An in Vitro Hydroxyl Radical Generation Assay for Microdialysis Sampling Calibration
Transcript of An in Vitro Hydroxyl Radical Generation Assay for Microdialysis Sampling Calibration

Analytical Biochemistry 306, 40–49 (2002)
An in Vitro Hydroxyl Radical Generation Assayfor Microdialysis Sampling Calibration
Rui Chen and Julie A. Stenken1
Department of Chemistry, Rensselaer Polytechnic Institute, Cogswell Laboratories,110 8th Street, Troy, New York 12180-3590
A xanthine oxidase hydroxyl radical (�OH)-generat-ing system was created for sustained in vitro produc-tion of �OH. This assay was coupled with microdialysissampling to elucidate the factors that influence micro-dialysis calibration during radical trapping. A �OHtrapping agent, 4-hydroxybenzoic acid, was includedeither in the microdialysis perfusion fluid or in themedium external to the microdialysis probe. Xanthineoxidase enzymatic activity was reproducible and hadan average activity measured by UV absorbance ofproduced uric acid of 0.037 � 0.005 �AU/min (n � 5). Aconsiderable amount of variance in the rate andamount of the product, 3,4-dihydroxybenzoic acid (3,4-DHBA), was observed when one microdialysis probewas placed in the reaction mixture. When two micro-dialysis probes were placed in the reaction mixture, agreater rate and amount of 3,4-DHBA was observed.Different concentrations of 3,4-DHBA were obtainedbetween quiescent and stirred systems. © 2002 Elsevier
Science (USA)
Key Words: microdialysis calibration; hydroxyl radi-cal trapping; 4-hydroxybenzoic acid.
Recently there has been an explosion of researchinterest in free radical production in biological sys-tems. Oxygen free radicals (OFRs)2 are suspected ofplaying a central role in pathological processes, includ-ing Parkinson’s disease, cancer, and aging (1, 2). Hy-droxyl radical (�OH) readily reacts with many impor-tant biomolecules, including DNA, membrane lipids,proteins, carbohydrates, and a variety of low-molecu-
1 To whom correspondence should be addressed. Fax: (518) 276-4887. E-mail: [email protected].
2 Abbreviations used: OFR, oxygen free radical; DHBA, dihydroxy-
tion; HX, hypoxanthine; XO, xanthine oxidase; AH2, ascorbic acid.
40
lar-weight species (3, 4). Reaction of �OH not only dam-ages these molecules, but also leads to the productionof cytotoxic species.
Despite the large amount of research literature im-plicating OFRs as initiators of various disease pro-cesses, there have been few accounts that give an ac-curate and thorough description of the quantitativerelationship between the concentration of OFRs andthe initiation of the disease process. A principal reasonis due to OFRs’ high reactivity in biological systemsand their low free concentrations in biological tissue.
To assess the damaging role of �OH, it is importantto be able to measure �OH concentration accurately.Hydroxyl radical is extremely reactive and its reactiv-ity is under diffusion control, which makes quantita-tive determination of �OH difficult. �OH reacts at diffu-sion-controlled rates (typical second-order rateconstant of 109 to 1010 M�1 s�1) with molecules in itspath. With a short half-life (10�10 s), �OH diffuses onlya few angstroms from its site of production before re-acting with neighboring molecules (4).
Direct detection of �OH can be achieved only by usingelectron spin resonance (ESR), but the transient andreactive nature of �OH makes direct detection unreal-istic (5, 6). Therefore, indirect detection by trappingwith a spin trap is the preferred method for �OH detec-tion. Indirect ESR detection of �OH can be extremelydifficult due to the insensitive detection (micromolarconcentrations of trapped radical species are often re-quired), expertise in operating the ESR equipment,potential toxicity of spin traps, and possibility for nu-merous secondary reactions of the �OH trapped spinadduct (6, 7).
Because ESR requires extensive expertise and canhave artifact problems, indirect methods of �OH detec-tion are commonly applied to free radical research. Acommon indirect method for �OH detection is aromatic
Received October 15, 2001; published online June 5, 2002
benzoic acid; HBA, hydroxybenzoic acid; EC, electrochemical detec-
doi:10.1006/abio.2001.5702
hydroxylation, in which an aromatic compound scav-
0003-2697/02 $35.00© 2002 Elsevier Science (USA)
All rights reserved.

enger is used to trap �OH. Salicylic acid is a commonlyused aromatic trap. Salicylic acid reacts with �OH toproduce the o-,p-directed hydroxylated products 2,3-dihydroxybenzoic acid (2,3-DHBA), 2,5-dihydroxyben-zoic acid (2,5-DHBA), and catechol in a ratio of 49:40:11%, respectively (8). One of the problems withsalicylic acid for in vivo �OH detection is that 2,5-DHBA is a cytochrome P450 metabolite of salicylic acid(9). Because of the oxidative metabolism that salicylicacid can undergo, 4-hydroxybenzoic acid offers an al-ternative to salicylic acid trapping. 4-Hydroxybenzoicacid (4-HBA) reacts with �OH to produce 3,4-dihydroxy-benzoic acid (3,4-DHBA) and other minor productssuch as hydroquinone and resorcinol (10–12). High-performance liquid chromatography coupled with elec-trochemical detection (HPLC-EC) is applied to sepa-rate and quantify the hydroxylated products (8, 13).Although this method is highly sensitive compared tothe ESR spin trapping method (nanomolar versus mi-cromolar detection limits), there are concerns associ-ated with its use in biological systems, including issuesof multiple reaction products from salicylic acid (cate-chol, 2,3-DHBA, and 2,5-DHBA) and the potential cel-lular toxicity of the trapping agent at concentrationsneeded for efficient reaction with �OH (14, 15).
