7 - Regulations.gov · inhalation assessments have been re-calculated using the updated HEC...
Transcript of 7 - Regulations.gov · inhalation assessments have been re-calculated using the updated HEC...

Permethrin
DATE:
SUBJECT:
Human Health Risk Assessment DP No. 414137
UNITED STATES ENVIRONMENT AL PROTECTION AGENCY W ASIDNGTON, D.C. 20460
30-JUN-2017
OFFICE OF CHEMICAL SAFETY AND POLLUTION PREVENTION
Permethrin: Human Health Draft Risk Assessment for Registration Review.
PC Code: 109701 Decision No.: 482012 Petition No.: NA
DP Barcode: D414137 Registration No.: NA Regulatory Action: Registration Review Case No.: 2510 Risk Assessment Type: Single Chemical/ Aggregate
TXRNo.: NA CAS No.: 52645-53-1 MRIDNo.: NA 40 CFR: §180.378
FROM:
Joshua Godshall, Biologist Risk Assessment Branch I ( HED (7509P)
THROUGH: Thomas Moriarty, Acting Branch Chie< ~~ / If=--~ . RAB VI/ HED (7509P) 7 ..,,.. / / Michael A. Doherty, Ph.D. , Senior Chemist . Monique M. Perron, Sc.D., Senior Toxicologist ~t.fu,( \JtV HED Risk Assessment Review Committee (RARC)
TO: Linsey Walsh, Chemical Review Manager Elizabeth Evans, Team Leader Kelly Sherman, Branch Chief Risk Management and Implementation Branch III Pesticide Re-Evaluation Division (PRD; 7508P)
As part of Registration Review, the Pesticide Re-evaluation Division (PRD) of the Office of Pesticide Programs (OPP) has requested that the Health Effects Division (HED) evaluate the
Page 1 of 129

Permethrin Human Health Risk Assessment DP No. 414137
Page 2 of 129
occupational exposure assessments to estimate the risks to human health that may result from the currently registered uses of permethrin. This memorandum serves as HED’s draft human health risk assessment of the dietary, residential, aggregate, and occupational exposures and risks from the registered uses of permethrin. The hazard characterization and endpoint selection were provided by Chris Schlosser; the occupational and residential exposure assessments were provided by Josh Godshall; and the residue chemistry, dietary, and risk assessments were provided by Julie Van Alstine. The drinking water assessment was previously provided by José Melendez of the Environmental Fate and Effects Division (EFED). The most recent quantitative human health risk assessment was performed in 2009 (C. Smith, D357566, 01-APR-2009) and the current risk assessment includes the following updates:
There is no longer a non-cancer dermal hazard for permethrin; There is no longer a chronic dietary endpoint for permethrin; Two acute oral neurotoxicity studies have been evaluated for permethrin; The human-equivalent concentration (HECs) for systemic effects that are used in the
inhalation assessments have been re-calculated using the updated HEC calculator; Updated tolerances are recommended for some commodities based on field trial data that
were received in response to the permethrin data call-in (GDCI-109701-26467); An updated dietary assessment was completed using U.S. Department of Agriculture
(USDA) Pesticide Data Program (PDP) data and percent crop treated (PCT) data; The registered residential uses of permethrin have been reevaluated using the updated
inhalation risk assessment point of departure and the revised Residential Standard Operating Procedures (SOPs);
The Residential Exposure Joint Venture (REJV) National Pesticide Survey (2012-2013) was used to refine the adult residential cancer assessment;
Updated acute and short-term aggregate exposure assessments were completed; An occupational exposure assessment for the registered uses was completed reflecting
recent updates to the permethrin risk assessment points of departure, HED’s SOPs, and policy changes for body weight assumptions.
A Tier II Incident and Epidemiology Report was completed. A summary of the findings and an assessment of human risk resulting from the registered uses of permethrin are provided in this document.

Permethrin Human Health Risk Assessment DP No. 414137
Page 3 of 129
Table of Contents 1.0 Executive Summary ............................................................................................................. 5 2.0 HED Conclusions................................................................................................................. 9
2.1 Data Deficiencies ............................................................................................................. 9 2.2 Tolerance Considerations ............................................................................................... 10 2.2.1 Enforcement Analytical Method ................................................................................ 10 2.2.3 International Harmonization ....................................................................................... 12 2.3 Label Recommendations ................................................................................................ 12
3.0 Introduction ........................................................................................................................ 13 3.1 Chemical Identity ........................................................................................................... 13 3.2 Physical/Chemical Characteristics ................................................................................. 13 3.3 Pesticide Use Pattern ...................................................................................................... 14 3.4 Anticipated Exposure Pathways ..................................................................................... 15 3.5 Consideration of Environmental Justice ........................................................................ 15
4.0 Hazard Characterization and Dose-Response Assessment ................................................ 15 4.1 Toxicology Studies Available for Analysis ................................................................... 16 4.3 Pyrethroid Pharmacokinetic and Pharmacodynamic Profile .......................................... 18
4.3.1 Pharmacokinetics (PK) ........................................................................................... 19 4.3.2 Pharmacodynamics (PD) ........................................................................................ 20 4.3.3 Critical Duration of Exposure ................................................................................. 22
4.4 Safety Factor for Infants and Children (FQPA Safety Factor) ....................................... 24 4.4.1 Completeness of the Toxicology Database ............................................................. 24 4.4.2 Evidence of Neurotoxicity ...................................................................................... 25 4.4.3 Evidence of Sensitivity/Susceptibility in the Developing or Young Animal ......... 25 4.4.4 Residual Uncertainty in the Exposure Database ..................................................... 26 4.5.1 Dose-Response Assessment .................................................................................... 26 4.5.2 Recommendation for Combining Routes of Exposure for Risk Assessment ......... 28 4.5.3 Cancer Classification and Risk Assessment Recommendation .............................. 28 HEC = human-equivalent concentration; HED = human-equivalent dose. NA = not applicable (the expected duration of the exposure scenario is less than the duration in the available inhalation toxicity studies; downward adjustments are not permitted). ................ 30 4.6 Endocrine Disruptor Screening Program .................................................................... 30
5.0 Dietary Exposure and Risk Assessment ............................................................................ 31 5.1. Residues of Concern Summary and Rationale ............................................................... 31 5.2 Food Residue Profile ...................................................................................................... 31 5.3 Water Residue Profile .................................................................................................... 32 5.4 Dietary Risk Assessment ................................................................................................ 33 5.4.1 Overview of Residue Data Used ................................................................................ 33 5.4.2 Percent Crop Treated Used in Dietary Assessment .................................................... 33 5.4.3 Acute Dietary Risk Assessment ................................................................................. 34 5.4.4 Average Dietary Exposure Assessment ...................................................................... 34 5.4.5 Cancer Dietary Risk Assessment ................................................................................ 34 5.4.6. Dietary Assessment Summary Tables ........................................................................... 35
6.0 Residential (Non-Occupational) Exposure/Risk Characterization ......................................... 35 6.1 Residential Handler Exposure/Risk Estimates ............................................................... 36 6.2 Residential Post-Application Exposure and Risk Estimates .......................................... 45

Permethrin Human Health Risk Assessment DP No. 414137
Page 4 of 129
6.3 Residential Risk Estimates for Use in Aggregate Assessment ...................................... 53 7.0 Aggregate Exposure/Risk Characterization ....................................................................... 54
7.1 Acute Aggregate Risk .................................................................................................... 54 7.2 Short-Term Aggregate Risk ........................................................................................... 55 7.3 Cancer Aggregate Risk ................................................................................................... 56
8.0 Non-Occupational Bystander Post-Application Inhalation Exposure and Risk Estimates ..... 56 9.0 Non-Occupational Spray Drift Exposure and Risk Estimates ................................................ 59 10.0 Cumulative Exposure/Risk Characterization ................................................................... 59 11.0 Occupational Exposure/Risk Characterization ........................................................... 60
11.1 Occupational Handler Exposure/Risk Estimates .............................................................. 60 11.2 Occupational Post-Application Exposure/Risk Estimates ................................................ 69 11.2.1 Occupational Post-Application Inhalation Exposure/Risk Estimates ........................ 69 11.2.2. Occupational Post-Application Risk Dermal Exposure/Risk Estimates ...................... 70
12.0 Incident and Epidemiological Data Review ...................................................................... 76 12.1 Acute Permethrin Incident Summary ................................................................................ 76 12.2 Epidemiological Studies from the Literature Summary ................................................... 77
13.0 References .......................................................................................................................... 77 13.0 Appendices ......................................................................................................................... 79
Appendix A. Toxicity Profiles. ................................................................................................ 80 Appendix B. Physical Chemical Properties. ............................................................................ 85 Appendix C. International Residue Limit Status Sheet. .......................................................... 86 Appendix D. Submission of Analytical Standards .................................................................. 91 Appendix E. Summary of RED Data Requirements and Data Reviewed as Part of Registration Review. ..................................................................................................................................... 92 Appendix F. Permethrin Use Pattern Tables............................................................................ 96 Appendix G. Summary of Assumptions Used in the Residential Post-Application Assessment.................................................................................................................................................. 111 Appendix H. Occupational Handler Non-Cancer and Cancer Risk Estimates. ...................... 119

Permethrin Human Health Risk Assessment DP No. 414137
Page 5 of 129
1.0 Executive Summary Permethrin [(3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-1-carboxylate] is a broad-spectrum pyrethroid insecticide that is currently registered in the U.S. with 121 companies on 582 product labels. Permethrin is registered to control insects in indoor and outdoor residential, institutional (e.g., hotels, theatres, restaurants, hospitals), and industrial settings (e.g., industrial buildings, poultry houses, warehouses); on agricultural crops, livestock and companion animals; for public health mosquito abatement programs; and is used as an insect repellent in impregnated fabrics. In addition, there is one registered Section 18 emergency exemption registration for application to military aircraft cabin, crew, and cargo areas with an aerosol space spray (currently expires 13-JUL-2017). Permethrin is formulated as emulsifiable concentrate (EC), dry flowable (DF), wettable powder (WP; including water soluble bags), granule (G), dust (D), as well as a number of ready to use (RTU) formulations (e.g., aerosol cans, foggers, trigger pump sprayers, ear tags, hose-end sprayers). Registered permethrin occupational labels require handlers to wear baseline attire (long-sleeved shirt, long pants, shoes, and socks) and chemical resistant gloves in consideration of potential exposure. Additional personal protection equipment (PPE) is required for some permethrin formulations (e.g., coveralls, National Institute of Occupational Safety and Health (NIOSH) approved respirators, etc.). Permethrin is a restricted use pesticide (RUP) due to toxicity to fish and aquatic organisms for all wide-area agricultural outdoor broadcast applications including agricultural crops, golf courses, and nurseries (does not apply to wide-area mosquito adulticide applications). All RUP product labels stipulate a restricted-entry interval (REI) of 12 hours with the exception of EPA Reg. No. 53883-72 which currently requires a 24-hour REI. The toxicology database for permethrin is considered complete with respect to guideline toxicity studies. Permethrin is a Type I pyrethroid, and, like other pyrethroids, causes neurotoxicity from interaction with sodium channels leading to clinical signs of neurotoxicity. The toxicity profiles for all the pyrethroids are very similarly marked by rapid absorption, metabolism, and time-to-peak effect. The single-dose and repeated-dose permethrin studies show that repeat exposures do not result in lower points of departure (PODs; i.e. there is no evidence of increasing toxicity with an increased duration of exposure). Therefore, the exposure assessments are conducted as a series of acute exposures, and these are protective of scenarios in which exposure occurs for multiple days. In conjunction with the completion of the pyrethroid cumulative risk assessment (K. Whitby, D394576, 10/4/2011, EPA-HQ-OPP-2011-0746-0003), HED determined that the Food Quality Protection Act Safety Factor (FQPA SF) can be reduced to 1X for adults and children ≥6 years old. The Agency is retaining a 3x FQPA Safety Factor to protect children <6 years of age based on the pyrethroid pharmacokinetic (PK) difference between adults and children <6 years old that leads to the increased quantitative juvenile susceptibility observed in high dose studies in the literature. The endpoint of decreased motor activity observed in the acute oral Wolansky study (an acute non-guideline study conducted for several pyrethroids; Wolansky, et al., 2006) was used for the dietary (acute) and incidental oral scenarios because it was considered to be the most robust data

Permethrin Human Health Risk Assessment DP No. 414137
Page 6 of 129
set for assessing permethrin exposure and risk. Due to the lack of increased hazard from repeated/chronic exposure to permethrin, the risk estimates derived from use of the acute study are protective of risk from repeated exposures. The endpoint of tremors and hypersensitivity observed in the 15-day inhalation study in the rat was used for the inhalation (residential and occupational) assessments. The incidental and adult oral levels of concern (LOC) are equal to a margin of exposure (MOE) of 300 for children <6 years old and 100 for adults and children ≥6 years old. The inhalation LOC is equal to an MOE of 100 for children <6 years old, and 30 for adults and children ≥6 years old. Since inhalation and incidental oral exposure routes share a common toxicological endpoint but have different LOCs, risk estimates have been combined as appropriate for those routes using an Aggregate Risk Index (ARI) approach. Non-cancer dermal endpoints were not selected for permethrin as no toxicity was observed in the rat dermal study identified up to the limit dose, which is the highest tested dose. This lack of toxicity is also supported by the low dermal absorption of permethrin (<5%). Low dermal absorption is consistent for the pyrethroid class as a whole. Permethrin is classified as “Likely to be Carcinogenic to Humans” based on lung tumors (adenomas and/or carcinomas combined) in female mice and liver tumors (hepatocellular adenomas) in male and female mice. The cancer potency factor or slope factor (Q1* (mg/kg/day)-1) is 9.567 x 10-3 and is based on lung tumors in female mice. The qualitative nature of the residue of permethrin in plants and livestock is understood. The residue of concern in plants and animals is permethrin (cis- and trans-isomers) for both tolerance enforcement and risk assessment purposes. Adequate storage stability, processed food, and magnitude of the residue data exist to support the currently established tolerances for residues of permethrin, except in kiwi. HED notes that the available labels include use information for several commodities that are not supported with field trial data and established tolerances. Residue data for these commodities and kiwi, per Office of Chemical Safety and Pollution Prevention (OCSPP) guideline 860.1500, are required to support these use patterns or the uses should be removed from the labels. Highly refined acute, average (chronic food and drinking water exposure only), and cancer dietary exposure and risk assessments were conducted for permethrin using the Dietary Exposure Evaluation Model software with the Food Commodity Intake Database (DEEM-FCID; Ver. 3.18). Modeled surface water estimated drinking water concentrations (EDWCs) were included in the assessments. The acute, average (chronic), and cancer assessments were refined using PDP monitoring data, field trial data, PCT data, and empirical processing factors. The acute dietary exposure and risk estimates do not exceed HED’s level of concern at the 99.9th exposure percentile for the general U.S. population [2.6% of the acute population-adjusted dose (aPAD)] and all population subgroups. The most highly exposed population subgroup is children 3-5 years old at 20% of the aPAD. For the cancer assessment, the most highly exposed adult population subgroup is adults 50-99 years old, with a cancer dietary (food and drinking water) exposure estimate of 0.000130 mg/kg/day. Applying the cancer potency factor (Q1*) of 9.567 x 10-3 (mg/kg/day)-1 to the exposure value results in a cancer dietary (food and drinking water) risk estimate of 1.3 x 10-6. Spinach, endive, turnip greens, kiwi, and drinking water were the main drivers in the cancer dietary (food and drinking water) exposure and risk assessment. The

Permethrin Human Health Risk Assessment DP No. 414137
Page 7 of 129
average (chronic food and drinking water) exposure assessment was conducted solely for the purpose of obtaining dietary exposure estimates for use in the aggregate assessment. The population subgroup with the highest average dietary (food and drinking water) exposure estimate is children 1-2 years old (0.000214 mg/kg/day). Residential handler and post-application exposures are anticipated from the use of permethrin products. A screening-level approach was used for the assessment of residential exposures by evaluating only the maximum registered application rates for all possible residential exposure scenarios of permethrin. Since there is no non-cancer dermal hazard for permethrin, the non-cancer handler assessment includes only inhalation exposures. For the cancer assessment, both dermal and inhalation exposures were assessed. All screening-level non-cancer residential handler inhalation risks estimates are not of concern, with MOEs ranging from 370 to 770,000 (adult inhalation LOC = 30). Residential handler cancer (dermal + inhalation) risk estimates range from 3×10-10 to 2×10-6 with the greatest cancer risk estimate resulting from mixing/loading/applying liquid applications with a backpack sprayer to gardens/trees/ornamentals. The majority of the non-cancer post-application risk estimates result in MOEs greater than the LOC and are not of concern (i.e., adult and children ≥6 years old inhalation MOEs are >30; children <6 years old inhalation MOEs are >100; adult and children ≥6 years old incidental oral MOEs are >100; and children <6 years old incidental oral MOEs are >300). However, children 3 to <6 years old present risk estimates of concern from inhalation exposures resulting from indoor barn misting systems following “initial cleanout” application rates with an inhalation MOE of 54 and an ARI of 0.54. For residential cancer assessments, HED considers all potential pathways of exposure (oral, inhalation, and/or dermal), depending on the use pattern. Adult residential post-application exposure cancer risk estimates range from 9.1×10-9 to 4.0×10-5 with the greatest cancer risk estimate resulting from contact with small cats treated with liquid formulations. A quantitative non-occupational spray drift assessment for permethrin is not required because the potential residues from direct applications to residential turf are greater than the potential calculated residues resulting from drift from nearby agricultural applications. There were no risks of concern for the residential turf assessment; therefore, the assessment to residues on turf is protective of exposure to the residue from spray drift. Acute and short-term aggregate (food, drinking water, and residential) risk assessments have been conducted for the registered uses of permethrin. A cancer aggregate assessment was not conducted at this time. The acute aggregate risk assessment combines exposures to permethrin in food and drinking water only and there were no risks of concern identified. An ARI approach was used for the adult, children 1 to <2 years old, and children 3 to <6 years old short-term aggregate assessments since the incidental and adult oral and inhalation endpoints have different LOCs. ARIs that are ≥1 are not of concern to HED. The short-term aggregate assessments for adults and children 1 to <2 years old resulted in aggregate ARIs of 2.5 and 1.0, respectively, and are not of concern to HED. An aggregate assessment was completed for children 3 to <6 years old for indoor barn misting systems using the lower “normal infestation” application rate that

Permethrin Human Health Risk Assessment DP No. 414137
Page 8 of 129
resulted in an ARI of 1.1, and is not of concern to HED. It should be noted that for children 3 to <6 years old, the residential post-application risk estimate for the higher “initial cleanout” application of permethrin from indoor barn misting systems was not considered in the aggregate assessment since it results in a risk estimate of concern. Occupational handler and post-application dermal and inhalation exposures are anticipated from the use of permethrin products; however, since there is no dermal hazard identified, a non-cancer quantitative dermal assessment was not conducted. Therefore, the non-cancer handler assessment includes only inhalation exposures. A screening-level approach was used for the assessment of occupational exposures by evaluation of the maximum application rate for all possible occupational exposure scenarios of permethrin. For the cancer assessment, both dermal and inhalation exposures were assessed. For occupational cancer assessments, HED considers all potential pathways of exposure (oral, inhalation, and/or dermal), depending on the use pattern. All screening-level non-cancer occupational handler inhalation risks estimated are not of concern using engineering controls (for aerial applicators) or baseline PPE and no respirator, with MOEs ranging from 31 to 240,000,000 (LOC <30). The cancer occupational handler risk estimates for the currently registered crops and crop groups ranged from 1×10-8 to 5×10-5 for private handlers (10 days of exposure/year) and 3×10-8 to 2×10-4 for commercial handlers (30 days of exposure/year). The cancer occupational handler risk estimates for non-agricultural use sites ranged from 2×10-9 to 1×10-3
for commercial handlers. The potential for dermal or inhalation occupational post-application exposure to mosquito adulticide applicators is anticipated to be negligible since they are not expected to be present in treated areas after application. Although there is potential for indirect dermal post-application exposure to re-entry workers in agricultural fields under the airspace receiving public health mosquito vector control treatment with permethrin, a non-cancer quantitative dermal assessment was not conducted since there is no dermal hazard identified. Based on the Agency's current practices, a quantitative occupational post-application inhalation exposure assessment was not performed for re-entry workers exposed to indirect residues of permethrin resulting from public health uses or registered agricultural uses. If new policies or procedures are put into place, the Agency may revisit the need for a quantitative occupational post-application inhalation exposure assessment for permethrin. Commercial applicators do not typically return to the treated areas after non-agricultural commercial pesticide applications (sites such as warehouses, food handling establishments, hotels, lawns/landscaping, etc.) and thus an occupational indoor post-application exposure assessment was not performed for commercial applicators. Occupational post-application cancer risk estimates for the registered agricultural uses ranged from 1×10-9 to 4×10-6 using the average 30-day dose. The forestry post-application activity of hand set irrigation result in the highest cancer risk estimate. This risk assessment relies in part on data from studies in which adult human subjects were intentionally exposed to a pesticide or other chemical. These data, which include studies from

Permethrin Human Health Risk Assessment DP No. 414137
Page 9 of 129
PHED 1.1; the AHETF database; and the Residential SOPs (Treated, Lawns/Turf, Indoor Environments, Insect Repellents, Outdoor Fogging and Misting Systems, Treated Paints and Preservatives, and Treated Pets), other registrant-submitted exposure monitoring studies (MRID: 448524-02, 448524-03, 437557-01, 449555-01, 48135326, 4407668-12, and 48135325); and the REJV National Pesticide Survey; are (1) subject to ethics review pursuant to 40 CFR 26, (2) have received that review, and (3) are compliant with applicable ethics requirements. For certain studies, the ethics review may have included review by the Human Studies Review Board. Descriptions of data sources, as well as guidance on their use, can be found at the Agency website1. 2.0 HED Conclusions Data deficiencies for permethrin are outlined in Section 2.1. HED notes residue data are not available to support the established tolerance for residues of permethrin kiwi, as indicated in Section 2.2.2. HED also notes that there are commodities included in currently registered labels that are not supported by established tolerances, as indicated in Section 2.3. Acute dietary exposure and risk estimates are not of concern to HED. The acute and short-term aggregate assessments are also not of concern for adults, children 1 to <2 years old, and children 3 to <6 years. HED notes that for children 3 to <6 years old, the residential post-application risk estimate for the “initial cleanout” application of permethrin from indoor barn misting systems results in a risk estimate of concern. All screening-level non-cancer occupational handler inhalation risks estimated are not of concern using engineering controls (for aerial applicators) or baseline PPE and no respirator, with MOEs ranging from 31 to 240,000,000 (LOC <30). A cancer aggregate assessment was not conducted at this time. Refer to Section 5.4.6 for the highest cancer dietary exposure estimate for adults and to Tables 6.1.2. and 6.2.2 for the residential handler and residential post-application cancer exposure estimates for adults. Additionally, a summary of the occupational cancer exposure and risk estimates can be found in Appendix H.
2.1 Data Deficiencies
Analytical reference standards are currently available for permethrin (expires 31-JUL-2019); however, standards are currently unavailable for cis-permethrin and trans-permethrin. Standards should be submitted, as outlined in Appendix D.
HED notes that data have not been submitted to support the established kiwi tolerance (2.0 ppm). Additionally, HED notes that the available labels include use information for chicory, blueberry, okra, pecan, raspberry (black and red), and strawberry; however, tolerances are not established for residues in these commodities. Residue data, per OCSPP guideline 860.1500, are required to support these use patterns.
HED notes that field trial data have been reviewed as part of Registration Review to support the established cabbage tolerance. These data are considered adequate, provided the registrant confirms that the cabbage samples included wrapper leaves (MRID 48598601).
1 http://www.epa.gov/pesticides/science/handler-exposure-data.html and http://www.epa.gov/pesticides/science/post-app-exposure-data.html

Permethrin Human Health Risk Assessment DP No. 414137
Page 10 of 129
2.2 Tolerance Considerations 2.2.1 Enforcement Analytical Method Adequate gas chromatography (GC) electron capture detection (GC/ECD) methods are available for enforcing tolerances of permethrin per se and are listed in PAM Vol. II (Section 180.378). Method I is a GC/ECD method for determining permethrin in plant matrices and has a limit of quantitation (LOQ) of 0.05 ppm for each isomer. Method II is a GC/ECD method for determining permethrin in animal matrices that has a LOQ of 0.01 ppm for each isomer. In addition, permethrin is completely recovered using FDA Multiresidue Methods (PAM Vol. I Sections 302 and 304). 2.2.2 Recommended and Established Tolerances HED notes that the tolerance expressions for permethrin in 40 CFR §180.378 require revision to state the following:
(a) General. Tolerances are established for residues of permethrin, including its metabolites and degradates, in or on the commodities in the table below. Compliance with the tolerance levels specified below is to be determined by measuring only permethrin [(3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate], as the sum of its cis- and trans- isomers in or on the commodity. (c) Tolerances with regional registrations. Tolerances with regional registrations, as defined in §180.1(l), are established for residues of permethrin, including its metabolites and degradates, in or on the commodities in the table below. Compliance with the tolerance levels specified below is to be determined by measuring only permethrin [(3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate], as the sum of its cis- and trans- isomers in or on the commodity.
Additionally, revisions are recommended at this time to include updated crop group definitions, updated tolerances levels (based on residue data submitted in response to the permethrin data call-in; GDCI-109701-26467), as well as increased tolerance levels for international harmonization. Only the recommended tolerance revisions are presented in Table 2.2.4; for a complete list of the U.S., Canadian, and Codex tolerances/ Maximum Residue Limits (MRLs), refer to Appendix C.
Table 2.2.2. HED-Recommended Tolerance Revisions for Registration Review.
Commodity Currently
Established Tolerance (ppm)
Recommended Tolerance (ppm)
Comments (Correct Commodity Definition)
40 CFR §180.378 (a) General Almond 0.05 0.1 Harmonization with Codex MRL Arugula2 20 (as part of 4A) 50 Crop group conversion/revision Cabbage1 6.0 8.0 New residue data Celtuce2 5.0 (as part of 4B) 5.0 Crop group conversion/revision Cress, garden2 20 (as part of 4A) 50 Crop group conversion/revision

Permethrin Human Health Risk Assessment DP No. 414137
Page 11 of 129
Table 2.2.2. HED-Recommended Tolerance Revisions for Registration Review.
Commodity Currently
Established Tolerance (ppm)
Recommended Tolerance (ppm)
Comments (Correct Commodity Definition)
Cress, upland2 20 (as part of 4A) 50 Crop group conversion/revision Eggplant 0.50 1.0 Harmonization with Codex MRL Florence fennel2 5.0 (as part of 4B) 5.0 Crop group conversion/revision Fruit, pome, group 11
0.05 --
Tolerance should be revoked upon establishment of Fruit, pome, group
11-10 tolerance. Crop group conversion/revision
Fruit, pome, group 11-10
-- 0.05 Crop group conversion/revision
Hog, meat byproducts 0.05 0.1 Harmonization with Codex MRL
Kiwifruit 2.0 -- Residue data should be submitted to
support the tolerance
Leaf petioles subgroup 4B
5.0 --
Tolerance should be revoked upon establishment of Leaf petiole
vegetable subgroup 22B, celtuce, and Florence fennel tolerances. Crop group conversion/revision
Leaf petiole subgroup 22B2 -- 5.0 Crop group conversion/revision
Leafy greens subgroup 4A
20 -- Tolerance should be revoked upon
establishment of Leafy greens subgroup 4-16A
Leafy greens subgroup 4-16A
-- 50 Crop group conversion/revision
Lettuce, head 20 -- Tolerance should be revoked upon
establishment of Leafy greens subgroup 4-16A
Milk, fat (reflecting 0.88 ppm in whole milk)
3.0 -- Tolerance should be revoked upon establishment of milk and milk, fat
tolerances Milk -- 0.90 Update commodity definitions to
reflect current policy Milk, fat -- 3.0 Poultry, meat 0.05 0.1 Harmonization with Codex MRL Poultry, meat byproducts
0.05 0.1 Harmonization with Canadian MRL
Spinach 20 -- Tolerance should be revoked upon
establishment of Leafy greens subgroup 4-16A
40 CFR §180.378 (c) Tolerances with regional registrations Collards1 15 30 New residue data Grass, forage1 15 3.0 New residue data Grass, hay1 15 10 New residue data
1 HED is recommending for the revision of these tolerance levels based on the field trial data submitted in response to the permethrin data call-in (GDCI-109701-26467; Memo, J. Van Alstine, 23-JUN-2017; D440981).
2 The Phase IV crop group revision significantly re-structured the existing Crop Groups 4 and 5 to create new Crop Groups 4-16, 5-16 and Subgroup 22B. Crop Group 4-16 has the same representative commodities as Crop Subgroups 4A (lettuce and spinach) and 5B (mustard greens); Crop Group 5-16 has the same representative commodities as Crop Subgroup 5A (cabbage, broccoli and cauliflower); and Crop Group 22 has the same representative commodities as Crop Subgroup 4B (celery) and a new representative commodity, asparagus.

Permethrin Human Health Risk Assessment DP No. 414137
Page 12 of 129
Several commodities in Crop Groups 4 and 5 are better represented by a different commodity or commodities. As a result, Swiss chard (Subgroup 4B member represented by celery) moved to Subgroup 4-16A (represented by lettuces and spinach). Arugula, garden cress and upland cress (Subgroup 4A members represented by lettuces and spinach), as well as Chinese broccoli (Subgroup 5A member represented by cabbage, and broccoli or cauliflower) moved to Subgroup 4-16B (represented by mustard greens). Celtuce and Florence fennel (Subgroup 4B members represented by celery), as well as kohlrabi (Subgroup 5A member represented by cabbage, and broccoli or cauliflower) moved to Subgroup 22A (represented by asparagus).
Tolerances are currently established for residues of permethrin in Leaf petioles subgroup 4B at 5.0 ppm. As part of the crop group revisions, the commodities in crop group 4B are being moved to crop groups 4-16A, 22A, and 22B. A tolerance is being recommended for residues of permethrin in Leaf petiole subgroup 22B commodities. The commodities of celtuce and Florence Fennel (which are commodities that are included in crop subgroup 4B) are being moved to stalk and stem vegetable subgroup 22A (representative commodity asparagus). Although a tolerance is currently established for residues of permethrin in asparagus at 2.0 ppm, a tolerance for residues in stalk and stem vegetable subgroup 22A is not being recommended since the celtuce and Florence fennel application rates are higher than the asparagus application rate. HED is recommending for individual tolerances of 5.0 ppm for celtuce and Florence fennel based on the currently established tolerance for these commodities as part of crop group 4B.
Tolerances are currently established for residues in Leafy greens subgroup 4A at 20 ppm. A tolerance of 50 ppm is being recommended for residues in Leafy greens subgroup 4-16A commodities as part of the crop group conversion. The increased tolerance level is due to data that were received in response to the data requests in the permethrin data call-in (GDCI-109701-26467; see Memo, J. Van Alstine, 23-JUN-2017; D440981). Additionally, as part of the crop group conversion, arugula, garden cress, and upland cress have moved to crop group 4-16B. HED is recommending for individual tolerances for residues in these commodities at 50 ppm to ensure that previously-established tolerances associated with phase four revisions are not inadvertently lost during crop group conversion requests.
2.2.3 International Harmonization U.S. permanent tolerances (listed in 40 CFR §180.378) and Canadian and Codex MRLs are summarized in Appendix C. The U.S., Canadian, and Codex residue definitions are harmonized; however not all tolerance/MRL levels are harmonized. When the U.S. tolerance is higher, harmonization is not feasible because the tolerances are based on field trial data that resulted in residues that necessitated the higher limit. However, in some cases, the U.S. tolerance is lower and can be increased to harmonize with the Codex and Canadian MRLs. HED is recommending to increase the following tolerance levels in order to harmonize with the Codex and Canadian MRLs (Table 2.2.4): almond (from 0.05 ppm to 0.1 ppm); eggplant (from 0.50 ppm to 1.0 ppm); hog meat byproducts (from 0.05 ppm to 0.10 ppm); poultry meat (from 0.05 ppm to 0.10 ppm); and poultry meat byproducts (from 0.05 ppm to 0.10 ppm). For some cases, such as corn grain, HED has not recommended to harmonize the U.S. tolerance (0.05 ppm) with the Codex tolerance (2 ppm) due to differences in use patterns. 2.3 Label Recommendations HED notes that residue data have not been submitted to support the established kiwi tolerance (2.0 ppm). Additionally, HED notes that the available labels include use information for chicory, blueberry, okra, pecan, raspberry (black and red), and strawberry; however, tolerances are not established for residues in these commodities. Residue data, per OCSPP guideline 860.1500, are required to support these uses or they should be removed from the labels.

Permethrin Human Health Risk Assessment DP No. 414137
Page 13 of 129
HED also notes that permethrin can be applied for wide area public health mosquito abatement; however, a separate tolerance has not been established for this use pattern since the available labels limit mosquitocide applications over agricultural crops to commodities that already have tolerances established based on direct agricultural application of permethrin. No specific occupational or residential label recommendations are being made, however, HED notes that there are several occupational handler and post-application scenarios for registered uses that have cancer risk estimates which may impact potential mitigation. 3.0 Introduction 3.1 Chemical Identity Table 3.1. Test Compound Nomenclature. Chemical structure
Common name
Permethrin
Molecular Formula
C21H20Cl2O3
Molecular Weight
391.3
IUPAC name
3-phenoxybenzyl (1RS)-cis-trans-3-(2,2-dichlorovinyl)-2,2- dimethylcyclopropanecarboxylate
CAS name
(3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane carboxylate
CAS #
52645-53-1
PC Code
109701
Current Food/Feed Site Registration
Indoor and outdoor residential, institutional (e.g., hotels, theatres, restaurants, hospitals), and industrial settings (e.g., industrial buildings, poultry houses, warehouses); on agricultural crops, livestock and companion animals; for public health mosquito abatement programs; and is used as an insect repellent in impregnated fabrics. In addition, there is one registered Section 18 emergency exemption registration for application to military aircraft cabin, crew, and cargo areas with an aerosol space spray (currently expires 13-JUL-2017).
3.2 Physical/Chemical Characteristics Permethrin is a persistent pyrethroid in the environment, and was immobile in several soils tested, both sterile and viable (Koc >5000). It is slow to hydrolyze and biodegrade. The relatively low water solubility and hydrophobic nature of permethrin leads to strong soil adsorption and a tendency to partition to sediment in aquatic systems. The high octanol/water partition coefficient suggests that permethrin will bioconcentrate in aquatic organisms. Permethrin has a vapor pressure of 2.15x10-8 mm Hg, water solubility of 0.0055 mg/L, and an estimated Henry’s law constant of 1.4x10-6 atm-m3/mol. Based upon its Henry’s law constant and vapor pressure, permethrin is expected to have a relatively low potential for volatilization from soil and water surfaces. Permethrin’s potential for volatilization is also reduced

Permethrin Human Health Risk Assessment DP No. 414137
Page 14 of 129
significantly because it adsorbs strongly to soils and suspended solids or sediment in the water column. A table of physical/chemical properties for permethrin are included in Appendix B. 3.3 Pesticide Use Pattern Permethrin is registered to control insects in indoor and outdoor residential, institutional (e.g., hotels, theatres, restaurants, hospitals), industrial settings (e.g., industrial buildings, poultry houses, warehouses), and on agricultural crops; and is registered as a seed treatment and for public health uses. It can be used indoors as a direct spot treatment (with some residential site restrictions), crack and crevice application, aerosol space spray, and total release fogger. Outdoor applications can be made as a direct or spot treatment to buildings/household perimeters, landscaping, or lawns via aerosol cans, handheld equipment, and trigger sprays. Outdoor applications may also be applied via ultra-low volume (ULV) thermal fogger and automatic spraying systems. Agricultural crop applications can be made as a broadcast spray or spot treatment via ground, air, and handheld equipment (e.g., aerial, airblast, backpack, chemigation, groundboom, manually/mechanically pressurized handgun, tractor drawn spreader, and truck mounted fogger). In addition, there is a registered Section 18 emergency exemption registration for application to military aircraft cabin, crew, and cargo areas with an aerosol space spray (currently expires 13-JUL-2017). Permethrin is also registered for direct use on fabric (e.g., personal clothing, camping gear, mattresses), dogs, horses, and livestock (including beef/dairy cattle, goats, sheep, poultry, and swine), and as factory-treated permethrin clothing products. Permethrin products are formulated as ECs, DFs, WPs (including water soluble bags), granules, dusts, as well as a number of RTU formulations (e.g., aerosol cans, foggers, trigger pump sprayers, ear tags, hose-end sprayers). Permethrin may be applied as an ULV vector mosquito adulticide by ground (truck mounted fogger), aerial, and handheld equipment. These mosquito vector control products are only to be applied by federal, state, tribal, or local government officials responsible for public health and adult mosquito control. Registered occupational labels require handlers wear baseline attire (long-sleeved shirt, long pants, shoes, and socks) and chemical-resistant gloves in consideration of potential exposure. Additional PPE is required for some permethrin formulations (e.g., coveralls, NIOSH approved respirators, etc.). Permethrin is a RUP for all wide area agricultural outdoor broadcast applications including agricultural crops, golf courses, and nurseries. It may not be applied directly to water, or to areas where surface water is present or to intertidal areas below the mean water mark. With the exception of drain/sewer specific label directions, application is prohibited directly into sewers or drains, or to any area like a gutter where drainage to sewers, storm drains, water bodies, or aquatic habitat can occur. Re-entry restrictions are found on the registered labels for indoor aerosol sprays and foggers which direct applicators to exit treated areas immediately and remain outside the treated area until aerosols and vapors have dispersed. Adults, children, and pets should not enter treated

Permethrin Human Health Risk Assessment DP No. 414137
Page 15 of 129
areas until sprays have dried or vapors, mist, and aerosols have dispersed and rooms are ventilated. A summary of the representative registered food end-use products and use sites with the highest application rates or percent ai is provided in Appendix F (Table F.1). A summary of the representative registered non-food/non-crop end use products and use sites with the highest application rates or percent ai is provided in Appendix F (Table F.2). 3.4 Anticipated Exposure Pathways Humans may be exposed to permethrin in food and drinking water since permethrin may be applied directly to growing crops and application may result in residues of permethrin reaching sources of drinking water. Adults and children may be exposed to permethrin in residential settings due to the currently registered uses. Non-occupational bystanders may be exposed to spray drift from occupational applications. Based on the registered use pattern for permethrin, workers may be exposed to permethrin while mixing/loading/applying and conducting post-application activities after application of this pesticide. All these relevant exposure pathways have been included in this risk assessment. 3.5 Consideration of Environmental Justice Potential areas of environmental justice concerns, to the extent possible, were considered in this human health risk assessment, in accordance with U.S. Executive Order 12898, "Federal Actions to Address Environmental Justice in Minority Populations and Low-Income Populations," (http://www.archives.gov/federal-register/executive-orders/pdf/12898.pdf). As a part of every pesticide risk assessment, OPP considers a large variety of consumer subgroups according to well-established procedures. In line with OPP policy, HED estimates risks to population subgroups from pesticide exposures that are based on patterns of that subgroup’s food and water consumption, and activities in and around the home that involve pesticide use in a residential setting. Extensive data on food consumption patterns are compiled by the USDA under the NHANES/WWEIA and are used in pesticide risk assessments for all registered food uses of a pesticide. These data are analyzed and categorized by subgroups based on age and ethnic group. Additionally, OPP is able to assess dietary exposure to smaller, specialized subgroups and exposure assessments are performed when conditions or circumstances warrant. Whenever appropriate, non-dietary exposures are also evaluated based on home use of pesticide products which includes calculating associated risks for adult applicators and for toddlers, youths, and adults entering or playing in previously treated areas. Spray drift can also potentially result in exposure and it was also considered in this analysis. Further considerations are currently in development as OPP has committed resources and expertise to the development of specialized software and models that consider exposure to bystanders and farm workers as well as lifestyle and traditional dietary patterns among specific subgroups. 4.0 Hazard Characterization and Dose-Response Assessment Permethrin is a member of the pyrethroid class of insecticides. Pyrethroids have historically been classified into two groups, Type I and Type II, based on chemical structure and

Permethrin Human Health Risk Assessment DP No. 414137
Page 16 of 129
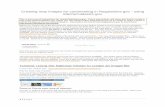
toxicological effects. Type I pyrethroids, which lack an alpha-cyano moiety, induce in rats a syndrome consisting of aggressive sparring, altered sensitivity to external stimuli, hyperthermia, and fine tremor progressing to whole-body tremor and prostration (T-syndrome). Type II pyrethroids, which contain an alpha-cyano moiety, in rats produce a syndrome that includes pawing, burrowing, salivation, hypothermia, and coarse tremors leading to choreoathetosis (CS-syndrome) (Verschoyle and Aldridge, 1980; Lawrence and Casida, 1982). Permethrin is a Type I pyrethroid. Structurally, permethrin lacks the alpha-cyano moiety. The adverse outcome pathway (AOP, based on the Bradford-Hill criteria) shared by pyrethroids involves the ability to interact with voltage-gated sodium channels (VGSCs) in the central and peripheral nervous systems, leading to changes in neuron firing and, ultimately, neurotoxicity (Figure 4.0).
Figure 4.0. Adverse Outcome Pathway for Pyrethroids
4.1 Toxicology Studies Available for Analysis The database of experimental toxicology studies available for permethrin provides a robust characterization of the hazard potential for children 6 years old and older as well as for adults. With respect to CFR Part 158, all data requirements have been met except for developmental neurotoxicity (DNT) and immunotoxicity studies. However, both the DNT (TXR# 0055306, E. Scollon, 20-JAN-2010) and immunotoxicity (TXR# 0056729, U. Habiba, 12-AUG-2013) studies have been waived for the pyrethroids as a class. In addition, there are ongoing efforts to develop data to inform the potential sensitivity of infants and young children to pyrethroids as a class (see Section 4.4). Despite the missing studies and the ongoing scientific efforts, HED has chosen points of departure and uncertainty factors in this risk assessment that are protective of the effects associated with exposure to permethrin. Data from the following studies were used to evaluate the hazard potential of permethrin:
-Wolansky (Wolansky, et al., 2006) Acute Oral Study in the Rat -WIL Laboratory (Weiner, et al., 2009) Acute Oral Study in the Rat -21-Day Dermal -Studies in the Rat and Rabbit -Subchronic Toxicity Studies in the Rat and Dog -Chronic/Carcinogenicity Studies in the Rat, Mouse, and Dog -Developmental Studies in the Rat and Rabbit -Reproduction Study in the Rat -Acute and Subchronic Studies in the Rat -Mutagenicity Battery of Studies -Metabolism study in the Rat -Dermal penetration studies in the Rat
Target Tissue Dose
VGSC Alterations
In Vivo Clinical Signs
Altered Neuronal
Excitability

Permethrin Human Health Risk Assessment DP No. 414137
Page 17 of 129
The studies available for consideration of permethrin toxicity provide a comprehensive database. Since the last risk assessment, two acute oral neurotoxicity studies have been evaluated for permethrin. The Wolansky and WIL studies are acceptable acute oral non-guideline studies that measure locomotor and functional observational battery (FOB) activity, respectively, and provide robust data to evaluate the hazard potential of permethrin. In addition, numerous studies from the scientific literature conducted over several decades describe the pharmacodynamic (PD) and pharmacokinetic (PK) profile of the pyrethroids in general (see Section 4.3 for more detail) and have been considered for risk assessment. This scientific literature has been reviewed by several groups (Soderlund, et al., 2002; Shafer, et al., 2005; Wolansky and Harrill, 2008). 4.2 Toxicological Profile Permethrin has been evaluated for a variety of toxicological effects in guideline experimental toxicity studies. Behavioral changes characteristic of Type I pyrethroids, such as muscle tremors, hyperthermia, aggression and hypersensitivity, were seen in most of the experimental toxicology studies including the acute and subchronic neurotoxicity studies, the subchronic studies in the rat and dog, the chronic carcinogenicity study in the rat, the developmental studies in the rat and rabbit, the WIL and Wolansky studies in the rat, and the two-generation reproduction study in the rat. Tremors were the most common indication of neurotoxicity; however, decreased motor activity from the Wolansky study provides the most sensitive endpoint. Neurotoxic effects were the primary and most sensitive effects seen in the toxicity database and are protective of all other effects observed, such as decreased body weight, developmental effects, lung toxicity, and liver toxicity. Permethrin has been evaluated for potential developmental effects in the rat and rabbit. No evidence of increased quantitative and/or qualitative susceptibility was seen in these studies. Maternal toxicity included neurological effects such as tremors in the rat and decreased body weights in the rat and rabbit. Increased post-implantation loss, decreased offspring size, and decreased ossification were observed in developmental studies in the rat and rabbit. All effects occurred at maternally toxic doses or above. The potential reproductive toxicity of permethrin was examined in a two-generation reproduction study in the rat. No evidence of increased quantitative and/or qualitative susceptibility was seen in the study. All of the findings in pups were seen at the same doses that caused clinical signs of toxicity and mortality in dams, and the severity of treatment-related effects did not increase in offspring relative to parental animals. The only effect noted in pups was decreased size, occurring at the same dose as tremors in maternal animals. Increased lung and liver adenomas and carcinomas were observed in the mouse carcinogenicity studies; no increase in tumors was observed in the rat. With respect to acute lethality studies, permethrin exhibits low acute toxicity via the oral and dermal routes (Toxicity Categories III). Permethrin is a mild eye irritant (Toxicity Category III), but does not cause dermal irritation in rabbits or skin sensitization in guinea pigs.

