Aberrant type I and type III collagen gene expression in human breast cancerin vivo
Transcript of Aberrant type I and type III collagen gene expression in human breast cancerin vivo

J. Pathol. 186: 262–268 (1998)
ABERRANT TYPE I AND TYPE III COLLAGEN GENEEXPRESSION IN HUMAN BREAST CANCER IN VIVO
1,2, ä2, 3, 4 1*1Department of Medical Biochemistry, University of Oulu, Oulu, Finland
2Department of Pathology, University of Oulu, Oulu, Finland3Department of Clinical Chemistry, University of Oulu, Oulu, Finland
4Department of Oncology, University of Oulu, Oulu, Finland
SUMMARY
Increased synthesis and degradation of extracellular matrix components are associated with breast cancer development. This studyevaluated type I and type III procollagen mRNA expression and the corresponding protein synthesis and maturation, as well as the tissuedistribution of these collagens, in benign breast lesions, infiltrating ductal carcinomas, and their metastases by in situ hybridization andimmunohistochemistry. In the benign lesions, the type I and type III collagen bundles were regularly organized and the expression of thecorresponding mRNA was weak, indicating a relatively slow collagen turnover. In the malignant tumours, increased expression of typeI and type III procollagen mRNAs was observed in the fibroblastic cells of the stroma; the malignant epithelial cells did not participate.The staining of corresponding newly-synthesized pN-collagens showed aberrant bundles in the invasive front of the malignant tumours.Newly-synthesized type I and type III procollagens were occasionally observed in fibroblastic cells, particularly in grade 2 and grade 3tumours. Metastases of breast carcinoma resembled poorly differentiated primary tumours with respect to their collagen synthesis anddeposition. The increased synthesis of fibrillar type I and type III procollagens may serve as a pathway for tumour invasion. Theenhanced synthesis is associated with the formation of aberrant collagen bundles, which may be more readily degradable and may thusfacilitate breast tumour invasion. ? 1998 John Wiley & Sons, Ltd.
KEY WORDS—type I collagen; type III collagen; breast cancer; desmoplasia; fibroproliferative reaction; immunohistochemistry; in situhybridization
INTRODUCTION
Breast cancer is the most common cancer in women ofthe western world, with persistently rising incidencerates.1,2 The progression of this tumour involves severalalterations in the composition and function of the extra-cellular matrix.3 Extracellular proteolysis is importantfor tumour cell invasion through the surroundingstroma.4–6 On the other hand, desmoplasia, the forma-tion of excessive dense connective tissue around theinvasive tumours, is characteristic of the ductal form ofbreast carcinoma.7
The main interstitial collagen types in the soft tissuesare type I and type III collagens, which are synthesizedintracellularly as procollagens with propeptide domainsat both ends.8 These propeptides are cleaved off extra-cellularly and the collagen molecules are associated intocollagen fibres, in which intermolecular cross-linkingreactions take place.9 Sometimes the amino-terminalpropeptides of type I and type III collagens remainattached, particularly in the case of type III collagen,yielding type I and type III pN-collagen molecules,which can be found on the surface of collagen fibrils.10
In the present study we have characterized the type Iand type III collagen expression patterns from mRNA
*Correspondence to Dr Leila Risteli, Department of ClinicalChemistry, University of Oulu, Kajaanintie 50, FIN-90220 Oulu,Finland. E-mail: [email protected]
Contract/grant sponsors: Finnish Cancer Foundations; MedicalResearch Council of the Academy of Finland; Oulu UniversityHospital
CCC 0022–3417/98/110262–07 $17.50? 1998 John Wiley & Sons, Ltd.
synthesis to corresponding protein synthesis and matu-ration using in situ hybridization and immunohisto-chemistry in benign breast tumours and in the differentgrades and stages of infiltrating ductal carcinoma. Ourresults suggest that the expression of fibrillar collagens isclosely linked to the development of the malignanttumour in breast tissue.
MATERIALS AND METHODS
Tissue samples
Samples from 16 patients with mammary disease wereobtained at operation, fixed in 10 per cent bufferedformalin, and embedded in paraffin. In addition to theprimary tumours, samples of metastases (one in skin andtwo in lung tissue) from three of these patients wereincluded. Of the primary tumours, one was a benignfibroadenoma and 15 were malignant tumours. In mostsamples, several types of lesions could be seen.
