5'-Terminal Cap Structure EucaryoticMessenger …ture, function, and biosynthesis of messenger...
Transcript of 5'-Terminal Cap Structure EucaryoticMessenger …ture, function, and biosynthesis of messenger...

MICROBIOLOGICAL REVIEWS, June 1980, p. 175-205 Vol. 44, No. 20146-0749/80/02-0175/31$02.00/0
5'-Terminal Cap Structure in Eucaryotic MessengerRibonucleic AcidsAMIYA K. BANERJEE
Department of Cell Biology, Roche Institute ofMolecular Biology, Nutley, New Jersey 07110
NqTRODUCTION ............................................................. 1755'-TERMINAL CAP STRUCTURE IN MESSENGER RIBONUCLEIC ACID
(mRNA)............................................................... 176Discovery ................................................................. 176Isolation and Characterization of the Cap Structures ...... .................. 177
Viral mRNA's ............................................................ 177(i) Phosphatase-resistant 5'-terminal phosphates in methylated
mRNA's .......................................................... 177(ii) Structure of the blocked 5' termini ................................. 177
(iii) 5'-5' Inverted linkage of the terminal nucleotides ...... ............ 178Cellular mRNA's ........................................................... 178
Properties ofCap Structures .............. .................................. 178Distribution of Capped mRNA's in Nature ........ .......................... 179
Viral mRNA's ............................................................. 179Cellular mRNA's......................................................... 182
MECHANISM OF THE CAPPING REACIION ................................ 183ViralmRNA's ............................................................... 183
Reovirus................................................................. 183Vaccinia virus............................................................ 184Cytoplasmic polyhedrosis virus ........................................... 184Vesicular stomatitis virus .............. .................................. 185Influenza virus........................................................... 185
Cellular mRNA's ............................................................ 186ENZYMES INVOLVED IN THE CAPPING REACTIONS ...... ................ 187mRNA Guanylyltransferase and mRNA (Guanine-7-)Methyltransferase ..... 1882'-O-Methyltransferase ...................................................... 188Guanylyltransferases and Methyltransferases from Eucaryotic Cells ..... ... 189Enzymes That Cleave the Cap Structure ........ ............................ 190
BIOLOGICAL ROLE OF THE CAP STRUCTURE ....... ...................... 190Protein Synthesis........................................................... 190mRNA Stability ............................................................. 194mRNA Biosynthesis......................................................... 194
CONCLUSIONS ............................................................... 196LITERATURE CITED......................................................... 196
INTRODUCTION
Over the past 10 years the study of the struc-ture, function, and biosynthesis of messengerribonucleic acid (mRNA) in eucaryotic cells hasbeen an area of intense biochemical research invarious laboratories. Gratifyingly, these studieshave produced fascinating discoveries whichhave enabled us to approach an understandingof the regulation of eucaryotic gene expression.Unlike procaryotic mRNA's, eucaryoticmRNA's are synthesized in the nucleus of a cellas part of a long transcriptional precursor unit(commonly referred to as heterogeneous nuclearRNA [hnRNA]). These precursors are appar-ently subjected to various post-transcriptionalmodifications and tailoring before maturemRNA's are finally transported to the cyto-
plasm (48). These post-transcriptional processesinclude cleavage, 5'-terminal capping, methyla-tion, 3'-terminal polyadenylation, and splicing.Although the mRNA's in the cytoplasm carryingthe hallmarks ofthese modifications can be stud-ied easily, the precise molecular events whichoccur within the nucleus and lead to these mod-ifications are not yet clearly understood. Themain stumbling blocks to such investigationshave been the difficulties in radiolabeling RNAswithin nuclei in satisfactory amounts, character-izing the enzymes involved in the modificationprocesses, and finally developing a well-definedisolated nuclear system for the in vitro study oftranscription and RNA modification. Recently,great progress has been made in this directionbecause viruses have been used as tools in thestudy of cellular mRNA biosynthesis. Many vi-
175
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

176 BANERJEE
ruses contain virion-associated RN}ases which synthesize mRNA's incarry modifications identical to thosemRNA's (26). In addition, many de,cleic acid (DNA)-containing viruses r
cell nuclei, and in transformed celinomes become part of the cellular nintegration (57). Virus-specific mRNIthesized in the nucleus, processed, motransported to the cytoplasm, presithe same mechanism as cellular mRbthe recent understanding of mRNAand function has been facilitated by Iuing search into the processes involvmRNA biosynthesis.
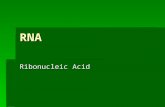
This review deals primarily with cthe important modifications ofmRNA's, namely 5'-terminal cappingmajority of eucaryotic mRNA's have]to contain a novel 5'-terminal structi5'-cap a guanosine residue blocks thepenultimate base of the mRNAunique 5'-5' linkage containing threegroups (Fig. 1). The blocking guanosuniversally contains a methyl groupposition, and the 5'-terminal penultiand its adjoining residue are alsomethylated in the ribose moiety orpenultimate residue is an adenosineposition as well. The unique 5'-terstructure of eucaryotic mRNA's cleientiates them from the polycistronic ImRNA's, which contain unblocked triends (7). These observations also in(the mode of mRNA biosynthesis inyotic system is more complex than icaryotic system; this is commensuralincreased complexity of the genetic oiof eucaryotic species.
Since the discovery of the cap st
FIG. 1. Structure of 5' cap. When bassine, it is methylated at the N-6 position.
v polymer-vitro thatof cellularoxyribonu-replicate ins their ge-naterial byk's are syn-dified, andumably byWA's. Thus,i structurethe contin-Ted in viral
nly one ofeucaryotic(211). Thebeen foundure. In thisr L__ __!___1- I
1974, a great deal of work has been done toelucidate its structure, synthesis, and functionalrole. I have attempted to review as much aspossible from both the published and the unpub-lished data available to me, highlighting thesignificant findings rather than describing in de-tail all of the papers published to date. Clearly,there are omissions, for which the responsibilityis mine. The excellent reviews by Shatkin (224)and Filipowicz (70) have dealt specifically withthe cap structures, and the reviews by Rottman(210), Adams (7), and Busch et al. (34) havedealt with these structures in their summarieson eucaryotic mRNA structure and function.
5'-TERMINAL CAP STRUCTURE INMESSENGER RIBONUCLEIC ACID
(mRNA)
Discovery5-termmial The existence of a 5'-terminal blocked struc-through a ture at the 5' terminus of an RNA molecule wasphosphate first shown by Reddy et al. (196). These re-,me residue searchers studied the low-molecular-weight nu-at the N-7 clear RNAs (4 to 8S) derived from Novikoffimate base hepatoma cells. The primary sequence analysissometimes of one of the RNA species revealed a blocked 5'when the terminus with a hypermethylated guanosine res-in the N-6 idue (2,2,7-trimethylguanosine) linked to a 2'-O-rminal cap methylated adenosine residue through a 5'-5'arly differ- pyrophosphate (PPJ) linkage. Since the preciseprocaryotic functions of the small nuclear RNA species wereiphosphate not clear, the importance of the cap structure indicate that these RNAs was not immediately evident. Thethe eucar- 5'-terminal structures of eucaryotic mRNA'sin the pro- were believed to be different from those of pro-te with the caryotic mRNA's since they were resistant torganization phosphorylation by polynucleotide kinase after
prior treatment with alkaline phosphatase (211),tructure in a property shared with the low-molecular-
weight nuclear RNAs. The discovery of the capstructure in mRNA's was achieved through a
BASEI(CH3A) series of original observations made by variousresearchers. Perry and Kelly (186) reported theexistence of methylated mRNA's in mouse L-cells. They reported the presence of approxi-mately 2.2 methyl groups per 1,000 nucleosides
O(CH3) in polyadenylic acid [poly(A)]-containing L-cellBASE2 mRNA. In addition, a portion of the methyl
5-CH2 0 label was found to be present in alkali-resistantoligonucleotides, suggesting that some of themethyl groups may be located in the ribose
0 O(CH3) moieties in adjacent nucleotides or at the 5'O=P-O termini of the mRNA molecules. Similarly, the
°- existence of methylated mRNA's in Novikoff3e, is adeno- hepatoma cells was reported by Desrosiers et al.
(52). Miura et al. (161, 162) observed that the 5'
MICROBIOL. REV.
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

5'-TERMINAL CAP STRUCTURES IN mRNA's 177
termini of the double-stranded genome RNAs ofcytoplasmic polyhedrosis virus (CPV) and reo-virus were scarcely labeled at their 5' terminiwith [y-32P]adenosine triphosphate (ATP) bythe polynucleotide kinase procedure. However,after sequential oxidation, fl-elimination, andphosphomonoesterase digestion, both of the 5'ends of CPV and reovirus were successfully la-beled with 32P. These authors also showed thatone of the strands of each of the 10 double-stranded genome RNAs of reovirus appeared tocontain blocked 5' termini containing 2'-O-meth-ylated guanosine (Gm). Furuichi (75), also work-ing with CPV, showed that the virion-associatedRNA polymerase requires S-adenosylmethio-nine (AdoMet) for transcription of the genomeRNA in vitro. Moreover, all of the mRNA spe-cies synthesized in vitro were methylated as partof the initiation step of transcription, indicatingthat the purified virions of CPV contain a meth-yltransferase activity that transfers the methylgroup of AdoMet to mRNA probably at or nearthe 5' end of nascent transcripts. Similar meth-yltransferase activities were later shown to bepresent in purified reovirus (68, 223), vacciniavirus (263), and vesicular stomatitis virus (VSV)(198). The actual identification of the site ofmethylation in mRNA and the eventual eluci-dation of the 5'-terminal methylated cappedstructure shown in Fig. 1 were reported inde-pendently by Furuichi et al. (79), Wei and Moss(264), and Furuichi and Miura (78), who usedreovirus, vaccinia virus, and CPV, respectively.These results were followed quickly by a seriesof reports which demonstrated the presence ofthe cap structure in viral and eucaryoticmRNA's; these included reports on vaccinia vi-rus by Urushibara et al. (256), VSV by Abrahamet al. (2), mouse L-cells by Perry et al. (188, 189),rat hepatoma cells by Desrosiers et al. (53),HeLa cells by Furuichi et al. (81) and Wei et al.(261, 262), and mouse myeloma cells by Adamsand Cory (8).
Isolation and Characterization of the CapStructures
Viral mRNA's. The detailed method of iso-lation and characterization of the cap structuresin mRNA's was established through studies ofmethylated mRNA's synthesized in vitro by thevirion-associated polymerases of reovirus, vac-cinia virus, CPV, and VSV (2, 78, 79, 264). Theessential steps involved in the isolation and sub-sequent characterization of the cap structure, asexemplified for reovirus mRNA's (79), are de-scribed below.
(i) Phosphatase-resistant 5'-terminal
phosphates in methylated mRNA's. Undernormal in vitro transcription conditions (i.e., inthe absence of AdoMet) in the presence of [f,-y-32P]guanosine triphosphate (GTP), the reovi-rus mRNA's terminate with 5' ppGpC (83) (anasterisk denotes a labeled phosphate). However,in the presence of AdoMet, it was observed that54% of the 5'-terminal labeled phosphate fromthe initiating nucleotide GTP was resistant toremoval from the RNA by treatment with al-kaline phosphatase. These results indicated thata population of the mRNA molecules synthe-sized in the presence of AdoMet must contain5'-terminal ppGpC in a blocked structure.
(ii) Structure of the blocked 5' termini.To study the nature of the phosphate-protectinggroup, mRNA synthesized in vitro in the pres-ence of [/B-y-32P]GTP and [methyl-3H]AdoMetwas digested with nuclease P1, an enzyme thatcleaves phosphodiester linkages in nucleic acidsto yield 5'-mononucleotides (74). All of themethyl-3H- and 32P-labeled material migratedtogether between pA and pG markers on paperelectrophoresis. The molar ratio of 32P to methyl-3H was 1:2. The electrophoretic migration andthe net negative charge (-3) of this materialwere not altered after digestion with alkalinephosphatase, indicating that the phosphate moi-ety was resistant to the enzyme. However, whenthe phosphatase-resistant structure was treatedwith a combination of alkaline phosphataseand nucleotide pyrophosphatase, all of the3p was converted to inorganic orthophosphate(Pi) and the 3H radioactivity was divided equallybetween material that migrated with theauthentic markers 7-methylguanosine (7mG)and Gm. From these results, together with otherconfirmatory data, it appeared that the 5'-ter-minal blocked structure of reovirus mRNA's invitro was 7mGpfpGmp.... The sequence of thereactions which are described above and led tothe elucidation of the structure is as follows:
pppG + CTP + UTP + ATP
+ [methyl-3H]AdoMet - 7mGpfpGip...
+ PPi + AdoHcy (1)
7mGpppG pCpUpXp + nuclease P1
- 7mGp*pG' + nPi + pC + pU + pX (2)
7mGp*pG' + nucleotide pyrophosphatase
*7mGp+ pG' + Pi (3)
7mGp*pGm + nucleotide pyrophosphatase
+ alkaline phosphatase 7mG + Gm + Pi (4)
VOL. 44, 1980
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

178 BANERJEE
where CTP is cytidine triphosphate, UTP isuridine triphosphate, and AdoHcy is S-adenosyl-L-homocysteine.
(iii) 6'-5' Inverted linkage of the terminalnucleotides. It is apparent from reaction 3, inwhich the products of nucleotide pyrophospha-tase treatment of the cap structure are 5' 7mGpand pG, that a 5'-5' inverted linkage exists be-tween the blocking guanosine base and the pe-
nultimate residue in the RNA. A nucleotidelinkage of this type contains a 2',3'-cis-diol sim-ilar to the 3'-terminal nucleotides of RNA.
Cellular mRNA's. Isolation and characteri-zation of the cap structures of cellular mRNA'shave been carried out essentially by the follow-ing two methods. (i) The RNAs of cells were
labeled uniformly in vivo with 32Pi and purifiedfrom the cytoplasmic fraction. The poly(A)-con-tamiing mRNA's were purified by repeated chro-matography on oligodeoxythymnidylic acid-cel-lulose columns (186). The purified mRNA's were
then digested with ribonuclease (RNase) T2, andthe products were fractionated by electropho-resis on cellulose acetate at pH 3.5 and then on
diethylaminoethyl paper at the same pH (8). Aseries of capped and methylated oligomers wereobtained; these were further characterizedchemically and enzymatically as describedabove. Using this method, Cory and Adams (45)identified a minimum of 27 oligonucleotides con-
taining a capped structure in mRNA's isolatedfrom mouse myeloma cells. (ii) The cap struc-tures in mRNA's were labeled with methyl-3Hby exposing exponentially growing cells to[methyl-3H]-methionine in medium containinga reduced concentration of methionine and sup-plemented with sodium formate, adenosine, andguanosine to prevent incorporation of methio-nine methyl groups via the one-carbon pool(186). The poly(A)-containing mRNA's were an-
alyzed for cap structures by various enzymaticprocedures, as described above. A similar ap-
proach was taken to analyze cap structures inmRNA's isolated from virus-infected cells (172).mRNA's were purified by chromatography on
oligo-deoxythymidylic acid-cellulose or, in cer-
tain cases, by hybridization to viral genomeRNA or deoxyribonucleic acid (DNA) (138).The cap structures isolated from eucaryotic
mRNA's and subsequently characterized havethe general structure 7mG(5')ppp(5')NlmpN2m-pN3P..-, where N is a purine or a pyrimidine.mG is universally present as the capping base,and the 5'-penultimate nucleoside (N1) and itsadjacent nucleoside (N2) can also be methylated.The methylation of the latter two mncleosidesmay vary, generating the following types of
structures: 7mG(5')ppp(5')N,pN2p... (cap 0),7mG(5')ppp(5')NlmpNp... (cap 1), and 7mG(5')-ppp(5')NlmpN2mp...(cap 2). Various RNaseshave different modes of action on the cap-con-taining regions of the mRNA's. RNase T2, anonspecific RNase, does not cleave the phospho-diester bond adjacent to a 2'-0-methylated nu-cleoside. Thus, after RNase T2 digestion,mRNA's containing cap 0, cap 1, and cap 2 yield7mGpppN,p, 7mGpppNimpN2p, and 7mGppp-NimpN2mpN3p, respectively, in addition to theinternal mononucleotides. Similar products arealso generated by alkaline hydrolysis ofmRNA'scontaining the corresponding cap species. Nu-clease P1, on the other hand, generates thestructures 7mG(5')ppp(5)N1 (referred to as thecap core) from cap 0-containing mRNA's and7mG(5')ppp(5')Nim from both cap 1- and cap 2-containing mRNA's. RNase A and RNase T,digestions of mRNA's generate cap-containingoligonucleotides terminated with 3'-pyrimidineand guanosine, respectively. Thus, by using var-ious RNases, different cap-containing oligonu-cleotides of mRNA's can be isolated (for exam-ple, by binding of the oligonucleotides to dihy-droxyborate cellulose [85a, 197]), and they canbe further characterized by electrophoresis (45,79), chromatography on diethylaminoethyl-cel-lulose (2, 264), or high-pressure liquid chroma-tography (52).
Properties of Cap Structures
The cap structures have some interesting,characteristic features that impart special prop-erties to the 5'-terminal structures and thus tothe mRNA's. These are described below.
(i) The capping base is universally 7mG, exceptfor Sindbis virus mRNA, which contains a lowlevel of 2'2'7m3G at the 5' terminus (see below).So far, no other terminal base besides 7mG hasbeen found in the cap structure of eucaryoticmRNA's. Although 7mG is present intemally intransfer RNA, it is absent in ribosomal RNA,hnRNA, and cytoplasmic mRNA. In the lattertwo RNA species 7mG is present only as part ofthe 5'-terminal cap. It is interesting to note thatthe N-7 atom of guanine is more susceptible tothe action of alkylating compounds, and theconversion of guanine to 7-alkylguanine in nu-cleic acid is considered to be an important eventunderlying the mutagenic, cytotoxic, carcino-genic, and carcinostatic activities of biologicalalkylating agents (139). Alkylation of the N-7atom of guanine in a nucleic acid also introducesa positive charge in the imidazole ring, whichhas been implicated in erroneous base pairing
MICROBIOL. REV.
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