Microdialysis sampling is a well-known in vivo sam-pling technique that gives protein-free samples thatcan be directly analyzed by various analytical separa-tion techniques (16–18). Microdialysis sampling pro-vides a relative measure of the analyte concentrationwithin the vicinity of the microdialysis probe. Therehave been numerous reports that have coupled in vivomicrodialysis sampling to studies of radical trappingvia aromatic hydroxylation for studies in neuroscience(19–24). However, there have been no attempts to fullyreconcile or understand the calibration of the microdi-alysis probe during a radical trapping event.
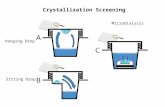
During microdialysis sampling coupled with indirectdetection of �OH via aromatic hydroxylation, the trap-ping agent can be placed either in the microdialysisperfusion fluid, and locally applied to the extracellularfluid space, or external to the microdialysis probe via asystemic injection as shown in Fig. 1. From the per-spective of microdialysis sampling, these two experi-mental protocols are entirely different with respect tothe calibration of the microdialysis probe (25, 26). InFig. 1B, the trapping agent (4-hydroxybenzoic acid forexample) is included in the microdialysis perfusionfluid and locally delivered to the sample space externalto the probe. The amount of material that is locallydelivered to that site will be a function of the extractionefficiency, E d, of the microdialysis probe described inEq. [1]:
Ed �Cinlet � Coutlet
Cinlet � Csample� 1 � exp��
1Qd�Rd � Rm � Rs�
� .
[1]
E d is highly dependent upon probe and sample pa-rameters including flow rate (Q d) and mass transportresistances through the dialysate, membrane, andsample (R d, Rm, and R s, respectively) (27). Mass trans-port resistances are highly dependent upon the diffu-sion and kinetic properties of the sample. During atrapping experiment, illustrated in Fig. 1B, theamount of trapping agent delivered to the implantationsite will be highly dependent upon the tissue propertiessuch as uptake kinetics and the microdialysis perfu-sion fluid flow rate. In this scenario, the trapping agenthas to be delivered to the local site, react with �OH, andthen diffuse back to the microdialysis probe. In otherexperiments in which a local delivery is performedduring microdialysis experiments, the amount of con-
FIG. 1. Diagram of microdialysis trapping with �OH generation external to the microdialysis probe. The trapping agent 4-HBA can beincluded in either (A) the external medium or (B) the perfusion fluid.
41MICRODIALYSIS CALIBRATION FOR HYDROXYL RADICAL TRAPPING

verted material during microdialysis sampling is verysmall and often less than 1% of the infused concentra-tion for enzymatic conversions of hypoxanthine viaxanthine oxidase in vitro (26) and substance P in vivo(28). In addition to this limitation, the concentration oftrapping agent that is released to the sample spaceexternal to the microdialysis probe is related to probeE d. Probe E d is highly influenced by perfusion fluid flowrates and membrane lengths. This is in contrast to thesetup illustrated in Fig. 1A in which the concentrationof the trapping agent would be finite at an infinitedistance from the probe. Moreover, the concentrationprofile of the trapping agent around the microdialysisprobe will be different between Fig. 1A and Fig. 1B.
Figure 2A shows the concentration profiles aroundthe probe when the radical trap is included in thesample medium (bolus injection of trapping agent) orin the microdialysis perfusion fluid (25). When thetrapping agent is included in the sample medium, itsconcentration is high away from the probe and thendecreases near the probe. On the other hand, when thetrapping agent is included in the perfusion fluid, itsconcentration is high near the probe and diminishedfar away from the probe. Figure 2B shows normalizedconcentration profiles for the products between thetrapping agent and the hydroxyl radical. In this case,the product concentration profile for a trap that isincluded in the external medium has the same profileas the trap itself. However, the product concentrationprofile for a delivery of trapping agent shows a differ-ent profile. In this case, product can diffuse either awayfrom the probe or back into the probe, thus giving aconcentration profile that is maximized slightly awayfrom the microdialysis probe.
The trapping of hydroxyl radical via reaction with atrapping agent such as salicylic acid is becoming awell-established procedure in the field of microdialysissampling. There has been no empirical or theoreticalanalysis published that relates the concentration of theindirectly trapped products to an approximate level ofoxidative stress at the site external to the microdialysisprobe. In many papers, only qualitative informationabout �OH based on the concentration change of thetrapped products without specific information relatingto the recovery of the analyte is reported. Microdialysissampling is a technique whose calibration is highlydependent upon the fluid parameters; different envi-ronments (tissue) will affect the local concentration(distribution profile) of the radical as well as clearanceof the trapping agent and product, thus making theestimation of �OH level rather difficult.
In this paper we have described in vitro methods toaddress this microdialysis calibration issue during �OHproduction and trapping. A hypoxanthine/xanthine ox-idase (HX/XO) system provided sustained productionof �OH. Different concentrations of the trapping agent,4-HBA, were included either in the perfusion fluid or inthe medium external to the microdialysis probe.
MATERIALS AND METHODS
Chemicals
4-HBA, 3,4-DHBA, allantoin, hypoxanthine, xan-thine, uric acid, Chelex-100 resin, and 1-octanesulfonicacid were purchased from Sigma Chemical (St. Louis,MO). Ascorbic acid was purchased from Fisher Scien-tific (Pittsburgh, PA). XO suspension (20 unit/ml) wasfrom Roche Molecular Biochemicals (Indianapolis, IN).