Well and moderately differentiated infiltrating ductalcarcinoma, grade 1–2, was present in ten specimens,while poorly differentiated infiltrating ductal carcinoma,grade 3, was seen in seven specimens. In nine samples ofmalignant tumour, benign fibroadenomatous changeswere also present. Fibrocystic lesions and morphologi-cally unaffected, normal breast tissue were present infour samples of malignancy.
The clinical data were obtained for 13 of the 16patients and the clinical stages, defined according to theUICC (International Union Against Cancer, 1987)
Received 7 October 1997Revised 23 January 1998
Accepted 1 July 1998

263TYPE I AND TYPE III COLLAGENS IN BREAST CANCER
classification, were compared with the type I collagená1-chain mRNA synthesis.
cRNA probes and in situ hybridization
Riboprobes for the type I procollagen and type IIIprocollagen á1-chain gene transcripts were prepared tolocalize the corresponding mRNAs. Selected 300–400 bpfragments of the cDNA covering part of the carboxy-terminal propeptide domain of the á1-chain of humantype I procollagen and part of the triple-helical domaintogether with the carboxy-terminal telopeptide and partof the carboxy-terminal propeptide of the á1-chain ofhuman type III procollagen,11–13 respectively, were sub-cloned into the polylinker site of pGEM1 vectors(Promega, Madison, WI, U.S.A.).
A riboprobe transcription kit (Promega) was used.The transcripts were labelled with 35S-UTP. Antisenseprobes were used to detect specific binding to corre-sponding mRNA and sense probes for control sectionsto detect non-specific binding. All the solutions andglassware used with the RNA probes were pretreatedwith 0·1 per cent diethylpyrocarbonate (Sigma ChemicalCo, St Louis, MO, U.S.A.).
In situ hybridization was carried out as we havedescribed previously,14 with minor modifications of themethod used by Autio-Harmainen et al.15 Pretreatedslides were hybridized overnight at 50)C in 50 per centformamide. The slides were then washed under highlystringent conditions and treated with ribonuclease A(Sigma Chemical Co) to remove non-specific binding ofthe probe. The hybrids were visualized by emulsionautoradiography. The slides were dipped into NTB-3nuclear track emulsion (Eastman Kodak, Rochester,NY, U.S.A.) diluted 1:1 with 1 per cent glycerol. Theywere then exposed for 10–14 days, developed in KodakD-19 developer, and fixed in Kodak AGEFIX. Theslides were counterstained with haematoxylin and eosin.
For examining the distribution of the signal in in situhybridization, the stromal elements were divided intotwo parts: the adjacent stroma, i.e., the stroma in thevicinity of the tumour cells, and the distant stroma.The intensity of the signal was graded as none,slight, moderate, or strong, on the basis of visualestimation.
Antibodies and immunohistochemical methods
Three monospecific antibodies were used to character-ize the forms of type I and type III (pro)collagensdeposited in the tissue. Anti-PINP detects the amino-terminal propeptide of type I procollagen. In the tissue,staining with this antibody is an indicator for newlysynthesized type I collagen still retaining the amino-terminal propeptide of the procollagen. i.e., the so-calledtype I pN-collagen. Anti-ICTP detects the carboxy-terminal telopeptide part of the type I collagen moleculeproper, when involved in intermolecular trivalent cross-linking in collagen fibres, and thus indicates the presenceof mature, cross-linked type I collagen. Anti-PIIINPis directed to the amino-terminal propeptide of typeIII procollagen. This antibody detects both newly
? 1998 John Wiley & Sons, Ltd.
synthesized and partially matured type III pN-collagen,as the propeptide remains on the surface of practicallyall type III collagen fibres in the tissues. All thesepolyclonal antibodies were raised in rabbits as describedpreviously16–18 and purified by immunoadsorption onthe relevant antigens coupled to Sepharose 4B, aftercross-adsorption with several other connective tissueantigens.19,20
The immunohistochemical staining was carried outwith the avidin–biotin–immunoperoxidase technique, asdescribed elsewhere.19,20 The slides were counterstainedwith haematoxylin. The distribution of the stainingand the staining intensity were evaluated according toprinciples similar to those described above.
