Animal models and the search for anti- rheumatic drugs Objectives To understand the problems with...
-
Upload
frank-west -
Category
Documents
-
view
213 -
download
0
Transcript of Animal models and the search for anti- rheumatic drugs Objectives To understand the problems with...
Animal models and the search for anti-rheumatic drugs
ObjectivesTo understand the problems with existing therapies
for RAThe reasoning behind screening cascades and the
position of in vivo pharmacologyTo appreciate the specific problems related to animal
experimentationTo understand how to manipulate in vivo models
(immune/non immune etc)To give an example of a tertiary model of arthritis its
similarities and differences to RA
Diagnosis of rheumatoid arthritis
Morning stiffness 1h
Three or more joints involved
Arthritis of hand joints
Symmetric arthritis
Rheumatoid nodules
Rheumatoid factor (positive < 5% normal subjects)
Radiographic changes (must show erosion/decalcification)
Present for 6wk
Any 4 of the following must be present to allow diagnosis of RA:-
Exper
imental
therapy
High dose
corticosteroids
Cyclophosphamide
Methotrexate, azathiaprine
Hydroxychloroquine
Sulphasalazine
Gold
salts
NSAIDs Low dose prednisone
Patient
education
Physical
therapy
Occupational
therapy
High dose
salicylates
Sur
gery
Intra-articular corticosteroids
Traditional pyramid for treatment of RA
Assumptions:-
1) RA relatively benign disease
2) NSAIDs less toxic than DMARDs
Sawtooth strategy for treatment of RA
0 5 10 15 20 25
Dis
ab
il ity
i nd
ex
Years
Typical course of disease
Sawtooth course of disease
DMARD
1 4 532 6 7
Current therapies: Treatment cascade & limitations
NSAIDs
Volterol, Vioxx, Celebrex(GI ulceration & Bleeding. Not DMARD)
DMARDs
Methotrexate, Leflunamide, HydroxychloroquineEfficacy (refractory), Safety – Myelosuppression, Hepatic toxicity
BIOLOGICALSEnbrel, infliximab, Adalimumab, Anakinra (IL-1r)Expensive, inconvenient Admin, Partial/Non-responders?,
Safety – TB, Op infection, CHF, Demyelinating Disease, Lymphoma
NCEs and NBEs
• NCEs generally work well across species– tend to target well-conserved sites eg enzyme
active site
• NBEs are often species specific– Large molecular interactions are less likely to be
well-conserved– May need parallel reagent for animal models with
final testing of human reagent in primates– Use human transgenic animal– Use human explant tissue
Screening cascade for NCEIsolated protein
Functional cell assay
Ex vivo assay
In vitro toxicology
CYP induction/inhibition
Mini Ames
Micronucleus test
In vitro Metabolism
Microsomal stability
CaCO-2 studies
In vivo PK
In vivo efficacy
Primary model
Secondary model
Tertiary model
Counter screens for selectivity
In vitro studies isolated target and murine cell systems
Single dose PK
Medium term secondary models
Tertiary disease model
Furry test tube
Primary model
Multidose PK
Screening cascade for NBEs: concept ofparallel reagents
In vitro studies isolated target and human cell systems
Human explant studies?
Furry test tube?
Hu-Mouse transgenic?
Primate studies
Murine parallel reagent Human therapeutic
Clinical trial
Animal models: considerations for use and interpretation of data
• Ethical concerns: When to use. Distress scoring.
• Legal: Licensing issues.
• Practical concerns: Is this a good experiment?
• Statistical concerns: Group sizes. Most appropriate
analysis. What to do with data from culled animals.
Effects of compound on plasma TNF 90 mins after challenge with LPS
0 3 10 300
10
20
30
mg/kg p.o.
***
Pla
sma
TN
F n
g/m
l
Paw oedema
•Sub plantar injection of irritant eg carrageenan
•Reaction usually maximal by 3 hours
•Readily quantified by plethysmometry
•Not an arthritis
•Not amenable to histological or biochemical assay
•End point is crude
•Very popular in industry!
