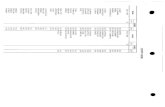
2015 2016 2017 Oct-17 · (d) submit to a criminal backgrOlUld check pursuant to Subsection 58-31...
Transcript of 2015 2016 2017 Oct-17 · (d) submit to a criminal backgrOlUld check pursuant to Subsection 58-31...

Administrative Fil ings
Criminal FilinglFelony
Letter of Concern
Referred to Diversion
PRiOutreach
Cases Received
Case Assigned
Closed Cases
Citations Issued
Pharmacy Inspections
Pharmacy Alerts
NOTES Pharmacy Group
Administrative Action
Administrative Action
Administrative Action
Citation
Citation
Pharmacy Board 11282017 October 2017
2015 2016 2017 Oct-17
33 22 20 3 3 0 0 0
98 110 60 6 0 I 0
3 0
666 483 349 41 659 481 348 41 624 607 428 47
64 30 29 3
316 310 185 29 220 303 130 25
Pharmacist in Charge signed a Surrender Stipulation and Order surrendering his license to practice
Pharmcy Technician signed a Stipulation and Order revoking her license to practice
Pharmacist took 809 tablets of Tylenol 4 over a ten month period from his employer and filled his own prescription for Tramadol from
his employers drug stock prescription was expired The Pharmacist signed a Stipulation and Order placing his license on Probation
A random inspection was conducted by the Division and violations
were found A check of five Schedule II controlled substances were
made against the pharmacys Schedule II controlled substance perpetual inventory Discrepancies of greater than 100 were found
with four of the five medications checked A fine was issued in the
amount of $125000
A random inspection was conducted by the Division and violations
were found No annual inventory was conducted for Schedule II
controlled substances for the years 2014 and 2015 none of the
annual inventories except the Schedules III IV and V controlled substance inventory for 2016 indicated the time the inventory was conducted and no reconciliation was conducted of the pharmacys Schedule II controlled substance perpetual inventory at the time of the
annual inventories A fine was issued in the amount of $105000
Pharmacy Board 11282017 October 2017
Citation A random inspection was conducted by the Division and violations were found A total of 108 medications were found in the pharmacys stock that wre either expired or had indeterminate expiration dates A fine was issued in the amount of $1 05000
(5) (a) Any license that is not renewed may be reinstated
(i) upon submission of an application for reinstatement payment of the renewal fee together with a reinstatement fee determined by the department under Section 63]-1-504 and upon submission of documentation showing completion of or compliance with renewal qualifications and
(ii) (A) at any time within two years after nonrenewal or
(B) between two years and five years after nonrenewal if established by rule made by the division in consultation with the applicable licensing board in accordance with Title 63G Chapter 3 Utah Administrative Rulemaking Act
(b) The application procedures specified in Subsection 58-1-301 (2) apply to the reinstatement applications to the extent they are not in conflict with this section
(c) Except as otherwise provided by rule a license that is reinstated no later than 120 days after it expires shall be retroactively reinstated to the date it expired
(6) (a) Except as provided in Subsection (5)(a) if not reinstated within two years the holder may obtain a license only if the holder meets requirements provided by the division by rule or by statute for a new license
(b) Each licensee under this title who has been active in the licensed occupation or profession while in the fullshytime employ of the United States government or under license to practice that occupation or profession in any other state or territory of the United States may reinstate the licensees license without taking an examination by submitting an application for reinstatement paying the current annual renewal fee and the reinstatement fee and submitting documentation showing completion of or compliance with any renewal qualifications at any time within six months after reestablishing domicile within Utah or terminating fullshytime government service
Amended by Chapter 238 2016 General Session
bull R156-1-308a Renewal Dates bull R156-1-308b Renewal Periods - Adjustment of Renewal Fees for an Extended or Shortened Renewal
Period bull R156-1-308c Renewal of Licensure Procedures bull R156-1-308d Waiver of Continuing Education Requirements - Renewal Requirements bull R156-1-308e Automatic Expiration of Licensure Upon Dissolution of Licensee
bull R156-1-308f Denial of Renewal of Licensure - Classification of Proceedings - Conditional Renewal of Licensure During Adjudicative Proceedings - Conditional Initial Renewal or Reinstatement Licensure During Audit or Investigation
bull R156-1-308g Reinstatement of Licensure which was Active and in Good Standing at the Time of Expiration of Licensure - Requirements
bull R156-1-308h Reinstatement of Restricted Suspended or Probationary Licensure During Term of Restriction Suspension or Probation - Requirements
bull R156-1-308i Reinstatement of Restricted Suspended or Probationary Licensure After the Specified Term of Suspension of the License or After the Expiration of Licensure in a Restricted Suspended or Probationary Status - Requirements
bull R156-1-308j Relicensure Following Revocation of Licensure - Requirements bull R156-1-308k Relicensure Following Surrender of Licensure - Requirements bull R156-1-3081 Reinstatement of Licensure and Relicensure - Term of Licensure
Effective 5102016 58-1-308 Term of license -- Expiration of license -- Renewal of license -- Reinstatement of license __ Application procedures
(1) (a) Each license issued under this title shall be issued in accordance with a two-year renewal cycle established by rule
(b) A renewal period may be extended or shortened by as much as one year to maintain established renewal cycles or to change an established renewal cycle
(2) (a) The expiration date of a license shall be shown on the license
(b) A license that is not renewed prior to the expiration date shown on the license automatically expires
(c) A license automatically expires prior to the expiration date shown on the license upon the death of a licensee who is a natural person or upon the dissolution of a licensee that is a partnership corporation or other business entity
(d) If the existence of a dissolved partnership corporation or other business entity is reinstated prior to the expiration date shown upon the entitys expired license issued by the division the division shall upon written application reinstate the applicants license unless it flnds that the applicant no longer meets the qualifications for licensure
(e) Expiration of licensure is not an adjudicative proceeding under Title 63G Chapter 4 Administrative Procedures Act
(3) (a) The division shall notify each licensee in accordance with procedures established by rule that the licensees license is due for renewal and that unless an application for renewal is received by the division by the expiration date shown on the license together with the appropriate renewal fee and documentation showing completion of or compliance with renewal qualiflcations the license will not be renewed
(b) Examples of renewal qualiflcations which by statute or rule the division may require the licensee to document completion of or compliance with include
(i) continuing education
(ii) continuing competency
(iii) quality assurance
(iv) utilization plan and protocol
(v) fInancial responsibility
(vi) certiflcation renewal and
(vii) calibration of equipment
(4) (a) (i) An application for renewal that complies with Subsection (3) is complete
(ii) A renewed license shall be issued to applicants who submit a complete application unless it is apparent to the division that the applicant no longer meets the qualiflcations for continued licensure
(b) (i) The division may evaluate or verify documentation showing completion of or compliance with renewal requirements on an entire population or a random sample basis and may be assisted by advisory peer committees
(ii) If necessary the division may complete its evaluation or veriflcation subsequent to renewal and if appropriate pursue action to suspend or revoke the license of a licensee who no longer meets the qualifications for continued licensure
(c) The application procedures specified in Subsection 58-1-301 (2) apply to renewal applications to the extent they are not in conflict with this section
(d) submit to a criminal backgrolUld check pursuant to Subsection 58-31 b-302(5) and Section R156-31 b-30 19 (3) ~ apph~anl who holds a current LPN license in an interstate compact state shall apply for a license within 90 days of
estabhshmg reSidency In Utah and complete all requirements pursuant to R 156-31 b-30 I a(2) (4) An applicant who has been licensed previously in Utah but whose license has expired or lapsed shall (a) if the applicant has not practiced as a nurse for up to five years document current compliance with the continuing competency
requirements as established in SubseL1ion R 156-31b-303(3)
(b) if the applicant has not practiced as a nurse for more than five years but less than eight years (I) pass the NCLEX-PN examination within 60 days following the date of application or (ii) successfully complete an approved re-entry program (c) if the applicant has not practiced as a nurse for more than eight years but less than 10 years (i) successfully complete an approved re-entry program and (ii) pass the NCLEX-PN examination within 60 days following the date of application or (d) if the applicant has not practiced as a nurse for 10 years or more comply with this Subsection (1) (5) An applicant who has been licensed in another state or country but whose license has expired or lapsed shall (a) comply with this Subsection (2)(b) and (b) comply with this Subsection (4) a~ applicable and (c) submit to a criminal background check pursuant to Subsection 58-31 b-302(5) and Section R156-31 b-30 I g
R156-31b-301b RN License- Education Examination and Experience Requirements (I) An applicant who has never obtained a license in any state or country shall (a) demonstrate that the applicant ha~ successfully completed an RN prelicensing education program that (i) meets the requirements of Section 58-31 b-60 I or (ii) is equivalent to an approved program under Section 58-3 I b-60 I (b) pass the NCLEX-RN examination purslant to Section R 156-31 b-30 I e and (c) submit to a climinal background check pursuant to Subsection 58-31 b-302(5) and Section R155-31 b-301g (2) An applicant who holds a current RN license issued by another country or state shall (a) demonstrate that the license issued by the other jurisdiction is current active and in good standing as of the date of
application (b)(i) demonstrate that the applicant has graduated from an RN prelicensing education program and (ii) if a foreign education program demonstrate that the program meets all requirements outlined in Section R 156-31 b-30 I d (c) pass the NCLEX-RN examination pursuant to Section RI56-31b-301e and (d) submit to a criminal backgrOlUld check pursuant to Subsection 58-31 b-302(5) and Sel-1ion R156-31 b-30 I g (3) An applicant who holds a current RN license in an interstate compact state shall apply for a license within 90 days of
establishing residency in Utah and complete all requirements pursuant to R156-31 b-301 b(2) (4) An applicant who has been licensed previously in Utah but whose license has expired or lapsed shall (a) if the applicant has not practiced as a nurse for up to five years document current compliance with the continuing competency
requirements as established in Subsection R 156-31 b-303(3) (b) if the applicant has not practiced as a nurse for more than five years but less than eight years (i) pass the NCLEX-RN examination within 60 days following the date of application or (ii) successfully complete an approved re-entry program (c) if the applicant has not practiced as a nurse for more than eight years but less than 10 years (i) successfully complete an approved re-entry program and (ii) pass theNCLEX-RN examination within 60 days following the date of application or (d) if the applicant ha~ not practiced as a nurse for 0 years or more comply with this Subsection (1) (5) An applicant who has been licensed in another state or country but whose license ha~ expired or lapsed shall (a) comply with this Subsection (2)(b) (b) comply with this Subsection (4) as applicable and (c) submit to a criminal background check pursuant to Subsection 58-31 b-302(5) and Section R156-3l b-30 I g
R156-31b-301c APRN Lkense - Education Examination and Experience Requirements (I) An applicant who is 110t currently and validly licensed alt an APRN in any state or country shall (a) demonstrate that the applicant holds a current active RN license in good standing (b) demonstrate that the applicant has successfully completelt an APRN prelicensing education program that meets the
requirements of Subsection 58-31b-601(1) and Subsection 58-31b-302(4)(e) (c) pass a national certitication examination consistent with the applicants educational specialty pursuant to Section R156-31 bshy
30 I e and administered by one of the following credentialing bodies (i) the American Nurses Credentialing Center Certification
4
PHARMACIST COLLABORATIVE PRACTICE AGREEMENTS
KEY ELEMENTS FOR LEGISLATIVE AND REGULATORY AUTHO RITY
A REPORT OF THE COLLABORATIVE PRACTICE WORKGROUP
CONVENED BY THE NATIONAL ALLIANCE OF STATE PHARMACY ASSOCIATIONS
PHARMACIST COLLABORATIVE PRACTICE AGREEMENTS KEY ELEMENTS FOR LEGISLATIVE AND REGULATORY AUTHORITY
PROJE CT OVERVIEW
Collaborative practice agreements (CPAs) create a formal practice relationship between pharmacists and other health care practitioners whereby the pharmacist assumes responsibility for specific patient care functions that are otherwise beyond their typical scope of practice but aligned with their education and training These patient care services can include initiation and modification of drug therapy The extent of the services authorized under the collaborative agreement depends on the states statutory and regulatory provisions for collaborative practice authority as well as the terms of the specific agreement between the pharmacist and other health care practitioners
State laws and regulations authorizing CPAs are highly variable Some states specify the practitioners
able to participate in CPAs restrict the services that may be provided under a CPA or include extensive
logistical barriers that limit the utility of such agreements
In their 2015 paper The Expanding Role of Pharmacists in a Transformed Health Care System the
National Governors Association (NGA) presented the following state policy considerations in regards to
collaborative practice provisions
bull Enact broad collaborative practice provisions that allow for specific provider functions to be
determined at the provider level rather than set in state statute or through regulation
bull Evaluate practice setting and drug therapy restrictions to determine whether pharmacists and
providers face disincentives that unnecessarily discourage collaborative arrangements
bull Examine whether CPAs unnecessarily dictate disease or patient specificity1
The National Alliance of State Pharmacy Associations (NASPAs) Executive Committee directed staff to
convene a workgroup to build upon the NGA recommendations with additional specificity The workgroup was charged with examining existing state CPA laws and regulations The workgroup was
tasked with developing recommendations for what elements of collaborative practice authority should
appropriately be defined under state law andor regulation and what elements are best left to be
determined between pharmacists and other practitioners when developing their specific collaborative
practice arrangement Using a modified Delphi method the Collaborative Practice Workgroup conducted
this work with two key questions in mind
bull Is this recommendation in the best interest of the patient receiving care under a collaborative
agreement
bull Is this recommendation aligned with pharmacists education and tra ining
The following is a report of the workgroups recommendations
1 Nati onal Governors Association The Expanding Role of Pharmacists in a Transformed Health Care System
JQ lwwwngaorgfiles llvesitesNGAfil esp d(20151 SOl Th eExp a ncli ngHo l e(jfPh~ rm cists~ Accessed
61515 1
WORKGROUP RECOMMENDATIONS The workgroup took the approach that rapid innovation in education training technology and evidence-based gUidelines necessitate a collaborative practice framework that is flexible and facilitates innovation in care delivery Thus the following statements include two levels of recommendations
1 Elements of collaborative practice authority that should be codified in state law andor state regulations and
2 Elements that are more appropriately determined by the parties at the practice level who voluntarily enter into a CPA and thus for which the laws and regulations should be silent
The workgroup views both levels of recommendations as needed and synergistic State law andor regulations if too restrictive can impede innovative team-based care models
COLLABORATIVE PRACTICE AGREEMENT PARTICIPANTS
RECOMMENDED ELEMENTS FOR INCLUSION IN STATE LAWS ANDOR REGULATIONS
Collaborative practice laws andor regulations should specify that
bull Any practitioner with prescriptive authority may collaborate with pharmacists using a CPA
bull CPAs may be between a single or multiple pharmacists and a single or multiple prescribers
bull CPAs may apply to a single patient multiple patients or patient populations as specified in the agreement
ELEMENTS THAT MAY BE DETERMINED AT THE PRACTITIONER LEVEL
Individual CPAs may address the below elements but state laws andor regulations should be silent
bull CPAs should specify which patient(s) andor patient population(s) can receive services under the agreement
bull Depending on the complexity of the services being provided under a CPA it may be appropriate for the pharmacist to have additional credentials or training beyond what is required for licensure
bull CPAs should specify which pharmacist(s) may provide services under the CPA A pharmacists practice setting should not be a barrier to their ability to enter into a CPA
COLLABORATIVE PRACTICE AGREEIVlEIlT AUTHORIZED SERVICES
RECOMMENDED ELEMENTS FOR INCLUSION IN STATE LAWS ANDOR REGULATIONS
Collaborative practice laws andor regulations should specify that
bull The initiation and modification of drug therapy may be authorized under a CPA with a prescriber
ELEMENTS THAT MAY BE DETERMINED AT THE PRACTITIONER LEVEL
Individual CPAs may address the below elements but state laws andor regulations should be silent
bull In some situations the use of an evidence-based protocol can ensure optimal care when pharmacists are initiating or modifying drug therapy and may be included in the CPA though they may not be needed or appropriate in others
2