RESULTS
In situ hybridization with antisense probes for themRNAs for the pro-á1 chains of type I and type IIIprocollagens revealed positive signalling in eachspecimen studied. However, in the benign lesions thiswas often quite indistinct. The sense probes were used todetect non-specific background signals. The procollagenmRNAs were exclusively detected in the spindle-shapedfibroblastic stromal cells, whereas the cells of epithelialorigin did not reveal any discernible mRNA signalling inthe specimens studied. Both type I and type III pro-collagen mRNAs and the corresponding proteindistributions were evaluated in relation to the histo-pathological diagnosis; the results are summarized inTables I and II, respectively. In general, the intensity ofprocollagen mRNA expression increased with increasinggrade of malignancy. In Table III, the intensity of type Iprocollagen mRNA expression is compared with theclinical stages of 13 patients. There seems to be noassociation between clinical stage and procollagenexpression.
Normal breast tissue and benign lesions
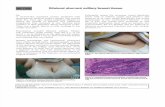
The structures in the benign areas of breast tissuesshowed light microscopically organized collagenousstructures. Both type I and type III procollagen pro-á1-chain gene transcripts were rather indistinct in thefibroblastic cells throughout the stroma (see Table I andII and Fig. 1a). Next to the involuted epithelial struc-tures, staining for type I and type III pN-collagens wasoften distinct, even though the corresponding mRNAswere not observed (Figs 1b and 1d). The distant stromashowed very weak anti-PINP staining, Mature cross-linked type I collagen (staining with anti-ICTP) wasobserved only in the stroma further away from theepithelia in these areas (Fig. 1c). The collagen bundlesrevealed by staining with any of the antibodies were thinand regularly arranged.
In most benign lesions, anti-PINP staining and type Iprocollagen mRNA were co-localized in the sameareas. Anti-ICTP staining was often similar to that ofanti-PINP, showing distinct well-organized bundles(Table I). Staining for type III pN-collagen varied fromnon-existent to strong in the benign tissues (Table II).
J. Pathol. 186: 262–268 (1998)

Intensity: 0 to+ + + (0=none; + =slight; + + =moderate; + + + =strong).
264 S. KAUPPILA ET AL.
Infiltrating ductal breast carcinoma
The stroma of infiltrating ductal carcinomas generallyexhibited irregular and dispersed collagenous structures.There seemed to be increased numbers of fibroblasticcells in the stroma, particularly in the areas adjacent tothe epithelial tumour cells.
? 1998 John Wiley & Sons, Ltd.
The mRNA for the pro-á1-chain of type I procollagenwas abundant in the fibroblastic cells both in the distantstroma and adjacent to the epithelial tumour cells. Theoverall expression patterns in the well, moderately, andpoorly differentiated tumours were generally similar(Table I). mRNA signalling was accentuated in thefibroblastic cells around the tumour cell islets, almost
Table I—Distribution and intensity of type I collagen mRNA expression, protein synthesis (PINP), and maturation (ICTP) in breasttumours
Lesion
Type I procollagená1-chain mRNA
Type I pN-collagen(PINP)
Mature cross-linkedtype I collagen (ICTP)
AS DS AS DS AS DS
Normal breast + + +to+ + + +to+ + + +Fibrocystic disease + + +to+ + + + + +Fibroadenoma + + + + + + + + + + +Infiltrating ductal carcinoma, gr 1–2 + +to+ + + + + + +to+ + + + + + + + + +Infiltrating ductal carcinoma, gr 3 + + + + +to+ + + + + + + + + + +Metastatic tumours + +to+ + + +to+ + + + + + + + +to+ + + +to+ + +
gr=grade; AS=adjacent stroma next to the epithelial cells; DS=stroma distant from the epithelial cells.
Table II—Distribution and intensity of type III collagen mRNA expression and protein deposition(PIIINP) in breast tumours
Lesion
Type III procollagen á1-chain mRNA expression
Type III pN-collagen(PIIINP)
AS DS AS DS
Normal breast 0 to+ 0 to+ 0 to+ + + 0 to+ +Fibrocystic disease + 0 to+ +to+ + +to+ +Fibroadenoma + + + + + +Infiltrating ductal carcinoma, gr 1–2 +to+ + + + + + + + + +Infiltrating ductal carcinoma, gr 3 + +to+ + + + + + +to+ + + + +Metastatic tumours + +to+ + + + + + + + + + +
gr=grade; AS=adjacent stroma next to the epithelial cells; DS=stroma distant from the epithelial cells.Intensity: 0 to+ + + (0=none; + =slight; + + =moderate; + + + =strong).
