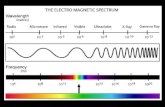
Absorption and Emission
-
Upload
faris-alarshani -
Category
Documents
-
view
215 -
download
0
Transcript of Absorption and Emission
-
8/13/2019 Absorption and Emission
1/15
-
8/13/2019 Absorption and Emission
2/15
for theoretical studies on the structure of matter and for
qualitative and quantitative analyses. ecently, however, the
definition has broadened as new techniques have been
developed that utili!e not only visible light, but many other
forms of electromagneticand non"electromagnetic radiation#
microwaves, radiowaves, x"rays, electrons,phonons(sound
waves) and others. Impedance spectroscopyis a study of
frequency responsein alternating current.
Spectroscopy is often used inphysicaland analytical chemistry
for the identification of substances through the spectrum emitted
from them or absorbed in them. $ device for recording a
spectrum is a spectrometer. Spectroscopy can be classifiedaccording to the physical quantity which is measured or
calculated or the measurement process.
Spectroscopy is also heavily used in astronomyand remote
sensing. %ost large telescopeshave spectrographs, which are
used either to measure the chemical composition and physical
properties of astronomical ob&ects or to measure their velocities
from the 'oppler shiftof spectral lines.
http://en.wikipedia.org/wiki/Electromagnetichttp://en.wikipedia.org/wiki/Radiationhttp://en.wikipedia.org/wiki/Microwavehttp://en.wikipedia.org/wiki/Radio_frequencyhttp://en.wikipedia.org/wiki/X-rayhttp://en.wikipedia.org/wiki/Electronhttp://en.wikipedia.org/wiki/Phononhttp://en.wikipedia.org/wiki/Soundhttp://en.wikipedia.org/wiki/Wavehttp://en.wikipedia.org/wiki/Frequency_responsehttp://en.wikipedia.org/wiki/Physical_chemistryhttp://en.wikipedia.org/wiki/Analytical_chemistryhttp://en.wikipedia.org/wiki/Spectrometerhttp://en.wikipedia.org/wiki/Astronomyhttp://en.wikipedia.org/wiki/Remote_sensinghttp://en.wikipedia.org/wiki/Remote_sensinghttp://en.wikipedia.org/wiki/Telescopehttp://en.wikipedia.org/wiki/Doppler_shifthttp://en.wikipedia.org/wiki/Electromagnetichttp://en.wikipedia.org/wiki/Radiationhttp://en.wikipedia.org/wiki/Microwavehttp://en.wikipedia.org/wiki/Radio_frequencyhttp://en.wikipedia.org/wiki/X-rayhttp://en.wikipedia.org/wiki/Electronhttp://en.wikipedia.org/wiki/Phononhttp://en.wikipedia.org/wiki/Soundhttp://en.wikipedia.org/wiki/Wavehttp://en.wikipedia.org/wiki/Frequency_responsehttp://en.wikipedia.org/wiki/Physical_chemistryhttp://en.wikipedia.org/wiki/Analytical_chemistryhttp://en.wikipedia.org/wiki/Spectrometerhttp://en.wikipedia.org/wiki/Astronomyhttp://en.wikipedia.org/wiki/Remote_sensinghttp://en.wikipedia.org/wiki/Remote_sensinghttp://en.wikipedia.org/wiki/Telescopehttp://en.wikipedia.org/wiki/Doppler_shift -
8/13/2019 Absorption and Emission
3/15
Contents
hysical quantity measured
* %easurement processo *. +hree main types of spectroscopy
o *.* ommon types of spectroscopy
*.*. -lame Spectroscopy
*.*.* isible spectroscopy
*.*./ 0ltraviolet spectroscopy
*.*.1 2nfrared spectroscopy *.*.3 +hermal infrared
spectroscopy
*.*.4 5uclear magnetic
resonance spectroscopy
*.*.6 hotoemission
spectroscopy
o *./ 7ess frequently used 8 combined
spectroscopy
o *.1 9uadratic ompression
onversion (9) $lgorithm
o
*.3 :ac;ground Subtraction / See also
1 External lin;s
http://en.wikipedia.org/wiki/Spectroscopy#Physical_quantity_measured%23Physical_quantity_measuredhttp://en.wikipedia.org/wiki/Spectroscopy#Measurement_process%23Measurement_processhttp://en.wikipedia.org/wiki/Spectroscopy#Three_main_types_of_spectroscopy%23Three_main_types_of_spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Common_types_of_spectroscopy%23Common_types_of_spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Flame_Spectroscopy%23Flame_Spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Visible_spectroscopy%23Visible_spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Ultraviolet_spectroscopy%23Ultraviolet_spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Infrared_spectroscopy%23Infrared_spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Thermal_infrared_spectroscopy%23Thermal_infrared_spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Thermal_infrared_spectroscopy%23Thermal_infrared_spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Nuclear_magnetic_resonance_spectroscopy%23Nuclear_magnetic_resonance_spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Nuclear_magnetic_resonance_spectroscopy%23Nuclear_magnetic_resonance_spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Photoemission_spectroscopy%23Photoemission_spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Photoemission_spectroscopy%23Photoemission_spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Less_frequently_used_.2F_combined_spectroscopy%23Less_frequently_used_.2F_combined_spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Less_frequently_used_.2F_combined_spectroscopy%23Less_frequently_used_.2F_combined_spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Quadratic_Compression_Conversion_.28QCC.29_Algorithm%23Quadratic_Compression_Conversion_.28QCC.29_Algorithmhttp://en.wikipedia.org/wiki/Spectroscopy#Quadratic_Compression_Conversion_.28QCC.29_Algorithm%23Quadratic_Compression_Conversion_.28QCC.29_Algorithmhttp://en.wikipedia.org/wiki/Spectroscopy#Background_Subtraction%23Background_Subtractionhttp://en.wikipedia.org/wiki/Spectroscopy#See_also%23See_alsohttp://en.wikipedia.org/wiki/Spectroscopy#External_links%23External_linkshttp://en.wikipedia.org/wiki/Spectroscopy#Physical_quantity_measured%23Physical_quantity_measuredhttp://en.wikipedia.org/wiki/Spectroscopy#Measurement_process%23Measurement_processhttp://en.wikipedia.org/wiki/Spectroscopy#Three_main_types_of_spectroscopy%23Three_main_types_of_spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Common_types_of_spectroscopy%23Common_types_of_spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Flame_Spectroscopy%23Flame_Spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Visible_spectroscopy%23Visible_spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Ultraviolet_spectroscopy%23Ultraviolet_spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Infrared_spectroscopy%23Infrared_spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Thermal_infrared_spectroscopy%23Thermal_infrared_spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Thermal_infrared_spectroscopy%23Thermal_infrared_spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Nuclear_magnetic_resonance_spectroscopy%23Nuclear_magnetic_resonance_spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Nuclear_magnetic_resonance_spectroscopy%23Nuclear_magnetic_resonance_spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Photoemission_spectroscopy%23Photoemission_spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Photoemission_spectroscopy%23Photoemission_spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Less_frequently_used_.2F_combined_spectroscopy%23Less_frequently_used_.2F_combined_spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Less_frequently_used_.2F_combined_spectroscopy%23Less_frequently_used_.2F_combined_spectroscopyhttp://en.wikipedia.org/wiki/Spectroscopy#Quadratic_Compression_Conversion_.28QCC.29_Algorithm%23Quadratic_Compression_Conversion_.28QCC.29_Algorithmhttp://en.wikipedia.org/wiki/Spectroscopy#Quadratic_Compression_Conversion_.28QCC.29_Algorithm%23Quadratic_Compression_Conversion_.28QCC.29_Algorithmhttp://en.wikipedia.org/wiki/Spectroscopy#Background_Subtraction%23Background_Subtractionhttp://en.wikipedia.org/wiki/Spectroscopy#See_also%23See_alsohttp://en.wikipedia.org/wiki/Spectroscopy#External_links%23External_links -
8/13/2019 Absorption and Emission
4/15
Physical quantity measured
+he type of spectroscopy depends on the physical quantity
measured. 5ormally, the quantity that is measured is an amount
or intensity of something.
+he intensity of emitted
electromagnetic radiationand the
amount of absorbed
electromagnetic radiationare
studied by electromagnetic
spectroscopy(see also cross
section). +he amplitude of macroscopic
vibrations is studied by acoustic
spectroscopyand dynamic
mechanical spectroscopy.
-
8/13/2019 Absorption and Emission
5/15
spectrum is usually called cross
section.
