A modular, computer-controlled system for olfactory stimulation in the MRI environment
Click here to load reader
-
Upload
jaime-meneses -
Category
Documents
-
view
214 -
download
1
Transcript of A modular, computer-controlled system for olfactory stimulation in the MRI environment

A modular, computer-controlled system for olfactorystimulation in the MRI environment
Patrice Andrieu & Vincent Bonnans & Jaime Meneses &
Jean-Louis Millot & Thierry Moulin & Tijani Gharbi
# Psychonomic Society, Inc. 2013
Abstract Although the cerebral networks involved in sen-sory perception are of general interest in neuroscience, reg-istration of the effects of olfactory stimulation, especially in amagnetic resonance imaging (MRI) environment, presentsparticular problems and constraints. This article presents de-tails of a reliable and portable system for olfactory stimula-tion that is modular in design and based on microcontrollertechnology. It has the following characteristics: (1)It is undersoftware control; (2)the presentation of olfactory stimulationcan be synchronized with respiration; (3)it can be manuallycontrolled; and (4)it is fully compatible with an MRI envi-ronment. The principle underlying this system is to direct anodor to the subject’s nostrils by switching airflow to differentodor diffusers. The characteristics of this system wereestablished using (1)ultraviolet (UV) spectroscopy, to mea-sure its response time, and (2)gas chromatography, to mea-sure the repeatability of odor presentation in terms of gas
concentration. A response time of 200± 25 ms was obtainedfor the system, and the standard deviations of the gas con-centration delivered during stimulation ranged from 1.5% to22%, depending on the odor, the airflow, and the dilution ofthe odor used. Since it is portable, controlled by software,and reliable, on the basis of the results we obtained, thissystem will lend itself to a wide range of applications inolfactory neuroscience.
Keywords fMRI . Olfactometer . Microcontroller .
Modular . Computer-controlled
As compared with other neuroimaging methods, func-tional magnetic resonance imaging (fMRI) presents dis-tinct advantages in terms of its spatial resolution andnoninvasive nature. For these reasons, it is widely usedin neuroimaging research (Sobel, Johnson, Mainland,and Yousem, 2003; Volkow et al. 1997). In humanolfactory research, however, the use of fMRI presentsspecial problems in terms of the control of olfactorystimulation, and several attempts at developing MRI-compatible olfactometers have been described. Thesesystems suffer disadvantages, either in terms of their high cost(Doty and Kobal 1995; Johnson and Sobel 2007) or their lackof temporal accuracy with regard to stimulus presentation(Lowen and Lukas 2006; Sommer et al. 2012), which prohibitevent-related paradigms. As a compromise solution, we de-veloped a system for olfactory stimulation based on principlesalready used for suprathreshold odor presentation (Lundströmet al. 2010; Sobel et al. 1997) at a reasonable cost. Inspired bythe olfactometer proposed by Lorig, Elmes, Zald, and Pardo(1999), we designed a modular olfactometer based on a mi-crocontroller with the following criteria in mind: It should besoftware-controlled and capable characterization: the first to
P. Andrieu (*) : J.<L. Millot : T. MoulinBesançon Laboratory of Integrative and ClinicalNeuroscience (EA-481), Franche-Comté University,25030 Besançon, Cedex, Francee-mail: [email protected]
P. Andrieu : T. GharbiNanomedecine Laboratory, CHU Besançon,25030 Besançon, Cedex, France
V. Bonnans : T. MoulinClinical Investigation Center CIC-IT 808 INSERM,CHU Besançon, 25030 Besançon, Cedex, France
J. MenesesGrupo de Optica y Tratamiento de Seãles, Escuela de Física,Universidad Industrial de Santander, A. 678 Bucaramanga, Colombia
P. Andrieu : T. Moulin : T. GharbiFunctional Imaging Research Department, CHU Besançon,25030 Besançon, Cedex, France
Behav ResDOI 10.3758/s13428-013-0362-x

measure system latency, and the second to measure the re-peatability of stimulation in terms of odor concentration.