Table III—Type I procollagen mRNA expression compared with the clinical stage of the disease accordingto UICC (International Union Against Cancer) in patients with breast tumours
No. Clinical stagePredominant histopathological
diagnosisType I procollagen á1-
chain mRNA expression
1 — Fibroadenoma +2 I Infiltrating ductal carcinoma, gr 2 + +3 I Infiltrating ductal carcinoma, gr 2 + + +4 I Infiltrating ductal carcinoma, gr 3 + + +5 II Infiltrating ductal carcinoma, gr 1 + +6 II Infiltrating ductal carcinoma, gr 1 + +7 II Infiltrating ductal carcinoma, gr 2 + + +8 II Infiltrating ductal carcinoma, gr 3 + + +9 III Infiltrating ductal carcinoma, gr 2 + +
10 III Infiltrating ductal carcinoma, gr 3 + + +11 IV Metastasis + + +12 IV Metastasis + + +13 IV Metastasis + + +
Intensity: 0 to + + + (0=none; + =slight; + + =moderate; + + + =strong).gr=grade.
J. Pathol. 186: 262–268 (1998)

265TYPE I AND TYPE III COLLAGENS IN BREAST CANCER
forming a barrier-like structure (Fig. 2a), where thestroma around ductal carcinoma cell islets also showedabnormally stained PINP positive material (Fig. 2b).ICTP staining showed both the aberrant accumulationof disrupted collagen bundles and often fairly denseICTP positive bundles (Fig. 2c). Signalling for type Iprocollagen mRNA was also abundantly distributed inthe stromal cells in the distant stroma, though in a lessconcentrated manner. Often the abundant signalling wasseen in the fibroblastic cells admixed with tumour cells inthe areas where anti-PINP showed poorly organizedtype I pN-collagen bundles around the edges of invadingepithelial cancer cells (Figs 2d and 2e).
Type III procollagen mRNA expression was similar tothat of the pro-á1-chain of type I procollagen in poorlydifferentiated tumours (Fig. 2f), but less pronouncedin the well and moderately differentiated tumours(Table II). The staining with anti-PIIINP showedaberrant type III pN-collagen bundles exhibiting astaining pattern similar to that seen with anti-PINP.Strikingly, both anti-PINP and anti-PIIINP stainingwere observed intracellularly in the spindle-shapedfibroblastic cells of the stroma of several grade 2 andgrade 3 tumours (Fig. 2g).
? 1998 John Wiley & Sons, Ltd.
Metastatic tumours
Metastases from three of the patients were analysed.As in the malignant primary tumours, the fibroblasticcells seemed to be present in increased density and thecollagen bundles were irregularly arranged and discon-tinuous. Also, the transcripts for the pro-á1-chains ofboth type I and type III procollagen appeared asabundant as in the primary tumours (Tables I and II).The transcripts were accentuated in the fibroblastic cellsadjoining the epithelial structures (Fig. 2h). Immuno-histochemistry revealed aberrant type I and type IIIpN-collagen staining (Fig. 2i) and a disrupted pattern ofmature, cross-linked type I collagen bundles in theseareas (Fig. 2j).
DISCUSSION
Our present findings indicate that the major fibrillarcollagens have a relatively slow metabolic turnoverin histologically normal breast tissue and in benignmammary lesions, at least in the age group studied here,as evidenced by the weak expression of the mRNAs for
Fig. 1—Interstitial type I and type III collagens in normal breast tissue. (a) An insignificant amount of the mRNA for the pro-á1-chainof type I procollagen and (b) abundant PINP staining next to the involuted epithelial structures (star) showing regularly organized typeI pN-collagen positive bundles. (c) ICTP staining shows regularly stained maturely cross-linked type I collagen bundles in the distantstroma (star), whereas the vicinity of the epithelial cells is negative for ICTP. (d) PIIINP staining shows type III pN-collagen positivebundles next to the involuted epithelial structures (star)
J. Pathol. 186: 262–268 (1998)

266 S. KAUPPILA ET AL.
?