5'-TERMINAL CAP STRUCTURES IN mRNA's 179
with thymine in DNA (140). Moreover, alkyla-tion of the N-7 atom facilitates rapid exchangeof the H-8 atom with water, and the imidazolering undergoes fision when treated with alkali(107). The 9-monosubstituted guanine deriva-tives, however, are resistant even when treatedwith hot strong alkali (107). Thus, alkylating ofthe N-7 atom in guanine imparts some specialchemical properties to the capped 5' termini ofmRNA's, but the precise functions of these arenot clear at the present time.
(ii) The unique 5'-5' phosphate linkage in thecap structure results in the retention of a 2',3'-cis-diol on the 5'-terminal 7mG. In this respect,the 3' and 5' ends of the mRNA appear to beidentical. The presence of the cis-diol at the 5'end permits special chemical reactions usuallyrestricted to the 3' end, such as periodate oxi-dation followed by labeling with [3H]borohy-dride (173). The capping base may also be selec-tively removed by fl-elimination by treating theoxidized nucleoside with aniline (173). The re-active dialdelyde may be reduced with cyano-borohydride in the presence of protein, yieldingcovalently linked protein-RNA conjugates (234).It is also possible to isolate 5'-terminal fragmentsof mRNA by using affinity chromatography onresins containing covalently bound borate,which were originally developed for selecting 3'-terminal fragments of RNA (208a).
(iii) Nuclear magnetic resonance studies (54,126, 127) of the capped structure 7mGpppAmpAphave revealed that the three bases form a closelystacked array, resulting in increased stability. Itappears that 7mG5'p has enough flexibility sothat it can intercalate between the two adenineresidues. The 2'-O-methyl group present in thepenultimate nucleoside induces sufficient con-formational strain that the backbone of theAmpA segment assumes an extended spatial con-figuration, enabling the 7mG5'p residue to inter-calate between the adenine bases (111). Thepresence of a long triphosphate bridge wouldallow 7mG5'p to bend and intercalate betweenthe adenines. Thus, it appears that the overallspatial configuration of the mRNA terminus7mG5'ppp5'AmpA is a self-intercalating onewhich imparts a unique feature to the terminus.These features may affect stability, allow inter-action with specialized proteins in ribosomes,etc.
Distribution of Capped mRNA's in Nature
After the discovery of cap structures in reo-virus, vaccinia virus, CPV, and VSV, a searchwas made for such structures in a variety ofother viral and cellular mRNA's. As Tables 1
and 2 show, cap structures are present almostuniversally in eucaryotic mRNA's, with only afew exceptions. Capped mRNA's are present inyeasts, molds, plants, insects, and higher orga-nisms. The only reported exceptions among cel-lular mRNA's are the poly(A)-containingmRNA's isolated from HeLa cell mitochondria(90, 248). These mRNA's may contain ppA atthe 5' termini. The 5' termini of maternalmRNA's obtained from developing oocytes ofthe tobacco hornworm (Manduca sexta) appar-ently contain a cap structure but lack methyla-tion of the capping guanosine (117). In plant andanimal viruses almost all mRNA's of both theDNA- and RNA-containing viruses contain acap structure, except picornaviruses (108, 142)and three plant viruses, satellite tobacco necrosisvirus (144, 269) and its helper virus (143) andcowpea mosaic virus (CMV) (50, 241). In thecase of picornaviruses, including poliovirus andencephalomyocarditis virus, it has been shownthat a protein (molecular weight, 12,000) is con-nected covalently to the 5'-terminal uracil of theviral genome through a phosphodiester linkageto a tyrosine residue in the protein (88, 108). Asimilar protein-linked genome has also been de-scribed for CMV (50, 241). Satellite tobacconecrosis virus, however, contains a di- or tri-phosphate (p)ppA at the 5' end (144), which issimilar to bacterial and bacteriophage mRNA's(144, 269).
In a survey of cap structures present in eucar-yotic mRNA's (Tables 1 and 2), an interestingpattern emerges with regard to the cap compo-sition of various types of mRNA's. These fea-tures can be summarized as follows.Viral mRNA's. All mRNA's contain 7mG as
the capping base except the few viral mRNA'sthat do not contain a cap (Table 1). Only themRNA strand (i.e., the positive strand) iscapped, whereas the antisense strand, such asthe genome of VSV and influenza virus and onestrand of the duplex genome RNA of reovirusand CPV, usually has a di- or triphosphate 5'end rather than a cap. This indicates that cap-ping is probably restricted to mRNA's. The ex-ceptions are the low-molecular-weight nuclearRNAs (46, 196, 202) which are capped but donot act as messengers in in vitro protein synthe-sizing systems (195). The 5'-penultimate base ofviral mRNA's is always a purine, which may ormay not be methylated. This may indicate thatin viruses initiation of RNA synthesis or proc-essing ofRNA may be purine specific. When the5'-penultimate base is adenosine, it exists in themajority of cases in a dimethylated form, N6-methyl-2'-O-methyladenosine (N6mAm) (260).Plant viruses usually contain a cap 0 structure,
VOL. 44, 1980
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

180 BANERJEE MICROBIOL. REV.
_l O
N.Cl.- -- C( C
N 4 Cs - '5,lw
*- C4 C i
o0_ c
-0
2P-
et- " _
C400
t-
.C
IV usf
00 C
,- Cl- so
Cl 00 Cl
ClsOC4
cl N- 00- C-l ClCl -
00 6 R - -0oo
0 _2 CD Ot- '5, -- v -- -4 m Cq C
Cu*o .. .¢0 0 o o o 0 oo ooo o 0 000E ZZZEZZZ Z Z Z° z ZZZZZZZZ ZZZ
<~~~~~~~~0 E
E ¢ OXEC) 3
-
-
=Z EO EQ E zPz zXX
E na ,Z,6i6 ,, zz ,, z zzzzzzz z zzz
lg_-
-o < 22
z z 0. E EE-m .& & &e cooozzzzz zz¢zz L - -CL Q m
~~M. , . ...~~~~~
0 0E- ~ ~ Z
C.
x 0I 0z z 2 - &EE &--0I<Cu &Q C.LZV=,,' CL *CC.L A ,Q,Z, C.. Z Q L
ci~~~~~~~~~~~~~c
o .OOO.O.O.OO. a.)a).> ) a,) O
QQQQQQQZQQQ ZZZ Z O
b+ + + + + + I+l + + + + + +++
rZZZZZz ZZ ZZZZ Z z ZzzzzZ Z ZZZE azak X ak a e aza aq z ax azug
Wzaza) X ak ak a)az cna) ~ ~ cnW)W)W)W)WeWWnXa )a t r u XXa ea n6
*5clacnco
5.-.~ ~ ~ ~~ ~~~~~~~.- -;c 0 > C > .C
Cu (A > 'oC
ico 0) cecd> C/2 14 0~~~~~~~(
a)
Cu~a)a)~
0ccq
L oM'= Cq -4 --
L.2z
CsL1.
-4
V)
a)
co~
C.)
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

5'-TERMINAL CAP STRUCTURES IN mRNA's 181
.'
I;A'
01t. .3
0C9C-
r~~~~-0E
2 z z zEE2 2
z~~~~~~~~~.0.
O= 0.
z -E E E Z E
z 2
E~ ~~~2~,
P' .,
20~~~~~~~~
1. 0. 'iE* - z0. Q(
OV~~~~~~~~"
~0. ZI 4-Z
0
Cao 2mm
00
la
(A
:r
ON
00.~~~~~~~~~~~i
.0
.0
'
2 E z a z
-C ci t ;& - %
VOL. 44, 1980
C)
0
I.
04go
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

TABLE 2. 5'- Terminal cap structures in cellular mRNA 'sCap structure' Intenal meth-
Cells ylation Reference(s)Cap 0 Cap 1 Cap 2 ylto
Human HeLa cells None N1 = Pum, Pym, m6Am N2 = Pum, Pym m6A 81, 261, 262,266
HeLa cell histone None N, = Am, m6Am, Gm N2 = Pum, Py None 167, 242Mouse myeloma None N, = Pum, Pym, mSAm N2 = Pum, Pym m6A 8, 45Mouse erythroid None N, = pum, pym, m6Atm N2 = Pum, Pym m6A 101Mouse L-cells None N, = pum, pym, m6Am N2 = Pum, Pym m6A 188Mouse kidney None N1 = Nm N2 = Nm Present 182, 183Rat hepatoma None N, = pum, pym, m6Am N2 = Pum, Pytm m6A 52, 53Hamster kidney (BHK-21) None N1 = Pum, Pym, mSAm N2 = Pum, Pytm m6A, m5C 61, 172Monkey kidney (BSC-1) None N, = pum, pym, m6Am N2 = Pum, Pym m6A 138Mouse immunoglobulin None N, = Gi, m6Am N2 = Am m6A 47, 156Human globin None N, = m6Am, Am N2 = Cm None 140Mouse globin None N1 = m6Am, Am N2 = Cm None 39Rabbit globin None N1 = mSAm N2 = Cm None 47, 148, 150Duck globin None N, = Nm NDb None 190Ovalbumin N, = A N1 = m6AAm, Am N2 = PYm ND 154Protamine Contains cap 86Drosophila N, = C N1 = Pym> Pum N2 = Pum, Pym ND 147Silk fibroin None N1 = Am N2 = Um m6A 274Aedes albopictus None N, = Pum, Pym N2 -- Pum, Pym m6A 113Tobacco hornworm oocyte GpppN 117Brine shrimp N, ND ND ND 174Sea urchin embryo None N1 = Pum > Pytm None m6A 69, 244Slime mold N = A > G N1 = Am (10%) None None 58Neurospora N = A > G None None None 220Yeast N = A > G None None None 51, 152, 240Wheat embryo N, None None None 98, 125, 213Maize N, None None None 178Soybean seeds N, N, = Nm None None 247
"Cap 0 denotes7mGpppN1pN2p...; cap 1 denotes 7mGpppNitmpN2p.. .; and cap 2 denotes7mGpppNimpN2mp....N1 and N2 are purine (Pu) or pyrimidine (Py) ribonucleosides.
b ND, Not determined.
but among animal viruses cap 0 structures arerare, with the exception of Sindbis virus genomeRNA (103) and Newcastle disease virus mRNAsynthesized in vitro (44). The majority of viralmRNA's contain a cap 1 (in vitro mRNA's) orcap 2 (in vivo mRNA's) structure. In addition tomethyl groups in the cap structure at the 5'terminus of an mRNA, N6-methyladenosine(N6mA) is often present internally in a varietyof mRNA's. Viruses that replicate in the cyto-plasm of infected cells (such as VSV, reovirus,and vaccinia virus) do not contain internalN6mA residues, whereas those replicating in thenucleus (simian virus 40, adenovirus, etc.) con-tain N6mA located internally. The exception isSindbis virus, in which N6mA is present in invivo mRNA's but not in genome RNA. Theseresults may indicate that the methylation ofinternal adenosines in mRNA is a nuclear event.5-Methylcytidine (m5C) also has been shown tobe present internally in adenovirus (233) andSindbis virus (59) mRNA's. In the latter case,m5C represents the only internal methylatedbase. Interestingly, a fraction of the Sindbis virus
mRNA (26S) molecules from infected cells con-tain, in addition to the usual 7mG, the hyper-modified congeners m227G, and m32'2'7G (112),similar to the low-molecular-weight nuclearRNAs (46, 202).Cellular mRNA's. A variety of 5'-terminal
capped sequences were found in higher eucar-yotic mRNA's, including those of HeLa cells,myeloma cells, baby hamster kidney cells, etc.In the well-studied mouse myeloma cells (MPC-11-66) (45), a minimum of 27 5'-terminal se-quences were reported. Unlike viral mRNA's,the 5'-penultimate base can be any of the fourstandard bases or NemAm. The second base canalso be any one of the standard four. mRNA'scontaining 7mG(5')ppp(5')CmpUp appear to bepresent in relatively high abundance (19%), com-pared with other sequences, which occur at lev-els of less than 1%. It is not known whether thedifferent termini are associated with specificclasses of mRNA or have any functional signifi-cance. In this respect, cellular mRNA's are morecomplex than viral mRNA's. The presence ofpyrimidines in the 5'-penultimate position in
182 BANERJEE MICROBIOL. REV.
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

5'-TERMINAL CAP STRUCTURES IN mRNA's 183
cellular mRNA's suggests the possibility thatthey may not be primary transcripts, assumingthat all RNA chains are initiated by purines(153). On the other hand, it is quite possible thatinitiation may occur by pyrimidines (209). Abetter understanding of the precise nature ofinitiation in eucaryotic systems will help to clar-ify these observations.The degrees of methylation in the cap struc-
tures are also different in cellular and viralmRNA's (Tables 1 and 2). Unlike viral mRNA's,cap 1 and cap 2 structures are present in allcellular mRNA's, although the cap 1 structureis about five times more abundant than the cap2 structure (187). As in viral mRNA's, 5'-penul-timate adenosine residues are predominantlyN6mAm. The only exception is in Drosophilamelanogaster mRNA's (147), which contain cap0 structures in addition to cap 1 and cap 2structures. Moreover, these mRNA's do not con-tain N6mAm. Like higher mammal mRNA's,Drosophila cell mRNA's contain purines andpyrimidines at the N1 bases of the cap 1 struc-tures. However, the majority ofpoly(A)-contain-ing cytoplasmic RNA molecules from heat-shocked Drosophila cells contain purine almostexclusively as the N1 base of cap 1. These resultsare consistent with other results, indicating thata special set of mRNA's is turned on during heatshock. It was also observed in DrosophilamRNA's that two RNase T2-resistant dinucleo-tides were present in the fingerprint (147). Thesedinucleotides are not capped and may or maynot be methylated; their precise compositionsare not known. Interestingly, similar spots in thefingerprints of mouse myeloma cell mRNA'swere also found (45), but they were not detectedin sea urchin or Dictyostelium mRNA's (58).As Table 2 shows, the diversity of cap struc-
tures is restricted to certain eucaryotes. A com-parison of the 5'-terminal sequences of mam-malian mRNA's and those of lower eucaryotemRNA's reveals phylogenetic trends in the capstructures. For example, only cap 0 is found inyeast mRNA and predominates in the mRNA ofthe unicellular slime mold Dictyostelium discoi-deum. Moreover, the N, base is a purine in bothof these organisms. Studies of sea urchin mRNAhave identified only cap 1 structures, with apurine in more than 80% of the N1 bases. Inmetazoan cell mRNA (Drosophila) three vari-eties of caps are found, with a random frequencyof purines and pyrimidines but no N6mAm in theN1 base. In the fibroin mRNA of the silkwormand in higher organisms, cap 1 and cap 2 struc-tures predominate, and N6mAm appears in theN1 position. These observations indicate thatthe cap structure and its adjacent bases increase
in complexity in the more developed genomestructures of higher eucaryotes. The precisefunction of these modified structures in relationto gene regulation is not known.The internal N6-methylation ofeucaryotic cel-
lular mRNA's also has an interesting pattern.The mRNA's isolated from higher eucaryoticorganisms, such as HeLa cells (human), L-cells(mouse), and BSC cells (monkey), invariablycontain N6mA internally. The striking excep-tions are the globin and histone mRNA's, whichdo not contain this methylated base internally(167, 190). In contrast to the findings describedabove, the mRNA's isolated from lower eucar-yotic organisms, such as slime molds, yeasts,etc., do not contain internal N6mA. Althoughthe reasons for these differences are not known,they may reflect some basic differences amongeucaryotic organisms that may directly relate tothe mode of mRNA biosynthesis. The methyla-tion of internal adenosine appears to occur atspecific sites in RNA. In HeLa cells and L-cellstwo methylated internal sequences were de-tected, namely, Gpm6ApC and Apm6ApC (217,266). Interestingly, in avian sarcoma virus ge-nome (55), Rous sarcoma virus (24), and simianvirus 40 late mRNA's (36) identical sequenceswere found. Like some animal viruses, culturedhamster kidney cells and NovikoffmRNA's havebeen shown to contain internal m5C at levels of20 and 3%, respectively, of the total, the remain-der being N6mA.
MECHANISM OF THE CAPPINGREACTION
Viral mRNA'sElucidation of the various steps involved in
the fonnation of the cap structure was madepossible through studies of animal viruses thatcarry virion-associated RNA polymerase. Underappropriate reaction conditions, mRNA's syn-thesized in vitro by virion-associated RNA po-lymerase may contain 5'-terminal uncappedstructures, capped but not methylated struc-tures, cap 0 structures, or cap 1 structures, dem-onstrating that these viruses contain all of theenzymes necessary for the synthesis of cap. Themechanisms of capping that have been well elu-cidated by studying reovirus (83), vaccinia virus(168), CPV (76), VSV (17), influenza virus (133),etc., are summarized below.Reovirus. Virion-associated RNA polymer-
ase transcribes in vitro the 10 double-strandedgenome RNAs into 10 mRNA species containingpredominantly the 5'-terminal sequenceppGpC... (224). In the presence of the methyldonor AdoMet, the predominant 5'-terminalstructure is 7mGpppG'pC... (79). Ifmethylation
VOL. 44, 1980
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

184 BANERJEE
is prevented by AdoHcy, 5'-terminal ppGpC...predominates (79). Reovirus cores incubatedin the presence of GTP, cytidine triphosphate,and AdoMet synthesize the cap structure7mGpppGmpC (83). The dinucleotide ppGpCfunctions as a substrate for a core-associatedguanylyltransferase and is converted toGpppGpC by the addition of pG from GTP. Foroptimal conversion, both a diphosphate termi-nus and a phosphodiester bond are required inthe acceptor. pGpC is not a substrate, butpppGpC is utilized after removal of the y-phos-phate by a core-associated nucleotide phospho-hydrolase. GpppGpC is hydrolyzed by the coresto ppGpC in the presence of PPi; however, thishydrolysis is markedly decreased by methyla-tion at N-7 of guanosine. It was further shownthat the ,--y-imido analog of GTP, GMPPNHP,can replace GTP for transcription; under theseconditions, the 5'-terminal structure ispNHppGpC (84). Thus, hydrolysis of the f8-ybond of GTP in the acceptor RNA molecule isneeded for cap formation. Based on these obser-vations, the following reaction series was pro-posed for the synthesis of reovirus mRNA caps:
RNApppG + pppC -N pppGpC + PP1
polymeraseNucleotide
pppGpC ppGpC + Piphosphohydrolase
ppGpC + pppG <__Guanylyltransferase
GpppGpC + PPiGpppGpC
+ AdoMet Methyltransferase I
7mGpppGpC + AdoHcy7mGpppGpC
+ AdoMet Methyltransferase II
7mGpppGmpC + AdoHcy
Thus, it appears that in reovirus mRNA's thea- and fl-phosphates of GTP and the a-phos-phate of the capping GTP are retained in thecap structure and that capping and initiation ofRNA synthesis occur concurrently.Vaccinia virus. mRNA's synthesized in
vitro by vaccinia virus in the presence ofAdoMet contain two 5'-terminal structures,7mGpppGmp... and 7mGpppAmp... (264). Thevirion contains guanylyltransferase and methyl-transferase activities, which modify the 5' endsof mRNA's. The mechanism of the capping re-action is similar to the reovirus system, and thefollowing scheme has been established (168):
p,BG + WBa' .~af'a' +BpppG ppN- GpppN- VA
GpppN- + AdoMet -- 7mGpppN- + AdoHcy7mGpppN- + AdoMet - 7mGpppNm- + AdoHcy
At limited concentrations of AdoMet, meth-ylation occurs at the N-7 G position, whereas athigher concentrations ribose methylation of thepenultimate N, base occurs. (See below for adetailed description of the properties of the vac-cinia virus guanylyltransferase and methyltrans-ferases which have been solubilized and purifiedfrom the virion in an active form.) Although theproperties of the purified enzymes have beenwell characterized, it is still not clear whetherthe specificities of the enzymes within the viruscore are the same as those of the highly purifiedenzymes.Cytoplasmic polyhedrosis virus. Like reo-
virus, the RNA polymerase of CPV transcribes10 genome segments in vitro (78). In contrast toreovirus and vaccinia virus, the synthesis ofmRNA in vitro is stimulated considerably bythe addition of AdoMet; the resulting mRNAcontains the 5'-terminal capped structure7mGpppAmpGp... (78). Moreover, the competi-tive inhibitor of methylation AdoHcy also effec-tively stimulates mRNA synthesis in vitro, re-sulting in the synthesis of viral mRNA contain-ing 5'-terminal GpppA and ppA. Thus, it appearsthat CPV may have an additional regulatorymechanism(s) by which AdoMet and AdoHcy"switch on" mRNA synthesis, probably as allo-steric effectors that influence the RNA polym-erase and capping enzymes (85) (see below). Itwas further shown that the f,,y-imido analog ofATP (AMPPNHP) failed to support methyla-tion and RNA synthesis, whereas similar analogsof GTP, cytidine triphosphate, and uridine tri-phosphate were effective substrates for RNAsynthesis and methylation (76). These resultswere interpreted as due to the inability ofAMPPNHP to replace 5'-ATP because it cannotbe processed by phosphohydrolase, an enzymeinvolved in cap formation (see above). It wasshown that during partial reactions in vitro withATP and GTP as substrates, GpppA was pro-duced and subsequently methylated to7mGpppAmp in the presence of AdoMet (227).However, neither the 5'-capped compounds norother dinucleotides, such as pppApG andppApG, stimulated mRNA synthesis in vitro inthe absence of AdoMet or AdoHcy (Y. Furuichi,personal communication). These results may im-ply that CPV RNA polymerase, for its full func-tion, may require that some functional barrierbe removed by an unknown process mediated bythe presence of AdoMet or AdoHcy (see below).
MICROBIOL. REV.
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

