic
aneurysm is a full-thickness dilatation involving all layers of the artery wall. The
pathophysiology of aortic aneurysms can be characterized by four main events.
The first and most frequently documented event is the degeneration of the aortic
media and adventitia elastin and collagen by proteases, which comprises
metalloproteinases, plasmin and cathepsin S and K. The cause of this event is
largely debated but remains elucidated. The second recognized event is the
infiltration of the vessel wall by inflammatory cells, such as CD4-positive
lymphocytes and macrophages [5, 7]. Aneurysmal size has had a positive
association discovered with inflammatory markers such as C-reactive protein
(CRP) and interlueukin-6 (IL-6) [10]. Infiltration by inflammatory cells is thought to
be involved with the third event, which is apoptosis of vascular smooth muscles
resulting in thinning of the media layer [5, 7]. The fourth event in degeneration of
the arterial wall is neovascularization, which is considered to play a critical role in
development and rupture of an AAA [9].
Approximately 85% of all AAA’s are infrarenal and often involve the Common Iliac
Arteries (CIA) [7]. Only 5% of infrarenal AAA’s include the renal arteries [5]. Most
are asymptomatic with 30% of cases discovered when pulsatility of the abdomen
is felt, on palpation, during a routine physical examination. In addition to this,
auscultation of the abdomen may reveal the presence of a bruit however body
habitus is a major obstacle to accuracy in both circumstances [6]. Non-modifiable
risk factors include age, race, gender and family history. Men are 10 times more
likely to have an AAA with rates of prevalence increased from the age of 55 years
advancing by 10% between the ages of 65-79 years [10]. Modifiable risk factors
include hypertension, hyperlipidemia, atherosclerotic disease and tobacco use.
Smoking can increase the risk of developing an AAA by approximately 7% and is
associated with causing incremental increase in AAA growth by 0.4mm per year
[5, 6].
-
2
Case presentation
Patient C Characteristic
Age: 75yrs
Gender: Male
Relevant case history:
Hyperlipidaemia
Hypertension
Clinical diagnosed prostate cancer
Controlled schizophrenia
Socioeconomic background:
Patient C is a retired non-smoking gentleman living with his wife.
Clinical details as documented by treating physician:
The earliest documented report of Patient C’s AAA dated 6th February 2004 notes
an incidental finding of an asymptomatic 3.8cm abdominal aortic aneurysm.
Regular surveillance of the AAA was to be conducted with surgical repair to be
considered once the AAA was > 5cm.
The latest vascular surgeon report, dated 19th September 2014 reveals that
Patient C remains asymptomatic with ultrasound today measuring an infrarenal
AAA of 48mm X 51mm, which has grown 1mm over the previous 12 months.
He has good pulses in his femoral, dorsalis pedis and posterior tibial distally. He
has no sequelae of thrombus emboli or ischemic disease. On examination he
does have a prominent right popliteal artery for further investigation. Conservative
management is to continue with repeat duplex Aorto-iliac scan and duplex arterial
right leg in one year’s time.
-
3
Test requested
1. Aorto-Iliac Arterial Duplex Scan (Appendix 1 Worksheet/ Appendix 3
Report)
Duplex imaging of the Abdominal Aorta and Iliac arteries was performed using two-
dimensional (B-mode imaging system) spectral pulse wave Doppler and colour flow
imaging with a 4C (9-14MHz) curved linear array transducer. The patient was
positioned supine throughout the test. In Patient C’s case body habitus or
comorbidity did not require repositioning throughout the test from supine to either
right or left lateral decubitus, or perhaps virtually prone, as can commonly be
required. The Abdominal Aorta is viewed in transverse and longitudinal planes from
the celiac trunk distally past the iliac arteries. Assessment of both Aortic diameter
and basic structure is performed using B-mode imaging. Flow velocities and areas of
possible stenoses are evaluated using pulsed Doppler. Colour flow Doppler was
used to assess for branches and the extent of Mural thrombus present in the
aneurysmal sac [11].