1998 John Wiley & Sons, Ltd. J. Pathol. 186: 262–268 (1998)
267TYPE I AND TYPE III COLLAGENS IN BREAST CANCER
the pro-á1-chains of type I and type III procollagens.Next to the involuted normal epithelial structures, therewas strong staining for the type I and type IIIpN-collagens, even though the corresponding mRNAswere absent. This indicates the well-known delayedcleavage of the amino-terminal propeptides of theseprocollagens.10 We have previously described a similardissociation between mRNA expression and amino-propeptide staining in the epithelial–stromal junction ofbenign ovarian tissue.14,19 The lack of ICTP staining inthese areas indicated that the type I collagen moleculeshad not reached the level of trivalent cross-linking.However, the stromal areas further away from theepithelial structures stained strongly for maturely cross-linked type I collagen fibres. The fibroadenomas, lesionswith distinct stromal proliferation, nevertheless showedhardly detectable mRNAs for the type I and type IIIprocollagens. This emphasizes the role of the depositionof mature, cross-linked collagen fibres in these benignneoplasms.
Expression of both type I and type III procollagensincreased with the increasing grades of malignancy.Accentuated type III procollagen mRNA expressionwas characteristic of more anaplastic tumours, ratherthan type I procollagen; we have previously observeda similar phenomenon for serous ovarian cystadeno-carcinomas.14
Human breast cancer cells have been suggested to con-tribute to the desmoplastic reaction in vivo by producingcollagen and prolyl hydroxylase, an enzyme involved inthe post-translational modification of procollagen.21 Inthe present study, we found no indication of either of theprocollagens or the corresponding mRNAs in the malig-nant epithelial cells themselves, suggesting that desmo-plasia, the fibroproliferative reaction typical of breasttumours, is produced by the stromal cells. In thisrespect, the breast tumours seem to differ from anaplas-tic ovarian epithelial tumours, where some malignantcells contain type I or type III procollagen mRNAand/or protein.14,19
Enhanced mRNA expression was observed not onlynext to the epithelial tumour cells, but also in the moredistant stromal locations. Interactions between themalignant epithelium and the surrounding stroma areconsidered essential for tumourigenicity. The matrix ofbreast tumour cells influences the host stromal cells incell culture conditions,22 and fibroblasts have also beenshown to promote breast cancer cell growth.23,24 Theenhanced synthesis of procollagen mRNA noted hereparticularly at the edge of the invasive front of the
? 1998 John Wiley & Sons, Ltd.
tumour could be explained in these terms. In otherlocations, the large amounts of procollagen mRNAs inthe fibroblastic cells occasionally gave the impression ofa barrier-like structure around the tumour cell islets.
We observed peculiar intracellular staining for bothPINP and PIIINP in the stromal fibroblastic cells in themalignant primary tumours. In principle, this may justindicate that the mRNAs indeed direct the correspond-ing protein synthesis. However, such an intracellularaccumulation of the procollagens is an interestingphenomenon that has not been previously seen in thestromal cells of serous ovarian cystadenocarcinomas14
or in murine mammary cancer.25 This accumulationmay be an inherent characteristic of the fibroblastic cellsinvolved in the procollagen production, or it could berelated to the chemical nature of the proteins produced.There is some evidence that in breast carcinoma,unusual variants of type I/type III procollagen areproduced.26 Our recent findings indicating an excess ofthe amino-terminal propeptide over the carboxy-terminal propeptide of type I procollagen in the bloodof patients with progressive breast carcinoma couldalso be explained by the synthesis of such an unusualcollagen.27
In the highly malignant tumours, our immunohisto-chemical studies suggested aberrant collagen bundleorganization in the tumour stroma. The invasiveness ofinfiltrating ductal breast carcinoma is associated withthe lack of expression of lysyl oxidase, an enzymeinvolved in the cross-linking of collagen,28 which shouldlead to a delayed and poor cross-linking process. Onthe other hand, faulty organization of the collagenmolecules within a fibre may also hinder the normalcross-linking mechanism. We have recently found that inmalignant ovarian tumours there is a shift frommaturely to only partially cross-linked type I andtype III collagens in the collagen fibres (unpublishedexperiments).