5'-TERMINAL CAP STRUCTURES IN mRNA's 185
Vesicular stomatitis virus. The virion-as-sociated RNA polymerase ofVSV synthesizes invitro five mRNA species that are capped andhave the 5' sequence GpppApApCpApG (3,197).These 5' ends are quantitatively methylated to7mGpppAmpApCpApGp... in the presence ofAdoMet (2). The interesting features of the capstructures of VSV mRNA that distinguish themfrom the other viral mRNA cap structures de-scribed above are that (i) the a-phosphate ofATP and the a- and ,f-phosphates of GTP areincorporated into the cap structure (2), (ii) ex-ogenously added ppA-containing mRNA's or
GpppA cannot be capped or methylated, respec-tively, in the in vitro system (1), and (iii) themethyltransferase reaction takes place as follows(250):
GpppA- + AdoMet (<0.1 ,iM)methyltransferase
,.GpppAmp-
GpppAmp- + AdoMet (>5 /iM)methyltransferase 7mGpppAmp
These reactions are different from those in thereovirus and vaccinia virus systems, in which 7-methylation precedes 2'-O-methylation even atlow AdoMet concentrations. It appears that cap-ping and methylation in the VSV system aretightly coupled to transcription (1). The forma-tion of the three-phosphate bridge in the VSV
mRNA's, as GCp/ a A, indicated that themRNA's may have been synthesized by a proc-essing mechanism in which capping involved
Gafly and 5,_aA ends from newly processedppp p
mRNA. Alternatively, the mRNA species mayindeed be initiated RNA molecules with pppAends, and capping with GTP may proceed byremoval of the 8,-y-phosphates of adenosine andthe -y-phosphate of guanosine. This reaction, ifoperative, is clearly different from the reactionsin the reovirus, vaccinia virus, and CPV systemsdescribed above, which have such capped struc-
tures as G a/ ON. Recently, 5'-ppA-ended
VSV mRNA fragments have been detected invitro (D. Testa, P. K. Chanda, and A. K. Baner-jee, unpublished data), suggesting that in theVSV system removal of the ,B-y-phosphates ofadenosine and the y-phosphate of guanosinemay occur at least in vitro. It is interesting tonote that unlike reovirus and vaccinia virus, thecapping of VSV mRNA in vitro is not inhibitedby PPi and Pi (17). Moreover, the mRNA syn-
thesized in the presence of GMPPNHP (replac-
ing GTP) contains the normal cap, Gp /p A
(18). It was later confirmed that purified VSVcontains a phosphohydrolase that can convertGTP to guanosine diphosphate (GDP) and thatthe latter can be converted to GTP by a virion-associated nucleoside diphosphate kinase (251).Recently, by using a fish rhabdovirus it wasshown that, unlike VSV, in the presence ofGMPPNHP the cap structure was Gpy/PA,indicating that an alternate capping reactionmay be operative in this system (96). Like CPV,AMPPNHP is not used in place of ATP in thein vitro reaction for RNA synthesis. This isprobably because initiation of VSV mRNA in-volves the synthesis of a leader RNA which isnot capped and contains the 5'ppACG... se-quence. This step may require ATP containinghydrolyzable ,8,y-phosphates (251).Influenza virus. The mechanism by which a
5'-terminal cap is added to influenza virusmRNA appears to be unique. It was observedthat the mRNA's synthesized in vitro by thevirion-associated RNA polymerase in the pres-ence of AdoMet with or without primers, suchas ApG (193), did not contain cap structures. Incontrast, the viral mRNA's isolated from in-fected cells were capped and methylated andcontained the sequences 7mGpppAmp...,7mGpppN6mAmp..., and 7mGpppGmp... (134).The predominant species of caps containedmethylated adenosine. These results suggestedthat complementary RNA synthesis in infectedcells initiates preferentially with adenosine andthat the RNA polymerase in purified virions,unlike the RNA polymerases in other RNA vi-ruses, did not associate with the enzymes for capstructure formation. This apparent paradox wasresolved when it was found totally unexpectedlythat the virion-associated RNA polymerase re-action is stimulated by exogenously added glo-bin, reovirus, and other capped mRNA's (31, 32)and that a portion of the 5'-terminal region ofthe mRNA (approximately 10 to 15 nucleotides)is donated to the influenza virus mRNA in vitro.Thus, it appeared that the purified virion ofinfluenza virus contains enzymes that can incor-porate a specific 5'-terminal portion of cappedand methylated mRNA's into the nascent tran-scripts (192) either by splicing onto the influenzavirus-specific mRNA synthesized in vitro or bycleavage and extension, the added mRNA actingas a primer for RNA synthesis. This uniqueenzyme system can recognize only cappedmRNA's containing 7mG since chemically de-capped globin mRNA (32) and blocked but un-methylated reovirus mRNA's do not act as
VOL. 44, 1980
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

186 BANERJEE
primers for in vitro mRNA synthesis. Recently,it has been shown that a similar mechanismprobably also operates in vivo (133). Influenzavirus-specific complementary RNA from in-fected cells contains a nonviral stretch of 10 to15 nucleotides cannabilized from cellularmRNA's. These results suggest that host cellmRNA's serve as primers for viral RNA tran-scription in infected cells, accounting for thepreviously observed requirement of functionalhost nuclear RNA polymerase II for influenzavirus replication (133).
It is clear from the studies on the viral systemsdescribed above that the presence of a virion-associated RNA polymerase greatly facilitatedelucidation of the capping reaction. In thoseviruses that do not contain a virion polymerase,such as adenovirus, simian virus 40, polyomavirus, herpesvirus, etc., the mechanism of thecapping reaction has been studied by nucleotidesequence determination of complementaryRNAs pulse-labeled in vivo. By direct sequencedeterminations of the late promoter regions andthe 5'-terminal portions of the nuclear and cy-toplasmic transcription products formed duringlate times after adenovirus infection, Ziff andEvans (278) precisely mapped the transcripts onthe promoter region. It was concluded that thecapped termini of nuclear and cytoplasmicRNAs and the late promoter map together, sug-gesting that the initiating residues ofthe primarytranscript are precursors ofthe capped terminus.From similar studies with simian virus 40 (37)and polyoma virus (72) late mRNA's, the viraltranscripts also appear to be initiated moleculesthat are capped subsequently. In contrast to theadenovirus system, the mRNA's of these twoviruses contain highly heterogeneous 5'-terminalsequences (37, 72, 89, 97). These results indicatethat theRNA polymerase may initiate transcrip-tion over a range of nucleotides on the promoterregion. The precise reasons for this heterogene-ity in the 5'-terminal cap sequence are notknown. Recently, similar heterogeneity in capsequences has been shown in the early mRNA'sof adenovirus-infected cells (99). Interestingly,one of the six early promoter sequences contains7mGpppU'pN2 as the cap structure, indicatingthat a pyrimidine can be present in the N1position in viral mRNA's (100).
Celilular mRNA'sAs described above, cellular mRNA's contain
the cap structure 7mGpppNim, where N1 is Gm,N6`mA, Am, methylcytidine, or methyluridine.It has been generally assumed that purines inthe N1 position indicate that caps are put on
initiated RNA chains and that when the N1 base
is a pyrimidine, caps are fonned on cleavedmolecules (218). This assumption is mainly de-rived from the observation that in procaryoticsystems, the RNA polymerase initiates chainspredominantly with purines (153). In fact, allbases are used for initiation, but purines arefavored 5 to 10 to 1 over pyrimidines, dependingon the DNA template (153). Thus, RNA polym-erase II of eucaryotic cells would also presum-ably initiate mainly or exclusively with purines.This assumption may no longer be tenable sinceinitiation with pyrimidines has now been shownto occur in at least in one bacteriophage operon(209) and at a second promoter for ribosomalRNA synthesis in Escherichia coli (275). Never-theless, it has not been demonstrated conclu-sively in eucaryotes whether RNA chains mayindeed initiate with pyrimidines. A detailedanalysis of the 5'-terminal structure of thehnRNA from mouse L-cells (the presumed pre-cursor of mRNA) revealed the presence of pu-rine tri- and diphosphates, such as pppG, pppA,ppG, and ppA (219), as well as the four varietiesof cap structures described above. These resultsindicate that the 5' portions of some of theprimary transcripts (those starting with purines)are directly converted to caps. Similar uncapped5' termini have also been found in pre-mRNAmolecules of Ehrlich carcinoma celLs (15). TheRNA chains containing pyrimidines may be gen-erated by either (i) an initiation process or (ii)cleavage at specific sites on hnRNA. In theformer case, capping and initiation may be con-current, and in the latter capping may occur onRNAs containing monophosphates at the 5' ter-mini. In an earlier report, Schibler and Perry(218) found large amounts of pUp in addition toppUp and ppCp at the 5' termini of the hnRNA.It was proposed that the mRNA segments de-rived by cleavage could be converted by a cel-lular kinase action to diphosphate-terminatedpyrimidines, which would then condense withGTP to form a pyrimidine-containing cap struc-ture. Later it was found that no pyrimidinediphosphates were present in the hnRNA, thusnegating this model (219). Although the biosyn-thesis ofthe pyrinidine-containing cap structureremains unclear, the existence of enzyme activ-ities in mammalian cells which form caps bycondensation of GTP with diphosphate-termi-nated RNA lends credence to the idea thatprobably all capped RNAs (including those withpyrimidine-containing caps) are initiated, pri-mary transcripts (see below).Recent experiments (214) have shown that in
cells labeled by brief exposure to [methyl-3H]-AdoMet the majority of the labeled 5'-terminalcap 1 structures were in hnRNA molecules hav-
MICROBIOL. REV.
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

5'-TERMINAL CAP STRUCTURES IN mRNA's 187
ing a chain length of about 750 nucleotides or
less. After longer labeling times, the proportionof cap 1 structures in hnRNA molecules longerthan mRNA molecules was increased to more
than 50% of the total. The cap structures in bothlong and short hnRNA chains contained all four2'-O-methylated nucleotides in the N1 positionin about the same proportion as in mRNA.These results may indicate that hnRNA chainscan indeed initiate with pyrimidines and thatcapping occurs very close to or at the start ofchain formation.The mechanism of capping in cellular and
viral systems has also been approached by usingisolated nuclei to study transcription and cap-ping in vitro. Groner and Hurwitz (94) reportedthat the RNA synthesized in vitro in thepresence of AdoMet by HeLa cell nuclei con-
tained a variety of capped and methylated 5'-terminal structures, such as 7mGppNmp,7mGppNlmpN2m]p..., 7mGpppN2mp..., and7mGpppN,pN2mp. The relative amounts of Nimwere as follows: guanosine, 45%; adenosine, 25%;uridine, 20%; and cytosine, 10%. Thus, in the invitro system, in addition to the cap 1 structures,diphosphate-containing capped structures andcap 2 structures were also found. In contrast, inthe hnRNA isolated from the nucleus, only cap1 structures were found, and cap 2 structureswere obtained only in the mRNA's isolated fromthe cytoplasm (73, 187, 215). Clearly, in contrastto intracellular nuclei, in isolated nuclei meth-yltransferases involved in methylating N1 andN2 bases are present and active. Using a subcel-lular system isolated from mouse L-cell nuclei,Winicov and Perry (270, 271) have also shownthat cap 1 and cap 2 structures were present inthe in vitro transcripts. It was further shownthat cap formation in large nuclear RNA speciessynthesized in vitro was closely associated withtranscription, as indicated by a-amanitin sensi-tivity and a requirement for the presence of allfour ribonucleoside triphosphates. Recently,Groner et al. (92) showed that [,B-32P]ATP can
be incorporated into the 5'-terminal cappedstructure of RNA synthesized in vitro by HeLacell nuclear homogenates. Approximately 10% ofthe RNA chains were initiated in vitro with [,f-32P]ATP, and this value decreased considerablywhen a-amanitin was present in the reactionmixture. These results indicate that, at least forthose RNA chains containing adenosine in theN1 position, initiation and capping occur by con-
densation of pG from GTP on either a diphos-phate or triphosphate 5' end. It was furtherobserved that in contrast to triphosphate caps,
in which the N1 base is any one of the four 2'-O-methylated derivatives, the diphosphate caps
(7mGppNlp) contain exclusively Gm at the N,position. It is not clear whether these cap-con-taining RNA species are artifacts of the in vitroreaction or whether capping enzymes present inthe nuclei may carry out reactions which havenot been observed in vivo.
Using isolated nuclei from adenovirus-in-fected cells, Manly et al. (155) demonstratedthat adenovirus-specific RNA was synthesizedin vitro and that the RNA species originated ata map position expected for late mRNA in vivopromoters (i.e., map position 16.5). Moreover, itwas observed that the 5' termini of the RNAswere capped with 7mG and that the amount ofradioactivity in 7-methylguanosine monophos-phate was equivalent to the amount of radioac-tivity in any other single nucleotide in the 5'-terminal RNase T, fragment. Since a largeamount of the transcription observed arises fromelongation of nascent chains initiated in vivo,the possibility existed that [a-32P]GTP was in-corporated into the 5' termini of the RNA chainsalready initiated in vivo. Under these conditions,a greater than equimolar amount ofradioactivityin 7-methylguanosine monophosphate wouldhave been obtained; thus, the results indicatethat post-transcriptional capping does not occurat detectable levels and that the capping andtranscription initiation processes are tightlylinked.ENZYMES INVOLVED IN THE CAPPING
REACTIONSFrom the structure of the 5' cap and the
mechanism of its formation, it became apparentthat multienzyme systems are involved in theformation of fully methylated capped 5' ends.The enzymes involved in the cap formation areas follows. (i) mRNA guanylyltransferase, in-volved in the first step of the capping reaction,catalyzes the formation of the cap structure byusing GTP that is linked through a triphosphatebridge to the 5' end of the RNA. To carry outthis reaction, two other enzymatic activities ap-pear to be involved; these are (a) an enzyme thatconverts 5'-phosphate-terminated RNA to 5'-di-and triphosphate-terminated RNAs, which inturn can be used for capping with GTP (239)and (b) an RNA triphosphatase activity thatconverts triphosphate-terminated RNA to di-phosphate-terminated RNA (the latter is thenused as a substrate for capping) (257). (ii) mRNA(guanine-7-)methyltransferase catalyzes thetransfer of a methyl group from AdoMet to theposition 7 of the capping guanosine residue. (iii)2'-O-Methyltransferase activity methylates the2'-O-ribose moieties of the N, nucleotides of themRNA's. (iv) A methyltransferase activity spe-
VOL. 44, 1980
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

188 BANERJEE
cifically catalyzes the transfer of a methyl groupfrom AdoMet to the N' position of a 2'-O-meth-yladenosine residue at the N1 position of thecapped 5' end of mRNA.
All of the above-described enzymes have beenpurified from vaccinia virions by Moss and hisco-workers (19, 20, 65, 157-159) with the excep-tion of the last enzyme, which was purified bythem from HeLa cells (120). RNA (guanine-7-)methyltransferase was also purified fromHeLa cells (66). Purifications of some of theseenzymes have also been carried out in the labo-ratory of Hurwitz (164, 165). Recently, bothguanylyltransferase and methyltransferase ac-tivities were purified from rat liver nuclei (163).The properties and specificities of these enzymesare described below.
mRNA Guanylyltransferase and mRNA(Guanine-7-)Methyltransferase
Martin et al. (159) first reported the isolationand purification of these enzymes from vacciniavirions. They took advantage of the selectivebinding of these activities to homopolyribonu-cleotides to achieve a 200-fold increase in specificactivity. Both activities remain together duringaffinity or ion-exchange chromatography. Thepurified enzyme has a molecular weight of127,000 and contains two polypeptides of molec-ular weights 95,000 and 31,400 in a molar ratioclose to 1:1, indicating that both activities arecomponents of an enzyme system. This systemspecifically catalyzes modification of the diphos-phate-containing 5' termini of mRNA, poly(A),or polyguanidylic acid (157, 158):
ppN- + GTP = GpppN + PPi
GpppN + AdoMet --*7mGpppN + AdoHcyThe enzyme prefers Mg2+ over Mn2+, and the
optimum pH is 7.8. Studies on the donor andsubstrate specificities of this enzyme complexrevealed that diphosphate-terminated polyribo-nucleotides (such as pp[N]n, where N can be anybase) are preferred and that monophosphatetermini are not used. Although mononucleotides(ppN) are capped, trinucleotides (ppNpNpN)are better substrates, and slightly longer polyri-bonucleotides might be optimal (157). GTP, in-osine triphosphate, and deoxyguanosine triphos-phate, but not 7mGTP, can be used as donors.Monroy et al. (164) reported an approximately
10,000-fold purification of the same activitiesfrom purified vaccinia virus. Slightly differentproperties were observed. The molecular weightof the native protein is 120,000; a major band ofmolecular weight 95,000 is released in sodiumdodecyl sulfate-polyacrylamide gel electropho-
resis, along with minor bands having molecularweights of 59,000 and 28,000. However, when thenative guanylyltransferase is purified by nonde-naturing polyacrylamide gel electrophoresis, re-covered, iodinated, and electrophoresed on apolyacrylamide gel containing sodium dodecylsulfate, a major polypeptide band of molecularweight 59,000 is observed. This enzyme specifi-cally uses 5'-triphosphate-ended RNA chains[ppp(A)n] (165). The apparent Km for termini ofppp(A)n is 0.2 ,uM, indicating a high affinity ofthe enzyme for the substrate. During guanyly-lation of ppp(A)n, Pi and PPi are both releasedin a 1:1 stoichiometry with cap formation. Thereason for the apparent discrepancy betweenthese results and those described by Moss andhis colleagues (157, 158) are presently unclear.However, Venkatesan and Moss (257) have re-cently detected an additional enzyme activity(RNA triphosphatase) in purified vaccinia viri-ons. This activity also appears to be a compo-nent ofthe guanylyltransferase enzyme complex.In addition, this enzyme activity is involved inthe removal of y-phosphate from triphosphate-ended RNA chains, converting the 5' end to adiphosphate, which in turn is used by the en-zyme as the principal substrate. A similar mech-anism has been proposed previously for the cap-ping of reovirus mRNA's in vitro (83).Spencer et al. (239) have reported isolation of
a 5'-phosphate-phosphate polyribonucleotide ki-nase activity from vaccinia virus cores; this ac-tivity catalyzes the conversion of 5'-phosphateand 5'-diphosphate termini of RNAs to 5'-tri-phosphate forms. 5'-Hydroxyl RNA or DNA and5'-phosphorylated DNA are not phosphorylatedby this enzyme. In a system that coupled 5'-phosphate polyribonucleotide kinase and guan-ylyltransferase, p(A)n and pp(A)n were capped.The guanylyltransferase by itself, however, canonly cap ppp(A)n. The precise role of the 5'-polyribonucleotide kinase activity in the cappingprocess is not known, but this enzyme may beinvolved in the capping of mRNA's that areformed by cleavage of pre-mRNA (219) to form5'-phosphate termini, which are then convertedto capped molecules.
2'-O-MethyltransferaseThis activity has been purified from vaccinia
virions approximately 350-fold. It catalyzes thetransfer of a methyl group from AdoMet to the2'-OH of the penultimate nucleoside of thecapped mRNA, such as conversion of 7mGpppGin brome mosaic virus RNA to 7mGpppGm (19).The molecular weight of the enzyme is 38,000.RNAs ending on pN, ppN, or GpppN are inef-fective substrates for this enzyme (20). poly(A)
MICROBIOL. REV.
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