bull Performing physical assessments as well as ordering performing or interpreting laboratory tests (eg CLiA-waived tests) may be included in a CPA to help identify or refer patients for services however a pharmacist may also perform these services without a CPA as these activities should fall within pharmacists standard scope of practice
bull Specific disease states may be included in a CPA at the participating practitioners discretion
COLLABORATIVE PRACTICE AGREEMENT REQUIREMENTS AND RESTRICTIONS
RECOMMENDED ELEMENTS FOR INCLUSION IN STATE LAWS ANDOR REGULATIONS
Collaborative practice laws andor regulations should specify that
bull All prescription drugs including controlled substances may be included within pharmacists collaborative practice authority
bull CPAs should be maintained by the pharmacist(s) and collaborating prescriber(s) and be available upon request or inspection
ELEMENTS THAT MAY BE DETERMINED AT THE PRACTITIONER LEVEL
Individual CPAs may address the below elements but state laws andor regulations should be silent
bull Pharmacist(s) and prescriber(s) may specify the level of patient involvement in the CPA Depending on the level of service elements such as informed consent written consent or optshyout provisions may be appropriate as determined by the parties to the agreement
bull Agreements should not be required to be sent to or approved by a state regulatory board or other agency such requirements create unnecessary paperwork burden and slow the efficiency of care delivery
bull Collaborating practitioners are encouraged to review andor renew their CPAs within a timeframe that is clinically appropriate
bull Collaborating practitioners should conform to evidence-based guidelines and the agreed upon process of care with regards to the documentation requirements and the collaborating practitioners responsibility for review of services provided under the agreement
bull Practitioners may consider liability insurance provisions and the appropriateness of articulating these in their voluntary agreement
bull It is the professional duty of all healthcare professionals to stay current in the clinical areas in which they practice If individual practitioners determine that continuing education requirements are appropriate for their clinical arrangement they may be specified in the agreement
3
APPENDIX A WORKGROUP PARTICIPANTS
The individuals listed below were appointed to participate in the Collaborative Practice Workgroup by one of two methods NASPA invited all Joint Commission of Pharmacy Practitioners (JCPP) member organizations CEOs to appoint a representative from their organization to participate (an invitation for an appointment was also extended to the National Association of Chain Drug Stores who currently is not a member of JCPP) It was recommended that professional affairs staff be considered for this work
State representatives were nominated by state pharmacy associations and appointed by the NASPA Executive Committee The selection process was intended to produce a group of participants who had experience with CPAs and practice in a variety of settings
Of note participants were only asked to represent their own opinions Participants from the national pharmacy associations were not acting as representatives of their organizations but rather as individuals whose experiences with their various memberships provide them with an informed perspective
Name StateNationa I OrganizationState
Alex Adams National NACDS
Jennifer Bacci State Pennsylvania
Lynette Bradley-Baker National AACP
Anne Burns National APhA
Carolyn Ha National NCPA
Julie Johnson State Minnesota
Sandra Leal State Arizona
Christine Lee-Wilson State Maryland
Dianne Miller State Michigan
Susan Oh National AMCP
Anthony Pudlo State Iowa
Kelly Ridgway State Arizona
Scotti Russell National NABP
Douglas Scheckel hoff National ASHP
L Michelle Vaughn State Alaska
Pete Vlasses National ACPE
Ed Webb National ACCP
Bryan Ziegler State South Carolina
4
APPENDIX B MODIFIED DELPHI METHOD
The goal of the workgroup was to reach consensus on each of the elements discussed To do this a modified Delphi method was used A survey was sent to all participants to collect their initial thoughts on each of the elements identified in currently existing collaborative practice authority laws andor regulations Participants were given the current variations of each element as a multiple-choice selection with the opportunity to answer in free form text if the desired option was not listed After completion of the survey the workgroup discussed all questions where consensus was not already reached via conference call The conference call discussions were structured to have a defined period of time for discussion followed by a summary of the current options being discussed and a roll call vote by each of the participants The item was included on the next survey if consensus was not reached on the conference call This process was repeated a total of three times before the group reached consensus on all items being considered See below for a diagram of the process used
1 Level-setting conference call
2 Distribution of survey with 3 weeks to complete
5
Task Force on
Collaborative Practice Agreements
Members Present
Sharlea M Leatherwood Chair (MO) Michael J Ayotte (VA) Winifred A Landis (IN) Jeffery Lindoo (NIN) C Ann Perry (GA) Charles R Young (MA)
Others Present
Joseph A Whaley Jr Executive Committee Liaison Carmen A Catizone NABP Executive DirectorSecretary Melissa Madigan NABP Staff
Introduction
The Task Force on Collaborative Practice Agreements (TFCPA) met December 3 1998 at the Marriott Suites Hotel in Rosemont Illinois The Task Force was established by the NABP Executive Corrunittee in response to recent increases in the number of prescribers and pharmacists entering into collaborative practice agreements and requests that boards of pharmacy define the elements of an appropriate collaborative practice agreement
Charge of the Task Force on Collaborative Practice Agreements
Task Force members reviewed their charge and proposing no changes accepted it as follows
Review the available literature and information pertaining to collaborative practice agreements between prescribers and pharmacists
bull Develop national model guidelines for writing and implementing uniform collaborative practice agreements that can be utilized by the state boards of pharmacy
TFCPA Recommendation 1
The Task Force on Collaborative Practice Agreements recorrunends to the Executive Committee and the Corrunittee on Law EnforcementLegislation that the following language be adopted for incorporation into the NABP Model State Pharmacy Act and Model Rules
Article I
Title Purpose Definition
Section 105 Definitions
(g) Collaborative Pharmacy Practice is that Practice of Pharmacy whereby a one or more Pharmacist~ has jointly agreed on a voluntary basis to work in conjunction with one or more Practitioners under protocol whereby the Pharmacist may perform certain patient care functions authorized by the Practitioner or Practitioners under certain specified conditions andor limitations
NATIONAL ASSOCIA nON OF BOARDS OF PHARMACYmiddot (P) 847391-4406 bull (F) 847391-4502 bull wwwnabpnel
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
(h) Collaborative Pharmacy Practice Agreement is a written and signed agreement between one or more Pharmacists and one or more Practitioners that provides for Collaborative Pharmacy Practice for the purpose of Drug Therapy Management of patients
(r) Drug Therapy Management means the review of Drug therapy regimen(s) of patients by one or more Pharmacists for the purpose of evaluating and rendering advice to one or more Practitioners regarding adjustment of the regimen Decisions involving Drug Therapy Management shall be made in the best interest of the patient Drug Therapy Management may include
(1) Implementing modifying and managing Drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement
(2) Collecting and reviewing patient Drug histories (3) Obtaining and checking vital signs including pulse temperature blood pressure
and respiration (4) Ordering and evaluating the results of laboratory tests directly relating to Drug
therapy when performed in accordance with approved protocols applicable to the practice setting and
(5) Such other patient care services as may be allowed by law
Pharmacists Scope of Practice Pursuant to the Collaborative Pharmacy Practice Agreement means those duties and limitations of duties placed upon one or more Pharmacists by the collaborating Practitioner or Practitioners the Board and applicable law and includes the limitations implied by the specialty practiced by the collaborating Practitioner or Practitioners
Model Rules for Pharmaceutical Care
Section 3 Pharmacy Practice
Collaborative Pharmacy Practice
(1) Collaborative Pharmacy Practice Agreement A Pharmacist planning to engage in Collaborative Pharmacy Practice shall have on file at his or her place of practice the written Collaborative Pharmacy Practice Agreement The existence and termination of such Agreement shall be reported to the Board and such Agreements shall be made available to the Board for review upon request The Agreement may allow the Pharmacist within the Pharmacists Scope of Practice Pursuant to the Collaborative Pharmacy Practice Agreement to conduct Drug Therapy Management activities approved by the Practitioner The collaboration that the Practitioner agrees to conduct with the Pharmacist must be within the scope of the Practitioners current practice Patients who receive services from one or more collaborating Pharmacists shall receive notification of receipt of such services
(2) Contents The Collaborative Pharmacy Practice Agreement shall include
(a) Identification of the Practitioner(s) and Pharmacist(s) who are parties to the Agreement
(b) The types of Drug Therapy Management decisions that the Pharmacist is allowed to make which may include
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpnet 2
Report of the Task Force on Collaborative Practice Agreements - NABP 9Smiddoth Annual Meeting Proceedings
(i) A detailed description of the types of diseases Drugs or Drug categories involved and the type of Drug Therapy Management allowed in each case
(ii) A detailed description of the methods procedures decision criteria and plan the Pharmacist is to follow when conducting Drug Therapy ~anagement and
(iii) A detailed description of the activities the Pharmacist is to follow in the course of conducting Drug Therapy Management including documentation of decisions made and a plan or appropriate mechanism for conununication feedback and reporting to the Practitioner concerning specific decisions made In addition to the Agreement documentation shall occur on the prescription record patient profile a separate log book or in some other appropriate system
(c) A method for the Practitioner to monitor compliance with the Agreement and clinical outcomes where Drug Therapy Management by the Pharmacist has occurred and to intercede where necessary
(d) A provision that allows the Practitioner to override a collaborative practice decision mad by the Pharmacist whenever he or she deems it necessary or appropriate
(e) A provision that allows either party to cancel the agreement by written notification
(f) An effective date and (g) Signatures of all collaborating Pharmacists and Practitioners who are party to the
agreement as well as the date of signing Amendments to a Collaborative Pharmacy Practice Agreement must be documented signed and dated
(3) Initiation of the Collaborative Pharmacy Practice Agreement The Collaborative Pharmacy Practice Agreement must be coupled with a medical order from the Practitioner to initiate Drug Therapy Management for any particular patient
(4) Documentation of Drug Therapy Management Docwnentation of Drug Therapy ~anagement must be kept as part of the patients permanent record and be readily available to other health care professionals providing care to that patient and who are authorized to received it Docwnentation of drug Therapy Management shall be considered Confidential Information
(5) Review At a minimum the written agreement shall be reviewed and renewed and if necessary revised every year
[NOTE the above new subsections would be inserted with sequential relettering of all subsequent subsections]
Background
Task Force members listened to a brief presentation by American College of Clinical Pharmacy (ACCP) Executive Director Robert Elenbaas and Immediate Past President Jerry Bauman regarding ACCPs position on collaborative practice agreements and later discussed their charge to develop national guidelines for writing and implementing uniform collaborative practice agreements for use by state boards of pharmacy They agreed that the best way to fulfill their charge was to create model regulations addressing the issue
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI3
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sb Annual Meeting Proceedings
To assist in developing the model regulations members looked to several sources of information including current state pharmacy laws and regulations as well as the information provided by Drs Elenbaas and Bauman The Task Force felt that the Idaho State Board of Pharmacys regulations on collaborative practice encompassed much of what they wanted to include in the model regulation therefore they were used as a blueprint
A brainstorming session brought issues and concerns regarding state regulation of collaborative pharmacy practice to the forefront Topics that members felt needed to be addressed in a model regulation included
Contents of collaborative pharmacy practice agreements
Practitioner override authority
Board approval of agreements
Phannacists documentation of care
Patient consentnotification
Confidentiality of patient information
Periodic review of agreement
Phannacist competencequalifications to provide drug therapy management services and
Quality assurance review of practice
Task Force members concurred on a majority of these topics and incorporated their determinations into the model recommendation Members agreed that certain key elements should be included in collaborative phannacy practice agreements so that such agreements may guide both the pharmacists and collaborating practitioners decisions and conduct Key elements incorporated included a description of the pharmacists activities a method describing a practitioner monitoring methods and a practitioner override clause
Members also agreed that it should not be necessary for boards of pharmacy to take on the responsibility for approving agreements or even requiring their submission but that boards should require parties to notify them of the existence of such agreements and to keep a copy at the practice site On this issue members concluded that the individual practitioners were the persons most qualified to approve the agreements noting that minimal government involvement in the practice of the phannacist and collaborating practitioner was the most desirable In order for the board to have some handle on the situation however it was determined that agreements should be made available for board inspection upon request Regarding he issue of pharmacist competence to provide drug therapy management services Task Force members concluded that the subject should not be addressed in the model regulations They agreed that he determination of competence should be left up to the individuals who are party to the agreement Members felt certain that most if not al1 collaborating practitioners would only delegate certain aspects of their patient care authority upon demonstration by the pharmacist of his or her abilities to provide such care Further members felt that the majority ofphannacists would not take on the responsibility for providing such care without being competent to do so
TFCP A Recommendation 2
The Task Force on Collaborative Practice Agreements recognized that the issue of quality care assurance is not still developing and at present cannot be addressed with regard to collaborative pharmacy practice The Task Force recommends that the NABP Executive Committee closely
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI4
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
follow this issue and should it be determined that language addressing the subject should be incorporated into the model regulation take further action at that time
Background
Task Force members were uncertain at this time of how to address the issue of quality care assurance Members noted that most states addressing collaborative pharmacy practice in their laws or regulations did not include a provision on this subject It was noted however that tat least one state placed the responsibility for quality assurance on the Principal pharmacist and practitioner and that ACCP urged that an appropriate body be responsible for such activities
Member postulated that collaborative practice activities would likely be subject to quality assurance review via physician peer-review processes Additionally they suggested that those parties practicing in institutional settings would follow the quality assurance policies guidelines andor requirements of the institution The Task Force felt it was premature to make a recommendation regarding those parties who practice in ambulatory settings and who may not be subject to quality assurance review Members observed that the work ofNABPs Task Force on Patient Outcomes Regulation should be reviewed before a recommendation was made
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406 (F) 847391-4502middot wwwnabpnet 5
58-1-107 Applicability -- Relationship to specific chapters under title The provisions of this chapter LJniformly apply to the administration and enforcement of this title However
unless expressly prohibited in this chapter any provision of this chapter may be supplemented or altered by specific chapters of this title
R1S6-1-107 Organization of Rules - Content Applicability and Relationship of Rules
(1) The rules and sections in Title R156 shall to the extent practicable follow the numbering and
organizational scheme of the chapters in Title 58
(2) Rule R156-1 shall contain general provisions applicable to the administration and enforcement of all
occupations and profeSSions regulated in Title 58
(3) The provisions of the other rules in Title R156 shall contain specific or unique provisions applicable to
particular occupations or professions
(4) Specific rules in Title R156 may supplement or alter Rule R156-1 unless expressly provided otherwise
in Rule R156-1
R1S6-17b-l04 Organization - Relationship to Rule R1S6-1
The organization of this rule and its relationship to Rule R156-1 is as described in Section R156-1-107
58-17b-50G Petitioning for reinstatement of licensure Any person whose license to practice pharmacy in this state has been revoked suspended or surrendered
voluntarily or by action of the division shall have the right at reasonable intervals to petition the division for reinstatement of such license Such petition shall be made in writing and in the form prescribed by the division Upon investigation and hearing the division may in its discretion grant or deny such petition or it may modify its driginal finding to reflect any circumstances that have changed sufficiently to warrant such modifications Themiddotdivision also at its discretion may require such person to pass an examination or examinations for reshyentry into the practice of pharmacy Enacted by Chapter 280 2004 General Session