Materials and method
Overall system function
The system can be described in terms of a number ofinterconnected subsystems, as follows. First, as is shown inFig. 1, there is intercommunication (data transfer) between thecontroller and the computer. The olfactometer both sends datato the computer, indicating the state of the system, and re-ceives data from it in the form of commands to run andexecute tasks. The controller in turn drives another subsystem,the pneumatic system (PS), fed by compressed air, whichregulates airflow and directs it to different odor diffusers bymeans of solenoid valves. Depending on the valve enabled bythe controller, an odor is presented to the subject. Anothersubsystem, the breathing detector (BD, described in detailbelow), measures respiration and synchronizes with the
controller. The electronic parts are placed in a control roomto avoid radio-frequency interference with the scanner. Theodor diffusers are placed near the subject (10 cm away) andare linked to the PS by Tygon tubing (10 m, Ø1.6–3.2 mm;available from Fisher Scientific, www.fishersci.com). Nasal/oral cannula (#P1319; from Pro-Tech, www.pro-tech.com),connected to the BD by Tygon tubing, are used both to registerthe subject’s respiration and to present odors. The nasal side ofthe cannula is used to measure pressure, and its oral side isconnected to the odor diffusers via PTFE tubing (10 cm,Ø1.6–3.2 mm; www.fishersci.com) inserted into the oral seg-ment (Fig. 1). The airflow enters the nostrils, and the BDcloses the part of the cannula passing over the ears, and thusprevents air flow (and odors) in this direction. Some odors canbe captured by the cannula and thus persist after stimulation(e.g., isovaleric acid). In this case, it is preferable to direct theairflow toward the nostrils without passing through the can-nulas. To achieve this, the outlet of the PTFE tubing is placed2 cm from the nostrils. Alternatively, it is possible to useanother model of nasal cannula (#P1317-20; www.pro-tech.com) that does not include an oral side.
Fig. 1 Schematic diagram shows global functioning of the olfactometer, and in particular its modular aspect
Behav Res

Breathing detector
The power supply for the BD is enclosed in metal in order toavoid electromagnetic disturbance, and all electronic partsare enclosed in a Faraday cage. Respiration is measuredusing a pressure sensor (SKU #14830; SleepSense, www.sleepsense.com) connected to the nasal cannula. Changes inpressure due to breathing into the nasal cannula correspondto the velocity of air, hence to the flow rate. This methodprovides a high temporal resolution and good flexibility inthe MRI environment (Johnson et al. 2006). The analogsignal representing respiration is first amplified usingAD620 amplifiers (Analog Devices, www.analog.com) andsubjected to low-pass filtering in order to retain only that partof the signal corresponding to the respiration frequency (0.2–0.5 Hz), with high-frequency noise removed. The circuitdepends on a pressure sensor. For more details, please referto the AD620 data sheet. Filtering is processed by a resistor–capacitor circuit (RC circuit). The analog signal is thendigitized for further processing and displayed (Fig. 2), usinga microcontroller (#PIC16F877; Microchip Technology,Inc., www.microchip.com) that codes the signal in eight bits,which is sufficient to represent the respiratory cycle andsimplifies the program. The signal is acquired every 10 msand compared to two thresholds using a Schmitt trigger. The
result of the comparison is written as a digital output, allowingthe controller to be synchronized with the respiratory cycle.This part of the program is prioritized, and the rest of theprogram is placed in an infinite loop for display and keyboardmanagement. Both the thresholds and the signal gain can beset and LCD-displayed, with a resolution of 128 × 64 pixels(#64128MCOG; Displaytech Ltd., www.displaytech.com.hk).An output is provided for connecting an acquisition card torecord the amplified signal of the subject’s respiration.