Permethrin Human Health Risk Assessment DP No. 414137
Page 18 of 129
The permethrin PK profile is similar to the general PK profile of other pyrethroids, i.e., rapid absorption and clearance and extensive metabolism. Data are available to allow for use of the triple pack approach, including an in vivo rat dermal penetration study and an in vitro dermal absorption study using both rat and human skin. The in vivo dermal penetration study in rats (MRID 43169001) indicated a dermal absorption factor of 21.7%, at 10 hours after administration. The comparative in vitro dermal penetration study using human and rat skin (MRID 47514801) showed that 18% of an applied dose was absorbed through rat skin and 2.3% through human skin, which indicates that in vitro rat skin is 6.6 times more permeable than in vitro human skin. Therefore, a dermal absorption factor of 21.7/6.6 = 3.3% is considered appropriate for risk assessment. 4.3 Pyrethroid Pharmacokinetic and Pharmacodynamic Profile OPP is making best use of the extensive scientific knowledge about the AOP of pyrethroids in the risk assessments for this class of pesticides. In this way, information on a subset of pyrethroids can be used to help interpret and understand the toxicological profile for other members of the class. In that regard, a group of pesticide registrants and product formulators known as the Council for the Advancement of Pyrethroid Human Risk Assessment (CAPHRA) has been conducting multiple experiments with permethrin and deltamethrin as model Type I and Type II compounds, respectively, in order to develop an initial extensive database of in vitro and in vivo toxicology studies and highly refined physiologically-based pharmacokinetic (PBPK) models. In addition to the efforts of the CAPHRA, the extensive body of scientific literature on the pyrethroids provides insight into the contributions of PK and PD to the general toxicity profile of this class of chemicals. This information also provides valuable insight into the potential age-related differences in toxicity for the pyrethroids. This scientific literature has been reviewed by several groups (Soderlund, et al., 2002; Shafer, et al., 2005; Wolansky and Harrill, 2008) and the following sections of the risk assessment discuss the specific issues related to pyrethroid PK, pyrethroid PD, and age-related differences in pyrethroid toxicity. Furthermore, the Agency will be updating its literature review for pyrethroids in 2017 as described below prior to completion of the revised risk assessments. In recent years, the National Academies’ National Research Council (NRC) has encouraged the Agency to move towards systematic review processes to enhance the transparency of scientific literature reviews that support chemical-specific risk assessments to inform regulatory decision making (NRC 2011, 2014). The NRC defines systematic review as “a scientific investigation that focuses on a specific question and uses explicit, pre-specified scientific methods to identify, select, assess, and summarize the findings of similar but separate studies” (NRC 2014). According to the NRC, systematic reviews “have several common elements: transparent and explicitly documented methods, consistent and critical evaluation of all relevant literature, application of a standardized approach for grading the strength of evidence, and clear and consistent summative language.” EPA’s OCSPP is currently developing systematic review policies and procedures. The Agency is currently working with EPA reference librarians to

Permethrin Human Health Risk Assessment DP No. 414137
Page 19 of 129
develop a systematic review for the pyrethroids. This analysis is still on-going and will be incorporated in the revised risk assessment for permethrin.
4.3.1 Pharmacokinetics (PK) PK can be defined as what the body does to the chemical; in this case, how pyrethroids are distributed and eliminated following exposure. Specific to pyrethroids, PK refers to the process(es) that determine(s) the concentration of the pyrethroids reaching sodium channels. The underlying PK of pyrethroids is an important determination of their toxicity because the concentration of pyrethroid at the sodium channel relates to the extent of toxicity; greater pyrethroid concentration translates as increased neurotoxicity. Physiological processes that significantly contribute to the PK include metabolism, protein binding, and partitioning. Carboxylesterases and cytochrome P450 enzymes are the two major enzyme families responsible for the metabolism of pyrethroids. It is the ontogeny of these enzymes that accounts for the age-related sensitivity observed after pyrethroid exposures, as described below in more detail. In terms of partitioning, pyrethroids tend to distribute into fat. However, pyrethroid residues in fatty tissue are not available to interact with the VGSCs in vital tissues and, therefore, do not contribute to overall toxicity. Age-dependent PK differences have been identified for several pyrethroids; that is, there are differences in the ability of adults and juveniles to metabolize pyrethroids. The enzymes that metabolize and detoxify pyrethroids are present in rats and humans at birth (Koukouritaki, et al., 2004; Yang, et al., 2009). As a result, both juveniles and adults are able to tolerate low doses of pyrethroids when the internal dose, or the amount of pyrethroid at the sodium channel, is low. However, the expression, and therefore activity, of these enzymes increases with age, conveying in adults a greater capacity than juveniles to detoxify pyrethroids (Anand, et al., 2006; de Zwart, et al., 2008; Yang, et al., 2009). For example, the rate of in vitro metabolism of deltamethrin by plasma carboxylesterases, plus hepatic carboxylesterases and cytochrome P450s (microsomes) is at least 6 times as high for postnatal day (PND) 90 rats as for PND 10 rats (Anand, et al., 2006). In humans, expression of hepatic carboxylesterases is significantly lower in infants <3 weeks old but then increase to near adult levels (Hines, et al., 2016). Similar information is also available for the major human P450s involved in pyrethroid metabolism (CYP2C8, CYP2C19, and CYP3A4). CYP2C19 levels are approximately 80% of adult values from >5 months to 10 years, CYP3A4 reaches near adult levels by 1-2 years, and CYP2C8 levels are comparable to adult levels after 6 months of age (Koukouritaki, et al., 2004; Stevens, et al., 2003; Song, et al., 2015). As a consequence, higher internal doses (i.e., those associated with high doses in experimental toxicology studies) overwhelm the clearance mechanisms in juveniles, but because adults have greater enzyme activity, they are able to tolerate higher doses prior to the onset of toxicity. As a matter of perspective, the anticipated exposures from typical dietary or residential activities are not expected to overwhelm the premature metabolic systems in juveniles. To better understand the role of PK and reduce uncertainty associated with extrapolating across species (i.e., rat to human) and life stages, the Agency developed PBPK models designed to predict pyrethroid concentration in tissues following in vivo exposure. The Agency has determined that the important PK properties relevant to the metabolism and distribution of pyrethroids in the body are sufficiently similar for members of this class such that using a

Permethrin Human Health Risk Assessment DP No. 414137
Page 20 of 129
‘generic’ or family model structure for this class is scientifically appropriate. In other words, because of the similarities in the PK profiles of pyrethroids, a single model structure is able to predict the tissue dose based on the PK of every member of the class. The family modeling approach was primarily developed based on PBPK modeling performed with deltamethrin and was presented to, and supported by, the Federal Insecticide, Fungicide, and Rodenticide Scientific Advisory Panel (FIFRA SAP; USEPA, 2007). The initial deltamethrin PBPK model presented to the SAP was developed in the adult male Sprague Dawley (SD) rat (Mirfazaelian, et al., 2006). The deltamethrin PBPK model was further refined based on oral bioavailability and disposition studies in rats and included estimates for target tissue concentrations in humans (Godin, et al., 2010). The initial PBPK model was also extended by accounting for age-dependent changes in physiological and biochemical parameters (Tornero-Velez, et al., 2010) to address juvenile sensitivity in rats. This model predicts that, compared to adult rats (i.e., 90-days old), equivalent brain concentrations of deltamethrin would be achieved with a 3.8x fold lower oral dose in 10-day old rats and 2.5x lower dose in 21-day old rats. For example, the internal dose from an administered dose of 1 mg/kg in the adult is equivalent to the internal dose from an administered dose of 0.26 mg/kg (≈1 mg/kg ÷ 3.8 mg/kg) in the 10-day old rat and to an administered dose of 0.4 mg/kg (≈1 mg/kg ÷ 2.5mg/kg) in the 21-day old rat. As a result, the Agency concludes that juvenile rats are three times as sensitive as adult rats with respect to pyrethroid PK. At this time, the Agency considers that the differences in the PK profile observed in the rat are relevant to humans. Therefore, the PK contribution to the FQPA Safety Factor is 3x for children less than 6 years old and 1x for children 6 years of age or older and for adults. Further information regarding the decision to retain the FQPA Safety Factor and the choice of age groups it applies to can be found in the Re-Evaluation of the FQPA Safety Factor of Pyrethroid Pesticides memo (D381210, E. Scollon, 06/27/2011). The CAPHRA is collecting metabolism and tissue dosimetry data from rats and human tissues across different life stages. These data will be used to inform the development of PBPK models for the pyrethroids. The CAPHRA initially presented its experimental data and proposed path forward to the SAP on May 19, 2015 (USEPA, 2015). Based on the comments from the SAP, the CAPHRA continued to pursue its research efforts and gather additional data. Currently, data from CAPHRA to develop PBPK models for deltamethrin and permethrin have been submitted to the Agency and are scheduled to be reviewed by the SAP in October of 2017. 4.3.2 Pharmacodynamics (PD) PD can be defined as the changes that chemicals cause to the body, in this case, how pyrethroids interact with the sodium channels. Substantial evidence from in vitro and in vivo studies support the AOP illustrated in Figure 4.0 and the disruption of sodium channels by pyrethroids as an early key event (Lund and Narahashi, 1982; Salgado, et al., 1989; Song and Narahashi, 1996; Tabarean and Narahashi, 1998; Soderlund, et al., 2002). There are several studies that provide specific information for permethrin. Choi and Soderlund (2006) examined interactions of several pyrethroids, including permethrin, with mammalian VGSCs expressed in Xenopus oocytes (i.e., frog oocytes). With respect to altered neuronal excitability, Type I pyrethroids cause slight prolongations of the sodium current tails (e.g. ~20 ms), often resulting in long trains

Permethrin Human Health Risk Assessment DP No. 414137
Page 21 of 129
of action potentials. In contrast, Type II pyrethroids significantly prolong sodium current tails (e.g., 200 ms to minutes) typically resulting in increased resting membrane potential and ultimately causing depolarization dependent action potential block. Permethrin produced modifications of sodium channel kinetics intermediate between Type I and Type II compounds (Figure 4.3). Specifically, permethrin caused slow activation and inactivation, like Type II compounds, but also caused rapidly-decaying monoexponential tail currents like Type I compounds. Cao, et al. (2011a) measured sodium influx in primary cultures of mammalian (mouse) neurons and demonstrated that permethrin caused increases in sodium influx in this model; this confirms the ability of permethrin to interact with VGSC in intact mammalian neurons. An additional study by Cao, et al. (2011b) demonstrated that the interaction of permethrin with VGSC caused changes in neuronal excitability that resulted in calcium influx into intact mouse neurons. As this effect of permethrin was entirely blocked by the VGSC blocker tetrodotoxin, it provides evidence that the changes in sodium channel function lead to changes in neuronal excitability, as illustrated in Figure 4.3.
Figure 4.3. Resting modification of rat Nav1.8 sodium channels by permethrin expressed in Xenopus oocytes. Channel current vs time traces from individual representative oocytes in the absence or presence (*) of 100 µM permethrin were obtained during and after 40-ms depolarizations from 100 mV to 10 mV. Calibration bars: 20 ms for the x-axis and 500 nAmp on the y-axis. Figure 4.3 was extracted from Figure 3 in Choi and Soderlund (2006). HED would prefer to use an early key event in the AOP for pyrethroids in selection of points of departure, such as sodium channel modification. However, in vivo techniques used to detect VGSC alteration and altered neuronal excitability are not practical for use in risk assessment at this time, and approaches for extrapolating in vitro findings to in vivo measures are not yet developed. As such, the Agency is focusing its efforts for all pyrethroids in hazard characterization and identification on the apical endpoint (i.e., changes in neurobehavior in laboratory animals). Neurotoxicity resulting from pyrethroids is generally characterized by tremors, hyper- or hypothermia, heightened response to stimuli, salivation, reduced locomotor activity, or convulsions (Soderlund, et al., 2002; Wolansky and Harrill, 2008; Weiner, et al., 2009). In addition, results from the study by Wolansky, et al. (2006) indicated that motor activity is a sensitive and robust measure of neurotoxicity for this class of compounds. The changes in motor activity observed were not specific to either of the syndromes described for pyrethroids and were observed with both Type I and Type II pyrethroids. In contrast to the age-related PK differences identified in the 2011 analysis, PD contributions to pyrethroid toxicity are not age-dependent, even though there are several variations of sodium

Permethrin Human Health Risk Assessment DP No. 414137
Page 22 of 129
channels, called isoforms, that are differentially expressed by tissue and age. Because of the nature of the interaction of pyrethroids with sodium channels, it is difficult to obtain dynamic information in vivo. To date, a readily useable biomarker of in vivo pyrethroid interaction with sodium channels has not been identified, making it impractical to determine the isoform combinations that are present and being acted upon by pyrethroids. Therefore, much of the information available to the Agency to characterize the PD relationship between pyrethroids and sodium channels has been derived from in vitro studies using frog oocytes or neuronal cells cultured in defined media. These in vitro techniques do not provide a direct quantitative measure of in vivo pyrethroid activity. However, these techniques consistently and qualitatively demonstrate that sodium channel isoforms expressed in juveniles are not more sensitive to pyrethroid perturbation compared to isoforms expressed in adults and that, pharmacodynamically, the rat is a conservative model for humans. For example, Meacham, et al. (2008), expressed adult and juvenile isoforms of rat sodium channels in frog oocytes and compared their sensitivity following exposure to deltamethrin. The isoforms had comparable responses at environmentally relevant concentrations (<500 nM) of deltamethrin, suggesting a lack of PD difference between juveniles and adults at low exposure levels. In addition, in a direct comparison of a homologous rat and human VGSC isoform, NaV1.3, the rat isoform was 4-fold more sensitive than the equivalent human sodium channel to the pyrethroid tefluthrin (Tan and Soderlund 2009). This observation suggests that the rat is a highly sensitive model, and extrapolations from the rat would be protective of human health. The occurrence and ontogeny of VGSCs in humans are not as well characterized as those of the rat. However, based on the comparable function and distribution of sodium channels between the species, the rat is an appropriate surrogate for the evaluation of human PD (Goldin, et al., 2000; Goldin, 2002). As a result, the Agency concludes that juvenile rats are not more sensitive than adults with respect to pyrethroid PD based on sodium channel data. Therefore, the PD contribution to the FQPA factor is 1x. 4.3.3 Critical Duration of Exposure One of the key elements in risk assessment is the appropriate integration of temporality between the exposure and hazard assessments. Following a single oral gavage dose, permethrin is absorbed quickly in rats. Effects such as tremors and hypersensitivity were observed within 1 and 2 hours following dosing in the rat developmental and acute neurotoxicity (ACN) studies. Rats typically recover within 24 hours without any persisting neurotoxic effects at doses near the LOAEL value. These observations are consistent with the toxicity profiles for other pyrethroids that are marked by rapid absorption, metabolism, and elimination, with a time to peak effect for neurobehavioral effects ranging from 4 to 8 hours. The time to peak effect is approximately 1.5 hours for permethrin (Wolansky and Harrill, 2008; Weiner, et al., 2009; Scollon, et al., 2011). The combination of rapid absorption, metabolism, and elimination precludes accumulation and increased potency following repeated dosing. Therefore, for most pyrethroids, the acute toxicity studies typically result in neurotoxicity at lower doses than in the repeat-dose studies. In the case of permethrin, the NOAELs and LOAELs for tremors established from results of experimental toxicity studies are remarkably consistent across durations of exposure, ranging from a single dose up to 2 years of dosing (Table 4.3.3). The Wolansky motor activity study is the most sensitive in the database. While the ACN provides a lower POD at the NOAEL, this is

Permethrin Human Health Risk Assessment DP No. 414137
Page 23 of 129
considered to be an artifact of dose spacing. The benchmark dose level (BMDL) in the Wolansky study is protective of the ACN LOAEL. Additionally, there is more confidence in the benchmark dose (BMD) modeling from the Wolansky study. The WIL acute study has a POD 2-3 fold higher than the POD from the Wolansky acute study (Table 4.3.3). It is notable that both the ACN and WIL study used relatively high vehicle volume of 5 mL/kg, compared to a vehicle volume of 1 mL/kg in the Wolansky acute study, which may account for the higher POD. For some pyrethroids, the gavage volume can play an important role in potency because the volume impacts the absorption, distribution, metabolism, and elimination (ADME) characteristics (e.g., timing and amount; Crofton, et al., 1995). In the WIL and ACN studies, it is likely that the relatively high corn oil volume caused a reduction in the peak level of permethrin, resulting in lower toxicity, thereby increasing the POD.
Table 4.3.3. Permethrin NOAEL and LOAEL Values versus Treatment Time.
Study Duration Study findings
NOAEL (mg/kg/day)
LOAEL (mg/kg/day)
Wolansky (2006)-Rat (gavage 1 mL/kg)
Acute, single exposure
ABMDL1SD = 44.0 BMD1SD = 63
WIL-Rat (gavage 5 mL/kg
Acute, single exposure
NA BMD20 = 156
Acute neurotoxicity-Rat (gavage 5 mL/kg)
Acute, single exposure
25 75
Developmental-Rat (gavage 1 mL/kg)
10 days 50 150
Developmental-Rabbit2
(gavage 0.5 mL/kg) 10 days 600 1200
Subchronic neurotoxicity-Rat
(dietary) 90 days 100 200
Sub-chronic-Dog (dietary)
90 days 100 364
Sub-chronic-Rat (dietary)
90 days 110 221
Reproductive toxicity-Rat (dietary)
120 days 50 125
Chronic-Dog (dietary)
1-year 100 1000
Chronic-carcinogenicity-Rat
(dietary) 2-years 36.9 91.5
A BMD1SD is the central estimate of the dose that results in decreased motor activity compared to control animals based upon a 1 standard deviation using Benchmark Dose Analysis. BMDL1SD is the 95% lower confidence limit of the central estimate. Data extrapolated from Wolansky (2006), MRID 47885701. B BMD20 is the central estimate of the dose that results in a 20% difference in FOB scores compared to control animals using Benchmark Dose Analysis. BMDL is the 95% lower confidence limit of the central estimate. Data and analysis can be found in MRID 48714301. In general, for pyrethroids, rat dietary studies tend to have higher NOAELs/LOAELs than gavage studies because, as rats feed continuously, pyrethroids are metabolized and excreted from

Permethrin Human Health Risk Assessment DP No. 414137
Page 24 of 129
the system relatively quickly, and the overall systemic concentration in the rat remains low. In contrast, bolus/gavage dosing results in greater maximal plasma concentrations immediately after dosing. The results from permethrin studies are similar in this respect, with dietary studies generally resulting in higher LOAELs than gavage studies. The exceptions to this are the WIL study, the ACN study, and the repeat dose dog studies. The WIL study and the ACN study involved relatively high gavage volumes. In the repeat dose dog studies, the dogs tend to eat their food all at once in a manner that is very similar to bolus dosing, in contrast to rats which tend to feed continuously over the course of the day. Comparing the POD established from the Wolansky acute study and the repeat dosing studies, it is apparent that repeat exposures result in either higher or very similar PODs (Table 4.3.3). This observation is consistent with the general kinetic profile for pyrethroids. Therefore, the endpoint from the Wolansky acute study is protective of the endpoints from the repeat dosing studies and, for the purposes of endpoint selection and exposure assessment, only single-day risk assessments need to be conducted. 4.4 Safety Factor for Infants and Children (FQPA Safety Factor)2 There was no evidence of increased qualitative or quantitative susceptibility in guideline developmental toxicity studies in the rat and rabbit and a three-generation reproductive toxicity study in the rat. After reviewing the extensive body of peer-reviewed literature on pyrethroids, the Agency has no residual uncertainties regarding age-related sensitivity for women of child bearing age as well as for all adult populations and children ≥6 years of age, based on the absence of prenatal sensitivity observed in 76 guideline studies for 24 pyrethroids and the scientific literature. Additionally, no evidence of increased quantitative or qualitative susceptibility was seen in the pyrethroid scientific literature related to PD. However, the Agency is retaining a 3x FQPA Safety Factor for children <6 years of age based on the increased quantitative susceptibility seen in studies on pyrethroid PKs and the increased quantitative juvenile susceptibility observed in high dose studies in the literature. There is no residual uncertainty in the exposure database because adequate residue data are available. In addition, many conservative assumptions were made in the dietary and residential exposure and risk assessments. As a result, exposure will not be underestimated. 4.4.1 Completeness of the Toxicology Database The toxicology database for permethrin is complete with respect to CFR part 158 data requirements. The Agency is expecting additional in vitro and in vivo data. In 2010, the Agency requested proposals for study protocols that could identify and quantify potential juvenile sensitivity and received a single response from the Pyrethrin and Pyrethroids Technical Working Group (PPTWG), a conglomerate of pyrethroid registrants. The PPTWG protocol was reviewed during a July 2010 FIFRA SAP meeting. Based on comments from the SAP, the initial study proposal was refined, and the CAPHRA submitted its updated research to the SAP on May 19, 2015
2 HED’s standard toxicological, exposure, and risk assessment approaches are consistent with the requirements of EPA’s children’s environmental health policy (https://www.epa.gov/children/epas-policy-evaluating-risk-children).

Permethrin Human Health Risk Assessment DP No. 414137
Page 25 of 129
(USEPA, 2015). Based on the SAP’s most recent comments, the CAPHRA is continuing to: 1) develop rat and human PBPK models, including additional PK data, and 2) conduct in vivo behavioral testing using auditory startle testing in rats and planning to submit additional data to the Agency throughout 2017 However, at the time of the preparation for this risk assessment, the data available from the CAPHRA were not useful for deriving PODs or for species extrapolation, so the CAPHRA data have not been included in the current draft risk assessment. 4.4.2 Evidence of Neurotoxicity There are no residual uncertainties with regard to evidence of neurotoxicity for permethrin. As with other pyrethroids, permethrin causes neurotoxicity from interaction with sodium channels leading to clinical signs of neurotoxicity. These effects are well characterized and adequately assessed by the available guideline and non-guideline studies. 4.4.3 Evidence of Sensitivity/Susceptibility in the Developing or Young Animal Based on the permethrin-specific data from the guideline toxicity studies, there is no indication of increased juvenile qualitative or quantitative sensitivity. There are, however, high-dose studies in the scientific literature indicate that younger animals are more susceptible to the toxicity of pyrethroids. For example, Sheets, et al. (1994) found increased brain deltamethrin levels in young rats (PND 11 and 21) relative to adult rats (PND 72). These age-related differences in toxicity are principally due to age-dependent PK. The activity of enzymes associated with the metabolism of pyrethroids increases with age (Anand, et al., 2006). However, in context, normal dietary or residential exposures of juveniles are not expected to overwhelm their ability to metabolize pyrethroids. In support, at a dose of 4.0 mg/kg deltamethrin (near the Wolansky study LOAEL value of 3.0 mg/kg for deltamethrin), the change in the acoustic startle response was similar between adult and young rats (Sheets, et al., 1994). In addition, EPA’s Office of Research and Development (ORD) recently developed an age-dependent PBPK model for deltamethrin (Tornero-Velez, et al., 2010) that predicts a 3–fold increase of pyrethroid in neuronal tissue in younger animals compared to adults. There are several studies (in vitro and in vivo) that indicate that PD contributions to pyrethroid toxicity are not age-dependent. Examination of specific VGSCs has demonstrated that there is a lack of increased sensitivity in either juvenile specific isoforms (Meacham, et al., 2008) or in human isoforms compared to rat variants (Tan and Soderlund, 2009). After reviewing the extensive body of peer-reviewed literature on pyrethroids, the Agency has no residual uncertainties regarding age-related sensitivity for women of child bearing age as well as for all adult populations and children ≥6 years of age, based on the absence of prenatal sensitivity observed in 76 guideline studies for 24 pyrethroids and the scientific literature. Additionally, no evidence of increased quantitative or qualitative susceptibility was seen in the pyrethroid scientific literature related to PD. The Agency is retaining a 3x FQPA Safety Factor to protect children <6 years of age based on the pyrethroid pharmacokinetic (PK) difference between adults and children <6 years old that leads to the increased quantitative juvenile susceptibility observed in high dose studies in the literature. Further information regarding the

Permethrin Human Health Risk Assessment DP No. 414137
Page 26 of 129
decision to retain the FQPA Safety Factor and the choice of age groups it applies to can be found in Section 4.3.1. 4.4.4 Residual Uncertainty in the Exposure Database
There are no residual uncertainties in the permethrin dietary exposure database. Although refinements were incorporated into the dietary exposure assessments, EPA is confident that the aggregate exposure from exposure to permethrin in food, drinking water, and residential pathways will not be underestimated. PDP and PCT data were used for the majority of commodities in the acute, average, and cancer dietary exposure assessments. Although the dietary exposure and risk assessments included refinements, the use of such refinements are aimed at reducing the chance of overestimating potential dietary exposure, while still ensuring that actual exposures and risks from residues in food are not underestimated. The drinking water assessment utilized water concentration values generated by models incorporating parameters designed to produce conservative, health protective, estimates of water concentrations.
In addition, the residential exposure assessments are based on the 2012 Residential SOPs employing surrogate study data, including conservative exposure assumptions based on Day 0 dermal and oral contact to previously treated turf and surfaces. These data are not expected to underestimate risks to adults or children. The Residential SOPs are based upon reasonable assumptions, maximum registered application rates, and are not expected to underestimate exposure. Chemical-specific turf transferable residue (TTR) data are available and have been adjusted and reflect the maximum application rates for permethrin.
4.5 Toxicity Endpoint and Point of Departure Selections 4.5.1 Dose-Response Assessment Based on the existing use patterns for permethrin, the expected exposure profile includes dietary (food and drinking water); residential dermal, inhalation, and incidental oral exposures; occupational dermal and inhalation exposures; and dermal and incidental oral exposures resulting from spray drift. However, based on the toxicity profile, HED is not conducting non-cancer dermal assessments (see below for a more detailed rationale). A chronic dietary risk assessment is also not being conducted because there is no apparent increase in hazard from repeated/chronic exposures to permethrin. Therefore, the acute dietary exposure assessment is protective of chronic dietary exposures. Additionally, incidental oral and inhalation exposures are expected to be short-, intermediate-, or long-term. However, due to rapid toxicokinetics and toxicity profile of pyrethroids these assessments are conducted as a series of acute exposures. Therefore, the same endpoint is used regardless of duration. As previously indicated, the toxicity endpoints in the permethrin database are consistently based on clinical signs of neurotoxicity and, more specifically, tremors. These studies include multiple species, study designs, and durations (Table 4.3.3). Moreover, the acute exposure or bolus dosing studies generally result in lower NOAELs compared to longer term dietary administration studies, consistent with other pyrethroids in this class. Because uncertainty associated with the POD is propagated throughout the risk assessment, one of the key factors in POD selection is the

Permethrin Human Health Risk Assessment DP No. 414137
Page 27 of 129
robustness of the dose-response data. The guideline experimental toxicology studies available for permethrin are generally high quality and were considered in the POD selection process (Appendix A.3) and in the weight of the evidence evaluation. In addition to the typical guideline studies, data from two special studies evaluating neurobehavioral outcomes are available for permethrin (Wolansky study on motor activity, Wolansky, et al. 2006; and the WIL FOB study, Weiner, et al., 2009). Wolansky, et al. measured motor activity at the time of peak effect after exposure to 11 pyrethroids, including permethrin, although other neurobehavioral parameters, such as tremors, were not assessed. Dose-response relationships were determined using 6-11 doses per pyrethroid (9 doses used for permethrin) and 3-18 rats per dose group (3-12 animals/group used for permethrin) minimizing variability and increasing the confidence in the BMD estimates determined from this study. Moreover, each pyrethroid was evaluated by the same scientist, thus decreasing some of the variability associated with neurobehavioral measures. The Agency conducted a BMD analysis for all the pyrethroids included in the Wolansky study (MRID 48714301). Overall, because of the large number of doses and high quality measurements, the BMD analysis yielded high confidence results. In performing BMD analysis, a benchmark response (BMR) must be selected. As a general approach, it is preferable to use a combination of biological and statistical factors in the BMR selection. In the case of motor activity data, the scientific community has not established a specific level of change that would be considered to be adverse. Therefore, OPP has elected to use one standard deviation (1SD) from the control group, as suggested for continuous endpoints in the Agency’s BMD guidance (USEPA, 2012) as the BMR. OPP has estimated both the BMD1SD and the BMDL1SD (where the BMDL1SD is the lower 95% confidence limit of the BMD1SD). The BMD1SD and the BMDL1SD for permethrin are 63 mg/kg and 44 mg/kg, respectively. As a matter of science policy, EPA uses the BMDL, not the BMD, for deriving PODs. Therefore, the BMDL1SD of 44 mg/kg is being used as the dose for acute dietary risk assessment. Acute Dietary (All Age Groups): Quantitation of the dietary risks was performed using the acute oral Wolansky study, with a BMD1SD value of 63 mg/kg and a BMDL1SD value of 44 mg/kg based on decreased locomotor activity. The acute RfD is 0.44 mg/kg/day, and the aPAD is 0.147 mg/kg/day. Short-term Incidental Oral: The oral BMDL1SD of 44 mg/kg from the Wolansky acute rat study is being used for this endpoint because of the overall robust nature of the study, and it is protective of all effects observed in the toxicology database. Short-term Dermal: A non-cancer dermal assessment is not being conducted for permethrin. No toxicity was observed in the rat dermal study up to the limit dose. This lack of toxicity is also supported by the low dermal absorption of permethrin (<5%). Low dermal absorption is consistent with the pyrethroid class as a whole. Additionally, using an oral study with the available 3.3% dermal absorption factor, the point of departure would be above the limit dose and is protective of those exposure to children < 6. Short-term Inhalation: A 15-day inhalation study in rat resulted in a NOAEL and LOAEL of 0.042 and 0.583 mg/L based on clinical signs (tremors and hypersensitivity). This study was

Permethrin Human Health Risk Assessment DP No. 414137
Page 28 of 129
selected as it is route-specific and the calculated Human Equivalent Dose inhalation exposure is protective of the effects observed in the permethrin following oral exposure. The methods and dosimetry equations described in EPA’s reference concentration (RfC) guidance (1994) are suited for calculating HECs based on the inhalation toxicity point of departure (NOAEL, LOAEL, or benchmark dose level (BMDL)) for use in MOE calculations. The regional-deposited-dose ratio (RDDR), which accounts for the particulate diameter (mass median aerodynamic diameter [MMAD] and geometric standard deviation [g] of aerosols), can be used to estimate the different dose fractions deposited along the respiratory tract. The RDDR is also based on interspecies differences in ventilation and respiratory-tract surface areas. Thus, the RDDR can be used to adjust an observed inhalation particulate exposure of an animal to the predicted inhalation exposure for a human. For the inhalation toxicity study with permethrin, a extrarespiratory RDDR was estimated at 3.142 based extra respiratory effects using female body weights. The traditional uncertainty factors of 10X for inter-species extrapolation and 10X for intra-species variability were applied to the dietary and incidental oral assessments. For children <6 a 3x FQPA factor was applied. Therefore, the total uncertainty factors for dietary and incidental oral assessments are 100 for adults and children ≥6, and 300 for children <6. For inhalation risk assessments, the inter-species extrapolation factor was reduced to 3X based on the use of the human-equivalent doses (HEDs) to assess inhalation exposure and risk, which accounts for pharmacokinetic (not pharmacodynamics) interspecies differences. Therefore, the inhalation LOC for adults and children >6 years old is equal to an MOE of 30 and for children <6 is equal to 100. 4.5.2 Recommendation for Combining Routes of Exposure for Risk Assessment The incidental oral and inhalation routes of exposure are being assessed based on the same endpoint (signs of neurotoxicity) and therefore can be combined. No dermal endpoint was selected since no dermal hazard was identified for permethrin. 4.5.3 Cancer Classification and Risk Assessment Recommendation Permethrin is classified as “Likely to be Carcinogenic to Humans” based on lung tumors (adenomas and/or carcinomas combined) in female mice and liver tumors (hepatocellular adenomas) in male and female mice. The cancer potency factor (Q1* (mg/kg/day)-1) is 9.567 x 10-3 and is based on lung tumors in female mice.

Permethrin Human Health Risk Assessment DP No. 414137
Page 29 of 129
4.5.4 Summary of Points of Departure and Toxicity Endpoints
Table 4.5.4.1. Summary of Permethrin Endpoints. Exposure Scenario
Point of Departure
Uncertainty/FQPA Safety Factors
RfD, PAD, Level of Concern for Risk
Assessment
Study and Toxicological Effects
Acute Dietary- (≥6 years old)
Wolansky BMDL1SD = 44 mg/kg
UFA = 10X UFH = 10X FQPA SF = 1X
Acute RfD = 0.44 mg/kg aPAD =0.44 mg/kg/day
Wolansky BMD1SD = 63 mg/kg based on decreased motor activity
Acute Dietary- (<6 years old)
Wolansky BMDL1SD = 44 mg/kg
UFA = 10X UFH = 10X FQPA SF = 3X
Acute RfD = 0.44 mg/kg aPAD =0.147 mg/kg/day
Wolansky BMD1SD = 63 mg/kg based on decreased motor activity
Incidental Oral (All durations)
Wolansky BMDL1SD = 44 mg/kg
UFA = 10X UFH = 10X FQPA SF = 3X
LOC for Adult Aggregate = 100
Wolansky BMD1SD = 63 mg/kg based on decreased motor activity
Residential LOC for Children <6 = 300
Dermal (short-term; all populations)
No assessment recommended. No effects observed in the dermal toxicity study, and low dermal absorption based on dermal penetration studies. Using the oral data with the dermal penetration factor would lead to a POD near the limit dose.
Inhalation (All durations; ≥6 years old)
Inhalation NOAEL= 0.042 mg/l
UFA = 3X UFH = 10X FQPA SF = 1X
Occupational/Residential LOC = 30
15-Day Inhalation Toxicity (rat) LOAEL = 0.583 mg/l based on body tremors and hypersensitivity to noise.
Inhalation (All durations; <6 years old)
Inhalation NOAEL= 0.042 mg/l
UFA = 3X UFH = 10X FQPA SF = 3X
Residential LOC = 100 15-Day Inhalation Toxicity (rat) LOAEL = 0.583 mg/l based on body tremors and hypersensitivity to noise.
Cancer (oral, dermal, inhalation)
Classification: Permethrin is classified as “Likely to be Carcinogenic to Humans” based on lung tumors (adenomas and/or carcinomas combined) in female mice and liver tumors (hepatocellular adenomas) in male and female mice. The Q1* (mg/kg/day)-1 = 9.567 x 10-3 is based on lung tumors in female mice.
Point of Departure (POD) = A data point or an estimated point that is derived from observed dose-response data and used to mark the beginning of extrapolation to determine risk associated with lower environmentally relevant human exposures. NOAEL = no observed adverse effect level. LOAEL = lowest observed adverse effect level. UF = uncertainty factor. UFA = extrapolation from animal to human (interspecies). UFH = potential variation in sensitivity among members of the human population (intraspecies). FQPA SF = FQPA Safety Factor. PAD = population adjusted dose (a = acute). RfD = reference dose. MOE = margin of exposure. LOC = level of concern.