Agenda Board of Pharmacy
Collaborative Practice Agreement Discussion 100 PM - 200 PM
-~ Agenda Item Overview - I
Welcome and Introductions Paige Patterick and Carrie Dunford
Discuss the wording of 58-17b-l 02( 17) (17) Collaborative pharmacy practice agreement means a written and signed agreement between one or more pharmacists and one or more practitioners that provides for collaborative pharmacy practice for the purpose of drug therapy management of patients and prevention of disease of human subjects
Discuss the definition of Drug Therapy Management in R 156-17b-l 02 (19)
(J 9) Drug therapy management means the review of a drug therapy regimen of a patient by one or more pharmacists for the purpose of evaluating and rendering advice to one or more practitioners regarding adjustment of the regimen
Resources
NABP https llnabppharmacywp-contentuploads20 1607 ITFCollaborativePracticeAgreements AM95 Dec 1998 pdf
Operating Standards Drug therapy management R 156-1 7b-61 I
R 156-l7b-611 Operating Standards - Drug Therapy Management (I) In accordance with Subsections 58-17b-l 02( 17) and 58-l7b-60 I( I) decisions involving drug therapy management shall be made in the best interest of the patient Drug therapy management may include (a) implementing modifying and managing drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement (b) collecting and reviewing patient histories (c) obtaining and checking vital signs including pulse temperature blood pressure and respiration (d) ordering and evaluating the results of laboratory tests directly applicable to the drug therapy when perfolmed in accordance with approved protocols applicable to the practice setting and (e) such other patient care services as may be allowed by rule (2) For the purpose of promoting therapeutic appropriateness a pharmacist shall at the time of dispensing a prescription or a prescription drug order review the patients medication record Such review shall at a minimum identify clinically significant conditions situations or items such as (a) inappropriate drug utilization (b) therapeutic duplication (c) drug-disease contraindications (d) drug-drug interactions (e) incorrect drug dosage or duration of drug treatment (f) drug-allergy interactions and (g) clinical abuse or misuse (3) Upon identifying any clinically significant conditions situations or items listed in Subsection (2) above the pharmacist shall take appropriate steps to avoid or resolve the problem including consultation with the prescribing practitioner
Note In compliance with the Americans with Disabilities Act individuals needing special accommodations (including auxiliary communicative aids and services) during this meet ing should not ify Dave Taylor ADA Coordinator at least three working days prior to the meeting Division of Occupational amp Professional Licensing 160 East 300 South Salt Lake City Utah 84115 801-530-6628 or toll-free in Utah only 866-275-3675 Posted to Bulletin Board on 11-21-2017

Pharmacy Board 11282017 October 2017
Citation A random inspection was conducted by the Division and violations were found A total of 108 medications were found in the pharmacys stock that wre either expired or had indeterminate expiration dates A fine was issued in the amount of $1 05000
(5) (a) Any license that is not renewed may be reinstated
(i) upon submission of an application for reinstatement payment of the renewal fee together with a reinstatement fee determined by the department under Section 63]-1-504 and upon submission of documentation showing completion of or compliance with renewal qualifications and
(ii) (A) at any time within two years after nonrenewal or
(B) between two years and five years after nonrenewal if established by rule made by the division in consultation with the applicable licensing board in accordance with Title 63G Chapter 3 Utah Administrative Rulemaking Act
(b) The application procedures specified in Subsection 58-1-301 (2) apply to the reinstatement applications to the extent they are not in conflict with this section
(c) Except as otherwise provided by rule a license that is reinstated no later than 120 days after it expires shall be retroactively reinstated to the date it expired
(6) (a) Except as provided in Subsection (5)(a) if not reinstated within two years the holder may obtain a license only if the holder meets requirements provided by the division by rule or by statute for a new license
(b) Each licensee under this title who has been active in the licensed occupation or profession while in the fullshytime employ of the United States government or under license to practice that occupation or profession in any other state or territory of the United States may reinstate the licensees license without taking an examination by submitting an application for reinstatement paying the current annual renewal fee and the reinstatement fee and submitting documentation showing completion of or compliance with any renewal qualifications at any time within six months after reestablishing domicile within Utah or terminating fullshytime government service
Amended by Chapter 238 2016 General Session
bull R156-1-308a Renewal Dates bull R156-1-308b Renewal Periods - Adjustment of Renewal Fees for an Extended or Shortened Renewal
Period bull R156-1-308c Renewal of Licensure Procedures bull R156-1-308d Waiver of Continuing Education Requirements - Renewal Requirements bull R156-1-308e Automatic Expiration of Licensure Upon Dissolution of Licensee
bull R156-1-308f Denial of Renewal of Licensure - Classification of Proceedings - Conditional Renewal of Licensure During Adjudicative Proceedings - Conditional Initial Renewal or Reinstatement Licensure During Audit or Investigation
bull R156-1-308g Reinstatement of Licensure which was Active and in Good Standing at the Time of Expiration of Licensure - Requirements
bull R156-1-308h Reinstatement of Restricted Suspended or Probationary Licensure During Term of Restriction Suspension or Probation - Requirements
bull R156-1-308i Reinstatement of Restricted Suspended or Probationary Licensure After the Specified Term of Suspension of the License or After the Expiration of Licensure in a Restricted Suspended or Probationary Status - Requirements
bull R156-1-308j Relicensure Following Revocation of Licensure - Requirements bull R156-1-308k Relicensure Following Surrender of Licensure - Requirements bull R156-1-3081 Reinstatement of Licensure and Relicensure - Term of Licensure
Effective 5102016 58-1-308 Term of license -- Expiration of license -- Renewal of license -- Reinstatement of license __ Application procedures
(1) (a) Each license issued under this title shall be issued in accordance with a two-year renewal cycle established by rule
(b) A renewal period may be extended or shortened by as much as one year to maintain established renewal cycles or to change an established renewal cycle
(2) (a) The expiration date of a license shall be shown on the license
(b) A license that is not renewed prior to the expiration date shown on the license automatically expires
(c) A license automatically expires prior to the expiration date shown on the license upon the death of a licensee who is a natural person or upon the dissolution of a licensee that is a partnership corporation or other business entity
(d) If the existence of a dissolved partnership corporation or other business entity is reinstated prior to the expiration date shown upon the entitys expired license issued by the division the division shall upon written application reinstate the applicants license unless it flnds that the applicant no longer meets the qualifications for licensure
(e) Expiration of licensure is not an adjudicative proceeding under Title 63G Chapter 4 Administrative Procedures Act
(3) (a) The division shall notify each licensee in accordance with procedures established by rule that the licensees license is due for renewal and that unless an application for renewal is received by the division by the expiration date shown on the license together with the appropriate renewal fee and documentation showing completion of or compliance with renewal qualiflcations the license will not be renewed
(b) Examples of renewal qualiflcations which by statute or rule the division may require the licensee to document completion of or compliance with include
(i) continuing education
(ii) continuing competency
(iii) quality assurance
(iv) utilization plan and protocol
(v) fInancial responsibility
(vi) certiflcation renewal and
(vii) calibration of equipment
(4) (a) (i) An application for renewal that complies with Subsection (3) is complete
(ii) A renewed license shall be issued to applicants who submit a complete application unless it is apparent to the division that the applicant no longer meets the qualiflcations for continued licensure
(b) (i) The division may evaluate or verify documentation showing completion of or compliance with renewal requirements on an entire population or a random sample basis and may be assisted by advisory peer committees
(ii) If necessary the division may complete its evaluation or veriflcation subsequent to renewal and if appropriate pursue action to suspend or revoke the license of a licensee who no longer meets the qualifications for continued licensure
(c) The application procedures specified in Subsection 58-1-301 (2) apply to renewal applications to the extent they are not in conflict with this section
(d) submit to a criminal backgrolUld check pursuant to Subsection 58-31 b-302(5) and Section R156-31 b-30 19 (3) ~ apph~anl who holds a current LPN license in an interstate compact state shall apply for a license within 90 days of
estabhshmg reSidency In Utah and complete all requirements pursuant to R 156-31 b-30 I a(2) (4) An applicant who has been licensed previously in Utah but whose license has expired or lapsed shall (a) if the applicant has not practiced as a nurse for up to five years document current compliance with the continuing competency
requirements as established in SubseL1ion R 156-31b-303(3)
(b) if the applicant has not practiced as a nurse for more than five years but less than eight years (I) pass the NCLEX-PN examination within 60 days following the date of application or (ii) successfully complete an approved re-entry program (c) if the applicant has not practiced as a nurse for more than eight years but less than 10 years (i) successfully complete an approved re-entry program and (ii) pass the NCLEX-PN examination within 60 days following the date of application or (d) if the applicant has not practiced as a nurse for 10 years or more comply with this Subsection (1) (5) An applicant who has been licensed in another state or country but whose license has expired or lapsed shall (a) comply with this Subsection (2)(b) and (b) comply with this Subsection (4) a~ applicable and (c) submit to a criminal background check pursuant to Subsection 58-31 b-302(5) and Section R156-31 b-30 I g
R156-31b-301b RN License- Education Examination and Experience Requirements (I) An applicant who has never obtained a license in any state or country shall (a) demonstrate that the applicant ha~ successfully completed an RN prelicensing education program that (i) meets the requirements of Section 58-31 b-60 I or (ii) is equivalent to an approved program under Section 58-3 I b-60 I (b) pass the NCLEX-RN examination purslant to Section R 156-31 b-30 I e and (c) submit to a climinal background check pursuant to Subsection 58-31 b-302(5) and Section R155-31 b-301g (2) An applicant who holds a current RN license issued by another country or state shall (a) demonstrate that the license issued by the other jurisdiction is current active and in good standing as of the date of
application (b)(i) demonstrate that the applicant has graduated from an RN prelicensing education program and (ii) if a foreign education program demonstrate that the program meets all requirements outlined in Section R 156-31 b-30 I d (c) pass the NCLEX-RN examination pursuant to Section RI56-31b-301e and (d) submit to a criminal backgrOlUld check pursuant to Subsection 58-31 b-302(5) and Sel-1ion R156-31 b-30 I g (3) An applicant who holds a current RN license in an interstate compact state shall apply for a license within 90 days of
establishing residency in Utah and complete all requirements pursuant to R156-31 b-301 b(2) (4) An applicant who has been licensed previously in Utah but whose license has expired or lapsed shall (a) if the applicant has not practiced as a nurse for up to five years document current compliance with the continuing competency
requirements as established in Subsection R 156-31 b-303(3) (b) if the applicant has not practiced as a nurse for more than five years but less than eight years (i) pass the NCLEX-RN examination within 60 days following the date of application or (ii) successfully complete an approved re-entry program (c) if the applicant has not practiced as a nurse for more than eight years but less than 10 years (i) successfully complete an approved re-entry program and (ii) pass theNCLEX-RN examination within 60 days following the date of application or (d) if the applicant ha~ not practiced as a nurse for 0 years or more comply with this Subsection (1) (5) An applicant who has been licensed in another state or country but whose license ha~ expired or lapsed shall (a) comply with this Subsection (2)(b) (b) comply with this Subsection (4) as applicable and (c) submit to a criminal background check pursuant to Subsection 58-31 b-302(5) and Section R156-3l b-30 I g
R156-31b-301c APRN Lkense - Education Examination and Experience Requirements (I) An applicant who is 110t currently and validly licensed alt an APRN in any state or country shall (a) demonstrate that the applicant holds a current active RN license in good standing (b) demonstrate that the applicant has successfully completelt an APRN prelicensing education program that meets the
requirements of Subsection 58-31b-601(1) and Subsection 58-31b-302(4)(e) (c) pass a national certitication examination consistent with the applicants educational specialty pursuant to Section R156-31 bshy
30 I e and administered by one of the following credentialing bodies (i) the American Nurses Credentialing Center Certification
4
PHARMACIST COLLABORATIVE PRACTICE AGREEMENTS
KEY ELEMENTS FOR LEGISLATIVE AND REGULATORY AUTHO RITY
A REPORT OF THE COLLABORATIVE PRACTICE WORKGROUP
CONVENED BY THE NATIONAL ALLIANCE OF STATE PHARMACY ASSOCIATIONS
PHARMACIST COLLABORATIVE PRACTICE AGREEMENTS KEY ELEMENTS FOR LEGISLATIVE AND REGULATORY AUTHORITY
PROJE CT OVERVIEW
Collaborative practice agreements (CPAs) create a formal practice relationship between pharmacists and other health care practitioners whereby the pharmacist assumes responsibility for specific patient care functions that are otherwise beyond their typical scope of practice but aligned with their education and training These patient care services can include initiation and modification of drug therapy The extent of the services authorized under the collaborative agreement depends on the states statutory and regulatory provisions for collaborative practice authority as well as the terms of the specific agreement between the pharmacist and other health care practitioners
State laws and regulations authorizing CPAs are highly variable Some states specify the practitioners
able to participate in CPAs restrict the services that may be provided under a CPA or include extensive
logistical barriers that limit the utility of such agreements
In their 2015 paper The Expanding Role of Pharmacists in a Transformed Health Care System the
National Governors Association (NGA) presented the following state policy considerations in regards to
collaborative practice provisions
bull Enact broad collaborative practice provisions that allow for specific provider functions to be
determined at the provider level rather than set in state statute or through regulation
bull Evaluate practice setting and drug therapy restrictions to determine whether pharmacists and
providers face disincentives that unnecessarily discourage collaborative arrangements
bull Examine whether CPAs unnecessarily dictate disease or patient specificity1
The National Alliance of State Pharmacy Associations (NASPAs) Executive Committee directed staff to
convene a workgroup to build upon the NGA recommendations with additional specificity The workgroup was charged with examining existing state CPA laws and regulations The workgroup was
tasked with developing recommendations for what elements of collaborative practice authority should
appropriately be defined under state law andor regulation and what elements are best left to be
determined between pharmacists and other practitioners when developing their specific collaborative
practice arrangement Using a modified Delphi method the Collaborative Practice Workgroup conducted
this work with two key questions in mind
bull Is this recommendation in the best interest of the patient receiving care under a collaborative
agreement
bull Is this recommendation aligned with pharmacists education and tra ining
The following is a report of the workgroups recommendations
1 Nati onal Governors Association The Expanding Role of Pharmacists in a Transformed Health Care System
JQ lwwwngaorgfiles llvesitesNGAfil esp d(20151 SOl Th eExp a ncli ngHo l e(jfPh~ rm cists~ Accessed
61515 1
WORKGROUP RECOMMENDATIONS The workgroup took the approach that rapid innovation in education training technology and evidence-based gUidelines necessitate a collaborative practice framework that is flexible and facilitates innovation in care delivery Thus the following statements include two levels of recommendations
1 Elements of collaborative practice authority that should be codified in state law andor state regulations and
2 Elements that are more appropriately determined by the parties at the practice level who voluntarily enter into a CPA and thus for which the laws and regulations should be silent
The workgroup views both levels of recommendations as needed and synergistic State law andor regulations if too restrictive can impede innovative team-based care models
COLLABORATIVE PRACTICE AGREEMENT PARTICIPANTS
RECOMMENDED ELEMENTS FOR INCLUSION IN STATE LAWS ANDOR REGULATIONS
Collaborative practice laws andor regulations should specify that
bull Any practitioner with prescriptive authority may collaborate with pharmacists using a CPA
bull CPAs may be between a single or multiple pharmacists and a single or multiple prescribers
bull CPAs may apply to a single patient multiple patients or patient populations as specified in the agreement
ELEMENTS THAT MAY BE DETERMINED AT THE PRACTITIONER LEVEL
Individual CPAs may address the below elements but state laws andor regulations should be silent
bull CPAs should specify which patient(s) andor patient population(s) can receive services under the agreement