Measurement process
'ifferent types of spectroscopy use different measurement
processes#
Three main types of spectroscopy
Absorption spectroscopyuses the range of electromagneticspectra in which a substance absorbs. 2n atomic absorption
spectroscopy, the sample is atomi!ed and then light of a
particular frequency is passed through the vapour. $fter
calibration, the amount of absorption can be related to the
concentrations of various metal ions through the :eer"7ambert
law. +he method can be automated and is widely used to
measure concentrations of ions such as sodiumand calciumin
blood. =ther types of spectroscopy may not require sampleatomi!ation. -or example, ultraviolet8visible (08 is)
absorption spectroscopyis most often performed on liquid
samples to detect molecular content and infrared (2)
spectroscopyis most often performed on liquid, semi"liquid
(paste,grease,and petroleum &elly), dried, or solid samples to
determine molecular information, including structural
information.
Emission spectroscopyuses the range of electromagnetic
spectra in which a substance radiates. +he substance first
absorbs energy and then radiates this energy as light. +his
energy can be from a variety of sources, including collision
(either due to high temperatures or otherwise), and chemical
reactions.
Scattering spectroscopymeasures certain physical properties
by measuring the amount of light that a substance scatters at
http://en.wikipedia.org/wiki/Absorption_cross_sectionhttp://en.wikipedia.org/wiki/Absorption_cross_sectionhttp://en.wikipedia.org/wiki/Absorption_spectroscopyhttp://en.wikipedia.org/wiki/Beer-Lambert_lawhttp://en.wikipedia.org/wiki/Beer-Lambert_lawhttp://en.wikipedia.org/wiki/Sodiumhttp://en.wikipedia.org/wiki/Calciumhttp://en.wikipedia.org/wiki/UV/Vis_spectroscopyhttp://en.wikipedia.org/wiki/UV/Vis_spectroscopyhttp://en.wikipedia.org/wiki/IR_spectroscopyhttp://en.wikipedia.org/wiki/IR_spectroscopyhttp://en.wikipedia.org/wiki/Emission_spectroscopyhttp://en.wikipedia.org/wiki/Absorption_cross_sectionhttp://en.wikipedia.org/wiki/Absorption_cross_sectionhttp://en.wikipedia.org/wiki/Absorption_spectroscopyhttp://en.wikipedia.org/wiki/Beer-Lambert_lawhttp://en.wikipedia.org/wiki/Beer-Lambert_lawhttp://en.wikipedia.org/wiki/Sodiumhttp://en.wikipedia.org/wiki/Calciumhttp://en.wikipedia.org/wiki/UV/Vis_spectroscopyhttp://en.wikipedia.org/wiki/UV/Vis_spectroscopyhttp://en.wikipedia.org/wiki/IR_spectroscopyhttp://en.wikipedia.org/wiki/IR_spectroscopyhttp://en.wikipedia.org/wiki/Emission_spectroscopy -
8/13/2019 Absorption and Emission
6/15
certain wavelengths, incident angles, and polari!ation angles.
Scattering spectroscopy differs from emission spectroscopy due
to the fact that the scattering process is much faster than the
absorption8emission process. =ne of the most useful
applications of light scattering spectroscopy is aman
spectroscopy.
Common types of spectroscopy
Spectrum of light from a fluorescent lampshowing prominent
mercury pea;s.
Fluorescence spectroscopy-luorescence spectroscopy uses
higher energyphotonsto excite a sample, which will then emit
lower energy photons. +his technique has become popular for its
biochemicaland medical applications, and can be used for
confocal microscopy, fluorescence resonance energy transfer,
and fluorescence lifetime imaging.
http://en.wikipedia.org/wiki/Raman_spectroscopyhttp://en.wikipedia.org/wiki/Raman_spectroscopyhttp://en.wikipedia.org/wiki/Fluorescent_lamphttp://en.wikipedia.org/wiki/Fluorescence_spectroscopyhttp://en.wikipedia.org/wiki/Photonshttp://en.wikipedia.org/wiki/Biochemicalhttp://en.wikipedia.org/wiki/Confocal_microscopyhttp://en.wikipedia.org/wiki/Fluorescence_resonance_energy_transferhttp://en.wikipedia.org/wiki/Fluorescence_lifetime_imaginghttp://en.wikipedia.org/wiki/Image:Fluorescent_lighting_spectrum_peaks_labelled.gifhttp://en.wikipedia.org/wiki/Image:Fluorescent_lighting_spectrum_peaks_labelled.gifhttp://en.wikipedia.org/wiki/Raman_spectroscopyhttp://en.wikipedia.org/wiki/Raman_spectroscopyhttp://en.wikipedia.org/wiki/Fluorescent_lamphttp://en.wikipedia.org/wiki/Fluorescence_spectroscopyhttp://en.wikipedia.org/wiki/Photonshttp://en.wikipedia.org/wiki/Biochemicalhttp://en.wikipedia.org/wiki/Confocal_microscopyhttp://en.wikipedia.org/wiki/Fluorescence_resonance_energy_transferhttp://en.wikipedia.org/wiki/Fluorescence_lifetime_imaging -
8/13/2019 Absorption and Emission
7/15
Xray spectroscopyand Xray crystallography>hen ?"rays
of sufficient frequency (energy) interact with a substance, inner
shell electrons in the atom are excited to outer empty orbitals, or
they may be removed completely, ioni!ing the atom. +he inner
shell @hole@ will then be filled by electrons from outer orbitals.