Permethrin Human Health Risk Assessment DP No. 414137
Page 30 of 129
Table 4.5.4.2: Summary of HEC/HED Values.
Population Scenario Toxicity duration
adjustment1 HEC HED2
(mg/kg-day) Daily Weekly mg/L mg/m3
Occupational Handler 0.75 1 0.099 98.973 9.366
Residential
Handler NA NA 0.132 131.964 3.122 Outdoor
post-application
NA NA 0.132 131.964 3.590
Indoor Post-application
NA 7 0.132 131.964 3.122
Bystander 24 7 0.033 32.991 -- HEC = human-equivalent concentration; HED = human-equivalent dose. NA = not applicable (the expected duration of the exposure scenario is less than the duration in the available inhalation toxicity studies; downward adjustments are not permitted). 1 Duration Adjustment: Daily Adjustment = 8-hour human exposure/6-hour rat exposure = 0.75; Weekly Adjustment = 5 days human exposure/5 days rat exposure = 1.
4.6 Endocrine Disruptor Screening Program As required by FIFRA and FFDCA, EPA reviews numerous studies to assess potential adverse outcomes from exposure to chemicals. Collectively, these studies include acute, subchronic and chronic toxicity, including assessments of carcinogenicity, neurotoxicity, developmental, reproductive, and general or systemic toxicity. These studies include endpoints which may be susceptible to endocrine influence, including effects on endocrine target organ histopathology, organ weights, estrus cyclicity, sexual maturation, fertility, pregnancy rates, reproductive loss, and sex ratios in offspring. For ecological hazard assessments, EPA evaluates acute tests and chronic studies that assess growth, developmental and reproductive effects in different taxonomic groups. As part of its reregistration decision for permethrin, EPA reviewed these data and selected the most sensitive endpoints for relevant risk assessment scenarios from the existing hazard database. However, as required by FFDCA section 408(p), permethrin is subject to the endocrine screening part of the Endocrine Disruptor Screening Program (EDSP). EPA has developed the EDSP to determine whether certain substances (including pesticide active and other ingredients) may have an effect in humans or wildlife similar to an effect produced by a “naturally occurring estrogen, or other such endocrine effects as the Administrator may designate.” The EDSP employs a two-tiered approach to making the statutorily required determinations. Tier 1 consists of a battery of 11 screening assays to identify the potential of a chemical substance to interact with the estrogen, androgen, or thyroid (E, A, or T) hormonal systems. Chemicals that go through Tier 1 screening and are found to have the potential to interact with E, A, or T hormonal systems will proceed to the next stage of the EDSP where EPA will determine which, if any, of the Tier 2 tests are necessary based on the available data. Tier 2 testing is designed to identify any adverse endocrine-related effects caused by the substance, and establish a dose-response relationship between the dose and the E, A, or T effect. Under FFDCA section 408(p), the Agency must screen all pesticide chemicals. Between October 2009 and February 2010, EPA issued test orders/data call-ins for the first group of 67 chemicals, which contains 58 pesticide active ingredients and 9 inert ingredients. A second list

Permethrin Human Health Risk Assessment DP No. 414137
Page 31 of 129
of chemicals identified for EDSP screening was published on June 14, 20133 and includes some pesticides scheduled for registration review and chemicals found in water. Neither of these lists should be construed as a list of known or likely endocrine disruptors4. Permethrin is on List 1 for which EPA has received all of the required Tier 1 assay data. The Agency has reviewed all of the assay data received for the appropriate List 1 chemicals and the conclusions of those reviews are available in the chemical-specific public dockets (see Docket # EPA-HQ-OPP-2011-0039 for permethrin). For further information on the status of the EDSP, the policies and procedures, the lists of chemicals, future lists, the test guidelines and the Tier 1 screening battery, please visit our website.4
5.0 Dietary Exposure and Risk Assessment 5.1. Residues of Concern Summary and Rationale The qualitative nature of the residue in plants and livestock is understood based on the available metabolism data. The residue of concern in plants and animals is permethrin (cis- and trans- isomers) for purposes of both tolerance expression and risk assessment (Memo, S. Kinard, et al., 06-JUL-2004; TXR#0052775).
Table 5.1. Summary of Permethrin Residues of Concern.1
Matrix Residues included in Risk Assessment Residues included in Tolerance Expression Plants Parent only (both cis- and trans-) Parent only (both cis- and trans-) Livestock Parent only (both cis- and trans-) Parent only (both cis- and trans-) Drinking Water Parent only (both cis- and trans-) NA
1 Memo, S. Kinard, et al., 06-JUL-2004; TXR#0052775. 5.2 Food Residue Profile The existing residue chemistry database for permethrin is adequate for risk assessment purposes. Adequate methods are available for the enforcement of established tolerances. Adequate analytical methods exist for data collection. Adequate storage stability, processed food, and magnitude of the residue data exist to support the currently established tolerances for residues of permethrin, except in kiwi. The most recent residue chemistry chapter for permethrin identified several field trial, storage stability, and analytical methodology data deficiencies (Memo, S. Kinard, D313662, 17-MAR-2005) that were required in a permethrin DCI that was issued in 2009 (GDCI-109701-26467). The required studies have been submitted and reviewed and are considered adequate, provided the registrant confirms that the cabbage samples included wrapper leaves (MRID 48598601). A summary of the conclusions for the residue chemistry studies submitted in response to GDCI-109701-26467 can be found in Appendix E.
3 See http://www.regulations.gov/#!documentDetail;D=EPA-HQ-OPPT-2009-0477-0074 for the final second list of chemicals. 4 http://www.epa.gov/endo/

Permethrin Human Health Risk Assessment DP No. 414137
Page 32 of 129
Residue data have not been submitted to support the established kiwi tolerance (2.0 ppm). Additionally, the available labels include use information for chicory, blueberry, okra, pecan, raspberry (black and red), and strawberry; however, tolerances are not established for residues in these commodities. Residue data, per OCSPP guideline 860.1500, are required to support these use patterns or the uses should be removed from the labels. Other than these data gaps and the submission of analytical standards, there are no other outstanding residue chemistry data gaps, and the residue chemistry database is sufficient to support the currently established tolerances and the human health risk assessment for residues of permethrin. HED notes that permethrin may be applied in food/feed handling establishments; however, a tolerance has not been established based on this use pattern since the labels indicate that permethrin may not be applied in food areas of food handling establishments, restaurants, or other areas where food is commercially prepared or processed, or in serving areas while food is exposed or the facility is in operation5. As a result, residues in food are not anticipated based on the use pattern and this use was not included in the dietary assessments. 5.3 Water Residue Profile The residue of concern for risk assessment in drinking water is the parent (cis- and trans-permethrin). As a class of chemicals, the pyrethroids have low solubility in water and a high affinity to bind to soils. Given these physical/chemical properties, it is unlikely that oral exposure from drinking water will be a major pathway of exposure. However, in the absence of a comprehensive set of monitoring data with sufficiently low detection limits which could be used to derive exposure values for drinking water, the Agency has incorporated modeled drinking water values into the acute, chronic, and cancer dietary assessments. The EDWCs that are used in the acute, chronic, and cancer dietary assessments were previously provided by the Environmental Fate and Effects Division (EFED) in the following memorandum: “Second Revision Tier II Estimated Drinking Water Concentrations of Permethrin (PC Code # 109701; DP Barcode D324197)” (J. Melendez, D324197, 17-JAN-2006). The Tier II EDWCs of permethrin were calculated using the Pesticide Root Zone Model/Exposure Analysis Modeling System (PRZM/EXAMS; surface water) and the Screening Concentration in Ground Water (SCI-GROW; ground water) models. The EDWCs for permethrin were calculated based on a maximum application rate of 2.0 lb ai/A and were calculated using the “Georgia onion” scenario. The groundwater screening concentrations were lower than the surface water concentrations; therefore, the acute, chronic, and cancer dietary assessments used the surface water EDWCs of 0.00479 ppm, 0.000901 ppm, and 0.000751, respectively. HED notes that the water solubility of permethrin is 0.0055 ppm. Water residues were incorporated into the DEEM-FCID food categories of “water, direct, all sources” and “water, indirect, all sources.” The drinking water models and their descriptions are available at the EPA internet site: http://www.epa.gov/oppefed1/models/water/.
5 Permethrin Final HED Master Label Report 9-29-2010, the Summary of Labeling Changes for Permethrin (revised 8/29/2011) https://www.regulations.gov/document?D=EPA-HQ-OPP-2004-0385-0128

Permethrin Human Health Risk Assessment DP No. 414137
Page 33 of 129
5.4 Dietary Risk Assessment 5.4.1 Overview of Residue Data Used Highly refined acute, average, and cancer dietary exposure and risk assessments for cis- and trans-permethrin, calculated as total permethrin, were conducted using DEEM-FCID Version 3.18. This model uses 2003-2008 food consumption data from USDA’s NHANES/WWEIA. A chronic dietary endpoint has not been selected for permethrin because repeated exposure does not result in a point of departure lower than that resulting from acute exposure; therefore, the acute dietary risk assessment is protective of chronic dietary risk. However, since there are residential uses of permethrin, a highly refined dietary exposure assessment was conducted to calculate average dietary (food and drinking water) exposure estimates to support the permethrin aggregate risk assessment. Additionally, a commodity specific analysis (CSA) was completed for permethrin using PDP data and no risks of concern were identified. The acute, average, and cancer assessments were refined using PDP monitoring data, field trial data, PCT data, and empirical processing factors. If monitoring data were not available for a particular commodity (e.g., endive), but were available for a similar commodity (e.g., spinach), the available data were translated to the similar crop and the PCT was adjusted, as appropriate. Tolerance-level values (acute assessment) and/or field trial values (chronic and cancer assessments) were used for horseradish, turnip roots, broccoli raab, crabapple, loquat, quince, kiwi, artichoke, and watercress. PDP data and/or translation of PDP data were used for the remaining commodities in the assessment. The acute, chronic, and cancer dietary assessments used modeled surface water EDWCs of 0.00479 ppm, 0.000901 ppm, and 0.000751, respectively. 5.4.2 Percent Crop Treated Used in Dietary Assessment BEAD provided a Screening Level Usage Analysis (SLUA) for permethrin that is dated December 10, 2015. The acute dietary assessment used the following maximum PCT estimates: Almonds (20%); Apples (10%); Asparagus (35%); Avocados (2.5%); Broccoli (15%); Brussels Sprouts (15%); Cabbage (20%); Cantaloupes (40%); Cauliflower (30%); Celery (65%); Cherries (15%); Corn (2.5%); Cucumbers (15%); Garlic (15%); Hazelnuts (2.5%); Lettuce (75%); Nectarines (2.5%); Onions (20%); Peaches (20%); Pears (10%); Peppers (20%); Pistachios (80%); Potatoes (10%); Pumpkins (25%); Soybeans (2.5%); Spinach (90%); Squash (20%); Sweet Corn (10%); Tomatoes (10%); Walnuts (10%); and Watermelons (15%). 100% CT was used for the remaining commodities. The average and cancer dietary assessments used the following average PCT estimates: Almonds (5%); Apples (5%); Artichokes (35%); Asparagus (20%); Avocados (1%); Broccoli (5%); Brussels Sprouts (5%); Cabbage (15%); Cantaloupes (15%); Cauliflower (10%); Celery (50%); Cherries (5%); Corn (2.5%); Cucumbers (5%); Garlic (5%); Hazelnuts (2.5%); Lettuce (45%); Nectarines (2.5%); Onions (10%); Peaches (10%); Pears (2.5%); Peppers (5%); Pistachios (65%); Potatoes (5%); Pumpkins (15%); Soybeans (1%); Spinach (50%); Squash (10%); Sweet Corn (5%); Tomatoes (5%); Walnuts (5%); and Watermelons (5%). Additionally, a PCT value of 5% from almond, apple, and potato was used for all livestock commodities since

Permethrin Human Health Risk Assessment DP No. 414137
Page 34 of 129
almonds, apples, and potatoes have the highest PCT estimate of the commodities that may be fed to livestock. 100% CT was assumed for the remaining commodities. 5.4.3 Acute Dietary Risk Assessment The acute dietary (food and drinking water) exposure and risk estimates do not exceed HED’s level of concern (less than 100% of the aPAD) at the 99.9th exposure percentile for the general U.S. population (2.6% of the aPAD) and all population subgroups. The most highly exposed population subgroup is children 3-5 years old at 20% of the aPAD. The exposure and risk estimates are presented in Table 5.4.6. 5.4.4 Average Dietary Exposure Assessment An average dietary (food and drinking water) exposure assessment was conducted to support the permethrin aggregate risk assessment. The most highly exposed adult population subgroup was adults 50-99 years old, with an average dietary (food and drinking water) exposure estimate of 0.000133 mg/kg/day. The population subgroup with the highest average dietary (food and drinking water) exposure estimate is children 1-2 years old (0.000214 mg/kg/day). The exposure estimates are presented in Table 5.4.6. 5.4.5 Cancer Dietary Risk Assessment The most highly exposed adult population subgroup was adults 50-99 years old, with a cancer dietary (food and drinking water) exposure estimate of 0.000130 mg/kg/day. Applying the cancer potency factor (Q1*) of 9.567 x 10-3 (mg/kg/day)-1) to the exposure value results in a cancer dietary (food and drinking water) risk estimate of 1.3 x 10-6. Spinach (38% of total exposure), endive (translated from Spinach PDP data; 12% of total exposure), turnip greens (translated from Spinach PDP data; 8% of total exposure), kiwi (6% of total exposure; tolerance-level residue), and drinking water (12% of total exposure) were the main drivers in the cancer assessment. Although spinach PDP and PCT data were used in the assessments, 51% of samples had detectable residues. Additionally, spinach PDP data were translated to numerous commodities including endive, leafy amaranth, arugula, garland chrysanthemum, garden and upland cress, dandelion leaves, parsley leaves, radicchio, Swiss chard, turnip greens, cilantro leaves, and dillweed which may have led to overestimation of dietary exposure to residues of permethrin. The contributions from these commodities could be further refined with commodity-specific monitoring data and/or PCT data.

Permethrin Human Health Risk Assessment DP No. 414137
Page 35 of 129
5.4.6. Dietary Assessment Summary Tables
Table 5.4.6. Summary of Dietary (Food and Drinking Water) Exposure and Risk for Permethrin.1
Population Subgroup
Acute Dietary (99.9th Percentile)
Average (Chronic) Dietary
Cancer
Dietary Exposure
(mkd)2 % aPAD
Dietary Exposure
(mkd)2 % cPAD
Dietary Exposure
(mkd)2
Risk Estimate
General U.S. Population 0.011495 2.6 0.000120
NA3 NA4
All Infants (<1 year old) 0.010810 7.4 0.000138 Children 1-2 years old 0.016142 11 0.000214 Children 3-5 years old 0.029784 20 0.000154 Children 6-12 years old 0.009542 2.2 0.000084 Youth 13-19 years old 0.010087 2.3 0.000070 Adults 20-49 years old 0.010570 2.4 0.000121 Adults 50-99 years old 0.010135 2.3 0.000133 0.000130 1.3 x 10-6 Females 13-49 years old 0.011827 2.7 0.000127 NA4
1 The bolded values represent the most highly exposed population subgroups for each of the risk assessments. 2 mkd=mg/kg/day. 3 A chronic dietary endpoint has not been selected for permethrin because repeated exposure does not result in a point of departure lower than that resulting from acute exposure. Therefore, the acute dietary risk assessment is protective of chronic dietary risk. However, since there are residential uses of permethrin, a highly refined average dietary (food and drinking water) exposure assessment was conducted to calculate average dietary exposure estimates to support the permethrin aggregate risk assessment. 4 Cancer exposure and risk estimate are presented for the most highly exposed adult population subgroup (adults 50-99 years old for permethrin). 6.0 Residential (Non-Occupational) Exposure/Risk Characterization There are existing residential uses that have been reassessed in this document to reflect updates to HED’s 2012 Residential SOPs6 along with policy changes for body weight assumptions. The following changes have also been incorporated:
Residential incidental oral post-application exposure risk estimates resulting from vector mosquito control aerial and truck mounted fogger applications have been revised to incorporate the new off-target deposition rate of 8.7 percent of the application rate may be used to evaluate ground-based ULV applications;
Chemical-specific turf transferable residue (TTR) data are available and have been adjusted and reflect the maximum application rates for permethrin; and
The inhalation and incidental oral scenarios have been reevaluated to incorporate changes to the permethrin toxicity database and to provide a refined assessment of the end-use products.
Residential handler and post-application exposures are anticipated from the registered use of permethrin. In assessing these exposures, HED used the REJV National Pesticide Use Survey
6 Available: http://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/standard-operating-procedures-residential-pesticide

Permethrin Human Health Risk Assessment DP No. 414137
Page 36 of 129
(2012-2013), which underwent secondary review in 20167, to refine the cancer assessment. REJV data are proprietary and, thus, are subject to the data protection provisions of FIFRA. The revision of residential exposures will impact the human health cancer and non-cancer aggregate risk assessments for permethrin. 6.1 Residential Handler Exposure/Risk Estimates Several permethrin products require PPE to be worn by applicators. These labels were not considered to be marketed as consumer products and have been considered only for residential post-application exposure assessment as products with PPE requirements are assumed to imply applications are done by professionals. Permethrin product labels with residential use sites that do not require specific clothing (e.g., long-sleeved shirt/long pants) and/or PPE, have been considered in the residential handler assessment. Both non-cancer and cancer residential handler exposure assessments were performed for adult homeowners applying permethrin dusts/powders, dips, RTU products, and pump/trigger sprays products to cats and dogs. For spot-on applications to pets, inhalation exposure is negligible. Since there is no non-cancer dermal hazard for permethrin, the non-cancer handler assessment includes only inhalation exposures. For the cancer assessment, both dermal and inhalation exposures are assessed. The quantitative exposure/risk assessment developed for residential handlers is based on the following scenarios which correlate to the use pattern (Appendix F):
Application to: 1. Indoor Perimeter/Spot/Bedbug; Crack and Crevice Application with Bulb Duster 2. Outdoor Garden/Tree (Ornamental) Dust Application with Shaker Can 3. Indoor Perimeter/ Spot/ Bedbug (course application) with Aerosol Can 4. Fabric Directed Spray (insect repellent) Spot/Bedbug Application with Aerosol
Can 5. Outdoor Garden/Tree (Ornamental) Application with Aerosol Can 6. Outdoor Space/Perimeter Treatment with Aerosol Can 7. Indoor Perimeter/ Spot/ Bedbug (course application) with Trigger-Spray Bottle 8. Fabric Directed Spray (insect repellent) Spot/Bedbug Application with Trigger-
Spray Bottle 9. Outdoor Garden/Tree (Ornamental) Application with Trigger-Spray Bottle 10. Outdoor Lawn/Turf Treatment with Hose-end Sprayer 11. Outdoor Lawn/Turf Treatment with Push-type Rotary Spreader 12. Outdoor Lawn/Turf Treatment with Belly Grinder 13. Outdoor Lawn/Turf Treatment with Spoon 14. Outdoor Lawn/Turf Treatment with Cup 15. Outdoor Lawn/Turf Treatment Dispersed by Hand 16. Outdoor Perimeter Treatment with Shaker Can 17. Outdoor Paints/Preservative Wood Treatment with Airless Sprayer
7 Review of “Residential Exposure Joint Venture: National Pesticide Use Survey”, M. Crowley et. al., 21-JUL-2016; D433915

Permethrin Human Health Risk Assessment DP No. 414137
Page 37 of 129
18. Outdoor Paints/Preservative Wood Treatment with Brush 19. Outdoor Paints/Preservative Wood Treatment with Manually-pressurized
handwand 20. Outdoor Paints/Preservative Wood Treatment with Roller 21. Direct Application to Dogs with Dip Treatment 22. Direct Body Wipe Application to Dogs/Horses with Sponge/Towelette 23. Direct Application to Dogs/Horses with Trigger-Spray Bottle 24. Direct Application to Dogs with Aerosol Can 25. Direct Application to Dogs with RTU via Hand/Glove 26. Direct Spot-On Treatment to Dogs with RTU Applicator Tube 27. Direct Application to Dogs/Horses with Shaker Can
Mixing/Loading/Applying: 28. Indoor Perimeter/Spot/ Bedbug (coarse application); Perimeter /Spot/ Bedbug
(pinstream application); Crack and Crevice with Manually-pressurized handwand (w/ or w/o pin stream nozzle)
29. Indoor/Outdoor Garden/Tree/Ornamental Application with Manually-pressurized handwand
30. Outdoor Garden/Tree/Ornamental Application with Manually-pressurized handwand
31. Outdoor Lawn/Turf/Perimeter Treatment with Manually-pressurized handwand 32. Outdoor Garden/Tree/Ornamental Application with backpack 33. Indoor/Outdoor Garden/Tree/Ornamental Application with Backpack 34. Outdoor Lawn/Turf/Perimeter Treatment with Backpack
Residential Handler Exposure Data and Assumptions A series of assumptions and exposure factors served as the basis for completing the residential handler risk assessments. A screening-level approach was used for assessment of residential exposures by evaluation of the maximum application rate for all possible residential handler exposure scenarios of permethrin. The registered application rates used for the residential handler quantitative exposure/risk assessment are based on the scenarios listed in Appendix F. The algorithms used to estimate exposure and dose for residential handlers can be found in Memo, J. Godshall, 30-JUN-2017 (D440978) and in the 2012 Residential SOPs8. Unit Exposures and Area Treated or Amount Handled: Unit exposure values and estimates for area treated or amount handled were taken from HED’s 2012 Residential SOPs9, when available. For assessment of residential application to horses, it was assumed that 3 horses were treated per day. This recommendation is based upon data available from the American Veterinary Medical Association (AVMA) which references data from its U.S. Pet Ownership and Demographics Sourcebook (2012) that reports pet owners have an average of 2.7 horses per household.10
8 Available: http://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/standard-operating-procedures-residential-pesticide 9 Available: http://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/standard-operating-procedures-residential-pesticide 10 https://www.avma.org/KB/Resources/Statistics/Pages/Market-research-statistics-US-pet-ownership.aspx

Permethrin Human Health Risk Assessment DP No. 414137
Page 38 of 129
Exposure Duration: The toxicological profile of pyrethroids characterizes pyrethroids, including permethrin, as being rapid in onset and associated with acute, peak exposures. The single dose and repeat dosing studies show that repeat exposures do not result in lower PODs (i.e., there is no evidence of increasing toxicity with an increased duration of exposure). As such, due to the rapid toxicokinetics and toxicity profile of pyrethroids, the residential assessments are conducted as a series of acute exposures, and the same endpoint is used regardless of duration. Therefore, residential handler short-term exposure is considered protective of the longer durations since intermediate- and long-term exposure is expected, but due to the nature of this chemical only a short-term assessment is needed. Residential Handler Non-Cancer Exposure and Risk Estimate Equations The algorithms used to estimate exposure and dose for residential handlers can be found in the 2012 Residential SOPs11. Combining Exposures/Risk Estimates: Residential handler dermal and inhalation exposure is anticipated from registered permethrin uses, however there is no non-cancer dermal hazard for permethrin. Therefore, only non-cancer inhalation exposures have been quantitatively assessed and there are no additional routes to combine. For residential handlers, exposures from application to turf were not combined with exposures from treating gardens/trees because concurrent use of separate pesticide products that contain the same active ingredient to treat the same or different pests does not typically occur. Therefore, although the same products allow treatment of gardens/trees and turf, these exposures were not combined for residential handlers. Residential Handler Cancer Exposure and Risk Estimate Equations Cancer risk estimates were calculated using a linear low-dose extrapolation approach in which a Lifetime Average Daily Dose (LADD) is first calculated and then compared with a cancer potency factor (Q1*) that has been calculated for permethrin based on dose response data in the appropriate toxicology study (Q1* = 9.567 x 10-3 (mg/kg/day)-1). Absorbed average daily dose (ADD) levels were used as the basis for calculating the LADD values. Dermal and inhalation ADD values were first added together to obtain combined ADD values. LADD values were then calculated and compared to the cancer potency factor (Q1*) to obtain cancer risk estimates. While no dermal hazard was identified for the non-cancer quantitative assessment, the cancer potency factor (Q1*) was used in coordination with the DAF of 3.3% (as detailed in Section 4.2). Days per Year of Exposure: As the days of exposure per year is needed to calculate the LADD and the maximum number of applications/re-treatment intervals provided on the permethrin labels are not considered prescriptive for efficacy, the label-stated number of treatments per year may not be representative of actual usage. Therefore, the maximum days per year of exposure for residential
11 Available: http://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/standard-operating-procedures-residential-pesticide

Permethrin Human Health Risk Assessment DP No. 414137
Page 39 of 129
handlers have been refined using data on application frequencies from the Residential Exposure Joint Venture (REJV) National Pesticide Use Survey (2012-2013) which underwent secondary review in 201612. Use site- and method-specific application information specific to permethrin registrations was compiled, however, the data were not subset for permethrin-specific products. The results, as they relate to the permethrin use pattern, are presented as a range in Table 6.1.1, including the average and 95th percentile for the number of applications made per year, as well as the maximum number of applications made per year. These application frequencies reflect responses for a single year per household (the extent of the survey), not an average of multiple years. Pest pressures and product usage are expected to vary over the course of a lifetime, and consumers are not expected to continuously, year-after-year, apply products at the high frequencies reflected in high percentile and maximum survey results. Therefore, for the purposes of representing the average use of permethrin products over a lifetime by residential handlers for the residential handler cancer assessment, the average data have been used.
Table 6.1.1. Residential Exposure Joint Venture Results for Number of Applications per Year.1
Formulation Exposure Scenario Average2 95th percentile3 Maximum
Dust
1. Indoor Perimeter/Spot/Bedbug; Crack and Crevice Application with Bulb Duster
3.03 14 30
2. Outdoor Garden/Tree (Ornamental) Dust Application with Shaker Can
2.99 9 18
27. Direct Application to Dogs/Horses with Shaker Can 2.05 4 6
RTU
3. Indoor Perimeter/ Spot/ Bedbug (coarse application) with Aerosol Can
6.49 21 259
4. Fabric Directed Spray (insect repellent) Spot/Bedbug Application with Aerosol Can
2.71 10 39
5. Outdoor Garden/Tree (Ornamental) Application with Aerosol Can
2.83 9 32
6. Outdoor Space/Perimeter Treatment with Aerosol Can 2.13 7 10
7. Indoor Perimeter/ Spot/ Bedbug (course application) with Trigger-Spray Bottle
3.75 11 107
8. Fabric Directed Spray (insect repellent) Spot/Bedbug Application with Trigger-Spray Bottle
2.71 10 39
9. Outdoor Garden/Tree (Ornamental) Application with Trigger-Spray Bottle
2.83 9 32
10. Outdoor Lawn/Turf Treatment with Hose-end Sprayer 2.53 5 65
23. Direct Application to Dogs/Horses with Trigger-Spray Bottle
6.09 28 170
24. Direct Application to Dogs with Aerosol Can 3.04 9 24
25. Direct Application to Dogs with RTU via Hand/Glove 5.11 17 56
26. Direct Spot-On Treatment to Dogs with RTU Applicator Tube
5.49 15 32
Other: Indoor Fogger Application 2.02 5 16
Other: Outdoor Aerosol Space Spray 2.57 6 76
Other: Pressurized liquid application to Mattress 3.66 20 32
Granular 11. Outdoor Lawn/Turf Treatment with Push-type Rotary Spreader
2.04 5 31
12. Outdoor Lawn/Turf Treatment with Belly Grinder 1.93 5 14
12 Review of “Residential Exposure Joint Venture: National Pesticide Use Survey”, M. Crowley et. al., 21-JUL-2016; D433915

Permethrin Human Health Risk Assessment DP No. 414137
Page 40 of 129
Table 6.1.1. Residential Exposure Joint Venture Results for Number of Applications per Year.1
Formulation Exposure Scenario Average2 95th percentile3 Maximum 13. Outdoor Lawn/Turf Treatment with Spoon 3.67 12 47
14. Outdoor Lawn/Turf Treatment with Cup 3.67 12 47
15. Outdoor Lawn/Turf Treatment Dispersed by Hand 3.10 11 34
16. Outdoor Perimeter Treatment with Shaker Can 3.67 12 47
Paints/ Preservatives/ Stains
17. Outdoor Paints/Preservative Wood Treatment with Airless Sprayer
3.30 10 40
18. Outdoor Paints/Preservative Wood Treatment with Brush 3.30 10 40
19. Outdoor Paints/Preservative Wood Treatment with Manually-pressurized handwand
2.79 8 34
20. Outdoor Paints/Preservative Wood Treatment with Roller 3.30 10 40
Liquid Concentrate
21. Direct Application to Dogs with Dip Treatment 3.10 9 30
22. Direct Body Wipe Application to Dogs/Horses with Sponge/Towelette
5.52 15 32
28. Indoor Perimeter/Spot/ Bedbug (course application); Perimeter /Spot/ Bedbug (pinstream application); Crack and Crevice with Manually-pressurized handwand (w/ or w/o pin stream nozzle)
2.12 6 17
29. Indoor/Outdoor Garden/Tree/Ornamental Application with Manually-pressurized handwand
3.29 10 48
30. Outdoor Garden/Tree/Ornamental Application with Manually-pressurized handwand
3.29 10 48
31. Outdoor Lawn/Turf/Perimeter Treatment with Manually-pressurized handwand
2.67 8 23
32. Outdoor Garden/Tree/Ornamental Application with backpack
2.2 6 7
33. Indoor/Outdoor Garden/Tree/Ornamental Application with Backpack
2.2 6 7
34. Outdoor Lawn/Turf/Perimeter Treatment with Backpack 2.53 12 14
Other: Indoor Barn Misting System 2.61 7 49 1. Program search criteria and calculations are detailed in J. Godshall, 30-JUN-2017, D440978, Appendix D Table D.1. 2. Sampling weighted averages were not used in this assessment. 3. 95th percentile was rounded to the closest number of applications.
Lifetime Expectancy: Life expectancy values are from the Exposure Factors Handbook 2011 Edition Table 18-1 (U.S. EPA, 2011). The table shows that the overall life expectancy is 78 years based on life expectancy data from 2007. In 2007, the average life expectancy for males was 75 years and 80 years for females. Based on the available data, the recommended value for use in cancer risk assessments is 78 years. Years per Lifetime of Exposure: It is assumed that residential handlers would be exposed for 50 years out of a 78-year lifespan. Summary of Residential Handler Non-Cancer Exposure and Risk Estimates All registered residential uses were reassessed using the revised 2012 Residential SOPs and policy changes for body weights. All screening-level residential handler non-cancer inhalation risks estimated are not of concern with MOEs ranging from 370 to 770,000 (adult inhalation LOC = 30). The residential handler exposure risk estimates are summarized in Table 6.1.2.

Permethrin Human Health Risk Assessment DP No. 414137
Page 41 of 129
Summary of Residential Handler Cancer Exposure and Risk Estimates The cancer exposure and risk estimates for permethrin residential handler scenarios are presented in Table 6.1.2. Residential handler cancer (dermal + inhalation) risk estimates range from 3×10-
10 to 2×10-6 with the greatest cancer risk estimate for liquid applications to outdoor gardens/trees/ornamentals with a backpack sprayer (Table 6.1.2).

Permethrin Human Health Risk Assessment DP No. 414137
Page 42 of 129
Table 6.1.2. Residential Handler Non-Cancer and Cancer Exposure and Risk Estimates for Permethrin.
Formulation Exposure Scenario
Unit Exposure (mg/lb ai) Maximum
Application Rate1
Area Treated or Amount Handled
Daily2
Non-Cancer Cancer
Inhalation REJV6
(apps per year)
Route Specific LADD8
Cancer Risk
Estimate9 Dermal Inhalation
Dose (mg/kg/day)4
MOE5 Inhalation Dermal7
Applicator
Dust/Powder
1. Indoor Perimeter/Spot/Bedbug; Crack and Crevice Application with Bulb Duster
250 1.7 0.01 lb ai/lb dust 0.25 lb dust 0.000053 59000 3.03 2.822E-07 1.385E-06 1.59E-08
2. Outdoor Garden/Tree (Ornamental) Dust Application with Shaker Can
4300 18 0.0025 lb ai/can 2 cans 0.0011 2800 2.99 5.785E-06 4.68E-05 5.03E-07
RTU
3. Indoor Perimeter/ Spot/ Bedbug (course application) with Aerosol Can
370 3.0 0.00438 lb ai/16 oz
can 0.5 – 16 oz can
(8 oz) 0.000082 38,000 6.46 9.298E-07 3.742E-06 4.47E-08
4. Fabric Directed Spray (insect repellent) Spot/Bedbug Application with Aerosol Can
N/A 3.0 0.0075 lb ai/can 1 can 0.00028 11,000 3.27 1.61E-06 Negligible 1.54E-08
5. Outdoor Garden/Tree (Ornamental) Application with Aerosol Can
370 3.0 0.0025 lb ai/can 2 cans 0.00019 17,000 3.19 1.065E-06 4.261E-06 5.10E-08
6. Outdoor Space/Perimeter Treatment with Aerosol Can 370 3.0 0.225 lb ai/can 1 can 0.0084 370 2.13 3.146E-05 0.0001273 1.52E-06
7. Indoor Perimeter/ Spot/ Bedbug (course application) with Trigger-Spray Bottle
85.1 0.061 0.043 lb ai/bottle 0.5 bottle 0.000017 190,000 3.75 1.119E-07 5.067E-06 4.96E-08
8. Fabric Directed Spray (insect repellent) Spot/Bedbug Application with Trigger-Spray Bottle
N/A 0.061 0.0075 lb ai/bottle 1 bottle 0.0000057 550,000 3.27 3.277E-08 Negligible 3.14E-10
9. Outdoor Garden/Tree (Ornamental) Application with Trigger-Spray Bottle
85.1 0.061 0.043 lb ai/bottle 2 bottles 0.000066 48,000 3.19 3.701E-07 1.682E-05 1.64E-07
10. Outdoor Lawn/Turf Treatment with Hose-end Sprayer 6.26 0.034 0.45 lb ai/acre 0.5 acres 0.000096 33,000 2.53 4.264E-07 2.576E-06 2.87E-08
Granular
11. Outdoor Lawn/Turf Treatment with Push-type Rotary Spreader
0.81 0.0026 0.65 lb ai/acre 0.5 acres 0.000011 300,000 2.04 3.943E-08 3.943E-07 4.15E-09
12. Outdoor Lawn/Turf Treatment with Belly Grinder 360 0.039 0.0003125 lb ai/ft2 1200 ft2 0.00018 17,000 1.93 6.113E-07 0.0001902 1.83E-06
13. Outdoor Lawn/Turf Treatment with Spoon 6.2 0.087 0.0003125 lb ai/ft2 100 ft2 0.000034 92,000 3.67 2.189E-07 5.152E-07 7.02E-09
14. Outdoor Lawn/Turf Treatment with Cup 0.11 0.013 0.00156 lb ai/ft2 100 ft2 0.000025 120,000 3.67 1.61E-07 4.572E-08 1.98E-09
15. Outdoor Lawn/Turf Treatment Dispersed by Hand 160 0.38 0.0003125 lb ai/ft2 100 ft2 0.00015 21,000 3.10 8.159E-07 1.142E-05 1.17E-07
16. Outdoor Perimeter Treatment with Shaker Can 0.11 0.013 0.0008 lb ai/ft2 100 ft2 0.000013 240,000 3.67 8.371E-08 2.318E-08 1.02E-09
Paints/ Preservatives
/ Stain
17. Outdoor Paints/Preservative Wood Treatment with Airless Sprayer
160 0.56 0.04 lb ai/gal 5 gal 0.0014 2,200 3.30 8.113E-06 7.533E-05 7.98E-07
18. Outdoor Paints/Preservative Wood Treatment with Brush
450 0.20 0.04 lb ai/gal 2 gal 0.0002 16,000 3.30 1.159E-06 8.692E-05 8.43E-07
19. Outdoor Paints/Preservative Wood Treatment with 63 0.018 0.04 lb ai/gal 3 gal 0.000027 120,000 2.79 1.323E-07 1.52E-05 1.47E-07

Permethrin Human Health Risk Assessment DP No. 414137
Page 43 of 129
Table 6.1.2. Residential Handler Non-Cancer and Cancer Exposure and Risk Estimates for Permethrin.
Formulation Exposure Scenario
Unit Exposure (mg/lb ai) Maximum
Application Rate1
Area Treated or Amount Handled
Daily2
Non-Cancer Cancer
Inhalation REJV6
(apps per year)
Route Specific LADD8
Cancer Risk
Estimate9 Dermal Inhalation
Dose (mg/kg/day)4
MOE5 Inhalation Dermal7
Manually-pressurized handwand 20. Outdoor Paints/Preservative Wood Treatment with
Roller 450 0.20 0.04 lb ai/gal 1 gal 0.0002 16,000 3.30 1.159E-06 8.692E-05 8.43E-07
Liquid Concentrate
21. Direct Application to Dogs with Dip Treatment 100 0.027 0.006 lb ai/gal 2 animals 0.0000041 770,000 3.02 2.174E-08 2.652E-06 2.56E-08
22. Direct Body Wipe Application to Dogs/Horses with Sponge/Towelette
1600 0.21 0.0062 lb ai/animal 3 horses
(protective of 2 dogs)
0.000049 64,000 5.52 4.753E-07 0.0001164 1.12E-06
RTU
23. Direct Application to Dogs/Horses with Trigger-Spray Bottle
820 3.3 0.007 lb ai/animal 3 animals 0.00087 3,600 6.09 9.304E-06 7.593E-05 8.15E-07
24. Direct Application to Dogs with Aerosol Can 820 3.3 0.000538 lb ai/16
oz can
2 animals
0.000044 70,000 3.04 2.348E-07 1.921E-06 2.06E-08
25. Direct Application to Dogs with RTU via Hand/Glove 2000 0.29 0.0014 lb ai/animal 0.00001 310,000 5.11 8.976E-09 2.065E-05 1.98E-07
26. Direct Spot-On Treatment to Dogs with RTU Applicator Tube
120 negligible 0.006 lb ai/animal Negligible Negligible 5.49 Negligible 5.691E-06 5.44E-08
Dust 27. Direct Application to Dogs/Horses with Shaker Can 4300 18 0.0625 lb ai/animal 3 animals 0.00011 29,000 2.05 3.956E-07 5.754E-07 9.29E-09
Mixer/Loader/Applicator
Liquid Concentrates
28. Indoor Perimeter/Spot/ Bedbug (course application); Perimeter /Spot/ Bedbug (pinstream application);
Crack and Crevice with Manually-pressurized handwand (w/ or w/o pin stream nozzle)
69 1.1 0.042 lb ai/gal 0.5 gal 0.00029 11,000 2.12 1.078E-06 2.23E-06 3.16E-08
29. Indoor/Outdoor Garden/Tree/Ornamental Application with Manually-pressurized handwand
63 0.018 0.041 lb ai/gal 5 gal 0.000046 68,000 3.29 2.662E-07 3.067E-05 2.96E-07
30. Outdoor Garden/Tree/Ornamental Application with Manually-pressurized handwand
63 0.018 0.00078 lb ai/ft2 1200 ft2 0.00021 15,000 3.29 1.215E-06 0.0001389 1.34E-06
31. Outdoor Lawn/Turf/Perimeter Treatment with Manually-pressurized handwand
63 0.018 0.78 lb ai/gal 5 gal 0.000045 69,000 2.67 2.108E-07 2.436E-05 2.35E-07
32. Outdoor Garden/Tree/Ornamental Application with backpack
130 0.14 0.00078 lb ai/ft2 1200 ft2 0.0016 1,900 2.20 6.182E-06 0.0001932 1.91E-06
33. Indoor/Outdoor Garden/Tree/Ornamental Application with Backpack
130 0.018 0.041 lb ai/gal 5 gal 0.00036 8,700 2.20 1.391E-06 4.25E-05 4.20E-07
34. Outdoor Lawn/Turf/Perimeter Treatment with Backpack
130 0.14 0.78 lb ai/gal 5 gal 0.0068 8,900 2.53 1.556E-06 4.89E-05 4.83E-07
1 Based on registered labels in Appendix F - Table F.1 and F.2. 2 Based on HED’s 2012 Residential SOPs (http://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/standard-operating-procedures-residential-pesticide).

Permethrin Human Health Risk Assessment DP No. 414137
Page 44 of 129
4 Inhalation Dose = Inhalation Unit Exposure (mg/lb ai) × Application Rate (lb ai/acre or gal) × Area Treated or Amount Handled (A/day or gallons/day) ÷ BW (80kg). 5 Inhalation MOE = Inhalation NOAEL (mg/kg/day) ÷ Inhalation Dose (mg/kg/day). 6 See Table 6.1.1 for application percentile ranges. 7 Dermal Dose = Dermal Unit Exposure (mg/lb ai) × Application Rate (lb ai/acre or gal) × Area Treated or Amount Handled (A/day or gallons/day) ÷ BW (80kg). 8 Route specific LADD = Dermal or Inhalation dose (mg/kg/day) × (REJV days of exposure per year (days/yr) ÷ 365 days/year] × [Years per lifetime of exposure (50 yrs) ÷
Lifetime expectancy (78 yrs)]. 9 Cancer risk estimates = Total LADD (Dermal LADD + Inhalation LADD) * Q1*, where Q1* = 9.567×-3 (mg/kg/day)-1.

Permethrin Human Health Risk Assessment DP No. 414137
Page 45 of 129
6.2 Residential Post-Application Exposure and Risk Estimates There is the potential for post-application exposure for individuals exposed as a result of being in an environment that has been previously treated with permethrin. As there is no dermal hazard identified for the non-cancer assessment, there are few adult post-application scenarios where exposure would be anticipated (i.e., no adult dermal or incidental oral exposure assessed). The quantitative non-cancer exposure/risk assessment for residential post-application exposures is based on the following scenarios:
Children 1 to <2 years old incidental oral (hand-to-mouth and object-to-mouth) post-application exposures from contact with treated carpet and hard flooring following indoor crack and crevice, total release fogger, and perimeter/spot/bedbug applications;
Children 1 to <2 years old incidental oral (hand-to-mouth and object-to-mouth) post-application exposures from contact with treated turf following broadcast application (liquid formulation application rates presented are protective of granular formulation application rates);
Children 1 to <2 years old incidental oral (hand-to-mouth) post-application exposures from contact with treated pets;
Children 1 to <2 years old incidental oral (hand-to-mouth and object-to-mouth) post-application exposures from contact with outdoor treated wood (paints);
Children 1 to <2 years old incidental oral (hand-to-mouth and object-to-mouth) post-application exposures from contact with treated/impregnated fabrics (permethrin specific study value used for fraction transferred)
Adult inhalation post-application exposures from activities following outdoor residential misting system and barn misting system usage;
Children 3 to <6 years old inhalation and incidental oral (hand-to-mouth) post-application exposures from activities outdoors following outdoor residential misting system and barn misting system usage;
Adult inhalation post-application exposures from activities following public health use ULV mosquito foggers (aerial and truck-mounted);
Children 1 to <2 years old inhalation and incidental oral (hand-to-mouth) post-application exposures from activities following public health use ULV mosquito foggers (aerial and truck-mounted).
In addition, the quantitative cancer exposure/risk assessment for residential post-application exposures is based on the following scenarios:
Adult dermal and inhalation post-application exposures from activities following: o Indoor animal barn misting system applications (normal infestation and initial
application rates); o Outdoor residential misting system applications; o Outdoor aerosol space sprays; o Public health use ULV mosquito foggers (aerial and truck-mounted);
Adult dermal only post-application exposures from activities following: o Indoor coarse and pin-stream perimeter/spot/bedbug treatments to carpet and hard
surfaces;

Permethrin Human Health Risk Assessment DP No. 414137
Page 46 of 129
o Indoor crack and crevice applications to carpets and hard surfaces; o Fogger applications to carpets and hard surfaces; o Liquid and granular formulation broadcast applications to turf (high contact lawn
activities, mowing,); o Liquid broadcast applications to golf courses; o Broadcast applications to gardens/trees; o Liquid and solid formulation direct applications to dogs and cats (small, medium,
and large); o Clothing/fabric treatments (residential and military battle dress uniforms), and; o Mattress treatments.
Post-application inhalation exposures are not anticipated following surface directed and fogger applications as product labels state a reentry restriction in addition to ventilation of the treated area after use. The lifestages selected for each post-application scenario are based on an analysis provided as an Appendix in the 2012 Residential SOPs13. While not the only lifestage potentially exposed for these post-application scenarios, the lifestage that is included in the quantitative assessment is health protective for the exposures and risk estimates for any other potentially exposed lifestage. Residential exposures are expected to be short-, intermediate-, or long-term in duration. The single dose and repeat dosing permethrin studies show that repeat exposures do not result in lower PODs (i.e., there is no evidence of increasing toxicity with an increased duration of exposure). As such, the residential assessments are conducted as a series of acute exposures, and the same endpoint is used regardless of duration. Therefore, residential handler short-term exposure is considered protective of the longer durations since intermediate- and long-term exposure is expected, but due to the nature of this chemical, only a short-term assessment is needed. Therefore, for purpose of exposure assessments, the residential assessments were conducted for permethrin as a series of acute exposures, and these are protective of scenarios in which exposure occurs for multiple days. A series of assumptions and exposure factors served as the basis for completing the residential post-application risk assessment. A screening-level approach was used for assessment of residential exposures by evaluation of the maximum application rate for all possible residential post-application exposure scenarios of permethrin. The maximum rates for all registered uses of permethrin are summarized in Appendix F. The assumptions, factors, and algorithms used to estimate residential post-application exposures and doses are detailed in the 2012 Residential SOPsError! Bookmark not defined.. In addition to the Residential SOPs, a number of pyrethroid-specific assumptions and inputs were selected for use in the residential post-application scenarios. These inputs are generic to pyrethroids but diverge from those recommended in the Residential SOPs. In conjunction with the pyrethroid-specific inputs, permethrin specific DFR, TTR, pet residue, and impregnated materials data were also used. The assumptions used for the post-application
13 Available: http://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/standard-operating-procedures-residential-pesticide

Permethrin Human Health Risk Assessment DP No. 414137
Page 47 of 129
residential assessment are summarized in Appendix G and in Memo, J. Godshall, 30-JUN-2017; D440978. Combining Exposure and Risk Estimates Residential post-application exposures are anticipated via the dermal, inhalation, and incidental oral routes for permethrin. There is no dermal hazard for permethrin, so a quantitative non-cancer dermal assessment has not been conducted. Since the remaining exposure routes do share common neurologic toxicological effects (decreased motor activity, body tremors, and hypersensitivity to noise) risk estimates have been combined for those routes. The incidental oral scenarios (i.e., hand-to-mouth and object-to-mouth) should be considered inter-related and it is likely that they occur interspersed amongst each other across time. Combining these scenarios would be overly-conservative because of the conservative nature of each individual assessment. The post-application exposure scenarios, hand-to-mouth and inhalation exposures, for children 1 to <2 years old and 3-6 years old were combined for each lifestage. This combination should be considered a protective estimate of children’s exposure. In order to combine these exposure, an ARI was used since the LOCs for children’s hand-to-mouth exposure (300) and inhalation exposure (100) are different. The target ARI is 1; therefore, ARIs of less than 1 are risk estimates of concern. The ARI was calculated as follows. Aggregate Risk Index (ARI) = 1÷ [(Incidental Oral LOC ÷ Incidental Oral MOE) + (Inhalation LOC ÷ Inhalation MOE)] Summary of Residential Post-Application Non-Cancer Exposure and Risk Estimates The majority of screening level residential post-application risks are not of concern and resulted in MOEs greater than their respective LOCs (adult inhalation MOE ≥30; child incidental oral MOE ≥300; and child inhalation MOE ≥100). Children’s inhalation + incidental oral exposure following treatment with the higher “initial treatment” application rate (0.50 oz/1,000ft3/day) for barn misting systems is of concern with an ARI of 0.54 (driven by the inhalation MOE of 54). Normal infestation applications for barn misting systems however are not of concern (0.25 oz/1,000ft3/day). A summary of the residential post-application exposure risk estimates which represent the existing residential uses with the highest application rates or percent ai is provided in Table 6.2.
Table 6.2. Residential Post-Application Non-Cancer Exposure and Risk Estimates for Permethrin.
Lifestage Post-application Exposure Scenario
Application Rate1 Dose
(mg/kg/day)2 MOEs3
Combined Risk Use Site Route of Exposure ARI4
Children 1 to <2
Years Old
Indoor Perimeter/Spot/Bedbug Application (Coarse) - Carpet
Hand-to-Mouth
0.50%
0.0051 8,600
NA
Object-to-Mouth 0.0012 37,000
Indoor Perimeter/Spot/Bedbug Application (Coarse) - Hard Flooring
Hand-to-Mouth 0.0019 23,000 Object-to-Mouth 0.0009 50,000
Indoor Perimeter/Spot/Bedbug (Pin Stream) Application - Carpet
Hand-to-Mouth 0.0029 15,000 Object-to-Mouth 0.0003 150,000
Indoor Perimeter/Spot/Bedbug (Pin Stream) Application - Hard Flooring
Hand-to-Mouth 0.0011 40,000 Object-to-Mouth 0.0002 200,000
Indoor Crack and Crevice - Carpet Hand-to-Mouth 0.00079 56,000
Object-to-Mouth 0.0001 560,000

Permethrin Human Health Risk Assessment DP No. 414137
Page 48 of 129
Table 6.2. Residential Post-Application Non-Cancer Exposure and Risk Estimates for Permethrin.
Lifestage Post-application Exposure Scenario
Application Rate1 Dose
(mg/kg/day)2 MOEs3
Combined Risk Use Site Route of Exposure ARI4
Indoor Crack and Crevice - Hard Flooring
Hand-to-Mouth 0.00029 150,000 Object-to-Mouth 0.0001 750,000
Indoor Fogger - Carpet Hand-to-Mouth
0.58%
0.012 3,600
Object-to-Mouth 0.0016 27,000
Indoor Fogger – Hard Flooring Hand-to-Mouth 0.0046 9,500
Object-to-Mouth 0.0012 36,000
Lawn / Turf
Hand-to-Mouth 0.87 lb ai/A [0.04 lb ai/gal]
Liquid formulations
0.0084 5,300
NA Object-to-Mouth 0.00025 170,000
Soil Ingestion 0.000026 1,700,000
Granule Ingestion 0.65 lb ai/A
[5% permethrin] 0.14 320
Contact with Treated Pets
Hand-to-Mouth (liquid formulations i.e., pour-on, trigger
spray bottle)
3.18 g ai/ small dog5
(0.007 lb ai/animal) 0.027 1,600
NA
3.18 g ai/ small cat5
(0.007 lb ai/animal) 0.052 840
Hand-to-Mouth (solid formulations
i.e., dust)
0.0013 oz ai/ small dog (< 20 lbs)
0.073 600
0.0025 oz ai/ medium dog > 20 lbs
0.062 700
0.0013 oz ai/ small cat5 (all cats assumed < 20 lbs)
0.15 300
Outdoor treated paints Hand-to-Mouth 0.081 mg ai/cm2
(7.21 lb ai/A) 0.11 410
NA Impregnated Fabric – Custom
(Military BDU Study) Hand-to-Mouth
0.125 mg ai/cm2 0.017 2,600
Object-to-Mouth 0.0082 5,400 Adult
Outdoor Residential Misting System Inhalation 0.25 g/ 1,000 ft3 / day
(0.0088 oz ai/ 1,000 ft3 per day)
0.004 5,800 NA
Children 1 to <2 Years Old
0.014 1,500 14.25
Hand-to-Mouth 0.0031 86,000 Adult
Outdoor Aerosol Space Spray Inhalation 0.007 lb ai/A
(0.225% ai/16 oz can)
0.0015 2,400 NA
Children 1 to <2 Years Old
0.0057 630 5.44
Hand-to-Mouth 0.0038 12,000 Adult
Indoor Animal Barn Misting System
Inhalation Initial application 0.50 oz/ 1,000 ft3 / day
0.042 75 NA
Children 3 to <6 Years Old
0.058 54 0.54
Hand-to-Mouth 0.0020 22,000 Adult
Inhalation Normal infestation 0.25 oz/ 1,000 ft3 / day
0.021 150 NA
Children 3 to <6 Years Old
0.029 110 1.09
Hand-to-Mouth 0.0010 43,000 Adult
Public Health Use Truck Mounted ULV Mosquito Fogger
Inhalation
0.007 lb ai/A
N/A (0.00090 mg/m3)
150,000 NA
Children 1 to <2 Years Old
150,000 1,490
Hand-to-Mouth 0.00000067 66,000,000 Adult
Public Health Use Aerial ULV Mosquito Fogger
Inhalation NA (0.0014
mg/m3) 94,000 NA
Children 1 to <2 Years Old
94,000 902
Hand-to-Mouth 0.0000066 6,700,000 1 Based on registered labels presented in Appendix F, Table F.1. Values in red result in risks of concern. 2 Dose (mg/kg/day) algorithms provided in 2012 Residential SOPs (http://www.epa.gov/pesticide-science-and-assessing-pesticide-
risks/standard-operating-procedures-residential-pesticide). 3 MOE = POD (mg/kg/day) ÷ Dose (mg/kg/day). 4 Aggregate Risk Index (ARI) = 1÷ [(Hand-to-Mouth LOC (300) ÷ Hand-to-Mouth MOE) + (Inhalation LOC (100) ÷ Inhalation MOE)], where
applicable. 5 The same application rate was used regardless of animal size (small, medium, or large). The small dog/cat is presented since this size results in the greatest risk potential.