2. Bilateral Peripheral Leg Arterial Duplex Scan (Appendix 2 Worksheet/
Appendix 4 Report)
Duplex imaging of the right leg arteries was performed using two-dimensional (B-
mode imaging system) spectral pulse wave Doppler and colour flow imaging with a
9L (4-10MHz) linear array transducer. The patient was positioned supine with a 20-
30 degree reverse Trendelenburg throughout the test. The subsequent imaging
evaluated clinical findings of a prominent right popliteal pulse with interest to
assessing for an aneurysm. The presence of vascular disease of the arteries can be
assessed for areas of stenosis and occlusion as well as dilatation. The worksheet in
appendix 2 illustrates observed pathological changes.
Interpretation of the test results
Aorto-Iliac Arterial Duplex Scan – Abdominal Aorta (Illustrated in appendix 1-
Report appendix 3)
The Infra-renal Aorta was significantly dilated to a maximal diameter of 50mm X
53mm from a baseline 23mm X 23mm. This is a 2mm increase over the last 12
-
4
months, which is consistent with growth rates represented in appendix 5.4. The neck
length measured at 78mm with a saccular length of 73mm. The walls of the AAA
were mildly calcific with evidence of mural thrombus and no associated flow
disturbances on Doppler sampling. Characteristics of mural thrombi are described as
a permanent dynamic interface with circulating blood components. Recent evidence
suggests that a relationship exists between the presence of mural thrombi and
evolution of aneurysmal dilation towards rupture. The extent of involvement of the
mural thrombus encompasses thinner arterial walls, increased elastolysis, a lower
density of smooth muscle cells in the media layer and a higher level of immune-
inflammatory of the adventitia [12]. Mural thrombi increases Patient C’s
predisposition to distal embolization [8].
Aorto-Iliac Arterial Duplex Scan – Iliac Arteries (Illustrated in appendix 1- Report
appendix 3)
Minor thickening of the right iliac artery was noted with no associated flow
disturbance on Doppler sampling. The right Common Iliac Artery (CIA) was ectatic
with a maximal diameter measured of 17mm. The right External Iliac Artery (EIA)
had a maximal diameter of 11mm. The left CIA and EIA were ectatic and measure
maximal diameters of 17mm and 15mm, respectively. Normal triphasic flow was
demonstrated bilaterally in the Iliac arteries on Doppler sampling. The CIA diameter
reference range of 1.1-1.4cm is considered normal [13]. A CIA diameter of
for men and for women is considered ectatic [2].
Right Leg Arterial Duplex Scan (Illustrated in appendix 2- Report appendix 4)
Minor plaque was observed in the common Femoral, Profunda Femoris, Superficial
Femoral and Popliteal arteries. Normal triphasic flow noted throughout on Doppler
sampling. The Femoro-Popliteal arteries showed evidence of ectasia but were not
aneurysmal. The Popliteal artery measured a maximum diameter of 11.0mm along
its length. The calf arteries demonstrated normal triphasic flow on Doppler sampling.
Patient C was investigated for right Popliteal artery aneurysm after a Popliteal
prominence was revealed on clinical examination. No evidence to support clinical
findings of prominent Popliteal artery aneurysm, however it was found to be ectatic.
Studies reveal 62% of patients with a Popliteal aneurysm will also have an AAA.
-
5
Dissimilarly to this only 14% of patients with an AAA will develop a Femoral or
Popliteal artery aneurysm. Hence justifying the need for further investigation when a
prominence is identified on clinical examination [5].
Management and treatment outcomes
Elective AAA repair should be considered in men with aneurysm 5.5cm or when
rate of expansion is 0.6cm-0.8cm per year [1, 10]. Duplex ultrasound on Patient C
revealed a 2mm growth in the last year of his AAA thus this measurement criteria in
isolation suggests continued management by surveillance. Conservative treatment
by risk modification is limited as the main modifiable risk factor and leading
contributor to AAA growth is smoking and Patient C is known to be a non-smoker.
However Patient C’s comorbidities of hypertension and hyperlipidaemia should
continue to be aggressively managed with antihypertensives and statins. There are
current debates to the additional benefit of statin use for reduction of AAA size.