bull Depending on the complexity of the services being provided under a CPA it may be appropriate for the pharmacist to have additional credentials or training beyond what is required for licensure
bull CPAs should specify which pharmacist(s) may provide services under the CPA A pharmacists practice setting should not be a barrier to their ability to enter into a CPA
COLLABORATIVE PRACTICE AGREEIVlEIlT AUTHORIZED SERVICES
RECOMMENDED ELEMENTS FOR INCLUSION IN STATE LAWS ANDOR REGULATIONS
Collaborative practice laws andor regulations should specify that
bull The initiation and modification of drug therapy may be authorized under a CPA with a prescriber
ELEMENTS THAT MAY BE DETERMINED AT THE PRACTITIONER LEVEL
Individual CPAs may address the below elements but state laws andor regulations should be silent
bull In some situations the use of an evidence-based protocol can ensure optimal care when pharmacists are initiating or modifying drug therapy and may be included in the CPA though they may not be needed or appropriate in others
2
bull Performing physical assessments as well as ordering performing or interpreting laboratory tests (eg CLiA-waived tests) may be included in a CPA to help identify or refer patients for services however a pharmacist may also perform these services without a CPA as these activities should fall within pharmacists standard scope of practice
bull Specific disease states may be included in a CPA at the participating practitioners discretion
COLLABORATIVE PRACTICE AGREEMENT REQUIREMENTS AND RESTRICTIONS
RECOMMENDED ELEMENTS FOR INCLUSION IN STATE LAWS ANDOR REGULATIONS
Collaborative practice laws andor regulations should specify that
bull All prescription drugs including controlled substances may be included within pharmacists collaborative practice authority
bull CPAs should be maintained by the pharmacist(s) and collaborating prescriber(s) and be available upon request or inspection
ELEMENTS THAT MAY BE DETERMINED AT THE PRACTITIONER LEVEL
Individual CPAs may address the below elements but state laws andor regulations should be silent
bull Pharmacist(s) and prescriber(s) may specify the level of patient involvement in the CPA Depending on the level of service elements such as informed consent written consent or optshyout provisions may be appropriate as determined by the parties to the agreement
bull Agreements should not be required to be sent to or approved by a state regulatory board or other agency such requirements create unnecessary paperwork burden and slow the efficiency of care delivery
bull Collaborating practitioners are encouraged to review andor renew their CPAs within a timeframe that is clinically appropriate
bull Collaborating practitioners should conform to evidence-based guidelines and the agreed upon process of care with regards to the documentation requirements and the collaborating practitioners responsibility for review of services provided under the agreement
bull Practitioners may consider liability insurance provisions and the appropriateness of articulating these in their voluntary agreement
bull It is the professional duty of all healthcare professionals to stay current in the clinical areas in which they practice If individual practitioners determine that continuing education requirements are appropriate for their clinical arrangement they may be specified in the agreement
3
APPENDIX A WORKGROUP PARTICIPANTS
The individuals listed below were appointed to participate in the Collaborative Practice Workgroup by one of two methods NASPA invited all Joint Commission of Pharmacy Practitioners (JCPP) member organizations CEOs to appoint a representative from their organization to participate (an invitation for an appointment was also extended to the National Association of Chain Drug Stores who currently is not a member of JCPP) It was recommended that professional affairs staff be considered for this work
State representatives were nominated by state pharmacy associations and appointed by the NASPA Executive Committee The selection process was intended to produce a group of participants who had experience with CPAs and practice in a variety of settings
Of note participants were only asked to represent their own opinions Participants from the national pharmacy associations were not acting as representatives of their organizations but rather as individuals whose experiences with their various memberships provide them with an informed perspective
Name StateNationa I OrganizationState
Alex Adams National NACDS
Jennifer Bacci State Pennsylvania
Lynette Bradley-Baker National AACP
Anne Burns National APhA
Carolyn Ha National NCPA
Julie Johnson State Minnesota
Sandra Leal State Arizona
Christine Lee-Wilson State Maryland
Dianne Miller State Michigan
Susan Oh National AMCP
Anthony Pudlo State Iowa
Kelly Ridgway State Arizona
Scotti Russell National NABP
Douglas Scheckel hoff National ASHP
L Michelle Vaughn State Alaska
Pete Vlasses National ACPE
Ed Webb National ACCP
Bryan Ziegler State South Carolina
4
APPENDIX B MODIFIED DELPHI METHOD
The goal of the workgroup was to reach consensus on each of the elements discussed To do this a modified Delphi method was used A survey was sent to all participants to collect their initial thoughts on each of the elements identified in currently existing collaborative practice authority laws andor regulations Participants were given the current variations of each element as a multiple-choice selection with the opportunity to answer in free form text if the desired option was not listed After completion of the survey the workgroup discussed all questions where consensus was not already reached via conference call The conference call discussions were structured to have a defined period of time for discussion followed by a summary of the current options being discussed and a roll call vote by each of the participants The item was included on the next survey if consensus was not reached on the conference call This process was repeated a total of three times before the group reached consensus on all items being considered See below for a diagram of the process used
1 Level-setting conference call
2 Distribution of survey with 3 weeks to complete
5
Task Force on
Collaborative Practice Agreements
Members Present
Sharlea M Leatherwood Chair (MO) Michael J Ayotte (VA) Winifred A Landis (IN) Jeffery Lindoo (NIN) C Ann Perry (GA) Charles R Young (MA)
Others Present
Joseph A Whaley Jr Executive Committee Liaison Carmen A Catizone NABP Executive DirectorSecretary Melissa Madigan NABP Staff
Introduction
The Task Force on Collaborative Practice Agreements (TFCPA) met December 3 1998 at the Marriott Suites Hotel in Rosemont Illinois The Task Force was established by the NABP Executive Corrunittee in response to recent increases in the number of prescribers and pharmacists entering into collaborative practice agreements and requests that boards of pharmacy define the elements of an appropriate collaborative practice agreement
Charge of the Task Force on Collaborative Practice Agreements
Task Force members reviewed their charge and proposing no changes accepted it as follows
Review the available literature and information pertaining to collaborative practice agreements between prescribers and pharmacists
bull Develop national model guidelines for writing and implementing uniform collaborative practice agreements that can be utilized by the state boards of pharmacy
TFCPA Recommendation 1
The Task Force on Collaborative Practice Agreements recorrunends to the Executive Committee and the Corrunittee on Law EnforcementLegislation that the following language be adopted for incorporation into the NABP Model State Pharmacy Act and Model Rules
Article I
Title Purpose Definition
Section 105 Definitions
(g) Collaborative Pharmacy Practice is that Practice of Pharmacy whereby a one or more Pharmacist~ has jointly agreed on a voluntary basis to work in conjunction with one or more Practitioners under protocol whereby the Pharmacist may perform certain patient care functions authorized by the Practitioner or Practitioners under certain specified conditions andor limitations
NATIONAL ASSOCIA nON OF BOARDS OF PHARMACYmiddot (P) 847391-4406 bull (F) 847391-4502 bull wwwnabpnel
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
(h) Collaborative Pharmacy Practice Agreement is a written and signed agreement between one or more Pharmacists and one or more Practitioners that provides for Collaborative Pharmacy Practice for the purpose of Drug Therapy Management of patients
(r) Drug Therapy Management means the review of Drug therapy regimen(s) of patients by one or more Pharmacists for the purpose of evaluating and rendering advice to one or more Practitioners regarding adjustment of the regimen Decisions involving Drug Therapy Management shall be made in the best interest of the patient Drug Therapy Management may include
(1) Implementing modifying and managing Drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement
(2) Collecting and reviewing patient Drug histories (3) Obtaining and checking vital signs including pulse temperature blood pressure
and respiration (4) Ordering and evaluating the results of laboratory tests directly relating to Drug
therapy when performed in accordance with approved protocols applicable to the practice setting and
(5) Such other patient care services as may be allowed by law
Pharmacists Scope of Practice Pursuant to the Collaborative Pharmacy Practice Agreement means those duties and limitations of duties placed upon one or more Pharmacists by the collaborating Practitioner or Practitioners the Board and applicable law and includes the limitations implied by the specialty practiced by the collaborating Practitioner or Practitioners
Model Rules for Pharmaceutical Care
Section 3 Pharmacy Practice
Collaborative Pharmacy Practice
(1) Collaborative Pharmacy Practice Agreement A Pharmacist planning to engage in Collaborative Pharmacy Practice shall have on file at his or her place of practice the written Collaborative Pharmacy Practice Agreement The existence and termination of such Agreement shall be reported to the Board and such Agreements shall be made available to the Board for review upon request The Agreement may allow the Pharmacist within the Pharmacists Scope of Practice Pursuant to the Collaborative Pharmacy Practice Agreement to conduct Drug Therapy Management activities approved by the Practitioner The collaboration that the Practitioner agrees to conduct with the Pharmacist must be within the scope of the Practitioners current practice Patients who receive services from one or more collaborating Pharmacists shall receive notification of receipt of such services
(2) Contents The Collaborative Pharmacy Practice Agreement shall include
(a) Identification of the Practitioner(s) and Pharmacist(s) who are parties to the Agreement
(b) The types of Drug Therapy Management decisions that the Pharmacist is allowed to make which may include
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpnet 2
Report of the Task Force on Collaborative Practice Agreements - NABP 9Smiddoth Annual Meeting Proceedings
(i) A detailed description of the types of diseases Drugs or Drug categories involved and the type of Drug Therapy Management allowed in each case
(ii) A detailed description of the methods procedures decision criteria and plan the Pharmacist is to follow when conducting Drug Therapy ~anagement and
(iii) A detailed description of the activities the Pharmacist is to follow in the course of conducting Drug Therapy Management including documentation of decisions made and a plan or appropriate mechanism for conununication feedback and reporting to the Practitioner concerning specific decisions made In addition to the Agreement documentation shall occur on the prescription record patient profile a separate log book or in some other appropriate system
(c) A method for the Practitioner to monitor compliance with the Agreement and clinical outcomes where Drug Therapy Management by the Pharmacist has occurred and to intercede where necessary
(d) A provision that allows the Practitioner to override a collaborative practice decision mad by the Pharmacist whenever he or she deems it necessary or appropriate
(e) A provision that allows either party to cancel the agreement by written notification
(f) An effective date and (g) Signatures of all collaborating Pharmacists and Practitioners who are party to the
agreement as well as the date of signing Amendments to a Collaborative Pharmacy Practice Agreement must be documented signed and dated
(3) Initiation of the Collaborative Pharmacy Practice Agreement The Collaborative Pharmacy Practice Agreement must be coupled with a medical order from the Practitioner to initiate Drug Therapy Management for any particular patient
(4) Documentation of Drug Therapy Management Docwnentation of Drug Therapy ~anagement must be kept as part of the patients permanent record and be readily available to other health care professionals providing care to that patient and who are authorized to received it Docwnentation of drug Therapy Management shall be considered Confidential Information
(5) Review At a minimum the written agreement shall be reviewed and renewed and if necessary revised every year
[NOTE the above new subsections would be inserted with sequential relettering of all subsequent subsections]
Background
Task Force members listened to a brief presentation by American College of Clinical Pharmacy (ACCP) Executive Director Robert Elenbaas and Immediate Past President Jerry Bauman regarding ACCPs position on collaborative practice agreements and later discussed their charge to develop national guidelines for writing and implementing uniform collaborative practice agreements for use by state boards of pharmacy They agreed that the best way to fulfill their charge was to create model regulations addressing the issue
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI3
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sb Annual Meeting Proceedings
To assist in developing the model regulations members looked to several sources of information including current state pharmacy laws and regulations as well as the information provided by Drs Elenbaas and Bauman The Task Force felt that the Idaho State Board of Pharmacys regulations on collaborative practice encompassed much of what they wanted to include in the model regulation therefore they were used as a blueprint
A brainstorming session brought issues and concerns regarding state regulation of collaborative pharmacy practice to the forefront Topics that members felt needed to be addressed in a model regulation included
Contents of collaborative pharmacy practice agreements
Practitioner override authority
Board approval of agreements
Phannacists documentation of care
Patient consentnotification
Confidentiality of patient information
Periodic review of agreement
Phannacist competencequalifications to provide drug therapy management services and
Quality assurance review of practice
Task Force members concurred on a majority of these topics and incorporated their determinations into the model recommendation Members agreed that certain key elements should be included in collaborative phannacy practice agreements so that such agreements may guide both the pharmacists and collaborating practitioners decisions and conduct Key elements incorporated included a description of the pharmacists activities a method describing a practitioner monitoring methods and a practitioner override clause
Members also agreed that it should not be necessary for boards of pharmacy to take on the responsibility for approving agreements or even requiring their submission but that boards should require parties to notify them of the existence of such agreements and to keep a copy at the practice site On this issue members concluded that the individual practitioners were the persons most qualified to approve the agreements noting that minimal government involvement in the practice of the phannacist and collaborating practitioner was the most desirable In order for the board to have some handle on the situation however it was determined that agreements should be made available for board inspection upon request Regarding he issue of pharmacist competence to provide drug therapy management services Task Force members concluded that the subject should not be addressed in the model regulations They agreed that he determination of competence should be left up to the individuals who are party to the agreement Members felt certain that most if not al1 collaborating practitioners would only delegate certain aspects of their patient care authority upon demonstration by the pharmacist of his or her abilities to provide such care Further members felt that the majority ofphannacists would not take on the responsibility for providing such care without being competent to do so
TFCP A Recommendation 2
The Task Force on Collaborative Practice Agreements recognized that the issue of quality care assurance is not still developing and at present cannot be addressed with regard to collaborative pharmacy practice The Task Force recommends that the NABP Executive Committee closely
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI4
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
follow this issue and should it be determined that language addressing the subject should be incorporated into the model regulation take further action at that time
Background
Task Force members were uncertain at this time of how to address the issue of quality care assurance Members noted that most states addressing collaborative pharmacy practice in their laws or regulations did not include a provision on this subject It was noted however that tat least one state placed the responsibility for quality assurance on the Principal pharmacist and practitioner and that ACCP urged that an appropriate body be responsible for such activities
Member postulated that collaborative practice activities would likely be subject to quality assurance review via physician peer-review processes Additionally they suggested that those parties practicing in institutional settings would follow the quality assurance policies guidelines andor requirements of the institution The Task Force felt it was premature to make a recommendation regarding those parties who practice in ambulatory settings and who may not be subject to quality assurance review Members observed that the work ofNABPs Task Force on Patient Outcomes Regulation should be reviewed before a recommendation was made
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406 (F) 847391-4502middot wwwnabpnet 5
58-1-107 Applicability -- Relationship to specific chapters under title The provisions of this chapter LJniformly apply to the administration and enforcement of this title However
unless expressly prohibited in this chapter any provision of this chapter may be supplemented or altered by specific chapters of this title
R1S6-1-107 Organization of Rules - Content Applicability and Relationship of Rules
(1) The rules and sections in Title R156 shall to the extent practicable follow the numbering and