Permethrin Human Health Risk Assessment DP No. 414137
Page 49 of 129
Residential Post-Application Cancer Exposure and Risk Estimate Equations Post-application cancer risk estimates for adults were calculated using a linear low-dose extrapolation approach in which a LADD is first calculated and then compared with a cancer potency factor (Q1*) that has been calculated for permethrin based on dose response data in the appropriate toxicology study (Q1* = 9.567 x 10-3 (mg/kg/day)-1). Some of the inputs for the post-application cancer calculations may be different from the handler cancer calculations and are detailed below. Cancer Specific Residential Post-Application Exposure Data and Assumptions REJV National Survey Data The 2012-2013 REJV survey was used to provide the most recent dataset to determine the typical number of times per year that permethrin broadcast indoor applications are used. The REJV survey is a 12 month long longitudinal survey that examined pesticide use in a residential environment. The data evaluated by HED in this analysis were collected in 2012 to 2013. Deposited Residues & Dermal Dose Estimates To determine the average dermal dose over the course of a year, HED combined the starting permethrin depositions (deposited residues) identified for each scenario in Table 6.2.2 and input a daily dissipation each day until the next application took place. The following assumptions were incorporated into the assessment:
Chemical/pyrethroid specific TTR and DFR dissipation (i.e., 11%) rates were used for relevant outdoor scenarios.
A 8.79% dissipation rate per day was used for pet scenarios as detailed in the non-cancer assumptions above (A. Rivera-Lupianez, 14-DEC-2010, D380194). For most indoor and outdoor uses, a typical re-treatment interval (RTI) of 30 days was assumed to represent one treatment per month. RTIs of 1 and 7 days were also considered; however, the deposited residue estimates were found to be within relatively similar to the 30 day RTI deposited residue estimate. Some RTIs (e.g., outdoor residential misting systems = 3 day RTI) used shorter intervals as directed by representative labels and REJV data was not appropriate for the use scenario.
Currently, HED has no chemical specific indoor dissipation data, therefore, for this draft approach HED is conservatively assuming a 10% dissipation rate per day (this value was determined via assumptions from the RED in addition to a rounded average dissipation rate from DFR/TTR/pet dissipation rates).
A dermal absorption factor of 3.3% was used in the post-application cancer assessment. To represent longer-term exposure to permethrin in indoor environments, the assessment incorporated levels of permethrin in house dust from a study entitled, “Children’s Total Exposure to Persistent Pesticides and Other Persistent Organic Pollutants (CTEPP).”14 This study was performed by the EPA’s office of Research and Development and it was designed to determine what commonly used chemicals are found in home and/or daycare environments. A total of 129
14 Morgan, M.K., Sheldon, L.S., Croghan, C.W., 2004. A Pilot Study of Children’s Total Exposure to Persistent Pesticides and other Persistent Organic Pollutants (CTEPP). EPA/600/R-041/193, vol. I: Final Report. US Environmental Protection Agency, Research Triangle Park, NC.

Permethrin Human Health Risk Assessment DP No. 414137
Page 50 of 129
dust samples were collected in OH and NC homes and 100% of these samples contained some level of permethrin. For this assessment, HED also assumed that an individual could be exposed to permethrin found in house dust (from the CTEPP study) the remaining days of the year after estimated dissipation (10% per day) reduces the initial concentration equal to or below the dust study value of 0.001283 µg/cm2 (samples labeled as, “home children at home” were used). To calculate the average daily permethrin exposure value, the surface residues were averaged over the course of 365 days. As a result of these considerations, the average deposited residue and dermal LADD equation was updated and calculated as follows: Yearly Average deposited residue (mg/kg/day) = (∑ Day-0 deposited residue to Day 365 deposited residue) ÷ 365
when Day X deposited residue = previous days deposited residue × e^ [ -(daily dissipation rate) × number of days since most recent application]
Dermal LADD = Yearly Average dermal dose (mg/kg/day) × [days of post-app exposure (365 days) ÷ days in a year (365)] × [years of exposure (50 years) ÷ average lifespan (78 years)] Days Per Year of Exposure (Inhalation): Some formulations (e.g., automatic misting systems and public health uses) are automatically released following pre-programed settings or conducted by local government for insect control and intended to remain suspended in the air for efficacy purposes. However, as previously detailed in the non-cancer portion of the assessment, residues are expected to settle within 7 minutes of the application and volatilization is not expected to occur. Therefore, the days of post-application exposure are limited to the number of applications made per year (as identified by REJV survey data, the use pattern table, submitted data, or representative labels). Scenarios in which post application inhalation exposure is expected to occur, the number of days exposed is detailed below:
Indoor animal barn misting systems: 3 days or applications (REJV); Outdoor residential misting systems: 25 days or applications (EPA Reg. No. 73748-1); Outdoor aerosol space spray: 3 days or applications (REJV); and For public health uses, 8 days of peak deposition exposure was expected (i.e., 1
application every other week) in summer over the course of 4 months. Days Per Year of Exposure (Dermal):
High contact activities on carpet and hard surfaces following indoor applications (including barns): 365 days per year was assumed when considering an averaged typical dose using REJV survey data.
High contact lawn activities and public health uses: 120 days (4 months of exposure during warm weather/summer).
Mowing Turf: 17 days (assuming that mowing takes place once per week over 120 days during warm weather/summer).

Permethrin Human Health Risk Assessment DP No. 414137
Page 51 of 129
Golfing activities: 52 days (assuming 1 game per week with 52 total exposures over 365 days).
Gardening and fruit and nut tree activities following outdoor applications: 120 days per year (4 months of exposure during warm weather/summer).
Pet Treatments: 180 days per year when considering an averaged typical dose using REJV survey data.
Personal Clothing: 30 days per year when considering an averaged typical dose using REJV survey data.
Military Clothing: 250 days per year (work days in one year). Mattresses: 365 days per year when considering an averaged typical dose using REJV
survey data. For scenarios with multiple sets of applicable average REJV applications the scenario with the greatest number of applications, rounded to the nearest whole number, was used to calculate the deposited residue. For example, 6 total applications per year was used to calculate post-application exposure to pets;
i.e., dust applications to dogs/horses (2.05 applications/year) < direct application to dogs/horses with trigger-spray bottle (6.09 applications/year)
Years Per Lifetime of Exposure: It is assumed that adults would be exposed for 50 years out of a 78-year lifespan. Summary of Residential Post-Application Cancer Exposure and Risk Estimates Indoor Permethrin Exposure: Estimated adult post-application cancer risk estimates range from 3.8×10-8 to 3.1×10-6, with the highest cancer risk estimate resulting from indoor animal barn misting system applications (initial application rate). Outdoor Permethrin Exposure: Estimated adult post-application cancer risk estimates range from 9.1×10-9 to 1.2×10-6, with this highest cancer risk estimate resulting from high contact lawn activities treated with granular formulations. Treated Pet Permethrin Exposure: Estimated adult post-application cancer risk estimates range from 3.3×10-6 to 4.0×10-5 with the highest cancer risk estimate resulting from contact with small cats treated with liquid formulations. Treated Fabric/Clothing Permethrin Exposure: Estimated adult post-application cancer risk estimates range from 6.7×10-6 to 4.9×10-7 with the highest cancer risk estimate resulting from contact with treated mattresses. Adult residential post-application dermal cancer risk estimates are presented in Table 6.2.2 below.

Permethrin Human Health Risk Assessment DP No. 414137
Page 52 of 129
Table 6.2.2. Residential Post-Application Cancer Exposure and Risk Estimates for Permethrin.
Adult Post-Application Exposure Scenario Route of Exposure
Application Rate
Days of Exposure per Year
Typical Deposited Residue /
Exposure1,2
(ug/cm2)
Absorbed Daily Dose mg/kg/day
LADD3
(mg/kg/day) Cancer Risk
Estimate4
Indoors
Indoor Animal Barn Misting Systems
Normal Infestation Dermal
0.25% 120 0.10 3.3E-05 2.10E-05
1.26E-06 Inhalation 3 2.61 mg/m3 0.042 1.10E-04
Initial Application Dermal
0.50% 120 0.12 1.7E-04 1.05E-04
3.13E-06 Inhalation 3 5.22 mg/m3 0.021 2.20E-04
Perimeter/Spot/Bedbug Treatment (course)
Carpet – high contact activities Dermal 0.50%
365
0.32 1.4E-04 9.13E-05 8.74E-07
Hard Surface – high contact activities
5.3E-05 3.43E-05 3.28E-07
Perimeter/Spot/Bedbug Treatment (Pin Stream)
Carpet – high contact activities Dermal 0.50% 0.14
4.0E-05 2.58E-05 2.46E-07 Hard Surface – high contact
activities 1.5E-05 9.66E-06 9.24E-08
Crack and Crevice Carpet – high contact activities
Dermal 0.50% 0.04 1.7E-05 1.06E-05 1.02E-07
Hard Surface – high contact activities
6.2E-06 3.99E-06 3.81E-08
Fogger Carpet – high contact activities
Dermal 0.50% 0.28 1.3E-04 8.19E-05 7.83E-07
Hard Surface – high contact activities
4.8E-05 3.07E-05 2.94E-07
Outdoors
Outdoor residential misting system Dermal
0.25 g ai/1000 ft3/day
120 6.8E-07 lb
ai/ft2 3.7E-04 7.85E-05 1.01E-06
Inhalation 25 1.53
mg/m3 6.2E-04 2.71E-05
Outdoor aerosol space spray Dermal
0.225% ai/16 oz can
120 3.0E-7 1.12E-07 2.35E-08 3.76E-07
Inhalation 2 0.12
mg/day 0.0015 5.31E-06
Public health use – Truck Mounted ULV Fogger Dermal
0.007 lbs ai/A 120 1.00E-06 1.12E-07 2.4E-08
6.04E-09 Inhalation 8
29.18 mg/day
4.32E-05 6.1E-07
Public health use – Aerial ULV Mosquito Fogger Dermal
0.007 lbs ai/A 120 1.3E-04 9.50E-06 2.0E-06
2.14E-08 Inhalation 8
0.0014 mg/m3
1.68E-05 2.36E-07
High Contact Lawn Activities - Liquid
Dermal
0.87 lbs ai/A 120
0.0048
5.4E-04 1.13E-04 1.08E-06 High Contact Lawn Activities - Granular 0.65 lbs ai/A 120 6.0E-04 1.25E-04 1.20E-06
Mowing Turf – Liquid 0.87 lbs ai/A 17 1.1E-05 3.27E-07 3.13E-09 Mowing Turf – Granular 0.65 lbs ai/A 17 1.1E-05 3.26E-07 3.12E-09
Golfing (Liquid Only) 0.79 lbs ai/A 52 0.0044 3.8E-05 3.50E-06 3.35E-08
Gardening Activities (Esfenvalorate DFR data) 0.0036 lbs
ai/gal 120 0.045 3.5E-04 7.26E-05 6.95E-07
Fruit and Nut Trees (Permethrin Peach DFR data) 0.2 lbs ai/gal 120 0.074 5.2E-05 1.10E-05 1.05E-07 Pets
Dog (liquids)
small
Dermal
0.007 lbs ai/animal (3175 mg ai/animal)
180
0.0040 mg/cm2 6.64E-03
0.00210 2.01E-05
medium 0.0017 mg/cm2
2.85E-03 0.00090 8.61E-06
large 0.0011 mg/cm2
1.81E-03 0.00057 5.48E-06
Cats (liquids) small
180
0.0080 mg/cm2
1.33E-02 0.00420 4.02E-05
medium 0.0048 mg/cm2
7.97E-03 0.00252 2.41E-05

Permethrin Human Health Risk Assessment DP No. 414137
Page 53 of 129
Table 6.2.2. Residential Post-Application Cancer Exposure and Risk Estimates for Permethrin.
Adult Post-Application Exposure Scenario Route of Exposure
Application Rate
Days of Exposure per Year
Typical Deposited Residue /
Exposure1,2
(ug/cm2)
Absorbed Daily Dose mg/kg/day
LADD3
(mg/kg/day) Cancer Risk
Estimate4
large 0.0030 mg/cm2
4.98E-03 0.00157 1.51E-05
Dog (solids)
small (< 20 lbs rate) 35.4 mg ai/animal
180
0.000045 mg/cm2
1.99E-03 0.00063 6.02E-06
medium (> 20 lbs rate) 70.85 mg ai/animal
0.000038 mg/cm2
1.71E-03 0.00054 5.16E-06
large (> 20 lbs rate) 0.000024 mg/cm2
1.09E-03 0.00034 3.28E-06
Cats (solids)
small (< 20 lbs rate)
35.4 mg ai/animal
180
0.00009 mg/cm2
3.98E-03 0.00126 1.20E-05
medium (< 20 lbs rate) 0.000054 mg/cm2
2.39E-03 0.00076 7.23E-06
large (< 20 lbs rate) 0.000034 mg/cm2
1.49E-03 0.00047 4.52E-06
Fabric/Clothing
Clothing Military Battle Dress Uniform
Dermal 0.125
mg ai/cm2
250 0.0047 mg/cm2 1.1E-04 4.75E-05 4.54E-07
Clothing Jacket/pants/shirt 30 0.012
mg/cm2 1.3E-04 7.04E-06 6.74E-08
Bedding/Mattresses 15 µg/cm2 365 1.74 8.02E-05 5.13E-05 4.91E-07 1 REJV program search criteria and calculations are detailed in J. Godshall, 30-JUN-2017; D440978, Appendix D, Table D.1. 2 Example of calculated yearly average residue calculations are detailed in J. Godshall, 30-JUN-2017; D440978, Appendix D, Table D.2 3 LADD (mg/kg/day) = Average (dermal or inhalation) dose (mg/kg/day) × [days of post-app exposure (days) ÷ 365 days/year] × [Years per
lifetime of exposure (50 yrs) ÷ Lifetime expectancy (78 yrs)]. 3 Cancer risk estimates = Total LADD × Q1
*, where Q1* = 9.567 x 10-3 (mg/kg/day)-1.
6.3 Residential Risk Estimates for Use in Aggregate Assessment Non-Cancer Aggregate Assessment: Table 6.4 reflects the residential risk estimates that are recommended for use in the aggregate assessment for permethrin. The exposure scenario for the higher “initial application” rate from barn misting systems results in risks of concern for children 3 to <6 years old (however the lower “normal infestation” rate was considered as there were no risks of concern); therefore, this scenario is not recommended for inclusion in the aggregate assessment. The following residential risk estimates are recommended for the aggregate assessment of permethrin:
The recommended residential exposure for use in the adult aggregate assessment is from the post-application exposure following indoor barn misting system applications.
The recommended residential exposure for use in the children 3 to <6 years old aggregate assessment is for inhalation and hand-to-mouth exposures from post-application exposure following indoor barn misting system applications at the normal infestation rate.
The recommended residential exposure for use in the children 1 to <2 years old aggregate assessment is exposure following incidental oral hand to mouth exposure to small cats previously treated with solid/dust formulations.

Permethrin Human Health Risk Assessment DP No. 414137
Page 54 of 129
Table 6.3. Recommendations for the Residential Exposures for the Permethrin Aggregate Assessment.
Lifestage Exposure Scenario Dose (mg/kg/day)1 MOE2
Inhalation Oral Total Inhalation Oral Total
Adult
Post-application inhalation exposure
following indoor barn misting system initial
application
0.04177 N/A 0.04177 75 N/A 75
Children 1 to <2
years old
Post-application incidental oral hand
to mouth exposure to small cats
N/A 0.1457 0.1457 N/A 300 300
ARI3
Children 3 to <6
years old
Post-application inhalation exposure
following indoor barn misting system
normal infestation application rate
0.02885 0.0010149 0.02986 110 43,000 1.2
1 Dose = the highest dose for each applicable lifestage of all residential scenarios assessed. Total = dermal + inhalation + incidental oral (where applicable).
2 MOE = the MOEs associated with the highest residential doses. Total = 1 ÷ (1/Dermal MOE) + (1/Inhalation MOE) + (1/Incidental Oral MOE), where applicable. Inhalation LOC = 30.
3 ARI = 1 ÷ [(Inhalation LOC 100/Inhalation MOE) + (Incidental oral LOC 300/Incidental Oral MOE)].
Cancer Aggregate Assessment The following reflects the residential risk estimate that is recommended for use in the adult cancer aggregate assessment for permethrin.
The greatest residential cancer risk estimate reflects post-application dermal exposure from contact with small cats treated with liquid formulations of permethrin which results in a LADD of 0.0042 mg/kg/day and a cancer risk estimate of 4.0×10-5. See Table 6.2.2 for additional information.
7.0 Aggregate Exposure/Risk Characterization In accordance with the FQPA, HED must consider and aggregate (add) pesticide exposures and risks from three major sources: food, drinking water, and residential exposures. In an aggregate assessment, exposures from relevant sources are added together and compared to quantitative estimates of hazard (e.g., a NOAEL or PAD), or the risks themselves can be aggregated. When aggregating exposures and risks from various sources, HED considers both the route and duration of exposure. A chronic aggregate assessment was not conducted since single dose and repeat dosing permethrin studies show that repeat exposures do not result in lower PODs (i.e. there is no evidence of increasing toxicity with an increased duration of exposure). Therefore, only acute and short-term aggregate risk assessments need to be conducted for permethrin, and these are protective of scenarios in which exposure occurs for multiple days. A cancer aggregate assessment was not conducted at this time. 7.1 Acute Aggregate Risk The acute aggregate risk assessment combines exposures to permethrin in food and drinking water only. The acute dietary exposure and risk estimates do not exceed HED’s level of concern

Permethrin Human Health Risk Assessment DP No. 414137
Page 55 of 129
(less than 100% of the aPAD) at the 99.9th exposure percentile for the general U.S. population (2.6% of the aPAD) and all population subgroups. The most highly exposed population subgroup is children 3-5 years old at 20% of the aPAD (see Section 5.4.6). 7.2 Short-Term Aggregate Risk Short-term aggregate risk assessments are necessary for both adults and children since there is the potential for both short-term handler exposure and short-term post-application exposure from the residential uses of permethrin. For the short-term aggregate risk assessment, potential residential post-application exposures (Table 6.4) were combined with average food and drinking water exposures (Table 5.4.6). For adults, post-application exposure following indoor barn misting system applications resulted in the highest short-term exposures; therefore, this exposure estimate was aggregated with food and drinking water exposures. Children 3 to <6 years old had the highest post-application residential exposure estimates due to inhalation and hand-to-mouth exposures following indoor barn misting system applications. Post-application exposures to this use pattern were assessed for initial application (0.50 oz/1,000 ft3/day) and normal infestation (0.25 oz/1,000 ft3/day). The initial application assessment indicated that there are risks of concern for children 3 to <6 years old. The normal infestation application did not result in risks of concern for children 3 to <6 years old and was identified as the post-application scenario with the highest exposure estimate that did not result in risks of concern for all child lifestages. Therefore, an aggregate (food, drinking water, and residential) exposure assessment was conducted for this exposure scenario using the normal infestation application rate. Children 1 to 2 years old had the highest average (chronic) dietary (food and drinking water) exposure estimate; therefore, an aggregate assessment was also conducted for children 1 to <2 years old because there are residential exposure scenarios specific to this lifestage. The highest residential exposure for children 1 to <2 years old is from post-application incidental oral hand-to-mouth exposure from small cats treated with solid formulations; an aggregate (food, drinking water, and residential) exposure assessment was conducted for this exposure scenario. An ARI approach was used for the short-term aggregate assessments since the incidental oral and inhalation endpoints have different LOCs. ARIs that are ≥1 are not of concern. The aggregate assessment for children 1 to <2 years old was conducted using the ARI approach for consistency purposes, even though only average (chronic) dietary (food and drinking water) and oral post-application exposures are anticipated. The short-term aggregate (food, water, residential exposures) assessment for adults resulted in an ARI of 2.5. The short-term aggregate (food, drinking water, and residential exposure) ARIs for children 1 to <2 years old and 3 to <6 years old were 1.0 and 1.1, respectively. Since the ARIs are ≥1, there are no aggregate risks of concern, excluding the initial application assessment for barn missing systems that indicated that there are risks of concern for children 3 to <6 years old.

Permethrin Human Health Risk Assessment DP No. 414137
Page 56 of 129
Table 7.2. Short-Term Aggregate Risk Calculations for Permethrin.1
Population NOAEL2 Dietary Exposure2 Oral Residential Exposure3
Inhalation Residential Exposure4
Aggregate ARI (food, water, and
residential)5,6 Mg/kg/day MOE ARI Mg/kg/day MOE ARI Mg/kg/day MOE ARI
Adults 44 0.000133 330000 3300 -- -- -- 0.04177 75 2.5 2.5 Children 1 to <2 years old
44 0.000214 205000 690 0.1457 302 1.0 -- -- -- 1.0
Children 3 to <6 years old
44 0.000154 285000 950 0.0010149 43300 140 0.02885 108 1.1 1.1
1 Risk estimates equal to or greater than 1 are not of concern. (ARI = Aggregate Risk Index). 2 MOE dietary = [(short- term oral BMDL1SD (44 mg/kg/day))/(chronic dietary exposure)]. ARI dietary = [(MOE dietary)/(MOE target=300 for <6 years old and 100 for ≥6 years old)]. See Table 5.4.6 for dietary exposure estimates. 3 MOE oral = [(short-term oral BMDL1SD (44 mg/kg/day)/(hand-to-mouth residential exposure)]. ARI oral = [(MOE oral)/(MOE target=300 for <6 years old and 100 for ≥6 years old)]. See Table 6.4. 4 MOE inhalation = [(short-term inhalation HED (3.122 mg/kg/day handlers and indoor post-application))/(high end inhalation residential exposure)]. ARI inhalation = [(MOE inhalation)/(MOE target=30 for ≥6 years old and 100 for <6 years old)]. 5 ARI Aggregate = 1/[(1/ARI dietary) + (1/ARI oral) + (1/ARI dermal) + (1/ARI inhalation)]. 6 No effects were observed in the dermal toxicity study and there was low dermal absorption based on dermal penetration studies; therefore, dermal assessments were not conducted. 7.3 Cancer Aggregate Risk Aggregate (food, drinking water, and residential exposure) cancer assessments are conducted for adult lifestages only. A cancer aggregate assessment was not conducted at this time. Refer to Section 5.4.6 for the highest cancer dietary exposure estimate for adults and to Tables 6.1.2. and 6.2.2 for the residential handler and residential post-application cancer exposure estimates for adults.
8.0 Non-Occupational Bystander Post-Application Inhalation Exposure and Risk Estimates Volatilization of pesticides may be a source of post-application inhalation exposure to individuals nearby pesticide applications. The Agency sought expert advice and input on issues related to volatilization of pesticides from its Federal Insecticide, Fungicide, and Rodenticide Act Scientific Advisory Panel (SAP) in December 2009, and received the SAP’s final report on March 2, 2010 (http://www.regulations.gov/#!documentDetail;D=EPA-HQ-OPP-2009-0687-0037). The Agency has evaluated the SAP report and has developed a Volatilization Screening Tool and a subsequent Volatilization Screening Analysis (http://www.regulations.gov/#!docketDetail;D=EPA-HQ-OPP-2014-0219). During Registration Review, the Agency will utilize this analysis to determine if data (i.e., flux studies, route-specific inhalation toxicological studies) or further analysis is required for permethrin. The Agency has developed a preliminary bystander volatilization inhalation exposure assessment for permethrin utilizing the currently available inhalation toxicity and air monitoring data. Permethrin was detected in multiple ambient air studies. Reported detections include:

Permethrin Human Health Risk Assessment DP No. 414137
Page 57 of 129
Report of Ambient Air Monitoring for Pesticides in Lompoc, California Report for the Application (Butte County) and Ambient (Monterey County) Air
Monitoring of Permethrin Pesticide Air Monitoring in Parlier, CA Air Monitoring Network Results for 2011: Volume 1
Application site air monitoring (i.e., also known as field volatility) refers to the collection of air samples around the edges of a treated field during and after a pesticide application. Samples are generally collected for short intervals (e.g., <8 hours), for at least the first day or two after application with subsequent samples increasing in duration. In this type of study, it is typically known when an application occurred, the equipment used for the application, and the application rate. Application site monitoring data represents an exposure to vapors at or near the field edge resulting from an application. Ambient air monitoring typically is focused on characterizing the airborne pesticide levels within a localized airshed or community structure of some definition (e.g., city, township, or municipality). This type of monitoring effort also can be focused on capturing chronic background levels or other temporal characteristics of interest such as focusing on seasonal pesticide use patterns. Typically, samples are generally taken for 24 consecutive hours and collected at the same site over an extended period of time (e.g., several weeks or months). In contrast to application site air monitoring, information on the precise timing and location of pesticide applications are rarely collected in ambient air monitoring studies. However, this does not mean that an application did not occur near an ambient sampler during the monitoring period. The permethrin bystander volatilization inhalation exposure assessment compares the maximum 24-hour air concentration detected in each of the monitoring studies to the HEC for residential bystanders (32.991 mg/m3). This comparison was done to represent a potential resident who lives next to a treated field and may be exposed to the peak concentration of permethrin volatilizing off the field over a 24-hour period. In addition, the arithmetic mean permethrin air concentration from each study was compared to the HEC for residential bystanders. This comparison was done to represent a potential seasonal exposure. The toxicological profile of pyrethroids characterizes pyrethroids, including permethrin, as being rapid in onset and associated with acute, peak exposures. The single dose and repeat dosing studies show that repeat exposures do not result in lower PODs (i.e. there is no evidence of increasing toxicity with an increased duration of exposure). As such, the assessments are conducted as a series of acute exposures, and the same endpoint is used regardless of duration. Typically, maximum concentrations are compared to acute PODs, but in this case the acute and short-term PODs are the same, therefore the short-term POD was used for both. For the purposes of the post-application bystander inhalation quantitative assessment, only acute 24-hour post application ambient air concentrations were incorporated into Table 8.1 below, which provides permethrin volatilization MOE calculations for each site. None of the air concentrations results in risks of concern.

Permethrin Human Health Risk Assessment DP No. 414137
Page 58 of 129
Table 8.1: Residential Bystander Preliminary Volatilization MOE Analysis of Permethrin.
Study Year of Study
Level of Detection (ng/m3)
Level of Quantification
(ng/m3)
Duration of
samples
Maximum Air Concentration
(ng/m3)a
Acute MOEsb (LOC = 30)
Ambient Air Data (CDPR and CARB)
Lompoc, CA 2003 1.4 7.2 24-hour trace (4.3) 7,700,000
(CDPR) Monterey, CA
1998 0.10 0.33 24-hour trace (0.215) 153,400,000
(CDPR and CARB) Parlier, CA
2009 N/A 46.3 24-hour trace (26.8) 1,200,000
(CDPR AMN) Salinas
2015
7.2 23.1
24-hour
not detected (3.6)
9,200,000
(CDPR AMN) Shafter
7.2 23.1 not detected
(3.6) 9,200,000
(CDPR AMN) Ripon
7.2 23.1 trace (15.2) 2,200,000
(CDPR AMN) Salinas
2014
7.2 23.1
24-hour
not detected (3.6)
9,200,000
(CDPR AMN) Shafter
7.2 23.1 trace (15.2) 2,200,000
(CDPR AMN) Ripon
7.2 23.1 trace (15.2) 2,200,000
(CDPR AMN) Salinas
2013
7.2 23.1
24-hour
not detected (3.6)
9,200,000
(CDPR AMN) Shafter
7.2 23.1 not detected
(3.6) 9,200,000
(CDPR AMN) Ripon
7.2 23.1 trace (15.2) 2,200,000
(CDPR AMN) Salinas
2012
7.2 23.1
24-hour
not detected (3.6)
9,200,000
(CDPR AMN) Shafter
7.2 23.1 not detected
(3.6) 9,200,000
(CDPR AMN) Ripon
7.2 23.1 not detected
(3.6) 9,200,000
(CDPR AMN) Salinas
2011
7.2 23.1
24-hour
not detected (3.6)
9,200,000
(CDPR AMN) Shafter
7.2 23.1 trace (7.9) 4,200,000
(CDPR AMN) Ripon
7.2 23.1 trace (7.9) 4,200,000
Application Study Data Butte, CA (CDPR)
1998 0.10 0.33 5-hour 0.57 57,900,000
a. All non-detects and trace concentrations reported as identified in the individual study reports. For non-detects, assumed 1/2 Limit of Detection (LOD). For trace concentrations, assumed concentration halfway between LOD and Limit of Quantitation of (LOQ) unless otherwise indicated by the study.
b. Acute MOE = Residential Bystander HEC (32,991,000,000 ng/m3) / Study maximum air concentration (ng/m3). LOC = 30
Some of the limitations and considerations that have been identified that should be considered in the interpretation of these results include:
Most of the data utilized in this preliminary assessment are 24-hour air samples. When these data are used, an assumption is made that an individual is exposed to the same air concentration for 24-hours every day. However, this is not always the case as real world time-activity data indicate that many parts of the population move from site to site on a daily basis (e.g., go to work and back).

Permethrin Human Health Risk Assessment DP No. 414137
Page 59 of 129
This assessment is only representative of outdoor concentrations (i.e., the exposure and risk estimates assume an individual is outdoors all the time). It does not take into account potential effects of air conditioning systems and similar air filtration systems which could potentially reduce air concentrations of permethrin indoors. The Agency believes that indoor concentrations will be at worst equivalent to outdoor concentrations and may potentially be lower.
All of the data used for this analysis have been generated in California; however,
permethrin is used in many regions of the country. Therefore, the results based on the limited available air monitoring data were used to represent the rest of the country due to a lack of adequate information for any other region. It is unclear what potential impacts this extrapolation might have on the risk assessment. Factors such as meteorology and cultural practices may impact the overall amounts of permethrin that volatilize from a treated field as well as the rate at which it volatilizes.
The residential bystander estimated exposure should not be included in the human health
risk assessment aggregate due to the fact that this is only a preliminary assessment and is not considered a refined assessment for the reasons noted above. There are limitations associated with the air monitoring data that are available, such as the fact that most are 24-hour air samples and that the measurement techniques do not distinguish between aerosols and vapors. In addition, as noted in the above bullet, this assessment assumes residents are outdoors during the entire exposure duration.
9.0 Non-Occupational Spray Drift Exposure and Risk Estimates A quantitative spray drift assessment for permethrin is not required because the maximum application rate to a crop/target site (1.6 lbs ai/A for forestry applications) multiplied by the adjustment factor for drift of 0.26 is less than the maximum direct spray residential turf application rate (0.87 lb ai/A) for any permethrin products (1.6 lbs ai/A * 0.26 = 0.416 lbs ai/A < 0.87 lbs ai/A). There were no risks of concern for the residential turf assessment; therefore, the assessment for exposure to residues on turf is protective of exposure to the residue from spray drift. 10.0 Cumulative Exposure/Risk Characterization The Agency is required to consider the cumulative risks of chemicals sharing a common mechanism of toxicity. The Agency has determined that the pyrethroids and pyrethrins share a common mechanism of toxicity (http://www.regulations.gov; EPA-HQ-OPP-2008-0489-0006). The members of this group share the ability to interact with voltage-gated sodium channels ultimately leading to neurotoxicity. The cumulative risk assessment for the pyrethroids/ pyrethrins was published on 11/09/2011 and is available at http://www.regulations.gov; EPA-HQ-OPP-2011-0746. No cumulative risks of concern were identified, allowing the Agency to consider new uses for pyrethroids. For information regarding EPA’s efforts to evaluate the risk

Permethrin Human Health Risk Assessment DP No. 414137
Page 60 of 129
of exposure to this class of chemicals, refer to http://www.epa.gov/oppsrrd1/reevaluation/pyrethroids-pyrethrins.html Since the 2011 CRA, the Agency has added multiple new pyrethroid/pyrethrin uses. In each of these instances, a qualitative screen has been conducted to evaluate any potential impacts on the CRA prior to those uses being granted. Prior to a final registration review decision for permethrin, the Agency will determine if a revisit of the 2011 CRA is warranted based on the availability of any new hazard, use, or exposure information that could potentially change the results of or otherwise impact the 2011 CRA. 11.0 Occupational Exposure/Risk Characterization 11.1 Occupational Handler Exposure/Risk Estimates Based on the anticipated use patterns and current labeling, types of equipment and techniques that can potentially be used, occupational handler exposure is expected from the registered uses. For impregnated materials treated with non-biocide pesticides (e.g., insecticides and repellents), exposure during the manufacturing process is not assessed by EPA. The quantitative exposure/risk assessment developed for occupational handlers is based on the following representative scenarios further detailed in Appendix F (Tables F.1 and F.2). Agricultural Uses
Mixing/loading: o Water Dispersible Granules/Dry Flowables for:
Aerial applications for orchard/vineyards and typical/high acreage crops, Airblast applications to orchard/vineyards, Chemigation applications to orchard/vineyards and typical/high acreage
crops, Groundboom applications to orchard/vineyards and typical/high acreage
crops, o Granules for:
Aerial applications to orchard/vineyards, o Liquid/Emulsifiable Concentrates and Wettable Powders for:
Aerial applications for orchard/vineyards and typical/high acreage crops, Impregnation/coating of dry bulk fertilizer (commercial and on-farm) Airblast applications to orchard/vineyards, Chemigation applications to orchard/vineyards and typical/high acreage
crops, Groundboom applications to orchard/vineyards and typical/high acreage
crops, Stationary fogger applications to mushroom houses
Applying: o Spray (all formulations):
Via aerial equipment orchard/vineyards and typical/high acreage crops, Via airblast equipment to orchard/vineyards, Via groundboom equipment orchard/vineyards and typical/high acreage

Permethrin Human Health Risk Assessment DP No. 414137
Page 61 of 129
crops, o Dry Bulk Fertilizer:
Via commercial treatment for typical/high acreage crops, Via on-farm treatment for typical/high acreage crops,
o Granules for: Aerial applications to orchard/vineyards,
Flagging: o All spray formulations for aerial applications to orchard/vineyards, and
typical/high acreage crops, o Granular applications for aerial applications to orchard/vineyards
Mixing/loading/applying: o Water Dispersible Granules/Dry Flowables for:
Backpack applications to orchard/vineyards Mechanically-pressurized handgun applications to orchard/vineyards and
typical field crops, o Liquid/ Emulsifiable Concentrates and Wettable Powder for:
Backpack applications to orchard/vineyards, Stationary fogger applications to mushroom houses, Manually-pressurized handgun applications to mushroom houses, Mechanically pressurized handgun to turf, orchard/vineyards, typical
acreage crops, Loading/Applying
o Granules for Backpack, belly grinder, and rotary spreader applications to
orchard/vineyards. Non-Agricultural Uses
Mixing/Loading o Water Dispersible Granules/Dry Flowables for:
Dip applications for livestock, Aerial applications for forestry, Chemigation and groundboom applications for greenhouse ornamentals,
o Liquid/ Emulsifiable concentrates for: Dip applications for livestock, Aerial applications for aquatic and terrestrial vector control (mosquito
adulticide public health uses) and forestry applications, Truck mounted fogger applications for aquatic and terrestrial vector
control, Groundboom applications for golf courses, greenhouses, and field-grown
ornamentals, Boom sprayer applications for aquatic vector control, Automatic misting systems for barns and outdoor residential areas, Stationary foggers for warehouses and indoor barns,
o Wettable Powders for: Aerial applications for forestry, Chemigation and groundboom applications for greenhouse ornamentals,

Permethrin Human Health Risk Assessment DP No. 414137
Page 62 of 129
Applying o RTU Dusts for:
Dust bag applications for livestock, Shaker can applications for landscaping, livestock, and domestic animals,
o Spray (all formulations) for: Aerial applications for aquatic and terrestrial vector control (mosquito
adulticide public health uses) and forestry applications, Truck mounted fogger applications for terrestrial vector control, Groundboom applications for golf courses and greenhouse ornamentals, Boom sprayer applications for aquatic vector control,
o RTU Liquids for: Dip applications to domestic animals, Pour-in/on applications to livestock/domestic animals, Shampoo applications to domestic animals, Sponge applications to horses and domestic animals, Spot-on applications to domestic animals, Trigger spray bottle applications to horses, domestic animals, indoor
surfaces (crack and crevice), and landscaping, Wipe/towelette applications to domestic animals and horses,
o RTU Granular: Shaker can applications to insect nests/mounds,
o RTU Pressurized Liquid: Aerosol can applications to military aircraft (cabin, crew, and cargo areas),
domestic animals, foundations/perimeters, indoor living spaces (crack and crevice), outdoor residential spaces, and landscaping areas,
Total release fogger applications to warehouses, o RTU Solid:
Ear-tag applications to livestock, Mixing/Loading/Applying
o Water Dispersible Granules/Dry Flowables for: Backpack applications to Christmas tree farms, conifer orchards, Manually pressurized handwand applications to Christmas tree farms, Mechanically pressurized handgun applications to Christmas tree farms
and greenhouse ornamentals, o Liquid/Emulsifiable concentrates for:
Backpack applications to greenhouse ornamentals, wildlife management areas, Christmas tree farms, forestry areas, landscaping areas (trees, shrubs, lawns, turf), termiticide structural uses, industrial areas, barns/feedlots, livestock, foundations/perimeters, and aquatic vector control,
Injector applications to structures for termiticide uses, Manually pressurized handwand applications to greenhouse ornamentals,
wildlife management areas, Christmas tree farms, landscaping areas (interior/exterior, trees, shrubs, lawns, turf), food handling establishments, industrial areas, warehouses, barns/feedlots, livestock, foundations/perimeters, and insect mounds/nests,

Permethrin Human Health Risk Assessment DP No. 414137
Page 63 of 129
Mechanically pressurized handgun applications to golf courses, Christmas tree farms, landscaping lawns/turf, livestock, and aquatic vector control,
o Water Soluble Packets for: Backpack, manually pressurized handwand, and mechanically pressurized
handwand applications to Christmas tree farms, conifer orchards, and greenhouse ornamentals,
Loading/applying o Granules for:
Belly grinder applications to turf, Cup applications to insect mounds/nests,
o Paint/stain for: Airless sprayer and brush/roller applications to residential and commercial
structures. Additionally, there are seed treatment uses with a quantitative exposure and risk assessment for occupational handlers based on the following scenarios:
On-Farm Seed Treatment: Permethrin on-farm seed treatment utilizes planter and hopper box seed treatments only and are physically mixed by a worker with a stick or paddle.
Planting Treated Seed (Planters): Potential occupational exposure scenarios from the use of permethrin as a seed treatment include planting treated seed (secondary handler). Planting treated seed consists of the farmer placing the seed in the hopper and applying seed to fields and is considered a secondary handler exposure scenario.
Occupational Handler Non-Cancer Exposure Data and Assumptions A series of assumptions and exposure factors served as the basis for completing the occupational handler risk assessments. Each assumption and factor is detailed below on an individual basis. A screening-level approach was used for this assessment of occupational exposures by evaluation of the maximum application rate for all possible occupational handler exposure scenarios of permethrin. Unit Exposures: It is the policy of HED to use the best available data to assess handler exposure. Sources of generic handler data, used as surrogate data in the absence of chemical-specific data, include PHED 1.1, AHETF database, the ORETF database, or other registrant-submitted occupational exposure studies. Some of these data are proprietary (e.g., AHETF data), and subject to the data protection provisions of FIFRA. The standard values recommended for use in predicting handler exposure that are used in this assessment, known as “unit exposures”, are outlined in the “Occupational Pesticide Handler Unit Exposure Surrogate Reference Table15”, which, along with additional information on HED policy on use of surrogate data, including descriptions of the various sources, can be found at the Agency website16. Seed treatment unit exposures were based on ExpoSAC Policy 14.
15 Available: http://www.epa.gov/opp00001/science/handler-exposure-table.pdf 16 Available: http://www.epa.gov/pesticides/science/handler-exposure-data.html