We also observed active synthesis of type I and typeIII procollagen mRNAs and the corresponding proteinsin the metastatic tumours. In addition, the ICTP stain-ing pattern showed aberrant, disrupted collagen bundlesin the invasion front of the tumour. This aberrantstaining may be due to the extracellular proteolysisof pre-existing collagen necessary for invasion,4–6 or tothe altered synthesis and processing of collagen.
In conclusion, infiltrating ductal carcinoma of thebreast induces diverse changes in the metabolism of themost abundant extracellular matrix proteins, involvingboth increased synthesis and disruption of the stroma.
Fig. 2—Type I and type III collagens in infiltrating ductal carcinoma of the breast (a–g) and in the lung metastasis of a primary breast tumour(h–j). (a) Type I procollagen mRNA signalling is seen in the stromal cells forming a barrier-like structure (arrow) around the well-differentiatedepithelial tumour cells, (b) with aberrant PINP staining showing newly-formed type I pN-collagen positive bundles (arrow) and intracellularstaining in some fibroblastic cells. (c) ICTP staining shows dense collagen bundles surrounding the tumour cells (star). (d) Newly-formed type IpN-collagen forms aberrant, scanty collagen bundles (arrow) admixed with the invasive tumour cells in less differentiated neoplasms. (e) Type Iprocollagen mRNA expression is seen in the stromal cells at the invasive front of the tumour next to the malignant epithelial cells. (f ) Abundanttype III procollagen mRNA expression in the stromal cells surrounding the poorly differentiated tumour cells; (g) PIIINP staining is seenintracellularly in fibroblastic cells (arrow). (h) Type I procollagen mRNA expression is seen in the fibroblastic cells of a metastasis, (i) wherePINP staining and (j) ICTP staining show an aberrant and destructive pattern of newly-formed and mature cross-linked type I collagen bundles,respectively
J. Pathol. 186: 262–268 (1998)

268 S. KAUPPILA ET AL.
ACKNOWLEDGEMENTS
This study was supported in part by grants from theFinnish Cancer Foundations, the Medical ResearchCouncil of the Academy of Finland, and the OuluUniversity Hospital. The cDNAs for probes were a kindgift of Professor Eero Vuorio, Department of MedicalChemistry, University of Turku, Finland. We thank MsAnnikki Huhtela, Ms Riitta Karvonen, Ms TuulaLujala, Mr Manu Tuovinen, and Ms Mirja Vahera forexcellent technical assistance.
REFERENCES1. Garfinkel L, Boring CC, Heath CW Jr. Changing trends. An overview of
breast cancer incidence and mortality . Cancer 1994; 74 (Suppl): 222–227.2. Kelsey JL, Horn-Ross PL. Breast cancer: magnitude of the problem and
descriptive epidemiology. Epidemiol Rev 1993; 15: 7–16.3. Lochter A, Bissell MJ. Involvement of extracellular matrix constituents in
breast cancer. Semin Cancer Biol 1995; 6: 165–173.4. Furcht LT, Skubitz APN, Fields GB. Tumor cell invasion, matrix metallo-
proteinases, and the dogma. Lab Invest 1994; 70: 781–783.5. Stetler-Stevenson WG, Liotta LA, Kleiner DE. Extracellular matrix 6: role
of matrix metalloproteinases in tumor invasion and metastasis. FASEB J1993; 7: 1434–1441.
6. Liotta LA, Rao CN, Barsky SH. Tumor invasion and the extracellularmatrix. Lab Invest 1983; 49: 636–649.
7. Barsky SH, Rao CN, Grotendorst GR, Liotta LA. Increased content oftype V collagen in desmoplasia of human breast carcinoma. Am J Pathol1982; 108: 276–283.
8. Kühn K. The classical collagens: types I, II, III. In: Mayne R, Burgeson R,eds. Structure and Function of Collagen Types. Orlando, FL: AcademicPress, 1987; 1–42.
9. Yamauchi M, Mechanic GL. Cross-linking of collagen. In: Nimni ME, ed.Collagen. Boca Raton: CRC Press, 1988; 157–172.
10. Fleischmajer R, Perlish JS, Timpl R. Collagen fibrillogenesis in human skin.Ann NY Acad Sci 1985; 460: 246–257.
11. Vuorio T, Mäkelä JK, Kähäri V-M, Vuorio E. Coordinated regulation oftype I and type III collagen production and mRNA levels of proá1(I) andproá2(I) collagen in cultured morphea fibroblasts. Arch Dermatol Res 1987;279: 154–160.