5'-TERMINAL CAP STRUCTURES IN mRNA's 189
and polyinosinic acid ending in 7mGpppN areexcellent substrates, whereas capped polyguano-sinic acid, polyuridylic acid, and polycytidylicacid are poor substrates for the enzyme. The lowability of the enzyme to use dinucleotides assubstrates (for example, 7mGpppG) suggeststhat a longer polyribonucleotide chain is re-quired. This activity is extremely sensitive toAdoHcy. With only 1 ,iM AdoHcy in the pres-ence of 2.5 ,uM AdoMet, the activity is virtuallyabolished. This is in contrast to the VSV system,in which 2'-O-methyltransferase activity is moreresistant to AdoHcy than (guanine-7-)methyl-transferase (250).
Guanylyltransferases andMethyltransferases from Eucaryotic CellsLike the enzymes in vaccinia virus, the en-
zymes involved in the capping reaction in eucar-yotic cells have also been detected and partiallypurified. A soluble extract prepared from HeLacell nuclei has been shown to catalyze the 5'-terminal modification of RNA and syntheticpolyribonucleotides to form 7mGpppA and7mGpppG structures (66, 265). The substrateRNA molecule appears to require the presenceof at least two terminal phosphates. A polyri-bonucleotide with only a single 5'-terminal phos-phate is not capped unless a second phosphateis added. Thus, it appears that the first step ofthe capping reaction in eucaryotic cells may besimilar to that in the vaccinia virus and reovirussystems. More recently, Venkatesan et al. (S.Venkatesan, A. Gershowitz, and B. Moss, per-sonal communication) have purified this enzyme1,000-fold from HeLa cell nuclei. It has a molec-ular weight of 48,500 and shows optimal activityat pH 7.5 in the presence of Mn2" or Mg2". Usingthis purified enzyme, Venkatesan and Moss haveshown that RNA with ppC at the 5' end (atranscription product of phage Xc17 DNA [209])is also capped by purified mRNA guanylyltrans-ferase, indicating that pyrimidine-ended as wellas purine-ended RNAs can serve as acceptors.This result is consistent with the presence of apyrimidine in the N1 base of the cap in eucar-yotic mRNA's and also suggests that pyrimi-dine-containing caps may also be part of initi-ated RNA molecules. Recently, an mRNA guan-ylyltransferase purified from wheat germ hasalso been shown to use only diphosphate-endedRNA (J. Keith, A. Gershowitz, S. Venkatesan,and B. Moss, personal communication).
Unlike vaccinia virus, the HeLa cell RNA(guanine-7-)methyltransferase is clearly separa-ble from the guanylyltransferase activity (66).This enzyme specifically methylates the 5'-ter-minal guanosine of RNA chains ending in
GpppG or GpppA. It differs from the vacciniavirus enzyme in the following respects. (i) TheHeLa cell enzyme exhibits exclusively methyl-transferase activity, whereas the vaccinia virusis part of an enzyme complex that also includesguanylyltransferase activity (see above). (ii)GTP and GDP are not substrates for HeLa cellenzymes, whereas these compounds can bemethylated to a small extent by the viral en-zyme. (iii) The HeLa cell enzyme has a molecu-lar weight of 56,000, whereas the molecularweight of the viral enzyme is 127,000.As discussed above, in viral and cellular
mRNA's synthesized in vivo (in contrast to invitro mRNA in the case of viruses) adenosineresidues in cap structures exist largely as theunusual dimethylated nucleoside N6 mAm (Ta-ble 1). Consistent with this observation, an en-zyme activity (molecular weight, 65,000) wasobserved in the postribosomal supernatant ofHeLa cells; this activity specifically methylatesthe N6 position of 2'-O-methylated adenosine inthe cap structure (120). Less activity was foundwith RNA ending in 7mGpppA, and barely de-tectable activity was found with RNAs endingin GpppA and ppA- or oligonucleotides of thetype 7mGpppAmpN or 7mGpppApN. The latterresults indicate that some extension of RNA isrequired before the methylation of the N-6 po-sition of adenosine may occur. The failure of thisenzyme to methylate internal RNA segmentsmakes it likely that a separate methyltransfer-ase, not isolated so far, catalyzes the formationof internal m6A in eucaryotic mRNA's (Table1).
Recently, separate guanylyltransferase andRNA (guanine-7-)methyltransferase activitieshave been purified from rat liver nuclei (163).They have molecular weights of approximately65,000 and 130,000, respectively. For the guany-lylation reaction, dithiothreitol is essential, andMn2" (2 mM) is twice as effective as Mg2+ (8mM). The capping reaction appears to involvecondensation of guanosine monophosphate to adiphosphate-terminated ribopolymer [such aspp(A)n, ppGpCpC, etc.]. The triphosphate-ter-minated ribopolymers are capped at much lowerrates than their diphosphate-terminated coun-terparts. In this respect, the mode of action ofthis enzyme is similar to the vaccinia virus en-zyme reported by Martin and Moss (157, 158).Recently, Bajszar and co-workers (16) have re-ported that 30S ribonucleoprotein particles fromrat liver nuclei contain (guanine-7-)methyltrans-ferase and 2'-O-methyltransferase in addition toguanylyltransferase.RNA (guanine-7-)methyltransferase has also
been partially purified from Neurospora crassa
VOL. 44, 1980
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

190 BANERJEE
(87). It catalyzes the transfer of a methyl groupfrom AdoMet to an RNA containing a 5'-termi-nal unmethylated capped structure. N. crassapoly(A)+ RNA and reovirus mRNA are equallygood as substrates. No 2'-O-methyltransferaseactivity is associated with this enzyme, which isconsistent with the absence of 2'-O-methylationin N. crassa mRNA (220). Although not puri-fied, a similar methyltransferase activity hasbeen demonstrated in the extracts ofwheat germ(173, 184; J. Keith and B. Moss, personal com-munication), Artemia salina (174), calf thymus(141), and embryonic chicken lens (136).Recently, two separate mRNA (nucleoside-2'-
O-)-methyltransferases from HeLa cells havebeen purified; one of these completes formationof cap 1 structures and the other yields cap 2structures with suitable acceptors (S. Langbergand B. Moss, personal communication).
Enzymes That Cleave the Cap StructureEnzymes that cleave the cap structure into
7mGp and pNm-containing mRNA have beenisolated and purified free from RNase from to-bacco tissue culture cells (228) and potato (276).The nucleotide pyrophosphatase purified frompotato has been used to remove the caps fromreovirus, rabbit globin, and A. salina mRNA'sfor studies on the effect of caps on mRNA trans-lation (276). The corresponding enzyme fromtobacco cells was used similarly to decap CPVmRNA (226, 229). Moreover, after removal ofthe cap from tobacco mosaic virus RNA, it wasshown that the ability of the RNA to reassemblewith the coat protein was unchanged, but infec-tivity was abolished (181). By using tobaccopyrophosphatase, the blocking nucleotide ofrab-bit globin mRNA was removed, and the mRNAwas subsequently sequenced by the polynucleo-tide kinase method, using y-ATP (63).An enzyme activity was also detected in HeLa
cell extracts; this activity specifically cleaved thedinucleotide 7mGpppGm to 7mGp and ppGm(180). It did not hydrolyze the correspondingunmethylated structure or ring-opened cap de-rivative. However, this enzyme failed to cleavethe cap structure present in mRNA. It appearsto require for activity capped oligonucleotidesshorter than 10 bases (179). The low-molecular-weight nuclear hypermethylated capped RNAswere not substrates for the enzyme. The functionof this enzyme in eucaryotic cells is not known.One of the proposed functions is as a scavengerby which accumulated cap structures are de-stroyed during degradation of mRNA's, thusavoiding inhibitory effects of caps on ribosomebinding and eventually facilitating translation ofcapped mRNA (179). A similar nucleotide py-
rophosphatase activity has also been detectedin embryonic chicken lens (135). It releases7mGp from the cap structure 7mGpppN (whereN is adenosine, Am, GmpCp, AmpApC, orAmpApCpApG) (137). Phage T4-induced poly-nucleotide kinase has also been reported tocleave the PPi bond from a cap structure, butthis has not been confirmed (4).
BIOLOGICAL ROLE OF THE CAPSTRUCTURE
The presence of the unconventional cap struc-ture in eucaryotic mRNA's prompted investiga-tions to determine its role in mRNA function. Itwas quickly recognized that the cap structurecarrying the polar 7mG moiety may play a sig-nificant role in the interaction of mRNA withribosomal RNA or its protein components dur-ing initiation of protein synthesis. Moreover, the5'-terminal cap structure of mRNA may be re-sistant to the action of 5'-exonuclease action,thus imparting stability to the mRNA's withinthe cell. Finally, the capping process may bedirectly involved in the biosynthesis of mRNA.These areas covering the possible roles of thecap structure have been investigated in greatdetail, and the results strongly indicate that capstructures may indeed play an important role inconferring higher translational ability and sta-bility to mRNA's and in addition may also bedirectly related to the transcription process.
Protein SynthesisBoth et al. (28) were the first to demonstrate
that the cap structure in mRNA was requiredfor efficient translation of VSV and reovirusmRNA's in a wheat germ cell-free protein-syn-thesizing system. The corresponding uncapped(reovirus) and capped and unmethylated (VSV)mRNA's were virtually untranslated in the sameextract. Similar results were obtained when thetranslational abilities of capped reovirus, VSV,and globin mRNA's were compared with thecorresponding mRNA's after chemical removalof the cap structures from their 5' ends (173).Subsequently, Both et al. (29) observed that thecap structure facilitates efficient binding of reo-virus mRNA's to 40S ribosomal subunits duringinitiation of protein synthesis. After these dis-coveries, a great deal of data have been accu-mulated over the years; the role of the capstructure in translation has been studied exten-sively by using a number of different mRNA'sfrom viral and eucaryotic sources and a varietyof cell-free protein-synthesizing systems. Therecent reviews by Filipowicz (70) and Kozak(130) have dealt comprehensively with the func-tions of the cap structure in protein synthesis
MICROBIOL. REV.
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

5'-TERMINAL CAP STRUCTURES IN mRNA's 191
and its possible involvement in the capacity ofribosomes to select the initiation regions inmRNA's. To avoid repetition, I have summa-rized below the observations already reviewedand added some new interesting findings regard-ing the role of mRNA cap structures in eucar-yotic protein synthesis.From a vast body of reports published over
the years concerning the function of the capstructure in mRNA translation, some distinctpatterns emerged.Uncapped or unmethylated mRNA's are
poor templates for protein synthesis in vi-tro. This was shown either by using standard invitro synthesized mRNA's that lacked the capstructure or by removing the cap structure fromthe mRNA by chemical or enzymatic methods.The translational abilities of these mRNA's andtheir abilities to bind to initiation complexeswere then tested in a variety of cell-free protein-synthesizing systems. The mRNA's tested werefrom reovirus (104, 146, 173, 175, 276), vacciniavirus (176, 185, 259), VSV (151, 175, 206, 254),CPV (226), tobacco mosaic virus (177, 226, 272),brome mosaic virus (225), globin (104, 149, 173,226, 272, 276), protamine (86), bovine parathy-roid (123), hen ovalbumin (222), and A. salina(174). The results indicated that virtually allmRNA's that lacked 7mG were ineffective tem-plates for protein synthesis. The observed trans-lational deficiency of mRNA lacking 7mG wasmore pronounced in wheat germ extract com-pared with reticulocyte lysates and ascite ex-tracts. It was further observed that wheat germextracts and reticulocyte lysates responded dif-ferently to changes in salt concentrations whentranslating uncapped mRNA's (25, 41, 124, 185,272). It is not clear whether there is an intrinsicdifference in the components used for chaininitiation or whether the extracts differ only inthe concentrations or activities ofsome commoninitiation components. Although other condi-tions, such as mRNA concentration (110, 146,225, 272), initiation factors (104, 116), and tem-perature (259), favorably affect the translationof uncapped mRNA's under optimum condi-tions, the importance of the cap for translationwas evident.
Recently, the role ofthe cap structure in trans-lation of procaryotic mRNA's in eucaryotic cell-free systems has been studied. Various procar-yotic mRNA's have been translated with accu-racy in both mammalian and wheat germ cell-free systems to produce functional protein prod-ucts (11), but the efficiency of translation wassignificantly higher in the procaryotic extract.Using vaccinia virus capping enzyme, Patersonand Rosenberg (184) were able to cap the ir
vitro mRNA's coding for A cro gene. The cappedmRNA's were translated at relatively high effi-ciencies in wheat germ extract (compared withcapped rabbit globin mRNA), and this efficienttranslation was absolutely dependent on themodification of the A transcripts by addition ofthe cap structure. Like other capped mRNA's,cro protein translation was inhibited by the capanalog 7mGpppA. These results suggest that theprocaryotic mRNA's contain all of the informa-tion necessary for efficient recognition and ini-tiation by eucaryotic translation components,except for the cap structure.7-Methylguanosine facilitates binding of
mRNA's to ribosomes. Although the transla-tion of capped and uncapped mRNA's in vitrodepended on various experimental parameters,the ribosome binding studies were unambiguous.In experiments with capped polynucleotides,such as 7GpppGmC(U). and 7mGpppGmC(A,C)n,it was shown that these polymers were able tobind more efficiently to 40S ribosomal subunitsthan the corresponding uncapped ribopolymers(30). Capped mRNA's were also more efficientthan their uncapped counterparts in binding toribosomes to form stable initiation complexes(151, 175, 176, 206, 259). The addition of a capstructure to uncapped mRNA by vaccinia virusenzyme increased the rate and extent of bindingof vaccinia virus mRNA to wheat germ ribo-somes (176). Moreover, in a mixture of mRNA'scontaining capped and uncapped mRNA's,capped mRNA was found to be rapidly andselectively associated with ribosomes. (175, 176).The notable exceptions are the mRNA's thatnormally lack the cap (e.g., poliovirus, satellitetobacco necrosis virus, cow pea mosaic virus)but nevertheless bind to ribosomes and aretranslated efficiently in in vitro systems. Un-capped satellite tobacco necrosis virus RNA wasapparently translated equally efficiently when itwas enzymatically capped (232). The latter ex-amples indicate that in these systems cellularfactors play a primary role in the translationprocess (see below).From experimental data relating binding of
the 40S and 80S initiation complexes to the 5'ends of a number of mRNA's, Kozak and Shat-kin (130, 132) postulated a "scanning model" inwhich the 40S ribosomal subunit first attachesto the 5' end of a message, irrespective of se-quence, and subsequently advances until it en-counters the first AUG triplet, at which pointthe 40S ribosome stops and the 60S subunitjoins. The presence of 7mG probably facilitatesthe initial binding with 40S ribosome. Thistranslation process in eucaryotic systems isclearly different from the polycistronic procar-
VOL. 44, 1980)
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

192 BANERJEE
yotic mRNA's, in which the ribosomes can at-tach to multiple AUG codons in the mRNA.Eucaryotic mRNA's, on the other hand, presum-ably contain one functional AUG codon to ac-
count for their monocistronic nature. This modelmay account for the inability of the ribosome toattach to an internal AUG codon and is alsocompatible with the observed variability in thelocation of the AUG codon within the 5'-proxi-mal regions of different messages with varyingneighboring sequences. The nucleotide se-
quences of the 5'-proximal regions of eucaryoticmRNA's (130) indicate that the initiator codonAUG is located at variable distances, rangingfrom 10 to 100 bases from the 5'-terminal cap.The interaction ofthe 5' terminus ofthe messagewith the 40S subunit appears to be more impor-tant than the position or location of the AUGcodon in the mRNA. In support of this model,Kozak (131) has recently demonstrated that eu-
caryotic ribosomes failed to attach to the AUGcodon when present in a circular mRNA. Cir-cularization of pUpG(pA)4o8o or of poly(A,U,G)by T4 RNA ligase virtually abolished the abilityof the template to bind to wheat germ or retic-ulocyte ribosomes. In control experiments, linearforms of each polymer were able to form initia-tion complexes in wheat germ and reticulocyteextracts, and the circular molecules that failedto bind to wheat germ or reticulocyte ribosomeswere able to bind to E. coli ribosomes. Theseresults are compatible with the proposed mech-anism by which eucaryotic ribosomes enter atthe open 5' terminus of a message by a threadingmechanism, with translation beginning at thefirst AUG codon encountered by the ribosome.The precise function of the cap structure in thisprocess is not clear since, as described above,uncapped mRNA's also attach to ribosomes andare translated, although at lesser efficiency.The role of 7mG in translation has also been
tested by using a polycistronic procaryoticmRNA. Rosenberg and Paterson (209) used a
high-molecular-weight polycistronic in vitro X
DNA transcript containing the 5'-terminal crogene in the RNA. When a cap structure was
added to the 5' end of the RNA before transla-tion in wheat germ extract, efficient translationof only the cro gene occurred. The inability todetect protein synthesis from any of the cistronslocated internally in the transcript suggests thatthe eucaryotic ribosome interacts with the cap
structure, which subsequently allows recognitionof the first AUG located close to the 5' end.Using a similar translation system, these inves-tigators have also shown that in the gal operon-derived polycistronic message only the cap-prox-imal cistron (galE) was translated in vitro. Fu-ture experiments in this area should give inter-
esting insights into the nature of the interactionsof the cap structures in procaryotic mRNA witheucaryotic ribosomes that allow the 5'-proximalAUG to be recognized in a polycistronic mes-sage.Chemical analogs of cap structures, such
as p7mG, 7mGDP, and 7mGpppNm, inhibittranslation and ribosome binding ofcapped mRNA. Under appropriate ionic con-ditions these cap analogs inhibited translation ofa variety of capped mRNA's in various cell-freeextracts (13, 38, 71, 93, 110, 203, 221, 245, 258,268). These studies indicate that some specialstructural features of the cap play a significantrole in the recognition process during the for-mation of the initiation complex of mRNA andribosomes. In a survey of the inhibitory effectsof different methylated nucleosides and nucleo-tides in translation and ribosome binding, someinteresting structural requirements for cap rec-ognition were observed. 7mG, GMP, 7-methyl-guanosine 2',3'-cyclic phosphate, and GpppNwere not inhibitory to translation, whereasp7mG, 7mGDP, 7mGTP, and 7mGpppNm werepotent inhibitors. These results indicate thatsome interaction between the polar group of7mG and the negatively charged phosphategroup(s) of these inhibitors is involved in alter-ing the binding of capped mRNA to ribosomes(71, 111). Replacement of the methyl group byethyl (7-ethyl)GDP or benzyl (7-benzyl)GDPdid not decrease the inhibitory capacity of7mGDP (6), indicating that the maintenance ofthe positive charge (but not necessarily a methylgroup at the N7 position) is essential for theinhibitory effect. This is also borne out by theobservation that 7-ethylguanosine-containingcapped mRNA's (mRNA's synthesized in vitroby reovirus-associated enzymes in the presenceof s-adenosyl-l-ethionine) were essentiallyequally active in translation and binding to ri-bosomes compared with their methylated coun-terparts (80).Cap-binding protein. Since ribosomal sub-
units from wheat germ or reticulocyte extractsbound efficiently to the 5'-terminal region ofmany mRNA's and protected cap-containing se-quences against RNase digestion (132), itseemed possible that initiation complexes in-cluded proteins capable of binding mRNA (cap-binding protein [CBP]), thus protecting the capfrom nuclease action. Activity of this type wasdetected in an A. salina ribosomal high-saltwash by assaying the binding of methyl-3H-la-beled 7mGpppGmC to membrane filters (71). Bythe same technique it was found that prepara-tions of the purified rabbit reticulocyte initiationfactors (for nomenclature, see Anderson et al.[12]) eIF-2, -4B, and -5 bound to mRNA (21, 22,
MICROBIOL. REV.
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