organizational scheme of the chapters in Title 58
(2) Rule R156-1 shall contain general provisions applicable to the administration and enforcement of all
occupations and profeSSions regulated in Title 58
(3) The provisions of the other rules in Title R156 shall contain specific or unique provisions applicable to
particular occupations or professions
(4) Specific rules in Title R156 may supplement or alter Rule R156-1 unless expressly provided otherwise
in Rule R156-1
R1S6-17b-l04 Organization - Relationship to Rule R1S6-1
The organization of this rule and its relationship to Rule R156-1 is as described in Section R156-1-107
58-17b-50G Petitioning for reinstatement of licensure Any person whose license to practice pharmacy in this state has been revoked suspended or surrendered
voluntarily or by action of the division shall have the right at reasonable intervals to petition the division for reinstatement of such license Such petition shall be made in writing and in the form prescribed by the division Upon investigation and hearing the division may in its discretion grant or deny such petition or it may modify its driginal finding to reflect any circumstances that have changed sufficiently to warrant such modifications Themiddotdivision also at its discretion may require such person to pass an examination or examinations for reshyentry into the practice of pharmacy Enacted by Chapter 280 2004 General Session
Agenda Board of Pharmacy
Collaborative Practice Agreement Discussion 100 PM - 200 PM
-~ Agenda Item Overview - I
Welcome and Introductions Paige Patterick and Carrie Dunford
Discuss the wording of 58-17b-l 02( 17) (17) Collaborative pharmacy practice agreement means a written and signed agreement between one or more pharmacists and one or more practitioners that provides for collaborative pharmacy practice for the purpose of drug therapy management of patients and prevention of disease of human subjects
Discuss the definition of Drug Therapy Management in R 156-17b-l 02 (19)
(J 9) Drug therapy management means the review of a drug therapy regimen of a patient by one or more pharmacists for the purpose of evaluating and rendering advice to one or more practitioners regarding adjustment of the regimen
Resources
NABP https llnabppharmacywp-contentuploads20 1607 ITFCollaborativePracticeAgreements AM95 Dec 1998 pdf
Operating Standards Drug therapy management R 156-1 7b-61 I
R 156-l7b-611 Operating Standards - Drug Therapy Management (I) In accordance with Subsections 58-17b-l 02( 17) and 58-l7b-60 I( I) decisions involving drug therapy management shall be made in the best interest of the patient Drug therapy management may include (a) implementing modifying and managing drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement (b) collecting and reviewing patient histories (c) obtaining and checking vital signs including pulse temperature blood pressure and respiration (d) ordering and evaluating the results of laboratory tests directly applicable to the drug therapy when perfolmed in accordance with approved protocols applicable to the practice setting and (e) such other patient care services as may be allowed by rule (2) For the purpose of promoting therapeutic appropriateness a pharmacist shall at the time of dispensing a prescription or a prescription drug order review the patients medication record Such review shall at a minimum identify clinically significant conditions situations or items such as (a) inappropriate drug utilization (b) therapeutic duplication (c) drug-disease contraindications (d) drug-drug interactions (e) incorrect drug dosage or duration of drug treatment (f) drug-allergy interactions and (g) clinical abuse or misuse (3) Upon identifying any clinically significant conditions situations or items listed in Subsection (2) above the pharmacist shall take appropriate steps to avoid or resolve the problem including consultation with the prescribing practitioner
Note In compliance with the Americans with Disabilities Act individuals needing special accommodations (including auxiliary communicative aids and services) during this meet ing should not ify Dave Taylor ADA Coordinator at least three working days prior to the meeting Division of Occupational amp Professional Licensing 160 East 300 South Salt Lake City Utah 84115 801-530-6628 or toll-free in Utah only 866-275-3675 Posted to Bulletin Board on 11-21-2017

(5) (a) Any license that is not renewed may be reinstated
(i) upon submission of an application for reinstatement payment of the renewal fee together with a reinstatement fee determined by the department under Section 63]-1-504 and upon submission of documentation showing completion of or compliance with renewal qualifications and
(ii) (A) at any time within two years after nonrenewal or
(B) between two years and five years after nonrenewal if established by rule made by the division in consultation with the applicable licensing board in accordance with Title 63G Chapter 3 Utah Administrative Rulemaking Act
(b) The application procedures specified in Subsection 58-1-301 (2) apply to the reinstatement applications to the extent they are not in conflict with this section
(c) Except as otherwise provided by rule a license that is reinstated no later than 120 days after it expires shall be retroactively reinstated to the date it expired
(6) (a) Except as provided in Subsection (5)(a) if not reinstated within two years the holder may obtain a license only if the holder meets requirements provided by the division by rule or by statute for a new license
(b) Each licensee under this title who has been active in the licensed occupation or profession while in the fullshytime employ of the United States government or under license to practice that occupation or profession in any other state or territory of the United States may reinstate the licensees license without taking an examination by submitting an application for reinstatement paying the current annual renewal fee and the reinstatement fee and submitting documentation showing completion of or compliance with any renewal qualifications at any time within six months after reestablishing domicile within Utah or terminating fullshytime government service
Amended by Chapter 238 2016 General Session
bull R156-1-308a Renewal Dates bull R156-1-308b Renewal Periods - Adjustment of Renewal Fees for an Extended or Shortened Renewal
Period bull R156-1-308c Renewal of Licensure Procedures bull R156-1-308d Waiver of Continuing Education Requirements - Renewal Requirements bull R156-1-308e Automatic Expiration of Licensure Upon Dissolution of Licensee
bull R156-1-308f Denial of Renewal of Licensure - Classification of Proceedings - Conditional Renewal of Licensure During Adjudicative Proceedings - Conditional Initial Renewal or Reinstatement Licensure During Audit or Investigation
bull R156-1-308g Reinstatement of Licensure which was Active and in Good Standing at the Time of Expiration of Licensure - Requirements
bull R156-1-308h Reinstatement of Restricted Suspended or Probationary Licensure During Term of Restriction Suspension or Probation - Requirements
bull R156-1-308i Reinstatement of Restricted Suspended or Probationary Licensure After the Specified Term of Suspension of the License or After the Expiration of Licensure in a Restricted Suspended or Probationary Status - Requirements
bull R156-1-308j Relicensure Following Revocation of Licensure - Requirements bull R156-1-308k Relicensure Following Surrender of Licensure - Requirements bull R156-1-3081 Reinstatement of Licensure and Relicensure - Term of Licensure
Effective 5102016 58-1-308 Term of license -- Expiration of license -- Renewal of license -- Reinstatement of license __ Application procedures
(1) (a) Each license issued under this title shall be issued in accordance with a two-year renewal cycle established by rule
(b) A renewal period may be extended or shortened by as much as one year to maintain established renewal cycles or to change an established renewal cycle
(2) (a) The expiration date of a license shall be shown on the license
(b) A license that is not renewed prior to the expiration date shown on the license automatically expires
(c) A license automatically expires prior to the expiration date shown on the license upon the death of a licensee who is a natural person or upon the dissolution of a licensee that is a partnership corporation or other business entity
(d) If the existence of a dissolved partnership corporation or other business entity is reinstated prior to the expiration date shown upon the entitys expired license issued by the division the division shall upon written application reinstate the applicants license unless it flnds that the applicant no longer meets the qualifications for licensure
(e) Expiration of licensure is not an adjudicative proceeding under Title 63G Chapter 4 Administrative Procedures Act
(3) (a) The division shall notify each licensee in accordance with procedures established by rule that the licensees license is due for renewal and that unless an application for renewal is received by the division by the expiration date shown on the license together with the appropriate renewal fee and documentation showing completion of or compliance with renewal qualiflcations the license will not be renewed
(b) Examples of renewal qualiflcations which by statute or rule the division may require the licensee to document completion of or compliance with include
(i) continuing education
(ii) continuing competency
(iii) quality assurance
(iv) utilization plan and protocol
(v) fInancial responsibility
(vi) certiflcation renewal and
(vii) calibration of equipment
(4) (a) (i) An application for renewal that complies with Subsection (3) is complete
(ii) A renewed license shall be issued to applicants who submit a complete application unless it is apparent to the division that the applicant no longer meets the qualiflcations for continued licensure
(b) (i) The division may evaluate or verify documentation showing completion of or compliance with renewal requirements on an entire population or a random sample basis and may be assisted by advisory peer committees
(ii) If necessary the division may complete its evaluation or veriflcation subsequent to renewal and if appropriate pursue action to suspend or revoke the license of a licensee who no longer meets the qualifications for continued licensure
(c) The application procedures specified in Subsection 58-1-301 (2) apply to renewal applications to the extent they are not in conflict with this section
(d) submit to a criminal backgrolUld check pursuant to Subsection 58-31 b-302(5) and Section R156-31 b-30 19 (3) ~ apph~anl who holds a current LPN license in an interstate compact state shall apply for a license within 90 days of
estabhshmg reSidency In Utah and complete all requirements pursuant to R 156-31 b-30 I a(2) (4) An applicant who has been licensed previously in Utah but whose license has expired or lapsed shall (a) if the applicant has not practiced as a nurse for up to five years document current compliance with the continuing competency
requirements as established in SubseL1ion R 156-31b-303(3)
(b) if the applicant has not practiced as a nurse for more than five years but less than eight years (I) pass the NCLEX-PN examination within 60 days following the date of application or (ii) successfully complete an approved re-entry program (c) if the applicant has not practiced as a nurse for more than eight years but less than 10 years (i) successfully complete an approved re-entry program and (ii) pass the NCLEX-PN examination within 60 days following the date of application or (d) if the applicant has not practiced as a nurse for 10 years or more comply with this Subsection (1) (5) An applicant who has been licensed in another state or country but whose license has expired or lapsed shall (a) comply with this Subsection (2)(b) and (b) comply with this Subsection (4) a~ applicable and (c) submit to a criminal background check pursuant to Subsection 58-31 b-302(5) and Section R156-31 b-30 I g
R156-31b-301b RN License- Education Examination and Experience Requirements (I) An applicant who has never obtained a license in any state or country shall (a) demonstrate that the applicant ha~ successfully completed an RN prelicensing education program that (i) meets the requirements of Section 58-31 b-60 I or (ii) is equivalent to an approved program under Section 58-3 I b-60 I (b) pass the NCLEX-RN examination purslant to Section R 156-31 b-30 I e and (c) submit to a climinal background check pursuant to Subsection 58-31 b-302(5) and Section R155-31 b-301g (2) An applicant who holds a current RN license issued by another country or state shall (a) demonstrate that the license issued by the other jurisdiction is current active and in good standing as of the date of
application (b)(i) demonstrate that the applicant has graduated from an RN prelicensing education program and (ii) if a foreign education program demonstrate that the program meets all requirements outlined in Section R 156-31 b-30 I d (c) pass the NCLEX-RN examination pursuant to Section RI56-31b-301e and (d) submit to a criminal backgrOlUld check pursuant to Subsection 58-31 b-302(5) and Sel-1ion R156-31 b-30 I g (3) An applicant who holds a current RN license in an interstate compact state shall apply for a license within 90 days of
establishing residency in Utah and complete all requirements pursuant to R156-31 b-301 b(2) (4) An applicant who has been licensed previously in Utah but whose license has expired or lapsed shall (a) if the applicant has not practiced as a nurse for up to five years document current compliance with the continuing competency
requirements as established in Subsection R 156-31 b-303(3) (b) if the applicant has not practiced as a nurse for more than five years but less than eight years (i) pass the NCLEX-RN examination within 60 days following the date of application or (ii) successfully complete an approved re-entry program (c) if the applicant has not practiced as a nurse for more than eight years but less than 10 years (i) successfully complete an approved re-entry program and (ii) pass theNCLEX-RN examination within 60 days following the date of application or (d) if the applicant ha~ not practiced as a nurse for 0 years or more comply with this Subsection (1) (5) An applicant who has been licensed in another state or country but whose license ha~ expired or lapsed shall (a) comply with this Subsection (2)(b) (b) comply with this Subsection (4) as applicable and (c) submit to a criminal background check pursuant to Subsection 58-31 b-302(5) and Section R156-3l b-30 I g
R156-31b-301c APRN Lkense - Education Examination and Experience Requirements (I) An applicant who is 110t currently and validly licensed alt an APRN in any state or country shall (a) demonstrate that the applicant holds a current active RN license in good standing (b) demonstrate that the applicant has successfully completelt an APRN prelicensing education program that meets the
requirements of Subsection 58-31b-601(1) and Subsection 58-31b-302(4)(e) (c) pass a national certitication examination consistent with the applicants educational specialty pursuant to Section R156-31 bshy
30 I e and administered by one of the following credentialing bodies (i) the American Nurses Credentialing Center Certification
4
PHARMACIST COLLABORATIVE PRACTICE AGREEMENTS
KEY ELEMENTS FOR LEGISLATIVE AND REGULATORY AUTHO RITY
A REPORT OF THE COLLABORATIVE PRACTICE WORKGROUP
CONVENED BY THE NATIONAL ALLIANCE OF STATE PHARMACY ASSOCIATIONS
PHARMACIST COLLABORATIVE PRACTICE AGREEMENTS KEY ELEMENTS FOR LEGISLATIVE AND REGULATORY AUTHORITY
PROJE CT OVERVIEW
Collaborative practice agreements (CPAs) create a formal practice relationship between pharmacists and other health care practitioners whereby the pharmacist assumes responsibility for specific patient care functions that are otherwise beyond their typical scope of practice but aligned with their education and training These patient care services can include initiation and modification of drug therapy The extent of the services authorized under the collaborative agreement depends on the states statutory and regulatory provisions for collaborative practice authority as well as the terms of the specific agreement between the pharmacist and other health care practitioners
State laws and regulations authorizing CPAs are highly variable Some states specify the practitioners
able to participate in CPAs restrict the services that may be provided under a CPA or include extensive
logistical barriers that limit the utility of such agreements
In their 2015 paper The Expanding Role of Pharmacists in a Transformed Health Care System the
National Governors Association (NGA) presented the following state policy considerations in regards to
collaborative practice provisions
bull Enact broad collaborative practice provisions that allow for specific provider functions to be
determined at the provider level rather than set in state statute or through regulation
bull Evaluate practice setting and drug therapy restrictions to determine whether pharmacists and
providers face disincentives that unnecessarily discourage collaborative arrangements
bull Examine whether CPAs unnecessarily dictate disease or patient specificity1
The National Alliance of State Pharmacy Associations (NASPAs) Executive Committee directed staff to
convene a workgroup to build upon the NGA recommendations with additional specificity The workgroup was charged with examining existing state CPA laws and regulations The workgroup was
tasked with developing recommendations for what elements of collaborative practice authority should
appropriately be defined under state law andor regulation and what elements are best left to be
determined between pharmacists and other practitioners when developing their specific collaborative
practice arrangement Using a modified Delphi method the Collaborative Practice Workgroup conducted
this work with two key questions in mind
bull Is this recommendation in the best interest of the patient receiving care under a collaborative
agreement
bull Is this recommendation aligned with pharmacists education and tra ining
The following is a report of the workgroups recommendations
1 Nati onal Governors Association The Expanding Role of Pharmacists in a Transformed Health Care System
JQ lwwwngaorgfiles llvesitesNGAfil esp d(20151 SOl Th eExp a ncli ngHo l e(jfPh~ rm cists~ Accessed
61515 1
WORKGROUP RECOMMENDATIONS The workgroup took the approach that rapid innovation in education training technology and evidence-based gUidelines necessitate a collaborative practice framework that is flexible and facilitates innovation in care delivery Thus the following statements include two levels of recommendations
1 Elements of collaborative practice authority that should be codified in state law andor state regulations and
2 Elements that are more appropriately determined by the parties at the practice level who voluntarily enter into a CPA and thus for which the laws and regulations should be silent