Nonetheless statins have been shown to be useful as a threefold reduction in risk for
cardiovascular death after repair [7, 10].
At 75 years of age Patient C has been clinically diagnosed with prostate cancer for
which he has decided to take a watchful waiting approach. As the AAA is yet to
reach ≥5.5cm and expansion rates are consistent with predicted values the vascular
surgeon and Patient C may choose to take the same approach. As represented in
appendix 5.3 AAA between 5.0-5.9cm in diameter have an increased risk of rupture
by 1-10%. Taking this into consideration, with the increase in aneurysmal diameter to
twice that of the year before, surveillance of Patient C by Duplex ultrasound should
increase in frequency from 12 monthly to 3 monthly (appendix 5.1). This
recommendation is also reflected in, appendix 5.2, AAA surveillance intervals by
country.
Outcomes vary between asymptomatic and symptomatic presentations of patients
with AAA. Asymptomatic cases are often found incidentally on clinical examination
when a palpable abdominal mass is present. Symptomatic cases can present with a
pulsatile and painful abdomen, pain in the chest, lower back pain and scrotum. One
-
6
study reported mortality rates in symptomatic patients that undergo elective repair
are as high as 26% compared with asymptomatic patients with a 5%rate [10].
-
7
Discussion
Rupture of an AAA is highly lethal with an estimated incidence of sudden death in
>50% of cases. The high mortality rates can be attributed to patient failure to reach a
hospital in time as well as the level of surgical experience [10]. Emergency repairs
hold mortality rates as high as 50-80%. Whereas mortality rates in elective repair are
much lower at less than 5% in some cases [5]. Infrarenal AAA can rupture into the
peritoneal cavity (anteriorly) or retroperitoneum (posteriorly). Peritoneal rupture has
the highest mortality rate as it is commonly accompanied by haemodynamic
collapse. A posterior rupture into the retroperitoneum can occur without significant
blood loss and initially be haemodynamically stable. This occurs due to the
containment abilities of the psoas muscle, adjacent periaortic and perivertebral
tissue [1].
Surgical intervention of an AAA encompasses two main approaches of repair. The
first of which is an open repair requiring an abdominal flank incision, control of
vessels above and below the aneurysm whilst the aneurysmal sac is opened and
interposition of a synthetic graft is implemented. The second approach is an
endovascular repair (EVAR), which can be performed percutaneously under local
anaesthetic [7]. Patients 70 years and older, as in Patient C’s case, tend to do better
with open repair regardless of the 30-day mortality risk between 4-5%[6].
Common postoperative complications associated with elective repair include
myocardial ischaemia, mild renal insufficiency (occurs in 6%), pulmonary disease
(pneumonia 5%), lower extremity ischaemia and postoperative bleeding. EVAR is
significantly less invasive than open surgical repairs requiring fewer postoperative
days in hospital care, including ICU, and typically result in shorter patient recovery
periods. EVAR is initially offered to patient deemed unfit for open repair due to its
invasive nature but is dependent on anatomical structures. Failure of the EVAR to
exclude blood flow from the aneurysmal sac completely leads to endoleaks. In
addition to this the occasional report of endograft migration, aneurysmal rupture and
aneurysmal enlargement has raised questions regarding longevity. Thus it is seen
that where open repair has varied rates of complication following elective repair
EVAR requires more post-repair interventions. The long-term durability EVAR has
-
8
not been as well established as open repair [1, 8]. Nevertheless the curve of long-
term survival rates with either treatment approach appears to converge after 2-
3years [5].
The value of conservative treatment by surveillance continues to be maintained in
the presence of surgical risk and varied long term survival rates. Studies have
determined that until the infrarenal AAA diameter exceeds 5.5cm it carries a
-
9
-
10
References
1. Arko, F., Smith, S., & Zarins, C. 2007, "Repair of Infrarenal Abdominal
Aortic Aneurysms", ACS Surgery: Principles and Practice, pp. 1-12.