Pneumatic system
The PS comprises six electrical inputs connected to thecontroller (A in Fig. 3), six push-buttons for manual controlof the system (B in Fig. 3) and pneumatic outputs (C inFig. 3): One delivers a continuous airflow, six deliver airflowon demand according to the state of the inputs or push-buttons (each corresponding to a pneumatic output), andthe last delivers compensatory airflow when the six previousoutputs are all switched off, in order to keep the total flowconstant. All buttons are connected to a mixer (#1005 allowsfor the use of four odors, and #1006 for the use of six;Omnifit, www.omnifit.com), placed near the subject, that islinked to the nasal cannula. One-way valves (#3050591;www.fishersci.com) are placed between the odor diffusersand the mixer in order to block any backflow of odorant intothe PS. Some odors, like isoamyl acetate, can deteriorate theone-way valves early on, reducing pressure increases and aswell as increasing system response time (after several uses,we measured a delay of 200 ms relative to first use). In theworst-case scenario, the one-way valves remain closed. It isthus necessary to check and change the valves regularly.Each flow delivered on demand passes through an odordiffuser before arriving in the mixer, so each electrical inputdrives a single odor channel. The continuous airflow reducesflow change due to opening and closing the valves. Thus, thechange between odorant and nonodorant conditions does not
Fig. 2 Breathing signal and thresholds are displayed on the screen. Theuser can move up or down the thresholds and adjust the signal gain
Fig. 3 Schematic of pneumatic system
Behav Res

produce any thermal or tactile cues. When no odor is presentedto the subject, the flow rate is maintained by compensatoryairflow that replaces the airflow transmitting the odor. Thecontinuous airflow is managed by a flowmeter regulator (Din Fig. 3; #P16A4-BA0A; Aalborg, www.aalborg.com). Analternative airflow is managed by another flowmeter (E inFig. 3) whose output supplies six normally closed manifoldvalves/selectors (F in Fig. 3), each of which corresponds to oneof the six odor channels. Additionally, a normally open valve(G in Fig. 3; #225T091 and #161T021 from NResearch, Inc.,www.nresearch.com) corresponds to the compensatory air-flow. These valves are driven by a specific driver circuit (Hin Fig. 3; #225D5X12, www.nresearch.com) in order to pro-long valve life and limit power consumption. Another elec-tronic circuit combines the push-button and electrical inputs,and manages the normally open valve. Each push-button isassociated with an electrical input to an OR logic gate (I inFig. 3). The normally open valve, which allows air to flowwhen no odor channel is selected, is controlled by an OR gate(J in Fig. 3) connected to the output of the other OR gate. Thisvalve is then automatically closed when one or more odorchannels is selected. Medical air is used to supply air for thePS, as well as air compressors that require two filters(#AC010ABFI and #AO010ABFX; Parker Hannifin Corp.,www.parker.com) and a pressure-reducing valve. The pneu-matic system was simplified in relation to Lorig et al.’s (1999)olfactometer: Only two flowmeter regulators were used. Thisairflow management ensures the same response time, what-ever odor channel is selected, but it does not enable theconcentration to be adjusted with the “air dilution” method.Consequently, a different method is described below (see theOdor Diffusion section).
Controller functioning
The controller is based on microcontroller PIC16F877(www.microchip.com), which acts as an interface betweenthe computer and the PS. It receives commands from thecomputer, runs the task required, and informs the computerin real time of changes in the system’s state. For ease of useand implementation, the controller uses the RS232 port toexchange data with the computer. The controller manages sixdigital outputs corresponding to each odor channel. To drivethe PS, a request is sent by the computer to the controller.The request is composed of four parameters:
& the number of outputs that need to be enabled (numberedfrom 1 to 6);
& the length of time during which the outputs need to beenabled, and the relevant time unit (seconds, millisec-onds, or number of respirations);
& information about the synchronization with respiration(enabled or disabled); and
& the task number (this is optional but can be useful inprocessing, e.g., fMRI or other data offline; the tasknumber appears in the data sent to the computer).