+he energy available in this de"excitation process is emitted as
radiation (fluorescence) or will remove other less"bound
electrons from the atom ($uger effect). +he absorption or
emission frequencies (energies) are characteristic of the specific
atom. 2n addition, for a specific atom small frequency (energy)
variations occur which are characteristic of the chemical
bonding. >ith a suitable apparatus, these characteristic ?"ray
frequencies or $uger electron energies can be measured. ?"rayabsorption and emission spectroscopy is used in chemistry and
material sciences to determine elemental composition and
chemical bonding.
?"ray crystallography is a scattering processA crystalline
materials scatter ?"rays at well"defined angles. 2f the
wavelength of the incident ?"rays is ;nown, this allows
calculation of the distances between planes of atoms within thecrystal. +he intensities of the scattered ?"rays give information
about the atomic positions and allow the arrangement of the
atoms within the crystal structure to be calculated.
Flame Spectroscopy
7iquid solution samples are aspirated into a burner or
nebuli!er8burner combination, desolvated, atomi!ed, and
sometimes excited to a higher energy electronic state. +he use of
a flame during analysis requires fuel and oxidant, typically in
the form of gases. ommon fuel gases used are acetylene
(Ethyne) or hydrogen. ommon oxidant gases used are oxygen,
air, or nitrous oxide. +hese methods are often capable of
analy!ing metallic element analytes in thepart per million,
billion, or possibly lower concentrationranges. 7ight detectors
are needed to detect light with the analysis information coming
from the flame.
http://en.wikipedia.org/wiki/X-ray_spectroscopyhttp://en.wikipedia.org/wiki/X-ray_crystallographyhttp://en.wikipedia.org/wiki/Acetylenehttp://en.wikipedia.org/wiki/Hydrogenhttp://en.wikipedia.org/wiki/Oxygenhttp://en.wikipedia.org/wiki/Earth's_atmospherehttp://en.wikipedia.org/wiki/Nitrous_oxidehttp://en.wikipedia.org/wiki/Part_per_millionhttp://en.wikipedia.org/wiki/Concentrationhttp://en.wikipedia.org/wiki/X-ray_spectroscopyhttp://en.wikipedia.org/wiki/X-ray_crystallographyhttp://en.wikipedia.org/wiki/Acetylenehttp://en.wikipedia.org/wiki/Hydrogenhttp://en.wikipedia.org/wiki/Oxygenhttp://en.wikipedia.org/wiki/Earth's_atmospherehttp://en.wikipedia.org/wiki/Nitrous_oxidehttp://en.wikipedia.org/wiki/Part_per_millionhttp://en.wikipedia.org/wiki/Concentration -
8/13/2019 Absorption and Emission
8/15
Atomic Emission Spectroscopy"
+his method uses flame excitationA
atoms are excited from the heat of
the flame to emit light. +his method
commonly uses a total consumption
burner with a round burning outlet.
$ higher temperature flame than
atomic absorption spectroscopy
($$) is typically used to produce
excitation of analyte atoms. Since
analyte atoms are excited by the
heat of the flame, no special
elemental lamps to shine into theflame are needed. $ high resolution
polychromatorcan be used to
produce an emission intensity vs.