FIG. 2. Normalized concentration profiles of trap and product during microdialysis sampling. (A) Concentration profile for the trap whenit is included on the outside of the microdialysis probe (solid line) or inside the microdialysis probe (dashed line). (B) Concentration profilefor the product when the trap is included outside the microdialysis probe (solid line) or inside the microdialysis probe (dashed line).
42 CHEN AND STENKEN

KH2PO4/KOH, pH 7.4, buffer (0.15 M) was treated withChelex-100 resin overnight before use. XO stock solu-tions (4 unit/ml) were prepared in a 0.02% sodiumsalicylate solution and kept in the refrigerator untiluse. A KH2PO4/KOH, pH 7.4, buffer (0.15 M) was usedto dilute the stock solution to 0.4 unit/ml each day.Ringer’s solution was prepared in-house and contains155 mM NaCl, 5.4 mM KCl, and 2.4 mM CaCl2. Allother chemicals were reagent grade or better.
Chromatographic Conditions
Dihydroxybenzoic acids in dialysate samples wereanalyzed by HPLC-EC. The HPLC-EC system con-sisted of a Hypersil C18 column (5 �m, 250 � 2.00 mm)with an Ultracarb ODS 20 (4 �m, 30 � 2.00 mm) guardcolumn (Phenomenex, Torrence, CA). The mobilephase was delivered at 0.2 ml/min and contained so-dium citrate (pH 2.6, 0.1 M) and methanol, 80/20 v/v%.Amperometric electrochemical detection was per-formed using a Decade detector (Antec, Leyden, TheNetherlands) with the potential set to 800 mV vs Ag/AgCl.
An HPLC-UV system was used to analyze the sub-strate and reaction products of xanthine oxidase, in-cluding hypoxanthine, xanthine, and uric acid, duringmicrodialysis experiments. For HPLC-UV analyses(ThermoSeparations Products, San Jose, CA) an AquaC18 column (5 �m, 150 � 2.00 mm; Phenomenex, Tor-rance, CA) was used. The mobile phase for HPLC-UVanalyses was a mixture of citric acid (30 mM) andsodium acetate (27.7 mM) adjusted to pH 4.75 withmethanol (95/5, v/v%). The flow rate was 0.2 ml/min.The UV detector was set to 254 nm.
Allantoin was measured by HPLC-UV using a mod-ification of a previously published method (29). A King-sorb C18 column (5 �m, 150 � 4.60 mm; Phenomenex)was used with a flow rate of 1.0 ml/min. The mobilephase consisted of a 5 mM potassium dihydrogen phos-phate buffer containing 5 mM 1-octanesulfonic acidand adjusted to pH 3.1 using phosphoric acid. The UVdetector was set to 210 nm.
Background Subtraction of 3,4-DHBA
Injections of control dialysates containing 4-HBA,but not subjected to �OH generation, gave backgroundlevels of 3,4-DHBA. Background concentrations of 3,4-DHBA were determined daily by triplicate injection ofcontrol dialysates. Control dialysates vary from exper-iment to experiment because of the different types ofprotocols used in these studies. For example, when4-HBA was included in the perfusion fluid prior to �OHgeneration experiments, the controls consisted of infus-ing the 4-HBA through a solution containing all the�OH generation reagents except the reagents needed
for the enzymatic reaction (hypoxanthine and xanthineoxidase). When experiments were performed with4-HBA external to the probe, the control experimentincluded all the �OH generation reagents plus 4-HBA,but without hypoxanthine. The graphs depicting theresults of 3,4-DHBA production during �OH generationstudies have the 3,4-DHBA control background concen-tration subtracted.
Ascorbate Test for Catalytic Metals
To determine the presence of catalytic metals in var-ious buffers and mobile phases used in our samplepreparation, an ascorbate test method described byBuettner was used (30). To perform this assay, 3.8 �lascorbic acid solution (0.1 M) was added to 3.00 ml ofthe sample solution to be tested. Absorbance of thissolution is observed for 15 min at 265 nm using aHitachi U-2000 spectrophotometer. For a successfullydemetaled buffer, the loss of absorbance in 15 minshould be within 0.5% (30). A greater loss in absor-bance indicates that a significant concentration of cat-alytic metal remains.
Xanthine Oxidase Activity Monitoring
The increase in absorbance at 290 nm, caused by theoxidation of xanthine to uric acid, is a measure of thecatalytic activity of xanthine oxidase (31). To measurethis activity 950 �l KH2PO4/KOH, pH 7.4, buffer (0.15M), 50 �l xanthine oxidase solution (0.4 unit/ml from 4unit/ml stock), and 500 �l sample solution containingeither distilled–deionized water for control or 0.15 mMxanthine solution were pipetted into a quartz cuvette.The increase in absorbance at 290 nm was monitoredusing a Hitachi U-2000 spectrophotometer. �AU/minwas calculated using data obtained within the first 5min (linear range).
Hypoxanthine/Xanthine Oxidase �OH GeneratingSystemHydroxyl radical was produced by using a previously
described method with xanthine oxidase (32, 33). Tocreate this �OH-generating system, solutions prepareddaily consisting of 30 �l EDTA (5 mM), 30 �l FeCl3 (5mM), 150 �l HX (2 mM), 150 �l 4-HBA (20 mM), and1.11 ml KH2PO4/KOH, pH 7.4, buffer were mixed in a1.5-ml microcentrifuge tube. The reaction was initiatedby addition of 30 �l XO solution (0.4 unit/ml). Thesolution was gently agitated and a microdialysis probewas immersed into it. Reactions were carried out atambient temperature, which averages between 22 and23°C.