5'-TERMINAL CAP STRUCTURES IN mRNA's 193
221). The binding of eIF-4B to capped mRNA,but not to encephalomyocarditis virus RNA,which is uncapped, was inhibited by the capanalog p7mG. On this basis, it was suggested thateIF-4B recognizes and binds to the cap structure(221). By using the same experimental approach,it was shown that eIF-2 also recognizes the capstructure in mRNA (116). These researchersused the filter binding technique, which may notpermit unequivocal identification of polypep-tides with cap-binding activity, especially if thecap-binding component is a contaminant with astrong affinity for caps. To detect protein withcap-binding activity, Sonenberg and Shatkin(237) used a cross-linking method by which pro-teins in the proximity of the 5' end of mRNA ininitiation complexes can be detected. Using thismethod and polyacrylamide gel electrophoresis,Sonenberg et al. (234) detected a 24,000-daltonpolypeptide in initiation factors prepared byhigh-salt washes of rabbit reticulocyte, mouseascites, and human HeLa cell ribosomes. TheCBP can be specifically cross-linked to methyl-3H-labeled capped viral mRNA as defined by7mGDP inhibition. The 24,000-dalton CBP wasdetected only in purified eIF-3 and -4B amongthe many factors tested. The strong cap inter-action of the CBP was not limited to reovirusmRNA but was also observed with reticulocyteeIF-3 cross-linked to VSV, Newcastle diseasevirus, and vaccinia virus capped mRNA's (235).The failure to obtain nonspecific binding andcross-linking to the CBP in the presence of7mGDP suggests that this polypeptide has asingle site available for cross-linking and thatthis site recognizes the 7mG nucleotide. Re-cently, the CBP from reticulocyte ribosomes hasbeen purified to homogeneity by chromato-graphic passage through an affinity resin pre-pared by coupling the levulinic acid 02"3'-acetalof7mGDP to AH-Sepharose 4B (236). The trans-lation of capped mRNA's from Sindbis virus,reovirus, and rabbit globin was stimulated bypurified CBP in HeLa cell extracts. The trans-lation of uncapped mRNA's from encephalo-myocarditis virus and satellite tobacco necrosisvirus was not stimulated under the same condi-tions (236), indicating that the CBP may interactwith the 7mG in mRNA before the formation ofprotein synthesis initiation complexes.Based on these results, Sonenberg et al. (235)
proposed a model for the role of CBP in thepromotion of translation of capped mRNA's.The first step in protein synthesis involves theformation of a pre-initiation complex betweenthe 5' end of capped mRNA and the CBP. Sincethe CBP has a high affinity for eIF-3 (234), thenext step is the interaction of the pre-initiationcomplex containing CBP with the 40S ribosomal
subunits containing eIF-3. After initiation com-plex formation, the CBP is released for recycling,and its interaction with the 5' end of mRNA isreplaced by eIF-3 subunits. This model predictsthat eIF-3 or some of its subunits would bepresent in the 80S initiation complexes, eitheron the ribosome or on the 5' terminus of themRNA. Further characterization of the proteinsthat can be cross-linked to the cap structures ofthe mRNA's in initiation complexes should clar-ify the roles ofCBP and eIF-3 subunits in cappedmRNA translation.
Recently, CBP has been shown to have re-storing activity for capped mRNA translation incell extracts from poliovirus-infected cells. Infec-tion with poliovirus leads to inactivation of thecap-dependent recognition mechanism ofmRNA's during protein synthesis initiation. Itwas observed that the translation of cappedmRNA's (e.g., in VSV) was markedly decreasedin extracts of poliovirus-infected HeLa cells,whereas poliovirus RNA, which is not capped,continued to function well in vitro (208). Theaddition of only purified eIF-4B and not otherinitiation factors to infected cell extracts re-stored capped mRNA translation without in-creasing poliovirus synthesis. These results wereinterpreted as selective inactivation of eIF-4Bby poliovirus during infection and thus blockingof host cell protein synthesis (i.e., the translationof capped mRNA's). In view of the stimulatoryeffect of CBP in translation of capped mRNA'scompared with uncapped mRNA's, it was con-firmed that the observed restoring activity ofeIF-4B was indeed due to the presence of con-taminating CBP in the initiation factor (255).The restoring activity was purified from a high-salt wash of rabbit reticulocyte ribosomes, whichcorresponded to the CBP with regard to electro-phoretic mobility, tryptic peptide pattern, andability to cross-link to the 5'-terminal cap inmRNA. Thus, it appears that at least in thepoliovirus system cellular protein synthesis maybe inhibited by inactivation of some crucialproperty of the CBP.
Helentjaris and Ehrenfeld (105) have alsoshown that a salt wash from uninfected cellsstimulated translation of both cell (endogenoustranslation system derived from HeLa cells) andpoliovirus mRNA's, whereas a salt wash frominfected cells stimulated the translation only ofviral mRNA. This mRNA-discriminating activ-ity was found to be tightly associated with eIF-3 (106). Since CBP has a high affmiity for eIF-3(234), it is possible that the mRNA discriminat-ing activity may be similar, if not identical, tothe CBP.
Interesting results were obtained when similarexperiments were carried out in reovirus-in-
VOL. 44, 1980
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

194 BANERJEE
fected cell extracts. Skup and Miliward (231)have shown that extracts from uninfected L-cellstranslated capped reovirus mRNA at high effi-ciencies and that the translation was sensitive to7mGTP. The same extracts translated uncappedreovirus mRNA at low efficiencies. In contrast,extracts from infected L-cells translated un-capped reovirus mRNA at higher efficiencies,and the translation was not sensitive to 7mGTP.These results suggest that like the poliovirussystem, reovirus infection renders a compo-nent(s) of the protein synthesis machinery in-active, such that translation of capped mRNA'sis blocked. However, reovirus mRNA, in con-trast to poliovirus mRNA, is capped, and itstranslation continues unabated during infection.It has been claimed that reovirus mRNA's lateafter infection are not capped but contain pGpat the 5' end (277). Enzyme activity in L-cell S10extract rapidly converted diphosphate-endedmRNA's to monophosphate-ended mRNA's.Subsequently, it was shown (230) that the cap-ping and methyltransferase activities becomemasked in subviral particles isolated from cellsat late times after reovirus infection. Thus, themechanism of host cell shutoff by reovirus infec-tion may be similar to that in the poliovirussystem, except that additional controlling fea-tures may exist in the former that convertcapped viral mRNA to its uncapped form duringinfection.
mRNA StabilityOne of the features that distinguishes eucar-
yotic mRNA's from procaryotic mRNA's is theirlonger half-lives. The short-lived procaryoticmRNA's are degraded exonucleolytically fromthe 5' end during translation (7). Modification atthe 5' end of the eucaryotic mRNA's possiblyimparts stability by virtue of conferring resist-ance to the action of such nucleases. One of thereasons for the observed inefficient translationofuncapped mRNA's in in vitro cell-free extractsmay thus be due to preferential degradation ofuncapped compared with capped mRNA's. Sev-eral experiments indicated that the cap structurestabilizes mRNA both in vivo and in cell-freeextracts. Furuichi et al. (77) injected cappedreovirus mRNA's into Xenopus laevis oocytesand observed that capped mRNA's were morestable than their uncapped counterparts. It wasalso found that uncapped reovirus mRNA's werepreferentially degraded in cell-free extracts iso-lated from wheat germ or mouse L-cells. Rabbitreticulocyte lysate contained little or no nucleaseactivity, which is consistent with its loss of tran-scriptional machinery during differentiation and
possibly accounts for the increased efficiency oftranslation of uncapped mRNA's in this system.Similar findings confirming the importance ofthe cap structure in stabilizing mRNA duringeucaryotic protein synthesis have been obtainedwith the mRNA's of tobacco mosaic virus, glo-bin, protamine, and A. salina (see above). Ineach case, the capped mRNA species remainedintact, but when they were decapped, they losttheir translation capabilities (>90%) in wheatgerm cell-free extracts and were degraded ex-onucleolytically during translation. Whencapped and uncapped globin mRNA's were in-jected into frog oocytes, more than 90% of thetranslatability of uncapped globin mRNA waslost over a 96-h period (149). The capped globinmRNA, on the other hand, was apparently sta-ble since it continued to be translated. Theseresults clearly indicate that the cap structureprotects mRNA from degradation and thus in-creases its stability. There is one unconfirmedreport (5) in which removal of 7mG from globinmRNA by T4-polynucleotide kinase did not al-ter the translational ability of the mRNA inwheat germ extract.
In contrast to the above-described results, un-capped RNAs, such as those in poliovirus (208)and satellite tobacco necrosis virus (145), whichfunction as messengers in infected cells, are sta-ble and are translated in various model systems.One interpretation of this finding is that at leastin the case of poliovirus, the protein linked tothe 5' end of the RNA may protect it fromnuclease action. However, this argument isweakened by the finding that polysome-associ-ated poliovirus mRNA does not contain the 5'-terminal protein. It is removed from the genomeRNA by endogenous proteases present in cell-free extracts. In the case of satellite tobacconecrosis virus, the stabilizing role may be playedby a secondary structure at the 5' end of theRNA (232). Although the precise reasons for thestability of these uncapped mRNA's are notknown, some special interaction of proteins withthe 5' termini may account for their resistanceto degradation in infected cells.
mRNA BiosynthesisCap structures have been detected in early
stages of hnRNA synthesis in nuclei (48). Con-version of precursor transcripts to cytoplasmicmRNA's apparently involves conservation ofthecap structure during several processing steps,including 3'-terminal addition of poly(A) andsplicing ofintervening nucleotide sequences (48).It is still not clear whether cap fornation isinvolved or required for the initiation ofmRNA
MICROBIOL. REV.
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

5'-TERMINAL CAP STRUCTURES IN mRNA's 195
synthesis. An answer to this question has notbeen obtained because of the difficulties in con-trolling nuclear RNA synthesis in order to iso-late the primary RNA products in high yields.As discussed above, the mechanisms of cap for-mation have been studied in detail in viral sys-tems, and in some cases capping and mRNAsynthesis are tightly coupled. For example, VSVmRNA's synthesized ip vitro are always capped,and efforts to find conditions for uncappedmRNA formation have failed (17). Thus, it ap-pears that in the VSV system capping may beessential for the synthesis and eventual chaincompletion of mRNA's. Although not obliga-tory, influenza virus may use capped primers formRNA synthesis in vitro (32), but in vivo (133)capping appears somehow directly related to thebiosynthesis of influenza virus mRNA and thusto viral replication.CPV is a unique system, in which the capping
process appears to be directly involved in mRNAtranscription. The addition of AdoMet stimu-lates in vitro mRNA synthesis by the virion-associated RNA polymerase. More interestingly,AdoHcy, a competitive inhibitor ofAdoMet, alsostimulates RNA synthesis to the same extent asAdoMet (76, 160). Methylation of the cap ap-parently is not required for mRNA synthesis,but some structural features of AdoMet appearto be involved in the transcription complex andcause activation of RNA polymerase at the ini-tiation site of the genome. This hypothesis issupported by the finding that the apparent Kmfor ATP for the initiation of CPV mRNA syn-thesis is greatly reduced by increasing concen-trations of AdoMet. It has been proposed thatAdoMet may interact with a methylase subunitin the CPV transcription complex, resulting inan allosteric conformational change that lowersthe apparent Km for initiating ATP and activa-tion of the RNA polymerase (85). Recently,Wertheimer et al. (267) have studied the effectsof various AdoMet analogs on CPV transcriptionin vitro in an effort to understand which struc-tural features of AdoMet are important for stim-ulation of RNA synthesis. It appears that thoseanalogs that generally inhibit methyltransferasealso stimulate RNA synthesis. Furthermore, an-alogs which do not inhibit methyltransferase failto stimulate mRNA synthesis. These findingsalso indicate that some interaction betweenAdoMet and the methyltransferase turns onCPV mRNA synthesis. Stimulatory effects ofAdoMet on in vitro transcription of a fish rhab-dovirus have also been reported (212). Moredetailed studies in these viral systems shouldeludicate further the role of capping in mRNAsynthesis.
Inhibitors of methyltransferases have beenused to understand the role of methylation inmRNA synthesis, transport, and translation invivo. Kaehler et al. (115) have shown that invivo S-tubercidinyl homocysteine effectively in-hibits ribose 2'-O-methylation of the N, nucleo-side of the cap and base N' adenosine methyla-tion in internal positions of Novikoff hepatomamRNA's. Methylation of 5' blocking guanosinewas not affected, and the cytoplasmic mRNA'scontained predominantly cap 0 structures. Sincethis inhibitor did not affect the viability of thecell, it was concluded that ribose methylation orinternal adenosine methylation was not neededfor mRNA transport from the nucleus or sub-sequent translation in cytoplasm. Similarly, Di-mock and Stoltzfus showed that cycloleucine, acompetitive inhibitor of ATP:L-methionine S-adenosyltransferase, blocks ribose methylationand internal methylation (but not 7-methylationof guanosine) of avian sarcoma virus genomeRNA in chicken embryo cells (56). Viral repli-cation was virtually unaffected under these con-ditions, indicating that ribose and internal meth-ylation are not required for avian sarcoma virusRNA synthesis, processing, and transport. Sim-ilar results were obtained by Bachellerie et al.(14) in Chinese hamster ovary cells treated withcycloleucine. In contrast, Robert-Gero et al.(201) reported that 5'-deoxy-5'-isobutyryladen-osine, an analog of AdoHcy, inhibited by morethan 90% the production of the Schmidt-Ruppinstrain of Rous sarcoma virus. These results sug-gest that 5'-deoxy-5'-isobutyryladenosine mayinhibit the 7-methylguanosine methyltransfer-ase which may be essential for mRNA synthesis.In fact, Jacquemont and Huppert (114) haveshown that 5'-deoxy-5'-isobutyryladenosine in-hibited 7mG methylation of viral mRNA,whereas m6A methylation was not affected inherpes simplex virus type 1-infected cells. Re-cently, the antibiotic Sinefungin has been shownto inhibit Newcastle disease virus replicationand also the 7-methylguanine and 2'-O-methyl-nucleoside methyltransferases of vaccinia virusin vitro (194). Cycloleucine has also been shownto inhibit VSV replication in baby hamster kid-ney and Chinese hamster ovary cells (35, 243a),indicating that methylation may play an impor-tant role in viral mRNA synthesis in vivo. How-ever, inhibition of methylation (AdoHcy) in vitrohas virtually no effect on VSV mRNA transcrip-tion, although it affects the polyadenylationprocess. poly(A) tails 2,000 bases long (approxi-mately 10 times the normal length) were addedto VSV mRNA's when mRNA synthesis wascarried out in vitro in the presence of AdoHcy(207).
VOL. 44, 1980
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

196 BANERJEE
CONCLUSIONS
The appearance of the cap structure in eucar-yotic mRNA's is clearly an important step in theevolution of living organisms. Presumably, bio-synthesis of guanylyltransferase and mRNA (7-guanine)methyltransferase and the appearanceof these enzymes in the nuclei of primitive eu-caryotic cells marked the onset of the first pro-duction of capped mRNA's. Ribose methylationand internal methylations of mRNA are lateevolutionary events, the roles of which are stillnot clear. The methylation of the blocking gua-nosine base at position 7 is also an importantprocess because it renders the cap structurepolar, thus allowing interaction with chargedmacromolecules. In addition, the triphosphatebridge confers a freedom of movement of 7mGfor similar interactions. Moreover, the structureappears to be resistant to the 5'-exonucleaseaction and thus may increase the half-life of themessage. The involvement of the cap in mRNAtranslation has been documented adequately.More extensive studies on the CBP with regardto its structure and function will certainly helpto elucidate the involvement of the cap in ribo-some recognition. The roles of ribose methyla-tion, N6mAm, and internal N6mA in mRNA's arenot well understood. Possibly, they are involvedin fine tuning during initiation complex forma-tion (121, 175), mRNA processing, and transport(23). It is not clear whether 7mG has any role inthe transcription of the embryonic genome dur-ing preimplantation development ofmammalianembryos. There is one report (275a) describingthe appearance of 7mG in the RNA of mouseone-cell embryos 3 h after fertilization. This incontrast to the echinoderms, in which maternalmRNA's are capped and their translation afterfertilization does not require attachment of 7mGto the 5' terminus (69, 109,224). The mechanismof capping in the viral systems has been wellelucidated, and the enzymes involved in thecapping reactions have now been purified fromboth viral and eucaryotic sources. On the otherhand, an understanding of capping mechanismsin cellular mRNA is still not clear. With thecontinued use of more refined in vitro nuclearsystems, more light should be shed on this proc-ess in the near future. Elucidation of this reac-tion will be of great help in understanding themore general process of mRNA biosynthesis ineucaryotic cells in general. Future experimentswill clearly be directed toward a more preciseunderstanding of the function of the cap in eu-caryotic gene expression.
ACKNOWLEDGMENTSI am grateful to Aaron J. Shatkin for his valuable
commments for improving the manuscript. I thank
Yasuhiro Furuichi for many useful discussions. I alsothank my numerous colleagues who kindly suppliedtheir reprints and preprints. I am indebted to DianeAndriola and Edna Pearlman for excellent secretarialassistance.
LITERATURE CITED
1. Abraham, G., and A. K. Banerjee. 1976. Thenature of the RNA products synthesized invitro by subviral components of vesicular sto-matitis virus. Virology 71:230-241.
2. Abraham, G., D. P. Rhodes, and A. K. Ba-nerjee. 1975. The 5'-terminal structure of themethylated messenger RNA synthesized in vi-tro by vesicular stomatitis virus. Cell 5:51-58.
3. Abraham, G., D. P. Rhodes, and A. K. Ba-nerjee. 1975. Novel initiation of RNA synthe-sis in vitro by vesicular stomatitis virus. Nature(London) 255:37-40.
4. Abraham, K. A., and J. R. Lillehaug. 1976.Enzymic cleavage of m7GDP from eucaryoticmRNA. FEBS Lett. 71:49-52.
5. Abraham, K. A., and A. Pihl. 1977. Translationof enzymically decapped messenger RNA. Eur.J. Biochem. 77:589-593.
6. Adams, B. L., M. Morgan, S. Muthukrishnan,S. M. Hecht, and A. J. Shatkin. 1978. Theeffect of "cap" analogs on reovirus mRNAbinding to wheat germ ribosomes. J. Biol.Chem. 253:2589-2595.
7. Adams, J. M. 1977. Messenger RNA, p. 81-128.In P. R. Stewart, and D. S. Letham (ed.), Theribonucleic acids. Springer-Verlag, New York.
8. Adams, J. M., and S. Cory. 1975. Modifiednucleosides and bizarre 5'-termini in mousemyeloma mRNA. Nature (London) 255:28-33.
9. Agranovsky, A. A., V. V. Dolja, V. K. Kagra-manova, and J. G. Atabekov. 1979. Thepresence of a cap structure at the 5'-end ofbarley stripe mosaic virus RNA. Virology 95:208-210.
10. Aloni, Y. 1975. Methylated SV-40 messengerRNA. FEBS Lett. 54:363-367.
11. Anderson, C. W., J. F. Atkins, and J. J.Dunn. 1976. Bacteriophage Ti and T7 earlyRNAs are translated by eukaryotic 80S ribo-somes: active phage T, coded S-adenosylmeth-ionine cleaving enzyme is synthesized. Proc.Natl. Acad. Sci. U.S.A. 73:2752-2756.
12. Anderson, W. F., L. Bosch, W. E. Cohn, H.Lodish, W. C. Merrick, H. Weissbach, H.G. Wittman, and I. G. Wool. 1977. Interna-tional symposium on protein synthesis: meet-ing report. FEBS Lett. 76:1-10.
13. Asselbergs, F. A. M., W. Peters, W. J. VanVenrooij, and H. Bloemendal. 1978. Dimin-ished sensitivity of re-initiation of translationto inhibition by cap analogs in reticulocytelysates. Eur. J. Biochem. 88:483-488.
14. Bachellerie, J. P., F. Amalric, and M. Ca-boche. 1978. Biosynthesis and utilization ofextensively undermethylated poly(A) RNA inCHO cells during a cycloleucine treatment.Nucleic Acids Res. 5:2927-2943.
15. Bajszar, G., 0. P. Samarina, and G. P. Geor-
MICROBIOL. REV.
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