2. Arunkumar Mistry, K., Bashir, O. & et al. 2015, “Iliac artery aneurysm”,
Radiopedia viewed 09 September 2015, via radiopedia database.
3. Bown, M.J., Sweeting, M.J., Brown, L.C., Powell, J.T., Thompson, S.G. &
RESCAN Collaborators 2013, "Surveillance Intervals for Small Abdominal
Aortic Aneurysms: A Meta-analysis", JAMA, vol. 309, no. 8, pp. 806-813.
4. Grant SW, Sperrin M, Carlson E. & National Library of Medicine 2015,
Calculating when elective abdominal aortic aneurysm repair improves
survival for individual patients: development of the Aneurysm Repair
Decision Aid and economic evaluation, NIHR Journals Library.
5. Jacob, A.D., Barkley, P.L., Broadbent, K.C. & Huynh, T.T.T. 2015,
"Abdominal aortic aneurysm screening", Seminars in roentgenology, vol.
50, no. 2, pp. 118-126.
6. Keisler, B. & Carter, C. 2015, "Abdominal aortic aneurysm", American
Family Physician, vol. 91, no. 8, pp. 538.
7. Kent, K.C. 2014, "Clinical practice. Abdominal aortic aneurysms", The New
England journal of medicine, vol. 371, no. 22, pp. 2101.
8. Robinson, D., Mees, B., Verhagen, H. & Chuen, J. 2013, "Aortic aneurysms:
Screening, surveillance and referral", Australian Family Physician, vol. 42,
no. 6, pp. 364-369.
-
11
9. Sano, M., Sasaki, T., Hirakawa, S., Sakabe, J., Ogawa, M., Baba, S., Zaima,
N., Tanaka, H., Inuzuka, K., Yamamoto, N., Setou, M., Sato, K., Konno, H.
& Unno, N. 2014, "Lymphangiogenesis and Angiogenesis in Abdominal
Aortic Aneurysm: e89830", PLoS One, vol. 9, no. 3.
10. Skow, G. 2011, "Abdominal aortic aneurysm", Journal of Men's Health, vol.
8, no. 4, pp. 306-312.
11. Stickley, S. & Meier, G. 2013, " The Role of Color Duplex Ultrasound in Patients with Abdominal Aortic Aneurysms and Peripheral Aneurysms",
Noninvasive Vascular Diagnosis: A Practical Guide to Therapy, Springer-
Verlag London.
12. Thrush, A. & Hartshorne, T. 2005, Peripheral vascular ultrasound: how,
why, and when, 2nd edn, Churchill Livingstone, New York; Edinburgh.
13. Touat, Z., Ollivier, V., Dai, J., Huisse, M., Bezeaud, A., Sebbag, U.,
Palombi, T., Rossignol, P., Meilhac, O., Guillin, M. & Michel, J. 2006,
"Renewal of Mural Thrombus Releases Plasma Markers and Is Involved in
Aortic Abdominal Aneurysm Evolution", The American Journal of
Pathology, vol. 168, no. 3, pp. 1022-1030.
-
12
Appendix 1 – Aorto-Iliac Arterial Duplex Worksheets (August 2015)
-
13
Appendix 2 – Right Leg Peripheral Arterial Duplex Worksheets
-
14
Appendix 3 – Report – Aorto-Iliac Arterial Duplex Scan (August 2015)
-
15
Appendix 4 – Report - Peripheral Leg Arterial Duplex Scan
-
16
Appendix 5 5.1 Suggested surveillance for patients with stable AAA [8]
5.2 Abdominal Aortic Aneurysm Surveillance Intervals by Country [3]
5.3 Risk of rupture for AAA [8]
Reprinted from AUSTRALIAN FAMILY PHYSICIAN VOL. 42, NO. 6, JUNE 2013 365
growth and rupture have been promising in animal models, these benefits
have not been consistently reproduced in human studies. 21,22 Optimal
cardiovascular risk factor management should include smoking cessation,
a statin, antiplatelet and antihypertensive agents to improve life
expectancy by reducing cardiovascular mortality. In addition to preventing
aneurysm related mortality, screening for AAA will identify patients with
small aneurysms who are at increased risk of cardiovascular events and
who will benefit from cardiovascular risk management.
