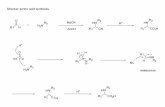
Alfa Aminonitriles
-
Upload
ledaivanova -
Category
Documents
-
view
25 -
download
0
description
Transcript of Alfa Aminonitriles
-
REVIEW 1941
The Chemistry of Deprotonated a-AminonitrilesDeprotonated a-AminonitrilesTill Opatz*Institut fr Organische Chemie, Universitt Hamburg, Martin-Luther-King-Platz 6, 20146 Hamburg, GermanyFax +49(40)428383834; E-mail: [email protected] 9 March 2009
SYNTHESIS 2009, No. 12, pp 19411959xx.xx.2009Advanced online publication: 29.05.2009DOI: 10.1055/s-0029-1216839; Art ID: E23609SS Georg Thieme Verlag Stuttgart New York
Abstract: This review highlights various aspects of the chemistryof the anions of a-aminonitriles. Their structural features and theircharacteristic reactivity are discussed, along with various methodsfor their preparation. Special emphasis is given to synthetic applica-tions of deprotonated a-aminonitriles which have been used as valu-able and readily accessible synthetic equivalents of acyl anions anda-aminocarbanions.
1 Introduction2 Structure and Reactivity of Deprotonated a-Aminonitriles3 Alkylations4 Opening of Epoxides5 1,2-Additions6 Acylations7 1,4-Additions8 Reactions of Unsaturated a-Aminonitriles9 Other Reactions10 Deprotonation of N-Monosubstituted and N-Unsubstituted
a-Aminonitriles11 Deprotonated a-Aminonitriles in Asymmetric Synthesis12 ConclusionsKey words: aminonitriles, carbanions, asymmetric synthesis,amines, ketones
1 Introduction
The chemistry of a-aminonitriles was first described byAdolph Strecker in 1850.1 Although more than 150 yearsold, his famous three-component condensation of amines,carbonyl compounds and hydrocyanic acid is still the fast-est and most widely used access to this highly versatilecompound class.2 The combination of an amino and a ni-trile group, and thus of a latent imine or carbonyl function,accounts for the usefulness of a-aminonitriles as startingmaterials for the preparation of a multitude of mono- ordifunctional compounds. Acid hydrolysis of a-aminoni-triles furnishes a-amino acids, and numerous methods forthe preparation of enantiomerically pure a-aminonitrileshave been described.317 Nucleophilic displacement of thenitrile group by hydride or a carbanion (Bruylants reac-tion) furnishes amines,1822 full reduction of the nitrilegroup yields 1,2-diamines23,24 and the preparation of a-aminoaldehydes via partial hydrogenation and hydrolysishas been described.25 The action of organometallics on a-aminonitriles may also lead to nucleophilic attack on thenitrile carbon.2628 Enamines are accessible by dehydrocy-
anation 2931 and various methods for the reversal of theStrecker reaction have been described, normally convert-ing a-aminonitriles back into their parent carbonyl com-pounds (Scheme 1).3238
Scheme 1 Transformations of a-aminonitriles
Aminonitriles can also serve as starting materials for thepreparation of nitrogen heterocycles.3942 Although theanion-stabilizing effect of the nitrile group in Streckerproducts derived from aldehydes was first employed byvon Miller and Plchl as early as 1898 (see section 10),43it took more than 60 years before the metallation of a-ami-nonitriles found wider application. The groups of Boekel-heide and Popp recognized the synthetic potential ofdeprotonated Reissert compounds in the 1950s and report-ed on their alkylations and on direct and vinylogous addi-tions.4457 In 1960, Hauser, Taylor, and Ledford describedthe a-alkylation of the potassium salts of N,N-disubstitut-ed a-arylaminonitriles and the subsequent base-inducedelimination of HCN from the substitution products toform enamines as well as their acidic hydrolysis to formketones.29 In the following two decades, deprotonatedaminonitriles experienced a boom and were extensivelyemployed as reactive, readily available and inexpensiveacyl anion equivalents. They turned out to be more versa-tile than O-protected cyanohydrins5860 or the widely used1,3-dithianes,6165 and even sterically hindered groupscould be introduced in high yield.30,35 While nucleophilicacylations using deprotonated N,N-dialkylaminonitrilesas well as various aspects of a-aminonitrile chemistryhave already been reviewed by Albright in 1983,66 byShafran, Bakulev, and Mokrushin in 1989,67 and by End-ers and Shilvock in 2000,68 the present review focuses on
NR2R1
CN
NR2R1
CN
1 2
substitution
oraddition R
1
NC NR2
R2
3
R1
HO2C NR2
R2
10
H3O+[red]
R1
NR2
R2
9
H2N
CN
R1 R2
4
NR2H2OR
1 R2
5
O
R1
6
NR2
R2'
7R1
H NR2
R28
R1
R4 NR2
R2
H R4M
R1
NR2
R2
11
R3NH
H+
H+
R3M
-
1942 T. Opatz REVIEW
Synthesis 2009, No. 12, 19411959 Thieme Stuttgart New York
the general chemistry of metallated a-aminonitriles andtheir synthetic applications.
2 Structure and Reactivity of Deprotonated a-Aminonitriles
The structures of the lithium salts of the a-aryl-substituteda-aminonitriles 13, 15, and 16 have been determined byX-ray crystallography.69 All three salts form dimers of N-lithiated a-cyanocarbanions containing a central Li2N2ring with exact or approximate Ci symmetry. The bondlengths of the C1C2 and the C1N bond (136138 pmand 117120 pm) are almost exactly intermediate be-tween the values expected for single/double and double/triple bonds, respectively. Similar values have been ob-tained for aggregates of lithiated phenylacetonitrile.70,71As would be expected, the aryl rings in 1316 are alignedparallel to the C2C1N axis to maximize charge delocal-ization and the nitrogen lone pairs are oriented in an anti-periplanar fashion to reduce the repulsive interaction withthe negatively charged conjugated system (Figure 1). IRspectroscopic studies of tetrahydrofuran solutions of 12,14, and 16 at 25 C revealed the presence of dimers andmonomers in equilibrium while the freezing point depres-sion of tetrahydrofuran solutions of 16 indicated a mono-meric structure at 108.5 C.72 The ambident character oflithiated aminonitriles allows reactions with electrophilesto take place at either carbon or nitrogen.Soft electrophiles like methyl iodide, acetaldehyde or cy-clohexenone react exclusively at carbon, while hard elec-trophiles like acetyl chloride, trimethylsilyl chloride ortrimethylsilyl triflate furnish predominantly the N-substi-tuted ketene imines which may slowly rearrange to thethermodynamically more stable C-substituted products.69This parallels findings obtained with metallated silyl cy-anohydrins.73
3 Alkylations
The first reported alkylation of a metallated acyclic N,N-disubstituted a-aminonitrile dates back to 1960, whenHauser, Taylor, and Ledford investigated reactions simi-lar to their previously published a-alkylation of diphe-
nylacetonitrile (19).29,74 They found that a-(dimethyl-amino)phenylacetonitrile (22) gave essentially the sameresults, including the base-induced b-elimination of HCN,in similar yield. The only significant difference was theheat sensitivity of aminonitrile 23, from which enamine24 was obtained upon distillation or attempted recrystalli-zation as a mixture of geometrical isomers. This behaviorcan be attributed to the electron-donating properties of thedimethylamino group which promotes the elimination ofcyanide under formation of an iminium ion. Enamine 24was converted into desoxybenzoin (26) upon acid hydro-lysis, while its reduction with sodium in liquid ammoniafurnished amine 25, demonstrating the use of the anion of22 as both an acyl anion and an a-aminocarbanion equiv-alent (Scheme 2).75 These reactions were later extended toaminonitriles derived from aliphatic aldehydes.30
The same authors subsequently reported on the exploita-tion of the latent iminium ion reactivity of aminonitriles27 obtained by a-alkylation of a-(dimethylamino)phenyl-acetonitrile to convert them into a-quaternary amines 29in a Bruylants reaction with organomagnesium ha-
Till Opatz was born in BadHomburg, Germany, in1973. He obtained his diplo-ma degree in 1997 with Pro-fessor Johann Mulzer at theUniversity of Frankfurt andhis doctorate in 2001 withProfessor Horst Kunz at the
University of Mainz. After apostdoctoral stay with Pro-fessor Rob Liskamp, Uni-versity of Utrecht (TheNetherlands), he completedhis Habilitation at the Uni-versity of Mainz in 2006.Since 2007, he is professor
of organic chemistry at theUniversity of Hamburg. Hisresearch interests are newsynthetic methods, the syn-thesis of biologically activecompounds and the chemis-try of natural products.
Biographical Sketch
Figure 1 Lithiated a-aminonitriles and their structures
CN
N
12
CN
N
13O
O
NMe
CN
14
Li Li
Li
O
O
NMe
CN
15
Li
O
O
NMe
CN
16
Li
MeO
NRR
N N
RR
NLi
Li
Solv
Solv17
N
R2
R1R1
N
Li
18
-
REVIEW Deprotonated a-Aminonitriles 1943
Synthesis 2009, No. 12, 19411959 Thieme Stuttgart New York
lides.18,76 The Bruylants reaction is thought to involve themetal-assisted elimination of cyanide from the aminoni-trile which is followed by nucleophilic addition of themetal organyl to the resulting iminium ion.21 While pri-mary Grignard reagents gave the desired substitutionproducts, the application of secondary or tertiary organo-magnesium halides resulted in Grignard reduction underformation of the benzylic amines 30 instead. Moreover,the acid-induced retro-Strecker reaction of the alkylationproducts 27 was used to prepare phenyl ketones 28 in ap-preciable yield (Scheme 3).
Scheme 3 Hydrolysis, Bruylants reaction and Grignard reduction ofalkylated a-aminonitriles
While Strecker products derived from aromatic aldehydesfurnish anions stabilized by additional delocalization, a-alkyl- and especially a-unsubstituted dialkylaminonitrileshave been reported to undergo undesired side reactionsand with most electrophiles, cleaner reactions are ob-served with a-aryl or a-hetaryl substituted nucleophiles.The anions of (dialkylamino)acetonitriles (formyl anionequivalents) are prone to self-condensation35 unless thesteric hindrance of the N-substituents becomes prohibi-tive. Remarkably, the lithium salt of (dimethylamino)ace-tonitrile has been reported to be unstable, while its diethylanalogue did not cause such problems: Two consecutivealkylations of (diethylamino)acetonitrile without isolationof the monoalkylated intermediate have been employed
by Stork et al. to prepare cyclopentenone 34, an interme-diate in the synthesis of cis-jasmone and methyl jas-monate.35 The dialkylated product was hydrolyzed toketone 32 under mild conditions using copper sulfate pen-tahydrate in ethanol.37 Cleavage of the cyclic acetal withoxalic acid and intramolecular aldol condensation underbasic conditions furnished 34 in 76% overall yield(Scheme 4).