5'-TERMINAL CAP STRUCTURES IN mRNA's 197
giev. 1976. On the nature of 5'-termini in nu-clear pre-mRNA of Ehrlich carcinoma cells.Cell 9:323-332.
16. Bajszar, G., G. Szabo, A. Simoncsits, and J.Molnar. 1978. Methylated cap formation byenzymes bound to nuclear informer particles.Mol. Biol. Rep. 4:93-96.
17. Banerjee, A. K., G. Abraham, and R. J. Co-lonno. 1977. Vesicular stomatitis virus: modeof transcription. J. Gen. Virol. 34:1-8.
18. Banerjee, A. K., R. J. Colonno, D. Testa, andM. T. Franze-Fernandez. 1978. Mechanismof RNA synthesis in vitro by vesicular stoma-titis virus, p. 249-259. In B. W. J. Mahy and R.D. Barry (ed.), Negative strand viruses and thehost cell. Academic Press Inc., New York.
19. Barboea, E., and B. Moss. 1978. mRNA (nu-cleoside-2'-)-methyltransferase from vacciniavirus: purification and physical properties. J.Biol. Chem. 253:7692-7697.
20. Barbosa, E., and B. Moss. 1978. mRNA (nu-cleoside-2'-)-methyltransferase from vacciniavirus: characteristics and substrate specificity.J. Biol. Chem. 253:7698-7702.
21. Barrieux, A., and M. C. Rosenfeld. 1977. Com-parison ofmRNA binding by met-tRNAf bind-ing protein and mRNA-associated proteins. J.Biol. Chem. 252:392-398.
22. Barrieux, A., and M. G. Rosenfeld. 1977. Char-acterization of GTP-dependent met-tRNAfbinding protein. J. Biol. Chem. 252:3843-3847.
23. Bartkoski, M. J., and B. Roizman. 1978. Reg-ulation of herpesvirus macromolecular synthe-sis. VII. Inhibition of internal methylation ofmRNA late in infection. Virology 85:146-156.
24. Beemon, K., and J. Keith. 1977. Localization ofNW-methyladenosine in the Rous sarcoma virusgenome. J. Mol. Biol. 113:165-179.
25. Bergmann, J. E., and H. F. Lodish. 1979.Translation of capped and uncapped vesicularstomatitis virus and reovirus mRNAs: sensitiv-ity to 7mGpppAn' and ionic conditions. J. Biol.Chem. 254:459-468.
26. Bishop, D. H. L. 1977. Virion polymerases, p.117-278. In H. Fraenkel-Conrat and R. R. Wag-ner (ed.), Comprehensive virology, vol. 10.Plenum Press, New York.
27. Black, D. N., J. N. Burroughs, T. J. R. Harris,and F. Brown. 1979. The structure and repli-cation of calcivirus RNA. Nature (London)274:614-615.
28. Both, G. W., A. K. Banerjee, and A. J. Shat-kin. 1975. Methylation-dependent translationof viral messenger RNAs in vitro. Proc. Natl.Acad. Sci. U.S.A. 72:1189-1193.
29. Both, G. W., Y. Furuichi, S. Muthukrishnan,and A. J. Shatkin. 1975. Ribosome binding toreovirus mRNA in protein synthesis requires5'-terminal 7-methylguanosine. Cell 6:185-195.
30. Both, G. W., Y. Furuichi, S. Muthukrishnan,and A. J. Shatkin. 1976. Effect of 5'-terminalstructure and base composition on polyribo-nucleotide binding to ribosomes. J. Mol. Biol.104:637-658.
31. Bouloy, M., M. Morgan, A. J. Shatkin, and R.
M. Krug. 1979. Cap and internal nucleotidesof reovirus mRNA primers are incorporatedinto influenza viral complementary RNA dur-ing transcription in vitro. J. Virol. 32:895-904.
32. Bouloy, M., S. J. Plotch, and R. M. Krug.1978. Globin mRNAs are primers for the tran-scription of influenza viral RNA in vitro. Proc.Natl. Acad. Sci. U.S.A. 75:4886-4890.
33. Burroughs, J. N., and F. Brown. 1978. Pres-ence of a covalently linked protein on calcivirusRNA. J. Gen. Virol. 41:443-446.
34. Bush, H., F. Hirsch, K. K. Gupta, M. Rao, W.Spohn, and B. C. Wu. 1976. Structural andfunctional studies on the "5'-cap": a surveymethod for mRNA. Prog. Nucleic Acid Res.Mol. Biol. 19:39-61.
35. Caboche, M., and C. La Bonnardiere. 1979.Vesicular stomatitis virus mRNA methylationin vivo: effect of cycloleucine, an inhibitor ofS-adenosylmethionine biosynthesis, on viraltranscription and translation. Virology 93:547-557.
36. Canaani, D., C. Kahana, S. Lavi, and Y.Groner. 1979. Identification and mapping ofN6-methyladenosine containing sequences insimian virus 40 RNA. Nucleic Acids Res. 6:2879-2899.
37. Canaani, D., C. Kahana, A. Mukamel, and Y.Groner. 1979. Sequence heterogeneity at the5'-termini of late simian virus 40 19S and 16SmRNAs. Proc. Natl. Acad. Sci. U.S.A. 76:3078-3082.
38. Canaani, D., M. Revel, and Y. Groner. 1976.Translational discrimination of "capped" and"non-capped" mRNAs: inhibition by a series ofchemical analogs of m7GpppX. FEBS Lett. 64:326-331.
39. Cheng, T.-C., and H. H. Kazazian, Jr. 1977.The 5'-terminal structures of murine alpha glo-bin and beta globin messenger RNA. J. Biol.Chem. 252:1758-1763.
40. Cho, S., T.-C. Cheng, and H. H. Kazazian, Jr.1978. Characterization of 5'-terminal methyla-tion of human alpha and beta globin mRNAs.J. Biol. Chem. 253:5558-5561.
41. Chu, L.-Y., and R. E. Rhoads. 1978. Transla-tional recognition of the 5'-terminal 7-methyl-guanosine of globin messenger RNA as a func-tion of ionic strength. Biochemistry 17:2450-2454.
42. Cleaves, G. R., and D. T. Dubin. 1978. Meth-ylation status of Dengue virus 40S RNA. Fed.Proc. 37:173.
43. Colonno, R. J., and H. 0. Stone. 1975. Meth-ylation of messenger RNA of Newcastle diseasevirus in vitro by a virion associated enzyme.Proc. Natl. Acad. Sci. U.S.A 72:2611-2615.
44. Colonno, R. J., and H. 0. Stone. 1976. New-castle disease virus messenger RNA lacks 2'-O-methylated nucleotides. Nature (London) 261:611-614.
45. Cory, S., and J. M. Adams. 1975. The modified5'-terminal sequences in messenger RNA ofmouse myeloma cells. J. Mol. Biol. 99:519-547.
46. Cory, S., and J. M. Adams. 1975. Modified 5'-
VOL. 44, 1980
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

198 BANERJEE
termini in small nuclear RNAs of mouse mye-loma cells. Mol. Biol. Rep. 2:287-294.
47. Cory, S., C. Genin, and J. M. Adams. 1976.Modified nucleosides and 5'-end groups in pu-rified mouse immunoglobulin light chainmRNA and rabbit globin mRNA detected byborohydride labelling. Biochim. Biophys. Acta454:248-262.
48. Darnell, J. E., Jr. 1979. Transcription units formRNA production in eukaryotic cells and theirDNA viruses. Prog. Nucleic Acid Res. Mol.Biol. 22:327-353.
49. DasGupta, R., F. Harada, and P. Kaesberg.1976. Blocked 5' termini in brome mosaic virusRNA. J. Virol. 18:260-267.
50. Daubert, S. D., G. Bruening, and R. C. Naja-rian. 1978. Protein bound to the genome RNAsof cowpea mosaic virus. Eur. J. Biochem. 92:45-51.
51. De Kloet, S. R., and B. A. G. Andrean. 1976.Methylated nucleosides in polyadenylate-con-taining yeast messenger ribonucleic acid.Biochim. Biophys. Acta 425:401-408.
52. Desrosiers, R., K. Friderici, and F. Rottman.1974. Identification of methylated nucleosidesin messenger RNA from Novikoff hepatomacells. Proc. Natl. Acad. Sci. U.S.A. 71:3971-3975.
53. Desrosiers, R. C., K. H. Friderici, and F. M.Rottman. 1975. Characterization of Novikoffhepatoma mRNA methylation and heteroge-neity in the methylated 5'-terminus. Biochem-istry 14:4367-4374.
53a. Desrosiers, R., G. C. Sen, and P. Lengyl.1976. Difference in 5'-terminal structure be-tween the mRNA and the double-stranded vir-ion RNA of reovirus. Biochem. Biophys. Res.Commun. 73:32-39.
54. Dhingra, M. M., and R. H. Sarma. 1978. NMRstudies and minimum energy conformation cal-culations on the 5'-terminus of mammalianmRNA. Quantum Chem. Biochem. 11:31-39.
55. Dimock, K., and C. M. Stoltzfus. 1977. Se-quence specificity of internal methylation inB77 avian sarcoma virus RNA subunits. Bio-chemistry 16:471-478.
56. Dimock, K., and C. M. Stoltzfus. 1978. Cyclo-leucine blocks internal and 5'-terminal meth-ylations of avian sarcoma virus genome RNA.Biochemistry 17:3627-3632.
57. Doerfler, W. 1977. Animal virus-host genomeinteraction, p. 279-399. In H. Fraenkel-Conratand R. R. Wagner (ed.), Comprehensive virol-ogy. vol. 10. Plenum Press, New York.
58. Dottin, R. P., A. M. Weiner, and H. F. Lodish.1976. 5'-Terminal nucleotide sequences of themessenger RNAs of Dictyostellium discoi-deum. Cell 8:233-244.
59. Dubin, D. T., and V. Stollar. 1975. Methylationof Sindbis virus 26S messenger RNA. Biochem.Biophys. Res. Commun. 66:1373-1379.
60. Dubin, D. T., V. Stollar, C.-C. Hsu-Chen, K.Timko, and G. M. Guild. 1977. Sindbis virusmessenger RNA: the 5'-termini and methyl-ated residues of 26 and 42S RNA. Virology 77:457-470.
61. Dubin, D. T., and R. H. Taylor. 1975. Themethylation state of poly A-containing messen-ger RNA from cultured hamster cells. NucleicAcids Res. 2:1653-1668.
62. Dubin, D. T., K. Timko, S. Gillies, and V.Stollar. 1979. The extreme 5'-terminal se-quences of Sindbis virus 26 and 42S RNA.Virology 98:131-141.
63. Efstratiadis, A., J. N. Vournakis, H. Donis-Keller, G. Chaconas, D. K. Dougall, and F.C. Kafatos. 1977. End labelling of enzymati-cally decapped mRNA. Nucleic Acids Res. 4:4165-4174.
64. Ehresmann, D. W., and F. L. Schaffer. 1979.Calcivirus intercellular RNA: fractionation of18-22 S RNA and lack of typical 5'-methylatedcaps on 36 S and 22 S San Miguel sea lion virusRNAs. Virology 95:251-255.
65. Ensinger, M. J., S. A. Martin, E. Paoletti, andB. Moss. 1975. Modification of the 5'-terminusof mRNA by soluble guanylyl and methyltransferases from vaccinia virus. Proc. Natl.Acad. Sci. U.S.A. 72:2525-2529.
66. Ensinger, M. J., and B. Moss. 1976. Modifica-tion of the 5' terminus of mRNA by an RNA(guanine-7-)methyltransferase from HeLacells. J. Biol. Chem. 251:5283-5291.
67. Faust, M., K. E. M. Hastings, and S. Mill-ward. 1975. 'mG(5')ppp(5')Gn'pCpUp at the5'-terminus of reovirus messenger RNA. Nu-cleic Acids. Res. 2:1329-1343.
68. Faust, M., and S. Millward. 1974. In vitromethylation of nascent reovirus mRNA by avirion-associated methyl transferase. NucleicAcids Res. 1:1739-1752.
69. Faust, M., S. Millward, A. Duchastel, and D.Fromson. 1976. Methylated constituents ofpoly(A)- and poly(A)+ polyribosomal RNA ofsea urchin embryos. Cell 9:597-604.
70. Filipowicz, W. 1978. Function of the 5'-terminal7mG cap in eukaryotic mRNA. FEBS Lett. 96:1-11.
71. Filipowicz, W., Y. Furuichi, J. M. Sierra, S.Muthukrishnan, A. J. Shatkin, and S.Ochoa. 1976. A protein binding the methylated5'-terminal sequence, 7mGpppN, of eukaryoticmessenger RNA. Proc. Natl. Acad. Sci. U.S.A.73:1559-1563.
72. Flavell, A. J., A. Cowie, S. Legon, and R.Kamen. 1979. Multiple 5'-terminal cap struc-tures in late polyoma virus RNA. Cell 16:357-371.
73. Friderici, K., M. Kaehler, and F. Rottman.1976. Kinetics of Novikoff cytoplasmic messen-ger RNA methylation. Biochemistry 15:5234-5241.
74. Fujimoto, M., A. Kuninaka, and H. Yoshino.1974. Substrate specificity of nuclease P,. Agr.Biol. Chem. 38:1555-1561.
75. Furuichi, Y. 1974. Methylation-coupled tran-scription by virus-associated transcriptase ofcytoplasmic polyhedrosis virus containing dou-ble-stranded RNA. Nucleic Acids Res. 1:802-809.
76. Furuichi, Y. 1978. "Pretranscriptional capping"in the biosynthesis of cytoplasmic polyhedrosis
MICROBIOL. REV.
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

5'-TERMINAL CAP STRUCTURES IN mRNA's 199
virus mRNA. Proc. Natl. Acad. Sci. U.S.A. 75:1086-1090.
77. Fuichi, Y., A. LaFinandra, and A. J. Shat-kin. 1977. 5'-Terminal structure and mRNAstability. Nature (London) 266:235-239.
78. Furuichi, Y., and K.-I. Miura. 1975. A blockedstructure at the 5'-terminus of messenger RNAfrom cytoplasmic polyhedrosis virus. Nature(London) 253:374-375.
79. Furuichi, Y., M. Morgan, S. Muthukrishnan,and A. J. Shatkin. 1975. Reovirus messengerRNA contains a methylated, blocked 5'-termi-nal structure, m7G(5')ppp(5')GpCp. Proc. Natl.Acad. Sci. U.S.A. 72:362-366.
80. Furuichi, Y., M. Morgan, and A. J. Shatkin.1979. Synthesis and translation of mRNA con-taining 5'-terminal 7-ethylguanosine cap. J.Biol. Chem. 254:6732-6738.
81. Furuichi, Y., M. Morgan, A. J. Shatkin, W.Jelinek, M. Salditt-Georgieff, and J. E.Darneli. 1975. Methylated, blocked 5' terminiin HeLa cell mRNA. Proc. Natl. Acad. Sci.U.S.A. 72:1904-1908.
82. Furuichi, Y., S. Muthukrishnan, and A. J.Shatkin. 1975. 5'-Terminal 7mG(5')ppp(5')-G'p in vivo: identification in reovirus genomeRNA. Proc. Natl. Acad. Sci. U.S.A. 72:742-745.
83. Furuichi, Y., S. Muthukrishnan, J. Tomasz,and A. J. Shatkin. 1976. Mechanism of for-mation of reovirus mRNA 5'-terminal blockedand methylated sequence, 7mGpppGmC. J.Biol. Chem. 251:5043-5053.
84. Furuichi, Y., and A. J. Shatkin. 1977. 5'-Ter-mini of reovirus mRNA. Ability of viral coresto form caps post-transcriptionally. Virology77:566-578.
85. Furuichi, Y., and A. J. Shatkin. 1979. Stimu-lation of CPV mRNA synthesis in vitro by S-adenosyl methionine and 5'-capping during ini-tiation, p. 351-360. In E. Usdin, R. T. Bor-chardt, and C. R. Creveling (ed.), Transmeth-ylation. Elsevier/North-Holland, New York.
85a. Furuichi, Y., A. J. Shatkin, E. Stavnezer,and J. M. Bishop. 1975. Blocked, methylated5'-terminal sequence in avian sarcoma virusRNA. Nature (London) 257:618-620.
86. Gedamu, L., and G. H. Dixon. 1978. Effect ofenzymatic "decapping" on protamine mRNAtranslation in wheat germ S-30. Biochem. Bio-phys. Res. Commun. 85:114-124.
87. Germershausen, J., D. Goodman, and E. W.Somberg. 1978. 5' Cap methylation of homol-ogous poly A(+) RNA by a RNA (guanine-7-)methyltransferase from Neurospora crassa.Biochem. Biophys Res. Commun. 82:871-878.
88. Golini, F., A. Nomoto, and E. Wimmer. 1979.The genome-linked protein of picornaviruses.IV. Difference in the VPg's of encephalomyo-carditis virus and poliovirus as evidence thatthe genome-linked proteins are virus-coded.Virology 89:112-118.
89. Gosh, P. K., V. B. Reddy, J. Swinscoe, P.Lebowitz, and S. M. Weissman. 1978. Het-erogeneity and 5'-terminal structures of the
late RNA's of simian virus 40. J. Mol. Biol.126:813-846.
90. Grohmann, K., F. Amahric, S. Crews, and G.Attardi. 1978. Failure to detect "cap" struc-tures in mitochondrial DNA-coded poly(A)-containing RNA from HeLa cells. NucleicAcids Res. 5:637-651.
91. Groner, Y., P. Carmi, and Y. Aloni. 1977.Capping structures of simian virus 40 19S and16S mRNAs. Nucleic Acids Res. 4:3959-3968.
92. Groner, Y., E. Gilboa, and H. Aviv. 1978.Methylation and capping of RNA polymeraseII primary transcripts by HeLa nuclear homog-enates. Biochemistry 17:977-982.
93. Groner, Y., H. Grosfeld, and U. Z. Littaur.1976. 5'-Capping structures of Artemia salinamRNA and the translational inhibition by capanalogs. Eur. J. Biochem. 71:281-293.
94. Groner, Y., and J. Hurwitz. 1975. Synthesis ofRNA containing a methylated blocked 5'-ter-minus by HeLa nuclear homogenates. Proc.Natl. Acad. Sci. U.S.A. 72:2930-2934.
95. Guilly, H., and J. P. Briand. 1978. Nucleotidesequence of turnip yellow mosaic virus coatprotein mRNA. Cell 15:113-122.
96. Gupta, K. C., and P. Roy. 1980. Alternate cap-ping mechanisms for transcription of springviremia carp virus: evidence for independentmRNA initiation. J. Virol. 33:292-303.
97. Haegeman, G., and W. Fiers. 1978. Localiza-tion of the 5'-terminus of late SV40 mRNA.Nucleic Acids Res. 5:2359-2371.
98. Haffner, M. H., M. B. Chin, and B. G. Lane.1978. Wheat embryo ribonucleates. XII. For-mal characterization of terminal and penulti-mate nucleoside residues at the 5'-ends of"capped" RNA from imbibing wheat embryos.Can. J. Biochem. 56:729-733.
99. Hashimoto, S., and M. Green. 1976. Multiplemethylated cap sequences in adenovirus type2 early mRNA. J. Virol. 20:425-435.
100. Hashimoto, S., and M. Green. 1979. Methyl-ated 5'-terminal caps of adenovirus type 2 earlymRNA: evidence for at least six 5'-termini.Virology 94:254-272.
101. Heckle, W. L., Jr., R. G. Fenton, T. G. Wood,C. G. Merkel, and B. J. Lingrel. 1977. Meth-ylated nucleosides in globin mRNA frommouse nucleated erythroid cells. J. Biol. Chem.252:1764-1770.
102. Hefti, E., and D. H. L. Bishop. 1975. The 5'-nucleotide sequence of vesicular stomatitisviral RNA. J. Virol. 15:90-96.
103. Hefti, E., D. H. L. Bishop, D. T. Dubin, andV. Stollar. 1976. 5'-Nucleotide sequence ofSindbis viral RNA. J. Virol. 17:149-159.
104. Held, W. A., K. West, and J. F. Gallagher.1977. Importance of initiation factor prepara-tions in the translation of reovirus and globinmessenger RNA lacking a 5' terminal 7 meth-ylguanosine. J. Biol. Chem. 252:8489-8497.
105. Helentiaris, T., and E. Ehrenfeld. 1978. Con-trol of protein synthesis in extracts from polio-virus-infected cells. I. mRNA discrimination bycrude initiation factors. J. Virol. 26:510-521.
VOL. 44, 1980
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