MicrodialysisMicrodialysis experiments were performed using a
nonmetal syringe (Hamilton Syringes, Reno, NV) with
43MICRODIALYSIS CALIBRATION FOR HYDROXYL RADICAL TRAPPING

nonmetal microdialysis probes with either 2 (BR-2) or4 mm (BR-4) of exposed membrane (Bioanalytical Sys-tems, Inc., West Lafayette, IN). The use of nonmetalparts during microdialysis sampling is extremely im-portant and has been described in the literature (21).The probes were perfused with either Ringer’s solutionor Ringer’s supplemented with 4-HBA. The flow ratewas 1.0 �l/min.
The microdialysis probes were calibrated at ambienttemperature in unstirred media. To calibrate theprobes 3,4-DHBA, hypoxanthine, xanthine, or uric acidat 10 �M was included in the sample medium externalto the probe.
The 4-HBA trapping agent was included either inthe �OH-generating system or in the perfusion fluid asshown in Fig. 1. In these experiments with only onemicrodialysis probe in the �OH-generating system, a2-mm probe was used. During experiments with4-HBA included in the �OH-generating system, the4-HBA concentration was 2 mM and the perfusion fluidconsisted of Ringer’s solution. In other experimentswith the �OH-generating system, 4-HBA was includedin the perfusion fluid to a final concentration of 20 mM.All collected dialysates were then diluted 1:10 withRinger’s solution and directly injected into the HPLC-EC. Concentrations of 3,4-DHBA were not corrected forthe relative recovery of the microdialysis probe.
In some experiments, two microdialysis probes withdifferent membrane lengths were placed into the �OH-generating system such that both probes were touchingas shown in Fig. 3. This gives a distance of 6 mmbetween the cannulas of both probes. Dialysates ob-tained from one probe were diluted 1:10 and wereanalyzed by HPLC-EC for 3,4-DHBA content. Dialy-sates obtained from the other probe were analyzed byHPLC-UV for hypoxanthine, xanthine, and uric acidcontent. Note that although hydroquinone can be pro-
duced during hydroxyl radical attack of 4-HBA (11, 12),production of hydroquinone was not observed with thisassay.
RESULTS AND DISCUSSION
An important analytical consideration during hy-droxyl radical trapping with microdialysis sampling isto ensure that nonspecific production of dihydroxyben-zoic acids is either eliminated or significantly reduced(21). This nonspecific production of dihydroxybenzoicacids during microdialysis sampling can come frommetals leached from syringe tips or cannulas. To pre-vent artifactual 3,4-dihydroxybenzoic acid productionduring trapping with 4-hydroxybenzoic acid, nonmetalsyringes and nonmetal microdialysis probes were used.Stringent tests were performed to ensure that catalyticmetal ions were not present in significant quantities inour experimental system. To ensure this, we used anascorbic acid assay previously described by Buettner(30). This assay is based upon the knowledge thatascorbic acid (AH2) is a diacid with pK a values of 4.2and 11.6. As long as a solution is not very acidic (muchless than pH 3.2) or extremely basic (greater than12.6), AH2 will have an equilibrium concentration ofAH� in solution. AH� rapidly decomposes in the pres-ence of metals, thus driving the chemical reaction forthe decomposition of AH2.
Table 1 shows the absorbance changes at 265 nm(�AU265 nm) for distilled–deionized water (Nanopure),Ringer’s solution, the phosphate buffers, and the mo-bile phase before and after it enters the HPLC column.Both Nanopure water and Ringer’s solution werewithin the 0.5% limit as defined by Buettner (30) andcould be used without treatment of the cation-ex-change resin, Chelex-100. The results of the ascorbicacid test for the HPLC mobile phase indicate thatartifactual production of 3,4-DHBA may occur on-col-umn. The potassium phosphate buffer had significantactivity with respect to the ascorbic acid test and hadpercentages as high as 2%. After an overnight Chelex-100 resin treatment, some of the catalytic metals were
TABLE 1
The Ascorbic Acid Oxidation Test Data
Sample Absorbance change % (n � 3)
Nanopure water �0.20 � 0.45Ringer’s solution �0.24 � 0.15Mobile phase, before column �1.53 � 0.12Mobile phase, after column �2.03 � 0.32Buffer (no treatment) �1.67 � 0.25Buffer (overnight resin treatment) �1.80 � 0.52
Note. A positive sign indicates essentially no change in ascorbicacid reduction. A negative sign indicates the anticipated change inascorbic acid reduction.
FIG. 3. Diagram of the two-probe microdialysis system. The per-fusion fluid flow rate through both probes is 1.0 �l/min. Dialysatesfrom Probe A are diluted 1:10 with Ringer’s and analyzed by HPLC-EC. Dialysates from Probe B are directly injected into an HPLC-UVsystem.
44 CHEN AND STENKEN

removed from the phosphate buffer and the �AU265 nm
was reduced to less than 2%, which was not within the0.5% limit suggested by Buettner. After a second over-night treatment with Chelex-100 resin, the change inabsorbance was not further reduced, which could causepotential difficulty with backgrounds of 3,4-DHBA inthe samples.