Scheme 4 Double alkylation of (diethylamino)acetonitrile by Storkand co-workers
N-Phenyl-2,6-dicyanopiperidine 35, which is readilyavailable from the bisulfite adduct of glutaraldehyde,aniline, and potassium cyanide, can be dialkylated in highyield. Depending on the conditions for hydrolysis of thedialkylation products, either d-diketones 37 or cyclohex-enones 38 are formed, the latter resulting from an intramo-lecular aldol condensation (Scheme 5).77
Scheme 5 Takahashis double alkylation of N-phenyl-2,6-dicya-nopiperidine
Intramolecular alkylations using deprotonated a-aminoni-triles have been utilized to construct three- to six-mem-bered heterocycles.7880 A recent example is thepreparation of azetidines from aminoacetonitriles contain-ing a suitable leaving group.81 Since these substrates areprone to the formation of aziridinium ions, rearrangementto their thermodynamically favored isomers may occurduring the preparation.82 Starting from the chiral aminoalcohols 39 obtained by reduction of a-amino acids, theN-benzylated oxazolidines 40 were prepared by reductive
Scheme 2 Alkylation of deprotonated aminonitriles by Taylor,Hauser, and Ledford
19
Ph
PhCN
1) KNH2 NH3 (l)2) BnBr
Ph
Ph
20 (96%)
Ph KNH2
NH3 (l)
Ph
Ph
Ph
21 (94%)
22
Me2N
PhCN
Me2N
Ph23 (91%)
PhKNH2NH3 (l)
or
Me2N
Ph
Ph
24 (8492%)
H3O+
O
PhPh
26
1) Na, NH3 (l)2) NH4Cl
NMe2
PhPh
25
NC
NC
1) KNH2 NH3 (l)2) BnBr
22
Me2N
Ph
CN1) KNH2 NH3 (l)
2) R1XMe2N
Ph
R1CN HCl
Ph
R1O
28 (7483%)R1 = Me, Et, iPr, nBu
27 (7089%)R1 = Me, Et, iPr, nBu
iPrMgXor
tBuMgX Me2N
Ph
R1
30 (1676%)5 examples
R2MgX(R2 = Me, Et, nBu, Bn)
Me2N
Ph
R1
29 (2185%)10 examples
R2
31
CNEt2N
1) LDA, HMPA2)
3) LDA, HMPA4)
5) CuSO45H2O
Br O
O
I
OO
O 32
(CO2H)2
O
OHC33 (83% overall)
NaOH (aq)O
34 (91%)
35 (73%)
OHC CHO1) NaHSO32) PhNH2, H2SO33) KCN
NPh
CNNC
1) LDA (4 equiv)2) R1X (3.5 equiv)
NPh
R1 R1CNNC
36 (96100%)R1 = Me, Bn, All
CuSO45H2OR1
O
R1
O
37 (7174%)R1 = Me, Bn O
R2
R138 (62100%)
R2 = H, Ph
HCl (aq)
-
1944 T. Opatz REVIEW
Synthesis 2009, No. 12, 19411959 Thieme Stuttgart New York
benzylation and reaction with formaldehyde. Ring open-ing with citric acid and potassium cyanide furnished ami-nonitriles 41 which were converted into the correspondingchlorides using thionyl chloride. In case of the phenylgly-cinol derivative, quantitative rearrangement took place.Deprotonation with lithium hexamethyldisilazide fur-nished the azetidine carbonitriles in high yield but low di-astereoselectivity (Scheme 6).83
Scheme 6 Intramolecular alkylations to azetidines by Couty and co-workers
A combination of an a-alkylation with the trapping of animinium ion generated by elimination of cyanide from thesubstitution product with an allylsilane was chosen by theMartin group to prepare the tricyclic compound 53.84 Cy-clization of the imine obtained from the amino-function-alized allylsilane 47 and glutaric aldehyde dimethyl acetalin the presence of trifluoroacetic acid and subsequent ad-dition of sodium cyanide gave the bicyclic aminonitrile 50in 89% yield as an 88:12 epimeric mixture. Lithiation andalkylation with the functionalized tosylate 51 gave 52(62%), the treatment of which with silver triflate led to theintermediate formation of the iminium ion,85 which cy-clized diastereoselectively to the spirocyclic product 53 in81% yield. Thus, the short reaction sequence allows theefficient construction of a product of considerable com-plexity (Scheme 7).
4 Opening of Epoxides
An early example of epoxide opening with deprotonatedaminonitriles was reported by Stork and co-workers whoreacted lithiated (diethylamino)acetonitrile with styreneoxide or heptene oxide. The intermediate g-amino alco-hols were not isolated but rather hydrolyzed under elimi-nation with aqueous oxalic acid to furnishcinnamaldehyde and trans-oct-2-enal (Scheme 8, top).35 Katagiri et al. used an intriguing combination of an ep-oxide opening and a consecutive intramolecular alkyationin their synthesis of a trifluoromethylated 1-aminocyclo-propane-1-carboxylic acid.86 Starting from the protected
glycine nitrile 54, alcohol 55 was converted into the tosy-late, the deprotonation of which furnished cyclopropane56. The latter, after recrystallization in order to increasethe enantiomeric excess, was converted into the targetcompound 57 by oxidative degradation of the pyrrole ringto the N-acetyl compound and double hydrolysis. Re-markably, the cyclization proved to be completely diaste-reoselective (Scheme 8, middle). Laurent et al. utilized an intramolecular epoxide openingfor a quick access to the eastern half of the potent b-lactamantibiotic thienamycin. The homochiral epoxy amide 60was deprotonated with lithium hexamethyldisilazide andfurnished an almost equimolar mixture of the diastereo-meric products 61 (Scheme 8, bottom).87
5 1,2-Additions
The synthesis of b-amino alcohols by 1,2-addition ofdeprotonated dialkylaminonitriles to aldehydes was de-scribed by Stork et al. in 1979. While the reaction of thelithium salt of 2-(dimethylamino)propionitrile (62) withbenzaldehyde and subsequent reduction of the primaryaddition product with sodium borohydride furnished pre-dominantly the syn-configured product N-methylpseu-doephedrine (63) in 93% yield, a similar reaction of N-benzoylpiperidine-2-carbonitrile with aromatic or aliphat-ic aldehydes gave the anti-diastereomers.88 The authorsexplain this difference with the dipole repulsion in theiminium salt resulting from elimination of cyanide fromthe N,N-dialkylated addition product. A special feature ofthe reaction with N-acylated aminonitriles to aldehydes isthe N-to-O migration of the acyl residue in the alcoholate67. Nucleophilc attack leads to formation of the tetrahe-dral intermediate, from which imine 69 is formed upon
R
OH
NH2
1) PhCHO2) NaBH43) CH2O
N
O
RBn40
citric acid
KCNR
OH
N CNBn 41
SOCl2R = Bn
R = PhBn
Cl
N CNBn
Cl
N CNBn
Ph
LHMDS
42
45
NBn Bn
CN
NBn
CNPh
43(91%, syn/anti 1:1)
46(94%, syn/anti 3:7)
39Ph Bn(R = , )
LHMDSN
Bn
CNCl
Ph 44
SN2Scheme 7 Allylsilaneiminium spirocyclization
SiMe3H2N47
+ OHCOMe
OMe
1) 4 MS2) CF3CO2H3) NaCN
N
H
CN50(89%, dr 88:12)
1) LDA2)
SiMe3TsO N
H
Me3Si
NC
52(62%)
AgOTf, 4 MS
N
H
53(81%, singlediastereomer)
51
NMe3Si
OMe
OMeH
48
N
H
49
-
REVIEW Deprotonated a-Aminonitriles 1945
Synthesis 2009, No. 12, 19411959 Thieme Stuttgart New York
elimination of cyanide. Reduction of 69 furnishes ester70, which is finally solvolyzed by the methanolic sodiumborohydride solution to amino alcohol 71 (Scheme 9).The same principle had already been employed earlier bythe groups of McEwen and Popp for the synthesis of hy-droxyalkylated isoquinolines from Reissert com-pounds.46,47,8991 In the case of the N-acylated startingmaterials 72, the primary addition products undergo acylmigration under formation of the aromatic esters 73(Scheme 10, top). If, however, the N-alkoxycarbonylcompounds 75 are used as the pronucleophiles, elimina-tion of alkoxide from the tetrahedral intermediates resultsin formation of tricyclic oxazolidinones 76, from whichHCN can be eliminated to form oxazolinones of type 77(Scheme 10, bottom).92 The analogous 1,2-addition tobenzalanilines has also been described. This is one of therare instances where aldimines were chosen as the reac-tion partners for deprotonated a-aminonitriles (see alsosection 10).93 Lithiated (dialkylamino)nitriles have been utilized as acylanion equivalents to prepare a-hydroxy ketones 81 and 82from aliphatic aldehydes and cyclic ketones in moderateto high yield (Scheme 11, top).94 Enders and Lotter com-bined the same reaction with the thermal dehydrocyana-tion of the addition products 84 for the preparation of a-(dimethylamino) ketones 86.31 After elimination of cya-
nide from 84, the iminium ion loses a proton to form theenamine, which tautomerizes to the thermodynamicallymore stable product 86 (Scheme 11, bottom).In contrast to the considerable number of reports on the di-astereoselective aldolizations of metallated arylacetoni-triles,9598 the respective chemistry of aminonitriles hasnot been investigated in great detail.88,99 The 1,2-additionof lithiated (dialkylamino)acetonitriles to aliphatic alde-hydes shows a strong dependence on the substituents atnitrogen with regard to the diastereoselectivity.100 While(dibenzylamino)acetonitrile (87) gave good anti/syn ra-tios only in combination with pivalaldehyde, the morebulky tert-butyl benzyl derivative 89 produced high selec-tivities with both unhindered and hindered aliphatic aswell as aromatic aldehydes (Scheme 12). Unfortunately,the addition to a-chiral aldehydes was not accompaniedby a pronounced facial selectivity.Depending on the reaction conditions, aminoacetonitrilescan also be condensed with carbonyl compounds to forma,b-unsaturated a-aminonitriles 92 which can be hydro-lyzed with aqueous acid to yield carboxylic acids 93. Thissequence can be employed for the homologation of aro-
Scheme 8 Inter- and intramolecular opening of epoxides with de-protonated a-aminonitriles
Et2N CN
1) LDA2)
3) (CO2H)2
OR
R = Ph
31 R = nC5H11
PhCHO
(65%)
nC5H11CHO
(50%)
O
F3C (75% ee)N
CN54
NaHMDS
N
CNF3C
OH
55 (73%,ca. 30% de)
TsCl, NaH
F3C
CN
N
56 (82%,>99% de, 75% ee)
1) recrystallization2) RuCl3 (cat.), NaIO43) HCl (aq)
F3C
CO2H
NH3ClH2O
57 (33%,>99% de, >99% ee)
Ph
NHPh
CN
+O
CO2Na58 59
(COCl)2pyridine
O
O
N
CN
Ph
Ph
60 (41%)
LHMDSNO Ph
Ph
CNHOH
61 (47%,trans/cis 52:48)
Scheme 9 1,2-Additions to aldehydes
NMe2
CN62
1) LDA2) PhCHO3) NaBH4, MeOH
NMe2
OH63 (93%)
syn/anti > 5:1
N CN
O
64
1) LDA2) RCHO3) NaBH4, MeOH
NH
OH
H
66 (77%)diastereomerically pure
after recrystallization
NH
H
OH
N
OMe65 (76%)anti/syn > 6:1
1) LDA2) PhCHO
N
O
67
CN ON
OO
CN
68N
O O
69NaBH4
NH
O O
70
H
NH
OH
H
71 (70%)anti/syn 6:1
CN
MeOH
(spontaneous)
-
1946 T. Opatz REVIEW
Synthesis 2009, No. 12, 19411959 Thieme Stuttgart New York
matic aldehydes but its application to aliphatic aldehydesfailed.101 The latter substrates were, however, brought toreaction in a one-pot sequence involving the trimethylsi-lylated aminonitrile generated in situ (Scheme 13), whichalso permitted the homologation of benzophenone.102
Scheme 13 Takahashis carbonyl homologations
6 Acylations
As already mentioned, acylation of deprotonated amino-nitriles can occur at the a-carbon as well as at the terminalnitrogen atom. According to an early observation byBoekelheide, deprotonated Reissert compounds may un-dergo 1,2-acyl migration to furnish the 1-substituted iso-quinolines after concomitant elimination of cyanide.44 It isunclear whether this reaction proceeds strictly in an in-tramolecular fashion.A Dieckmann-type cyclization of aminonitrile ester 98has been described by Uchibayashi103 as well as by Blakeet al.104 (Scheme 14). Remarkably, attack of the ester eno-late at the nitrile carbon has been observed for 98, if thedeprotonation was carried out under equilibrating condi-tions with ethanolic sodium ethanolate as the base. There-fore, 99 is not the thermodynamic reaction product in spiteof its CH-acidity.