Permethrin Human Health Risk Assessment DP No. 414137
Page 64 of 129
For the dry bulk fertilizer scenarios, HED assumes a closed mixing/loading scenario for commercial impregnation of dry bulk fertilizer, and an open mixing/loading scenario for grower-owned (i.e., on-farm) equipment impregnation of dry bulk fertilizer. For all applications of dry bulk fertilizer, HED assumes the use of an open-cab tractor spreader. As HED does not have aircraft-specific exposure data, the Pesticide Handlers Exposure Database Version 1.1 (PHED 1.1) indoor exposure data has been used to assess applications to military aircraft cabin, crew, and cargo areas for the purposes of this assessment. Area Treated or Amount Handled: The daily areas treated or amounts handled were defined for each handler scenario (in appropriate units) by determining the amount that can be reasonably treated by an individual in a single day. When possible, the assumptions for daily areas treated or amounts handled are taken from the HED’s ExpoSAC Policy 9.1: “Standard Values for Daily Acres Treated in Agriculture.” The amounts handled/treated for seed treatment were based on ExpoSAC Policy 15.1 and the BEAD memo: “Acres Planted per Day and Seeding Rates of Crops Grown in the United States” (J. Becker, et al.; March 2011). A literature article titled, “Demographic of United States Equine Population” (http://www.humanesociety.org/assets/pdfs/hsp/soaiv_07_ch10.pdf) indicates that the average number of horses boarded in a stable range from six to nineteen. HED assumed that a maximum of 25 horses would be treated per day. This is considered a conservative estimate to be protective of registered scenarios. HED does not have data regarding the mixing/loading or the application of permethrin-impregnated dry bulk fertilizer. The mixing/loading processing rate for commercial impregnation of dry bulk fertilizer has been estimated to be 960 tons of fertilizer processed per 8-hour day based on information supplied by a registrant concerning the chemical alachlor.17 Commercial/contract application of impregnated fertilizer is assessed assuming 320 acres/day (as determined by PHED Scenario 15/16). On-farm mixing/loading for, and application of, impregnation of dry bulk fertilizer is then assessed using an estimate of 160 acres/day. Agricultural crop inputs for area treated were based on information in ExpoSAC Policy 9.1 and include:
1200 acres for aerial applications on high acreage field crops; 960 tons for commercial impregnation/coating of dry bulk fertilizer; 350 acres for aerial application on typical acreage field crops and orchards/vineyards; 350 acres for chemigation on high and typical acreage field crops and orchards/vineyards; 320 acres for commercial impregnation/coating of dry bulk fertilizer; 200 acres for groundboom applications on high acreage field crops; 160 acres for on-farm impregnation/coating of dry bulk fertilizer; 80 acres for groundboom applications on typical acreage field crops; 40 acres for groundboom applications on orchards/vineyards; 40 acres for airblast applications on orchards/vineyards;
17 http://archive.epa.gov/pesticides/reregistration/web/pdf/0063fact.pdf

Permethrin Human Health Risk Assessment DP No. 414137
Page 65 of 129
1000 gallons sprayed via mechanically-pressurized handgun on typical acreage field crops; and
40 gallons sprayed via backpack sprayer on orchards/vineyards. Mushroom houses:
o Backpack/ manually pressurized handwands: 40 gallons/day o Foggers: 1,000,000 ft3
The following inputs are based on either the most recently conducted permethrin and TCVP occupational and residential exposure and risk assessment for similar use patterns18 or best professional judgment of product usage:
All livestock applications: 400 animals treated daily; Poultry livestock shaker can/dust bag: 1,000 birds or 1,000 square feet; Self-treating dust bags for livestock: 10 filled daily (assuming a 12.5 lb dust bag) or 400
animals treated daily; Veterinary/groomer domestic animal applications: 8 dogs treated daily (1 per hour in 8-
hour workday), 25 horses treated daily (10 gallons/day for dog dip); Veterinary/groomer aerosol can/trigger spray bottle: 2-16 oz cans/bottles; Christmas tree/conifer pine tree orchards manually pressurized handwand/backpack
applications: 5 acres; Christmas tree mechanically pressurized handwand applications: 125 acres19; Conifer pine tree orchard aerial applications: 125 acres; Indoor residential RTU Foggers: 8-6 oz foggers (negligible exposure); Indoor residential RTU Aerosol Cans: 8-16 oz cans; Indoor residential Trigger-spray bottles: 8 bottles; Indoor residential Dust applications: 10 lbs; Mounds/nests: 1000 linear ft/mounds; Termites: 2000 gallons for injectors; Paint/stain brush/roller applications: 5 gallons; Paint/stain airless sprayer applications: 40 gallons; Outdoor residential misting systems: 1000 gallons; Barn Misting Systems/Stationary Foggers: 200,000 cu ft; Barn manually pressurized handwand applications: 40 gallons; Barn mechanically pressurized handwand applications: 1000 gallons; Termites:
o manually pressurized handwand/backpack applications: 1000 linear feet; o injection: 2000 gallons.
18 C. Smith. Permethrin: Third Revision of the Occupational and Residential Exposure Assessment for the Reregistration Eligibility Decision Document. 4-APR-2006. D325428. W. Britton. Tetrachlorvinphos: Final Occupational and Residential Exposure Assessment for Registration Review. 21-DEC-2016. D436833. 19 Current PHED values: 1000 gallons (mechanically pressurized handgun) / 40 gallons (manually pressurized handwand) = 25:1 ratio. Therefore 5 acres (manually pressurized handwand) * 25 = 125 acres (mechanically pressurized handgun)

Permethrin Human Health Risk Assessment DP No. 414137
Page 66 of 129
For seed treatment uses, the amount of active ingredient handled depends on the application rate as well as the amount of seed handled. For primary handlers (mixers/loaders), the number of seeds treated in a day (8-hour work shift) was based on ExpoSAC Policy 15.1, with 339,500 lbs of corn seeds and 281,250 lbs of soybeans treated in a day. For secondary handlers (planters), the number of seeds planted in a day (8-hour work shift) was based on the BEAD memo: “Acres Planted per Day and Seeding Rates of Crops Grown in the United States” (J. Becker, et al.; March 2011), with 8,800 lbs of corn seeds (i.e., (59,739 seeds/acre/1,361 seeds/lb) * 200 acres/day), and 33,400 lbs of soybeans (i.e., (250,000 seeds/acre/1,500 seeds/lb) * 200 acres/day). For military aircraft applications, the amount handled could potentially vary year-to-year based on operational tempo and the number of military missions to countries which require disinsection to be performed inside an aircraft prior to arrival. As a conservative estimate for the non-cancer risk estimate, using the largest U.S. military aircraft20 (C-5M Super Galaxy) as the application site, HED assumed up to four 100-g canisters21 of product could be used. Exposure Duration: Occupational exposure is expected to be short- to intermediate term in duration. The single dose and repeat dosing permethrin studies show that repeat exposures do not result in lower PODs (i.e., there is no evidence of increasing toxicity with an increased duration of exposure). Therefore, the exposure assessments are conducted as a series of acute exposures, and these are protective of scenarios in which exposure occurs for multiple days. Mitigation/Personal Protective Equipment: Estimates of dermal and inhalation exposure were calculated for various levels of PPE. However, all results are presented for “baseline,” defined as a single layer of clothing consisting of a long-sleeved shirt, long pants, shoes plus socks, no protective gloves, and no respirator. The registered permethrin labels require baseline attire. Some labels require additional PPE depending on the use scenario and formulation22 which are summarized below:
For wettable powder, liquid, and dry flowable formulations: o Applicators using ULV cold foggers or fog/mist generators in indoor spaces must
wear: Coveralls over long-sleeved shirt and long pants, chemical-resistant gloves, chemical resistant footwear plus socks, and chemical-resistant headgear, if overhead exposure.
o Applicators using ULV cold foggers and/or fog/mist generators in outdoor spaces must wear: long-sleeved shirt and long pants, shoes plus socks, and chemical-resistant gloves.
o All other mixers, loaders, applicators, and other handlers must wear: long-sleeved shirt and long pants, shoes plus socks, chemical-resistant gloves for all handlers
20 http://www.af.mil/AboutUs/FactSheets/Display/tabid/224/Article/104492/c-5-abc-galaxy-c-5m-super-galaxy.aspx 21 A C-5M Super Galaxy has an approximate cargo hold volume of 880m3. One canister of product treats 285m3. Therefore, approximately 4 canisters are required to treat the aircrafts cargo hold (880 m3 / 285 m3 = 3.1 canisters + ~1 for cabin and crew areas) 22 Summary of Labeling Changes for Permethrin (Revised 8/29/2011) resulting from the Reregistration Eligibility Decision (RED)

Permethrin Human Health Risk Assessment DP No. 414137
Page 67 of 129
except for applicators using motorized ground equipment, pilots, and flaggers, chemical-resistant apron for mixers/loaders, persons cleaning equipment, and persons exposed to the concentrate and for handlers performing animal dip applications.
For granular formulations: o All loaders, applicators, and other handlers must wear: long-sleeved shirt and long
pants, shoes plus socks, and chemical-resistant gloves for all handlers except for applicators using motorized ground equipment, pilots, and flaggers.
For dust formulations: o Loaders, applicators, and other handlers must wear: long-sleeved shirt and long
pants, shoes plus socks, chemical-resistant gloves, and a NIOSH-approved respirator with: a dust/mist filter with MSHA/NIOSH approval number prefix TC-21C or any N, R, P, or HE filter.
For all engineering control scenarios: o Pilots must use an enclosed cockpit that meets the requirements listed in the
Worker Protection Standard (WPS) for agricultural pesticides [40 CFR 170.240(d)(6)].
For Section 18 Military Aircraft: o No PPE is required according to the labels; however, the Section 18 application
states, “No personal protective equipment is required for minor exposure. Applicators who may have moderate exposures should wear safety glasses, coveralls or long sleeve shirt and pants, rubber or nitrile gloves. Although unlikely for this type of application, applicators who might expect to have heavy exposures should wear a respirator if concentration of gas/particulates in the breathing zone approaches or exceeds the Occupational Exposure Standard.”
o As these exposure levels (minor, moderate, and heavy) are not defined on the label, all scenarios were assessed using baseline PPE defined as a long-sleeved shirt, long pants, shoes, socks, no gloves, and no respirator.
Combining Exposures/Risk Estimates Dermal and inhalation exposures are expected from the occupational handling of permethrin. However, since there is no dermal hazard from permethrin exposure, only inhalation non-cancer exposure has been quantitatively assessed. Occupational handler cancer risk estimates are quantified based on both dermal and inhalation exposures. This is because, despite the determination of the lack of dermal hazard for permethrin, dermal exposures from permethrin must be quantified for the purpose of cancer risk assessment. Summary of Occupational Handler Non-Cancer Exposure and Risk Estimates All screening-level occupational handler non-cancer inhalation risks estimates are not of concern using engineering controls (for aerial applicators) or no respirator, with MOEs ranging from 31 to 240,000,000 (LOC ≤30). Occupational Handler Cancer Exposure and Risk Equations Days per Year of Exposure: To assess cancer risk (both agricultural and non-agricultural uses), it is assumed that private growers would be exposed 10 days per year and commercial applicators would be exposed 30

Permethrin Human Health Risk Assessment DP No. 414137
Page 68 of 129
days per year. The term “private grower” means that the grower or one of the workers would apply the pesticides to land owned or operated by the grower. “Commercial applicators” means the applicators are completing multiple applications for multiple clients. Years per Lifetime of Exposure: It is assumed that handlers would be exposed for 35 years out of a 78-year lifespan. Lifetime Expectancy: Life expectancy values are from the Exposure Factors Handbook 2011 Edition Table 18-1 (U.S. EPA, 2011). The table shows that the overall life expectancy is 78 years based on life expectancy data from 2007. In 2007, the average life expectancy for males was 75 years and 80 years for females. Based on the available data, the recommended value for use in cancer risk assessments is 78 years. A DAF of 3.3% will be applied to estimate the dermal equivalent doses and inhalation absorption is considered equivalent to oral absorption (100%) for the quantitative cancer assessment. Cancer risk estimates were calculated using a linear low-dose extrapolation approach in which a LADD is first calculated and then compared with a cancer potency factor (Q1*) that has been calculated for permethrin based on dose response data in the appropriate toxicology study (Q1* = 9.567 x 10-3 (mg/kg/day)-1). ADD levels were used as the basis for calculating the LADD values. Dermal and inhalation ADD values were first added together to obtain combined ADD values. LADD values were then calculated and compared to the cancer potency factor (Q1*) to obtain cancer risk estimates. Summary of Occupational Handler Cancer Exposure and Risk Estimates Agricultural Uses The cancer occupational handler risk estimates for the registered crops and crop groups ranged from 1×10-8 to 5×10-5 to for private growers (10 days of exposure/year) and 3×10-8 to 2×10-4 for commercial applicators (30 days of exposure/year). Occupational handler manually-pressurized handwand applications (broadcast) to mushroom houses using liquid or wettable powder formulations result in the highest cancer risk estimate. The Agency matches quantitative occupational exposure assessment with appropriate characterization of exposure potential. While HED presents quantitative risk estimates for human flaggers where appropriate, agricultural aviation has changed dramatically over the past two decades. According the 2012 National Agricultural Aviation Association (NAAA) survey of their membership, the use of GPS for swath guidance in agricultural aviation has grown steadily from the mid 1990’s. Over the same time period, the use of human flaggers for aerial pesticide applications has decreased steadily from ~15% in the late 1990’s to only 1% in the most recent (2012) NAAA survey. The Agency will continue to monitor all available information sources to best assess and characterize the exposure potential for human flaggers in agricultural aerial applications. HED has no data to assess exposures to pilots using open cockpits. The only data available is for exposure to pilots in enclosed cockpits. Therefore, risks to pilots are assessed using the

Permethrin Human Health Risk Assessment DP No. 414137
Page 69 of 129
engineering control (enclosed cockpits) and baseline attire (long-sleeved shirt, long pants, shoes, and socks); per the Agency’s Worker Protection Standard stipulations for engineering controls, pilots are not required to wear protective gloves for the duration of the application. With this level of protection, there are currently no risk estimates of concern for applicators. Non-Agricultural Uses The cancer occupational handler risk estimates for the registered use sites ranged from 2×10-9 to 1×10-3 for commercial handlers. Occupational handler manually-pressurized handwand applications (spot) to insect mounds/nests using liquid formulations result in the highest cancer risk estimate. A detailed summary of occupational handler non-cancer and cancer risk estimates is presented in Appendix H, Tables H.1, H.2 and H.3. 11.2 Occupational Post-Application Exposure/Risk Estimates HED uses the term post-application to describe exposures that occur when individuals are present in an environment that has been previously treated with a pesticide (also referred to as re-entry exposure). Such exposures may occur when workers enter previously treated areas to perform job functions, including activities related to crop production, such as scouting for pests or harvesting. Post-application exposure levels vary over time and depend on such things as the type of activity, the nature of the crop or target that was treated, the type of pesticide application, and the chemical’s degradation properties. In addition, the timing of pesticide applications, relative to harvest activities, can greatly reduce the potential for post-application exposure. 11.2.1 Occupational Post-Application Inhalation Exposure/Risk Estimates Agricultural and Commercial Outdoor Uses: There are multiple potential sources of post-application inhalation exposure to individuals performing post-application activities in previously treated fields. These potential sources include volatilization of pesticides and resuspension of dusts and/or particulates that contain pesticides. The Agency sought expert advice and input on issues related to volatilization of pesticides from its Federal Insecticide, Fungicide, and Rodenticide Act Scientific Advisory Panel (SAP) in December 2009, and received the SAP’s final report on March 2, 2010 (http://www.epa.gov/scipoly/SAP/meetings/2009/120109meeting.html). The Agency has evaluated the SAP report and has developed a Volatilization Screening Tool and a subsequent Volatilization Screening Analysis (http://www.regulations.gov/#!docketDetail;D=EPA-HQ-OPP-2014-0219). During Registration Review, the Agency will utilize this analysis to determine if data (i.e., flux studies, additional route specific inhalation toxicity studies) or further analysis is required for permethrin. In addition, the Agency is continuing to evaluate the available post-application inhalation exposure data generated by the Agricultural Reentry Task Force. Given these two efforts, the Agency will continue to identify the need for and, subsequently, the way to incorporate occupational post-application inhalation exposure into the Agency's risk assessments.

Permethrin Human Health Risk Assessment DP No. 414137
Page 70 of 129
Furthermore, inhalation exposure during dusty mechanical activities such as shaking and mechanical harvesting is another potential source of post-application inhalation exposure. However, the airblast applicator scenario is believed to represent a reasonable worst case surrogate estimate of post-application inhalation exposure during these dusty mechanical harvesting activities. The non-cancer inhalation risk estimate for commercial airblast application is not of concern (i.e., MOE > 100) Public Health Uses: As post-application inhalation exposure for occupational workers would result in similar exposures as non-occupational bystanders and residential post application exposure scenarios, the AgDisp post-application assessment in section 6.2 is considered protective of any potential occupational post-application exposure from public health uses. Greenhouse Uses: The Worker Protection Standard for Agricultural Pesticides contains requirements for protecting workers from inhalation exposures during and after greenhouse applications through the use of ventilation requirements. [40 CFR §170.110, (3) (Restrictions associated with pesticide applications)] Seed Treatment Uses: A post-application inhalation exposure assessment is not required as exposure is expected to be negligible. Seed treatment assessments provide quantitative inhalation exposure assessments for seed treaters and secondary handlers (i.e., planters). It is expected that these exposure estimates would be protective of any potential low-level post-application inhalation exposure that could result from these types of applications. Non-Agricultural Commercial Uses: Commercial applicators do not typically return to the treated areas after non-agricultural commercial pesticide applications (sites such as warehouses, food handling establishments, military aircraft, hotels, lawns/landscaping, etc.) and thus an occupational post-application inhalation exposure assessment was not performed for commercial applicators. 11.2.2. Occupational Post-Application Risk Dermal Exposure/Risk Estimates Non-Cancer Occupational Post-Application Dermal Exposure/Risk Estimates No hazard was identified for dermal exposure for a quantitative non-cancer dermal post-application exposure assessment. In addition, commercial applicators do not typically return to the treated areas after a commercial pesticide application (sites such as warehouses, food handling establishments, military aircraft, hotels, etc.). Thus, a quantitative non-cancer occupational post-application dermal exposure assessment for non-agricultural uses was not performed for commercial applicators. Restricted Entry Interval Permethrin is classified as Toxicity Category III via the dermal route and Toxicity Category IV for skin irritation potential. It is not a skin sensitizer. Under 40 CFR §156.208 (c) (2), ai’s classified as Acute Toxicity Category III or IV for acute dermal, eye irritation and primary skin

Permethrin Human Health Risk Assessment DP No. 414137
Page 71 of 129
irritation are assigned a 12-hour REI. Therefore, the [156 subpart K] Worker Protection Statement interim REI of 12 hours is adequate to protect agricultural workers from post-application exposures to permethrin. HED would recommend a REI of 12 hours. This is the REI listed on the registered labels, and is considered protective of post-application exposure. Cancer Occupational Post-Application Dermal Exposure/Risk Estimates A series of assumptions and exposure factors served as the basis for completing the occupational post-application cancer risk assessments. Each assumption and factor is detailed below on an individual basis. Transfer Coefficients: It is the policy of HED to use the best available data to assess post-application exposure. Sources of generic post-application data, used as surrogate data in the absence of chemical-specific data, are derived from ARTF exposure monitoring studies, and, as proprietary data, are subject to the data protection provisions of FIFRA. The standard values recommended for use in predicting post-application exposure that are used in this assessment, known as “transfer coefficients”, are presented in the ExpoSAC Policy 323” which, along with additional information about the ARTF data, can be found at the Agency website24. Table 11.2.2.2 provides a summary of the anticipated post-application activities and associated transfer coefficients for the registered crops/use sites. Some scenarios assessed do not currently have available transfer coefficient data, as explained in the Non-Foliar Transfer Coefficient Table in ExpoSAC Policy 3, and are not quantitatively assessed herein:
Hand pruning: pome trees, citrus trees, and nut trees, o Transfer coefficients for dormant pruning are unavailable.
Hand harvesting: root vegetables (e.g., potatoes) o Harvesting occurs following defoliation, and exposure results via contact with
residues in the soil, for which transfer coefficients are currently unavailable. Mechanical sweeping and Windrowing: tree nuts
o Exposure during nut sweeping and windrowing results from contact with soil, for which transfer coefficients are currently unavailable.
Application Rate: A screening-level approach was used for the assessment of occupational exposures by evaluation of the maximum application rate for all possible exposure scenarios of permethrin. The registered application rates are based on the scenarios listed in Appendix F, Tables F.1 and F.2. Exposure Time: The average occupational workday is assumed to be 8 hours. Turf Transferable Residues: Post-application exposures from golf courses were assessed using 0-day residue data from a turf transferable residue study conducted with a liquid permethrin product (MRID 44955501). Corrected TTR values have been reassessed to incorporate current regression modeling into this
23 Available: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/occupational-pesticide-handler-exposure-data 24 Available: https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/occupational-pesticide-handler-exposure-data

Permethrin Human Health Risk Assessment DP No. 414137
Page 72 of 129
assessment resulting in day-0 TTR of 0.061 µg/cm2 at the study application rate of 0.87 lbs ai/acre. Additional summary information is available in section 6.2, Table 6.2.1. Dislodgeable Foliar Residues: For agricultural post-application scenarios, chemical-specific DFR data are available for four pyrethroids: cyfluthrin, fluvalinate, esfenvalerate, and permethrin. Most of these DFR data were collected on orchard crops (i.e., stone fruits, apples, oranges) or in greenhouses. The esfenvalerate DFR data underwent secondary review25 and included analysis of foliar residues on corn and broccoli and are considered most representative of potential field crops that could be found in an agricultural setting which are identified in Table 11.2.2.2. However, the permethrin DFR data26 included analysis of foliar residues on orchard crops (i.e., peaches) and are considered most representative of potential residues that could be found on fruit and nut tree foliage. Table 11.2.2.1 summarizes the available pyrethroid DFR data.
Table 11.2.2.1. Pyrethroid DFR Summary.
Chemical Study Sites Day 0 DFR
(ug/cm2)
Decay Constant (k)
Daily Dissipation
(%)
Half Life (days)
Esfenvalerate
Dissipation of Dislodgeable Foliar Residues of
Esfenvalerate from Broccoli Following Application of Asana® XL Insecticide in
the USA - Season 1997 (MRID 44852402)
CA (Trial 1) 0.191 -0.219 20% 3.2
CA (Trial 2) 0.123 -0.144 13% 4.8
Dissipation of Dislodgeable Foliar Residues of
Esfenvalerate from Sweet Corn Following Application of Asana® XL Insecticide in
the USA - Season 1998 (MRID 44852403)
CA (L) 0.221 -0.199 18% 3.5
PA (L) 0.157 -0.181 17% 3.8
Permethrin
Dissipation of Dislodgeable Foliar Residues of
Permethrin Applied to Orchards (Peaches) (MRID 437557-01)
CA (EC) 0.309 -0.029 3% 24.2 CA (W) 0.455 -0.025 2% 28.2 GA (W) 0.712 -0.060 6% 11.5 WA (W) 1.385 -0.047 5% 14.6
Average* 0.715 -0.040 4% 17.2 * Calculated as [CA (EC) + CA (W) + GA (W) + WA (W)] ÷ 4 **Bolded values were used to calculate typical doses for the cancer risk estimates.
The dermal dose used for the occupational post-application cancer risk estimate was calculated using a 30-day average dose. This was calculated by adding the Day-0 dermal dose with dermal doses from days 1 through 30 dissipated at the daily rate indicated in Table 11.2.2.1 above and then averaging the resulting value for each individual scenario. Days per Year of Exposure: To assess cancer risk, it is assumed that post-application scenarios could occur approximately 30 days a year at a 30-day average dose to calculate post-application risk estimates (B. Bobowiec, 16-OCT-2015; D429731).
25 B. O’Keefe 06-OCT-2003, D283191; B. O’Keefe 06-MAR-2003, D283188 26 Dissipation of Dislodgeable Foliar Residues of Permethrin Applied to Orchards (Peaches). EPA MRID 437557-01. T. Belcher, et. al., 20-JUL-1995

Permethrin Human Health Risk Assessment DP No. 414137
Page 73 of 129
Years per Lifetime of Exposure: HED assumes that post-application workers would be exposed for 35 years out of a 78-year lifespan. Lifetime Expectancy: Based on available data from EPA’s Exposure Factors Handbook 2011 Edition, the recommended lifespan for use in cancer risk assessments is 78 years. Life expectancy values are derived from the Exposure Factors Handbook 2011 Edition Table 18-1 (U.S. EPA, 2011). The table shows that the overall life expectancy is 78 years based on life expectancy data from 2007. In 2007, the average life expectancy for males was 75 years and 80 years for females. For the quantitative cancer assessment, a DAF of 3.3% has been applied to estimate the dermal equivalent doses, and inhalation absorption is considered equivalent to oral absorption (100%). Occupational Post-Application Cancer Dermal Exposure and Risk Equations As was done for occupational handlers, post-application cancer risk estimates were calculated using a linear low-dose extrapolation approach in which a LADD is first calculated and then compared with a cancer potency factor (Q1*) that has been calculated for permethrin based on dose response data in the appropriate toxicology study (Q1* = 9.567×10-3 (mg/kg/day)-1). Occupational Post-Application Cancer Dermal Risk Estimates The cancer post-application risk estimates for the registered crops and crop groups ranged from 1×10-9 to 4×10-6 using the average 30-day dose. The forestry post-application activity of hand set irrigation result in the highest cancer risk estimate.
Table 11.2.2.2. Occupational Post-Application Cancer Exposure and Risk Estimates for Permethrin.
Crop Grouping/Crop (Application Rate)
Activity Transfer
Coefficient (cm2/hr)
30-Day Average Dose Dermal LADD (mg/kg/day)1
Cancer Risk Estimate2
Permethrin Peach DFR Data (MRID 437557-01)
Papaya (0.15 lbs ai/acre)
orchard maintenance, hand weeding 100 1.90E-06 2E-08 scouting, hand pruning 580 1.10E-05 1E-07
hand harvesting 1400 2.66E-05 3E-07 transplanting 230 4.38E-06 4E-08
Cherry (0.2 lbs ai/acre)
orchard maintenance, hand weeding, bird control, and propping
100 2.54E-06 2E-08
Scouting, hand pruning, scouting, training 580 1.47E-05 1E-07 thinning fruit 3600 9.13E-05 9E-07
hand harvesting 1400 3.55E-05 3E-07 transplanting 230 5.84E-06 6E-08
Christmas Tree (0.2 lbs ai/acre)
irrigation (hand set) 1900 4.82E-05 5E-07 scouting, shaping 580 1.47E-05 1E-07
hand weeding, grading/tagging 100 2.54E-06 2E-08 hand harvesting 1400 3.55E-05 3E-07
transplanting 230 5.84E-06 6E-08
Pecans (0.2 lbs ai/acre)
mechanical harvesting (shaking) 190 4.82E-06 5E-08 poling, orchard maintenance, hand weeding 100 2.54E-06 2E-08
hand pruning, scouting 580 1.47E-05 1E-07 transplanting 230 5.84E-06 6E-08
Tree Nuts Almond, Hazelnut, Walnut
(0.25 lbs ai/acre)
orchard maintenance, poling, hand weeding 100 3.17E-06 3E-08 scouting 580 1.84E-05 2E-07
transplanting 230 7.29E-06 7E-08

Permethrin Human Health Risk Assessment DP No. 414137
Page 74 of 129
Table 11.2.2.2. Occupational Post-Application Cancer Exposure and Risk Estimates for Permethrin.
Crop Grouping/Crop (Application Rate)
Activity Transfer
Coefficient (cm2/hr)
30-Day Average Dose Dermal LADD (mg/kg/day)1
Cancer Risk Estimate2
Deciduous Fruit Trees Apple, Nectarine, Peach, Pear
(0.25 lbs ai/acre)
Scouting, hand pruning, training 580 1.84E-05 2E-07 orchard maintenance, propping, hand weeding 100 3.17E-06 3E-08
hand harvesting 1400 4.44E-05 4E-07 transplanting 230 7.29E-06 7E-08 thinning fruit 3600 1.14E-04 1E-06
Pistachio (0.3 lbs ai/acre)
orchard maintenance, hand weeding 100 3.81E-06 4E-08 hand harvesting (net) 1400 5.33E-05 5E-07
scouting 580 2.21E-05 2E-07 mechanical harvesting (shaking) 190 7.23E-06 7E-08
transplanting 230 8.75E-06 8E-08
Conifer pine seed orchard (1.6 lbs ai/acre)
harvesting seed cone (conifers) 1400 2.84E-04 3E-06 harvesting seedling production 6700 4.54E-05 4E-07
hand pruning (high/full), scouting 580 1.18E-04 1E-06 hand weeding 100 2.03E-05 2E-07
hand set irrigation 1900 3.86E-04 4E-06 transplanting 230 4.67E-05 4E-07
Esfenvalerate Broccoli and Sweet Corn DFR (MRID 448524-02 and 448524-03)
Kiwifruit (0.007 lbs ai/acre)
scouting, hand pruning, hand weeding, tying/training 640 4.11E-07 4E-09 hand harvesting 10100 6.48E-06 6E-08
transplanting 230 1.48E-07 1E-09
Asparagus (0.1 lbs ai/acre)
hand weeding 70 6.42E-07 6E-09 hand set irrigation 1900 1.74E-05 2E-07
scouting 210 1.93E-06 2E-08 hand harvesting 1100 1.01E-05 1E-07
transplanting 230 2.11E-06 2E-08
Head and Stem Brassica Brussel Sprouts, Cauliflower
(0.1 lbs ai/acre)
scouting (low/full), hand harvesting, topping, hand weeding, tying/training
4200 3.85E-05 4E-07
hand set irrigation 1900 1.74E-05 2E-07 scouting (low/min), thinning plants 330 3.03E-06 3E-08
transplanting 230 2.11E-06 2E-08 hand weeding (cauliflower low/min) 1400 1.28E-05 1E-07
Collards (0.15 lbs ai/acre
hand set irrigation 1900 2.61E-05 2E-07 scouting 210 2.89E-06 3E-08
hand harvesting 1100 1.51E-05 1E-07 hand weeding, thinning plants 70 9.63E-07 9E-09
transplanting 230 3.16E-06 3E-08
Field/Row Crop (tall) Corn (pop, field) (0.15 lbs ai/acre)
hand set irrigation 1900 2.61E-05 2E-07 scouting (high/full) 1100 1.51E-05 1E-07
scouting (low/min and low/full) 210 2.89E-06 3E-08 hand weeding 70 9.63E-07 9E-09
Fruiting Vegetables Eggplant
(0.15 lbs ai/acre)
hand harvesting 550 7.56E-06 7E-08 hand pruning, scouting, thinning fruit, hand weeding 90 1.24E-06 1E-08
hand set irrigation 1900 2.61E-05 2E-07 transplanting 230 3.16E-06 3E-08
Root Vegetables Turnip
(0.15 lbs ai/acre)
hand harvesting 1100 1.51E-05 1E-07 hand set irrigation 1900 2.61E-05 2E-07
scouting 210 2.89E-06 3E-08 hand weeding, thinning plants 70 9.63E-07 9E-09
Field/Row Crop (low/medium) Alfalfa, Soybean (0.2 lbs ai/acre)
hand set irrigation 1900 3.48E-05 3E-07 scouting 1100 2.02E-05 2E-07
hand weeding (soybean only) 70 1.28E-06 1E-08
Vine/Trellis Highbush Blueberry, Raspberry
(0.2 lbs ai/acre)
scouting, hand weeding, hand pruning, bird control, frost control, tying/training (high, low/min)
640 1.17E-05 1E-07
hand harvesting, tying/training (high/full) 1400 2.57E-05 2E-07 hand set irrigation 1900 3.48E-05 3E-07

Permethrin Human Health Risk Assessment DP No. 414137
Page 75 of 129
Table 11.2.2.2. Occupational Post-Application Cancer Exposure and Risk Estimates for Permethrin.
Crop Grouping/Crop (Application Rate)
Activity Transfer
Coefficient (cm2/hr)
30-Day Average Dose Dermal LADD (mg/kg/day)1
Cancer Risk Estimate2
transplanting 230 4.22E-06 4E-08
Head and Stem Brassica Broccoli, Cabbage
(0.2 lbs ai/acre)
scouting (low/full), hand harvesting, hand weeding (low/full)
4200 7.70E-05 7E-07
hand set irrigation 1900 3.48E-05 3E-07 scouting (low/min), thinning plants 330 6.05E-06 6E-08
transplanting 230 4.22E-06 4E-08 hand weeding (low/min), scouting (cabbage low/min),
hand harvesting (cabbage low/min), mechanically-assisted harvesting
1400 2.57E-05 2E-07
Leafy Vegetables Cabbage, Celery, Leafy Greens,
Lettuce, Spinach (0.2 lbs ai/acre)
transplanting 230 4.22E-06 4E-08
hand set irrigation 1900 3.48E-05 3E-07
Leafy Vegetables
(0.2 lbs ai/acre)
Cabbage
hand harvesting, scouting (low/full), hand weeding (low/min)
1400 2.57E-05 2E-07
scouting (low/min), thinning plants 330 6.05E-06 6E-08 hand weeding (low/full) 4200 7.70E-05 7E-07
Celery, Leafy Greens, Lettuce,
Spinach
scouting 210 3.85E-06 4E-08 hand harvesting 1100 2.02E-05 2E-07
hand weeding, thinning plants 70 1.28E-06 1E-08
Cucurbit Vegetables Cantaloupe, Cucumber,
Pumpkin, squash, watermelon (0.2 lbs ai/acre)
hand set irrigation 1900 3.48E-05 3E-07 scouting, thinning fruit, hand pruning, hand weeding 90 1.65E-06 2E-08 hand harvesting, mechanically-assisted harvesting,
training/turning 550
1.01E-05 1E-07 transplanting 230 4.22E-06 4E-08
Field/Row Crop (tall) Corn (sweet grain/processing)
(0.2 lbs ai/acre)
hand set irrigation 1900 3.48E-05 3E-07 scouting (high/full) 1100 2.02E-05 2E-07
scouting (low/full, low/min) 210 3.85E-06 4E-08 hand detasseling, hand harvesting 8800 1.61E-04 2E-06
hand weeding 70 1.28E-06 1E-08
Fruiting Vegetables Bell Pepper, Tomato
(0.2 lbs ai/acre)
hand harvesting, tying/training 1100 2.02E-05 2E-07 hand set irrigation 1900 3.48E-05 3E-07
scouting 210 3.85E-06 4E-08 hand weeding, hand pruning 70 1.28E-06 1E-08
transplanting 230 4.22E-06 4E-08 Root Vegetables
Potato (0.2 lbs ai/acre)
hand set irrigation 1900 3.48E-05 3E-07 scouting 210 3.85E-06 4E-08
hand weeding 70 1.28E-06 1E-08
Stem/Stalk Vegetables Artichoke
(0.3 lbs ai/acre)
hand harvesting 1100 3.03E-05 3E-07 hand pruning, hand weeding 70 1.93E-06 2E-08
hand set irrigation 1900 5.23E-05 5E-07 scouting 210 5.78E-06 6E-08
transplanting 230 6.33E-06 6E-08
Root Vegetables Onions
(0.3 lbs ai/acre)
hand set irrigation 1900 5.23E-05 5E-07 scouting, hand weeding (low/min) 1400 3.85E-05 4E-07
hand weeding (low/full) 4200 1.16E-04 1E-06 scouting, thinning plants 330 9.08E-06 9E-08
Permethrin TTR Data (MRID 449555-01) Golf Course
(0.79 lbs ai/acre) maintenance 3700 7.74E-06 7E-08
maintenance (greens only) 2500 5.23E-06 5E-08 1 Dermal LADD (mg/kg/day) = 30 day average dermal dose (mg/kg/day) × [Days per year of exposure (30 days/yr) ÷ 365 days/year] × [Years
per lifetime of exposure (35 yrs) ÷ Lifetime expectancy (78yrs)].
2 Cancer risk estimate = Dermal LADD (mg/kg/day) × Q1*, where Q1
* = 9.567×10-3 (mg/kg/day)-1.

Permethrin Human Health Risk Assessment DP No. 414137
Page 76 of 129
12.0 Incident and Epidemiological Data Review HED has prepared a Tier II Incident and Epidemiology Report for permethrin entitled “Permethrin: Tier II Incident and Epidemiology Report” (Memo, E. Evans, et al.; 27-JUN-2017; D440947). Prior to this memo, permethrin incidents and epidemiological publications were last reviewed in March 2011 (K. Oo, D386498, 3/1/2011). In 2011, HED prepared a preliminary Tier I human incident review of permethrin human incident reports by consulting the OPP Incident Data System (IDS) for reports of poisoning incidents. In 2011, a moderately large number of incidents were reported involving permethrin. At the time, given the frequency and relative severity, HED determined it would further evaluate permethrin acute poisoning event reporting and surveillance databases as well as a review of published literature as to the acute and chronic health effects associated with permethrin exposure by performing a Tier II review.27 The Permethrin Tier II Incident and Epidemiology Report reviews human observation data from a variety of sources including:
Human incident (poisoning) data from the following sources: o OPP’s IDS database, o The Center for Disease Control (CDC)/NIOSH Sentinel Event Notification
System for Occupational Risk (SENSOR)-Pesticides, o the Agency-sponsored National Pesticide Information Center (NPIC), and o California’s Pesticide Incident Surveillance Program (PISP),
Epidemiological studies from the literature. 12.1 Acute Permethrin Incident Summary HED found that the acute health effects reported to the incident databases queried are consistent with the previous incident report. These health effects primarily include dermal, neurological, and respiratory effects. HED did not identify any aberrant effects outside of those anticipated. These effects are generally mild/minor to moderate and resolve rapidly.
Across all the databases reviewed, homeowner application and post application exposures were responsible for most of the reported incidents. In addition, incidents occurred as a result of application of pet spot-on products to a pet were frequently reported to IDS.
Trends over time data from IDS (2006 to 2015) and SENSOR-Pesticides (1998 to 2013) data were reviewed. Based on IDS, which are primarily non-occupational cases, incidents appear to be decreasing over time. The SENSOR-Pesticide data represent both occupational and non-occupational cases. Residential case reports involving permethrin have increased over the 15 years of SENSOR-Pesticides data, but appear to be leveling off from 2007-2013. The occupational case reports appear to be decreasing over time. The high numbers of reported permethrin incidents are likely related to the fact that pyrethroids are now among the most commonly used pesticides in residential settings (Hudson, 2013). Due to the increase in the availability and usage of pyrethroids, the frequency of incident reports have
27 For this review, no medical case reports were investigated.

Permethrin Human Health Risk Assessment DP No. 414137
Page 77 of 129
also increased. While the permethrin incidents were predominately minor in severity, five deaths involving permethrin were identified in SENSOR-Pesticides, and a sixth fatality is under active investigation as of February 6, 2017. Incidents of severe outcome can signal the Agency to further investigate a particular chemical or product. However, all of the reported fatalities involved multiple active ingredients and therefore are less certain regarding information about the potential effects of exposure from permethrin. In addition, most of these fatal cases involved misuse, including: two suicidal ingestions, and two involved the excessive use of multiple products. However, these fatalities are of concern to EPA and permethrin incidents will be closely monitored moving forward. 12.2 Epidemiological Studies from the Literature Summary Epidemiological studies investigating the association of permethrin with various health outcomes were reviewed. The epidemiology review found that, overall, there was little substantive evidence to suggest a clear causal relationship between exposure to permethrin and the health outcomes investigated in the AHS studies reported here. The AHS is a high quality, prospective epidemiology study evaluating the link between pesticide use and various health outcomes including cancer. The AHS includes information on use of 50 different pesticide active ingredients commonly used in agriculture. Overall strengths of the AHS in regards to exposure assessment and outcome ascertainment are further discussed in the Framework for Incorporating Human Epidemiologic and Incident Data in Risk Assessments for Pesticides28. For multiple myeloma and childhood leukemia, the Agency will continue to monitor these two health endpoints in particular (in addition to all health endpoints) relative to permethrin exposure, since a significant positive association was observed. However, it should be noted that study limitations including recall bias due to the study design and a small number of exposed cases existed for the observed health outcomes, and likely effected the associations reported. The Agency will continue to monitor the incident data and epidemiology for permethrin, and if a concern is triggered, additional analysis will be conducted. 13.0 References USEPA, 2015, Meeting Minutes: FIFRA SAP Meeting on research to evaluate the potential for juvenile sensitivity to pyrethroids. Document ID: EPA-HQ-OPP-2015-0130-0019. Hines, R. N., P. M. Simpson, and D. G. McCarver (2016). “Age-Dependent Human Hepatic Carboxylesterase 1 (CES1) and Carboxylesterase 2 (CES2) Postnatal Ontogeny” Drug Metab Dispos 44 (7): 959-966. Stevens, J. C., R. N. Hines, C. Gu, S. B. Koukouritaki, J. R. Manro, P. J. Tandler, and M. J. Zaya (2003). “Developmental Expression of the Major Human Hepatic CYP3A Enzymes” J Pharmacol Exp Ther 307(2): 573-582.
28 Office of Pesticide Programs’ Framework for Incorporating Human Epidemiologic & Incident Data in Risk Assessments for Pesticides. https://www3.epa.gov/pesticides/EPA-HQ-OPP-2008-0316-DRAFT-0075.pdf

Permethrin Human Health Risk Assessment DP No. 414137
Page 78 of 129
Song, G., X. Sun, R. Hines, D. McCarver, B. Lake, T. Osimitz, M. Creek, H. Clewell, and M. Yoon (2015). “Age-Dependent Changes in Human Hepatic CYP2C8 and 1A2 Expression” Presented at the Society of Toxicology Annual Meeting, San Diega, CA, March 22-26, 2015. Shafer, T., Meyer, D., and Crofton, K. Developmental neurotoxicity of pyrethroid insecticides: critical review and future research needs. Environmental Health Perspectives. 2005 Feb; 113(2): 123–136. Soderlund, D., J. Clark, L. Sheets, L. Mullin, V. Piccirillo, D. Sargent, J. Stevens and M. Weiner. 2002. Mechanisms of pyrethroid neurotoxicity: Implications for cumulative risk assessment. Toxicology. 171(1): 3 - 59. Tan, J., and Soderlund, D, Human and rat Nav1.3 voltage-gated sodium channels differ in inactivation properties and sensitivity to the pyrethroid insecticide tefluthrin. Neurotoxicology. January 1, 2009; 30 (1): 81-9. Weiner, M., Nemec, M., Sheets, L., Sargent, D., Breckenridge, C. Comparative functional observational battery study of twelve commercial pyrethroid insecticides in male rats following acute oral exposure. NeuroToxicology 30S (2009) S1–S16 Whitby, K. 2011. Pyrethroid cumulative risk assessment. D394576. Wolanksy, M., C. Gennings, and K. Crofton. 2006. Relative potency for acute effects of pyrithroids on motor function in rats. Toxicological Sciences. 89 (1): 271-277. Memo, C. Smith, D357566, 01-APR-2009; Permethrin: Sixth Revision of the HED Chapter of the Reregistration Eligibility Decision Document (RED). Memo, S. Kinard, 17-MAR-2005; D313662. Permethrin. Revised Residue Chemistry Considerations for Reregistration Eligibility Decision (RED) Document. Memo, J. Van Alstine, 23-JUN-2017; D440981. Permethrin: Response to Health Effects Division (HED) Review of 17-MAR-2005 (D313662) and the Generic Data Call-In (GDCI). Memo, J. Van Alstine, 23-JUN-2017; D440977. Permethrin: Acute, Chronic, and Cancer Dietary (Food and Drinking Water) Exposure and Risk Assessments to Support Registration Review. Memo, J. Godshall, 30-JUN-2017; D440978. Permethrin. Occupational and Residential Exposure Assessment for Registration Review. Memo, E. Evans, et al.; 27-JUN-2017; D440947. Permethrin: Tier II Incident and Epidemiology Report.