The request is received on the PIC UART (universal asyn-chronous receiver/transmitter) module, and the program trans-lates the data, saves it, and confirms receipt of the request. Thetask is defined, in the program of the microcontroller, by fourparameters: the task number, the time unit, synchronizationenabled (or disabled), and the “time to live of task” (TTLT).When the output activation does not have to be synchronizedwith respiration, the program enables the output, returns feed-back to the computer, and informs the user via a message onthe liquid crystal display (LCD). When the output activationdoes need to be synchronized with respiration, the programwaits for the signal’s rising edge that is generated by the BDbefore activating the output. The user is informed via the LCDthat the output will be activated for the next respiratory cycle.The following process is the same as for synchronized tasks.As soon as the output is enabled, the TTLT decrements everysecond, millisecond, or respiratory cycle, according to thetime unit chosen. When the TTLT is equal to zero, the outputis switched off and a message is sent to the computer.
Technical specifications of the controller
We measured the execution time of the program functionsand calculated the response time, for both the most favorablecase and the worst case, at different serial-port bit rates.Since the response time is heavily dependent on the bit rate,we used a speed of 115,200 baud (the electronic componentsused did not allow a higher speed). We obtained responsetimes of 3.8 ms for the best case, and 9.9 ms for the worstcase. Temporal accuracy depends on the time unit selected.Indeed, three clocks can decrement the TTLT: one everymillisecond, another every second, and the last every BDsignal rising edge. The outputs are enabled as soon as pos-sible, in order to minimize response time (Fig. 4). Theprogram does not wait for the clock signal rising edge unlesstask activation is synchronized with respiration and the unitof time used is the number of inhalations. Hence, outputs areenabled during the request period increase by an error time of1 ms, 1 s, or one breathing period, according to the time unitused.
Odor diffusion
The odor diffusers comprise a filter holder (#SX0002500;www.fishersci.com) and an activated carbon felt (#FC1201;Pica, www.picacarbon.com) in the shape of a ring containingodorous liquid (Fig. 5). Carbon felt has a high specificsurface area (2,500 m2/g) that absorbs a high volume ofliquid. This shape provides a constant concentration of odor
Behav Res

over time. The felt is 2 mm thick, the radius of the larger ringis the same as that of the filter (22 mm), and the smallerradius measures 6 mm. To set a concentration, the liquidodorant is diluted into a neutral nonodorant solution(diethylphthalate). The volume of solution injected into thefelt was 400 μL in the present test.
Measuring response time of the olfactometer
To measure the system’s temporal resolution, we designed anapparatus measuring ultraviolet (UV) absorption (Johnsonet al. 2003) of the gas flow leaving the nasal cannula (Fig. 6).The gas used for this measurement is acetone, since itsextinction coefficient is high (12.4 L/mol.cm at 280 nm)and its vapor pressure is low (228 mbar at 20º), ensuring ahigh gas concentration. The UV source is a diode emittingspectral light centered at 260 nm. The beam is homogenizedand split in two. One of these beams is a reference whoseintensity is directly measured by a photodiode, and the otherpasses through the gas leaving the nasal cannula. The pho-todiode electric currents are converted into tensions with a
transimpedance OPA230. Both signals are acquired by aNational Instruments card. A MATLAB program drives the
Fig. 5 Photo of an odor diffuser opened. At the top is the body of thehold filter, at the bottom, the backing of the felt and the felt itself , bothin the shape of ring
Fig. 6 This apparatus measures the concentration at the outlet of thenasal cannula. The ultraviolet (UV) beam of a light-emitting diode(LED) is split in two: One beam is a reference, and the second passesthrough the gas. For this measure, we replace the odor by acetone,which has a high absorption in UV
Fig. 7 Delays (on the left) and temporal resolution as standard errors(on the right) of the olfactometer, depending on the flow rates of boththe odorant air and the continuous odorless airflow
Fig. 4 The temporal resolution of the controller depends on the unittime chosen. The output selected is switched on as soon as possible.Consequently, when the output is switched on between two clock ticks,the period of activation is longer than the period request
Behav Res

pneumatic system via the controller and acquires photodiodesignals via the National Instruments card. We obtained the gasconcentration by using the Beer–Lambert law: C = (−1/lε)log(I/I0). The apparatus frame, in aluminum, was connected toground, the amplifier circuit was housed in a Faraday cage,and the shielded cable was used to limit electromagneticinterference.