Scheme 14 Acyl migration and Dieckmann condensation
The first intermolecular C-acylation of a deprotonatedaminonitrile was reported by Hauser and co-workers whotreated the sodium salt of (dimethylamino)acetonitrile(100) with methyl benzoates to obtain the aroyl-substitut-ed products 101 in 4565% yield (Scheme 15, top).105 Thesame reaction can be adopted for the preparation of a-ke-
Scheme 10 1,2-Additions of deprotonated Reissert compounds
72
N
CN O
R1) nBuLi
2) PhCHO N
OPh 73
75
N
CN O
OR N
76O
O
Ph
NC HCN
N
O
O
Ph
1) nBuLi
2) PhCHO
77
O
R
1) nBuLi2)
Ar1 NAr2
N
N
O
Ar1NC
Ar278
HCN N
N
O
Ar1 Ar279
1) nBuLi
2)Ar1 N
Ar2 N
NAr1
O
RAr2 74
Scheme 11 Synthesis of a-hydroxy ketones and a-amino ketones
NMe2
CN
83
1) LDA
2) nBuCHO
NC NMe2
OH84
HCN
OH
NMe2
85O
NMe2
86
R1
CN
NR22
1) LDA
80R1 = Me, EtR2 = Me, Et
2) R3CHO R3 = nAlkyl3) HCl aq.
2)
3) HCl (aq)
O
n
R1
O
OH
R3
81(8 examples,
5772%)
R1
OOH
n
82(8 examples,
5578%)
Scheme 12 Diastereoselective aldolization of a-aminonitriles byMangeney and co-workers
87
CNBn2N1) LDA2) RCHO NC
NBn2
OH
R88(6 examples, 6575%,anti/syn 50:50 to 91:9)
N CNBn
89
1) LDA2) RCHO NC
N
OH
R90(6 examples, 7386%,anti/syn > 95:5)
Bn
N CNPh
Me
91
KH
ArCHO
92(4 examples, 6889%)
N CNPh
Me
Ar
CO2HAr
93(8096%)
HCl (aq)
1) KH2) TMSCl3) KH4)
R1 R2
O
N CNPh
Me
R2R1
94(4 examples, 53100%)
CO2HR1
95(3678%)
HCl (aq)
R2
EtO2C NCO2Et
CN
98
NaH (Uchibayashi)
orKOtBu (Blake et al.) N
CN
O
CO2Et99
N
CN96 O
Ph
NaH,
NaCN N
PhO97
-
REVIEW Deprotonated a-Aminonitriles 1947
Synthesis 2009, No. 12, 19411959 Thieme Stuttgart New York
toamides 106 if the anions of the primary acylation prod-ucts are oxidized with sodium hypochlorite (Scheme 15,middle).106 This process presumably involves the elimina-tion of HCN from the a-hydroxylated acylaminonitrile, acyanohydrin. Recent work by Mangeney, Vrancken and co-workersdemonstrates that the reduction of acylaminonitriles withsodium borohydride in the presence of magnesium bro-mide proceeds with high diastereoselectivity to affordsyn-b-hydroxy-a-aminonitriles 107 in moderate to highoverall yield (Scheme 15, bottom).107
7 1,4-Additions
Probably the earliest report on the a-substitution of adeprotonated N,N-disubstituted a-aminonitrile involvinga vinylogous addition is the synthesis of 1-skatylisoquin-oline (110) by Boekelheide and Ainsworth.45 The Reissertcompound 96 and gramine (108) reacted in boiling xylenein the presence of a small piece of metallic sodium. Underevolution of dimethylamine, the substitution product 109was formed in 46% yield. This reaction presumably pro-ceeds via the intermediate formation of the electrophilic3-methyleneindolenine (111), which then reacts with theanion of 96. Hydrolytic removal of the benzoyl group un-der basic conditions and concomitant elimination of cya-nide furnished 110 in quantitative yield (Scheme 16, top).1,4-Additons of deprotonated Reissert compounds toacrylonitrile are followed by ring closure to pyrroloiso-quinolines due to nucleophilic attack of the resulting carb-anion on the amide carbon.108 Surprisingly, it is not thenitrile but instead the corresponding amide that is ob-tained as the the final product (Scheme 16, bottom).109
Scheme 16 Vinylogous additions of metallated Reissert com-pounds
The addition of deprotonated N-acyl-a-aminonitriles(open-chain Reissert compounds) of type 114 to vinyl-triphenylphosphonium bromide provides a direct andefficient access to 1,2,5-trisubstituted pyrroles as demon-strated by Cooney and McEwen. The phosphonium ylideformed upon vinylogous addition undergoes an intramo-lecular Wittig reaction with the amide carbonyl under clo-sure of the five-membered ring (Scheme 17).110,111
Scheme 17 Pyrroles from N-acyl-a-aminonitriles
Whereas the former reactions do not suffer from the riskof a competing 1,2-addition, the regioselectivity may bean issue for reactions of aminoketeneiminates withenones, a,b-usaturated aldehydes or even unsaturated es-ters. The substituents on the aminonitrile are often crucialfor determining the regioselectivity, but solvents andcounterions are also known to play an important role. Ar-omatic substituents bound to nitrogen or the a-center of adeprotonated aminonitrile lead to a more stabilized
Scheme 15 Intermolecular C-acylations of deprotonated a-amino-nitriles
Me2N CN100
1) NaNH22)
CO2MeR R
ONMe2
CN101
(3 examples, 4565%)
R2N CN
102
HetO
OMe+
103
NaHMDS(2.5 equiv)
Het
ONR2
O 106(12 examples, 4073%)
Het
ONaNR2
CN104
NaOClHet
ONR2
OHNC105
HCN
Bn2N CN87
1) LDA2) RCOX3) NaBH4, MgBr24) NH4Cl
NC
NBn2R
OH107 (9 examples,
3888%, >92% de)R = aryl, alkyl
N
CNBz
96
+
NH
NMe2
108
NBz
NC
NH
109 (46%)
Na (cat.), NHMe2
NaOH (aq)N
NH
N
via intermediate formation of
110 (100%)111
N
CNBz
96
1) PhLi2)
CN
NPh
OLi
NC
CN112
NPh
H2O
ONH2
113 (76%)
1) NaH2)N
NC Bz
R2
R1
114PPh3 Br
NR2
R1Ph
O
PPh3
NC
115
HCN, Ph3PON Ph
R2
R1
116(11 examples, 50100%)
-
1948 T. Opatz REVIEW
Synthesis 2009, No. 12, 19411959 Thieme Stuttgart New York
carbanion and increase its tendency towards vinylogousaddition.112,113 For instance, Ahlbrecht and Kompter ob-served that the lithium salts of Strecker products derivedfrom N-methylaniline and aliphatic aldehydes underwentclean 1,4-additions to cyclic enones and methyl vinyl ke-tone when hexamethylphosphoramide was added to thereaction medium and the electrophiles were activated bycomplexation with lithium bromide.114 If no further pre-cautions were taken, the same combination of reactantsresulted in 5060% deprotonation of the electrophile bythe lithium keteneiminate. Acid hydrolysis of the additionproducts furnished 1,4-dicarbonyl compounds 121 inmoderate to high yield.115 As an alternative to the classicalgeneration of the nucleophiles by deprotonation, the au-thors used the Michael addition of lithium organyls to thea,b-unsaturated aminonitrile 118 (Scheme 18).116
Scheme 18 Synthesis of 1,4-dicarbonyl compounds
Wakamatsu et al. studied the influence of the reactiontemperature on the regioselectivity of the addition of thelithiated (2,6-dimethylpiperidin-1-yl)acetonitrile (122) tocyclohexenone.117 While at 78 C the 1,2-adduct waspredominantly formed, warming to ambient temperatureresulted in a preponderant formation of the 1,4-adduct dueto the reversibility of the 1,2-addition. The best 1,4-selec-tivity (97:3) was observed when a higher reaction temper-ature was combined with the addition ofhexamethylphosphoramide (Scheme 19). The somewhat unusual N-substituent was chosen in orderto suppress the undesired self-condensation of the keten-iminate.118 Remarkably, the addition of 122 to cyclopen-tenone under various conditions yielded the 1,4-additionproduct exclusively. Vinylogous addition of acyl anionequivalents to a,b-unsaturated nitriles furnishes g-ketoni-triles, the hydrogenation of which yields 2-substitutedpyrrolidines. This strategy has been employed by Leete ina short synthesis of two Nicotiana alkaloids.119121 (Mor-pholin-4-yl)-3-pyridylacetonitrile (125) reacted withacrylonitrile and methanolic potassium hydroxide in tert-butanol to furnish dinitrile 126 in 90% yield (Scheme 20).Acid hydrolysis to the ketone and catalytic hydrogenationgave nornicotine (129, 60%) along with myosmine (128,30%).
Trapping of the enolates generated in 1,4-additions ofdeprotonated aminonitriles to various electrophiles hasbeen described.122 Wartski, Posner, and Nierlich havecombined this reaction with an iminium ion allylsilane cy-clization to a [1+2+3]-annulation sequence.123 [2-(Iodo-methyl)-2-propenyl]trimethylsilane served as thebifunctional electrophile and silver-induced decyanationof the intermediate 130 gave the bicyclic product 131 in33% overall yield as a single diastereomer of unknownrelative configuration at the newly formed benzylic qua-ternary stereocenter (Scheme 21). The allylsilane moietyturned out to be crucial for the cyclization since the unsub-stituted methallyl residue was not attacked.124
8 Reactions of Unsaturated a-Aminonitriles
b,g-Unsaturated a-dialkylaminonitriles form ambident an-ions which can react with electrophiles in either the a- orthe g-position. As for the addition of simple deprotonateda-aminonitriles to a,b-unsaturated carbonyl compounds,the regioselectivity depends on the electrophile, on thesubstitution of the nitrile, and on the reaction conditions.While the morpholino derivative 132 is predominantlyalkylated in the a-position, the lithium salt of the sterically
R1
R2N
CNPh
Me
117
LDAR1
R2N
Ph
Me
NLiR1
N
CNPh
Me
118
1) HMPA2)
LiBr
3) H2O
119
O
R4R3
R1
R2
R3 O
R4
NNCPh
Me120
R1
R2
R3 O
R4
O
HCl (aq)
121(8 examples, 4287%)
R2Li
Scheme 19 Regioselectivity of the addition to cyclohexenone
122
N
CN
1) LDA2) O
N
CN
O 124123
OHN
NC +
78 C
conditions combinedyield 123 124
51% 10 90
78 C 25 C 47% 70 3078 C 25 C,
HMPA 70% 97 3
78 C, HMPA 72% 37 63
Scheme 20 Synthesis of myosmine and nornicotine by Leete
N
CN
NO
125
CN
KOHN
N
NCCN
O
126 (90%) AcOHH2O
N
O
CN
127 (90%)
H2Raney-NiNH3
N
N
myosmine(128, 30%)
N
NH
nornicotine(129, 60%)
-
REVIEW Deprotonated a-Aminonitriles 1949
Synthesis 2009, No. 12, 19411959 Thieme Stuttgart New York
more demanding cyclohexyl derivative 134 reacts exclusive-ly in the less hindered g-position (Scheme 22, top).125,126For the reaction of the aminonitriles 136 with cyclic ke-tones, the a-addition to form 137 is kinetically controlled,while the g-addition product 138 is obtained under ther-modynamic reaction conditions. Congruously, the formercan be converted into the latter upon treatment with lithi-um diisopropylamide (Scheme 22, bottom). Thus, sub-strates 136 permit an easy switch between acyl anion andhomoenolate reactivity. The addition of zinc chloride in-hibits the undesired aldolization of the ketone and increas-es the overall yield. Acid hydrolysis of the g-additionproducts leads to spirolactones 139 in moderate to goodyield.127 These compounds can in turn be converted intothe annulated cyclopentenones 140 upon treatment withpolyphosphoric acid or phosphorus pentoxide and meth-anesulfonic acid. Pierre and Enders described the a-selective 1,2-additionof the lithium salts of aminonitriles 141 to various alde-hydes.128,129 The resulting aminonitriles 142 were trans-formed into a-hydroxyenones 143 upon treatment withaqueous silver nitrate (Scheme 23, top). An earlier reportby Fang on both 1,2-additions and alkylations of the di-lithium salt of aminonitrile 144 derived from ephedrineindicated exclusive reaction in the g-position (Scheme 23,bottom).130 The stereochemical control over the g-centeris moderate and little influence can be imposed on theconfiguration of the carbinol center.Grierson, Husson and co-workers utilized 1,2,5,6-tetrahy-dropyridine-2-carbonitriles of type 149 for the prepara-tion of several mono- or polycyclic alkaloids containingthe piperidine ring.131136 The starting materials werereadily prepared from pyridinium salts like 146 by meansof partial reduction to the corresponding 1,2,5,6-tetrahy-dropyridine, N-oxidation, regioselective PotierPolonovski rearrangement137 to the 1-substituted 5,6-di-hydropyridinium ion, and, finally, addition of cyanide.131In the case of compound 149, the overall yield amountedto 35%. Deprotonation with lithium diisopropylamide and
a-alkylation furnished 150 (97%), which was then sub-jected to a Bruylants reaction with phenyl magnesiumchloride, followed by Grewe cyclization138 to the benzo-morphan derivative 152 (Scheme 24).139 In contrast to theclassical addition of a benzylic Grignard reagent to the py-ridinium salt, this technique permits the facile introduc-tion of an angular substituent.
