Stellar Atmospheres: Emission and Absorption 1 Emission and Absorption
Absorption
-
Upload
sr-drug-laboratories -
Category
Education
-
view
152 -
download
3
description
Transcript of Absorption

Absorption Study
Group MembersUsha Rijal JoshiRajan Ghimire
Prashant BasnetOm Prakash
Sajan Maharjan

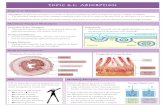
Absorption is the movement of a drug from its site of administration to the systemic circulation by crossing biological membrane.
DEFINITION

Factors Affecting Absorptions
Related to Drugs Lipid water solubility Molecular size Particle size Degree of ionization Physical forms Chemical nature Dosage form Formulation, concentration
Related to Body Area of Absorptive surface Vascularity PH Presence of other substances GI motility Diseases

ABSORPTION STUDY • An absorption study should be conducted using the route of
administration as the rate and efficiency of absorption depend on the route of administration.
Routes of Drug Administration
Enteral Parenteral Topical Oral Intradermal Dermal Sublingual Subcutaneous Inhalation Buccal Intramuscular Mucous membrane Rectal Intravenous Nasal
OphthalmicVaginal
• Regardless of the route of administration, information on changes in blood levels (concentration in whole blood, plasma, or serum) of the drug substances is necessary.

STUDY METHOD
Subjects• Studies with humans must be carefully evaluated with a concern
that there is an optimal risk/benefit ratio.• Studies should be conducted under well-controlled conditions
usually with an appropriate number of healthy volunteers.• If investigation of a drug poses a significant risk to healthy
volunteers, the studies should be conducted in patients with the target disease.

Factors of concern1.Health• Each subject will act as their own control. So, it is usually best
to have subjects of similar kinetic characteristic so that major variations are not introduced. Thus healthy volunteers are often preferred.
2. Age• Elderly patients and children can have quite different kinetics
compared with young adults. In the interest of a better matched group, subjects between the ages of 18 to 35 years are preferred.
3. Weight• In overweight or underweight subjects the Volume of
distribution may be somewhat different. So, to match the subjects, normal weights are preferred.

4. Enzyme status• Smokers or subjects taking certain other drugs may have altered
kinetics for the drug of interest. This can be caused by alteration of enzyme activity or by drug-drug interactions. These effects add complications to a study and an attempt is usually made to minimize these factors.
5. Number• The number of subjects included in the study should be sufficient to
see any real differences in the study. Usually 10 - 20 subjects are used in these studies.

Types of Study1.Standard Pharmacokinetic study• subjects are given a single dose or repeated doses of an
investigational drug.• Then, blood and urine samples are collected in compliance with a
fixed schedule. Fecal samples may also be necessary.• Then, the concentration of the investigational drug and its
metabolites is measured in these samples and the pharmacokinetic profile of the investigational drug is evaluated.
2. Population Pharmacokinetic Approach• a large number of subjects participate in the study, while the
number of samples collected from each subject can be small.• includes less inconvenience and stress on the subjects involved.

Sampling• The number of blood samples should be large enough and the
timing must be appropriate to allow an adequate determination of the absorption phases.
• Plasma concentrations in the post-absorptive phase should, whenever possible, be determined over at least two or three half-lives to avoid confusion between distribution, and elimination half-lives.
• If urinary data are obtained, the urine should be collected until there is no further detectable excretion of parent substance or metabolites within the limits of the method used.

Parameters to be considered
• For the drugs that show efficacy via the systemic circulation, pharmacokinetic parameters such as the absorption ratio, bioavailability, and absorption rate should be estimated.
• In cases of oral administration, comparison with results from intravenous administration is useful in order to estimate the absorption ratio and bioavailability, and to clarify the extent of first-pass effects.
• In the case of the drugs that are intended to be used locally, absorption from the application sites should be investigated.
As absorption from the gastrointestinal tract is likely to be affected by meals, the effects of a meal on gastrointestinal absorption should be evaluated for the drugs that are administered orally.

References:• Clinical Pharmacokinetic Studies of Pharmaceuticals (an
informal translation of the official text that was promulgated in Japanese on 1 June 2001 by Ministry of Health, Labour, and Welfare)
• Pharmacokinetic Studies in Man, Guidance formulated as per Directive 75/318/EEC.
• Biopharmaceutics and Pharmacokinetics, by David W.A. Bourne, B.Pharm., Ph.D. ( www.boomer.org )

THANK YOU!