Results and discussion
Olfactometer response time
A MATLAB routine was programmed to measure the re-sponse time of the system. This routine runs 40 cycles,switching between 4 s of clean air and 4 s sending acetone.The response time of the olfactometer depends on two pa-rameters: namely, the value of constant airflow and the value
of odor-transmitting airflow. Therefore, measurements weremade for several flows: the constant-airflow rate from 0 to1.8 L/min, with a step of 0.2 L/min, and the odor-transmitting airflow rate from 0.2 to 1.8 L/min with a stepof 0.2 L/min. The delay (latency) was measured between thetime at which feedback was returned by the controller to theMATLAB program and the time when the concentrationexceeded 95% of nominal concentration. The nominal con-centration was the mean concentration obtained during the2.5-s period that followed the acetone transmission.
Response time was observed to decrease when the odorairflow rate increased (Fig. 7). We obtained the same resultwhen the flow rate of the constant airflow was increased, butthe effect was less salient.
Thus, for a given total flow rate, the shortest response timewas obtained with the lowest constant stream flow rate, butthis flow rate had to be high enough to ensure that the subjectdid not perceive a tactile cue from the valve switching. Weobserved that for a flow rate of odorant air higher than1 L/min, the response time of the olfactometer was less than0.2 s, with a time resolution less than 25 ms. For an odorantair flow rate higher than 0.4 L/min, the response time wasless than 0.3 s, with a resolution less than 50 ms. Thus,thanks to the synchronization of odor presentation with thesubject’s inhalations, we could determine with high temporalprecision the beginning of stimulation with typical flow rate.
Repeatability of stimulation
To confirm that repeated presentations of odor resulted in thesame concentration of gas, we used a variable delay betweensuccessive odor presentations and measured a quantity of gassent during the second presentation using gas chromatogra-phy. The odors were presented for 15 s.
Only the airflow that transmitted odor was used, with aflow rate of 1 L/min. Six pause durations (10, 20, 40, 80,
Table 1 Means and standard errors of gas concentration measuredduring several odor presentations separated by a variable delay
Odorant Dilution (%) Concentration (ppm) Standard Error (%)
acetate 100 333 2.6
50 219 5.4
25 117 10.4
12.5 60 9.9
pyridine 100 1,932 1.5
50 586 16
25 226 20
12.5 61 20.8
butanol 100 764 3.1
50 399 5.4
25 312 21.7
12.5 106 21.8
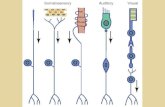
Fig. 8 Brain activations in response to olfactory stimulations (phenylethyl alcohol and isoamyl acetate) contrasted with the odorless airflowcondition. On the left, bilateral activations of the orbitofrontal cortex are
observed [Talairach coordinates: (21, 34, –5) and (−26, 33, –2)]. On theright, bilateral activations of the piriform cortex are observed [Talairachcoordinates: (19, 1, –7) and (−20, –2, –11)]
Behav Res

160, 320 s) were used, and for each of them the measure wasrepeated five times. The 30 measures were released succes-sively, and the order of pause durations was randomized. Thegas concentration delivered during odor presentation wasmeasured using the gas chromatography method. Threeodors were used: n-butanol, isoamyl acetate, and pyridine.These odorants are currently used in olfactory psychophys-ical studies. A lower dilution of the odorant corresponded tolower gas concentration and higher standard error (Table 1).These results show the limits of the odor diffusion method: Itcannot work with low concentrations of these three odorsclose to the olfactory detection threshold. However, it isappropriate for suprathreshold concentrations.
fMRI results
The olfactometer presented above was used in a previousfMRI study (Billot et al. 2011). In this study, two odors wereused with three different durations of presentation: over oneinhalation, three inhalations, and six inhalations. The olfac-tometer was synchronized with the inhalations and deliveredodors only during this phase.
The Fig. 8 shows the observed activations when theolfactory stimulation condition (one-inhalation duration)was contrasted with the odorless condition (p < .005corrected, qFDR). We identified two brain areas that arecharacteristic of olfactory perception (Sobel et al. 2003):the orbitofrontal cortex and the piriform cortex.