To ensure that nonspecific production of hydroxylradical or 3,4-DHBA was not occurring in our microdi-alysis setup, several control experiments were per-formed. In these experiments, 20 mM 4-HBA was per-fused through a microdialysis probe immersed in asolution containing the phosphate buffer and all thereagents except xanthine oxidase and hypoxanthine.Figure 4 shows the typical control data obtained duringa time period equivalent to that during hydroxyl radi-cal generation. This background concentration of 3,4-DHBA appears to be inherent to the 4-HBA used inthese experiments as this concentration level of 3,4-DHBA is similar to that of 4-HBA not perfused throughthe microdialysis probe. In three separate preparationsof 20 mM 4-HBA in the microdialysis perfusion fluid,the average percentage of the 3,4-DHBA impurity wasfound to be 0.0086 � 0.0006%. During these prepara-tions and throughout the experimental procedures carewas taken to ensure that catalytic metals were not inthe buffers by ion-exchange (Chelex) treatment and byspurious washing of glassware with nitric acid.
Production of �OH
There are a variety of methods that can be used togenerate �OH in aqueous solutions. These methods in-clude �-radiolysis, X rays, and Fenton chemistry. Be-cause �-radiolysis and X rays require special equip-ment, Fenton chemistry was used to generate �OH forthese experiments. Fenton chemistry requires hydro-gen peroxide (H2O2). The addition of H2O2 can beachieved by either direct addition of H2O2 or produc-tion of H2O2. H2O2 can be produced either electrochem-ically via the reduction of molecular oxygen to H2O2
(12, 34) or by using an oxidase enzymatic system.There are a variety of oxidase enzymes available. Weinitially chose to use glucose oxidase because it doesnot produce products that are known to scavenge �OH.Initial experiments with glucose oxidase showed no�OH production. For this reason, we chose to use xan-thine oxidase despite the possibility of �OH scavengingby uric acid. Xanthine oxidase has been widely used infree radical research as a means to produce �OH radi-cals (35, 36).
Xanthine oxidase produces a reliable source of su-peroxide radicals along with H2O2 as shown in Scheme1 (33, 37). Once superoxide is produced, it reacts withFe3� in the solution to produce Fe2�. Fe2� then reactswith H2O2 in the system via classic Fenton chemistryto produce �OH. As long as XO is able to turnoverhypoxanthine to xanthine or xanthine to uric acid andsubsequently produce superoxide and H2O2, this sys-tem will continuously produce �OH. Based on the stoi-chiometry of the reaction and the initial starting con-ditions of 200 �M (total) hypoxanthine, the maximumamount of hydroxyl radical anticipated would be 400�M assuming that hydroxyl radical reacts only withthe excess amount of trapping agent (4-HBA) in thesolution. However, this maximum amount is notachieved because of the considerations of competitionkinetics of �OH with other chemicals in the reactionsystem (38). This is especially true for uric acid, aknown hydroxyl radical scavenger (39).
In order to ensure a reliable hydroxyl radical produc-tion, the catalytic activity of XO was monitored each
SCHEME 1. Hydroxyl radical-generation system mechanism forxanthine oxidase.
FIG. 4. 3,4-DHBA control absolute concentrations during microdi-alysis perfusion (20 mM 4-HBA inside the probe) in a solution con-taining all reagents except xanthine oxidase. A single 2-mm probewas used with a 1.0 �l/min flow rate.
45MICRODIALYSIS CALIBRATION FOR HYDROXYL RADICAL TRAPPING

day prior to use. For five XO solutions of 0.4 unit/mlprepared on 5 consecutive days from the same 4unit/ml stock solution, the absorbance change as ameasure of enzyme activity was 0.037 � 0.005 �AU/min, indicating little change in enzyme activity usedfor a stock solution stored for a longer period of time.
With a reproducible turnover of the xanthine oxidasesystem from day to day, we used this system coupled
with radical trapping during microdialysis sampling.Figure 5 shows the compilation of three separate ex-periments for hydroxyl radical trapping with one mi-crodialysis probe immersed in the enzyme solution.One problem that was observed was that although theenzyme system reproducibly turned over to give thesame activity from run to run, the resultant concentra-tion increases and production kinetics of 3,4-DHBAwere different from run to run for more than the threedata sets illustrated in Fig. 5. The reason for thesediscrepancies may be due to stochastic interactionsbetween the hydroxyl radical and different componentsin the solution such as the hypoxanthine, xanthine, oruric acid. A second possibility for the lack of reproduc-ibility may be due to different localized vibrationsaround the quiescent solution from run-to-run. Theinitial concentration of hypoxanthine (200 �M) is 10%of the initial 4-HBA (2 mM) concentration, thus caus-ing competition between the trapping agent and theenzymatic substrates.
Figure 6A shows the time course of hypoxanthineloss and xanthine and uric acid production during anunstirred microdialysis radical trapping experiment.In this experiment, hypoxanthine, xanthine, and uricacid were collected in the microdialysis and analyzedby HPLC-UV. For this reason, the initial concentrationof hypoxanthine is not 200 �M because the relativerecovery across the microdialysis probe is not 100%.Figure 6A shows the decline of hypoxanthine and ini-tial formation of xanthine followed by subsequent for-mation of uric acid. However, uric acid concentrationsdo not reach those of the initial hypoxanthine concen-
FIG. 5. 3,4-DHBA absolute concentrations during three separateone-probe radical trapping experiments. A 2-mm probe was usedwith a 1.0 �l/min flow rate. 2 mM 4-HBA was included in themedium external to the microdialysis probe.