The workgroup views both levels of recommendations as needed and synergistic State law andor regulations if too restrictive can impede innovative team-based care models
COLLABORATIVE PRACTICE AGREEMENT PARTICIPANTS
RECOMMENDED ELEMENTS FOR INCLUSION IN STATE LAWS ANDOR REGULATIONS
Collaborative practice laws andor regulations should specify that
bull Any practitioner with prescriptive authority may collaborate with pharmacists using a CPA
bull CPAs may be between a single or multiple pharmacists and a single or multiple prescribers
bull CPAs may apply to a single patient multiple patients or patient populations as specified in the agreement
ELEMENTS THAT MAY BE DETERMINED AT THE PRACTITIONER LEVEL
Individual CPAs may address the below elements but state laws andor regulations should be silent
bull CPAs should specify which patient(s) andor patient population(s) can receive services under the agreement
bull Depending on the complexity of the services being provided under a CPA it may be appropriate for the pharmacist to have additional credentials or training beyond what is required for licensure
bull CPAs should specify which pharmacist(s) may provide services under the CPA A pharmacists practice setting should not be a barrier to their ability to enter into a CPA
COLLABORATIVE PRACTICE AGREEIVlEIlT AUTHORIZED SERVICES
RECOMMENDED ELEMENTS FOR INCLUSION IN STATE LAWS ANDOR REGULATIONS
Collaborative practice laws andor regulations should specify that
bull The initiation and modification of drug therapy may be authorized under a CPA with a prescriber
ELEMENTS THAT MAY BE DETERMINED AT THE PRACTITIONER LEVEL
Individual CPAs may address the below elements but state laws andor regulations should be silent
bull In some situations the use of an evidence-based protocol can ensure optimal care when pharmacists are initiating or modifying drug therapy and may be included in the CPA though they may not be needed or appropriate in others
2
bull Performing physical assessments as well as ordering performing or interpreting laboratory tests (eg CLiA-waived tests) may be included in a CPA to help identify or refer patients for services however a pharmacist may also perform these services without a CPA as these activities should fall within pharmacists standard scope of practice
bull Specific disease states may be included in a CPA at the participating practitioners discretion
COLLABORATIVE PRACTICE AGREEMENT REQUIREMENTS AND RESTRICTIONS
RECOMMENDED ELEMENTS FOR INCLUSION IN STATE LAWS ANDOR REGULATIONS
Collaborative practice laws andor regulations should specify that
bull All prescription drugs including controlled substances may be included within pharmacists collaborative practice authority
bull CPAs should be maintained by the pharmacist(s) and collaborating prescriber(s) and be available upon request or inspection
ELEMENTS THAT MAY BE DETERMINED AT THE PRACTITIONER LEVEL
Individual CPAs may address the below elements but state laws andor regulations should be silent
bull Pharmacist(s) and prescriber(s) may specify the level of patient involvement in the CPA Depending on the level of service elements such as informed consent written consent or optshyout provisions may be appropriate as determined by the parties to the agreement
bull Agreements should not be required to be sent to or approved by a state regulatory board or other agency such requirements create unnecessary paperwork burden and slow the efficiency of care delivery
bull Collaborating practitioners are encouraged to review andor renew their CPAs within a timeframe that is clinically appropriate
bull Collaborating practitioners should conform to evidence-based guidelines and the agreed upon process of care with regards to the documentation requirements and the collaborating practitioners responsibility for review of services provided under the agreement
bull Practitioners may consider liability insurance provisions and the appropriateness of articulating these in their voluntary agreement
bull It is the professional duty of all healthcare professionals to stay current in the clinical areas in which they practice If individual practitioners determine that continuing education requirements are appropriate for their clinical arrangement they may be specified in the agreement
3
APPENDIX A WORKGROUP PARTICIPANTS
The individuals listed below were appointed to participate in the Collaborative Practice Workgroup by one of two methods NASPA invited all Joint Commission of Pharmacy Practitioners (JCPP) member organizations CEOs to appoint a representative from their organization to participate (an invitation for an appointment was also extended to the National Association of Chain Drug Stores who currently is not a member of JCPP) It was recommended that professional affairs staff be considered for this work
State representatives were nominated by state pharmacy associations and appointed by the NASPA Executive Committee The selection process was intended to produce a group of participants who had experience with CPAs and practice in a variety of settings
Of note participants were only asked to represent their own opinions Participants from the national pharmacy associations were not acting as representatives of their organizations but rather as individuals whose experiences with their various memberships provide them with an informed perspective
Name StateNationa I OrganizationState
Alex Adams National NACDS
Jennifer Bacci State Pennsylvania
Lynette Bradley-Baker National AACP
Anne Burns National APhA
Carolyn Ha National NCPA
Julie Johnson State Minnesota
Sandra Leal State Arizona
Christine Lee-Wilson State Maryland
Dianne Miller State Michigan
Susan Oh National AMCP
Anthony Pudlo State Iowa
Kelly Ridgway State Arizona
Scotti Russell National NABP
Douglas Scheckel hoff National ASHP
L Michelle Vaughn State Alaska
Pete Vlasses National ACPE
Ed Webb National ACCP
Bryan Ziegler State South Carolina
4
APPENDIX B MODIFIED DELPHI METHOD
The goal of the workgroup was to reach consensus on each of the elements discussed To do this a modified Delphi method was used A survey was sent to all participants to collect their initial thoughts on each of the elements identified in currently existing collaborative practice authority laws andor regulations Participants were given the current variations of each element as a multiple-choice selection with the opportunity to answer in free form text if the desired option was not listed After completion of the survey the workgroup discussed all questions where consensus was not already reached via conference call The conference call discussions were structured to have a defined period of time for discussion followed by a summary of the current options being discussed and a roll call vote by each of the participants The item was included on the next survey if consensus was not reached on the conference call This process was repeated a total of three times before the group reached consensus on all items being considered See below for a diagram of the process used
1 Level-setting conference call
2 Distribution of survey with 3 weeks to complete
5
Task Force on
Collaborative Practice Agreements
Members Present
Sharlea M Leatherwood Chair (MO) Michael J Ayotte (VA) Winifred A Landis (IN) Jeffery Lindoo (NIN) C Ann Perry (GA) Charles R Young (MA)
Others Present
Joseph A Whaley Jr Executive Committee Liaison Carmen A Catizone NABP Executive DirectorSecretary Melissa Madigan NABP Staff
Introduction
The Task Force on Collaborative Practice Agreements (TFCPA) met December 3 1998 at the Marriott Suites Hotel in Rosemont Illinois The Task Force was established by the NABP Executive Corrunittee in response to recent increases in the number of prescribers and pharmacists entering into collaborative practice agreements and requests that boards of pharmacy define the elements of an appropriate collaborative practice agreement
Charge of the Task Force on Collaborative Practice Agreements
Task Force members reviewed their charge and proposing no changes accepted it as follows
Review the available literature and information pertaining to collaborative practice agreements between prescribers and pharmacists
bull Develop national model guidelines for writing and implementing uniform collaborative practice agreements that can be utilized by the state boards of pharmacy
TFCPA Recommendation 1
The Task Force on Collaborative Practice Agreements recorrunends to the Executive Committee and the Corrunittee on Law EnforcementLegislation that the following language be adopted for incorporation into the NABP Model State Pharmacy Act and Model Rules
Article I
Title Purpose Definition
Section 105 Definitions
(g) Collaborative Pharmacy Practice is that Practice of Pharmacy whereby a one or more Pharmacist~ has jointly agreed on a voluntary basis to work in conjunction with one or more Practitioners under protocol whereby the Pharmacist may perform certain patient care functions authorized by the Practitioner or Practitioners under certain specified conditions andor limitations
NATIONAL ASSOCIA nON OF BOARDS OF PHARMACYmiddot (P) 847391-4406 bull (F) 847391-4502 bull wwwnabpnel
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
(h) Collaborative Pharmacy Practice Agreement is a written and signed agreement between one or more Pharmacists and one or more Practitioners that provides for Collaborative Pharmacy Practice for the purpose of Drug Therapy Management of patients
(r) Drug Therapy Management means the review of Drug therapy regimen(s) of patients by one or more Pharmacists for the purpose of evaluating and rendering advice to one or more Practitioners regarding adjustment of the regimen Decisions involving Drug Therapy Management shall be made in the best interest of the patient Drug Therapy Management may include
(1) Implementing modifying and managing Drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement
(2) Collecting and reviewing patient Drug histories (3) Obtaining and checking vital signs including pulse temperature blood pressure
and respiration (4) Ordering and evaluating the results of laboratory tests directly relating to Drug
therapy when performed in accordance with approved protocols applicable to the practice setting and
(5) Such other patient care services as may be allowed by law
Pharmacists Scope of Practice Pursuant to the Collaborative Pharmacy Practice Agreement means those duties and limitations of duties placed upon one or more Pharmacists by the collaborating Practitioner or Practitioners the Board and applicable law and includes the limitations implied by the specialty practiced by the collaborating Practitioner or Practitioners
Model Rules for Pharmaceutical Care
Section 3 Pharmacy Practice
Collaborative Pharmacy Practice
(1) Collaborative Pharmacy Practice Agreement A Pharmacist planning to engage in Collaborative Pharmacy Practice shall have on file at his or her place of practice the written Collaborative Pharmacy Practice Agreement The existence and termination of such Agreement shall be reported to the Board and such Agreements shall be made available to the Board for review upon request The Agreement may allow the Pharmacist within the Pharmacists Scope of Practice Pursuant to the Collaborative Pharmacy Practice Agreement to conduct Drug Therapy Management activities approved by the Practitioner The collaboration that the Practitioner agrees to conduct with the Pharmacist must be within the scope of the Practitioners current practice Patients who receive services from one or more collaborating Pharmacists shall receive notification of receipt of such services
(2) Contents The Collaborative Pharmacy Practice Agreement shall include
(a) Identification of the Practitioner(s) and Pharmacist(s) who are parties to the Agreement
(b) The types of Drug Therapy Management decisions that the Pharmacist is allowed to make which may include
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpnet 2
Report of the Task Force on Collaborative Practice Agreements - NABP 9Smiddoth Annual Meeting Proceedings
(i) A detailed description of the types of diseases Drugs or Drug categories involved and the type of Drug Therapy Management allowed in each case
(ii) A detailed description of the methods procedures decision criteria and plan the Pharmacist is to follow when conducting Drug Therapy ~anagement and
(iii) A detailed description of the activities the Pharmacist is to follow in the course of conducting Drug Therapy Management including documentation of decisions made and a plan or appropriate mechanism for conununication feedback and reporting to the Practitioner concerning specific decisions made In addition to the Agreement documentation shall occur on the prescription record patient profile a separate log book or in some other appropriate system
(c) A method for the Practitioner to monitor compliance with the Agreement and clinical outcomes where Drug Therapy Management by the Pharmacist has occurred and to intercede where necessary
(d) A provision that allows the Practitioner to override a collaborative practice decision mad by the Pharmacist whenever he or she deems it necessary or appropriate
(e) A provision that allows either party to cancel the agreement by written notification
(f) An effective date and (g) Signatures of all collaborating Pharmacists and Practitioners who are party to the
agreement as well as the date of signing Amendments to a Collaborative Pharmacy Practice Agreement must be documented signed and dated
(3) Initiation of the Collaborative Pharmacy Practice Agreement The Collaborative Pharmacy Practice Agreement must be coupled with a medical order from the Practitioner to initiate Drug Therapy Management for any particular patient
(4) Documentation of Drug Therapy Management Docwnentation of Drug Therapy ~anagement must be kept as part of the patients permanent record and be readily available to other health care professionals providing care to that patient and who are authorized to received it Docwnentation of drug Therapy Management shall be considered Confidential Information
(5) Review At a minimum the written agreement shall be reviewed and renewed and if necessary revised every year
[NOTE the above new subsections would be inserted with sequential relettering of all subsequent subsections]
Background
Task Force members listened to a brief presentation by American College of Clinical Pharmacy (ACCP) Executive Director Robert Elenbaas and Immediate Past President Jerry Bauman regarding ACCPs position on collaborative practice agreements and later discussed their charge to develop national guidelines for writing and implementing uniform collaborative practice agreements for use by state boards of pharmacy They agreed that the best way to fulfill their charge was to create model regulations addressing the issue
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI3
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sb Annual Meeting Proceedings
To assist in developing the model regulations members looked to several sources of information including current state pharmacy laws and regulations as well as the information provided by Drs Elenbaas and Bauman The Task Force felt that the Idaho State Board of Pharmacys regulations on collaborative practice encompassed much of what they wanted to include in the model regulation therefore they were used as a blueprint
A brainstorming session brought issues and concerns regarding state regulation of collaborative pharmacy practice to the forefront Topics that members felt needed to be addressed in a model regulation included
Contents of collaborative pharmacy practice agreements
Practitioner override authority
Board approval of agreements
Phannacists documentation of care
Patient consentnotification
Confidentiality of patient information
Periodic review of agreement
Phannacist competencequalifications to provide drug therapy management services and
Quality assurance review of practice
Task Force members concurred on a majority of these topics and incorporated their determinations into the model recommendation Members agreed that certain key elements should be included in collaborative phannacy practice agreements so that such agreements may guide both the pharmacists and collaborating practitioners decisions and conduct Key elements incorporated included a description of the pharmacists activities a method describing a practitioner monitoring methods and a practitioner override clause
Members also agreed that it should not be necessary for boards of pharmacy to take on the responsibility for approving agreements or even requiring their submission but that boards should require parties to notify them of the existence of such agreements and to keep a copy at the practice site On this issue members concluded that the individual practitioners were the persons most qualified to approve the agreements noting that minimal government involvement in the practice of the phannacist and collaborating practitioner was the most desirable In order for the board to have some handle on the situation however it was determined that agreements should be made available for board inspection upon request Regarding he issue of pharmacist competence to provide drug therapy management services Task Force members concluded that the subject should not be addressed in the model regulations They agreed that he determination of competence should be left up to the individuals who are party to the agreement Members felt certain that most if not al1 collaborating practitioners would only delegate certain aspects of their patient care authority upon demonstration by the pharmacist of his or her abilities to provide such care Further members felt that the majority ofphannacists would not take on the responsibility for providing such care without being competent to do so
TFCP A Recommendation 2
The Task Force on Collaborative Practice Agreements recognized that the issue of quality care assurance is not still developing and at present cannot be addressed with regard to collaborative pharmacy practice The Task Force recommends that the NABP Executive Committee closely
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI4
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
follow this issue and should it be determined that language addressing the subject should be incorporated into the model regulation take further action at that time
Background
Task Force members were uncertain at this time of how to address the issue of quality care assurance Members noted that most states addressing collaborative pharmacy practice in their laws or regulations did not include a provision on this subject It was noted however that tat least one state placed the responsibility for quality assurance on the Principal pharmacist and practitioner and that ACCP urged that an appropriate body be responsible for such activities