Scheme 21 [1+2+3]-Annulation sequence according to Wartski,Posner, and Nierlich
Ph NMe2
CN 22
1) nBuLi2)
3)
O O
I
Me3Si
O
O
Ph
NMe2CNH
SiMe3
130(70%, dr = 7:3)
1) AgOTf2) H2O3) NaHCO3
O
O
H PhNMe2131
(47%, single diastereomer)
Scheme 22 Regioselectivity of alkylations and 1,2-additions of un-saturated aminonitriles
N
CN
O
1) LDA2) MeI
NO
NC
132 133(/ = 75:25)
N
CNMe 1) LDA
2) MeIN
CNMe
134 135(/ = 0:100)
R1
CN
NR22136
NR22 = NMe2,piperidin-1-yl
1) LDA2) ZnCl2,
78 C
0 C
n
R3 OH
NC NR22
R1
137
n
R3
R1OH
CN
NR22
138
LDA
n
R3 O
R1
O
n
R3H+ (CO2H)2
139(7 examples, 4371% from 136)
O
R1
140(5 examples, 7699%)
O
n
R3
Scheme 23 1,2-Additions of unsaturated aminonitriles according toEnders and Fang
R1
CN
NR22141
1) LDA
2) R3CHO R1NC NR22
R3
OH142
AgNO3
R1R3
OH
O
143(16 examples, 6183%)
N
CNMe
HO 1) LDA (2 equiv)2) PhCHO
N
CNMe
HO
Ph OH
145 (67%)4R-erythro/4R-threo/4S-threo = 60:25:15
144
R1 = Me, Ph, 2-furylNR22 = NMe2, piperidin-1-yl
-
1950 T. Opatz REVIEW
Synthesis 2009, No. 12, 19411959 Thieme Stuttgart New York
Scheme 24 Hussons benzomorphan synthesis
9 Other Reactions
Arnott et al. studied the spirocyclization of deprotonatedaminonitriles onto N-alkoxycarbonylpyridinium ions tofurnish diazaspiro[5.3]nonanes of type 154.140 Isonico-tinamides 153 were lithiated with lithium diisopropyl-amide and then N-acylated with methyl chloroformate todeliver the products in moderate yield (Scheme 25, top).An NMR study on a closely related glycine derivativeproved that even with this order of addition of the re-agents, spirocyclization does not take place unless the py-ridine has been N-acylated. While high stereoselectivitieshave been observed with N-linked benzylic auxiliaries inthe glycine ester series, the extension of the asymmetricvariant to glycine nitriles has not been reported. Underidentical conditions, the isomeric nicotinamides 155 gavethe aza-isoindolinones 156 in moderate yield (Scheme 25,bottom).
Couty et al. have developed a cyclopropanation reactionof Michael acceptors based on cyanide-stabilized azeti-dinium ylides like 160.141 The N-substituted azetidine-2-carbonitrile 158 was prepared by the intramolecular alkyl-ation methodology described in section 3. Quaternizationwith methyl triflate yielded the azetidinium triflate 159,which reacted with Michael acceptors in the presence oflithium hexamethyldisilazide to give cyclopropane carbo-nitriles 162 in moderate to high yield. After a-deprotona-tion, vinylogous addition to the a,b-unsaturated carbonylcompound produced the enolate, which opened the azeti-dinium ring under formation of the aminoethyl cyclopro-panes 162 (Scheme 26). Since free rotation about the a,b-bond in the enolate can occur, it is not surprising that di-ethyl maleate furnished the same product, albeit in signif-icantly lower yield. As expected for ammonium ylides,compound 160 reacts with aldehydes under formation ofepoxides.142
Scheme 26 Coutys cyclopropanation with azetidinium ylides
Deprotonated aminonitriles can be added to mono- anddisubstituted alkynes devoid of electron-withdrawingsubstituents.143 For instance, the addition of (dialkylami-no)phenylacetonitriles 163 to phenylacetylene gave thestyryl-substituted products as E/Z-isomeric mixtures.144When applied to 1-phenylpropyne, addition took place tofurnish products 164 in moderate to high yield but withcomplete E-selectivity.145 Remarkably, both reactionsonly required powdered potassium hydroxide in dimeth-ylsulfoxide in combination with the phase-transfer cata-lyst benzyltrimethylammonium chloride to deprotonatethe aminonitrile (Scheme 27, top). The addition of 163 toethoxyacetylene and (methylthio)acetylene has also beenachieved.144 An intramolecular variant of the reaction has been devisedby Wang et al., who cyclized the glycine nitriles 166 withlithium diisopropylamide to furnish cyanopyrrolines 167,which were directly isomerized in a deprotonationrepro-tonation sequence to the thermodynamically more stableD2-isomers 168.146 Dehydrogenation with 2,3-dichloro-5,6-dicyano-1,4-benzoquinone resulted in the pyrrole-2-carbonitriles 169 which were isolated in moderate yield(Scheme 27, bottom).
NMe I
146
1) NaBH42) H2O2
NMe O
147 (70%)
(CF3CO)2O
NMe
CF3CO2
148
KCNNMe
NC
149(50% from 147)
NMe
CN
OMe
1) LDA2) PMBCl
150 (97%)
PhMgClNMe
Ph
OMe
151 (64%)
HBr,
NMePh
OH152 (70%)
Scheme 25 Intramolecular additions to pyridinium salts by Arnottand co-workers
N
O
NR
CN153
R = tBu, CMe2Ph
1) LDA2) MeOCOCl N
N
MeO
O
O
CN
R
154R = tBu 53%
R = CMe2Ph 55%
N
O
NR
CN155
R = tBu, CMe2Ph
1) LDA2) MeOCOCl
NN
O
CN
RMeO
O
156R = tBu 62%
R = CMe2Ph 30%
OH
N CNBn
NBn
CNMeOTf
N
CN
BnMe
OTf
158 (78%) 159 (83%)
LHMDSN
CN
BnMe
EtO2CCO2Et
N BnMe
CO2EtEtO
O CN
157
160
CO2Et
EtO2C
CNN
Bn
Me
162 (80% from 159)161
-
REVIEW Deprotonated a-Aminonitriles 1951
Synthesis 2009, No. 12, 19411959 Thieme Stuttgart New York
Aryl and hetaryl halides susceptible to nucleophilic aro-matic substitution by means of an additioneliminationsequence may be subjected to nucleophilic acylationswith deprotonated Strecker products.147,148 Albright andMoran used their rugged lithiated (morpholin-4-yl)aryl-acetonitriles 170, which often give superior results com-pared to dialkylamino substituted nucleophiles,149,150 toeffect halide substitution on 1-benzyl-5-bromo-2-methyl-4-nitroimidazole.151 The primary products were subse-quently converted into the corresponding aryl ketones 172by treatment with copper sulfate pentahydrate(Scheme 28, top). The same type of nucleophile permittedthe conversion of 4-fluoronitrobenzene into 3-methoxy-4-nitrobenzophenone (Scheme 28, bottom).152Although heterocumulenes are highly reactive electro-philes, only a few publications have addressed their reac-tions with deprotonated aminonitriles. Notable exceptionsare reactions involving isocyanates and isothiocyan-ates.153 While chloroformate-derived Reissert compoundsof isoquinolines furnish addition products that cyclize toimidazo[5,1-a]isoquinolines of type 176, Reissert com-pounds of phthalazines yield open-chain adducts 178.154Their cyclization to imidazo[5,1-a]phthalazines 179 canbe effected by heating, albeit in low yield (Scheme 29).Among the further reactions involving deprotonated ami-nonitriles are [2,3]-sigmatropic rearrangements of nitrile-stabilized ammonium ylides,155163 ThorpeZiegler cy-clizations,164 additions to arynes,165 and trichloromethyla-tions.166 A more exotic example is the preparation ofazines by reaction of lithiated aminonitriles with sulfonyl-hydrazones reported by Takahashi and co-workers.167
10 Deprotonation of N-Monosubstituted and N-Unsubstituted a-Aminonitriles
Despite the fact that N-monosubstituted and N-unsubsti-tuted a-aminonitriles are susceptible to the base-inducedelimination of HCN, that is, the retro-Strecker reaction,the first reported reaction involving deprotonated amino-nitriles is the von MillerPlchl reaction published as ear-ly as 1898.43 In the presence of ethanolic potassiumhydroxide, a-phenyl toluidinoacetonitrile (180) reactswith cinnamaldehyde to form a trisubstituted pyrrole. Al-though the authors mistakenly identified the product asthe 1,2,5-trisubstituted isomer, their error was correctedby Bodforss, who recognized the 1,2,3-trisubstitution ofthe resulting pyrrole 182 (Scheme 30).168 In 1954, Treibsand Derra investigated the reaction in greater detail andfound out that only a,N-diaryl-substituted aminonitrilesare suitable pronucleophiles.169 Presumably, the high CH-acidity of these particular substrates protects them against
Scheme 27 Inter- and intramolecular additions to unactivated alky-nes
Ph
NC NR2
163
Ph
NaOH, DMSOBnNEt3Cl
Ph
NC NR2
Ph164
(4 examples, 7280%E-isomers only)
Ph
NaOH, DMSOBnNEt3Cl
Ph
NC NR2
Ph 165(5 examples, 4777%
E/Z = 12.1:1)
PhN
R
NCMe
166
LDA
N
Ph
R
Me
NC
167
H-shift
N
Ph
R
Me
NC
168
DDQ
N
Ph
R
Me
NC
169(3 examples, 4661%) Scheme 28 Nucleophilic aromatic substitution
N
NBr
O2N
Bn
R
N
CN
O
170
1) nBuLi
2)
N
N
O2N
BnNNC
O
R 171(7 examples, 2485%)
N
N
O2N
Bn
R172
O
CuSO45H2O
N
CN
O
173
1) NaH2)
3) AcOH F
O2N NO2
OMe
174 (70%)
O
OMe
Scheme 29 Addition of deprotonated Reissert compounds to iso-thiocyanates
175
N
CNCO2Me
1) NaH2) PhNCS
N
NPh
O
S
NC
176 (58%)
NN
CNCO2Me
1) NaH2) PhNCS
NN
CO2MeNCNHPhS
NN
NPh
O
S
NC177
178 (67%)
179 (20%)
-
1952 T. Opatz REVIEW
Synthesis 2009, No. 12, 19411959 Thieme Stuttgart New York
the impending retro-Strecker reaction under thermody-namic deprotonation conditions.For a long time, the von MillerPlchl reaction and theclosely related vinylogous addition to a,b-unsaturatedesters170 remained the only transformations involvingdeprotonated N-monosubstituted a-aminonitriles. Recentinvestigations on this topic have been carried out by theOpatz group. Using potassium hexamethyldisilazide intetrahydrofuran at low temperatures, Strecker productsderived from aromatic aldehydes and primary amines orammonia could be deprotonated and the resulting potassi-um keteneiminates were subjected to alkylations, 1,2-ad-ditions, and 1,4-additions. Upon reaction with a,b-unsaturated ketones and aldehydes, 2-cyano-5-hydroxy-pyrrolidines 185 result from cyclization of the primary ad-dition products. These compounds are thermally unstableand decompose under formation of pyrroles 186 accord-ing to the mechanism proposed by Treibs and Derra forthe von MillerPlchl synthesis.171 Twofold reduction ofthese double iminium ion equivalents with sodium cy-anoborohydride furnishes pyrrolidines 187 (Scheme 31).If a,b-unsaturated esters are used as the electrophiles, g-amino acid esters can be obtained after reductive decyana-tion of the primary addition products.172
Scheme 31 Pyrroles and pyrrolidines from N-monosubstituted ami-nonitriles
An intramolecular 1,4-addition can be employed for thepreparation of indoles from 2-aminocinnamic acid estersand amides. Their Strecker reaction with aromatic or a,b-unsaturated aldehydes produces aminonitriles of type 189,which cyclize in up to quantitative yield even under ther-modynamic deprotonation conditions to furnish indole-3-acetic acid derivatives like 190 (Scheme 32).173,174