200 BANERJEE
106. Helentjaris, T., E. Ehrenfeld, M. Brown-Luedi, and J. W. B. Hershey. 1979. Altera-tions in initiation factor activity from polio-virus-infected HeLa cells. J. Biol. Chem. 254:10973-10978.
107. Hendler, S., E. Fiirer, and P. R. Srinivasan.1970. Synthesis and chemical properties ofmonomers and polymers containing 7-methyl-guanine and an investigation of their substrateor template properties for bacterial deoxyri-bonucleic acid or ribonucleic acid polymerases.Biochemistry 9:4141-4153.
108. Hewlett, M. J., J. K. Rose, and D. Baltimore.1976. 5'-Terminal structure of poliovirus poly-ribosomal RNA is pUp. Proc. Natl. Acad. Sci.U.S.A. 73:327-330.
109. Hickey, E. D., C. Baglioni, and L. A. Weber.1976. Translation of RNA from unfertilizedsea-urchin eggs does not require methylationand is inhibited by 7-methyl GMP. Nature(London) 261:71-72.
110. Hickey, E. D., L. A. Weber, and C. Baglioni.1976. Inhibition of initiation of protein synthe-sis by 7-methylguanosine-5'-monophosphate.Proc. Natl. Acad. Sci. U.S.A. 73:19-23.
111. Hickey, E. D., L. A. Weber, C. Baglioni, C. H.Kim, and R. H. Sarma. 1977. A relation be-tween inhibition of protein synthesis and con-formation of 5'-phosphorylated 7-methyl-guanosine derivatives. J. Mol. Biol. 109:173-183.
112. Hsuchen, C.-C. H., and D. T. Dubin. 1976. Di-and trimethylated congeners of 7-methylgua-nine in Sindbis virus mRNA. Nature (London)264:190-191.
113. Hsuchen, C.-C. H., and D. T. Dubin. 1977.Methylated constituents of Aedes albopictuspoly(A)-containing messenger RNA. NucleicAcids Res. 4:2671-2682.
114. Jacquemont, B., and J. Huppert. 1977. Inhi-bition of viral RNA methylation in herpes sim-plex virus type 1-infected cells by 5',5-isobutyl-adenosine. J. Virol. 22:160-167.
115. Kaehler, M., J. Coward, and F. Rottman.1977. In vivo inhibition ofNovikoff cytoplasmicmessenger RNA methylation by S-tubercidi-nyl-homocysteine. Biochemistry 16:5770-5775.
116. Kaempfer, R., H. Rosen, and R. Israeli. 1978.Translational control: recognition of the meth-ylated 5'-end and an internal sequence in eu-caryotic mRNA by the initiation factor thatbinds methionyl-tRNAf et. Proc. Natl. Acad.Sci. U.S.A. 75:650-654.
117. Kastern, W. H., and S. J. Berry. 1976. Non-methylated guanosine as the 5' terminus ofcapped mRNA from insect oocytes. Biochem.Biophy. Res. Commun. 71:37-44.
118. Keith, J., and H. Fraenkel-Conrat. 1975. Iden-tification of the 5'-end of Rous sarcoma virusRNA. Proc. Natl. Acad. Sci. U.S.A. 72:3347-3350.
119. Keith, J., and H. Fraenkel-Conrat. 1975. To-bacco mosaic virus RNA carries 5' terminaltriphosphorylated guanosine blocked by 5'
linked 7-methylguanosine. FEBS Lett. 57:31-33.
120. Keith, J. M., M. J. Ensinger, and B. Moss.1978. HeLa cell RNA(2'-O-methyladenosine-N6-)-methyltransferase specific for the capped5'-end of messenger RNA. J. Biol. Chem. 253:5033-5041.
121. Keith, J. M., S. Muthukrishman, and B.Moss. 1978. Appendix: effect of methylation ofthe N6 position of the penultimate adenosineof capped mRNA on ribosome binding. J. Biol.Chem. 253:5039-5041.
122. Kelly, D. E., and R. P. Perry. 1975. Methylatedsequences at the 5' termini of heterogeneousnuclear RNA and messenger RNA. J. Cell Biol.76:205A.
123. Kemper, B. 1976. Inactivation of parathyroidhormone mRNA by treatment with periodateand aniline. Nature (London) 262:321-323.
124. Kemper, B., and L. Stolarsky. 1977. Depend-ence on potassium concentration of the inhi-bition of the translation of messenger ribonu-cleic acid by 7-methylguanosine 5'-phosphate.Biochemistry 16:5676-5680.
125. Kennedy, T. D., and B. G. Lane. 1975. Theprobable "capping" of wheat leaf messengerribonucleates by 7-methylguanosine. Can. J.Biochem. 53:1346-1348.
126. Kim, C. H., and R. H. Sarma. 1977. Spatialconfiguration of mRNA 5'-terminus. Nature(London) 270:223-227.
127. Kim, C. H., and R. H. Sarma. 1978. Spatialconfiguration of the bizarre 5'-terminus ofmammalian messenger RNA. J. Am. Chem.Soc. 100:1571-1590.
128. Klein, C., C. Fritsch, J. P. Braind, K. E. Rich-ards, G. Jonard, and L. Hirth. 1976. Physicaland functional heterogeneity in TYMV RNA:evidence for the existence of an independentmessenger coding for coat protein. NucleicAcids Res. 3:3043-3061.
129. Klootwijk, J., I. Klein, P. Zabel, and A. VanKammen. 1977. Cowpea mosaic virus RNAshave neither 7mGpppN.. nor mono-, di-, ortriphosphates at their 5' ends. Cell 11:73-82.
130. Kozak, M. 1978. How do eucaryotic ribosomesselect initiation regions in messenger RNA?Cell 15:1109-1123.
131. Kozak, M. 1979. Inability of circular mRNA toattach to eucaryotic ribosomes. Nature (Lon-don) 280:82-85.
132. Kozak, M., and A. J. Shatkin. 1978. Migrationof 40S ribosomal subunits on messenger RNAin the presence of edeine. J. Biol. Chem. 253:6568-6577.
133. Krug, R. M., B. A. Broni, and M. Bouloy.1979. Are the 5' ends of influenza viral mRNAssynthesized in vivo donated by host mRNAs?Cell 18:329-334
134. Krug, R. M., M. A. Morgan, and A. J. Shatkin.1976. Influenza viral mRNA contains internalN6-methyladenosine and 5'-terminal 7-meth-ylguanosine in cap structures. J. Virol. 20:45-53.
MICROBIOL. REV.
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

5'-TERMINAL CAP STRUCTURES IN mRNA's 201
135. Lavers, G. C. 1977. Cleavage of pm7G frommRNA 5' terminal cap structures by pyrophos-phatase activity in embryonic chick lens cells.Mol. Biol. Rep. 3:413-420.
136. Lavers, G. C. 1977. Detection of methyltransfer-ase activities which modify GpppG tom7GpppGm in embryonic chick lens. Mol. Biol.Rep. 3:275-281.
137. Lavers, G. C. 1979. Metabolism of m7GMP-con-taining 5' terminal mRNA cap sequences inextracts of embryonic chick lens cells. Exp. EyeRes. 28:299-309.
138. Lavi, S., and A. J. Shatkin. 1975. Methylatedsimian virus 40-specific RNA from nuclei andcytoplasm of infected BSC-1 cells. Proc. Natl.Acad. Sci. U.S.A. 72:2012-2016.
139. Lawley, P. D. 1966. Effects of some chemicalmutagens and carcinogens on nucleic acids.Prog. Nucleic Acid Res. Mol. Biol. 5:89-131.
140. Lawley, P. D., and P. Brookes. 1961. Acidicdissociation of 7:9-dialkylguanines and its pos-sible relation to mutagenic properties of alkyl-ating agents. Nature (London) 192:1081-1082.
141. Laycock, D. E. 1977. Purification of eukaryoticmRNA guanylyltransferase. Fed. Proc. 36:770.
142. Lee, Y. F., A. Nomoto, B. M. Detjen, and E.Wimmer. 1977. A protein covalently linked topoliovirus genome RNA. Proc. Natl. Acad. Sci.U.S.A. 74:59-63.
143. Lesnaw, J. A., and M. E. Reichmann. 1970.Identity of the 5'-terminal RNA nucleotidesequence of the satellite tobacco necrosis virusand its helper virus: possible role of the 5'-terminus in the recognition by virus-specificRNA replicase. Proc. Natl. Acad. Sci. U.S.A.66:140-145.
144. Leung, D. W., K. S. Browning, J. E. Heck-man, U. L. RajBhandary, and J. M. Clark.1979. Nucleotide sequence of the 5'-terminusof satellite tobacco necrosis virus ribonucleicacid. Biochemistry 18:1361-1366.
145. Leung, D. W., C. W. Gilbert, R. E. Smith, N.L. Sasavage, and J. M. Clark, Jr. 1974.Translation of satellite tobacco necrosis virusribonucleic acid by an in vitro system fromwheat germ. Biochemistry 15:4943-4950.
146. Levin, K. H., and C. E. Samuel. 1977. Biosyn-thesis of reovirus-specified polypeptides-ef-fect of methylation on the efficiency of reovirusgenome expression in vitro. Virology 77:245-259.
147. Levis, R., and S. Penman. 1978. 5'-Terminalstructures of poly(A)+ cytoplasmic messengerRNA and of poly(A)+ and poly(A)- heteroge-nous nuclear RNA of cells of the dipteranDrosophila melanogaster. J. Mol. Biol. 120:487-516.
148. Lockard, R. E. 1978. Different Cap I:Cap 2 ratiosin rabbit a and f, globin mRNA. Nature (Lon-don) 275:153-154.
149. Lockard, R. E., and C. Lane. 1978. Require-ment for 7-methylguanosine in translation ofglobin mRNA in vivo. Nucleic Acids Res. 5:3237-3247.
150. Lockard, R. E., and U. L. RajBhandary. 1976.
Nucleotide sequences at the 5'-termini of rab-bit a and / globin mRNA. Cell 9:747-760.
151. Lodish, H. F., and J. K. Rose. 1977. Relativeimportance of 7-methylguanosine in ribosomebinding and translation of vesicular stomatitisvirus messenger RNA in wheat germ and retic-ulocyte cell-free systems. J. Biol. Chem. 252:1181-1188.
152. Mager, W. H., J. Klootwijk, and I. Klein.1976. Minimal methylation of yeast messengerRNA. Mol. Biol. Rep. 3:9-17.
153. Maitra, U., and J. Hurwitz. 1965. The role ofDNA in RNA synthesis. IX. Nucleoside tri-phosphate termini in RNA polymerase prod-ucts. Proc. Natl. Acad. Sci. U.S.A. 54:815-822.
154. Malek, L. T., H.-J., Breter, G. M. Hellmann,K. H. Friderici, F. M. Rottman, and R. E.Rhoads. 1979. 5'-Terminal structure of henovalbumin messenger ribonucleic acid. J. Biol.Chem. 254:10415-10420.
155. Manley, J. L., P. A. Sharp, and M. L. Gefter.1979. RNA synthesis in isolated nuclei: in vitroinitiation of adenovirus 2 major late mRNAprecursor. Proc. Natl. Acad. Sci. U.S.A. 76:160-164.
156. Marcu, K. B., U. Schibler, and R. P. Perry.1978. The 5'-terminal sequence of immuno-globulin messenger RNAs of a mouse myeloma.J. Mol. Biol. 120:381-400.
157. Martin, S. A., and B. Moss. 1975. Modificationof RNA by mRNA guanylyltransferase andmRNA(guanine-7-)methyltransferase fromvaccinia virions. J. Biol. Chem. 250:9330-9335.
158. Martin, S. A., and B. Moss. 1976. mRNA guan-yltransferase and mRNA (guanine-7-)-meth-yltransferase from vaccinia virions. J. Biol.Chem. 251:7313-7321.
159. Martin, S. A., E. Paoletti, and B. Moss. 1975.Purification ofmRNA guanylyltransferase andmRNA(guanine-7-)methyltransferase fromvaccinia virions. J. Biol. Chem. 250:9322-9329.
160. Mertens, P. P. C., and C. C. Payne. 1978. S-adenosyl-L-homocysteine as a stimulator ofviral RNA synthesis by two distinct cytoplas-mic polyhedrosis viruses. J. Virol. 26:832-835.
161. Miura, K., K. Watanabe, and M. Sugiura.1974. 5'-Terminal nucleotide sequences of thedouble-stranded RNA of silkworm cytoplasmicpolyhedrosis virus. J. Mol. Biol. 86:31-48.
162. Miura, K.-I., K. Watanabe, M. Sugiura, andA. J. Shatkin. 1974. The 5'-terminal nucleo-tide sequences of the double-stranded RNA ofhuman reovirus. Proc. Natl. Acad. Sci. U.S.A.71:3979-3983.
163. Mizumoto, K., and F. Lipmann. 1979. Trans-methylation and transguanylylation in 5'-RNAcapping system isolated from rat liver nuclei.Proc. Natl. Acad. Sci. U.S.A. 76:4961-4965.
164. Monroy, G., E. Spencer, and J. Hurwitz. 1978.Purification of mRNA guanylyltransferasefrom vaccinia virions. J. Biol. Chem. 253:4481-4489.
165. Monroy, G., E. Spencer, and J. Hurwitz. 1978.Characteristics of reaction catalyzed by puri-fied guanylyltransferase from vaccinia virus. J.
VOL. 44, 1980
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

202 BANERJEE
Biol. Chem. 253:4490-4498.166. Moss, B., A. Gershowitz, J. R. Stringer, L. E.
Holland, and E. K. Wagner. 1977. 5'-Termi-nal and internal methylated nucleosides inherpes simplex virus type 1 messenger RNA.J. Virol. 23:234-239.
167. Moss, B., A. Gershowitz, L. A. Weber, and C.Baglioni. 1977. Histone mRNAs containblocked and methylated 5' terminal sequencesbut lack methylated nucleosides at internalpositions. Cell 10:113-120.
168. Moss, B., A. Gershowitz, C.-M. Wei, and R.Boone. 1976. Formation of the guanylylatedand methylated 5'-terminus of vaccinia virusmRNA. Virology 72:341-351.
169. Moss, B., J. M. Keith, A. Gershowitz, M. B.Ritchey, and P. Palese. 1978. Common se-quence at the 5' ends of the segmented RNAgenomes of influenza A and B viruses. J. Virol.25:312-318.
170. Moss, B., and F. Koczot. 1976. Sequence ofmethylated nucleotides at the 5' terminus ofadenovirus-specific RNA. J. Virol. 17:385-392.
171. Moyer, S. A., G. Abraham, R. Adler, and A.K. Banerjee. 1975. Methylated and blocked 5'termini in vesicular stomatitis virus in vivomRNAs. Cell 5:59-67.
172. Moyer, S. A., and A. K. Banerjee. 1976. In vivomethylation of vesicular stomatitis virus andits host-cell messenger RNA species. Virology70:339-351.
173. Muthukrishnan, S., C. W. Both, Y. Furuichi,and A. J. Shatkin. 1975. 5'-Terminal 7-meth-ylguanosine in eukaryotic mRNA is requiredfor translation. Nature (London) 255:33-37.
174. Muthukrishnan, S., W. Filipowicz, J. M.Sierra, G. W. Both, A. J. Shatkin, and S.Ochoa. 1975. Messenger RNA methylationand protein synthesis in extracts from embryosof brine shrimp, Artemia salina. J. Biol. Chem.250:9336-9341.
175. Muthukrishnan, S., M. Morgan, A. K. Baner-jee, and A. J. Shatkin. 1976. Influence of 5'terminal 7mG and 2'-O methylated residues onmessenger ribonucleic acid binding to ribo-somes. Biochemistry 15:5761-5768.
176. Muthukrishnan, S., B. Moss, J. A. Cooper,and E. S. Maxwell. 1978. Influence of 5'-ter-minal cap structure on the initiation of trans-lation of vaccinia virus mRNA. J. Biol. Chem.253:1710-1715.
177. Muthukrishnan, S., S. Szybiak, A. B. Lego-cki, and W. Filipowicz. 1978. 5'-Terminal 7-methylguanosine and mRNA function. The ef-fect of enzymatic decapping and of cap analogson translation of tobacco mosaic virus RNAand globin mRNA in vitro. Eur. J. Biochem.92:69-80.
178. Nichols, J. L. 1979. "Cap" structures in maizepoly(A)-containing RNA. Biochim. Biophys.Acta 563:490-495.
179. Nuss, D. L., and Y. Furuichi. 1977. Character-ization of the m7G(5')pppN-pyrophosphataseactivity from HeLa cells. J. Biol. Chem. 252:2815-2821.
180. Nuss, D. L., Y. Furuichi, G. Koch, and A. J.
Shatkin. 1975. Detection in HeLa cell extractsof a 7-methylguanosine specific enzyme activ-ity that cleaves m7GpppNm. Cell 6:21-27.
181. Ohno, T., Y. Okada, K. Shimotohno, K.Miura, H. Shinski, M. Miwa, and T. Sugi-mura. 1976. Enzymatic removal of the 5'-ter-minal methylated blocked structure of tobaccomosaic virus RNA and its effects on infectivityand reconstitution with coat protein. FEBSLett. 67:209-213.
182. Ouellette, A. J., D. Frederick, and R. A. Malt.1975. Methylated messenger RNA in mousekidney. Biochemistry 14:43614367.
183. Ouellette, A. J., S. L. Reed, and R. A. Malt.1976. Short-lived methylated messenger RNAin mouse kidney. Proc. Natl. Acad. Sci. U.S.A.73:2609-2613.
184. Paterson, M. B., and M. Rosenberg. 1979.Efficient translation of prokaryotic mRNAs ina eukaryotic cell-free system requires additionof a cap structure. Nature (London) 279:692-696.
185. Pelham, H. R. B., J. M. M. Sykes, and T.Hunt. 1978. Characteristics of a coupled cell-free transcription and translation system di-rected by vaccinia cores. Eur. J. Biochem. 82:199-209.
186. Perry, R. P., and D. E. Kelley. 1974. Existenceof methylated messenger RNA in mouse Lcells. Cell 1:3742.
187. Perry, R. P., and D. E. Kelley. 1976. Kineticsof formation of 5' terminal caps in mRNA. Cell8:433-442.
188. Perry, R. P., D. E. Kelley, K. Frederici, andF. Rottman. 1975. The methylated constitu-ents of L cell messenger RNA: evidence for anunusual cluster at the 5' terminus. Cell 4:387-394.
189. Perry, R. P., D. E. Kelley, K. H. Frederici,and F. M. Rottman. 1975. Methylated con-stituents of heterogeneous nuclear RNA: pres-ence in blocked 5' terminal structures. Cell 6:13-19.
190. Perry, R. P., and K. Scherrer. 1975. Methyl-ated constituents of globin mRNA. FEBS Lett.57:73-78.
191. Pinck, L. 1975. The 5'-end groups of alfalfa mo-saic virus RNA are 7mG(5')ppp(5')Gp. FEBSLett. 59:24-28.
192. Plotch, S. J., M. Bouloy, and R. M. Krug.1979. Transfer of 5'-terminal cap of globinmRNA to influenza viral complementary RNAduring transcription in vitro. Proc. Natl. Acad.Sci. U.S.A. 76:1618-1622.
193. Plotch, S. J., J. Tomasz, and R. M. Krug.1978. Absence of detectable capping and meth-ylating enzymes in influenza virions. J. Virol.28:75-83.
194. Pugh, C. S. G., R. T. Borchardt, and H. 0.Stone. 1978. Sinefungin, a potent inhibitor ofvirion mRNA (guanine-7-)methyltransferase,mRNA (nucleoside-'2-)methyltransferase, andviral multiplication. J. Biol. Chem. 253:4075-4077.
195. Rao, M. S., M. Blackstone, and H. Busch.1977. Effects of U-1 nuclear RNA on transla-
MICROBIOL. REV.
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