FIG. 6. Graph of hypoxanthine (�), xanthine (F), and uric acid (■ ) absolute concentrations during the formation of hydroxyl radical withthe xanthine oxidase system using a two-probe system. One probe was used to collect dialysates that were analyzed for 3,4-DHBA contentwhile the other was used to measure hypoxanthine, xanthine, and uric acid. Both probes were perfused at 1.0 �l/min. (A) 2 mM 4-HBA wasplaced into the solution external to the probes. (B) 20 mM 4-HBA was placed in both microdialysis probes.
46 CHEN AND STENKEN

trations. This cannot be attributed to differences inrelative recovery between these analytes because therelative recoveries of these three analytes are nearlyidentical at 1.0 �l/min. Typical relative recovery valuesacross a 2-mm microdialysis probe for hypoxanthine,xanthine, and uric acid were 23.4 � 1.8, 20.4 � 5.4, and21.4 � 1.9% (n � 3 collections from each probe), re-spectively.
A possible explanation for the lack of a mass balanceis that uric acid reacts with hydroxyl radical to formallantoin, which can be measured by HPLC (29). Addi-tional experiments were performed to determine if al-lantoin was produced in significant amounts duringthe xanthine oxidase experiments. These experimentsshowed that allantoin collected in the dialysate wasless than 2 �M, which does not account for the loss ofuric acid. These experiments do not rule out alternatereactions that may occur with allantoin or uric acidand hydroxyl radical. In separate experiments inwhich the concentrations of hypoxanthine, xanthine,and uric acid were measured with 20 mM 4-HBA in-cluded in the microdialysis perfusion fluid, signifi-cantly higher concentrations of uric acid were found(Fig. 6B). This suggests that uric acid may be unstableduring the conditions of radical trapping.
While performing the experiments with two probesin the xanthine oxidase system, we found that concen-trations of 3,4-DHBA entering the probe were actuallyhigher than those obtained when one probe was placedin the xanthine oxidase system. In this system, 4-HBA
was placed outside of the microdialysis probes. Figure7 shows the results of two separate experiments whentwo identical probes both perfused at 1.0 �l/min wereplaced in the radical generating system. For one exper-iment, the concentration of 3,4-DHBA first increasedsteadily with the rate equaling 15 nM/min and thenreached a plateau at 200 min (circles). This plateau isidentical to the point at which uric acid concentrationbegan to plateau as shown in Fig. 6A. In a separateexperiment (triangles), the rate of 3,4-DHBA produc-tion was approximately 30 nM/min and the plateauwas reached earlier and a lower concentration wasachieved.
It is important to note that the amount of 3,4-DHBAobtained with a two-probe setup was much larger thanthat obtained through a one-probe system. There maybe several reasons for this observation. The first is thatby introducing a microdialysis probe into any system,the probe can be considered to behave like an artificialblood capillary (40). By introducing the second probe,the concentration gradient for 3,4-DHBA around thefirst probe is significantly increased. The second possi-bility is that other chemicals existing in the mixture,including uric acid, a known radical scavenger, can beremoved more efficiently in the two-probe system. Byremoving the possibility of competition between 4-HBAand uric acid, the concentration of 3,4-DHBA would beexpected to increase. A third possibility is that additionof a second probe to the system makes it more repro-
FIG. 7. Concentration (absolute) of 3,4-DHBA in dialysates col-lected through a microdialysis probe when two identical probes wereimmersed into the hydroxyl radical-generating system. The probeswere both perfused at 1.0 �l/min. The concentration of 4-HBA was 2mM external to the probes. The symbols Œ and F denote two separateexperiments.
FIG. 8. Concentration (absolute) of 3,4-DHBA in dialysates col-lected through a two-probe quiescent radical-generating system with20 mM 4-HBA included in the perfusion fluid. The microdialysisprobes were perfused at 1.0 �l/min. The symbols Œ and F denote twoseparate experiments.
47MICRODIALYSIS CALIBRATION FOR HYDROXYL RADICAL TRAPPING

ducible by reducing the effects of random localizedvibrations.
Figure 8 shows the results of replicate experimentswhen 20 mM 4-HBA was included in the perfusionfluid of one probe while a second probe contained onlyRinger’s solution. The concentration of 3,4-DHBA col-lected when 20 mM 4-HBA was included in the perfu-sion fluid was higher than when 2 mM 4-HBA wasincluded on the outside of the microdialysis probe (Fig.7). The unstirred 4-HBA relative recovery for a 2-mmmicrodialysis probe is approximately 10%. The reasonfor the increase in 3,4-DHBA concentrations when4-HBA is included in the microdialysis perfusion fluidmay be due to a much higher localized concentration of4-HBA in the vicinity near the microdialysis probe.
Figure 9 shows the resulting 3,4-DHBA concentra-tion when 20 mM 4-HBA was included in the microdi-alysis perfusion fluid of two separate microdialysisprobes placed into the external enzyme system thatwas kept well-stirred. Three separate enzymatic exper-iments were performed. In Fig. 9, the concentrationsand production rate of 3,4-DHBA are quite reproduc-ible from run to run, indicating the ruggedness of theenzymatic �OH-generating system. Furthermore an in-crease in 3,4-DHBA concentration was observed overthat for the unstirred system. The concentration of3,4-DHBA is significantly higher than in the unstirredsystem, which is what would be expected during nor-mal microdialysis sampling.