Scheme 32 Cyclization to indoles
Aminonitrile 191, available in three steps from homover-atrylamine, can be deprotonated and a-alkylated with 3,4-dimethoxybenzyl bromide.175 The substitution productspontaneously eliminates HCN, yielding the 3,4-dihy-droisoquinoline 193, which can be subjected to an asym-metric transfer hydrogenation with Noyoris catalyst tofurnish (S)-norlaudanosine (194) in 93% ee and 78% yieldover three steps (Scheme 33).176 Various isoquinoline al-kaloids, as well as the pyrroloisoquinoline lamellarin U,have been prepared accordingly.175,177
1,2-Addition of the potassium keteniminates 195 to alde-hydes and imines and subsequent reduction in a one-potreaction sequence furnishes trisubstituted amino alcoholsand tetrasubstituted 1,2-diamines, respectively.178180 Ad-
Scheme 30 The von MillerPlchl pyrrole synthesis
p-TolHN Ph
CN+ Ph
CHO
KOH, EtOH
Np-Tol
Ph NPh
p-Tol
Ph
181 182 (69%)
180
Ph
(wrong structure, proposed by von Miller and Plchl, 1898)
(correct structure, proposed by Bodforss, 1931)
183
Ar NH
CNR1
1) KHMDS, THF, 78 C
2)R1 = H, alkyl R2 R3
O Ar
HNNC
R1
R2 O
R3
184
NR1
Ar R3
OHNC
R2
185
HCN H2O
NR1
Ar R3
R2
186
NR1
Ar R3
R2
NaCNBH3
187
NH2
O
N
NH
O
NN
CHO
KCN, AcOHEtOH
188
190 (95% from 188)
NC
N
KOtBu, EtOH
189
NH
N
O
N
Scheme 33 Catalytic asymmetric synthesis of tetrahydroisoquinoli-nes
191
NH
CN
1) KHMDS2)
MeO OMe
Br NHCN
HCN
192
MeO
MeO
MeO
MeO
N
MeO
MeO
193
NH
MeO
MeO(R,R)-Noyori
catalyst
HCO2H, Et3N
MeO
MeO
MeO
MeO
MeO
MeO
194 (78% from 191, 93% ee)
-
REVIEW Deprotonated a-Aminonitriles 1953
Synthesis 2009, No. 12, 19411959 Thieme Stuttgart New York
dition to imines followed by aerobic oxidation of the in-termediate enediamines yields 1,2-diimines 197, whichcan be cyclized to tetrasubstituted imidazolium salts 198.The latter are suitable as carbene precursors or can be re-duced to 1,2-diamines with high diastereoselectivity.180,181If the more reactive N-acylimines 200 are used as the elec-trophiles, the addition proceeds rapidly, even at 78 C.Elimination of HCN from the reaction products with 1,8-diazabicyclo[5.4.0]undec-7-ene in hot toluene furnishesthe a-amidoimines 202, which can be cyclized to tetrasub-stituted imidazoles 203 with phosphorus pentachloride.Acid hydrolysis of imines 202 yields the corresponding a-amido ketones 204, the cyclization of which results intrisubstituted oxazoles 205 (Scheme 34).182
11 Deprotonated a-Aminonitriles in Asymmet-ric Synthesis
Considering their versatility in carboncarbon-bond for-mations, the development of asymmetric reactions involv-
ing deprotonated a-aminonitriles is almostimperative.183,184 Husson and Royer have applied the ho-mochiral bicyclic aminonitriles 206 and 207 for the stere-oselective preparation of a variety of substitutedpiperidines, pyrrolidines and their bi- and tricyclic deriv-atives (Scheme 35).185206The starting materials are readily available from phenyl-glycinol and the corresponding a,w-dialdehyde. High dia-stereoselectivity was achieved in a-alkylations,reductions and Bruylants reactions. Moreover, the aminalmoiety could be used as an iminium ion equivalent. Thebenzylic stereodirecting substituent at nitrogen can ulti-mately be removed by hydrogenolysis. As an example ofthis powerful methodology, the synthesis of two tetrapon-erins, members of a series of ant venoms, is discussedhere. Starting with the diastereoselective a-alkylation ofthe aminonitrile moiety, the oxazolidine ring is reductive-ly opened and the auxiliary is removed by catalytic hydro-genation. The single stereogenic center in 209 thencontrols the formation of both the N,N-acetal and the a-aminonitrile. Another deprotonationalkylation sequencein combination with the reductive removal of the nitrilefunction by sodium in liquid ammonia gave T-8, while itsdiastereomer T-7 was obtained by Bruylants reaction of210 with n-pentylmagnesium bromide (Scheme 36).207 The synthesis of the tropane alkaloid ferruginine (215), anagonist of the nicotinic acetylcholine receptor that wasisolated from the bark of the arboreal species Darlingianaferruginea, was achieved starting from 207.208 In contrastto 206, its higher homologue, this material is convenientlyprepared and used as an inconsequential epimeric mix-ture. Deprotonation, alkylation with bromoacetaldehyde
Scheme 34 1,2-Additions of N-monosubstituted aminonitriles byOpatz and co-workers
Ar NH
R1
K
Ar NH
R1
KN
195
Ar
NHR1
OH
R2
(15 examples, 3278%,2.1:1 to 8.2:1 anti/syn)
196
Ar
NHR1
HN
R2
R3
(19 examples, 3268%,1:1 to 4.3:1 anti/syn)
199
Ar
NR1
N
R2
R3197(6 examples, 5573%)
MeOCH2Cl
N
NAr
R2
R1
R3
Cl
198
(6 examples2962% from 183)
BH3THF, NaBH4 (cat.)(5 examples, quant.,anti/syn up to 1:20)
or
BH3, phthalic acid(3 examples, quant.,anti/syn up to 27:1)
1) R2CHO2) BH3THF
1)2) NaCNBH3
1)2) O2
R2 NR3
R2 NR3
R2 N
O
R3
Ar
NHNC
HN
R2
O
R3R1
200
201
DBU
HCNAr
N
HN
R2
O
R3R1
202
Ar
O
HN
R2
O
R3
N
NArR1
R3
R2
PCl5 H3O+
203 204
PCl5
N
OArR3
R2205
(13 examples,5085% from 183)
(3 examples,4253% from 183)
Ar NH
CNR1
183
KHMDS
N
Scheme 35 Asymmetric synthesis of alkaloids using HussonsCN(R,S)-method
N O
Ph
NCN O
Ph
NC
206 207
HNR R H
NR R
N
N
RRN
R R
HN
R
R
R
R
HN
n
N
R
HNR
R
HNR
6784%ee > 99%de > 99% 3772%optically pure
dr = 8:2 to 7:33138%optically puredr = 8:2 to 7:3
-
1954 T. Opatz REVIEW
Synthesis 2009, No. 12, 19411959 Thieme Stuttgart New York
diethyl acetal and reductive removal of the cyano groupwith lithium in liquid ammonia furnishes 212, which isconverted into enone 213 upon acidolytic cleavage of theO,O-acetal and Horner reaction with dimethyl 2-oxopro-pylphosphonate. Under acidic conditions, the iminium iongenerated by opening of the oxazolidine ring attacks thea-position of the enone. In a concomitant and completelystereoselective addition of methanol to the b-position, thetropinone ring system is formed. The methoxy group iseliminated to restore the enone after the auxiliary has beenreplaced by a methyl group in a reductive methylation(Scheme 37).
Scheme 37 Hussons asymmetric synthesis of ferruginine
A slightly more complex but only monofunctional N-aux-iliary was developed by Enders, who employed lithiateda-aminonitriles derived from (S,S)-2,2-dimethyl-5-N-methylamino-4-phenyl-1,3-dioxane as chiral acyl anionequivalents (Scheme 38).209212 The auxiliary is the N-methyl derivative of the amine used by Weinges for thepreparation of chiral a-amino acids in an asymmetricStrecker reaction.8,213 The latter compound is formed as aside product in an industrial synthesis of the antibioticchloramphenicol. Outstandingly high enantio- and diaste-reoselectivities have been achieved in vinylogous addi-tions yielding 1,4 dicarbonyl compounds after eliminationof HCN from the primary addition products and hydroly-sis of the resulting ketimines. This procedure not only cir-cumvents the destructive removal of the auxiliary byperiodate cleavage but even permits its recycling.
Scheme 38 Asymmetric synthesis of 1,4-dicarbonyl compounds byEnders
In their synthesis of the lignans (+)-hinokinin and cubebindimethyl ether, Enders and Milovanovic used the vinylo-gous addition of the Strecker product obtained from aux-iliary 217 and piperonal to 5H-furan-2-one for theinstallation of the first asymmetric center.214,215 The prod-uct was obtained in >98% diastereomeric excess and wassubsequently a-alkylated with piperonyl bromide to fur-nish the trans-configured 2,3-disubstituted butanolide220, again in high diastereoselectivity.216 Removal of thechiral auxiliary under formation of the aryl ketone withaqueous silver nitrate and reductive removal of the carbo-nyl group in a reductionhydrogenation sequence yielded(+)-hinokinin (222), which could be converted into (S,S)-
Scheme 36 Auxiliary-controlled synthesis of two tetraponerins
N O
Ph
NC
206
1) LDA, THF2) RX3) NaBH4, EtOH
N
PhOH
O
O
208 (84%) H2, Pd/CMeOH
HNO
O
209 (91%)
1) HCl2)3) KCN
H2NOEt
OEt
N
NNCH H
210 (84%)
N
NH H
N
NH H
n-C5H11MgBrEt2O
1) LDA, THF2) n-C5H11I3) Na/NH3 (l)
(+)-T-7 (77%) (+)-T-8 (80%)
N O
Ph
NC
207
1) LDA, THF2)
BrOEt
OEt
N O
Ph
211 (86%)
NCEtO
OEt
Li/NH3
N O
Ph
212 (66%)
EtO
OEt
1) HCl2)
P(OMe)2O ON O
Ph
213 (86%)O
1) H2SO4, MeOH2) CH2O, H2, Pd/C, MeOH
MeN
O OMe
214 (61%)
TsOH, C6H6
MeN
O215 (68%)
O O
PhN
MeNC
R1
O
R1
O
O
O
R1
O
R2
OR3
OR1
O
R2
R4
OR1
O
R2
R3
O
R1
O
R1
216
(4771% from R1CHO,90 >96% ee)
(5488% from R1CHO,90 >96% ee)
48%, >96% ee
(2549% from R1CHO,>98% ee)
(4172% from R1CHO,>97 to >99% ee>98% de)
-
REVIEW Deprotonated a-Aminonitriles 1955
Synthesis 2009, No. 12, 19411959 Thieme Stuttgart New York
cubebin dimethyl ether (223) by reduction and O-methy-lation (Scheme 39). Several other lignans have been pre-pared using this strategy.217219
Along the same lines, the Enders group has developed anasymmetric nucleophilic glyoxylation by means of the vi-nylogous addition of a chiral 2-amino cyanoacetate to ni-troolefins.220 The starting material 224 was prepared byN-alkylation of auxiliary 217 with chloroacetonitrile, a-deprotonation and acylation of the keteneiminate with di-tert-butyl dicarbonate. Reaction of the potassium salt of224 with nitroalkenes furnished the Michael adducts 225in high diastereoselectivity, which could be further im-proved by a chromatographic separation. Again, the aux-iliary was removed by aqueous silver nitrate to give the a-keto-g-nitro esters 226 in high yield and with 9198%enantiomeric excess (Scheme 40, top). A related organocatalytic asymmetric nucleophilic glyox-ylation of a,b-unsaturated aldehydes with cyanoacetate227 was described by the same authors.221 Through imin-ium catalysis, the proline-derived organocatalyst 228 di-
rected the attack of the anion of 227 to the electrophile,resulting in 8387% enantiomeric excess or 4988% dia-stereomeric excess for the camphanoyl-substituted prod-ucts 231, respectively (Scheme 40, bottom).