Permethrin Human Health Risk Assessment DP No. 414137
Page 79 of 129
13.0 Appendices Appendix A. Toxicity Profiles. Appendix B. Physical Chemical Properties. Appendix C. International Residue Limit Status Sheet. Appendix D. Submission of Analytical Standards. Appendix E. Summary of RED Data Requirements and Data Reviewed for Registration Review. Appendix F. Permethrin Use Pattern Tables. Appendix G. Summary of Assumptions Used in the Residential Post-Application Assessment. Appendix H. Occupational Hander Non-Cancer and Cancer Risk Estimates.

Permethrin Human Health Risk Assessment DP No. 414137
Page 80 of 129
Appendix A. Toxicity Profiles. A.1 Toxicology Data Requirements The requirements (40 CFR 158.500) for the Food Use of permethrin are in Table 1. Use of the new guideline numbers does not imply that the new (1998) guideline protocols were used.
Test Technical
Required Satisfied 870.1100 Acute Oral Toxicity ....................................................... 870.1200 Acute Dermal Toxicity .................................................. 870.1300 Acute Inhalation Toxicity.............................................. 870.2400 Primary Eye Irritation ................................................... 870.2500 Primary Dermal Irritation .............................................. 870.2600 Dermal Sensitization .....................................................
yes yes yes yes yes yes
yes yes yes yes yes yes
870.3100 Oral Subchronic (rodent) ............................................... 870.3150 Oral Subchronic (nonrodent) ......................................... 870.3200 28-Day Dermal .............................................................. 870.3250 90-Day Dermal .............................................................. 870.3465 90-Day Inhalation ..........................................................
yes yes yes no yes
yes yes yes no
yes(a) 870.3700a Developmental Toxicity (rodent) .................................. 870.3700b Developmental Toxicity (nonrodent) ............................ 870.3800 Reproduction .................................................................
yes yes yes
yes yes yes
870.4100a Chronic Toxicity (rodent) .............................................. 870.4200a Oncogenicity (rat) .......................................................... 870.4200b Oncogenicity (mouse) ...................................................
yes yes yes
yes yes yes
870.5100 Mutagenicity—Gene Mutation - bacterial..................... 870.5300 Mutagenicity—Gene Mutation - mammalian ............... 870.5375 Mutagenicity—Structural Chromosomal Aberrations ... 870.5395 Mutagenicity—Other Genotoxic Effects ....................... 870.5550 Mutagenicity—Unscheduled DNA Synthesis
yes yes yes yes no
yes yes yes yes yes
870.6100a Acute Delayed Neurotox. (hen) .................................... 870.6200a Acute Neurotox. Screening Battery (rat) ...................... 870.6200b 90-Day Neuro. Screening Battery (rat) ........................ 870.6300 Develop. Neuro ............................................................
cr yes yes cr
no yes
yes no
870.7485 General Metabolism ...................................................... 870.7600 Dermal Penetration ........................................................ 870.7800 Immunotoxicity .............................................................
yes cr
yes
yes yes yes
(a) A 15 day non-guideline inhalation study is suitable for risk assessment based on the currently registered use pattern, and a 90 day inhalation study is not required at this time. cr = conditionally required

Permethrin Human Health Risk Assessment DP No. 414137
Page 81 of 129
A.2 Toxicity Profiles
Table A.2.1 Acute toxicity Profile – Permethrin. Guideline No. Type MRIDs Results Toxicity Category
870.1100 Acute oral toxicity in Rats 242899 LD50 = 3580 mg/kg (M)
2280 mg/kg (F) III
870.1200 Acute dermal toxicity in
Rabbits 242899 LD50 >2000 mg/kg III
870.2400 Acute eye irritation in Rabbits 242899 Irritation 24-48 hrs.
All cleared by 72 hrs. III
870.2500 Acute dermal irritation in
Rabbits 242899
All irritation cleared by 48 hrs
IV
870.2600 Skin sensitization in Guinea
Pigs NA Non-sensitizer Not Applicable
Table A.2.2 Subchronic, Chronic and Other Toxicity Profile for Permethrin. Guideline
No. Study Type
MRID No. (year)/ Classification /Doses
Results
870.3100
90-Day oral toxicity (rat)
00070577 00040491 00025914 00025915 00054737 0, 22.5, 46.0, 92.9 or 185 mg/kg/day for males 0, 27.5, 52.3, 110 or 221 mg/kg/day for females
NOAEL = 110 mg/kg/day LOAEL = 221 mg/kg/day based on tremors and hyper sensitivity.
870.3150
90-Day oral toxicity (dog)
00070576 00043722 00071951 00029832 Doses from multiple studies ranging from 10 to 1000 mg/kg/day.
NOAEL = 100 mg/kg/day LOAEL = 364 mg/kg/day based on nervous system effects including tremors.
870.3200
21-Day dermal toxicity (rat)
41143802 42653301 92142030 Acceptable/Guideline 0, 50, 150, or 500 mg/kg/day
NOAEL = 500 mg/kg/day No systemic effects were reported at the highest dose tested.
870.3465 15-day inhalation toxicity (rat)
00096713 Acceptable/non-guideline 0, 0.0061, 0.042, or 0.583 mg/L
NOAEL = 0.042 mg/L LOAEL = 0.583 mg/L based on based on body tremors and hypersensitivity to noise.

Permethrin Human Health Risk Assessment DP No. 414137
Page 82 of 129
Table A.2.2 Subchronic, Chronic and Other Toxicity Profile for Permethrin. Guideline
No. Study Type
MRID No. (year)/ Classification /Doses
Results
870.3700a
Prenatal developmental in (rat)
40943603 Acceptable/Guideline 0, 15, 50, or 150 mg/kg/day.
Maternal Systemic Toxicity NOAEL = 50 mg/kg/day Maternal Systemic Toxicity LOAEL = 150 mg/kg/day based on clinical signs of toxicity. Developmental Toxicity NOAEL = 600 mg/kg/day Developmental Toxicity LOAEL = 1200 mg/kg/day based on decrease in fetal body weights and an increase in the incidence rate of short length extra ribs.
870.3700b
Prenatal developmental in (rabbit)
92142091 40943602 92142036 Acceptable/Guideline 0, 600, 1200, or 1800 mg/kg/day
Maternal Systemic Toxicity LOAEL = 600 mg/kg/day based on decreased body weight gain. Developmental Toxicity NOAEL = 600 mg/kg/day Developmental Toxicity LOAEL = 1200 mg/kg/day based on increased post-implantation loss, greater numbers of early and late resorptions, and decreased ossification.
870.3800
Reproduction and fertility effects (rat)
00102108 00120271 92142092 92142037 Acceptable/Guideline 0, 25, 50, or 125 mg/kg/day
Parental NOAEL = 50 mg/kg/day Parental LOAEL = 125 mg/kg/day based on tremors. Reproductive NOAEL = 125 mg/kg/day Offspring NOAEL = 125 mg/kg/day
870.4100 Chronic Toxicity (Dog)
00129600 Acceptable/Guideline 0,5,100,1000 mg/kg/day (capsule)
NOAEL = 100 mg/kg/day LOAEL = 1000 mg/kg/day based on clinical signs and decreased body weight.
870.4200 Carcinogenicity (mouse)
00062806 92142033 Acceptable/guideline 0, 3, 71, 286 mg/kg/day (M) 0, 3, 357, 714 mg/kg/day (F)
There were statistically significant increases in liver adenoma at all doses for males and at mid- and high-doses for females with a significant dose-related trend in both sexes.

Permethrin Human Health Risk Assessment DP No. 414137
Page 83 of 129
Table A.2.2 Subchronic, Chronic and Other Toxicity Profile for Permethrin. Guideline
No. Study Type
MRID No. (year)/ Classification /Doses
Results
870.4200 Carcinogenicity (mouse)
00102110 92142032 Acceptable/guideline 0, 26.9, 110.5, 287.2 mg/kg/day (M). 0, 29.8, 124.2, 316.1 mg/kg/day (F)
There was no evidence of significant increase in unusual tumor types. A non-significant increase in lung adenomas in males and in lung adenomas plus carcinomas in females was seen at the highest dose.
870.4200 Carcinogenicity (mouse)
45597105 Acceptable/non-guideline 0, 5000 ppm (Females only) (0, 780-807 mg/kg/day)
There were significant increases in the incidences of lung bronchioloalveolar adenomas in mice. The increased incidences of basophilic hepatocellular adenoma did not show a relationship to the treatment duration. No progression to carcinoma was observed in the lung or liver.
870.4300
Combined Chronic Toxicity/Carcinogenicity (rat)
92142123 Acceptable/guideline 0, 500, 1000, or 2500 ppm 0, 19.4, 36.9, 91.5 mg/kg/day (M) 0, 19.1, 40.2, 104 mg/kg/day (F)
NOAEL = 36.9 mg/kg/day LOAEL = 91.5 mg/kg/day based on tremor and hypersensitivity.
870.5100 Gene Mutation (Ames Assay)
42005458 (1986) Acceptable/Guideline
Negative
870.5395 Micronucleus Assay
42005459 (1988) Acceptable/Guideline
Negative
870.5550 Unscheduled DNA Synthesis
42005461 (1987) Acceptable/Guideline
Negative
870.6200a
Acute neurotoxicity (rat)
43046301 45657401 Acceptable/Guideline 0, 25, 75 or 150 mg/kg/day
NOAEL = 25 mg/kg/day LOAEL = 75 mg/kg/day based on observations of clinical signs (i.e., aggression, abnormal and/or decreased movement) and increased body temperature.
870.6200b
Subchronic Neurotoxicity (rat)
00071952 Acceptable/non-guideline 0, 125, 150, 200, 225, 250, or 500 mg/kg/day
LOAEL = 125 mg/kg/day based on tremors and hypersensitivity.
870.6200b
Subchronic Neurotoxicity (rat)
40766807 Acceptable/non-guideline 0, 100, 200, or 400 mg/kg/day
NOAEL = 100 mg/kg/day LOAEL = 200 mg/kg/day based on tremors and irritability.

Permethrin Human Health Risk Assessment DP No. 414137
Page 84 of 129
Table A.2.2 Subchronic, Chronic and Other Toxicity Profile for Permethrin. Guideline
No. Study Type
MRID No. (year)/ Classification /Doses
Results
870.7485
Metabolism and Pharmacokinetics (rat)
00089006 00054719 92142041 92142042
Following a single oral dose of 6.5 mg/kg, most radioactivity (58-65%) from a single dose of the [14C-alcohol] permethrin was eliminated via the urine over a 7-day period with much of the remainder (29-43%) being excreted in the feces. Urinary excretion of radioactivity following a single dose of [14 C-acid] permethrin was slightly less and fecal excretion correspondingly greater. Results of tissue distribution and autoradiographic experiments showed that most radioactivity was associated with adipose tissue and, initially, with the gastrointestinal tract and organs/tissue associated with excretory function. Following multiple doses, radioactivity in adipose tissue appears to be greater for [14C-alcohol] permethrin than for [14C-acid] permethrin. This is also consistent with blood kinetics data showing lower radioactivity (Cmax) in the blood of rats receiving [14C-acid] permethrin.
870.7600 Dermal Penetration (rat)
43169001 4, 80, 860, or 9600 µg/cm2
Following 10 hours of exposure, a total of 21.7% permethrin was absorbed.
870.7800 Immunotoxicity (rat)
Not Required

Permethrin Human Health Risk Assessment DP No. 414137
Page 85 of 129
Appendix B. Physical Chemical Properties. Table B.1. Physicochemical Properties of the Technical Grade Test Compound: Permethrin. Parameter
Value
Reference
Boiling point
220 C (0.05 mm Hg; decomposes)
2001 Farm Chemicals Handbook
Melting point
31 C 35 C
RD D274107, 7/12/01, S. Mathur 2001 Farm Chemicals Handbook
pH
4.44 at 20 C
RD D274107, 7/12/01, S. Mathur
Density, bulk density, or specific gravity
1.229 g/cc 1.190-1.272 specific gravity at 20 C
RD D274107, 7/12/01, S. Mathur 2001 Farm Chemicals Handbook
Water solubility
0.21 mg/L at 20 C 1 ppm
RD D274107, 7/12/01, S. Mathur 2001 Farm Chemicals Handbook
Solvent solubility
258 mg/kg in methanol at 25 C >1000 g/kg in hexane at 25 C Miscible in most organic solvents except ethylene glycol; soluble in acetone, ethanol, ether, and xylene
RD D274107, 7/12/01, S. Mathur 2001 Farm Chemicals Handbook
Vapor pressure
0.07 mPa at 20 C 10 Torr at 50 C
RD D274107, 7/12/01, S. Mathur 2001 Farm Chemicals Handbook
Dissociation constant, pKa
Not applicable because permethrin is neither an acid nor a base.
Octanol/water partition coefficient
log POW = 4.19 at 20 C
RD D274107, 7/12/01, S. Mathur
UV/visible absorption spectrum
At pH 7 λmax 1 = 273 nm, 3.22 log ε λmax 2 = 207 nm, 4.55 log ε At pH 2 λmax 1 = 276 nm, 3.24 log ε λmax 2 = 209 nm, 4.43 log ε At pH >10 λmax 1 = 272 nm, 3.19 log ε λmax 2 = 212 nm, 4.99 log ε
RD D274107, 7/12/01, S. Mathur

Permethrin Human Health Risk Assessment DP No. 414137
Page 86 of 129
Appendix C. International Residue Limit Status Sheet. Table C.1. Summary of US and International Tolerances and Maximum Residue Limits. Residue Definition: US Canada Mexico Codex
40 CFR §180.378:
Plant/Livestock: Combined residues of the insecticide cis- and trans-permethrin isomers [cis-(3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane carboxylate] and [trans-(3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane carboxylate]
Plants/Livestock/Dairy commodities: (3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane carboxylate (For livestock/ dairy commodities calculated on the fat content)
Permethrin (sum of isomers) (fat- soluble)
Commodity1 Tolerance (ppm) /Maximum Residue Limit (mg/kg)
Established US
HED-Recommended
Canada Mexico Codex
40 CFR §180.378 (a) General Alfalfa, forage
20 20 100 Alfalfa fodder (dry
wt) Alfalfa, hay 45 45 Almond 0.05 0.1 0.1 Almond, hulls 20 20 Artichoke, globe 5.0 5.0 Arugula2 20 (as part
of 4A) 50
Asparagus2 2.0 2.0 1 Avocado 1.0 1.0 Broccoli 2.0 2.0 0.5 2 Brussels sprouts 1.0 1.0 0.5 1 Cabbage1
6.0 8.0 0.5
5 Cabbage, Savoy,
cabbage head,
Chinese cabbage
(type pe-tsai) Cattle, fat 1.5 1.5 0.1 Cattle, meat 0.10 0.10 0.1 Cattle, meat byproducts
0.10 0.10 0.1 0.1 3 Edible
offal (mammalian)
Cauliflower 0.5 0.5 0.5 Celtuce2 5.0 (as part
of 4B) 5.0
Cherry, sweet 4.0 4.0 2 Cherry, tart 4.0 4.0 2 Corn, field, forage 50 50 Corn, field, grain
0.05 0.05 0.05 2 Cereal
grains (Po5)

Permethrin Human Health Risk Assessment DP No. 414137
Page 87 of 129
Table C.1. Summary of US and International Tolerances and Maximum Residue Limits. Corn, field, stover
30 30 100 Maize
fodder (dry) dry wt
Corn, pop, grain 0.05 0.05
2 Cereal grains (Po)
Corn, pop, stover 30 30 Corn, sweet, forage 50 50 Corn, sweet, kernel plus cob with husks removed 0.10 0.10 0.1
0.1 Sweet corn (corn-on-the-cob)
Corn, sweet, stover 30 30 Cress, garden2 20 (as part
of 4A) 50
Cress, upland2 20 (as part of 4A)
50
Egg 0.10 0.10 0.1 Eggplant 0.50 1.0 1 Florence fennel2 5.0 (as part
of 4B) 5.0
Fruit, pome, group 11
0.05 -- 1.0 Apples, pears
50 Apple pomace,
Dry; 2 Pome fruits
Fruit, pome, group 11-10 -- 0.05 Garlic, bulb 0.10 0.10 Grain, aspirated fractions 0.50 0.50 Goat, fat 1.5 1.5 Goat, meat 0.10 0.10 Goat, meat byproducts
0.10 0.10 0.1 3 Edible
offal (mammalian)
Hazelnut 0.05 0.05 Hog, fat 0.05 0.05 Hog, meat 0.05 0.05 Hog, meat byproducts
0.05 0.1 0.1 Edible
offal (mammalian)
Horse, fat 1.5 1.5 Horse, meat 0.10 0.10 0.1 Horse, meat byproducts
0.10 0.10 0.1 0.1 Edible
offal (mammalian)
Horseradish 0.50 0.50 0.5 Kiwifruit 2.0 -- 2 Leaf petioles subgroup 4B 5.0 -- 5.0 Celery 2 Celery Leaf petiole subgroup 22B2 -- 5.0 Leafy greens subgroup 4A 20 -- 20 Leaf lettuce Leafy greens subgroup 4-16A -- 50 20 Leaf lettuce Lettuce, head 20 -- 10 2 Milk, fat (reflecting 0.88 ppm in whole milk)
3.0 -- 0.2 Milk; 0.2 Other
dairy products 0.1 F5
Milk -- 0.90 Milk, fat -- 3.0

Permethrin Human Health Risk Assessment DP No. 414137
Page 88 of 129
Table C.1. Summary of US and International Tolerances and Maximum Residue Limits. Mushroom 5.0 5.0 0.1 Onion, bulb 0.10 0.10 0.1 Peach
1.0 1.0 1.0
Peaches/Nectarines
2 (Stone Fruits)
Pepper, bell
0.50 0.50 0.5 Peppers
1 Peppers (bell and
non-bell); 10 Peppers
Chili, dried Pistachio
0.10 0.10 0.05
Pistachio nuts
Potato 0.05 0.05 0.05 0.05 Poultry, fat 0.15 0.15 0.1 Poultry, meat 0.05 0.1 0.1 0.1 Poultry, meat byproducts 0.05 0.1 0.1 Sheep, fat 1.5 1.5 0.1 Sheep, meat 0.10 0.10 0.1 Sheep, meat byproducts
0.10 0.10 0.1 0.1 Edible
offal (mammalian)
Soybean, seed
0.05 0.05
0.05 Soya bean (dry);
50 Soya bean fodder (dry
wt); 0.1 Soya bean oil,
Crude Spinach 20 -- 20 2 Tomato 2.0 2.0 0.5 1 Vegetable, cucurbit, group 9
1.5 1.5 0.5 Cucumbers
0.5 Cucumber, Gherkin, Squash
summer, Winter squash,
pumpkin; 0.1 Melons, except
watermelon Walnut 0.05 0.05 Watercress 5.0 5.0
40 CFR §180.378 (c) Tolerances with regional registrations Collards1 15 30 5 Grass, forage1 15 3.0 Grass, hay1 15 10 Papaya 1.0 1.0 Turnip, tops 10 10 Turnip, roots 0.20 0.20
MRLs with no US equivalent Beans 0.5
0.1 Beans
(dry)

Permethrin Human Health Risk Assessment DP No. 414137
Page 89 of 129
Table C.1. Summary of US and International Tolerances and Maximum Residue Limits. Blackberries 1 Carrot 0.1 Cereal Grains 2 Citrus fruits 0.5 Coffee beans 0.05 Common bean (pods and/or immature seeds)
1
Cotton seed 0.5 Cotton seed oil, Edible 0.1 Currants, Black, Red, White 2 Dewberries (including boysenberry and loganberry)
1
Gooseberry 2 Grapes 2.0 2.0 Hops, Dry 50 Kale (including among others:Collards, Curly kale, Scotch kale, thousand-headed kale; not including Marrow-stem
5
Kohlrabi 0.1 Leek 0.5 Meat (from mammals other than marine mammals)
1 (fat) 3
Olives 1 Peanut 0.1 Peas, Shelled (succulent seeds) 0.1 Plums 0.5 2 Radish, Japanese 0.1 Rape seed 0.05 Raspberries, Red, Black 1 Sorghum straw and fodder, Dry 20 Spring Onion 0.5 Stone fruits 2 Strawberry 1 Sugar beet 0.05 Sunflower seed 1 Sunflower seed oil, crude 1 Sunflower seed oil, edible 1 Tea, Green, Black (black, fermented and dried)
20
Wasabi 0.5 Wheat bran, Unprocessed 5 Wheat flour 0.5 Wheat germ 2 Wheat whole meal 2 Completed using Global MRL. 05-MAY-2017
*Commodities with different tolerance levels between U.S. Canada, Mexico, and Codex are bolded. Mexico adopts US tolerances and/or Codex MRLs for its export purposes.
1 HED is recommending for the revision of these tolerance levels based on the field trial data submitted in response to the RED (Memo, J. Van Alstine, 23-JUN-2017; D440981).
2 The Phase IV crop group revision significantly re-structured the existing Crop Groups 4 and 5 to create new Crop Groups 4-16, 5-16 and Subgroup 22B. Crop Group 4-16 has the same representative commodities as Crop

Permethrin Human Health Risk Assessment DP No. 414137
Page 90 of 129
Subgroups 4A (lettuce and spinach) and 5B (mustard greens); Crop Group 5-16 has the same representative commodities as Crop Subgroup 5A (cabbage, broccoli and cauliflower); and Crop Group 22 has the same representative commodities as Crop Subgroup 4B (celery) and a new representative commodity, asparagus.
Several commodities in Crop Groups 4 and 5 are better represented by a different commodity or commodities. As a result, Swiss chard (Subgroup 4B member represented by celery) moved to Subgroup 4-16A (represented by lettuces and spinach). Arugula, garden cress and upland cress (Subgroup 4A members represented by lettuces and spinach), as well as Chinese broccoli (Subgroup 5A member represented by cabbage, and broccoli or cauliflower) moved to Subgroup 4-16B (represented by mustard greens). Celtuce and Florence fennel (Subgroup 4B members represented by celery), as well as kohlrabi (Subgroup 5A member represented by cabbage, and broccoli or cauliflower) moved to Subgroup 22A (represented by asparagus).
Tolerances are currently established for residues of permethrin in Leaf petioles subgroup 4B at 5.0 ppm. As part of the crop group revisions, the commodities in crop group 4B are being moved to crop groups 4-16A, 22A, and 22B. A tolerance is being recommended for residues of permethrin in Leaf petiole subgroup 22B commodities. The commodities of celtuce and Florence Fennel (which are commodities that are included in crop subgroup 4B) are being moved to stalk and stem vegetable subgroup 22A (representative commodity asparagus). Although a tolerance is currently established for residues of permethrin in asparagus at 2.0 ppm, a tolerance for residues in stalk and stem vegetable subgroup 22A is not being recommended since the celtuce and Florence fennel application rates are higher than the asparagus application rate. HED is recommending for individual tolerances of 5.0 ppm for celtuce and Florence fennel based on the currently established tolerance for these commodities as part of crop group 4B.
Tolerances are currently established for residues in Leafy greens subgroup 4A at 20 ppm. A tolerance of 50 ppm is being recommended for residues in Leafy greens subgroup 4-16A commodities as part of the crop group conversion. The increased tolerance level is due to data that were received in response to the data requests in the permethrin data call-in (GDCI-109701-26467; see Memo, J. Van Alstine, 23-JUN-2017; D440981). Additionally, as part of the crop group conversion, arugula, garden cress, and upland cress have moved to crop group 4-16B. HED is recommending for individual tolerances for residues in these commodities at 50 ppm to ensure that previously-established tolerances associated with phase four revisions are not inadvertently lost during crop group conversion requests.
3 The MRL accommodates external animal treatment. 4 Po = postharvest treatment, such as treatment of stored grains. 5 F = measured in the milk fat.

Permethrin Human Health Risk Assessment DP No. 414137
Page 91 of 129
Appendix D. Submission of Analytical Standards Analytical reference standards are available for permethrin (expires 31-JUL-2019); however, standards are currently unavailable for cis-permethrin and trans-permethrin in EPA’s National Pesticide Standards Repository (NPSR), per email communication with G. Verdin of BEAD/ACB (18-APR-2017). The registrant is required to maintain reasonable amounts of the reference standards in the NPSR as long as tolerances remain published in 40CFR §180.378. When necessary, new reference standards, or updated certificates of analysis (COAs), should be sent to the ACB, which is located at Fort Meade, MD. It should be sent to the attention of either Theresa Cole or Thuy Nguyen at the address listed below, along with a letter of transmittal. Please note that the full 9-digit ZIP Code is required, or the mail will be returned to the registrant. USEPA National Pesticide Standards Repository Analytical Chemistry Branch/BEAD/OPP 701 Mapes Road Fort George G. Meade, MD 20755-5350 The letter of transmittal should include the assay of the standard, name of the analytical method used, a statement of principal impurities, purification procedures employed, storage requirements, and special precautions for safe handling. Replacement of standards, or updated certificates of analysis (COAs), may be required periodically if supplies are exhausted, if the standards expire, or if decomposition occurs during storage. Material Safety Data Sheets (MSDSs) must accompany all analytical standards as specified by the Occupational Safety and Health Administration (OSHA) in 29CFR §1910.1200.

Permethrin Human Health Risk Assessment DP No. 414137
Page 92 of 129
Appendix E. Summary of RED Data Requirements and Data Reviewed as Part of Registration Review.
Table E.1 Summary of RED Data Requirements and Data Reviewed as Part of Registration Review. RED Data Requirements Registration Review Comments
Commodity Data Requested in RED1 Additional
Data Needed?
MRID Comments/Conclusions
Field Trial Data
Cabbage
Due to the limited number of adequate field trials on cabbage at the current use rate and the substantial difference in residues observed in samples from the 0.1 and 0.2 lb ai/A/application rates, additional cabbage field trial data are required. A total of 8 new field trials should be conducted according to the current use directions using an EC formulation. Residue decline data are not required, and samples may be analyzed only for residues of permethrin.
Yes; Confirmati
on that cabbage samples included wrapper leaves.
48598601
The submitted cabbage field trial data are adequate, provided the registrant confirms that samples consisted of cabbage heads with wrapper leaves. An acceptable method was used for residue quantitation, an adequate number of field trials with geographical representation were submitted, and adequate data were submitted to support sample storage intervals and conditions. This deficiency is resolved.2
Collards
Confirmatory data are required from 1 test conducted in Region 6 depicting residues of permethrin in/on collards harvested 1 day following the last of eight foliar applications of permethrin (EC).
No 48639202
The submitted collards field trial data are adequate to support a regional registration. An acceptable method was used for residue quantitation, an adequate number of field trials with geographical representation are available to support a regional registration, and adequate data are available to support sample storage intervals and conditions. This is deficiency is resolved.2
Grasses (Rangeland)
Data are required depicting residues of permethrin in/on grass forage harvested immediately (0 day) following application of permethrin (EC) at 0.01 lb ai/A to rangeland. As the registrants are restricting this use to rangeland only in NM, a total of three tests conducted at 1x are required in NM at different locations with at least two samples per site. Alternatively, the registrants may conduct tests at two separate locations with each test site having 1x and 2x plots.
No 48598603
The submitted rangeland grass field trial data are adequate. An acceptable method was used for residue quantitation, an adequate number of field trials with geographical representation are available to support a regional registration, and adequate data were submitted to support sample storage intervals and conditions. This deficiency is resolved.2

Permethrin Human Health Risk Assessment DP No. 414137
Page 93 of 129
Table E.1 Summary of RED Data Requirements and Data Reviewed as Part of Registration Review. RED Data Requirements Registration Review Comments
Commodity Data Requested in RED1 Additional
Data Needed?
MRID Comments/Conclusions
Leaf Lettuce
A total of 8 new field trials should be conducted on leaf lettuce according to the current use directions using 10 foliar applications of permethrin (EC) at 0.2 lb ai/A/application (2 lb ai/A/season).
No 48598602
The submitted leaf lettuce field trial data are adequate. An acceptable method was used for residue quantitation, an adequate number of field trials with geographical representation were submitted, and adequate data were submitted to support sample storage intervals and conditions. This deficiency is resolved.2
Tomatoes
The registrants must either amend label directions for tomatoes to specify the supported use rate (12 applications at 0.1 lb ai/A, with at 0-day PHI), or submit a new set of tomato field trials (16 tests) to support the current use rate of 6 applications at 0.2 lb ai/A with a 0-day PHI. To support use on smaller tomato cultivars and tomatillos, at least 3 of the 16 requested field trials should be conducted on cherry type tomatoes.
No 48598604; 48639201
The submitted tomato field trial data are adequate. Although only 14 out of the 16 requested field trials were submitted, additional data are not required at this time. An acceptable method was used for residue quantitation, an adequate number of field trials with geographical representation were submitted, and adequate data are available to support sample storage intervals and conditions. This is deficiency is resolved. Tomato processing data were previously submitted and reviewed, and separate tolerances are not required for processed tomato commodities.2
Sweet Corn (FL only)
The higher use rate allowed on sweet corn grown in FL (0.25 lb ai/A/application; 2.0 lb ai/A/season) is not supported by the available residue data. This use should either be deleted from the EP labels, or the registrants should provide data from at least two field trials in FL supporting the higher application rate to sweet corn.
No --
The current labels have application rates of 0.2 lb ai/A for sweet corn that is supported by available residue data; therefore, additional data are not required at this time.2
Soybeans
Adequate data are available on soybeans provided the registrants amend use directions for soybeans to specify a minimum volume of 2 gal/A for aerial applications; otherwise, residue data supporting ULV applications to soybeans are required.
No --
The current labels indicate that a minimum volume of 2 gal/A is needed for aerial applications to soybeans; therefore, additional data are not required at this time.2
Food Handling Establishments
Labels for EPs registered to the basic producers prohibit applications in food areas or to service areas should be amended to "prohibit applications in food areas or to service areas while food is present."
No --
The current labels reflect the following restrictions for applications to food/feed handling and service areas: “Do not apply when food is present.” “Do not use in food areas of food handling establishments,

Permethrin Human Health Risk Assessment DP No. 414137
Page 94 of 129
Table E.1 Summary of RED Data Requirements and Data Reviewed as Part of Registration Review. RED Data Requirements Registration Review Comments
Commodity Data Requested in RED1 Additional
Data Needed?
MRID Comments/Conclusions
Confirmatory food residue data are still required at the present time to ensure no detectable residues in food when permethrin is applied in residential areas while food is present. The registrant should submit a protocol on the magnitude of the residue in various food products when permethrin is applied at maximum application rates to areas containing uncovered and covered food products.
restaurants, or other areas where food is commercially prepared or processed. Do not use in serving areas while food is exposed or facility is in operation. Serving areas are areas where prepared foods are served, such as dining rooms, but excluding areas where foods may be prepared or held. In the home, all food processing surfaces and utensils should be covered during treatment or thoroughly washed before use. Exposed food should be covered or removed.” Additional data are not needed at this time since the labels have been modified.2
Storage Stability Data
Mushroom Data are required depicting the frozen storage stability of permethrin in mushrooms stored for intervals of up to 1 year
No 48598606
The storage stability study with mushroom is considered scientifically acceptable. The data demonstrate that residues of cis- and trans-permethrin are stable during frozen storage (0 °C) for up to 12 months (374 days) in/on mushroom. This deficiency has been resolved.2
Tomato Additional information on sample storage intervals to upgrade the existing residue data on tomato.
No 48054603 The submitted data regarding the sample storage conditions/intervals for the tomato field trial study is adequate. This deficiency has been resolved.3
Pear Additional information on sample storage intervals to upgrade the existing residue data on pear.
No 48054602 The submitted data regarding the sample storage conditions/intervals for the pear field trial study is adequate. This deficiency has been resolved.3
Livestock Commodities
Data are required depicting the frozen storage stability of permethrin in representative livestock commodities stored for intervals of up to 1 year.
No 48598605
The storage stability study with livestock matrices (milk, cattle fat, egg, and poultry liver) is considered scientifically acceptable. The data demonstrate that residues of cis- and trans-permethrin are stable during frozen storage (0 °C) for up to 12 months (368-375 days) in milk, cattle fat and poultry liver, and during refrigerated storage (10 °C) in egg. This deficiency has been resolved.2
Analytical Methodology Data

Permethrin Human Health Risk Assessment DP No. 414137
Page 95 of 129
Table E.1 Summary of RED Data Requirements and Data Reviewed as Part of Registration Review. RED Data Requirements Registration Review Comments
Commodity Data Requested in RED1 Additional
Data Needed?
MRID Comments/Conclusions
Peach Additional information on the residue analytical method for peach are required.
No 48097701
The submitted addendum to the original field trial study report provides the additional analytical details that were required. HED concludes that methods GRAM ½ and GRAM -1/I are sufficiently similar in scope and practice. This deficiency has been resolved.3
1 Summarized from Memo, S. Kinard, 17-MAR-2005; D313662. 2 Memo, J. Van Alstine, 23-JUN-2017; D440981. 3 Memo, D. Wilbur, 26-JAN-2011; D382837.

Permethrin Human Health Risk Assessment DP No. 414137
Page 96 of 129
Appendix F. Permethrin Use Pattern Tables
Table F.1. Summary of Directions for Food Uses of Permethrin.
Crop/Use Site Application Type and
Equipment Formulation
Maximum Application Rate
Max # App. per Season
Max. Seasonal
Application Rate
Use Directions1
Agricultural Crops
Alfalfa Aerial, chemigation, groundboom,
tractor drawn spreader
EC, WP 0.20 lb ai/A 1 per
cutting 0.20 lb ai per
cutting RTI = 30 days DF 0.05 lb ai/A
RTU (ULV) 0.007 lb ai/A
Almond Aerial, airblast, backpack, chemigation, groundboom,
mechanically pressurized handgun
EC 0.0033 lbs ai/gal
0.0002 lbs ai/12 fl oz 0.25 lb ai/A 0.75 lb ai/A RTI = 10 days
G 0.25 lb ai/A RTU (ULV) 0.007 lbs ai/A
Amaranth, Chinese Aerial, chemigation, groundboom
EC 0.20 lb ai/A
L 0.0036 lbs ai/gal
RTU (ULV) 0.142 lb ai/A WP 0.20 lb ai/A
Apple Aerial, airblast, backpack, chemigation, groundboom,
mechanically pressurized handgun
EC 0.0033 lbs ai/gal
0.25 lb ai/A 0.0002 lbs ai/12 floz
0.5 lb ai/A RTI = 10 days
RTU (ULV) 0.007 lb ai/A
WDG, WP 0.25 lb ai/A
Artichoke Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader
EC, WDG, WP 0.30 lb ai/A 0.9 lb ai/A RTI = 10 days
RTU (ULV) 0.007 lb ai/A
Asparagus Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader
EC 0.10 lb ai/A
0.0027 lbs ai/gal 0.40 lb ai/A
RTI = 7 days RTU (ULV) 0.007 lb ai/A
WDG, WP 0.10 lb ai/A
Avocado Aerial, airblast, backpack, chemigation, groundboom,
mechanically pressurized handgun
EC, WDG, WP 0.2 lb ai/A 6 (RED) 0.80 lb ai/A RTI = 7 days
RTU (ULV) 0.007 lb ai/A
Blueberry Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader EC
0.20 lb ai/A 0.0036 lbs ai/gal
Broccoli (including chinese) EC, WDG, WP 0.20 lb ai/A
0.0018 lbs ai/ gal 5 (RED) 0.80 lb ai/A RTI = 5 days

Permethrin Human Health Risk Assessment DP No. 414137
Page 97 of 129
Table F.1. Summary of Directions for Food Uses of Permethrin.
Crop/Use Site Application Type and
Equipment Formulation
Maximum Application Rate
Max # App. per Season
Max. Seasonal
Application Rate
Use Directions1
Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader RTU (ULV) 0.007 lb ai/A
Brussel sprouts Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader
EC, WDG, WP 0.10 lb ai/A
0.0018 lbs ai/gal 4 (RED) 0.40 lb ai/A RTI = 5 days RTU (ULV) 0.007 lb ai/A
Cabbage (including Chinese) Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader
EC, WDG, WP 0.20 lb ai/A
0.0018 lbs ai/gal 2 (4 in HI)
0.40 lb ai/A (0.80 in HI)
RTI = 5 days RTU (ULV) 0.007 lb ai/A
Cantaloupe Aerial, groundboom EC, WP 0.20 lb ai/A 4
(6 in HI) 0.8
(1.20 in HI) RTI = 7 days
Cardoon Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader EC, WDG, WP 0.20 lb ai/A
Cauliflower Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader
EC, WDG, WP 0.1 lb ai/A
0.0018 lbs ai/gal 4 (6 in HI)
0.40 (0.60 in HI)
RTI = 5 days RTU (ULV) 0.007 lb ai/A
Celery Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader
EC, WDG, WP 0.20 lb ai/A
0.0036 lbs ai/gal 5 (6 in HI)
1.0 (1.2 in HI)
RTI = 7 days RTU (ULV) 0.007 lb ai/A
Celtuce, Swiss Chard, Chervil, Cress (Garden, Upland, Water),
Dandelion, Dock (sorrel), Fennel, Leafy Vegetables, Okra, Okra (Chinese), Parsley, Purslane (Garden, Winter), Rhubarb, Roquette (arugula), Spinach,
Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader
EC, WDG, WP 0.20 lb ai/A
0.0036 lbs ai/gal
RTU (ULV) 0.007 lb ai/A
Chayote, Chicory, Radicchio, Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader EC, WDG, WP 0.20 lb ai/A
Cherries: sour & sweet Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader
EC, WDG, WP 0.20 lb ai/A 0.6 lb ai/A RTI = 10 days
RTU (ULV) 0.007 lb ai/A
Cole Crops Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader
EC 0.15 lb ai/A
0.0018 lbs ai/gal RTU (ULV) 0.007 lb ai/A

Permethrin Human Health Risk Assessment DP No. 414137
Page 98 of 129
Table F.1. Summary of Directions for Food Uses of Permethrin.
Crop/Use Site Application Type and
Equipment Formulation
Maximum Application Rate
Max # App. per Season
Max. Seasonal
Application Rate
Use Directions1
Collards Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader EC, WDG, WP 0.15 lb ai/A 0.45 lb ai/A RTI = 3 days
Corn (field, popcorn, seed)
Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader, truck mounted fogger, backpack fogger
EC 0.15 lb ai/A
0.000015 lbs ai/linear ft
0.45 lb ai/A RTI = 7 days G
0.15 lb ai/A 0.000011 lbs ai/linear ft
RTU (ULV) 0.007 lb ai/A
WDG, WP 0.15 lb ai/A
0.000012 lb ai/linear ft
Sweet Corn (sweet: fresh & processed, unspecified)
Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader, truck mounted fogger, backpack fogger
EC, WDG, WP 0.20 lb ai/A
0.0027 lbs ai/gal 0.8 lb ai/A RTI = 3 days
G 0.20 lb ai/A
0.000015 lbs ai/linear ft RTU (ULV) 0.007 lb ai/A
Corn Salad (mache)
Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader, truck mounted fogger, backpack fogger
EC, WDG, WP 0.20 lb ai/A
0.0036 lbs ai/gal
Cucumbers
Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader, truck mounted fogger, backpack fogger
EC, WDG, WP 0.20 lb ai/A
0.0036 lbs ai/gal 1.2 lb ai/A RTI = 7 days RTU (ULV) 0.007 lb ai/A
Cucurbit Vegetables
Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader, truck mounted fogger, backpack fogger
EC, WDG, WP 0.20 lb ai/A
0.0036 lbs ai/gal
RTU (ULV) 0.007 lb ai/A
Eggplant
Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader, truck mounted fogger, backpack fogger
EC, WDG, WP 0.15 lb ai/A
0.0055 lbs ai/gal
0.6 lb ai/A (1.0 lb ai/A in
HI) RTI = 7 days
RTU (ULV) 0.007 lb ai/A
Endive (Escarole)
Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader, truck mounted fogger, backpack fogger
EC, WDG, WP 0.20 lb ai/A
0.0036 lbs ai/gal
RTU (ULV) 0.007 lb ai/A
Garlic Aerial, chemigation, groundboom, mechanically pressurized handgun,
EC, WDG, WP 0.2 lb ai/A 0.8 lb ai/A RTI = 10 days
RTU (ULV) 0.007 lb ai/A

Permethrin Human Health Risk Assessment DP No. 414137
Page 99 of 129
Table F.1. Summary of Directions for Food Uses of Permethrin.
Crop/Use Site Application Type and
Equipment Formulation
Maximum Application Rate
Max # App. per Season
Max. Seasonal
Application Rate
Use Directions1
tractor drawn spreader, truck mounted fogger, backpack fogger
Gherkin
Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader, truck mounted fogger, backpack fogger
EC, WDG, WP 0.20 lb ai/A
0.0036 lb/gal
Hazelnuts (Filberts)
Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader, truck mounted fogger, backpack fogger
EC, WDG, WP 0.25 lb ai/A 0.75 lb ai/A RTI = 10 days
RTU (ULV) 0.007 lb ai/A
Horseradish
Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader, truck mounted fogger, backpack fogger
EC, WDG, WP 0.15 lb ai/A
0.45 lb ai/A RTI = 10 days RTU (ULV) 0.007 lb ai/A
Kiwi Fruit Aerial, truck mounted fogger, non-
thermal backpack fogger RTU (ULV) 0.007 lb ai/A
Lettuce
Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader, truck mounted fogger, backpack fogger
EC, WDG, WP 0.20 lb ai/A
0.0036 lbs ai/gal
0.8 lb ai/A (1.2 lb ai/A in
HI) RTI = 7 days
RTU (ULV) 0.007 lb ai/A
Melons (Bitter, Cantaloupe, Citron, Honeydew, Mango,
Musk, Water, Winter)
Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader EC, WDG, WP
0.2 lb ai/A 0.0036 lbs ai/gal
1.2 lb ai/A RTI = 7 days
Mushrooms Aerial, truck mounted fogger, non-
thermal backpack fogger EC, RTU
(ULV) 0.007 lb ai/A
Nectarines Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader
EC, WDG, WP (EC-ULV)
0.25 lb ai/A 0.75 lbs ai/A
Onions Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader
EC, WDG, WP 0.30 lb ai/A 1.0 lb ai/A RTI = 7 days
RTU (ULV) 0.007 lb ai/A
Papaya
Aerial, airblast, chemigation, groundboom, mechanically
pressurized handgun, tractor drawn spreader
EC, WDG, WP 0.15 lb ai/A 0.75 lb ai/A RTI = 10 days
Peaches Aerial, airblast, chemigation, groundboom, mechanically
EC, WDG, WP 0.25 lb ai/A
0.0033 lbs ai/gal 0.0002 lb/12 floz
0.75 lb ai/A RTI = 10 days