wavelengthspectrum over a range
of wavelengths showing multiple
element excitation lines, meaning
multiple elements can be detected
in one run. $lternatively, amonochromatorcan be set at one
wavelength to concentrate on
analysis of a single element at a
certain emission line. lasma
emission spectroscopy is a more
modern version of this method. See
-lame emission spectroscopyfor
more details. Atomic absorption spectroscopy
(often called $$) " +his method
commonly uses a pre"burner
nebuli!er (or nebuli!ing chamber)
to create a sample mist and a slot"
shaped burner which gives a longer
pathlength flame. +he temperature
of the flame is low enough that the
http://en.wikipedia.org/wiki/Polychromatorhttp://en.wikipedia.org/wiki/Wavelengthhttp://en.wikipedia.org/wiki/Monochromatorhttp://en.wikipedia.org/wiki/Flame_emission_spectroscopyhttp://en.wikipedia.org/wiki/Atomic_absorption_spectroscopyhttp://en.wikipedia.org/wiki/Polychromatorhttp://en.wikipedia.org/wiki/Wavelengthhttp://en.wikipedia.org/wiki/Monochromatorhttp://en.wikipedia.org/wiki/Flame_emission_spectroscopyhttp://en.wikipedia.org/wiki/Atomic_absorption_spectroscopy -
8/13/2019 Absorption and Emission
9/15
-
8/13/2019 Absorption and Emission
10/15
-
8/13/2019 Absorption and Emission
11/15
widely expanded worldwide through production control
laboratories of foundries and steel mills.
$isible spectroscopy
%any atoms emit or absorb visible light. 2n order to obtain a
fine line spectrum, the atoms must be in a gas phase. +his means
that the substance has to be vaporised. +he spectrum is studiedin absorption or emission. isible absorption spectoscopy is
often combined with 0 absorption spectroscopy in 08is
spectroscopy.
%ltra&iolet spectroscopy
$ll atoms absorb in the 0 region because photons areenergetic enough to excite outer electrons. 2f the frequency is
high enough,photoionisationta;es place.
Infrared spectroscopy
Main article:Infrared spectroscopy
2nfrared spectroscopy offers the possibility to measure different
types of interatomic bond vibrations at different frequencies.
Especially in organic chemistrythe analysis of 2 absorption
spectra shows what type of bonds are present in the sample.
Thermal infrared spectroscopy
http://en.wikipedia.org/wiki/UV/Vis_spectroscopyhttp://en.wikipedia.org/wiki/UV/Vis_spectroscopyhttp://en.wikipedia.org/wiki/Photoionisationhttp://en.wikipedia.org/wiki/Infrared_spectroscopyhttp://en.wikipedia.org/wiki/Organic_chemistryhttp://en.wikipedia.org/wiki/UV/Vis_spectroscopyhttp://en.wikipedia.org/wiki/UV/Vis_spectroscopyhttp://en.wikipedia.org/wiki/Photoionisationhttp://en.wikipedia.org/wiki/Infrared_spectroscopyhttp://en.wikipedia.org/wiki/Organic_chemistry -
8/13/2019 Absorption and Emission
12/15
Main article: Thermal infrared
spectroscopy
+hermal infrared spectroscopy measures thermal radiation
emitted from materials and surfaces and is used to determine thetype of bonds present in a sample as well as their lattice
environment. +he techniques are widely used by organic
chemists, mineralogists, andplanetary scientists. +his type of
spectroscopy is great for small children and animals of
miniscule si!es.
'uclear magnetic resonance spectroscopy
Main article:NMR spectroscopy
5uclear magnetic resonance spectroscopy analy!es certain
atomic nuclei to determine different local environments of
hydrogen, carbon, or other atoms in the moleculeof an organic
compoundor other compound. +his is used to help determine
the structureof the compound.
Photoemission spectroscopy
(ess frequently used ) combined spectroscopy
aman spectroscopy uses the
inelastic scattering of light to
analyse vibrational and rotational
modes of molecules. +he resulting
CfingerprintsC are an aid to analysis.
2nelastic neutron scattering wor;s
li;e aman spectroscopy, with
neutronsinstead of light.