Member postulated that collaborative practice activities would likely be subject to quality assurance review via physician peer-review processes Additionally they suggested that those parties practicing in institutional settings would follow the quality assurance policies guidelines andor requirements of the institution The Task Force felt it was premature to make a recommendation regarding those parties who practice in ambulatory settings and who may not be subject to quality assurance review Members observed that the work ofNABPs Task Force on Patient Outcomes Regulation should be reviewed before a recommendation was made
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406 (F) 847391-4502middot wwwnabpnet 5
58-1-107 Applicability -- Relationship to specific chapters under title The provisions of this chapter LJniformly apply to the administration and enforcement of this title However
unless expressly prohibited in this chapter any provision of this chapter may be supplemented or altered by specific chapters of this title
R1S6-1-107 Organization of Rules - Content Applicability and Relationship of Rules
(1) The rules and sections in Title R156 shall to the extent practicable follow the numbering and
organizational scheme of the chapters in Title 58
(2) Rule R156-1 shall contain general provisions applicable to the administration and enforcement of all
occupations and profeSSions regulated in Title 58
(3) The provisions of the other rules in Title R156 shall contain specific or unique provisions applicable to
particular occupations or professions
(4) Specific rules in Title R156 may supplement or alter Rule R156-1 unless expressly provided otherwise
in Rule R156-1
R1S6-17b-l04 Organization - Relationship to Rule R1S6-1
The organization of this rule and its relationship to Rule R156-1 is as described in Section R156-1-107
58-17b-50G Petitioning for reinstatement of licensure Any person whose license to practice pharmacy in this state has been revoked suspended or surrendered
voluntarily or by action of the division shall have the right at reasonable intervals to petition the division for reinstatement of such license Such petition shall be made in writing and in the form prescribed by the division Upon investigation and hearing the division may in its discretion grant or deny such petition or it may modify its driginal finding to reflect any circumstances that have changed sufficiently to warrant such modifications Themiddotdivision also at its discretion may require such person to pass an examination or examinations for reshyentry into the practice of pharmacy Enacted by Chapter 280 2004 General Session
Agenda Board of Pharmacy
Collaborative Practice Agreement Discussion 100 PM - 200 PM
-~ Agenda Item Overview - I
Welcome and Introductions Paige Patterick and Carrie Dunford
Discuss the wording of 58-17b-l 02( 17) (17) Collaborative pharmacy practice agreement means a written and signed agreement between one or more pharmacists and one or more practitioners that provides for collaborative pharmacy practice for the purpose of drug therapy management of patients and prevention of disease of human subjects
Discuss the definition of Drug Therapy Management in R 156-17b-l 02 (19)
(J 9) Drug therapy management means the review of a drug therapy regimen of a patient by one or more pharmacists for the purpose of evaluating and rendering advice to one or more practitioners regarding adjustment of the regimen
Resources
NABP https llnabppharmacywp-contentuploads20 1607 ITFCollaborativePracticeAgreements AM95 Dec 1998 pdf
Operating Standards Drug therapy management R 156-1 7b-61 I
R 156-l7b-611 Operating Standards - Drug Therapy Management (I) In accordance with Subsections 58-17b-l 02( 17) and 58-l7b-60 I( I) decisions involving drug therapy management shall be made in the best interest of the patient Drug therapy management may include (a) implementing modifying and managing drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement (b) collecting and reviewing patient histories (c) obtaining and checking vital signs including pulse temperature blood pressure and respiration (d) ordering and evaluating the results of laboratory tests directly applicable to the drug therapy when perfolmed in accordance with approved protocols applicable to the practice setting and (e) such other patient care services as may be allowed by rule (2) For the purpose of promoting therapeutic appropriateness a pharmacist shall at the time of dispensing a prescription or a prescription drug order review the patients medication record Such review shall at a minimum identify clinically significant conditions situations or items such as (a) inappropriate drug utilization (b) therapeutic duplication (c) drug-disease contraindications (d) drug-drug interactions (e) incorrect drug dosage or duration of drug treatment (f) drug-allergy interactions and (g) clinical abuse or misuse (3) Upon identifying any clinically significant conditions situations or items listed in Subsection (2) above the pharmacist shall take appropriate steps to avoid or resolve the problem including consultation with the prescribing practitioner
Note In compliance with the Americans with Disabilities Act individuals needing special accommodations (including auxiliary communicative aids and services) during this meet ing should not ify Dave Taylor ADA Coordinator at least three working days prior to the meeting Division of Occupational amp Professional Licensing 160 East 300 South Salt Lake City Utah 84115 801-530-6628 or toll-free in Utah only 866-275-3675 Posted to Bulletin Board on 11-21-2017

Effective 5102016 58-1-308 Term of license -- Expiration of license -- Renewal of license -- Reinstatement of license __ Application procedures
(1) (a) Each license issued under this title shall be issued in accordance with a two-year renewal cycle established by rule
(b) A renewal period may be extended or shortened by as much as one year to maintain established renewal cycles or to change an established renewal cycle
(2) (a) The expiration date of a license shall be shown on the license
(b) A license that is not renewed prior to the expiration date shown on the license automatically expires
(c) A license automatically expires prior to the expiration date shown on the license upon the death of a licensee who is a natural person or upon the dissolution of a licensee that is a partnership corporation or other business entity
(d) If the existence of a dissolved partnership corporation or other business entity is reinstated prior to the expiration date shown upon the entitys expired license issued by the division the division shall upon written application reinstate the applicants license unless it flnds that the applicant no longer meets the qualifications for licensure
(e) Expiration of licensure is not an adjudicative proceeding under Title 63G Chapter 4 Administrative Procedures Act
(3) (a) The division shall notify each licensee in accordance with procedures established by rule that the licensees license is due for renewal and that unless an application for renewal is received by the division by the expiration date shown on the license together with the appropriate renewal fee and documentation showing completion of or compliance with renewal qualiflcations the license will not be renewed
(b) Examples of renewal qualiflcations which by statute or rule the division may require the licensee to document completion of or compliance with include
(i) continuing education
(ii) continuing competency
(iii) quality assurance
(iv) utilization plan and protocol
(v) fInancial responsibility
(vi) certiflcation renewal and
(vii) calibration of equipment
(4) (a) (i) An application for renewal that complies with Subsection (3) is complete
(ii) A renewed license shall be issued to applicants who submit a complete application unless it is apparent to the division that the applicant no longer meets the qualiflcations for continued licensure
(b) (i) The division may evaluate or verify documentation showing completion of or compliance with renewal requirements on an entire population or a random sample basis and may be assisted by advisory peer committees
(ii) If necessary the division may complete its evaluation or veriflcation subsequent to renewal and if appropriate pursue action to suspend or revoke the license of a licensee who no longer meets the qualifications for continued licensure
(c) The application procedures specified in Subsection 58-1-301 (2) apply to renewal applications to the extent they are not in conflict with this section
(d) submit to a criminal backgrolUld check pursuant to Subsection 58-31 b-302(5) and Section R156-31 b-30 19 (3) ~ apph~anl who holds a current LPN license in an interstate compact state shall apply for a license within 90 days of
estabhshmg reSidency In Utah and complete all requirements pursuant to R 156-31 b-30 I a(2) (4) An applicant who has been licensed previously in Utah but whose license has expired or lapsed shall (a) if the applicant has not practiced as a nurse for up to five years document current compliance with the continuing competency
requirements as established in SubseL1ion R 156-31b-303(3)
(b) if the applicant has not practiced as a nurse for more than five years but less than eight years (I) pass the NCLEX-PN examination within 60 days following the date of application or (ii) successfully complete an approved re-entry program (c) if the applicant has not practiced as a nurse for more than eight years but less than 10 years (i) successfully complete an approved re-entry program and (ii) pass the NCLEX-PN examination within 60 days following the date of application or (d) if the applicant has not practiced as a nurse for 10 years or more comply with this Subsection (1) (5) An applicant who has been licensed in another state or country but whose license has expired or lapsed shall (a) comply with this Subsection (2)(b) and (b) comply with this Subsection (4) a~ applicable and (c) submit to a criminal background check pursuant to Subsection 58-31 b-302(5) and Section R156-31 b-30 I g
R156-31b-301b RN License- Education Examination and Experience Requirements (I) An applicant who has never obtained a license in any state or country shall (a) demonstrate that the applicant ha~ successfully completed an RN prelicensing education program that (i) meets the requirements of Section 58-31 b-60 I or (ii) is equivalent to an approved program under Section 58-3 I b-60 I (b) pass the NCLEX-RN examination purslant to Section R 156-31 b-30 I e and (c) submit to a climinal background check pursuant to Subsection 58-31 b-302(5) and Section R155-31 b-301g (2) An applicant who holds a current RN license issued by another country or state shall (a) demonstrate that the license issued by the other jurisdiction is current active and in good standing as of the date of
application (b)(i) demonstrate that the applicant has graduated from an RN prelicensing education program and (ii) if a foreign education program demonstrate that the program meets all requirements outlined in Section R 156-31 b-30 I d (c) pass the NCLEX-RN examination pursuant to Section RI56-31b-301e and (d) submit to a criminal backgrOlUld check pursuant to Subsection 58-31 b-302(5) and Sel-1ion R156-31 b-30 I g (3) An applicant who holds a current RN license in an interstate compact state shall apply for a license within 90 days of
establishing residency in Utah and complete all requirements pursuant to R156-31 b-301 b(2) (4) An applicant who has been licensed previously in Utah but whose license has expired or lapsed shall (a) if the applicant has not practiced as a nurse for up to five years document current compliance with the continuing competency
requirements as established in Subsection R 156-31 b-303(3) (b) if the applicant has not practiced as a nurse for more than five years but less than eight years (i) pass the NCLEX-RN examination within 60 days following the date of application or (ii) successfully complete an approved re-entry program (c) if the applicant has not practiced as a nurse for more than eight years but less than 10 years (i) successfully complete an approved re-entry program and (ii) pass theNCLEX-RN examination within 60 days following the date of application or (d) if the applicant ha~ not practiced as a nurse for 0 years or more comply with this Subsection (1) (5) An applicant who has been licensed in another state or country but whose license ha~ expired or lapsed shall (a) comply with this Subsection (2)(b) (b) comply with this Subsection (4) as applicable and (c) submit to a criminal background check pursuant to Subsection 58-31 b-302(5) and Section R156-3l b-30 I g
R156-31b-301c APRN Lkense - Education Examination and Experience Requirements (I) An applicant who is 110t currently and validly licensed alt an APRN in any state or country shall (a) demonstrate that the applicant holds a current active RN license in good standing (b) demonstrate that the applicant has successfully completelt an APRN prelicensing education program that meets the
requirements of Subsection 58-31b-601(1) and Subsection 58-31b-302(4)(e) (c) pass a national certitication examination consistent with the applicants educational specialty pursuant to Section R156-31 bshy
30 I e and administered by one of the following credentialing bodies (i) the American Nurses Credentialing Center Certification
4
PHARMACIST COLLABORATIVE PRACTICE AGREEMENTS
KEY ELEMENTS FOR LEGISLATIVE AND REGULATORY AUTHO RITY
A REPORT OF THE COLLABORATIVE PRACTICE WORKGROUP
CONVENED BY THE NATIONAL ALLIANCE OF STATE PHARMACY ASSOCIATIONS
PHARMACIST COLLABORATIVE PRACTICE AGREEMENTS KEY ELEMENTS FOR LEGISLATIVE AND REGULATORY AUTHORITY
PROJE CT OVERVIEW
Collaborative practice agreements (CPAs) create a formal practice relationship between pharmacists and other health care practitioners whereby the pharmacist assumes responsibility for specific patient care functions that are otherwise beyond their typical scope of practice but aligned with their education and training These patient care services can include initiation and modification of drug therapy The extent of the services authorized under the collaborative agreement depends on the states statutory and regulatory provisions for collaborative practice authority as well as the terms of the specific agreement between the pharmacist and other health care practitioners
State laws and regulations authorizing CPAs are highly variable Some states specify the practitioners
able to participate in CPAs restrict the services that may be provided under a CPA or include extensive
logistical barriers that limit the utility of such agreements
In their 2015 paper The Expanding Role of Pharmacists in a Transformed Health Care System the
National Governors Association (NGA) presented the following state policy considerations in regards to
collaborative practice provisions
bull Enact broad collaborative practice provisions that allow for specific provider functions to be
determined at the provider level rather than set in state statute or through regulation
bull Evaluate practice setting and drug therapy restrictions to determine whether pharmacists and
providers face disincentives that unnecessarily discourage collaborative arrangements
bull Examine whether CPAs unnecessarily dictate disease or patient specificity1
The National Alliance of State Pharmacy Associations (NASPAs) Executive Committee directed staff to
convene a workgroup to build upon the NGA recommendations with additional specificity The workgroup was charged with examining existing state CPA laws and regulations The workgroup was
tasked with developing recommendations for what elements of collaborative practice authority should
appropriately be defined under state law andor regulation and what elements are best left to be
determined between pharmacists and other practitioners when developing their specific collaborative
practice arrangement Using a modified Delphi method the Collaborative Practice Workgroup conducted
this work with two key questions in mind
bull Is this recommendation in the best interest of the patient receiving care under a collaborative
agreement
bull Is this recommendation aligned with pharmacists education and tra ining
The following is a report of the workgroups recommendations
1 Nati onal Governors Association The Expanding Role of Pharmacists in a Transformed Health Care System
JQ lwwwngaorgfiles llvesitesNGAfil esp d(20151 SOl Th eExp a ncli ngHo l e(jfPh~ rm cists~ Accessed
61515 1
WORKGROUP RECOMMENDATIONS The workgroup took the approach that rapid innovation in education training technology and evidence-based gUidelines necessitate a collaborative practice framework that is flexible and facilitates innovation in care delivery Thus the following statements include two levels of recommendations
1 Elements of collaborative practice authority that should be codified in state law andor state regulations and
2 Elements that are more appropriately determined by the parties at the practice level who voluntarily enter into a CPA and thus for which the laws and regulations should be silent
The workgroup views both levels of recommendations as needed and synergistic State law andor regulations if too restrictive can impede innovative team-based care models
COLLABORATIVE PRACTICE AGREEMENT PARTICIPANTS
RECOMMENDED ELEMENTS FOR INCLUSION IN STATE LAWS ANDOR REGULATIONS
Collaborative practice laws andor regulations should specify that
bull Any practitioner with prescriptive authority may collaborate with pharmacists using a CPA
bull CPAs may be between a single or multiple pharmacists and a single or multiple prescribers
bull CPAs may apply to a single patient multiple patients or patient populations as specified in the agreement
ELEMENTS THAT MAY BE DETERMINED AT THE PRACTITIONER LEVEL
Individual CPAs may address the below elements but state laws andor regulations should be silent
bull CPAs should specify which patient(s) andor patient population(s) can receive services under the agreement
bull Depending on the complexity of the services being provided under a CPA it may be appropriate for the pharmacist to have additional credentials or training beyond what is required for licensure
bull CPAs should specify which pharmacist(s) may provide services under the CPA A pharmacists practice setting should not be a barrier to their ability to enter into a CPA
COLLABORATIVE PRACTICE AGREEIVlEIlT AUTHORIZED SERVICES
RECOMMENDED ELEMENTS FOR INCLUSION IN STATE LAWS ANDOR REGULATIONS
Collaborative practice laws andor regulations should specify that
bull The initiation and modification of drug therapy may be authorized under a CPA with a prescriber
ELEMENTS THAT MAY BE DETERMINED AT THE PRACTITIONER LEVEL
Individual CPAs may address the below elements but state laws andor regulations should be silent
bull In some situations the use of an evidence-based protocol can ensure optimal care when pharmacists are initiating or modifying drug therapy and may be included in the CPA though they may not be needed or appropriate in others
2
bull Performing physical assessments as well as ordering performing or interpreting laboratory tests (eg CLiA-waived tests) may be included in a CPA to help identify or refer patients for services however a pharmacist may also perform these services without a CPA as these activities should fall within pharmacists standard scope of practice