Permethrin Human Health Risk Assessment DP No. 414137
Page 100 of 129
Table F.1. Summary of Directions for Food Uses of Permethrin.
Crop/Use Site Application Type and
Equipment Formulation
Maximum Application Rate
Max # App. per Season
Max. Seasonal
Application Rate
Use Directions1
pressurized handgun, tractor drawn spreader
RTU (ULV) 0.007 lb ai/A
Pears: dormant & prebloom (combination)
Aerial, airblast, chemigation, groundboom, mechanically
pressurized handgun, tractor drawn spreader
EC, WDG, WP
0.25 lb ai/A (0.4 lb ai/A dormant
only) 0.0033 lbs ai/gal 0.0002 lb/12 fl oz
0.65 lb ai/A RTI = 10 days
RTU (ULV) 0.007 lb ai/A
Pecan
Aerial, airblast, chemigation, groundboom, mechanically
pressurized handgun, tractor drawn spreader
EC 0.20 lb ai/A
0.0033 lbs ai/gallon
Peppers, bell
Aerial, airblast, chemigation, groundboom, mechanically
pressurized handgun, tractor drawn spreader
EC, WDG, WP 0.20 lb ai/A
0.8 lb ai/A RTI = 5 days RTU (ULV) 0.007 lb ai/A
Pistachios
Aerial, airblast, chemigation, groundboom, mechanically
pressurized handgun, tractor drawn spreader
EC, WDG, WP 0.3 lb ai/A 0.9 lb ai/A RTI = 10 days
RTU (ULV) 0.007 lb ai/A
Potatoes Aerial , chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader
EC, WDG, WP 0.2 lb ai/A 0.8 lb ai/A RTI = 10 days
RTU (ULV) 0.007 lb ai/A
Pumpkins Aerial, chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader EC, WDG, WP
0.2 lb ai/A 0.0033 lbs ai/gal
1.2 lb ai/A RTI = 7 days
Rangeland Aerial, groundboom, mechanically pressurized handgun, tractor drawn
spreader
EC, WDG, WP 0.1 lb ai/A
RTU (ULV) 0.007 lb ai/A
Raspberry (Black, Red) Aerial, groundboom, mechanically
pressurized handgun EC
0.20 lb ai/A 0.0036 lbs ai/gal
Soybeans Aerial , chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader
EC, WDG, WP 0.2 lb ai/A 0.4 lb ai/A RTI = 10 days
RTU (ULV) 0.007 lb ai/A
Spinach, Orach (Mountain Spinach), spinach (New Zealand),
Aerial , chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader
EC, WDG, WP 0.2 lb ai/A 0.6 lb ai/A RTI = 3 days
RTU (ULV) 0.007 lb ai/A

Permethrin Human Health Risk Assessment DP No. 414137
Page 101 of 129
Table F.1. Summary of Directions for Food Uses of Permethrin.
Crop/Use Site Application Type and
Equipment Formulation
Maximum Application Rate
Max # App. per Season
Max. Seasonal
Application Rate
Use Directions1
Squash (summer, winter, spaghetti, butternut)
Aerial , chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader EC, WDG, WP
0.2 lb ai/A 0.0036 lbs ai/gal
1.2 lb ai/A RTI = 7 days
Strawberry Aerial , chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader EC
0.20 lb ai/A 0.0036 lb/gal
Tomatoes, Tomatillo Aerial , chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader
EC, WDG, WP 0.2 lb ai/A
0.0036 lb /gal
0.6 lb ai/A (0.8 lb ai/A in
HI) RTI = 7 days
RTU (ULV) 0.007 lb ai/A
Turnip (greens and roots) Aerial , chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader WDG, WP, EC 0.15 lb ai/A 0.45 lb ai/A RTI = 3 days
Walnuts Aerial , chemigation, groundboom, mechanically pressurized handgun,
tractor drawn spreader
EC, WDG, WP 0.25 lb ai/A 0.75 lb ai/A RTI = 10 days
RTU (ULV) 0.007 lb ai/A
Dry Bulk Fertilizer (Representative label: Permethrin EPA Reg. No.34704-873) Alfalfa, almonds, apples,
artichoke, asparagus, avocado, broccoli, Brussel sprouts,
cauliflower, cabbage, cantaloupes, celery, leafy
vegetables, cherries, collards, conifers, corn (field, pop, sweet,
seed), cucurbits, eggplants, filberts, horseradish, mushroom
(houses), onions, garlic, ornamentals, papaya, peaches, nectarines, pears, peppers, pine
seed orchards, pistachios, potatoes, pumpkins, range grass, soybeans, spinach, tomatoes, and
walnuts
Impregnation EC/L 0.3 lb ai/A
3.0 lb ai/ton
The listed crops corresponds to the crops listed on the agricultural labels. Apply using a minimum of 200 lbs dry bulk fertilizer/acre and a maximum of 450 lbs dry bulk fertilizer/acre Do not impregnate onto straight coated ammonium nitrate or straight limestone.
Seed Treatment (Representative label: Kernel Guard®/Vitavax® EPA Reg. No. 400-560)

Permethrin Human Health Risk Assessment DP No. 414137
Page 102 of 129
Table F.1. Summary of Directions for Food Uses of Permethrin.
Crop/Use Site Application Type and
Equipment Formulation
Maximum Application Rate
Max # App. per Season
Max. Seasonal
Application Rate
Use Directions1
Corn (field, sweet, and pop)
scoop/tube RTU
0.156 oz ai/ 42 lb seed
For application to corn (field, sweet, and pop) and soybeans. Do not bag or store excess treated seed beyond planting time. Do not use or mix treated seed with food or animal feed or process for oil. Do not mix with bare hands. PGI = 6 weeks
Soybeans 0.156 oz ai/ 50 lb seed
Residential Food Uses
Residential Fruit and Nut Trees
almond, filberts, pistachios
manually pressurized handwand, backpack
EC 53883-78
0.0036 lb ai/gal handler [29, 33]2
5 Do not make more than 2 applications during hull split PHI = 7 days
pears 2-dormant 3-summer
PHI = 14 days
apples 3 Do not apply after petal fall
peaches 8 PHI = 7 days
Residential Gardens
asparagus, broccoli, brussel sprouts, cabbage, cauliflower,
celery, cherries, cucurbits, eggplant, horseradish, leafy
vegetables, melons, potatoes, peppers, spinach, sweet corn,
tomatoes
hose end sprayer RTU 0.20 lb ai/gal handler [10]2
[EPA Reg. Nos. 53883-134, 1021-2695]
manually pressurized handwand, backpack
EC 0.02 ai/gal
handler [29, 33]2
shaker can, dust gun, puffer, rotary duster, bulbous duster
D 0.20 lb ai/A handler [2]2
3-4 (4 in HI)
N/A
[EPA Reg. No. 1021-2724] RTI = 5-10 days PHI = 0-22 days Do not apply to tomatoes less than 1 inch in diameter Do not use a power duster
1 REI: 12 hours on all occupational use crops, (24 hours for EC formulations on EPA Reg. No. 53883-72) 2 The number in brackets after “handler” indicate the exposure scenario each residential handler use was assessed under in Table 5.1.1. PPE- For liquids: All mixers, loaders, applicators, and other handlers must wear: baseline, gloves except for applicators using motorized ground equipment, pilots, and flaggers, chemical resistant aprons for mixer/loaders, cleaning equipment, and persons exposed to the concentrate and for handlers performing animal dip applications For granular: All mixers, loaders, applicators, and other handlers must wear: baseline, gloves except for applicators using motorized ground equipment, pilots, and flaggers, For Dust: All loaders, applicators, and other handlers must wear: baseline, gloves and a NIOSH respirator Application with aerial or motorized ground equipment is prohibited

Permethrin Human Health Risk Assessment DP No. 414137
Page 103 of 129
Table F.2. Non-Food and Non-Feed Use Patterns for Permethrin.
Crop/Use Site Application Type and
Equipment Formulation
Handler/Post-app Exposure Scenario(s)1 Maximum
Application Rate Use Directions
Residential2 Occupational Indoor uses2
Animal Premises
domestic animal premises [commercial and residential]
manually pressurized handwand liquid handler [28]
post-app handler 0.040 lb ai/gal
When used in dairy barns or facilities: Close milk bulk tank lids to prevent contamination. Indoor misting systems used in commercial barns, stables, and animal quarters: Not for use in outdoor residential misting systems
(indoor or outdoor). Do not apply this product in barns or stables where
animals intended for slaughter or human consumption will be maintained.
Do not apply when food, feed, or water is present. Do not apply directly to animals. When applying via a remote activation device, do not
apply when people and pets are present. If possible, when applying via automatic timer, set the timing for application when people and pets are unlikely to be present.
Direct nozzles to spray towards the target area and away from areas where people are typically present.
Do not use in an evaporative cooling system. Do not use in misters located within 3 feet of air vents,
air conditioner units, or windows. If used in a direct injection system, the pesticide
container must be locked. Securely attach the end use label to the pesticide container in a weather protected area or plastic sleeve. (These instructions not applicable to wettable powder products).
.
aerosol can PRL handler [3] post-app
handler 0.000538 lb ai/16 oz
can
kennels/sleeping quarters [commercial and residential]
manually pressurized handwand, backpack
liquid N/A handler
0.78 lb ai/1000 sq ft
non-thermal fogger EC 0.007 lb ai/acre
barns, dairies, feedlots, livestock buildings, poultry
houses, stables [commercial and residential]
manually pressurized handwand, backpack
EC, liquid N/A handler
0.04 lbs ai/gal 0.113 lb ai/1000 sq ft
compressed air-sprayer, non-thermal stationary fogger
[based on EPA Reg. No. 47000-103 as a representative label]
Initial cleanout 0.50 oz ai/1000 cu ft [0.031 lb ai/1000 cu
ft]
Normal infestations 0. 25 oz ai/1000 cu
ft [0.016 lb ai/1000 cu
ft]
Animals
treated pets (dogs and cats)
dip EC handler [21]
post-app 0.006 lb ai/gal
Do not use spot-on applications on cats. Use of handheld power duster equipment is prohibited.
spot treatments (tube) (not for use on cats)
RTU (1 to 5 cc applicator
tube)
handler [26] post-app
0.006 lb ai/animal
pour-on, trigger spray bottle EC handler [23]
post-app 0.007 lb ai/animal

Permethrin Human Health Risk Assessment DP No. 414137
Page 104 of 129
Table F.2. Non-Food and Non-Feed Use Patterns for Permethrin.
Crop/Use Site Application Type and
Equipment Formulation
Handler/Post-app Exposure Scenario(s)1 Maximum
Application Rate Use Directions
Residential2 Occupational [0.173 lb ai/gal
spray]
shaker can [EPA Reg. No(s) 1021-1749,
28296-126, 28296-352] D
handler [27] post-app
0.00016 lb ai/animal (0.0025 oz ai/animal) 70.85 mg ai/animal
>20 lbs 45.43 mg ai/animal
20 lbs
rubber gloves (hands)/shampoo RTU handler [25]
post-app 0.0014 lb ai/animal
aerosol/trigger spray bottle PRL handler [23,
24] post-app
0.000538 lb ai/16oz
can
dogs, horses body wipe (towelette/sponge) RTU handler [22]
post-app 0.0062 lb ai/animal
horses
dust bag, dust glove, shaker can D handler [27] 0.000031 lb
ai/animal
trigger spray bottle (body) RTU handler [23] 0.017 lb ai/animal
pour on, sponge L handler [22] 0.0062 lb ai/animal
livestock (beef cattle, dairy cattle,
goats, sheep)
ear tag RTU
N/A
0.0044 lb ai/animal
dust bag, shaker can, mechanical duster
D 0.000031 lb
ai/animal
pour-on (body) RTU 0.0017 lb ai/animal
manually pressurized handwand, backpack, dip
L 0.0023 lb ai/animal
poultry dust bag D 0.0025 lb ai/animal
high-pressure handwand L 0.00027 lb ai/animal
swine
shaker can, mechanical duster D 0.00016 lb ai/animal
manually pressurized handwand, backpack, dip
L 0.002 lb ai/animal
cup, spreader G 0.00156 lb ai/mound
Engines
vapor recovery systems tube RTU N/A 0.000189 lb ai/tube
Fabric

Permethrin Human Health Risk Assessment DP No. 414137
Page 105 of 129
Table F.2. Non-Food and Non-Feed Use Patterns for Permethrin.
Crop/Use Site Application Type and
Equipment Formulation
Handler/Post-app Exposure Scenario(s)1 Maximum
Application Rate Use Directions
Residential2 Occupational
personal clothing (shirt, pants, camping gear,
bed net, etc.) [residential]
aerosol, spray bottle RTU handler [4, 8]
post-app N/A
0.002 lb ai/shirt, pants & bed net
[0.0075 lb ai/24 oz bottle]
[0.5% ai/canister] From 2011 Table – Do not exceed an application rate equivalent to 1.25 grams of ai per square meter of fabric. All residential use liquid and RTU products labeled for surfaces must be formulated to no more than 0.5% ai.
military battle dress dip, handgun, manually pressurized
handwand, backpack, airblast L post-app N/A
0.00000011 lb ai/cm2 of fabric
personal/military clothing Impregnated
material post-app NA 0.125 mg ai/cm2
Human bedding/mattresses: [residential and commercial]
trigger spray bottle, manually pressurized handwand
L handler [28] post-app (HtM)
0.46 lb ai/1000 ft2
EC 0.036 lb ai/gal
Indoor Spaces
indoor residential fogger RTU post-app (HtM)
0.0023 lb ai/6 oz fogger
(each oz fogger treats 1000 ft3)
Do not use in aircraft cabins. Space spray or fog: Do not enter or allow others to enter until vapors, mists, and aerosols have dispersed and the treated area has been thoroughly ventilated. Total release foggers labeled for indoor use at residential sites must be formulated to contain no more than 0.58% permethrin. Note: If a higher concentration is proposed, the registrant must provide justification or data to demonstrate that an equivalent ISR of 5.6 ug.cm2 or less will result in a room of 2000 ft3 or less. For non-WPS use; stationary fogger- for 4 hours following applications, do not allow any persons to reenter treated areas. Total release fogger - Wait two (2) hours after application, then open windows, vents and doors for two hours. If an odor is still detected additional ventilation is required. Not for formulation into products for commercial indoor use applied with thermal or cold handheld foggers.
indoor commercial
fogger RTU
N/A
0.035 lb ai/oz fogger (each oz fogger treats
1000 ft3)
mechanical or compressed air equipment (non-thermal)
fogger
RTU 0.00036 lb ai/1000
cu ft
Indoor Surfaces

Permethrin Human Health Risk Assessment DP No. 414137
Page 106 of 129
Table F.2. Non-Food and Non-Feed Use Patterns for Permethrin.
Crop/Use Site Application Type and
Equipment Formulation
Handler/Post-app Exposure Scenario(s)1 Maximum
Application Rate Use Directions
Residential2 Occupational eating establishments (non-
food areas only), greenhouses, premises, refuse/solid waste sites,
storage, warehouse space, wood [commercial,
industrial and institutional]
crack and crevice, manually pressurized handwand, backpack
L
N/A
0.78 lb ai/1000 sq ft
0.037 lb ai/gal
Do not use in food areas of food handling establishments, restaurants, or other areas where food is commercially prepared or processed. Do not use in serving areas while food is exposed or facility is in operation. Serving areas are areas where prepared foods are served, such as dining rooms, but excluding areas where foods may be prepared or held. In the home, all food processing surfaces and utensils should be covered during treatment or thoroughly washed before use. Exposed food should be covered or removed. Do not apply when food is present. Do not apply as a broadcast treatment to indoor surfaces at residential sites, including nurseries, day care centers, schools, hospitals, and nursing homes. All residential use liquid and RTU products labeled for surfaces must be formulated to no more than 0.5% ai. Greater than 3% ai will be allowed when products are intended to be injected directly into crack and crevice if the registrant can provide justification will result in little to no exposure. Use of handheld power duster equipment is prohibited Do not enter or allow others to enter until sprays have dried and dusts have settled.
drainage systems [commercial, industrial,
institutional, and residential]
manually pressurized handwand, backpack
EC, L 0.46 lb ai/1000 sq ft
hospitals/medical institutions (human/veterinary)
crack and crevice EC 0.025 lb ai/gal
manually pressurized handwand, backpack
L 0.46 lb ai/1000 sq ft
households/domestic premises and contents
[commercial, industrial, institutional, and residential]
aerosol can, hand pressure sprayer [spot-on/perimeter treatment]
PRL handler [3, 7]
post-app
0.00438 lb ai/16oz can
trigger, pump, or other type of sprayer
[crack and crevice] RTU
handler [7] post-app
0.043 lb ai/gal
sprayer
hand trigger sprayer [spot-on/perimeter treatment]
EC handler [7,
28] post-app
0.042 lb ai/gal
dust bag, shaker can, mechanical duster
D handler [1] post-app
NA
0.01 lb ai/lb dust
1% ai (8oz product treats 100 sq ft)
mushroom house premises low-pressure handwand, fogger, EC, WP N/A 0.49 lb ai/A
0.267 lb ai/gal 0.0000018 lb ai/cu ft
Do not use high pressure handwands
Transportation
Vehicles [Automobiles, taxis,
limousines, recreational vehicles, and tires]
aerosol can, manually pressurized handwand
PRL handler 0.000189 lb ai/tube Do not use in aircraft cabins.
Military Aircraft (cabin, crew, and cargo
areas) EPA Reg. No. 88144-1
Aerosol Can (100g product, 2% permethrin)
RTU (PL) N/A handler
0.00441 lb ai/can where one can treats
285m3
Apply pre-flight – pre-embarkation. Do not spray directly on exposed food, food preparation areas or food utensils.

Permethrin Human Health Risk Assessment DP No. 414137
Page 107 of 129
Table F.2. Non-Food and Non-Feed Use Patterns for Permethrin.
Crop/Use Site Application Type and
Equipment Formulation
Handler/Post-app Exposure Scenario(s)1 Maximum
Application Rate Use Directions
Residential2 Occupational [35 g product/100m3]
Outdoor Uses3
Ants/Fire Ants
Ant mound [spot treatment]
manually pressurized handwand, backpack
EC, L handler [31,
34] 0.08 lb ai/mound
Do not water residential treated areas to the point of run-off. Do not make granular applications during rain.
cup, spreader G handler [11,
14] 0.00156 lb ai/mound
impregnated coasters & covers
RTU
handler
none stated impregnated gaskets for electrical wall plates, boxes, and plumbing
flanges N/A
Christmas Tree Farm
Christmas tree farm foliar
backpack, mechanically-pressurized handgun
DF, EC, L, WSP
N/A 0.2 lb ai/acre [DF,
EC, L] 0.02 lb ai/tree [EC]
Forest Trees
Conifer pine seed orchard: foliar
aerial, airblast, backpack, chemigation, groundboom,
manually pressurized handwand, mechanically pressurized handgun
EC, L
N/A
1.1 lb ai/acre 1.1 lb ai/100 gal
1.0 lb ai/tree
WDG, WP 1.6 lb ai/acre
1.6 lb ai/100 gal
Forest trees (excluding pine seed orchards): foliar
aerial, backpack EC, L 0.016 lbs ai/gal
0.6 lb ai/acre
Ground (low and high volume applications): Use 8-16 fuid oz of product/treated acre (0.2-0.4 lb ai/treated acre) using a final carrier solution of 25-400 gallons depending on the type of sprayer system being used. Make up to three applications per season. Air: Use 24 fluid oz of product/treated acre (0.6 lb ai/treated
acre). Apply in a minimum of 5 gallons of finished spray per acre. Apply once per season.
Public Health Uses/Wide Area Uses
outdoor/mosquitos
aerial, backpack, boom sprayer, mechanically pressurized
handwand, truck mounted ULV fogger
EC, L post-app 0.007 lb ai/acre Do not retreat site more than once in 3 days. Do not exceed 25 applications per season (Max seasonal application rate = 0.18 lb ai/acre)

Permethrin Human Health Risk Assessment DP No. 414137
Page 108 of 129
Table F.2. Non-Food and Non-Feed Use Patterns for Permethrin.
Crop/Use Site Application Type and
Equipment Formulation
Handler/Post-app Exposure Scenario(s)1 Maximum
Application Rate Use Directions
Residential2 Occupational outdoor barrier
spray/mosquitos backpack ULV fogger L (ULV) 0.007 lb ai/acre
Ornamentals
greenhouse [commercial and residential]
sprayer, sprinkler can EC handler [28] 0.0017 lb ai/gal
(0.0032 lb ai/mound)
greenhouse [commercial]
chemigation, mechanically pressurized handgun, groundboom
DF, WP N/A 0.2 lb ai/acre
indoor ornamentals [commercial, industrial,
institutional, and residential] manually pressurized handwand
L handler [29, 33]
0.017 lb ai/gal
EC 0.041 lb ai/gal
[1.03 lb ai/acre] indoor, outdoor
[commercial, industrial, institutional, and residential]
aerosol can PRL handler [3, 5] 0.0025 lb ai/16oz can
outdoor (trees, plants, shrubs, and vines)
[commercial, industrial, institutional, and residential]
manually pressurized handwand, backpack
L, EC handler [30, 32]
0.00078 lb ai/1000 sq
ft 0.20 lb ai/A
shaker can, mechanical duster D handler [2] 0.0025 lb ai/1 lb
container
Do not enter or allow others to enter the treated area until dusts have settled. If soil incorporation is required after the application, do not enter or allow others to enter the treated area (except those persons involved in the incorporation) until the incorporation is complete. If the incorporation is accomplished by watering-in, do not enter or allow others to enter the treated area until the surface is dry after the watering-in. Use of handheld power duster equipment is prohibited
trigger, pump, or other type of sprayer
RTU handler [9] 0.043 lb ai/gal
sprayer
Outdoor Spaces
commercial, industrial, institutional, and residential
aerosol RTU handler [6] post-app
0.007 lb ai/acre [0.225% ai/16oz
fogger]
Outdoor residential misting system use directions: Do not use in an evaporative cooling system. Direct nozzles to spray towards the target area and
away from swimming pools, water bodies, or eating and cooking areas.
Do not set nozzles to direct mist near outside air condition systems or other home air intakes.
If used in a direct injection system, the pesticide container must be locked. Securely attach the end use label to the pesticide container in a weather protected
automatic misting systems (including outdoor
residential misting systems) automatic misting system L post-app
0.25g ai/1000 ft3/day (0.00055 lb ai/1000
ft3/day)
[0.0023 lb ai/gal with 55 and 250 gal
drums]

Permethrin Human Health Risk Assessment DP No. 414137
Page 109 of 129
Table F.2. Non-Food and Non-Feed Use Patterns for Permethrin.
Crop/Use Site Application Type and
Equipment Formulation
Handler/Post-app Exposure Scenario(s)1 Maximum
Application Rate Use Directions
Residential2 Occupational area or plastic sleeve. (These instructions not applicable to wettable powder products).
If the system works on an automatic timer, set the timing for application when people, pets, and/or food are unlikely to be present.
If the system works when a person operates a remote activation device, then application of this pesticide when people, pets, and/or food are present is prohibited.
May only be used in systems that have been calibrated to apply no more than the maximum application rate of 0.25 grams per 1000 cubic feet per day.
Outdoor Surfaces
hedgerows, fencerows, equipment, outdoor premises, perimeter
treatments, rights-of-ways (soil and vegetation)
manually pressurized handwand, backpack
EC, L handler [31, 34]
0.78 lb ai/1000 sq ft
Do not enter or allow others to enter until sprays have dried. With the exception of outdoor fogging devices, all outdoor applications must be limited to spot or crack-and-crevice treatments only, except for the following permitted uses:
(1) Treatment to soil or vegetation around structures; (2) Applications to lawns, turf, and other vegetation; (3) Applications to building foundations, up to a
maximum height of 3 feet. Other than applications to building foundations, all outdoor applications to impervious surfaces such as sidewalks, driveways, patios, porches and structural surfaces (such as windows, doors, and eaves) are limited to spot and crack-and crevice applications, only. Do not water residential treated areas to the point of run-off. Do not make granular applications during rain.
aerosol can PRL/RTU handler [6] 0.035 lb ai/16oz can
crack and crevice, tube RTU handler [16] 0.0008 lb ai/1000 sq
ft
agricultural uncultivated areas
manually pressurized handwand, backpack
EC, L handler [19] 0.213 lb ai/acre [0.04 lb ai/gal]
refuse/solid waste sites [commercial, industrial,
institutional, and residential]
paintbrush, manually pressurized handwand, backpack
L N/A 0.04 lb ai/gal
[0.85 lb ai/1000 sq ft]
outdoor wood treatments (stored lumber/wood piles, pressure treatment, wood
surfaces, and wood protection)
[commercial, industrial, institutional, and residential]
paintbrush, roller, manually pressurized handwand, airless
sprayer
EC, L
handler [17, 18, 19,
20]
post-app
0.04 lb ai/gal [7.21 lb ai/acre]
[0.081 mg ai/cm2]
perimeter treatment (soil, vegetation, and lower
buildings)
backpack, manually pressurized handwand
EC, L handler [31, 34]
0.78 lb ai/gal
perimeter treatment (soil and vegetation)
shaker can G handler [16] 0.0008 lb ai/ft2
[0.8 lb ai/10 gallons treats 1000 sq ft]

Permethrin Human Health Risk Assessment DP No. 414137
Page 110 of 129
Table F.2. Non-Food and Non-Feed Use Patterns for Permethrin.
Crop/Use Site Application Type and
Equipment Formulation
Handler/Post-app Exposure Scenario(s)1 Maximum
Application Rate Use Directions
Residential2 Occupational Termites
termites: soil around underground utilities
handgun, backpack EC
N/A
33.2 lb ai/1000 linear
feet The treatment site must be covered prior to a rain event in order to prevent runoff of the pesticide into non-target areas. Do not treat soil that is water-saturated or frozen. Do not treat when raining. Do not allow treatment to runoff from the target area. Do not apply within 10 feet of storm drains. Do not apply within 25 feet of aquatic habitats (such as, but not limited to, lakes; reservoirs; rivers; permanent streams; marshes or ponds; estuaries; and commercial fish farm ponds).
termites: soil surrounding standing wood
injector EC 0.08 lb ai/gallon
termites: soil, under concrete slabs, stoops, porches,
structural voids, foam application RTU 4.25 lb ai/1000 sq ft
Wood Treatment: trees, telephone poles, fence posts:
nest opening
paintbrush, manually pressurized handwand, backpack
L 0.04 lb ai/gallon
Turf
turf [residential and commercial]
manually pressurized handwand, backpack
EC, L handler [31, 34] post-app
0.87 lb ai/acre [0.04 lb ai/gal]
Do not water residential treated areas to the point of run-off. Do not make granular applications during rain.
belly grinder, cup, hand dispersal, and spoon
G handler
[11 to 15] post-app
0.65 lb ai/acre [0.0003125 lb
ai/mound] 5% permethrin
hose end sprayer RTU handler [10]
post-app 0.45 lb ai/acre
golf course turf mechanically pressurized handgun,
groundboom EC N/A
0.79 lb ai/acre
commercial/industrial lawns manually-pressurized handwand,
mechanically pressurized handgun
0.87 lb ai/acre [0.04 lbs ai/gal]
1 Handler exposure includes inhalation exposure only unless otherwise indicated. Post-application exposure include Hand to Mouth (HtM)/Object to Mouth (OtM) unless otherwise indicated. 2 The number in brackets after “handler” indicate the exposure scenario each residential handler use was assessed under in Table 5.1.1. 3 With the exception of outdoor fogging devices, all outdoor applications must be limited to spot or crack-and-crevice treatments only, except for the following permitted uses: Treatment to
soil or vegetation around structures; Applications to lawns, turf, and other vegetation; Applications to building foundations, up to a maximum height of 3 feet.

Permethrin Human Health Risk Assessment DP No. 414137
Page 111 of 129
Appendix G. Summary of Assumptions Used in the Residential Post-Application Assessment. Residential Post-Application Exposure Data and Assumptions A series of assumptions and exposure factors served as the basis for completing the residential post-application risk assessment. Each assumption and factor is detailed in the 2012 Residential SOPs29. Application Rate: A screening-level approach was used for assessment of residential exposures by evaluation of the maximum application rate for all possible residential post-application exposure scenarios of permethrin. Appendix F, Table F.1 and Table F.2 of this document summarize the maximum rates for all registered uses of permethrin. Exposure Duration: Residential exposure is expected to be short-term in duration. The single dose and repeat dosing permethrin studies show that repeat exposures do not result in lower PODs (i.e. there is no evidence of increasing toxicity with an increased duration of exposure). Therefore, the exposure assessments are conducted as a series of acute exposures, and these are protective of scenarios in which exposure occurs for multiple days. Residential Post-Application Outdoor TTR Data: Post-application exposures from golf courses and treated turf were assessed using 0-day residue data from a turf transferable residue study conducted with a liquid permethrin product (MRID 44955501). The chemical-specific TTR data collected with the Modified California Roller Method are available for permethrin, and were summarized in the pyrethroid cumulative assessment30, and were determined to be acceptable for use in risk assessment. Corrected TTR values have been reassessed to incorporate current regression modeling into this assessment resulting in day-0 TTR of 0.061 µg/cm2 at the study application rate of 0.87 lbs ai/acre. The TTR Day-0 transfer residue did not require adjustment for liquid applications as the current maximum labeled application rate for permethrin is also 0.87 lb ai/acre. As there is no dermal hazard for a permethrin non-cancer assessment, only incidental oral and accidental ingestion scenarios have been qualitatively assessed for children 1 to <2 years old. Table G.1 summarizes the available pyrethroid TTR data.
Table G.1 Pyrethroid TTR Summary.
Chemical (study app rate)
Study Sites Day 0 TTR
(ug/cm2)
Average Day 0 TTR
(ug/cm2)
Daily Dissipation (%)
Permethrin (0.87 lbs ai/acre)
Transferable Turf Residue Study: Permethrin Residues in Turf Following Application of Dragnet® SFR Insecticide/Miticide (MRID
44955501)
PA (L) 0.051
0.061 11% CA (L) 0.073
GA (L) 0.058
Residential Post-Application Outdoor DFR Data:
29 Available: http://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/standard-operating-procedures-residential-pesticide 30 Pyrethrins/Pyrethroid Cumulative Risk Assessment. M. Crowley et. al.; 04-OCT-2003; D394576

Permethrin Human Health Risk Assessment DP No. 414137
Page 112 of 129
For the garden and ornamental use scenario, chemical-specific dislodgeable foliar residue (DFR) data are available for four pyrethroids: cyfluthrin, fluvalinate, esfenvalerate, and permethrin. Most of these DFR data were collected on orchard crops (i.e., stone fruits, apples, oranges) or in greenhouses. The esfenvalerate DFR data summarized in the pyrethroid cumulative document (K. Whitby, 04-OCT-2011; D394576) included analysis of foliar residues on corn and broccoli and are considered most representative of potential crops that could be found in a home garden. The permethrin DFR data included analysis of foliar residues on peach and are considered most representative of potential crops that could be found on residential fruit and nut trees. Table G.2 summarizes the available pyrethroid DFR data.
Table G.2. Pyrethroid DFR Summary.
Chemical Study Sites Day 0 DFR
(ug/cm2)
Average Day 0 DFR
(representative of a 0.05 lb
ai/A) (ug/cm2)
Max Label Application
Rate
Normalized Average Day 0
DFR1
(ug/cm2)
Esfenvalerate
Dissipation of Dislodgeable Foliar Residues of Esfenvalerate from Broccoli Following
Application of Asana® XL Insecticide in the USA -
Season 1997 (MRID 44852402)
CA1 (L) 0.157
0.132
0.2 lbs ai/acre (0.2 lbs ai/gal)
0.528
CA2 (L) 0.122
Dissipation of Dislodgeable Foliar Residues of
Esfenvalerate from Sweet Corn Following
Application of Asana® XL Insecticide in the USA -
Season 1998 (MRID 44852403)
FL (L) 0.072
PA (L) 0.177
Permethrin
“Dissipation of Dislodgeable Foliar
Residues of Permethrin Applied to Orchards (Peaches)” (MRID)
437557-01)
CA (Ambush
25W)
0.641
0.87
0.0036 lbs ai/gal
0.4 lbs ai/acre
0.87
CA (Pounce
3.2)
0.427
GA (Ambush
25W)
0.709
WA (Ambush
25W)
1.71
1 Normalized Average Day 0 DFR = Average Day 0 DFR × (Max Label Application Rate ÷ Study Application Rate)
Residential Post-Application Pet (Dog) Dislodgeable Residue Data: Post-application exposures from spot-on treated beagle dogs were assessed using 0-day residue data from a dislodgeable residue study conducted with a liquid permethrin product (MRID 48135326 and MRID 48135325) which underwent secondary review by HED (A. Rivera-Lupianez, 14-DEC-2010; D380194) after completion of the last assessment in 2009. The test substance, SCH 900560, was administered to 10 beagle dogs by topical application to the skin on the back shoulder blade area using plastic syringes in a spot-on procedure. Permethrin residues were measured on treated dogs after 25 petting simulations, with each simulation consisting of three strokes (75 strokes total). The strokes were conducted using a mannequin hand fitted with two cotton gloves over top of a nitrile glove. Residues were extracted from the nitrile and cotton gloves. Samples were collected from each dog at the following intervals: prior to treatment, at 4,

Permethrin Human Health Risk Assessment DP No. 414137
Page 113 of 129
and 8 hours after treatment and at 1, 2, 4, 7, 14, 21, and 28 days after treatment. The cotton and nitrile glove samples were analyzed for permethrin (SCH 169937). The cis- and trans- isomers of permethrin were analyzed separately and the results summed to provide total permethrin values. Total permethrin average residues from all three gloves combined increased from 9,686 µg/gloves (1.67% of applied dose and 2.98 µg/cm2) at 4 hours after application to a maximum of 11,125 µg/gloves (1.93% of applied dose and 3.43 µg/cm2) at 8 hours after application. Residues then declined to 821 µg/gloves (0.15% of applied dose and 0.26 µg/cm2) by Day 28 after application. This study data was incorporated for both liquid and solid formulations (e.g., spot on, aerosol cans, dusts, etc.). Based upon HED’s review of the permethrin dog residue transfer study, the maximum daily transfer was 1.93% (8 hours after application) corresponding to a fraction of the application rate available as transferable residue (FAR) value of 0.0193. Using the individual residue data for percentage of applied dose transferable calculations collected from 4 hours through day 28 after application, the daily dissipation was 8.79%. Post-application Inhalation and Incidental Oral Exposures from Residential Misting Systems: Residential post-application inhalation exposures are expected for adults and children following treatment with residential misting system. Incidental oral exposures are also expected for children 1 to <2 years old from contact with permethrin residues that have settled on turf following a pulse, or release, of the misting system. Post-application exposures from residential misting systems are assessed using the methodologies and inputs described in the 2012 Residential SOPs (Outdoor Fogging/Misting Systems SOP). The Outdoor Fogging/Misting Systems SOP recommends input of an active ingredient per single pulse application rate. This single pulse application rate is assumed to occur once hourly during the duration of time the exposed individual spends outdoors, 2.3 hours/day. For permethrin, only a daily maximum application rate (0.25 g/ 1,000 ft3/day) is provided on product labeling; i.e., the total amount of active ingredient to not be exceeded over the course of all release intervals in a day for all residential automatic misting system products for permethrin. The labels do not provide detail of how many pulses per day should be used to release this total, nor do they describe the time of day that the releases should occur. Typically, residential misting systems are designed so that pulses of active ingredient are released during the time of highest insect activity, during the early morning and late afternoon. In order to determine an active ingredient per pulse release rate appropriate for use with the SOPs, HED has assumed that the label maximum application rate is released over 6 intervals daily; 3 in the early morning and 3 in the early afternoon. HED has assessed the automated misting system use as though it were intended for residential application and presented the resulting risks which are not of concern (i.e., MOEs range from 1,500 to 86,000). Residential Post-Application Inhalation and Incidental Oral Exposure from All Surface Directed Indoor Uses (Crack and Crevice/Spot/Bed Bug): HED has received an Office of Research and Development (ORD) exposure study that was performed in the U.S. EPA’s Indoor Air Quality (IAQ) Research House. This study simulated crack and crevice applications of four pesticides; two emulsifiable concentrate products applied via a handheld sprayer (permethrin and cypermethrin), one aerosol can product (propoxur), and one gel bait product (fipronil). The application pattern used in this study is considered a

Permethrin Human Health Risk Assessment DP No. 414137
Page 114 of 129
reasonable representation of an indoor crack and crevice application and/or an indoor application for bed bugs. Air concentrations of all four chemicals were collected using stationary air samplers suspended 75 cm above the floor in the room of application (the living room) and two other rooms in the test house (the den and master bedroom). Air samples were collected during the application and 1, 1.5, 2, 2.5, 3, 7, 14, 21, 28, and 35 days after application. Permethrin and cypermethrin air concentrations were not found in any measurable quantities in any room in the research house. Although not all of the data is chemical specific, the Non-Dietary Exposure Task Force (NDETF) has performed an analysis of all the pyrethroid surface deposition and hand press exposure data that they produced. This analysis shows the exposure data for one pyrethroid can generally be used to represent the entire chemical class. Based on this NDETF analysis, HED believes it is appropriate to use the air concentration data from the ORD study as a surrogate for permethrin when applied as described on the registered label. HED does not have concerns for permethrin for the post-application inhalation exposure scenario given that all air concentration values were below the limit of quantitation in the ORD study. Post-Application Indoor Fraction of Residue Available for Transfer (Fai): Consistent with the 2011 Pyrethroid CRA, the assessment of indoor post-application exposures uses the average Fai for all pyrethroids. Chemical-specific data provided by the NDETF were used for the fraction of residue available for transfer (Selim, 2004a; Selim, 2003b; Selim, 2003c; Selim, 2000; Selim, 2002b; Selim, 2002c). The NDETF studies examined the transferability of residues from bare hand-presses on carpets and hard surfaces for deltamethrin, permethrin, and pyrethrins. For carpets, the fraction transferred was 0.03, 0.02, and 0.01 for pyrethrins, permethrin and deltamethrin, respectively. For hard surfaces, the fraction transferred was 0.04, 0.03, and 0.05 for pyrethrins, permethrin, and deltamethrin, respectively. Since there is chemical specific data available from these studies, the permethrin fraction transferred was used for this assessment: 0.02 for carpets and 0.03 for hard surfaces. The carpet Fai was also incorporated into the mattress assessment. Post-Application Impregnated Material/Clothing Exposure: Post-application exposures to treated fabric were assessed using transfer values from a study determining the transfer of impregnated permethrin products (MRID 4407668-12). Radiolabeled (14C) permethrin-treated fabric patches were applied to the backs of 22 male New Zealand white rabbits in four treatment groups based on environment (temperate vs. subtropical) and fabric type (cotton vs. 50:50 nylon/cotton blend). After seven days, the average percent migration to skin for each treatment group was estimated using the recovery of 14C from excreta and skin. Based on this approach, the overall fraction of ai transferred per day was 0.005 (0.5%) and ranged from an average ± standard deviation of 0.004 ± 0.09 fraction ai transferred per day in the subtropical/NYCO group to 0.0065 ± 0.10 fraction of ai transferred per day in the subtropical/cotton treatment group. For the purposes of this assessment, the 0.5% permethrin transferred per day was incorporated into the non-cancer incidental oral, and cancer dermal assessment. Additional information is available in the 2012 Residential SOPs. Residential Post-Application Indoor Inhalation Exposure from Fogger Applications: Post-application inhalation exposure to the use of indoor foggers is expected to be negligible since most fogger product labels typically state a period of no-entry following application (usually up to 4 hours), as well as a ventilation period before occupants can return. Permethrin

Permethrin Human Health Risk Assessment DP No. 414137
Page 115 of 129
residential fogger products include a 4-hour period of no entry following application. In addition, due to the low vapor pressure of pyrethroids in general, and the available air concentration data collected from the ORD test house following indoor applications of pyrethroids (D390098), HED does not have concerns for inhalation exposure following indoor fogger applications of permethrin. Residential Post-Application Indoor Deposited Residue (DepR) Values: Based on pyrethroid-specific data available in the 2012 SOPs, the following approaches/default values should be used. Note that it is not recommended to pull individual chemical-specific data from the SOPs, but rather to use the collective pyrethroid data available.
Perimeter/Spot/Bedbug applications (coarse): For coarse perimeter/spot/bedbug applications, the default deposited residue value, 2.6 µg/cm2, was used with no adjustment for percent ai. This value is a combination of the pyrethroid data from Keenan (2007) and esfenvalerate data from Selim (2008) for all pyrethroids.
Perimeter/Spot/Bedbug applications (pin stream): For pin stream perimeter/spot/bedbug applications, the default deposited residue, 1.5 µg/cm2, was used with no adjustment for percent ai. This value is a combination of the pyrethroid data from Keenan (2007) and the ORD Test house date (D390098) for all pyrethroids
Crack and crevice applications: For crack and crevice applications, the default deposited residue value, 0.4 µg/cm2, was used with no adjustment for percent ai. This value is a combination of the pyrethroid data from Keenan (2007), the esfenvalerate data from Selim (2008) and the ORD Test house date (D390098) for all pyrethroids.
Fogger applications: Data from the Non-Dietary Exposure Task Force (NDETF) were used to estimate deposited residue for the pyrethroids with registered indoor fogger uses (Rogers, 2000; Selim, 2002a; Selim, 2003a). The NDETF conducted three studies measuring the deposited residue following application of a 0.2% deltamethrin fogger, a 0.5% permethrin fogger, and a 0.5% pyrethrins fogger. In each study, the fogger was discharged in an experimental room and the resulting deposited residues were measured using deposition coupons. The average residue value (adjusted to 0.5% active ingredient, if necessary) from each study was 5.6 µg/cm2 for deltamethrin, 4.8 µg/cm2 for permethrin, and 5.8 µg/cm2 for pyrethrins. As permethrin specific data is available, the deposition rate of 4.8 µg/cm2 has been used in this assessment.
Dermal, Inhalation, and Incidental Oral (Children 1-2 Years Only) Post-Application Exposure Resulting from Horse End-Use Products: Based on current policy, post-application child dermal, inhalation, and incidental oral (children 1 to <2 years only) exposure is not quantitatively assessed for horses. Exposure is expected to be minimal because of the frequency of exposure is intermittent, and direct contact with the treated animal is limited.