Scheme 40 Enders asymmetric nucleophilic glyoxylation reac-tions
Asymmetric 1,2-additions of metallated a-aminonitrilescontrolled by auxiliaries derived from (S)-proline havebeen used for the preparation of chiral a-hydroxy ketoneswith up to 97% ee.222 In their asymmetric synthesis of thealkaloid (S)-(+)-coniine (238), Hurvois and co-workersused an electrochemical oxidation223 to convert piperidine233 into aminonitrile 234.224 Subsequent lithiation andalkylation with propyl iodide furnished 235 as a 9:1 mix-ture of diastereomers. The chiral benzylic auxiliary per-mitted control of the following hydride reduction,furnishing the diastereomeric amines 236 and 237 in 8%and 72% isolated yield, respectively. After chromato-graphic separation, the auxiliary was removed from 237by catalytic hydrogenation to give the target compound.Overall, this efficient procedure allowed the synthesis of(+)-coniine from commercially available (S)-1-phenyleth-ylamine in five steps and 35% yield (Scheme 41). Similarcyanationalkylation sequences have been employed bythe same authors for the preparation of various cyclic al-kaloids.225,226Scheme 39 Auxiliary-controlled synthesis of lignans
O O
HNMe
217
O
O
CHO
KCN, AcOH, MeOHPh
OO
N MePhNC
O
O
218 (81%)
1) LDA, THF2)
O
O
NMe
O
O
Ph
1) tBuLi, THF
2)
O
O
O
O
NC
219 (79%)de >98%
O
O
NMe
O
O
Ph
O
O
NC
OO
OO220 (79%)de >98%
AgNO3H2OTHF
O
OO
O
OO
O
221 (80%)de >98%ee >98%
O
OO
O
OO
1) NaBH4, MeOHCH2Cl22) H2, Pd/C, MeOH, HClO4
222 (88%)
O
O
OO
OMeOMe
223 (89%)
1) LiAlH4, THF2) NaH, MeI, THF
Br
O O
HNMe
217
Ph
OO
N MePhNC
CO2tBu224 (73%)
1) ClCH2CN Et3N, THF
2) Boc2O LDA, THF
1) KDA, THF2)
RNO2
O
O
NMe
Ph
tBuO2C NO2NC
R
(5 examples, 7584% 7596% de >98% de after chromatography)
AgNO3
H2OTHF
OtBuO
O RNO2
(5 examples5974%9198% ee)
226225
N
NC CO2tBu
+
R1
CHO
NH OTMS
PhPh
227
228
(20 mol%)
N
tBuO2C CHONC
R11) NaBH42) TBSCl or ()-camphanoyl chloride
N
tBuO2CNC
R1
229
OR2
230
AgNO3H2OTHF
O
R1
OR2tBuO
O 231
[8 examples, 2438%8387% ee (R2 = TBS)4988% de (R2 = camphanoyl)]
-
1956 T. Opatz REVIEW
Synthesis 2009, No. 12, 19411959 Thieme Stuttgart New York
Scheme 41 Synthesis of S-(+)-coniine by Hurvois and co-workers
12 Conclusions
In summary, deprotonated a-aminonitriles have proven tobe versatile building blocks that have been widely appliedas synthetic equivalents for acyl anions and a-aminocar-banions. Although the nitrile group has been replaced byother acidifying substituents such as the benzotriazolylmoiety,227231 its unparalleled anion-stabilizing capacity,combined with the convenient and economical prepara-tion of a-aminonitriles, strengthens their role as attractiveintermediates in the synthesis of a large variety of hetero-functionalized compounds. The minimal steric hindranceimposed by the rod-like cyano substituent and the high de-gree of stereofacial control exerted by some chiral auxil-iaries permits the efficient preparation of stericallyencumbered and enantiomerically pure products, respec-tively. The described advantages are likely to more thanoutweigh the manageable risk associated with the use ofthe toxic cyanide.
AcknowledgmentI thank my co-workers for their contributions. Our work was sup-ported by the Deutsche Forschungsgemeinschaft and the Fonds derChemischen Industrie.
References(1) Strecker, A. Justus Liebigs Ann. Chem. 1850, 75, 27.(2) Galatsis, P. In Name Reactions for Functional Group
Transformations; Li, J. J.; Corey, E. J., Eds.; Wiley: New York, 2007, 477.
(3) Connon, S. J. Angew. Chem. Int. Ed. 2008, 47, 1176.(4) Friestad, G. K.; Mathies, A. K. Tetrahedron 2007, 63, 2541.(5) Groeger, H. Chem. Rev. 2003, 103, 2795.(6) Yet, L. Angew. Chem. Int. Ed. 2001, 40, 875.(7) Kunz, H.; Sager, W. Angew. Chem. Int. Ed. 1987, 26, 557.
(8) Weinges, K.; Graab, G.; Nagel, D.; Stemmle, B. Chem. Ber. 1971, 104, 3594.
(9) Davis, F. A.; Reddy, R. E.; Portonovo, P. S. Tetrahedron Lett. 1994, 35, 9351.
(10) Corey, E. J.; Grogan, M. J. Org. Lett. 1999, 1, 157.(11) Dave, R. H.; Hosangadi, B. D. Tetrahedron 1999, 55, 11295.(12) Ishitani, H.; Komiyama, S.; Hasegawa, Y.; Kobayashi, S.
J. Am. Chem. Soc. 2000, 122, 762.(13) Kunz, H.; Sager, W.; Schanzenbach, D.; Decker, M. Liebigs
Ann. Chem. 1991, 649.(14) Takamura, M.; Hamashima, Y.; Usuda, H.; Kanai, M.;
Shibasaki, M. Chem. Pharm. Bull. 2000, 48, 1586.(15) Sigman, M. S.; Jacobsen, E. N. J. Am. Chem. Soc. 1998, 120,
5315.(16) Krueger, C. A.; Kuntz, K. W.; Dzierba, C. D.; Wirschun, W.
G.; Gleason, J. D.; Snapper, M. L.; Hoveyda, A. H. J. Am. Chem. Soc. 1999, 121, 4284.
(17) Wen, Y.; Gao, B.; Fu, Y.; Dong, S.; Liu, X.; Feng, X. Chem. Eur. J. 2008, 14, 6789.
(18) Bruylants, P. Bull. Soc. Chim. Belg. 1924, 33, 467.(19) Mattalia, J.-M.; Marchi-Delapierre, C.; Hazimeh, H.;
Chanon, M. ARKIVOC 2006, (iv), 90.(20) Sassaman, M. B. Tetrahedron 1996, 52, 10835.(21) Beaufort-Droal, V.; Pereira, E.; Thery, V.; Aitken, D. J.
Tetrahedron 2006, 62, 11948.(22) Bernardi, L.; Bonini, B. F.; Capito, E.; Dessole, G.; Fochi,
M.; Comes-Franchini, M.; Ricci, A. Synlett 2003, 1778.(23) Freifelder, M.; Hasbrouck, R. B. J. Am. Chem. Soc. 1960, 82,
696.(24) Rajagopalan, P.; Advani, B. G. Tetrahedron Lett. 1965,
2197.(25) Gaiffe, A.; Padovani, A. C. R. Seances Acad. Sci., Ser. C
1969, 269, 144.(26) Yoshimura, J.; Ogo, Y.; Sato, T. Bull. Chem. Soc. Jpn. 1965,
38, 1809.(27) Wasserman, H. H.; Dion, R. P.; Fukuyama, J. M.
Heterocycles 1989, 28, 629.(28) Zhu, J.; Quirion, J. C.; Husson, H. P. Tetrahedron Lett. 1989,
30, 5137.(29) Hauser, C. R.; Taylor, H. M.; Ledford, T. G. J. Am. Chem.
Soc. 1960, 82, 1786.(30) Ahlbrecht, H.; Raab, W.; Vonderheid, C. Synthesis 1979,
127.(31) Enders, D.; Lotter, H. Tetrahedron Lett. 1982, 23, 639.(32) Taillades, J.; Commeyras, A. Tetrahedron 1974, 30, 127.(33) Woodburn, H. M.; Lathroum, L. B. J. Org. Chem. 1954, 19,
285.(34) Welvart, Z. Bull. Soc. Chim. Fr. 1961, 1653.(35) Stork, G.; Ozorio, A. A.; Leong, A. Y. W. Tetrahedron Lett.
1978, 5175.(36) Reutrakul, V.; Nimgirawath, S.; Panichanun, S.;
Ratananukul, P. Chem. Lett. 1979, 399.(37) Bchi, G.; Liang, P. H.; Wst, H. Tetrahedron Lett. 1978,
2763.(38) Enders, D.; Amaya, A. S.; Pierre, F. New J. Chem. 1999,
261.(39) Bucherer, H. T.; Steiner, W. J. Prakt. Chem. 1934, 140, 291.(40) Chubb, F. L.; Edward, J. T. Can. J. Chem. 1981, 59, 2724.(41) Capp, C. W.; Cook, A. H.; Downer, J. D.; Heilbron, I. J.
Chem. Soc. 1948, 1340.(42) Parcher, B. W.; Erion, D. M.; Dang, Q. Tetrahedron Lett.
2004, 45, 2677.(43) von Miller, W.; Plchl, J. Ber. Dtsch. Chem. Ges. 1898, 31,
2718.(44) Boekelheide, V.; Weinstock, J. J. Am. Chem. Soc. 1952, 74,
660.
Ph
NH2
232
Br BrN
Ph233 (87%)
2 e, H+NaCN,LiClO4, MeOH
N
Ph
CN
234 (85%)
1) LDA
2) nPrIN
Ph
CN
235 (72%, dr = 9:1)
N
Ph
+N
Ph
236(8%, 99% ee)
237(72%, 99% ee)
1) NaBH42) chromatographic separation
H2
Pd/C NH238 (90%,
35% from 232)
-
REVIEW Deprotonated a-Aminonitriles 1957
Synthesis 2009, No. 12, 19411959 Thieme Stuttgart New York
(45) Boekelheide, V.; Ainsworth, C. J. Am. Chem. Soc. 1950, 72, 2134.