These results (adapted from Billot et al. 2011) demon-strate the use and the interest of this olfactometer for blockparadigms.
Conclusions
We have described the development of a versatile and mod-ular MRI-compatible olfactometer previously used for anfMRI study (Billot et al. 2011). The pneumatic system canbe used alone, with the operator manually driving the valves,or, when necessary, can be easily controlled by computersoftware and synchronized with the subject’s inhalationsusing the breathing detector. The modules are connectedonly when necessary, which can simplify the implementationof the system. Following extensive tests using the parametersdescribed above, we established the response time of thesystem and odor concentrations in different conditions. Weobtained a response time of less than 200 ms with a temporalresolution less than 25ms in typical conditions, which appears
convenient for fMRI event-related paradigms. The results ofstimulation repeatability obtained depended on the dilutionand odors used, but they were efficient for suprathresholdolfactory presentation, and so for a wide range of fMRIolfaction studies.
Author Note We are grateful to Holly Sandu for her language assis-tance with the manuscript.
References
Billot, P.-E., Comte, A., Galliot, E., Andrieu, P., Bonnans, V., Tatu,L., . . . Millot, J.-L. (2011). Time course of odorant- andtrigeminal-induced activation in the human brain: An event-related functional magnetic resonance imaging study.Neuroscience, 189, 370–376.
Doty, R. L., & Kobal, G. (1995). Current trends in the measurement ofolfactory function. In R. L. Doty (Ed.), Handbook of olfaction andgustation (Vol. 8, pp. 191–225). New York, NY: Dekker.
Johnson, B. N., Mainland, J. D., & Sobel, N. (2003). Rapid olfactoryprocessing implicates subcortical control of an olfactomotor sys-tem. Journal of Neurophysiology, 90, 1084–1094.
Johnson, B. N., Russell, C., Khan, R. M., & Sobel, N. (2006). Acomparison of methods for sniff measurement concurrent witholfactory tasks in humans. Chemical Senses, 31, 795–806.
Johnson, B. N., & Sobel, N. (2007). Methods for building an olfactom-eter with known concentration outcomes. Journal of NeuroscienceMethods, 160, 231–245.
Lorig, T. S., Elmes, D. G., Zald, D. H., & Pardo, J. V. (1999). Acomputer-controlled olfactometer for fMRI and electrophysiolog-ical studies of olfaction. Behavior Research Methods, Instruments,& Computers, 31, 370–375.
Lowen, S. B., & Lukas, S. E. (2006). A low-cost, MR-compatibleolfactometer. Behavior Research Methods, 38, 307–313.doi:10.3758/BF03192782
Lundström, J. N., Gordon, A. R., Alden, E. C., Boesveldt, S., &Albrecht, J. (2010). Methods for building an inexpensivecomputer-controlled olfactometer for temporally-precise experi-ments. International Journal of Psychophysiology, 78, 179–189.
Sobel, N., Johnson, B., Mainland, J., & Yousem, D. (2003). Functionalneuroimaging of human olfaction. In R. L. Doty (Ed.), Handbookof olfaction and gustation (2nd ed., Vol. chap.12). London, UK:Informa Healthcare. doi:10.1201/9780203911457.ch12
Sobel, N., Prabhakaran, V., Desmond, J. E., Glover, G. H., Sullivan, E.V., & Gabrieli, J. D. E. (1997). A method for functional magneticresonance imaging of olfaction. Journal of Neuroscience Methods,78, 115–123.
Sommer, J. U., Maboshe, W., Griebe, M., Heiser, C., Hörmann, K.,Stuck, B. A., & Hummel, T.(2012). A mobile olfactometer forfMRI-studies. Journal of Neuroscience Methods, 209, 189–194.doi:10.1016/j.jneumeth.2012.05.026
Volkow, N. D., Rosen, B., & Farde, L. (1997). Imaging the livinghuman brain: Magnetic resonance imaging and positron emissiontomography. Proceedings of the National Academy of Sciences,94, 2787–2788.
Behav Res