http://en.wikipedia.org/wiki/Thermal_infrared_spectroscopyhttp://en.wikipedia.org/wiki/Thermal_infrared_spectroscopyhttp://en.wikipedia.org/wiki/Mineralogyhttp://en.wikipedia.org/wiki/Planetary_sciencehttp://en.wikipedia.org/wiki/NMR_spectroscopyhttp://en.wikipedia.org/wiki/Hydrogenhttp://en.wikipedia.org/wiki/Carbonhttp://en.wikipedia.org/wiki/Moleculehttp://en.wikipedia.org/wiki/Organic_compoundhttp://en.wikipedia.org/wiki/Organic_compoundhttp://en.wikipedia.org/wiki/Chemical_compoundhttp://en.wikipedia.org/wiki/Chemical_structurehttp://en.wikipedia.org/wiki/Photoemission_spectroscopyhttp://en.wikipedia.org/wiki/Raman_spectroscopyhttp://en.wikipedia.org/wiki/Inelastic_neutron_scatteringhttp://en.wikipedia.org/wiki/Neutronhttp://en.wikipedia.org/wiki/Thermal_infrared_spectroscopyhttp://en.wikipedia.org/wiki/Thermal_infrared_spectroscopyhttp://en.wikipedia.org/wiki/Mineralogyhttp://en.wikipedia.org/wiki/Planetary_sciencehttp://en.wikipedia.org/wiki/NMR_spectroscopyhttp://en.wikipedia.org/wiki/Hydrogenhttp://en.wikipedia.org/wiki/Carbonhttp://en.wikipedia.org/wiki/Moleculehttp://en.wikipedia.org/wiki/Organic_compoundhttp://en.wikipedia.org/wiki/Organic_compoundhttp://en.wikipedia.org/wiki/Chemical_compoundhttp://en.wikipedia.org/wiki/Chemical_structurehttp://en.wikipedia.org/wiki/Photoemission_spectroscopyhttp://en.wikipedia.org/wiki/Raman_spectroscopyhttp://en.wikipedia.org/wiki/Inelastic_neutron_scatteringhttp://en.wikipedia.org/wiki/Neutron -
8/13/2019 Absorption and Emission
13/15
aman =ptical $ctivity
spectroscopyexploits aman
scattering and optical activity
effects to reveal detailed
information on chiral centres in
molecules.
$uger electron spectroscopy is a
method used to study surfaces of
materials on a micro"scale. 2t is
often used in connection with
electron microscopy.
-ourier transform is an efficient
method for collecting variousspectra. +he use of -ourier
transform in spectroscopy is called
-ourier transform spectroscopy.
5early all infrared spectroscopy
(-+2) and nuclear magnetic
resonance (5%) spectroscopy are
performed with -ourier transforms.
Spectroscopy of matter in situationswhere the properties are changing
with time is called +ime"resolved
spectroscopy.
Spectroscopy using an $-%"based
analytical technique is called -orce
spectroscopy.
'ielectric spectroscopy
ircular 'ichroism spectroscopy avity ring down spectroscopy
*uadratic Compression Con&ersion "*CC# Algorithm
http://en.wikipedia.org/wiki/Raman_optical_activityhttp://en.wikipedia.org/wiki/Raman_optical_activityhttp://en.wikipedia.org/wiki/Auger_electron_spectroscopyhttp://en.wikipedia.org/wiki/Electron_microscopyhttp://en.wikipedia.org/wiki/Fourier_transformhttp://en.wikipedia.org/wiki/Fourier_transform_spectroscopyhttp://en.wikipedia.org/wiki/Nuclear_magnetic_resonancehttp://en.wikipedia.org/wiki/Time-resolved_spectroscopyhttp://en.wikipedia.org/wiki/Time-resolved_spectroscopyhttp://en.wikipedia.org/wiki/Atomic_force_microscopehttp://en.wikipedia.org/wiki/Force_spectroscopyhttp://en.wikipedia.org/wiki/Force_spectroscopyhttp://en.wikipedia.org/wiki/Dielectric_spectroscopyhttp://en.wikipedia.org/wiki/Circular_Dichroismhttp://en.wikipedia.org/wiki/Cavity_ring_down_spectroscopyhttp://en.wikipedia.org/wiki/Raman_optical_activityhttp://en.wikipedia.org/wiki/Raman_optical_activityhttp://en.wikipedia.org/wiki/Auger_electron_spectroscopyhttp://en.wikipedia.org/wiki/Electron_microscopyhttp://en.wikipedia.org/wiki/Fourier_transformhttp://en.wikipedia.org/wiki/Fourier_transform_spectroscopyhttp://en.wikipedia.org/wiki/Nuclear_magnetic_resonancehttp://en.wikipedia.org/wiki/Time-resolved_spectroscopyhttp://en.wikipedia.org/wiki/Time-resolved_spectroscopyhttp://en.wikipedia.org/wiki/Atomic_force_microscopehttp://en.wikipedia.org/wiki/Force_spectroscopyhttp://en.wikipedia.org/wiki/Force_spectroscopyhttp://en.wikipedia.org/wiki/Dielectric_spectroscopyhttp://en.wikipedia.org/wiki/Circular_Dichroismhttp://en.wikipedia.org/wiki/Cavity_ring_down_spectroscopy -
8/13/2019 Absorption and Emission
14/15
-
8/13/2019 Absorption and Emission
15/15