(46) Popp, F. D.; McEwen, W. J. Am. Chem. Soc. 1957, 79, 3773.(47) Popp, F. D. Adv. Heterocycl. Chem. 1968, 9, 1.(48) Popp, F. D. Bull. Soc. Chim. Belg. 1981, 90, 609.(49) Popp, F. D. Adv. Heterocycl. Chem. 1979, 24, 187.(50) Popp, F. D. Heterocycles 1973, 1, 165.(51) Popp, F. D.; Buhts, R. E.; Chesney, D. K. J. Heterocycl.
Chem. 1978, 15, 429.(52) Popp, F. D.; Klinowski, C. W.; Piccirilli, R.; Purcell, D. H.
Jr.; Watts, R. F. J. Heterocycl. Chem. 1971, 8, 313.(53) Popp, F. D.; Purcell, D. H. Jr. Synthesis 1970, 591.(54) Piccirilli, R.; Popp, F. D. Can. J. Chem. 1969, 47, 3261.(55) Gibson, H. W.; Popp, F. D.; Noble, A. C. J. Heterocycl.
Chem. 1966, 3, 99.(56) Popp, F. D.; Gibson, H. W. J. Heterocycl. Chem. 1964, 1, 51.(57) Popp, F. D.; McEwen, W. E. J. Am. Chem. Soc. 1958, 80,
1181.(58) Deuchert, K.; Hertenstein, U.; Hnig, S. Synthesis 1973,
777.(59) Hnig, S.; Wehner, G. Synthesis 1975, 180.(60) Deuchert, K.; Hertenstein, U.; Hnig, S.; Wehner, G. Chem.
Ber. 1979, 112, 2045.(61) Corey, E. J.; Seebach, D. Angew. Chem., Int. Ed. Engl. 1965,
4, 1075.(62) Corey, E. J.; Seebach, D. Angew. Chem., Int. Ed. Engl. 1965,
4, 1077.(63) Seebach, D.; Corey, E. J. J. Org. Chem. 1975, 40, 231.(64) Smith, A. B. III.; Adams, C. M. Acc. Chem. Res. 2004, 37,
365.(65) Yus, M.; Najera, C.; Foubelo, F. Tetrahedron 2003, 59,
6147.(66) Albright, J. D. Tetrahedron 1983, 39, 3207.(67) Safran, Y. M.; Bakulev, V. A.; Mokrushin, V. S. Russ.
Chem. Rev. 1989, 58, 148.(68) Enders, D.; Shilvock, J. P. Chem. Soc. Rev. 2000, 29, 359.(69) Enders, D.; Kirchhoff, J.; Gerdes, P.; Mannes, D.; Raabe, G.;
Runsink, J.; Boche, G.; Marsch, M.; Ahlbrecht, H.; Sommer, H. Eur. J. Org. Chem. 1998, 63.
(70) Boche, G.; Marsch, M.; Harms, K. Angew. Chem., Int. Ed. Engl. 1986, 25, 373.
(71) Zarges, W.; Marsch, M.; Harms, K.; Boche, G. Angew. Chem., Int. Ed. Engl. 1989, 28, 1392.
(72) Raabe, G.; Zobel, E.; Fleischhauer, J.; Gerdes, P.; Mannes, D.; Mueller, E.; Enders, D. Z. Naturforsch., A: Phys. Sci. 1991, 46, 275.
(73) Cunico, R. F.; Kuan, C. P. J. Org. Chem. 1992, 57, 1202.(74) Hauser, C. R.; Brasen, W. R. J. Am. Chem. Soc. 1956, 78,
494.(75) Hauser, C. R.; Morris, G. F. J. Org. Chem. 1961, 26, 4740.(76) Taylor, H. M.; Hauser, C. R. J. Am. Chem. Soc. 1960, 82,
1960.(77) Takahashi, K.; Mikajiri, T.; Kurita, H.; Ogura, K.; Iida, H.
J. Org. Chem. 1985, 50, 4372.(78) Kant, J.; Popp, F. D. J. Heterocycl. Chem. 1984, 21, 425.(79) Badorrey, R.; Cativiela, C.; Diaz-De-Villegas, M. D.;
Galvez, J. A. Tetrahedron: Asymmetry 2000, 11, 1015.(80) Brion, F.; Marie, C.; Mackiewicz, P.; Roul, J. M.; Buendia,
J. Tetrahedron Lett. 1992, 33, 4889.(81) Couty, F.; Evano, G.; Vargas-Sanchez, M.; Bouzas, G.
J. Org. Chem. 2005, 70, 9028.(82) Sivaprakasam, M.; Couty, F.; Evano, G.; Srinivas, B.;
Sridhar, R.; Rao, K. R. Synlett 2006, 781.(83) Agami, C.; Couty, F.; Evano, G. Tetrahedron: Asymmetry
2002, 13, 297.(84) Amorde, S. M.; Judd, A. S.; Martin, S. F. Org. Lett. 2005, 7,
2031.
(85) Stork, G. Pure Appl. Chem. 1989, 61, 439.(86) Katagiri, T.; Irie, M.; Uneyama, K. Org. Lett. 2000, 2, 2423.(87) Laurent, M.; Belmans, M.; Kemps, L.; Ceresiat, M.;
Marchand-Brynaert, J. Synthesis 2003, 570.(88) Stork, G.; Jacobson, R. M.; Levitz, R. Tetrahedron Lett.
1979, 9, 771.(89) McEwen, W. E.; Cobb, R. L. Chem. Rev. 1955, 55, 511.(90) Popp, F. D.; Kant, J. J. Heterocycl. Chem. 1985, 22, 869.(91) Tyrell, J. A. III.; McEwen, W. E. J. Org. Chem. 1981, 46,
2476.(92) Popp, F. D.; Katz, L. E.; Klinowski, C. W.; Wefer, J. M.
J. Org. Chem. 1968, 33, 4447.(93) Kant, J. J. Heterocycl. Chem. 1990, 27, 2129.(94) Reutrakul, V.; Ratananukul, P.; Nimgirawath, S. Chem. Lett.
1980, 71.(95) Carlier, P. R.; Lo, K. M. J. Org. Chem. 1994, 59, 4053.(96) Carlier, P. R.; Lo, K. M.; Lo, M. M. C.; Williams, I. D.
J. Org. Chem. 1995, 60, 7511.(97) Carlier, P. R.; Lo, K. M.; Lo, M. M. C.; Lo, P. C. K.; Lo, C.
W. S. J. Org. Chem. 1997, 62, 6316.(98) Reich, H. J.; Biddle, M. M.; Edmonston, R. J. J. Org. Chem.
2005, 70, 3375.(99) Hnig, S.; Marschner, C. Chem. Ber. 1989, 122, 1329.
(100) Leclerc, E.; Vrancken, E.; Mangeney, P. J. Org. Chem. 2002, 67, 8928.
(101) Takahashi, K.; Masuda, T.; Ogura, K.; Iida, H. Synthesis 1983, 1043.
(102) Takahashi, K.; Shibasaki, K.; Ogura, K.; Iida, H. J. Org. Chem. 1983, 48, 3566.
(103) Uchibayashi, M. Yakugaku Zasshi 1958, 78, 845.(104) Blake, J.; Willson, C. D.; Rapoport, H. J. Am. Chem. Soc.
1964, 86, 5293.(105) Smith, R. E.; Morris, G. F.; Hauser, C. R. J. Org. Chem.
1968, 33, 2562.(106) Yang, Z.; Zhang, Z.; Meanwell, N. A.; Kadow, J. F.; Wang,
T. Org. Lett. 2002, 4, 1103.(107) Abdillah, F.; Almeras, L.; Leclerc, E.; Mangeney, P.;
Vrancken, E. Synlett 2005, 1033.(108) Boekelheide, V.; Godfrey, J. C. J. Am. Chem. Soc. 1953, 75,
3679.(109) Uff, B. C.; Budhram, R. S. Synthesis 1978, 206.(110) Cooney, J. V.; McEwen, W. E. J. Org. Chem. 1981, 46,
2570.(111) Cooney, J. V.; Beaver, B. D.; McEwen, W. E. J. Heterocycl.
Chem. 1985, 22, 635.(112) Zervos, M.; Wartski, L. Tetrahedron Lett. 1984, 25, 4641.(113) Taylor, H. M.; Hauser, C. R. J. Am. Chem. Soc. 1960, 82,
1790.(114) Ahlbrecht, H.; Kompter, H.-M. Synthesis 1983, 645.(115) Schick, H.; Theil, F.; Jablokoff, H.; Schwarz, S. Z. Chem.
1981, 21, 68.(116) Ahlbrecht, H.; Pfaff, K. Synthesis 1980, 413.(117) Wakamatsu, T.; Hobara, S.; Ban, Y. Heterocycles 1982, 19,
1395.(118) Wakamatsu, T.; Kondo, J.; Hobara, S.; Ban, Y. Heterocycles
1982, 19, 481.(119) Leete, E.; Chedekel, M. R.; Bodem, G. B. J. Org. Chem.
1972, 37, 4465.(120) Leete, E.; Leete, S. A. S. J. Org. Chem. 1978, 43, 2122.(121) Mueller, W.; Preuss, R.; Winterfeldt, E. Angew. Chem., Int.
Ed. Engl. 1975, 14, 357.(122) Zervos, M.; Wartski, L.; Seyden-Penne, J. Tetrahedron
1986, 42, 4963.(123) Roux, M. C.; Seyden-Penne, J.; Wartski, L.; Posner, G. H.;
Nierlich, M.; Vigner, D.; Lance, M. J. Org. Chem. 1993, 58, 3969.
-
1958 T. Opatz REVIEW
Synthesis 2009, No. 12, 19411959 Thieme Stuttgart New York
(124) Roux, M. C.; Wartski, L.; Nierlich, M.; Vigner, D.; Lance, M. Tetrahedron 1994, 50, 8445.
(125) Ahlbrecht, H.; Vonderheid, C. Synthesis 1975, 512.(126) Takahashi, K.; Honma, A.; Ogura, K.; Iida, H. Chem. Lett.
1982, 1263.(127) Jacobson, R. M.; Clader, J. W. Tetrahedron Lett. 1980, 21,
1205.(128) Pierre, F.; Enders, D. Tetrahedron Lett. 1999, 40, 5301.(129) Tran, V. H.; Kantharaj, R.; Roufogalis, B. D.; Duke, C. C.
Eur. J. Org. Chem. 2006, 2970.(130) Chang, C. J.; Fang, J. M.; Liao, L. F. J. Org. Chem. 1993, 58,
1754.(131) Grierson, D. S.; Harris, M.; Husson, H. P. J. Am. Chem. Soc.
1980, 102, 1064.(132) Bonin, M.; Romero, J. R.; Grierson, D. S.; Husson, H. P.
J. Org. Chem. 1984, 49, 2392.(133) Harris, M.; Grierson, D. S.; Husson, H. P. Tetrahedron Lett.
1981, 22, 1511.(134) Bonin, M.; Romero, J. R.; Grierson, D. S.; Husson, H. P.
Tetrahedron Lett. 1982, 23, 3369.(135) Bonin, M.; Besselievre, R.; Grierson, D. S.; Husson, H. P.
Tetrahedron Lett. 1983, 24, 1493.(136) Grierson, D. S.; Harris, M.; Husson, H. P. Tetrahedron 1983,
39, 3683.(137) Grierson, D. Org. React. 1990, 39, 85.(138) Grewe, R.; Mondon, A. Chem. Ber. 1948, 81, 279.(139) Jimonet, P.; Grierson, D. S.; Husson, H. P. Tetrahedron Lett.
1987, 28, 6179.(140) Arnott, G.; Clayden, J.; Hamilton, S. D. Org. Lett. 2006, 8,
5325.(141) Couty, F.; David, O.; Larmanjat, B.; Marrot, J. J. Org. Chem.
2007, 72, 1058.(142) Alex, A.; Larmanjat, B.; Marrot, J.; Couty, F.; David, O.