Permethrin Human Health Risk Assessment DP No. 414137
Page 116 of 129
Mosquito Adulticide Use: The post-application exposure potential from public health mosquito adulticide applications has been considered for ground-based truck foggers, backpack ULV foggers, and aerial applications. Chemical-specific exposure data have been submitted to support the permethrin mosquito adulticide use. Therefore, to assess the mosquito adulticide use, the algorithms and inputs presented in the 2012 Residential SOPs, Lawns/Turf section were used coupled with the permethrin TTR data described above. The deposition of permethrin from these applications are not based on the application rate alone, but also using the AgDISP (v8.2.6) model or empirical data to determine how much pesticide is deposited on residential lawns as a result of mosquito adulticide treatments at the maximum application rates for each. The TTR data are then used to determine the fraction of the total residue deposited following the mosquitocide application which can result in exposures to impacted individuals. Inhalation exposures are estimated using AgDISP (v8.2.6) for aerial applications, and a recently developed, Well Mixed Box (WMB) Model approach based on the Residential SOPs for outdoor foggers. Ground-based Truck-Mounted-Foggers In an analysis from 2013 (C. Peck, 28-MAR-2013; D407817), EFED reviewed eight published studies on ground ULV application in which deposition was measured. The studies varied in collection media (i.e., grass clippings and coupons), distance from application or spray head (ranging from 8 meters to 500 meters), and chemical measured (i.e., fenthion, malathion, naled, and permethrin). After considering the available data, HED has determined that an off-target deposition rate of 8.7 percent of the application rate may be used by HED to evaluate ground-based ULV applications (i.e., 8.7 percent of the target application rate deposits on turf). This value is the 90 percent upper confidence limit on the mean and is slightly higher than the mean values from all the data points observed in the studies (7.1%, n= 94). The adjusted application rate was then used to define TTR levels by scaling the available TTR data as appropriate. As chemical-specific TTR data are available for permethrin, its data was adjusted to reflect the maximum application rates (0.007 lb ai/A) for public health uses of permethrin. The adjusted TTR for permethrin is 4.9E-06 µg/cm2. In order to calculate airborne concentrations from ULV truck fogger applications, HED used the 2012 Residential SOPs for Outdoor Fogging/Misting Systems, with minimal modification to the WMB model. The WMB model allows for the estimation of air concentrations in the breathing zones of adults and children for use in calculating the post-application inhalation exposure to individuals residing in areas being treated by ground application of permethrin. For both adults and children, the exposure duration was adjusted to 6 hours as opposed to a default of 1.5 hours to mimic an exposure duration consistent with the 6-hour animal inhalation toxicity study used to define the endpoint and POD used as the basis of this assessment. The methodology more accurately accounts for dilution using the WMB model. Aerial Applications Deposition and airborne concentrations from aerial ULV applications, was modeled using the AGDISP (version 8.26) model to predict the motion of spray material released from aircraft, and determines the amount of application volume that remained aloft and the amount of the resulting droplets deposited on the surfaces in the treatment area as well as downwind from the treatment area. The 1-hour air concentration was calculated for a height of 5 feet resulting in an average

Permethrin Human Health Risk Assessment DP No. 414137
Page 117 of 129
air concentration of 0.0014 mg/ m3. The deposition fraction provided by AGDISP for permethrin was 0.85. The deposition fraction was then used to define TTR levels by scaling the available chemical specific TTR data as appropriate. A summary of data and calculations is available in J. Godshall 30-JUN-2017, D440978, Appendix B, Figures 5.2.1, and 5.2.2, and 5.2.3 presenting the estimated aerial permethrin residue fraction deposited on turf, an estimation of how permethrin deposition fluctuates over the spray block, and air born concentrations for the 1 hour following mosquito adulticide applications, respectively. The model also allows for the estimation of air concentrations in the breathing zones of adults and children for use in calculating the post-application inhalation risks to individuals residing in areas being treated by aerial application of permethrin. Post-application inhalation estimates resulting from aerial applications have been revised to incorporate the new HEC which is based on a 4-hour exposure duration. Post-application Inhalation Exposure resulting from Outdoor Aerosol Space Spray: In accordance with guidance for outdoor aerosol space sprays (OASS) in the Outdoor Fogging/Misting System Residential SOP, post-application exposure can result from activities performed following outdoor aerosol space spray pesticide applications. However, the SOP indicates that aerosolized pesticide exposure time is not a significant factor for calculation of inhalation exposure from outdoor aerosol space sprays due to the rapid dissipation of pesticide air concentrations. Based on the minimum airflow rate, the pesticide air concentration within the enclosed space (i.e., WMB) is virtually 0 after approximately 7 minutes. Therefore, since permethrin space sprays restrict entry until sprays have settled, which is protective of the air concentration after 7 minutes, a quantitative post-application inhalation exposure assessment is not required. Indoor Aerosol Space Spray Post-Application Inhalation Exposure: In accordance with Indoor Residential SOP, a quantitative post-application inhalation exposure assessment is not required for aerosol space sprays if the label has a reentry restriction/ ventilation requirement. Furthermore, the Summary of Labeling Changes for Permethrin (Revised 8/29/2011) requires all space spray labels to state, “do not enter or allow others to enter until vapors, mists, and aerosols have dispersed, and the treated area has been thoroughly ventilated.” Section 18 Military Aircraft Space Spray Residential Exposure: Residential handler exposure is not anticipated for the registered uses. Based on HED’s Residential SOPs31, for typical residential aerosol space sprays, it is assumed that there may be post-application dermal and incidental oral exposure to residues deposited on surfaces, and post-application inhalation exposure to pesticide aerosols that are still airborne after application. However, HED considers these exposures unlikely for the emergency exemption use for the reasons provided below:
A quantitative non-cancer dermal assessment is unnecessary since a dermal hazard has not been identified. Non-occupational inhalation exposure to aerosolized permethrin by the passengers is not expected as the aerosol spray is applied pre-flight – pre-embarkation
31 Available: http://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/standard-operating-procedures-residential-pesticide

Permethrin Human Health Risk Assessment DP No. 414137
Page 118 of 129
(before the passengers/crew board the aircraft). The use directions indicate the product is allowed to dry for at least 1 hour, during which
the aircraft is closed for 30 minutes after application, and then the aircraft exterior doors, to include cargo doors, are opened and the aircraft is allowed to passively ventilate for a minimum of 30 additional minutes prior to passengers and crew boarding the aircraft.
Passengers and crew generally consist of adults over the age of 18, but in rare circumstances, children may travel with families via ‘space available’ seating in military aircrafts. All passengers remain secured in their seats once they have boarded and while the aircraft is in transit, making incidental oral exposure unlikely for children.
Due to the limited mobility of residential passengers, negligible volatilization, permethrin’s lack of a dermal toxicological endpoint and low dermal penetration, HED considers residential post-application inhalation, incidental oral, and dermal exposure unlikely. Therefore, a quantitative residential post-application assessment is not necessary for non-cancer or cancer assessments. Dietary exposure is not expected as the label specifies not directly spraying food areas, and neither food or beverage services are provided on military aircraft. Residential Post-Application Non-Cancer Exposure and Risk Equations The algorithms used to estimate residential post-application exposure and dose can be found in the 2012 Residential SOPs32.
32 http://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/standard-operating-procedures-residential-pesticide

Permethrin Human Health Risk Assessment DP No. 414137
Page 119 of 129
Appendix H. Occupational Handler Non-Cancer and Cancer Risk Estimates.
Table H.1.1. Occupational Handler Non-Cancer and Cancer Exposure and Risk Estimates for Permethrin Agricultural Uses.
Exposure Scenario Crop or Target
Unit Exposure (μg/lb ai)1
Maximum Application Rate3
Area Treated or Amount Handled Daily4
Non-Cancer Cancer
Dermal Inhalation Inhalation Private Handler Commercial Applicator
Private Handler Commercial Applicator
PPE2 Dose (mg/kg/day)5
MOE6 Total LADD (mg/kg/day)7 Total Cancer Risk Estimate8
Mixer/Loader
WDG: Aerial
Orchard/Vineyard
227 8.96
0.4 lb ai/A 350 acres 0.016 600 0.000353 0.00106 3E-06 1E-05
Field Crop, Typical 0.3 lb ai/A 350 acres 0.012 790 0.000266 0.000797 3E-06 8E-06
Field Crop, High-acreage 0.2 lb ai/A 1200 acres 0.027 350 0.000607 0.00182 6E-06 2E-05
WDG: Airblast Orchard/Vineyard 0.4 lb ai/A 40 acres 0.018 5,200 0.0000404 0.000121 4E-07 1E-06
WDG: Chemigation
Orchard/Vineyard 0.4 lb ai/A 350 acres 0.016 600 0.000353 0.00106 3E-06 1E-05
Field Crop, Typical 0.3 lb ai/A 350 acres 0.012 790 0.000266 0.000797 3E-06 8E-06
Field Crop, High-acreage 0.2 lb ai/A 350 acres 0.078 1,200 0.000177 0.000531 2E-06 5E-06
WDG: Groundboom
Orchard/Vineyard 0.4 lb ai/A 40 acres 0.018 5,200 0.0000404 0.000121 4E-07 1E-06
Field Crop, Typical 0.3 lb ai/A 80 acres 0.027 3,500 0.0000607 0.000182 6E-07 2E-06
Field Crop, High-acreage 0.2 lb ai/A 200 acres 0.045 2,100 0.000101 0.000304 1E-06 3E-06
G: Aerial Orchard/Vineyard 8.4 1.7 0.25 lb ai/A 350 acres 0.0019 5,000 0.0000266 0.0000797 3E-07 8E-07
L/EC: Aerial Orchard/Vineyard 220 0.219 0.4 lb ai/A 350 acres 0.00038 24,000 0.000161 0.000483 2E-06 5E-06
L/EC: Impregnation Dry Bulk Fertilizer
Commercial Treatment
No Data 0.083
Engineering Controls
3 lb ai/ton 960 tons 0.0030 3,100 0.000162 0.000487 2E-06 5E-06
On-Farm
220 0.219
0.3 lb ai/acre 160 acres 0.00013 71,000 0.0000553 0.000166 5E-07 2E-06
L/EC: Aerial Field Crop, Typical 0.3 lb ai/A 350 acres 0.00029 33,000 0.000121 0.000362 1E-06 3E-06
Field Crop, High-acreage 0.2 lb ai/A 1200 acres 0.00066 14,000 0.000277 0.00083 3E-06 8E-06
L/EC: Airblast Orchard/Vineyard 0.4 lb ai/A 40 acres 0.000044 210,000 0.0000183 0.000055 2E-07 5E-07
L/EC: Chemigation
Orchard/Vineyard 0.4 lb ai/A 350 acres 0.00038 24,000 0.000161 0.000483 2E-06 5E-06
Field Crop, Typical 0.3 lb ai/A 350 acres 0.00029 33,000 0.000121 0.000362 1E-06 3E-06
Field Crop, High-acreage 0.2 lb ai/A 350 acres 0.00019 49,000 0.0000804 0.000241 8E-07 2E-06
L/EC: Groundboom Orchard/Vineyard 0.4 lb ai/A 40 acres 0.000044 210,000 0.0000183 0.000055 2E-07 5E-07
Field Crop, Typical 0.3 lb ai/A 80 acres 0.000066 140,000 0.0000277 0.000083 3E-07 8E-07

Permethrin Human Health Risk Assessment DP No. 414137
Page 120 of 129
Table H.1.1. Occupational Handler Non-Cancer and Cancer Exposure and Risk Estimates for Permethrin Agricultural Uses.
Exposure Scenario Crop or Target
Unit Exposure (μg/lb ai)1
Maximum Application Rate3
Area Treated or Amount Handled Daily4
Non-Cancer Cancer
Dermal Inhalation Inhalation Private Handler Commercial Applicator
Private Handler Commercial Applicator
PPE2 Dose (mg/kg/day)5
MOE6 Total LADD (mg/kg/day)7 Total Cancer Risk Estimate8
Field Crop, High-acreage 0.2 lb ai/A 200 acres 0.00011 85,000 0.000046 0.000138 4E-07 1E-06
L/EC: Stationary Fogger
Mushroom House 0.0000018 lbs ai/cu
ft 1,000,000 cu
ft 0.00000493 1,900,000 0.00000207 0.0000062 2E-08 6E-08
WP: Aerial
Orchard/Vineyard
77.7 2.75
0.4 lb ai/A 350 acres 0.075 120 0.000114 0.000343 1E-06 3E-06
Field Crop, Typical 0.3 lb ai/A 350 acres 0.057 170 0.0000858 0.000257 8E-07 2E-06
Field Crop, High-acreage 0.2 lb ai/A 1200 acres 0.013 73 0.000195 0.000586 2E-06 6E-06
WP: Airblast Orchard/Vineyard 0.4 lb ai/A 40 acres 0.0086 1,100 0.000013 0.0000391 1E-07 4E-07
WP: Chemigation
Orchard/Vineyard 0.4 lb ai/A 350 acres 0.075 120 0.000114 0.000343 1E-06 3E-06
Field Crop, Typical 0.3 lb ai/A 350 acres 0.057 170 0.0000858 0.000257 8E-07 2E-06
Field Crop, High-acreage 0.2 lb ai/A 350 acres 0.038 250 0.0000572 0.000171 5E-07 2E-06
WP: Groundboom
Orchard/Vineyard 0.4 lb ai/A 40 acres 0.0086 1,100 0.000013 0.0000391 1E-07 4E-07
Field Crop, Typical 0.3 lb ai/A 80 acres 0.013 730 0.0000195 0.0000586 2E-07 6E-07
Field Crop, High-acreage 0.2 lb ai/A 200 acres 0.022 440 0.0000327 0.0000981 3E-07 9E-07
WP: Stationary Fogger
Mushroom House 0.0000018 lbs ai/cu
ft 1,000,000 cu
ft 0.0000619 150,000 0.00000148 0.00000443 1E-08 4E-08
Applicator
Spray: Aerial
Orchard/Vineyard 2.08 Engineering
Controls
0.0049 Engineering
Controls
0.4 lb ai/A 350 acres 0.0000086 1,100,000 0.00000159 0.00000476 2E-08 5E-08
Field Crop, Typical 0.3 lb ai/A 350 acres 0.0000064 1,500,000 0.00000118 0.00000355 1E-08 3E-08
Field Crop, High-acreage 0.2 lb ai/A 1200 acres 0.000015 630,000 0.00000272 0.00000815 3E-08 8E-08
Spray: Airblast Orchard/Vineyard 1770 4.71 0.4 lb ai/A 40 acres 0.00094 9,900 0.000155 0.000465 1E-06 4E-06
Spray: Groundboom
Orchard/Vineyard
78.6 0.34
0.4 lb ai/A 40 acres 0.000068 140,000 0.00000723 0.0000217 7E-08 2E-07
Field Crop, Typical 0.3 lb ai/A 80 acres 0.00010 92,000 0.0000108 0.0000325 1E-07 3E-07
Field Crop, High-acreage 0.2 lb ai/A 200 acres 0.00018 55,000 0.0000181 0.0000542 2E-07 5E-07
Impregnated Dry Bulk Fertilizer
Field Crop, Typical Commercial Treatment
9.9 1.2 0.3 lb ai/A
320 acres 0.00144 6,500 0.0000225 0.0000675 2E-07 6E-07
Field Crop, Typical On-Farm Treatment
160 acres 0.00072 13,000 0.0000113 0.0000338 1E-07 3E-07
Field Crop, High Acreage 320 acres 0.00144 6,500 0.0000225 0.0000675 2E-07 6E-07

Permethrin Human Health Risk Assessment DP No. 414137
Page 121 of 129
Table H.1.1. Occupational Handler Non-Cancer and Cancer Exposure and Risk Estimates for Permethrin Agricultural Uses.
Exposure Scenario Crop or Target
Unit Exposure (μg/lb ai)1
Maximum Application Rate3
Area Treated or Amount Handled Daily4
Non-Cancer Cancer
Dermal Inhalation Inhalation Private Handler Commercial Applicator
Private Handler Commercial Applicator
PPE2 Dose (mg/kg/day)5
MOE6 Total LADD (mg/kg/day)7 Total Cancer Risk Estimate8
Commercial Treatment
Field Crop, High Acreage On-Farm Treatment
160 acres 0.00072 13,000 0.0000113 0.0000338 1E-07 3E-07
G: Aerial Orchard/Vineyard 1.7
Engineering Controls
1.3 Engineering
Controls 0.25 lb ai/A 350 acres 0.0014 6,500 0.0000183 0.000055 2E-07 5E-07
Flagger
Spray: Aerial
Orchard/Vineyard
11 0.35
0.4 lb ai/A
350 acres
0.00061 15,000 0.0000154 0.0000461 1E-07 4E-07
Field Crop, Typical 0.3 lb ai/A 0.00046 20,000 0.0000115 0.0000346 1E-07 3E-07
Field Crop, High-acreage 0.2 lb ai/A 0.00031 31,000 0.00000767 0.000023 7E-08 2E-07
G: Aerial Orchard/Vineyard 2.75 0.15 0.25 lb ai/A 0.00016 57,000 0.00000323 0.0000097 3E-08 9E-08
Mixer/Loader/Applicator
WDG: Backpack Orchard/Vineyard
(ground) 8260 2.58
0.0036 lb ai/gallon
40 gallons 0.0000047 2,000,000 0.0000061 0.0000183 6E-08 2E-07
WDG: Mechanically-pressurized Handgun
Orchard/Vineyard (foliar/ground)
6050 8.68 1000 gallons
0.00039 24,000 0.000115 0.000346 1E-06 3E-06
Field Crop, Typical (foliar/ground)
0.0055 lb ai/gallon 0.00060 16,000 0.000176 0.000527 2E-06 5E-06
L/EC: Backpack Orchard/Vineyard
(ground) 8260 2.58 0.0036 lb ai/gallon 40 gallons 0.0000047 2,000,000 0.0000061 0.0000183 6E-08 2E-07
L/EC: Fogging Equipment
Mushroom House No Data 8916 0.0000018 lbs ai/cu
ft 1,000,000 cu
ft 0.2 47 No Data No Data No Data No Data
L/EC: Manually-pressurized Handwand
Mushroom House 100000 30 0.267 lbs ai/gal 40 gallons 0.004 2300 0.00547 0.0164 5E-05 2E-04
L/EC: Mechanically-pressurized Handgun
Orchard/Vineyard (foliar/ground)
6050 8.68
0.0036 lb ai/gallon
1000 gallons
0.00039 24,000 0.000115 0.000346 1E-06 3E-06
Field Crop, Typical (foliar/ground)
0.0055 lb ai/gallon 0.00060 16,000 0.000176 0.000527 2E-06 5E-06

Permethrin Human Health Risk Assessment DP No. 414137
Page 122 of 129
Table H.1.1. Occupational Handler Non-Cancer and Cancer Exposure and Risk Estimates for Permethrin Agricultural Uses.
Exposure Scenario Crop or Target
Unit Exposure (μg/lb ai)1
Maximum Application Rate3
Area Treated or Amount Handled Daily4
Non-Cancer Cancer
Dermal Inhalation Inhalation Private Handler Commercial Applicator
Private Handler Commercial Applicator
PPE2 Dose (mg/kg/day)5
MOE6 Total LADD (mg/kg/day)7 Total Cancer Risk Estimate8
WP: Backpack Orchard/Vineyard
(ground) 8260 2.58 0.0036 lb ai/gallon 40 gallons 0.0000047 2,000,000 0.0000061 0.0000183 6E-08 2E-07
WP: Fogging Equipment
Mushroom House No Data 8916 0.0000018 lbs ai/cu
ft 1,000,000 cu
ft 0.2 47 No Data No Data No Data No Data
WP: Manually-pressurized Handwand
Mushroom House 100000 30 0.267 lbs ai/gal 40 gallons 0.004 2300 0.00547 0.0164 5E-05 2E-04
WP: Mechanically-pressurized Handgun
Orchard/Vineyard (foliar)
6050 8.68 0.0036 lb ai/gallon
1000 gallons
0.00039 24,000 0.000115 0.000346 1E-06 3E-06
Orchard/Vineyard (ground drench)
4310 3931 0.0036 lb ai/gallon 0.18 53 0.00226 0.00679 2E-05 7E-05
Field Crop, Typical (foliar)
6050 8.68
0.0055 lb ai/gallon
0.00060 16,000 0.000176 0.000527 2E-06 5E-06
Field Crop, Typical (ground drench)
4310 3931 0.27 35 0.000176 0.000527 2E-06 5E-06
Loader/Applicator
G: Backpack Orchard/Vineyard
(ground) 155 23.8
0.25 lb ai/A 1 acre
0.000074 130,000 0.00000111 0.00000333 1E-08 3E-08
G: Belly Grinder Orchard/Vineyard (broadcast)
10000 62 0.00019 48,000 0.000015 0.000045 1E-07 4E-07
G: Rotary Spreader 440 10 5 acres 0.00016 60,000 0.00000471 0.0000141 5E-08 1E-07 1 Based on the “Occupational Pesticide Handler Unit Exposure Surrogate Reference Table” (http://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/occupational-pesticide-handler-
exposure-data); Level of mitigation: Baseline PPE with no gloves and no respirator, Eng. Controls. 2 PPE = Baseline (i.e., long sleeved shirt, long pants, shoes plus socks) no gloves, and no respirator unless otherwise indicated 3 Based on registered uses listed in Appendix A, Tables 4.1 and 4.2. 4 Exposure Science Advisory Council Policy #9.1. 5 Inhalation Dose = Inhalation Unit Exposure (μg/lb ai) × Conversion Factor (0.001 mg/μg) × Application Rate (lb ai/acre or gal) × Area Treated or Amount Handled Daily (A or gal/day) ÷ BW (80
kg). 6 Inhalation MOE = Inhalation HED (mg/kg/day) ÷ Inhalation Dose (mg/kg/day). 7 Total LADD = Dermal LADD + Inhalation LADD 8 Total Cancer Risk Estimate = Total LADD × Q1
*, where Q1* = 9.567 x 10-3 (mg/kg/day)-1

Permethrin Human Health Risk Assessment DP No. 414137
Page 123 of 129
Table H.1.2. Occupational Handler Non-Cancer and Cancer Exposure and Risk Estimates for Permethrin Non-Agricultural Uses.
Exposure Scenario Formulation: Equipment
Crop or Target
Unit Exposure (μg/lb ai)1
Maximum Application Rate3
Area Treated or Amount Handled Daily4
Non-Cancer Cancer
Dermal Inhalation Inhalation Commercial Applicator
PPE2 Dose
(mg/kg/day)5 MOE6 Total LADD7 Cancer Risk
Estimate8
Mixer/Loader
L/EC: Dip Livestock
220 0.219 0.0023 lb ai/animal 400 animals
0.0000025 3,700,000 0.0000032 3E-08
DF/WDG: Dip 227 8.96 0.00010 91,000 0.0000070 7E-08
DF/WDG: Aerial Conifer Pine Seed
Orchard 227 8.96 1.6 lbs ai/acre 125 acres 0.022 420 0.00051 1E-05
DF/WDG: Chemigation Greenhouse Ornamentals
227 8.96 0.2 lbs ai/acre 60 acres 0.0014 6,900
0.000091 9E-07
DF/WDG: Groundboom 227 8.96 0.000091 9E-07
L/EC: Aerial
Aquatic Vector Control 220 0.219 0.007 lb ai/acre 250 acres 0.0000048 2,000,000 0.0000061 6E-08
Forestry 220 0.219 0.6 lb ai/acre
1,200 acres 0.0020 4,700 0.0025 2E-05
Forestry ULV/Wide Area 220 0.219 7,500 acres 0.012 760 0.016 1E-04
Terrestrial Vector Control: ULV
220 0.219 0.007 lbs ai/acre 7,500 acres 0.00014 65,000 0.00018 2E-06
L/EC: Truck-mounted Fogger
Terrestrial Vector Control: ULV
220 0.219 0.007 lbs ai/acre
3000 acres 0.000058 160,000 0.000073 7E-07
Terrestrial Vector Control
220 0.219 250 acres 0.0000048 2,000,000 0.0000066 6E-08
L/EC: Groundboom Golf course 220 0.219 0.79 lb ai/acre 40 acres 0.000087 110,000 0.00011 1E-06
Field-grown Ornamentals 220 0.219 0.2 lb ai/acre 40 acres 0.0000219 430,000 0.0000028 3E-08
L/EC: Boom Sprayer Aquatic Vector Control 220 0.219 0.007 lbs ai/acre 30 acres 5.8E-07 16,000,000 0.00000073 7E-09
L/EC: Automatic Misting System
Barn Misting System 220 0.219 0.000031 lb ai/cu ft 100,000 cu ft 0.000085 110,000 0.00011 1E-06
Residential Misting System
220 0.219 0.0023 lb ai/gal 1,000 gallons 0.0000063 1,500,000 0.0000079 8E-08
L/EC: Stationary Fogger Warehouse 220 0.219 0.00000036 lb ai/cu ft 100,000 cu ft 0.00000099 9,500,000 0.0000012 1E-08
Indoor Barnyard / Livestock House
220 0.219 0.000031 lb ai/cu ft 100,000 cu ft 0.000085 110,000 0.00011 1E-06
DF/WDG: Aerial Conifer Pine Seed
Orchard 77.7 2.75 1.6 lbs ai/acre 125 acres 0.0069 1,400 0.00049 5E-06
WP: Chemigation Greenhouse Ornamentals 77.7 2.75 0.2 lb ai/acre 60 acres 0.00041 23,000 0.000029 3E-07

Permethrin Human Health Risk Assessment DP No. 414137
Page 124 of 129
Table H.1.2. Occupational Handler Non-Cancer and Cancer Exposure and Risk Estimates for Permethrin Non-Agricultural Uses.
Exposure Scenario Formulation: Equipment
Crop or Target
Unit Exposure (μg/lb ai)1
Maximum Application Rate3
Area Treated or Amount Handled Daily4
Non-Cancer Cancer
Dermal Inhalation Inhalation Commercial Applicator
PPE2 Dose
(mg/kg/day)5 MOE6 Total LADD7 Cancer Risk
Estimate8
WP: Groundboom Greenhouse Ornamentals 77.7 2.75 0.000029 3E-07
Applicator
RTU (D): Dust Bag Livestock 227 8.96 0.0025 lb ai/animal 1000 poultry 0.000728 33,000 0.000019
2E-07
Spray: Aerial
Aquatic Vector Control
2.08 Engineering
Controls
0.0049 Engineering
Controls
0.007 lbs ai/acre 250 acres 0.00000011 88,000,000 5.9E-08 6E-10
Conifer Pine Seed Orchard
1.6 lbs ai/acre 125 acres 0.000012 760,000 0.0000068 0.0000068
Forestry 0.6 lbs ai/acre
1,200 acres 0.000044 210,000 0.000025 2E-07
Forestry ULV/Wide Area 7,500 acres 0.00028 34,000 0.00015 1E-06
Terrestrial Vector Control: ULV
0.007 lbs ai/acre 7,500 acres 0.0000032 2,900,000 0.0000018 2E-08
Spray: Truck-mounted Fogger
Terrestrial Vector Control: ULV
1770 4.71 0.007 lbs ai/acre
3,000 acres 0.0012 7,600 0.00061 6E-06
Terrestrial Vector Control
1770 4.71 250 acres 0.00010 91,000 0.000051 5E-07
Spray: Groundboom Golf Course 78.6 0.34 0.79 lbs ai/acre 40 acres 0.000134 70,000 0.000042 4E-07
Greenhouse Ornamentals 78.6 0.34 0.2 lbs ai/acre 60 acres 0.000051 180,000 0.000016 2E-07
Spray: Boom sprayer Aquatic Vector Control 78.6 0.34 0.007 lbs ai/acre 30 acres 0.00000089 10,000,000 0.00000028 3E-09
RTU (L): Dip Domestic Animal 54300 26.6 0.006 lb ai/gal 10 gallons 0.00002 470,000 0.000050 5E-07
RTU (L): Pour-in/on Livestock 220 0.219 0.0017 lb ai/animal 400 animals 0.0000019 5,000,000 0.0000024 2E-08
Domestic Animal 220 0.219 0.007 lb ai/animal 8 animals 0.00000015 61,000,000 0.00000019 2E-09
RTU (L): Shampoo Domestic Animal 2098000 292 0.0014 lbs ai/animal 8 animals 0.000041 230,000 0.00036 3E-06
RTU (L): Sponge Livestock (Horses) 844000 208 0.0062 ai/animal 25 animals 0.00040 23,000 0.0020 2E-05
Domestic Animal 844000 208 0.0062 ai/animal 8 animals 0.00013 73,000 0.00064 6E-06
RTU (D): Shaker Can
Landscaping (plants/flowers)
4042000 17500 0.0025 lb ai/1 lb
container 10 lbs 0.0055 1,700 0.0017 2E-05
Livestock 4042000 17500 0.000031 lb ai/animal 400 animals 0.0027 3,500 0.00086 8E-06
Domestic Animals 4042000 17500 0.00016 lb ai/animal 8 animals 0.00028 33,000 0.000089 9E-07

Permethrin Human Health Risk Assessment DP No. 414137
Page 125 of 129
Table H.1.2. Occupational Handler Non-Cancer and Cancer Exposure and Risk Estimates for Permethrin Non-Agricultural Uses.
Exposure Scenario Formulation: Equipment
Crop or Target
Unit Exposure (μg/lb ai)1
Maximum Application Rate3
Area Treated or Amount Handled Daily4
Non-Cancer Cancer
Dermal Inhalation Inhalation Commercial Applicator
PPE2 Dose
(mg/kg/day)5 MOE6 Total LADD7 Cancer Risk
Estimate8
RTU (G): Shaker Can Mounds/Nests 112 12.5 0.00156 lbs ai/mound 1000 mounds 0.00024 38,000 0.000012 1E-07
RTU (L): Spot-on Domestic Animal 112000 Negligible exposure
0.006 ai/animal 8 animals Negligible exposure
Negligible exposure
Negligible exposure
Negligible exposure
RTU (L): Trigger-spray Bottle
Livestock (Horses) 544000 3300 0.017 ai/animal 400 animals 0.018 540 0.0042 4E-05
Domestic Animal 544000 3300 0.000538 lbs ai/bottle 8 bottles 0.00018 53,000 0.000042 4E-07
Residential Indoor Surface (C&C)
3660 61.2 0.043 lb ai/bottle 8 bottles
0.00026 35,000 0.000029 3E-07
Landscaping (plants/flowers)
3660 61.2 0.00026 35,000 0.000029 3E-07
RTU (PL): Aerosol Can
Military Aircraft (using warehouse as surrogate)
190000 1300 0.00441 lbs ai/can 4 cans 0.00029 33,000 0.000062 6E-07
Domestic Animal 544000 3300 0.000538 lb ai/can
8 cans
0.00018 53,000 0.000042 4E-07
Foundations/perimeter 190000 1300 0.035 lb ai/16 oz can 0.0046 2,100 0.00098 9E-06
Residential Indoor Living Spaces
190000 1300 0.00438 lb ai/16 oz
can 0.00057 16,000 0.00012 1E-06
Residential Outdoor Spaces
190000 1300 0.007 lb ai/acre 0.00091 10,000 0.00020 2E-06
Landscaping (plants/flowers)
190000 1300 0.0025 lb ai/6 oz can 0.00033 29,000 0.000070 7E-07
RTU (PL): Total-release Fogger
Warehouse Negligible exposure
Negligible exposure
0.035 lbs ai/can 8 cans Negligible exposure
Negligible exposure
Negligible exposure
Negligible exposure
RTU (S): Ear Tag Livestock Negligible exposure
Negligible exposure
0.0044 lbs ai/eartag 400 eartags Negligible exposure
Negligible exposure
Negligible exposure
Negligible exposure
RTU (L): Wipe/Towelette
Domestic Animals 2380000 480 0.0062 8 animals 0.000298 31,000 0.0018 2E-05
Livestock (Horses) 2380000 480 0.0062 25 animals 0.00093 10,000 0.0056 5E-05
Mixer/Loader/Applicator
DF: Backpack Christmas Tree Farm 58400 69.1 0.2 lb ai/acre 5 acres 0.00086 11,000 0.00092 9E-06
Conifer Pine Seed Orchard
58400 69.1 0.016 lbs ai/gal 40 gallons 0.00055 17,000 0.00059 6E-06

Permethrin Human Health Risk Assessment DP No. 414137
Page 126 of 129
Table H.1.2. Occupational Handler Non-Cancer and Cancer Exposure and Risk Estimates for Permethrin Non-Agricultural Uses.
Exposure Scenario Formulation: Equipment
Crop or Target
Unit Exposure (μg/lb ai)1
Maximum Application Rate3
Area Treated or Amount Handled Daily4
Non-Cancer Cancer
Dermal Inhalation Inhalation Commercial Applicator
PPE2 Dose
(mg/kg/day)5 MOE6 Total LADD7 Cancer Risk
Estimate8
DF: Manually-Pressurized Handwand
Christmas Tree Farm 100000 30 0.2 lb ai/acre 5 acres 0.00038 25,000 0.0015 1E-05
DF: Mechanically-Pressurized Handgun
Greenhouse Ornamentals 3500 120 0.2 lb ai/gal 1000 gallons 0.30 31 0.0027 3E-05
Christmas Tree Farm 6050 8.68 0.2 ai/acre 125 acres 0.0027 3,500 0.0024 2E-05
L/EC: Backpack
Greenhouse Ornamentals 13200 140 0.037 lb ai/gal 40 gallons 0.0026 3,600 0.00039 4E-06
Wildlife Management 58400 69.1 69.1
0.04 lbs ai/gal 40 gallons 0.0014 6,700 0.0015 1E-05
Christmas Tree Farm 58400 0.2 lb ai/acre 5 acres 0.00086 11,000 0.00092 9E-06
Forestry (ground directed)
8260 2.58 0.016 lb ai/gal 40 gallons 0.000021 450,000 0.000081 8E-07
Forestry (foliar) 58400 69.1 0.016 lbs ai/gal 40 gallons 0.00055 17,000 0.00059 6E-06
Landscaping (trees/shrubs)
58400 69.1 0.2 lbs ai/acre 5 acres 0.00086 11,000 0.00092 9E-06
Landscaping (lawns/turf)
58400 69.1 0.04 lb ai/gal 40 gallons 0.0014 6,700 0.015 1E-04
Structural (termiticide) 2510 30 0.0332 lbs ai/1000 sq
ft 1000 linear ft 0.013 750 0.0017 2E-05
Industrial/commercial (tires, rail yards, junk
yards, etc.) 2510 30 0.0023 lb ai/gal 40 gallons 0.00056 17,000 0.000077 7E-07
Livestock 2510 30 0.0023 lb ai/animal 400 animals 0.00035 27,000 0.000048 5E-07
Poultry/livestock house/horse barn/feed lot
2510 30 0.113 lb ai/gal 40 gallons 0.0017 5,500 0.00024 2E-06
Foundations/perimeter 8260 2.58 0.78 lb ai/gal 40 gallons 0.0010 9,300 0.0040 4E-05
Aquatic Vector Control 8260 2.58 0.007 lb ai/acre 5 acres 0.0000011 8,300,000 0.0000044 4E-08
L/EC: Injector Structural (termiticide) 1300 2.2 0.08 lb ai/gal 2000 gallons 0.0044 2,100 0.0033 3E-05
L/EC: Manually Pressurized Handwand
Greenhouse Ornamentals 100000 30 0.037 lb ai/gal 40 gallons 0.00056 17,000 0.0023 2E-05
Wildlife Management 100000 30 0.04 lbs ai/gal 40 gallons 0.0006 16,000 0.0025 2E-05
Christmas Tree farm 100000 30 0.2 lb ai/acre 5 acres 0.00038 25,000 0.0015 1E-05
Landscaping (trees/shrubs)
100000 30 0.02 lbs ai/acre 125 acres 0.0094 1,000 0.038 4E-04

Permethrin Human Health Risk Assessment DP No. 414137
Page 127 of 129
Table H.1.2. Occupational Handler Non-Cancer and Cancer Exposure and Risk Estimates for Permethrin Non-Agricultural Uses.
Exposure Scenario Formulation: Equipment
Crop or Target
Unit Exposure (μg/lb ai)1
Maximum Application Rate3
Area Treated or Amount Handled Daily4
Non-Cancer Cancer
Dermal Inhalation Inhalation Commercial Applicator
PPE2 Dose
(mg/kg/day)5 MOE6 Total LADD7 Cancer Risk
Estimate8
Landscaping (lawns/turf)
100000 30 0.04 lb ai/gal 40 gallons 0.0006 16,000 0.025 2E-04
Industrial/commercial (tires, rail yards, junk
yards, etc.) 100000 30 0.037 lb ai/gal 40 gallons 0.00056 17,000 0.0023 2E-05
Food Handling Establishment
(broadcast) 29000 1100
0.037 lbs ai/gal 40 gallons 0.020 460 0.0014 1E-05
Food Handling Establishment (C&C)
29000 1100 0.020 460 0.0014 1E-05
Warehouse (broadcast) 29000 1100 0.037 lbs ai/gal 40 gallons
0.020 460 0.0014 1E-05
Warehouse (C&C) 29000 1100 0.020 460 0.014 1E-04
Poultry/livestock house/horse barn/feed lot
100000 30 0.113 lb ai/gal 40 gallons 0.0017 5,500 0.0069 7E-05
Livestock 100000 30 0.0023 lb ai/animal 400 animals 0.00035 27,000 0.0014 1E-05
Foundations/perimeter 100000 30 0.78 lb ai/gallon 40 gallons 0.012 800 0.048 5E-04
Mounds/Nests 100000 30 0.08 lbs ai/mound 1000 mounds 0.03 310 0.12 1E-03
Interior Landscaping 100000 30 0.041 lb ai/gallon 40 gallons 0.00062 15,000 0.0025 2E-05
L/EC: Mechanically-Pressurized Handgun
Golf course (fairways, tees, greens)
1140 1.9 0.87 lb ai/acre 5 acres 0.000094 100,000 0.000072 7E-07
Christmas Tree farm 6050 8.68 0.2 lb ai/acre 125 acres 0.0027 3,500 0.0024 2E-05
Landscaping (Lawns/Turf)
1140 1.9 0.87 lb ai/acre 5 acres 0.00010 91,000 0.000079 8E-07
Livestock 1800 79 0.00027 lbs ai/animal 400 animals 0.00011 88,000 0.0000069 7E-08
Aquatic Vector Control 6050 8.68 0.007 lb ai/acre 5 acres 0.0000038 2,500,000 0.0000034 3E-08
WP: Backpack Conifer Pine Seed
Orchard 58400 69.1 0.016 lbs ai/gal 40 gallons 0.00055 17,000 0.00059 6E-06
WP: Mechanically-Pressurized Handgun
Greenhouse Ornamentals 3500 120 0.2 lbs ai/gal 1000 gallons 0.3 31 0.0027 3E-05
WSP: Backpack Christmas Tree farm
58400 69.1 0.2 lb ai/acre 5 acres 0.00086 11,000 0.00092 9E-06
WSP: Manually- 100000 30 0.2 lb ai/acre 5 acres 0.00038 25,000 0.0015 1E-05

Permethrin Human Health Risk Assessment DP No. 414137
Page 128 of 129
Table H.1.2. Occupational Handler Non-Cancer and Cancer Exposure and Risk Estimates for Permethrin Non-Agricultural Uses.
Exposure Scenario Formulation: Equipment
Crop or Target
Unit Exposure (μg/lb ai)1
Maximum Application Rate3
Area Treated or Amount Handled Daily4
Non-Cancer Cancer
Dermal Inhalation Inhalation Commercial Applicator
PPE2 Dose
(mg/kg/day)5 MOE6 Total LADD7 Cancer Risk
Estimate8
Pressurized Handwand
WSP: Mechanically-Pressurized Handgun
6050 8.68 0.2 lb ai/acre 125 acres 0.0027 3,500 0.0024 2E-05
Loader/Applicator
G: Belly Grinder Landscaping (Lawns/Turf)
10000 62 0.65 lb ai/acre 1 acre 0.000504 19,000 0.00012 1E-06
G: Cup Mounds/Nests 112 12.5 0.00156 lb ai/mound 1000 mounds 0.000244 38,000 0.00016 2E-06
Paint/Stain: Airless Sprayer
Structural (warehouses, FHE, home bathrooms)
42600 560
0.04 lbs ai/gal 40 gallons
0.0112 840 0.0015 1E-05
Structural (bridges, shipyards, home decks,
foundations) 42600 560 0.0112 840 0.0015 1E-05
Paint/Stain: Brush/Roller
Structural (warehouses, FHE, home bathrooms)
180000 280
0.04 lbs ai/gal 5 gallons
0.0007 13,000 0.00058 6E-06
Structural (bridges, shipyards, home decks,
foundations) 180000 280 0.0007 13,000 0.00058 6E-06
1 Based on the “Occupational Pesticide Handler Unit Exposure Surrogate Reference Table” (http://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/occupational-pesticide-handler-exposure-data); Level of mitigation: Baseline PPE with no gloves and no respirator, Eng. Controls.
2 PPE = Baseline (i.e., long sleeved shirt, long pants, shoes plus socks) no gloves, and no respirator unless otherwise indicated 3 Based on registered uses listed in Appendix A, Tables 4.1 and 4.2. 4 Exposure Science Advisory Council Policy #9.1. 5 Inhalation Dose = Dermal Unit Exposure (μg/lb ai) × Conversion Factor (0.001 mg/μg) × Application Rate (lb ai/acre or gal) × Area Treated or Amount Handled Daily (A or gal/day) ÷ BW (kg). 6 Inhalation MOE = Inhalation NOAEL (mg/kg/day) ÷ Inhalation Dose (mg/kg/day). 7 Total LADD = Dermal LADD + Inhalation LADD 8 Total Cancer Risk Estimate = Total LADD × Q1
*, where Q1* = 9.567 x 10-3 (mg/kg/day)-1

Permethrin Human Health Risk Assessment DP No. 414137
Page 129 of 129
Table H.1.3. Occupational Handler Non-Cancer Exposure and Risk Estimates for Permethrin (Seed Treatment).
Crop or Target
Inhalation Unit Exposure1
(mg/lb ai)
Maximum Application Rate2
Amount of Seed Treated (T) or Planted (P) Per Day3
Inhalation (LOC = 30)
[Level of PPE] (lb ai/lb seed) (lb seed/day) MOE5 Mixer/Loader
Corn (field, pop, sweet) 0.0012 [No R]
0.0037 339,500 (T) 500 Soybeans 0.0031 281,250 (T) 720
Planters
Corn (field, pop, sweet) 0.0034 [No R]
0.0037 8,800 (P) 6,800 Soybeans 0.0031 33,400 (P) 2,100
1 Based on the Science Advisory Council for Exposure Policy 14 (May 2003); Level of mitigation: No R = No Respirator, PF5 Respirator, and PF10 Respirator. 2 Based on registered label (Reg. No. 400-560). Summarized in Appendix A, Table 4.1. 3 Based on highest pounds of seed treated per day (corn and soybean) from HED Exposure Science Advisory Council Interim Policy 15.1. 4 Inhalation MOE = Inhalation HED (mg/kg/day) ÷ Inhalation Dose (mg/kg/day). Inhalation Dose = Inhalation Unit Exposure (mg/lb ai) × Application Rate (lb ai/lb of seed) × Amount Handled Daily
(lb seed treated or planted/day) ÷ BW (80 kg).