Many patients are under the misapprehension that a diagnosis of
small AAA prohibits them from physical activity, or overseas travel.
There is no evidence that physical activity leads to aneurysm rupture.
The role of imaging
A number of imaging modalities are available for the assessment of
AAA. Ultrasound is a relatively cheap, non-invasive, widely available
and reliable tool for detecting and measuring AAAs. It is the modality
of choice for screening and surveillance.
Computed tomography angiography (CTA) is fast, reproducible
and accurately depicts aneurysm morphology. It is the investigation
of choice when considering potential surgical repair, and enables
multiplanar analysis. Limitations include the need for high doses of
intravenous iodinated contrast (potentially harmful in patients with
renal impairment), and the use of ionising radiation.
Magnetic resonance angiography has limited utility in AAA.
Digital subtraction angiography has largely been replaced by CTA for
preoperative planning. It is now predominantly reserved for therapeutic
purposes and is utilised during endovascular aneurysm repair (EVAR) to
accurately position the stent graft before deployment.
In AAA surveillance imaging, the most relevant dimension is the
maximum transverse diameter, ideally perpendicular to the line of
flow. Aneurysm length is often reported, but is not relevant, except in
planning for surgical repair. The presence of mural thrombus is also
generally not relevant unless the aorta is critically stenosed or there is
evidence of distal embolisation.
demonstrated to reduce aneurysm related mortality in four large trials,
including one performed in Western Australia.2–5,12 Considerable
variation exists between international screening protocols, with
concerns over value and effectiveness.13,14 Cost effectiveness may
be increased by screening those at particularly increased risk of AAA,
such as men with a history of smoking, or patients with atherosclerotic
risk factors or a family history of AAA. More rigorous risk scoring
systems are being explored.15
First degree family members of patients with a diagnosed AAA
are at higher lifetime risk of developing a AAA of up to 20%, 16–18
however, the genetic mechanisms for this are unclear.19 Previous
recommendations have included screening for men and women over 50
years of age with a family history of AAA.20
The majority of AAAs detected with screening are below the
threshold for elective repair. The management of patients with small
AAA most importantly includes cardiovascular risk management
with lifestyle advice, smoking cessation, pharmacotherapy (anti-
hypertensives, statins, beta-blockers), and ongoing aneurysm
surveillance. Suggested surveillance intervals are listed in Table 3,
although local protocols may vary based on accessibility to imaging,
patient preference and logistical factors.
While pharmacological interventions such as doxycycline, beta-
blockers, statins and angiotensin pathway inhibitors to reduce AAA
Ta b le 1. AAA r isk fa ct ors
Advancing age
Male gender
Smoking
Family history
Atherosclerosis
Hypertension
Hypercholesterolaemia
Other vascular aneurysm
Ta b le 2. 12 m on t h AAA ru p t u re r isk b y d ia m et er
AAA diameter (cm) Rupture risk (%/year)
3.0–3.9 0%
4.0–4.9 1%
5.0–5.9 1–10%
6.0–6.9 10–22%
>7.0 30–50%
Ta b le 3. Su g g est ed AAA su rveilla n ce in t erva ls
AAA diameter (cm) Surveillance interval (months)
3.0–3.9 24
4.0–4.5 12
4.6–5.0 6
>5.0 3
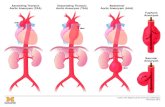
Figure 1. Anatomical reference terms for aor tic segments
Image cour tesy Cook Medical, Bloomington NH, USA
Ascending aorta, aortic root
Visceral segment suprarenal aorta
Iliac segment
Infrarenal aorta
Supra-aortic trunks and aortic arch
Descending thoracic aorta (distal to left subclavian artery)
Reprinted from AUSTRALIAN FAMILY PHYSICIAN VOL. 42, NO. 6, JUNE 2013 365
growth and rupture have been promising in animal models, these benefits
have not been consistently reproduced in human studies. 21,22 Optimal
cardiovascular risk factor management should include smoking cessation,
a statin, antiplatelet and antihypertensive agents to improve life
expectancy by reducing cardiovascular mortality. In addition to preventing
aneurysm related mortality, screening for AAA will identify patients with
small aneurysms who are at increased risk of cardiovascular events and
who will benefit from cardiovascular risk management.