A reproducible method for producing a continuousamount of hydroxyl radical during microdialysis sam-pling experiments has been achieved by using the en-zymatic reaction of hypoxanthine with xanthine oxi-dase as a source of hydrogen peroxide. Hydroxylradical can be trapped by including 4-HBA either inthe medium external to the microdialysis probe or inthe microdialysis perfusion medium. Addition of a sec-ond microdialysis probe to this small-volume systemcreated a more reproducible and stable assay. Withthis assay, the ability to test microdialysis calibrationof hydroxyl radical trapping under various conditionssuch as different perfusion fluid flow rates and trap-ping agent concentration will be possible.
ACKNOWLEDGMENT
We thank the National Science Foundation for its generous sup-port (NSF-CHE 9984150) of this work.
REFERENCES
1. Halliwell, B., and Gutteridge, J. M. C. (1989) Free Radicals inBiology and Medicine, 2nd ed., Clarendon Press, Oxford.
2. Zwart, L. L., Meerman, J. H., Commandeur, J. N., and Ver-meulen, N. P. (1999) Biomarkers of free radical damage: Appli-cations in experimental animals and in humans. Free RadicalBiol. Med. 26, 202–226.
3. Halliwell, B., and Aruoma, O. I. (1992) in Molecular Biology ofFree Radical Scavenging Systems (Scandalios, J. G., Ed.), pp.47–67, Cold Spring Harbor Laboratory Press, New York.
4. Acworth, I. N., Bogdanov, M. B., McCabe, D. R., and Beal, M. F.(1999) Estimation of hydroxyl free radical levels in vivo based onliquid chromatography with electrochemical detection. MethodsEnzymol. 300, 297–313.
5. Rosen, G. M., Britigan, B. E., Halpern, H. J., and Pou, S. (1999)Free Radicals: Biology and Detection by Spin Trapping. OxfordUniv. Press, New York.
6. Buettner, G. R., and Mason, R. P. (1990) Spin-trapping methodsfor detecting superoxide and hydroxyl free radicals in vitro andin vivo. Methods Enzymol. 186, 127–133.
7. Finkelstein, E., Rosen, G. M., and Rauckman, E. J. (1980) Spintrapping of superoxide and hydroxyl radical: Practical aspects.Arch. Biochem. Biophys. 200, 1–16.
8. Grootveld, M., and Halliwell, B. (1986) Aromatic hydroxylationas a potential measure of hydroxyl-radical formation in vivo.Identification of hydroxylated derivatives of salicylate in humanbody fluids. Biochem. J. 237, 499–504.
9. Ingelman-Sundberg, M., Kaur, H., Terelius, Y., Persson, J. O.,and Halliwell, B. (1991) Hydroxylation of salicylate by microso-mal fractions and cytochrome P-450. Lack of production of 2,3-dihydroxybenzoate unless hydroxyl radical formation is permit-ted. Biochem. J. 276, 753–757.
10. Ste-Marie, L., Boismenu, D., Vachon, L., and Montgomery, J.(1996) Evaluation of sodium 4-hydroxybenzoate as an hydroxylradical trap using gas chromatography–mass spectrometry andhigh-performance liquid chromatography with electrochemicaldetection. Anal. Biochem. 241, 67–74.
11. Anderson, R. F., Patel, K. B., and Stratford, M. R. L. (1987)Radical spectra and product distribution following electrophilicattack by the �OH radical on 4-hydroxybenzoic acid and subse-quent oxidation. J. Chem. Soc. Faraday Trans. I 83, 3177–3187.
FIG. 9. Concentration (absolute) of 3,4-DHBA in dialysates col-lected through a two-probe stirred radical-generating system with 20mM 4-HBA perfused through each probe. The microdialysis probeswere perfused at 1.0 �l/min. The symbols (�, F, ■ ) represent themean 3,4-DHBA concentrations for three separate �OH-generationexperiments. Error bars represent the range of 3,4-DHBA concen-tration obtained from two probes (one measurement from eachprobe) for each �OH-generation experiment.
48 CHEN AND STENKEN

12. Oturan, M. A., and Pinson, J. (1995) Hydroxylation by electro-chemically generated �OH radicals. Mono- and polyhydroxylationof benzoic acid: Products and isomers’ distribution. J. Phys.Chem. 99, 13948–13954.
13. Grootveld, M., and Halliwell, B. (1986) Aromatic hydroxylationas a potential measure of hydroxyl-radical formation in vivo.Identification of hydroxylated derivatives of salicylate in humanbody fluids. Biochem. J. 237, 499–504.
14. Li, B., Gutierrez, P. L., and Blough, N. V. (1997) Trace determi-nation of hydroxyl radical in biological systems. Anal. Chem. 69,4295–4302.
15. Sun, J. Z., Kaur, H., Halliwell, B., Li, X. Y., and Bolli, R. (1993)Use of aromatic hydroxylation of phenylalanine to measure pro-duction of hydroxyl radicals after myocardial ischemia in vivo.Direct evidence for a pathogenetic role of the hydroxyl radical inmyocardial stunning. Circ. Res. 73, 534–549.
16. Hansen, D. K., Davies, M. I., Lunte, S. M., and Lunte, C. E.(1999) Pharmacokinetic and metabolism studies using microdi-alysis sampling. J. Pharm. Sci. 88, 14–27.
17. Kehr, J. (1993) A survey on quantitative microdialysis: Theoret-ical models and practical implications. J. Neurosci. Methods 48,251–261.
18. Justice, J. B., Jr. (1993) Quantitative microdialysis of neuro-transmitters. J. Neurosci. Methods 48, 263–276.
19. McCabe, D. R., Maher, T. J., and Acworth, I. N. (1997) Improvedmethod for the estimation of hydroxyl free radical levels in vivobased on liquid chromatography with electrochemical detection.J. Chromatogr. B. Biomed. Sci. Appl. 691, 23–32.