Chem. Commun. 2007, 2500.(143) Jonczyk, A.; Lipiak, D.; Stepniewski, P.; Zdrojewski, T.
Bull. Soc. Chim. Belg. 1988, 97, 165.(144) Zdrojewski, T.; Jonczyk, A. Synthesis 1990, 224.(145) Zdrojewski, T.; Jonczyk, A. Liebigs Ann. Chem. 1993, 375.(146) Wang, J.-L.; Ueng, C.-H.; Yeh, M.-C. P. Tetrahedron Lett.
1995, 36, 2823.(147) Ezquerra, J.; Alvarez-Builla, J. J. Chem. Soc., Chem.
Commun. 1984, 54.(148) Higashino, T.; Kokubo, H.; Hayashi, E. Chem. Pharm. Bull.
1985, 33, 950.(149) Albright, J. D.; McEvoy, F. J.; Moran, D. B. J. Heterocycl.
Chem. 1978, 15, 881.(150) McEvoy, F. J.; Albright, J. D. J. Org. Chem. 1979, 44, 4597.(151) Albright, J. D.; Moran, D. B. J. Heterocycl. Chem. 1986, 23,
913.(152) Bellamy, F.; Horton, D.; Millet, J.; Picart, F.; Samreth, S.;
Chazan, J. B. J. Med. Chem. 1993, 36, 898.(153) Levy, R. S. Bull. Soc. Chim. Fr. 1967, 693.(154) Uff, B. C.; Budhram, R. S.; Ghaem-Maghami, G.; Mallard,
A. S.; Harutunian, V.; Calinghen, S.; Choudhury, N.; Kant, J.; Popp, F. D. J. Chem. Res., Synop. 1986, 206.
(155) Beall, L. S.; Padwa, A. Adv. Nitrogen Heterocycl. 1998, 3, 117.
(156) Sanders, E. B.; Secor, H. V.; Seeman, J. I. J. Org. Chem. 1976, 41, 2658.
(157) Sanders, E. B.; Secor, H. V.; Seeman, J. I. J. Org. Chem. 1978, 43, 324.
(158) Mander, L. N.; Turner, J. V. J. Org. Chem. 1973, 38, 2915.(159) Stella, L. Tetrahedron Lett. 1984, 25, 3457.(160) Bchi, G.; West, H. J. Am. Chem. Soc. 1974, 96, 7573.(161) Bryson, T. A.; Pye, W. E. J. Org. Chem. 1977, 42, 3214.(162) Vedejs, E.; Engler, D. A. Tetrahedron Lett. 1977, 1241.(163) Hiroi, K.; Nakazawa, K. Chem. Lett. 1980, 1077.
(164) Jackson, B. G.; Pedersen, S. W.; Fisher, J. W.; Misner, J. W.; Gardner, J. P.; Staszak, M. A.; Doecke, C.; Rizzo, J.; Aikins, J.; Farkas, E.; Trinkle, K. L.; Vicenzi, J.; Reinhard, M.; Kroeff, E. P.; Higginbotham, C. A.; Gazak, R. J.; Zhang, T. Y. Tetrahedron 2000, 56, 5667.
(165) Hauser, C. R.; Lednicer, D. J. Org. Chem. 1959, 24, 46.(166) Makosza, M.; Kwast, A.; Kwast, E.; Jonczyk, A. J. Org.
Chem. 1985, 50, 3722.(167) Takahashi, K.; Kurita, H.; Ogura, K.; Iida, H. Chem. Lett.
1983, 993.(168) Bodforss, S. Ber. Dtsch. Chem. Ges. B 1931, 64, 1111.(169) Treibs, A.; Derra, R. Justus Liebigs Ann. Chem. 1954, 589,
176.(170) Higginbotham, L.; Lapworth, A.; Simpson, C. J. Chem. Soc.,
Trans. 1924, 125, 2339.(171) Meyer, N.; Werner, F.; Opatz, T. Synthesis 2005, 945.(172) Bergner, I.; Opatz, T. Synthesis 2007, 918.(173) Opatz, T.; Ferenc, D. Org. Lett. 2006, 8, 4473.(174) Opatz, T.; Ferenc, D. Synthesis 2008, 3941.(175) Werner, F.; Blank, N.; Opatz, T. Eur. J. Org. Chem. 2007,
3911.(176) Uematsu, N.; Fujii, A.; Hashiguchi, S.; Ikariya, T.; Noyori,
R. J. Am. Chem. Soc. 1996, 118, 4916.(177) Liermann, J. C.; Opatz, T. J. Org. Chem. 2008, 73, 4526.(178) Kison, C.; Opatz, T. Eur. J. Org. Chem. 2008, 2740.(179) Kison, C.; Meyer, N.; Opatz, T. Angew. Chem. Int. Ed. 2005,
44, 5662.(180) Kison, C.; Opatz, T. Synthesis 2006, 3727.(181) Arduengo, A. J. III.; Krafczyk, R.; Schmutzler, R.; Craig, H.
A.; Goerlich, J. R.; Marshall, W. J.; Unverzagt, M. Tetrahedron 1999, 55, 14523.
(182) Kison, C.; Opatz, T. Chem. Eur. J. 2009, 15, 843.(183) Maigrot, N.; Mazaleyrat, J. P.; Welvart, Z. J. Org. Chem.
1985, 50, 3916.(184) Ruano, J. L. G.; Martin-Castro, A. M.; Tato, F.; Alonso, I.
J. Org. Chem. 2007, 72, 5994.(185) Husson, H. P. J. Nat. Prod. 1985, 48, 894.(186) Guerrier, L.; Royer, J.; Grierson, D. S.; Husson, H. P. J. Am.
Chem. Soc. 1983, 105, 7754.(187) Husson, H.-P.; Royer, J. Chem. Soc. Rev. 1999, 28, 383.(188) Royer, J.; Husson, H. P. Tetrahedron Lett. 1985, 26, 1515.(189) Grierson, D. S.; Royer, J.; Guerrier, L.; Husson, H. P. J. Org.
Chem. 1986, 51, 4475.(190) Lienard, P.; Royer, J.; Quirion, J. C.; Husson, H. P.
Tetrahedron Lett. 1991, 32, 2489.(191) Zhu, J.; Royer, J.; Quirion, J. C.; Husson, H. P. Tetrahedron
Lett. 1991, 32, 2485.(192) Ratovelomanana, V.; Vidal, L.; Royer, J.; Husson, H. P.
Heterocycles 1991, 32, 879.(193) Yue, C.; Royer, J.; Husson, H. P. J. Org. Chem. 1992, 57,
4211.(194) Francois, D.; Poupon, E.; Kunesch, N.; Husson, H.-P. Eur.
J. Org. Chem. 2004, 4823.(195) Gillaizeau-Gauthier, I.; Royer, J.; Husson, H.-P. Eur. J. Org.
Chem. 2002, 1484.(196) Freville, S.; Bonin, M.; Celerier, J.-P.; Husson, H.-P.;
Lhommet, G.; Quirion, J.-C.; Thuy, V. M. Tetrahedron 1997, 53, 8447.
(197) Yue, C.; Nicolay, F.; Royer, J.; Husson, H. P. Tetrahedron 1994, 50, 3139.
(198) Ribeiro, C. M. R.; de Melo, S. J.; Bonin, M.; Quirion, J.-C.; Husson, H.-P. Tetrahedron Lett. 1994, 35, 7227.
(199) Zhu, J.; Quirion, J. C.; Husson, H. P. J. Org. Chem. 1993, 58, 6451.
(200) Royer, J.; Husson, H. P. Janssen Chim. Acta 1993, 11, 3.(201) Lienard, P.; Quirion, J. C.; Husson, H. P. Tetrahedron 1993,
49, 3995.
-
REVIEW Deprotonated a-Aminonitriles 1959
Synthesis 2009, No. 12, 19411959 Thieme Stuttgart New York
(202) Huang, P.; Arseniyadis, S.; Husson, H. P. Xiamen Daxue Xuebao Ziran Kexueban 1992, 31, 646.
(203) Theodorakis, E.; Royer, J.; Husson, H. P. Synth. Commun. 1991, 21, 521.
(204) Yue, C.; Royer, J.; Husson, H. P. J. Org. Chem. 1990, 55, 1140.
(205) Zhu, J.; Quirion, J. C.; Husson, H. P. Tetrahedron Lett. 1989, 30, 6323.
(206) Huang, P. Q.; Arseniyadis, S.; Husson, H. P. Tetrahedron Lett. 1987, 28, 547.
(207) Yue, C.; Gauthier, I.; Royer, J.; Husson, H.-P. J. Org. Chem. 1996, 61, 4949.
(208) Gauthier, I.; Royer, J.; Husson, H.-P. J. Org. Chem. 1997, 62, 6704.
(209) Enders, D.; Gerdes, P.; Kipphardt, H. Angew. Chem., Int. Ed. Engl. 1990, 29, 179.
(210) Enders, D.; Mannes, D.; Raabe, G. Synlett 1992, 837.(211) Enders, D.; Kirchhoff, J.; Mannes, D.; Raabe, G. Synthesis
1995, 659.(212) Enders, D.; Shilvock, J. P.; Raabe, G. J. Chem. Soc., Perkin
Trans. 1 1999, 1617.(213) Weinges, K.; Klotz, K. P.; Droste, H. Chem. Ber. 1980, 113,
710.(214) Enders, D.; Milovanovic, M. Z. Naturforsch., B: Chem. Sci.
2007, 62, 117.(215) Enders, D.; Kirchhoff, J.; Lausberg, V. Liebigs Ann. 1996,
1361.(216) Roux, M.-C.; Patel, S.; Merienne, C.; Morgant, G.; Wartski,
L. Bull. Soc. Chim. Fr. 1997, 134, 809.
(217) Enders, D.; Lausberg, V.; Del Signore, G.; Berner, O. M. Synthesis 2002, 515.
(218) Enders, D.; Del Signore, G.; Berner, O. M. Chirality 2003, 15, 510.
(219) Enders, D.; Milovanovic, M.; Voloshina, E.; Raabe, G.; Fleischhauer, J. Eur. J. Org. Chem. 2005, 1984.
(220) Enders, D.; Bonten, M. H.; Raabe, G. Angew. Chem. Int. Ed. 2007, 46, 2314.
(221) Enders, D.; Bonten, M. H.; Raabe, G. Synlett 2007, 885.(222) Enders, D.; Lotter, H.; Maigrot, N.; Mazaleyrat, J. P.;
Welvart, Z. Nouv. J. Chim. 1984, 8, 747.(223) Le Gall, E.; Hurvois, J. P.; Renaud, T.; Moinet, C.; Tallec,
A.; Uriac, P.; Sinbandhit, S.; Toupet, L. Liebigs Ann./Recl. 1997, 2089.
(224) Girard, N.; Pouchain, L.; Hurvois, J.-P.; Moinet, C. Synlett 2006, 1679.
(225) Malassene, R.; Toupet, L.; Hurvois, J.-P.; Moinet, C. Synlett 2002, 895.
(226) Girard, N.; Hurvois, J.-P.; Toupet, L.; Moinet, C. Synth. Commun. 2005, 35, 711.
(227) Katritzky, A. R.; Yang, Z.; Lam, J. N. J. Org. Chem. 1991, 56, 6917.
(228) Katritzky, A. R.; Yang, H.; Singh, S. K. J. Org. Chem. 2005, 70, 286.
(229) Rys, V.; Couture, A.; Deniau, E.; Lebrun, S.; Grandclaudon, P. Tetrahedron 2005, 61, 665.
(230) Katritzky, A. R.; Qiu, G.; Yang, B.; Steel, P. J. J. Org. Chem. 1998, 63, 6699.
(231) Katritzky, A. R.; Cui, X.-L.; Yang, B.; Steel, P. J. J. Org. Chem. 1999, 64, 1979.