Many patients are under the misapprehension that a diagnosis of
small AAA prohibits them from physical activity, or overseas travel.
There is no evidence that physical activity leads to aneurysm rupture.
The role of imaging
A number of imaging modalities are available for the assessment of
AAA. Ultrasound is a relatively cheap, non-invasive, widely available
and reliable tool for detecting and measuring AAAs. It is the modality
of choice for screening and surveillance.
Computed tomography angiography (CTA) is fast, reproducible
and accurately depicts aneurysm morphology. It is the investigation
of choice when considering potential surgical repair, and enables
multiplanar analysis. Limitations include the need for high doses of
intravenous iodinated contrast (potentially harmful in patients with
renal impairment), and the use of ionising radiation.
Magnetic resonance angiography has limited utility in AAA.
Digital subtraction angiography has largely been replaced by CTA for
preoperative planning. It is now predominantly reserved for therapeutic
purposes and is utilised during endovascular aneurysm repair (EVAR) to
accurately position the stent graft before deployment.
In AAA surveillance imaging, the most relevant dimension is the
maximum transverse diameter, ideally perpendicular to the line of
flow. Aneurysm length is often reported, but is not relevant, except in
planning for surgical repair. The presence of mural thrombus is also
generally not relevant unless the aorta is critically stenosed or there is
evidence of distal embolisation.
demonstrated to reduce aneurysm related mortality in four large trials,
including one performed in Western Australia.2–5,12 Considerable
variation exists between international screening protocols, with
concerns over value and effectiveness.13,14 Cost effectiveness may
be increased by screening those at particularly increased risk of AAA,
such as men with a history of smoking, or patients with atherosclerotic
risk factors or a family history of AAA. More rigorous risk scoring
systems are being explored.15
First degree family members of patients with a diagnosed AAA
are at higher lifetime risk of developing a AAA of up to 20%, 16–18
however, the genetic mechanisms for this are unclear.19 Previous
recommendations have included screening for men and women over 50
years of age with a family history of AAA.20
The majority of AAAs detected with screening are below the
threshold for elective repair. The management of patients with small
AAA most importantly includes cardiovascular risk management
with lifestyle advice, smoking cessation, pharmacotherapy (anti-
hypertensives, statins, beta-blockers), and ongoing aneurysm
surveillance. Suggested surveillance intervals are listed in Table 3,
although local protocols may vary based on accessibility to imaging,
patient preference and logistical factors.
While pharmacological interventions such as doxycycline, beta-
blockers, statins and angiotensin pathway inhibitors to reduce AAA
Ta b le 1. AAA r isk fa ct ors
Advancing age
Male gender
Smoking
Family history
Atherosclerosis
Hypertension
Hypercholesterolaemia
Other vascular aneurysm
Ta b le 2. 12 m on t h AAA ru p t u re r isk b y d ia m et er
AAA diameter (cm) Rupture risk (%/year)
3.0–3.9 0%
4.0–4.9 1%
5.0–5.9 1–10%
6.0–6.9 10–22%
>7.0 30–50%
Ta b le 3. Su g g est ed AAA su rveilla n ce in t erva ls
AAA diameter (cm) Surveillance interval (months)
3.0–3.9 24
4.0–4.5 12
4.6–5.0 6
>5.0 3
Figure 1. Anatomical reference terms for aor tic segments
Image cour tesy Cook Medical, Bloomington NH, USA
Ascending aorta, aortic root
Visceral segment suprarenal aorta
Iliac segment
Infrarenal aorta
Supra-aortic trunks and aortic arch
Descending thoracic aorta (distal to left subclavian artery)
-
17
5.4 Growth rates for AAA [10]