20. Teismann, P., and Ferger, B. (2000) The salicylate hydroxylationassay to measure hydroxyl free radicals induced by local appli-cation of glutamate in vivo or induced by the Fenton reaction invitro. Brain Res. Brain Res. Protocols 5, 204–210.
21. Montgomery, J., Ste-Marie, L., Boismenu, D., and Vachon, L.(1995) Hydroxylation of aromatic compounds as indices of hy-droxyl radical production: A cautionary note revisited. Free Rad-ical Biol. Med. 19, 927–933.
22. Obata, T., Aomine, M., and Yamanaka, Y. (2000) Potassiumchloride depolarization enhances MPP�-induced hydroxyl radi-cal generation in the rat striatum. Brain Res. 852, 488–491.
23. Camp, D. M., Loeffler, D. A., and LeWitt, P. A. (2000) L-DOPAdoes not enhance hydroxyl radical formation in the nigrostriataldopamine system of rats with a unilateral 6-hydroxydopaminelesion. J. Neurochem. 74, 1229–1240.
24. Yang, C. S., Lin, N. N., Tsai, P. J., Liu, L., and Kuo, J. S. (1996)In vivo evidence of hydroxyl radical formation induced by eleva-tion of extracellular glutamate after cerebral ischemia in thecortex of anesthetized rats. Free Radical Biol. Med. 20, 245–250.
25. Stenken, J. A. (1999) Methods and issues in microdialysis cali-bration. Anal. Chim. Acta 379, 337–358.
26. Stenken, J. A., Holunga, D. M., Decker, S. A., and Sun, L. (2001)Experimental and theoretical microdialysis studies of in situmetabolism. Anal. Biochem. 290, 314–323.
27. Bungay, P. M., Morrison, P. F., and Dedrick, R. L. (1990) Steady-state theory for quantitative microdialysis of solutes and waterin vivo and in vitro. Life Sci. 46, 105–119.
28. Freed, A. L., Cooper, J. D., Davies, M. I., and Lunte, S. M. (2001)Investigation of the metabolism of substance P in rat striatum bymicrodialysis sampling and capillary electrophoresis with laser-induced fluorescence detection. J. Neurosci. Methods 109, 23–29.
29. Benzie, I. F. F., Chung, W., and Tomlinson, B. (1999) Simulta-neous measurement of allantoin and urate in plasma: Analyticalevaluation and potential clinical application in oxidant:antioxi-dant balance studies. Clin. Chem. 45, 901–904.
30. Buettner, G. R. (1990) Use of ascorbate as test for catalyticmetals in simple buffers. Methods Enzymol. 186, 125–127.
31. Kalckar, H. M. (1947) Differential spectrophotometry of purinecompounds by means of specific enzymes. I. Determination ofhydroxypurine compounds. J. Biol. Chem. 167, 429–443.
32. Cohen, G. (1985) in CRC Handbook of Methods for OxygenRadical Research (Greenwald, R. A., Eds.), pp. 55–64, CRCPress, Boca Raton, FL.
33. Rice-Evans, C., Diplock, A. T., and Symons, M. C. R. (1991)Techniques in Free Radical Research, pp. 81–85, Elsevier, NewYork.
34. Sawyer, D. T., Yamaguchi, K., and Calderwood, T. S. (1985) inCRC Handbook of Methods for Oxygen Radical Research (Green-wald, R. A., Ed.), pp. 65–69, CRC Press, Boca Raton, FL.
35. Halliwell, B., Grootveld, M., and Gutteridge, J. M. C. (1988)Methods for the measurement of hydroxyl radicals in biochemi-cal systems: Deoxyribose degradation and aromatic hydroxyla-tion. Methods Biochem. Anal. 33, 59–90.
36. Biondi, R., Xia, Y., Rossi, R., Paolocci, N., Ambrosio, G., andZweier, J. L. (2001) Detection of hydroxyl radicals by D-phenyl-alanine hydroxylation: A specific assay for hydroxyl radical gen-eration in biological systems. Anal. Biochem. 290, 138–145.
37. Hodges, G. R., Young, M. J., Paul, T., and Ingold, K. U. (2000)How should xanthine oxidase-generated superoxide yields bemeasured? Free Radical Biol. Med. 29, 434–441.
38. von Sonntag, C., and Schuchmann, H. P. (1994) Suppression ofhydroxyl radical reactions in biological systems: Considerationsbased on competition kinetics. Methods Enzymol. 233, 47–56.
39. Regoli, F., and Winston, G. W. (1999) Quantification of totaloxidant scavenging capacity of antioxidants for peroxynitrite,peroxyl radicals, and hydroxyl radicals. Toxicol. Appl. Pharma-col. 156, 96–105.
40. Ungerstedt, U. (1991) Microdialysis—Principles and applica-tions for studies in animals and man. J. Intern. Med. 230, 365–373.
49MICRODIALYSIS CALIBRATION FOR HYDROXYL RADICAL TRAPPING











![Improvement of the Mechanical Stability of Microdialysis Catheters · 2016-02-09 · The next step in the development of microdialysis was when Urban Ungerstedt [3] introduced a hollow](https://static.fdocuments.in/doc/165x107/5f2bf162a4336743450d5a4a/improvement-of-the-mechanical-stability-of-microdialysis-2016-02-09-the-next-step.jpg)