12. Mäkelä JK, Raassina M, Virta A, Vuorio E. Human proá1(I) collagen:cDNA sequence for the C-propeptide domain. Nucleic Acid Res 1988; 16:349.
? 1998 John Wiley & Sons, Ltd.
13. Loidl HR, Brinker JM, May M, et al. Molecular cloning and carboxyl-propeptide analysis of human type III procollagen. Nucleic Acid Res 1984;12: 9383–9394.
14. Kauppila S, Saarela J, Stenbäck F, Risteli J, Kauppila A, Risteli L.Expression of mRNAs for type I and type III procollagens in serous ovariancystadenomas and cystadenocarcinomas. Am J Pathol 1996; 148: 539–548.
15. Autio-Harmainen H, Hurskainen T, Niskasaari K, Höyhtyä M, TryggvasonK. Simultaneous expression of 70 kilodalton type IV collagenase and typeIV collagen á1(IV) chain genes by cells of early human placenta andgestational endometrium. Lab Invest 1992; 67: 191–200.
16. Melkko J, Kauppila S, Niemi S, et al. Immunoassay for intact aminotermi-nal propeptide of human type III procollagen. Clin Chem 1996; 42: 947–954.
17. Risteli J, Elomaa I, Niemi S, Novamo A, Risteli L. Radioimmunoassay forthe pyridinoline cross-linked carboxyterminal telopeptide of type I collagen:a new serum marker of bone collagen degradation. Clin Chem 1993; 39:635–640.
18. Risteli J, Niemi S, Trivedi P, Mäentausta O, Mowat AP, Risteli L. Rapidequilibrium radioimmunoassay for the aminoterminal propeptide of humantype III procollagen. Clin Chem 1988; 34: 715–718.
19. Zhu G-G, Risteli L, Mäkinen M, Risteli J, Kauppila A, Stenbäck F.Immunohistochemical study of type I collagen and type I pN-collagen inbenign and malignant ovarian neoplasms. Cancer 1995; 75: 1010–1017.
20. Zhu G-G, Stenbäck F, Risteli L, Risteli J, Kauppila A. Organization of typeIII collagen in benign and malignant ovarian tumors. Cancer 1993; 72:1679–1684.
21. Al-Adnani MS, Kirrane JA, McGee JO’D. Inappropriate production ofcollagen and prolyl hydroxylase by human breast cancer cells in vivo. Br JCancer 1975; 31: 653–660.
22. Kao RT, Hall J, Engel L, Stern R. The matrix of human breast tumor cellsis mitogenic for fibroblasts. Am J Pathol 1984; 115: 109–116.
23. van Roozendaal CEP, van Ooijen B, Klijn JGM, et al. Stromal influenceson breast cancer cell growth. Br J Cancer 1992; 65: 77–81.
24. Noël A, De Pauw-Gillet M-C, Purnell G, Nusgens B, Lapiere C-M, FoidartJ-M. Enhancement of tumorigenicity of human breast adenocarcinoma cellsin nude mice by matrigel and fibroblasts. Br J Cancer 1993; 68: 909–915.
25. Clavel C, Doco M, Lallemand A, Laurent M, Birembaut P. Detection by insitu hybridization of messenger RNAs of collagen types I and IV in murinemammary cancer. Int J Cancer 1989; 44: 548–553.
26. Pucci Minafra I, Minafra S, Tomasino RM, Sciarrino S, Tinervia R.Collagen changes in the ductal infiltrating (scirrhous) carcinoma of thehuman breast. J Submicrosc Cytol 1986; 18: 795–805.
27. Jukkola A, Tähtelä R, Thölix E, et al. Aggressive breast cancer leads todiscrepant serum levels of the type I procollagen propeptides PINP andPICP. Cancer Res 1997; 57: 5517–5520.
28. Peyrol S, Raccurt M, Gerard F, Gleyzal C, Grimaud JA, Sommer P. Lysyloxidase gene expression in the stromal reactions to in situ and invasiveductal breast carcinoma. Am J Pathol 1997; 150: 497–507.
J. Pathol. 186: 262–268 (1998)