Disadvantages
Measurement of paw oedema using plethysmometry
Results expressed as paw volume (ml)
or
change in paw volume (Δml)
Cavity models
•Peritonitis
•Pleurisy
•Air pouches
•Exudate volume
•Total and differential cell counts
•Amenable to biochemistry
Advantages
Effects of experimental compound on exudate volume recovered from a 4 hour carrageenan
pleurisy in the rat
- Vehicle 0.1 1 100.00
0.25
0.50
0.75
+1%carrageenan
(mg/kg)
**
***
***Ex
ud
ate
vo
lum
e(m
l)
Means s.e.m., n=8/9 per treated group, n=4 in untreated groupStatistical analysis: One way ANOVA followed by a Bonferroni's multiple comparisons test** p<0.01, ***p<0.001comparison with vehicle control
Vehicle= 10% DMSO and 90% LabrafilDrugs were administered 30 mins beforecarrageenan injection
Effects of experimental compound on total cells recovered from a 4 hour carrageenan
pleurisy in the rat
- Vehicle 0.1 1 100
10
20
+1%carrageenan
(mg/kg)
****
***
***
To
tal c
ells
(x1
06)
Means s.e.m., n=8/9 for treated groups, n=4 in untreated groupStatistical analysis: One way ANOVA followed by a Bonferroni's multiple comparisons testp<0.05, ***p<0.001comparison with vehicle control
Vehicle= 10% DMSO and 90% LabrafilDrugs were administered 30 mins beforecarrageenan injection
Air pouches
•No interfering visceral organs
•Pouch lining cells have similarities to synovial cells
•Prolonged inflammation can be induced
Disadvantages
•Preformed cavities (6-day-old-pouches) give best results
Accumulation of cells in a murine 6-day-old air pouch in response to LPS
0.000 0.025 0.050 0.075 0.100 0.125
0
5
10
LPS mg/air pouch
To
tal
cell
s x1
06
Manipulation of cavity models
•Non-immune models-complement dependent
-complement independent
•Immune models-active (sensitisation and challenge)
-passive (adoptive transfer of serum or cells)
Models of arthritis
•Polyarthritis
•Monoarticular arthritis
-Adjuvant arthritis-Collagen II arthritis
•Matrix degradation
-non-immune-Immune
Collagen induced arthritis
• Susceptibility linked to MHC
• immunologically mediated
• Erosions of cartilage & bone
• Autoimmunity to collagen seen in some RA patients
• Arthritic rather than multisystem
• Disease is induced• The model “resolves”• New bone formation
more marked• No synovial vasculitis• No cycles of relapse &
remission• No RF• No sex predilection
Similarities to RA Differences to RA
Collagen induced arthritis
•Animals (rats or mice) sensitised to collagen ll in adjuvant
•Booster injection (mice)
•Distress scoring
•Examine for clinical signs
•Measurement of hind paw volume (rats)
•Histology
Clinical assessmentDisease scoreBody weightAnalgesia
ImmunologyCII AbEx vivo lymphocyte proliferationHypersensitivity to CII
Serum markers of disease progressionAcute phase proteinCOMPBone sialoprotein
Assessment of CIA
Joint pathologyHistologyRadiographs
Drug specificPlasma levelsBioactivity markers
Clinical presentation of murine CIA
Score 1 2 3
Pictu
res Rem
i Ok
oye
NORMAL WRIST SWOLLEN WRIST + PAD SWOLLEN
WRIST + PAD + DIGITS SWOLLEN
0
Effects of an experimental compound onrat CIA
0 5 10 15 20 250.9
1.0
1.1
1.2
1.3
1.4 Vehicle0.03mg/kg0.3mg/kg3mg/kg10mg/kg
Days post sensitisation
Paw
vo
lum
e (m
l)
Vehicle 0.03 0.3 3 100
1
2
3
**
**
mg/kg u.i.d.
AU
C p
aw v
olu
me
(Day
s 14
-24)
Paw volumes Change in paw volumesAUC Days 14-24
Means s.e.m., n=10/groupStatistical analysis ANOVAWith Bonferroni post test**p<0.01 vs Vehicle
Effects of leflunomide on murine CIA
VehicleLeflunomide
Animals dosed day -1Leflunomide 3mg/kgPO once dailyVehicle = 1% MethylCelluloseDBA/1 mice N = 15
12 14 16 18 20 22 24 26 28 30 32 34 36 38 400
2
4
6
8
**
Day
Clin
ica
l sc
ore
P= <0.05 Day 31/32 *P= <0.01 Day 30/33-36**
Dosing strategies in CIA
Clin
ica
l sc
ore
SensitisationB
oost
Prophylactic dosing
Therapeutic dosing
Dosing through sensitisation period
Time
CIA Histopathology
Score 3 Score 0
0 normal1 inflammatory cell influx, 2 cell influx & focal erosion cartilage & bone 3 loss of joint architecture
Serological markers
• Anticollagen II antibodies and their isotypes
• Acute phase proteins
• Cartilage oligomeric matrix protein (COMP)
• Bone sialoprotein
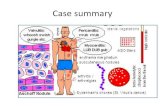
Summary
• There remains an unmet need in the treatment of RA
• In vivo models of inflammation are an essential part of drug discovery
• The strategy for NCEs and NBEs is different• Efficacy in models helps define species for
toxicology• Models can help with calculation of therapeutic
dose in man and with identifying surrogate markers of drug activity for use in the clinic