5'-TERMINAL CAP STRUCTURES IN mRNA's 203
tion of messenger RNA. Biochemistry 16:2756-2762.
196. Reddy, R., T. S. Ro-Choi, D. Henning, and H.Busch. 1974. Primary sequence of U-1 nuclearribonucleic acid of Novikoff hepatoma ascitescells. J. Biol. Chem. 249:6486-6494.
197. Rhodes, D. P., and A. K. Banerjee. 1976. 5'-Terminal sequence of vesicular stomatitis virusmessenger RNA synthesized in vitro. J. Virol.17:33-42.
198. Rhodes, D. P., S. A. Moyer, and A. K. Baner-jee. 1974. In vitro synthesis of methylated mes-
senger RNA by the virion-associated RNA po-
lymerase of vesicular stomatitis virus. Cell 3:327-333.
199. Rhodes, D. P., D. V. R. Reddy, R. MaCleod,L. M. Black, and A. K. Banerjee. 1977. Invitro synthesis of RNA containing7mG(5')ppp(5')Am by purified wound tumor vi-rus. Virology 76:554-559.
200. Richards, K. E., G. Jonard, M. Jacquemond,and H. Lot. 1978. Nucleotide sequence of cu-
cumber mosaic virus-associated RNA 5. Virol-ogy 89:395408.
201. Robert-Gero, M., F. Lawrence, G. Farrugia,A. Berneman, P. Blanchard, P. Vigier, andE. Lederer. 1975. Inhibition of virus-inducedcell transformation by synthetic analogues ofS-adenosyl homocysteine. Biochem. Biophys.Res. Commun. 65:1242-1249.
202. Ro-Choi, T. S., C. C. Yong, D. Henning, J.McCloskey, and H. Busch. 1975. Nucleotidesequence of U-2 ribonucleic acid. J. Biol. Chem.250:3921-3928.
203. Roman, R. J. D., S. N. Booker, and A. Mar-cus. 1976. Inhibition of the transition of a 40Sribosome-Met-tRNAne' complex to an 80S ri-bosome-Met-tRNA'et complex by 7-methyl-guanosine-5'-phosphate. Nature (London) 260:359-360.
204. Rose, J. K. 1975. Heterogenous 5'-terminalstructures occur on vesicular stomatitis virusmRNA's. J. Biol. Chem. 250:8098-8104.
205. Rose, J. K., W. A. Haseltine, and D. Balti-more. 1976. 5' Terminus of Moloney murineleukemia virus 35S RNA is 7mG(5')ppp(5')-Gn'pCp. J. Virol. 20:324-329.
206. Rose, J. K., and H. F. Lodish. 1976. Translationin vitro of vesicular stomatitis virus mRNAlacking 5'-terminal 7-methylguanosine. Nature(London) 262:32-37.
207. Rose, J. K., H. F. Lodish, and M. L. Brock.1977. Giant heterogenous polyadenylic acid on
vesicular stomatitis virus mRNA synthesizedin vitro in the presence of S-adenosylhomocys-teine. J. Virol. 21:683-693.
208. Rose, J. K., H. Trachsel, K. Leong, and D.Baltimore. 1978. Inhibition of translation bypoliovirus: inactivation of a specific initiationfactor. Proc. Natl. Acad. Sci. U.S.A. 75:2732-2736.
208a. Rosenberg, M. 1974. Isolation and sequence
determination of the 3'-terminal regions of is-otopically labeled RNA molecules. NucleicAcids Res. 1:653-671.
209. Rosenberg, M., and B. M. Paterson. 1979.
Efficient cap-dependent translation of polycis-tronic prokaryotic mRNAs is restricted to thefirst gene in the operon. Nature (London) 279:696-701.
210. Rottman, F. M. 1978. Methylation and polyad-enylation of heterogeneous nuclear and mes-senger RNA, p. 45-73. In B. F. C. Clark (ed).,Biochemistry of nucleic acids, vol 17. Univer-sity Park Press, Baltimore.
211. Rottman, F., A. J. Shatkin, and R. P. Perry.1974. Sequences containing methylated nucleo-tides at the 5' termini of messenger RNA:possible implications for processing. Cell 3:197-199.
212. Roy, P., and J. P. Clewley. 1978. Spring viremiaof carp virus RNA and virion-associated tran-scriptase activity. J. Virol. 25:912-916.
213. Saini, M. S., and B. G. Lane. 1977. Wheatembryo ribonucleates. VIII. The presence of 7-methylguanosine "cap structures" in the RNAof imbibing wheat embryos. Can. J. Biochem.55:819-824.
214. Salditt-Georgieff, M., M. Harpold, S. Chen-Kiang, and J. E. Darnell. 1980. The additionof 5'-cap structures occurs early in HnRNAsynthesis and prematurely terminated mole-cules are capped. Cell 19:69-78.
215. Salditt-Georgieff, M., W. Jelinek, J. E. Dar-nell, Y. Furuichi, M. Morgan, and A. Shat-kin. 1976. Methyl labeling of HeLa cellHnRNA: a comparison with mRNA. Cell 7:227-237.
216. Sangar, D. V., D. J. Rowlands, T. J. R. Har-ris, and F. Brown. 1977. Protein covalentlylinked to foot-and-mouth disease virus RNA.Nature (London) 268:648-650.
217. Schibler, U., D. E. Kelley, and R. P. Perry.1977. Comparison of methylated sequences inmessenger RNA and heterogeneous nuclearRNA from mouse L cells. J. Mol. Biol. 115:695-714.
218. Schibler, U., and R. P. Perry. 1976. Character-ization of the 5'-termini of HnRNA in mouse Lcells: implications for processing and cap for-mation. Cell 9:121-130.
219. Schibler, U., and R. P. Perry. The 5'-terminiof heterogenous nuclear RNA: a comparisonamong molecules of different sizes and ages.Nucleic Acids Res. 4:4133-4149.
220. Seidel, B. L., and E. W. Somberg. 1978. Char-acterization of Neurospora crassa polyaden-ylated messenger ribonucleic acid: structure ofthe 5'-terminus. Biochem. Biophys. Res. Com-mun. 187:108-112.
221. Shafritz, D. A., J. A. Weinstein, B. Safer, W.C. Merrick, L. A. Weber, E. D. Hickey, andC. Baglioni. 1976. Evidence for role of 7mGpgroup in recognition of eukaryotic mRNA byinitiation factor IF-M3. Nature (London) 261:291-294.
222. Sharma, 0. K., D. E. Hruby, and D. N. Bee-zley. 1976. Inhibition of ovalbumin mRNAtranslation by 7-methylguanosine-5'-phos-phate. Biochem. Biophys. Res. Commun. 72:1392-1398.
223. Shatkin, A. J. 1974. Methylated messenger
VOL. 44, 1980
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

204 BANERJEE
RNA synthesis in vitro by purified reovirus.Proc. Natl. Acad. Sci. U.S.A. 71:3204-3207.
224. Shatkin, A. J. 1976. Capping of eucaryoticmRNAs. Cell 9:645-653.
225. Shih, D. S., R. DasGupta, and P. Kaesberg.1976. 7-Methylguanosine and efficiency ofRNA translation. J. Virol. 19:637-642.
226. Shimotohno, K., Y. Kodama, J. Hashimoto,and K.-I. Miura. 1977. Importance of 5'-ter-minal blocking structure to stabilize mRNA ineukaryotic protein synthesis. Proc. Natl. Acad.Sci. U.S.A. 74:2734-2738.
227. Shimotohno, K., and K-I. Miura. 1976. Theprocess of formation of the 5'-terminal modi-fied structure in messenger RNA of cytoplas-mic polyhedrosis virus. FEBS Lett. 64:204-208.
228. Shinshi, H., M. Miwa, K. Kato, M. Noguchi,T. Matsushima, and T. Sugimura. 1976. Anovel phosphodiesterase from cultured tobaccocells. Biochemistry 15:2185-2190.
229. Shinshi, H., M. Miwa, T. Sugimura, K. Shi-motohno, and K. Miura. 1976. Enzyme cleav-ing the 5'-terminal methylated blocked struc-ture of messenger RNA. FEBS Lett. 65:254-257.
230. Skup, D., and S. Millward. 1980. mRNA cap-ping enzymes are masked in reovirus progenysubviral particles. J. Virol. 34:490-496.
231. Skup, D., and S. Millward. 1980. Reovirus in-duced modification of cap-dependent transla-tion in infected L-cells. Proc. Natl. Acad. Sci.U.S.A. 77:152-156.
232. Smith, R. E., and J. M. Clark. 1979. Effect ofcapping upon the mRNA properties of satellitetobacco necrosis virus ribonucleic acid. Bio-chemistry 18:1366-1371.
233. Sommer, S., M. Salditt-Georgieff, S. Bach-enheimer, J. E. Darnell, Y. Furuichi, M.Morgan, and A. J. Shatkin. 1976. The meth-ylation of adenovirus-specific nuclear and cy-toplasmic RNA. Nucleic Acids Res. 3:749-765.
234. Sonenberg, N., M. A. Morgan, W. C. Merrick,and A. J. Shatkin. 1978. A polypeptide ineukaryotic initiation factors that crosslinksspecifically to the 5'-terminal cap in mRNA.Proc. Natl. Acad. Sci. U.S.A. 75:4843-4847.
235. Sonenberg, N., M. A. Morgan, D. Testa, R. J.Colonno, and A. J. Shatkin. 1979. Interac-tion of a limited set of proteins with differentmRNAs and protection of 5'-caps against py-rophosphatase digestion in initiation com-plexes. Nucleic Acids Res. 7:15-29.
236. Sonenberg, N., K. M. Rupprecht, S. M. Hecht,and A. J. Shatkin. 1979. Eukaryotic mRNAcap binding protein: purification by affinitychromatography on Sepharose-coupled m7-GDP. Proc. NatI. Acad. Sci. U.S.A. 76:4345-4349.
237. Sonenberg, N., and A. J. Shatkin. 1977. Reo-virus mRNA can be covalently crosslinked viathe 5' cap to proteins in initiation complexes.Proc. Natl. Acad. Sci. U.S.A. 74:4288-4292.
238. Sonenberg, N., A. J. Shatkin, R. P. Ricciardi,M. Rubin, and R. M. Goodman. 1978. Anal-ysis of terminal structures of RNA from potato
virus X. Nucleic Acids Res. 5:2501-2512.239. Spencer, E., D. Loring, J. Hurwitz, and G.
Monroy. 1978. Enzymatic conversion of 5'-phosphate-terminated RNA to 5'-di- and tri-phosphate-terminated RNA. Proc. Natl. Acad.Sci. U.S.A. 75:4793-4797.
240. Sripati, C. E., Y. Groner, and J. R. Warner.1976. Methylated, blocked 5' termini of yeastmRNA. J. Biol. Chem. 251:2898-2904.
241. Stanley, J., P. Rottier, J. W. Davies, P. Zabel,and A. V. Kammen. 1978. A protein linked tothe 5' termini of both RNA components of thecowpea mosaic virus genome. Nucleic AcidsRes. 5:4505-4522.
242. Stein, J. L., G. S. Stein, and P. M. McGuire.1977. Histone messenger RNA from HeLa cells:evidence for modified 5'-termini. Biochemistry16:2207-2213.
243. Stoltzfus, C. M., and K. Dimock. 1976. Evi-dence for methylation of B-77 avian sarcomavirus genome RNA subunits. J. Virol. 18:586-595.
243a.Stoltzfus, C. M., K. Dimock, and S. A. Moyer.1979. Effect of an in vivo methylation inhibitor,cycloleucine, on viral and cellular mRNA struc-ture and function, p. 399-408. In E. Usdin, R.T. Borchardt, and C. R. Creveling (ed.), Trans-methylation. Elsevier/North-Holland, NewYork.
244. Surrey, S., and M. Nemer. 1976. Methylatedblocked 5' terminal sequences of sea urchinembryo messenger RNA classes containing andlacking poly(A). Cell 9:589-595.
245. Suzuki, H. 1977. Effect of 7mGppNm on therabbit globin synthesis. FEBS Lett. 79:11-14.
246. Symons, R. H. 1975. Cucumber mosaic virusRNA contains 7-methylguanosine at the 5'-ter-minus of all four RNA species. Mol. Biol. Rep.2:277-285.
247. Takagi, S., and T. Mori. 1979. The 5'-terminalstructure of poly(A)-containing RNA of soy-bean seeds. J. Biochem. 86:231-238.
248. Taylor, R. H., and D. T. Dubin. 1975. Themethylation status of mammalian mitochon-drial messenger RNA. J. Cell Biol. 67:428A.
249. Tazaki, Y., and K. I. Miura. 1977. Terminalstructure involving a single-stranded stretch inthe double-stranded RNA from Penicilliumchrysogenum virus. Virology 82:14-24.
250. Testa, D., and A. K. Banerjee. 1977. Two meth-yltransferase activities in the purified virionsof vesicular stomatitis virus. J. Virol. 24:786-793.
251. Testa, D., and A. K. Banerjee. 1979. Initiationof RNA synthesis in vitro by vesicular stoma-titis virus. J. Biol. Chem. 254:2053-2058.
252. Thomason, A. R., D. A. Brian, L. F. Velice,and F. M. Rottman. 1976. Methylation ofhigh-molecular-weight subunit RNA of felineleukemia virus. J. Virol. 20:123-132.
253. Thomason, A. R., K. H. Friderici, L. F. Veli-cer, and F. Rottman. 1978. Presence of 5'-terminal cap structures in virus-specific RNAfrom feline leukemia virus-infected cells. J. Vi-rol. 26:226-235.
254. Toneguzzo, F., and H. P. Ghosh. 1976. Char-
MICROBIOL. REV.
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from

5'-TERMINAL CAP STRUCTURES IN mRNA's 205
acterization and translation of methylated andunmethylated vesicular stomatitis virusmRNA synthesized in vitro by ribonucleopro-tein particles from vesicular stomatitis virus-infected L cells. J. Virol. 17:477-491.
255. Trachsel, H., N. Sonenberg, A. J. Shatkin, J.K. Rose, K. Leong, J. E. Bergman, J. Gor-don, and D. Baltimore. 1980. Purification ofa factor that restores translation of vesicularstomatitis virus mRNA in extracts from polio-virus-infected HeLa cells. Proc. Natl. Acad.Sci. U.S.A. 77:770-774.
256. Urushibara, T., Y. Furuichi, C. Nishimura,and K. Miura. 1975. A modified structure atthe 5'-terminus of mRNA of vaccinia virus.FEBS Lett. 49:385-389.
257. Venkatesan, S., A. Gershowitz, and B. Moss.1980. Modification of the 5' end of mRNA:association of RNA triphosphatase with theRNA guanyltransferase-RNA (guanine-7-)-methyltransferase complex from vaccinia virus.J. Biol. Chem., in press.
258. Weber, L. A., E. R. Feman, E. D. Hickey, M.C. Williams, and C. Baglioni. 1976. Inhibi-tion of HeLa cell messenger RNA translationby 7-methylguanosine 5'-monophosphate. J.Biol. Chem. 251:5657-5662.
259. Weber, L. A., E. D. Hickey, D. L. Nuss, andC. Baglioni. 1977. 5'-Terminal 7-methyl-guanosine and mRNA function: influence ofpotassium concentration on translation in vi-tro. Proc. Natl. Acad. Sci. U.S.A. 74:3254-3258.
260. Wei, C. M., A. Gershowitz, and B. Moss. 1975.N6,2'-O-dimethyladenosine, a novel methyl-ated ribonucleoside next to the 5'-terminal ofanimal cell and virus mRNAs. Nature (Lon-don) 257:251-253.
261. Wei, C. M., A. Gershowitz, and B. Moss. 1975.Methylated nucleotides block 5' terminus ofHeLa cell messenger RNA. Cell 4:379-386.
262. Wei, C. M.; A. Gershowitz, and B. Moss. 1976.5'-Terminal and internal methylated nucleo-tide sequences in HeLa cell mRNA. Biochem-istry 15:397-401.
263. Wei, C. M., and B. Moss. 1974. Methylation ofnewly synthesized viral messenger RNA by anenzyme in vaccinia virus. Proc. Natl. Acad. Sci.U.S.A. 71:3014-3018.
264. Wei, C. M., and B. Moss. 1975. Methylatednucleotides block 5'-terminus of vaccinia virusmessenger RNA. Proc. Natl. Acad. Sci. U.S.A.72:318-322.
265. Wei, C. M., and B. Moss. 1977. 5'-Terminalcapping of RNA by guanylyltransferase fromHeLa cell nuclei. Proc. Natl. Acad. Sci. U.S.A.74:3758-3761.
266. Wei, C. M., and B. Moss. 1977. Nucleotide se-quences at the N6-methyladenosine sites ofHeLa cell messenger ribonucleic acid. Bio-chemistry 16:1672-1676.
267. Wertheimer, A. M., S.-Y. Chen, R. T. Bor-chardt, and Y. Furuichi. 1980. S-adenosyl-
methionine and its analogs: structural featurescorrelated with synthesis and methylation ofmRNAs of cytoplasmic polyhedvirus virus. J.Biol. Chem., in press.
268. Willems, M., B. E. Wieringa, J. Mulder, A. B.Geert, and M. Gruber. 1979. Translation ofvitellogenin mRNA in the presence of 7-meth-ylguanosine 5'-triphosphate: cap analogs com-pete with mRNAs on the basis of affinity forinitiation-complex formation. Eur. J. Biochem.93:469-479.
269. Wimmer, E., A. Y. Chang, J. M. Clark, Jr.,and M. E. Reichmann. 1968. Sequence stud-ies of satellite tobacco necrosis virus RNA.Isolation and characterization of a 5'-terminaltrinucleotide. J. Mol. Biol. 38:59-73.
270. Winicov, I., and R. P. Perry. 1976. MessengerRNA and heterogeneous nuclear RNA meth-ylation in vitro. J. Cell Biol. 70:391A.
271. Winicov, I., and R. P. Perry. 1976. Synthesis,methylation and capping of nuclear RNA by asubcellular system. Biochemistry 15:5039-5045.
272. Wodnar-Filipowicz, A., E. Szczesna, M. Zan-Kowalozewska, S. Muthukrishnan, U.Szybiak, A. B. Legooki, and W. Filipowicz.1978. 5' Terminal 7 methylguanosine and mes-senger RNA function: the effect of enzymaticdecapping and of cap analogs on translation oftobacco mosaic virus RNA and globin messen-ger RNA in vitro. Eur. J. Biochem. 92:69-80.
273. Wold, W. S. M., M. Green, and T. W. Munns.1976. Methylation of late adenovirus 2 nuclearand messenger RNA. Biochem. Biophys. Res.Commun. 68:643-649.
274. Yang, N. S., R. F. Manning, and L. P. Gage.1976. The blocked and methylated 5' terminalsequence of a specific cellular messenger: themRNA for silk fibroin of Bombyx mori. Cell 7:339-347.
275. Yong, R. A., and J. A. Steitz. 1979. Tandempromoters direct E. coli ribosomal RNA syn-thesis. Cell 17:225-234.
275a.Young, R. J. 1977. Appearance of 7-methyl-guanosine-5'-phosphate in the RNA of mouse1-cell embryos three hours after fertilization.Biochem. Biophys. Res. Commun. 76:32-39.
276. Zan-Kowalczewska, M., M. Bretner, H. Sier-akowska, E. Szczesna, W. Filipowicz, andA. J. Shatkin. 1977. Removal of 5'-terminalm7G from eukaryotic mRNAs by potato nu-cleotide pyrophosphatase and its effect ontranslation. Nucleic Acids Res. 4:3065-3081.
277. Zarbl, H., D. Skup, and S. Millward. 1980.Reovirus progeny subviral particles synthesizeuncapped mRNA. J. Virol., in press.
278. Ziff, E. B., and R. M. Evans. 1978. Coincidenceof the promoter and capped 5'-terminus ofRNA from the adenovirus 2 major late tran-scription unit. Cell 15:1463-1475.
279. Zimmern, D. 1975. The 5' end group of tobaccomosaic virus RNA is ,,'G(5')ppp(5')Gp. NucleicAcids Res. 2:1189-1201.
VOL. 44, 1980
on June 30, 2020 by guesthttp://m
mbr.asm
.org/D
ownloaded from