bull Specific disease states may be included in a CPA at the participating practitioners discretion
COLLABORATIVE PRACTICE AGREEMENT REQUIREMENTS AND RESTRICTIONS
RECOMMENDED ELEMENTS FOR INCLUSION IN STATE LAWS ANDOR REGULATIONS
Collaborative practice laws andor regulations should specify that
bull All prescription drugs including controlled substances may be included within pharmacists collaborative practice authority
bull CPAs should be maintained by the pharmacist(s) and collaborating prescriber(s) and be available upon request or inspection
ELEMENTS THAT MAY BE DETERMINED AT THE PRACTITIONER LEVEL
Individual CPAs may address the below elements but state laws andor regulations should be silent
bull Pharmacist(s) and prescriber(s) may specify the level of patient involvement in the CPA Depending on the level of service elements such as informed consent written consent or optshyout provisions may be appropriate as determined by the parties to the agreement
bull Agreements should not be required to be sent to or approved by a state regulatory board or other agency such requirements create unnecessary paperwork burden and slow the efficiency of care delivery
bull Collaborating practitioners are encouraged to review andor renew their CPAs within a timeframe that is clinically appropriate
bull Collaborating practitioners should conform to evidence-based guidelines and the agreed upon process of care with regards to the documentation requirements and the collaborating practitioners responsibility for review of services provided under the agreement
bull Practitioners may consider liability insurance provisions and the appropriateness of articulating these in their voluntary agreement
bull It is the professional duty of all healthcare professionals to stay current in the clinical areas in which they practice If individual practitioners determine that continuing education requirements are appropriate for their clinical arrangement they may be specified in the agreement
3
APPENDIX A WORKGROUP PARTICIPANTS
The individuals listed below were appointed to participate in the Collaborative Practice Workgroup by one of two methods NASPA invited all Joint Commission of Pharmacy Practitioners (JCPP) member organizations CEOs to appoint a representative from their organization to participate (an invitation for an appointment was also extended to the National Association of Chain Drug Stores who currently is not a member of JCPP) It was recommended that professional affairs staff be considered for this work
State representatives were nominated by state pharmacy associations and appointed by the NASPA Executive Committee The selection process was intended to produce a group of participants who had experience with CPAs and practice in a variety of settings
Of note participants were only asked to represent their own opinions Participants from the national pharmacy associations were not acting as representatives of their organizations but rather as individuals whose experiences with their various memberships provide them with an informed perspective
Name StateNationa I OrganizationState
Alex Adams National NACDS
Jennifer Bacci State Pennsylvania
Lynette Bradley-Baker National AACP
Anne Burns National APhA
Carolyn Ha National NCPA
Julie Johnson State Minnesota
Sandra Leal State Arizona
Christine Lee-Wilson State Maryland
Dianne Miller State Michigan
Susan Oh National AMCP
Anthony Pudlo State Iowa
Kelly Ridgway State Arizona
Scotti Russell National NABP
Douglas Scheckel hoff National ASHP
L Michelle Vaughn State Alaska
Pete Vlasses National ACPE
Ed Webb National ACCP
Bryan Ziegler State South Carolina
4
APPENDIX B MODIFIED DELPHI METHOD
The goal of the workgroup was to reach consensus on each of the elements discussed To do this a modified Delphi method was used A survey was sent to all participants to collect their initial thoughts on each of the elements identified in currently existing collaborative practice authority laws andor regulations Participants were given the current variations of each element as a multiple-choice selection with the opportunity to answer in free form text if the desired option was not listed After completion of the survey the workgroup discussed all questions where consensus was not already reached via conference call The conference call discussions were structured to have a defined period of time for discussion followed by a summary of the current options being discussed and a roll call vote by each of the participants The item was included on the next survey if consensus was not reached on the conference call This process was repeated a total of three times before the group reached consensus on all items being considered See below for a diagram of the process used
1 Level-setting conference call
2 Distribution of survey with 3 weeks to complete
5
Task Force on
Collaborative Practice Agreements
Members Present
Sharlea M Leatherwood Chair (MO) Michael J Ayotte (VA) Winifred A Landis (IN) Jeffery Lindoo (NIN) C Ann Perry (GA) Charles R Young (MA)
Others Present
Joseph A Whaley Jr Executive Committee Liaison Carmen A Catizone NABP Executive DirectorSecretary Melissa Madigan NABP Staff
Introduction
The Task Force on Collaborative Practice Agreements (TFCPA) met December 3 1998 at the Marriott Suites Hotel in Rosemont Illinois The Task Force was established by the NABP Executive Corrunittee in response to recent increases in the number of prescribers and pharmacists entering into collaborative practice agreements and requests that boards of pharmacy define the elements of an appropriate collaborative practice agreement
Charge of the Task Force on Collaborative Practice Agreements
Task Force members reviewed their charge and proposing no changes accepted it as follows
Review the available literature and information pertaining to collaborative practice agreements between prescribers and pharmacists
bull Develop national model guidelines for writing and implementing uniform collaborative practice agreements that can be utilized by the state boards of pharmacy
TFCPA Recommendation 1
The Task Force on Collaborative Practice Agreements recorrunends to the Executive Committee and the Corrunittee on Law EnforcementLegislation that the following language be adopted for incorporation into the NABP Model State Pharmacy Act and Model Rules
Article I
Title Purpose Definition
Section 105 Definitions
(g) Collaborative Pharmacy Practice is that Practice of Pharmacy whereby a one or more Pharmacist~ has jointly agreed on a voluntary basis to work in conjunction with one or more Practitioners under protocol whereby the Pharmacist may perform certain patient care functions authorized by the Practitioner or Practitioners under certain specified conditions andor limitations
NATIONAL ASSOCIA nON OF BOARDS OF PHARMACYmiddot (P) 847391-4406 bull (F) 847391-4502 bull wwwnabpnel
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
(h) Collaborative Pharmacy Practice Agreement is a written and signed agreement between one or more Pharmacists and one or more Practitioners that provides for Collaborative Pharmacy Practice for the purpose of Drug Therapy Management of patients
(r) Drug Therapy Management means the review of Drug therapy regimen(s) of patients by one or more Pharmacists for the purpose of evaluating and rendering advice to one or more Practitioners regarding adjustment of the regimen Decisions involving Drug Therapy Management shall be made in the best interest of the patient Drug Therapy Management may include
(1) Implementing modifying and managing Drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement
(2) Collecting and reviewing patient Drug histories (3) Obtaining and checking vital signs including pulse temperature blood pressure
and respiration (4) Ordering and evaluating the results of laboratory tests directly relating to Drug
therapy when performed in accordance with approved protocols applicable to the practice setting and
(5) Such other patient care services as may be allowed by law
Pharmacists Scope of Practice Pursuant to the Collaborative Pharmacy Practice Agreement means those duties and limitations of duties placed upon one or more Pharmacists by the collaborating Practitioner or Practitioners the Board and applicable law and includes the limitations implied by the specialty practiced by the collaborating Practitioner or Practitioners
Model Rules for Pharmaceutical Care
Section 3 Pharmacy Practice
Collaborative Pharmacy Practice
(1) Collaborative Pharmacy Practice Agreement A Pharmacist planning to engage in Collaborative Pharmacy Practice shall have on file at his or her place of practice the written Collaborative Pharmacy Practice Agreement The existence and termination of such Agreement shall be reported to the Board and such Agreements shall be made available to the Board for review upon request The Agreement may allow the Pharmacist within the Pharmacists Scope of Practice Pursuant to the Collaborative Pharmacy Practice Agreement to conduct Drug Therapy Management activities approved by the Practitioner The collaboration that the Practitioner agrees to conduct with the Pharmacist must be within the scope of the Practitioners current practice Patients who receive services from one or more collaborating Pharmacists shall receive notification of receipt of such services
(2) Contents The Collaborative Pharmacy Practice Agreement shall include
(a) Identification of the Practitioner(s) and Pharmacist(s) who are parties to the Agreement
(b) The types of Drug Therapy Management decisions that the Pharmacist is allowed to make which may include
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpnet 2
Report of the Task Force on Collaborative Practice Agreements - NABP 9Smiddoth Annual Meeting Proceedings
(i) A detailed description of the types of diseases Drugs or Drug categories involved and the type of Drug Therapy Management allowed in each case
(ii) A detailed description of the methods procedures decision criteria and plan the Pharmacist is to follow when conducting Drug Therapy ~anagement and
(iii) A detailed description of the activities the Pharmacist is to follow in the course of conducting Drug Therapy Management including documentation of decisions made and a plan or appropriate mechanism for conununication feedback and reporting to the Practitioner concerning specific decisions made In addition to the Agreement documentation shall occur on the prescription record patient profile a separate log book or in some other appropriate system
(c) A method for the Practitioner to monitor compliance with the Agreement and clinical outcomes where Drug Therapy Management by the Pharmacist has occurred and to intercede where necessary
(d) A provision that allows the Practitioner to override a collaborative practice decision mad by the Pharmacist whenever he or she deems it necessary or appropriate
(e) A provision that allows either party to cancel the agreement by written notification
(f) An effective date and (g) Signatures of all collaborating Pharmacists and Practitioners who are party to the
agreement as well as the date of signing Amendments to a Collaborative Pharmacy Practice Agreement must be documented signed and dated
(3) Initiation of the Collaborative Pharmacy Practice Agreement The Collaborative Pharmacy Practice Agreement must be coupled with a medical order from the Practitioner to initiate Drug Therapy Management for any particular patient
(4) Documentation of Drug Therapy Management Docwnentation of Drug Therapy ~anagement must be kept as part of the patients permanent record and be readily available to other health care professionals providing care to that patient and who are authorized to received it Docwnentation of drug Therapy Management shall be considered Confidential Information
(5) Review At a minimum the written agreement shall be reviewed and renewed and if necessary revised every year
[NOTE the above new subsections would be inserted with sequential relettering of all subsequent subsections]
Background
Task Force members listened to a brief presentation by American College of Clinical Pharmacy (ACCP) Executive Director Robert Elenbaas and Immediate Past President Jerry Bauman regarding ACCPs position on collaborative practice agreements and later discussed their charge to develop national guidelines for writing and implementing uniform collaborative practice agreements for use by state boards of pharmacy They agreed that the best way to fulfill their charge was to create model regulations addressing the issue
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI3
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sb Annual Meeting Proceedings
To assist in developing the model regulations members looked to several sources of information including current state pharmacy laws and regulations as well as the information provided by Drs Elenbaas and Bauman The Task Force felt that the Idaho State Board of Pharmacys regulations on collaborative practice encompassed much of what they wanted to include in the model regulation therefore they were used as a blueprint
A brainstorming session brought issues and concerns regarding state regulation of collaborative pharmacy practice to the forefront Topics that members felt needed to be addressed in a model regulation included
Contents of collaborative pharmacy practice agreements
Practitioner override authority
Board approval of agreements
Phannacists documentation of care
Patient consentnotification
Confidentiality of patient information
Periodic review of agreement
Phannacist competencequalifications to provide drug therapy management services and
Quality assurance review of practice
Task Force members concurred on a majority of these topics and incorporated their determinations into the model recommendation Members agreed that certain key elements should be included in collaborative phannacy practice agreements so that such agreements may guide both the pharmacists and collaborating practitioners decisions and conduct Key elements incorporated included a description of the pharmacists activities a method describing a practitioner monitoring methods and a practitioner override clause
Members also agreed that it should not be necessary for boards of pharmacy to take on the responsibility for approving agreements or even requiring their submission but that boards should require parties to notify them of the existence of such agreements and to keep a copy at the practice site On this issue members concluded that the individual practitioners were the persons most qualified to approve the agreements noting that minimal government involvement in the practice of the phannacist and collaborating practitioner was the most desirable In order for the board to have some handle on the situation however it was determined that agreements should be made available for board inspection upon request Regarding he issue of pharmacist competence to provide drug therapy management services Task Force members concluded that the subject should not be addressed in the model regulations They agreed that he determination of competence should be left up to the individuals who are party to the agreement Members felt certain that most if not al1 collaborating practitioners would only delegate certain aspects of their patient care authority upon demonstration by the pharmacist of his or her abilities to provide such care Further members felt that the majority ofphannacists would not take on the responsibility for providing such care without being competent to do so
TFCP A Recommendation 2
The Task Force on Collaborative Practice Agreements recognized that the issue of quality care assurance is not still developing and at present cannot be addressed with regard to collaborative pharmacy practice The Task Force recommends that the NABP Executive Committee closely
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI4
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
follow this issue and should it be determined that language addressing the subject should be incorporated into the model regulation take further action at that time
Background
Task Force members were uncertain at this time of how to address the issue of quality care assurance Members noted that most states addressing collaborative pharmacy practice in their laws or regulations did not include a provision on this subject It was noted however that tat least one state placed the responsibility for quality assurance on the Principal pharmacist and practitioner and that ACCP urged that an appropriate body be responsible for such activities
Member postulated that collaborative practice activities would likely be subject to quality assurance review via physician peer-review processes Additionally they suggested that those parties practicing in institutional settings would follow the quality assurance policies guidelines andor requirements of the institution The Task Force felt it was premature to make a recommendation regarding those parties who practice in ambulatory settings and who may not be subject to quality assurance review Members observed that the work ofNABPs Task Force on Patient Outcomes Regulation should be reviewed before a recommendation was made
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406 (F) 847391-4502middot wwwnabpnet 5
58-1-107 Applicability -- Relationship to specific chapters under title The provisions of this chapter LJniformly apply to the administration and enforcement of this title However
unless expressly prohibited in this chapter any provision of this chapter may be supplemented or altered by specific chapters of this title
R1S6-1-107 Organization of Rules - Content Applicability and Relationship of Rules
(1) The rules and sections in Title R156 shall to the extent practicable follow the numbering and
organizational scheme of the chapters in Title 58
(2) Rule R156-1 shall contain general provisions applicable to the administration and enforcement of all
occupations and profeSSions regulated in Title 58
(3) The provisions of the other rules in Title R156 shall contain specific or unique provisions applicable to
particular occupations or professions
(4) Specific rules in Title R156 may supplement or alter Rule R156-1 unless expressly provided otherwise
in Rule R156-1
R1S6-17b-l04 Organization - Relationship to Rule R1S6-1
The organization of this rule and its relationship to Rule R156-1 is as described in Section R156-1-107
58-17b-50G Petitioning for reinstatement of licensure Any person whose license to practice pharmacy in this state has been revoked suspended or surrendered
voluntarily or by action of the division shall have the right at reasonable intervals to petition the division for reinstatement of such license Such petition shall be made in writing and in the form prescribed by the division Upon investigation and hearing the division may in its discretion grant or deny such petition or it may modify its driginal finding to reflect any circumstances that have changed sufficiently to warrant such modifications Themiddotdivision also at its discretion may require such person to pass an examination or examinations for reshyentry into the practice of pharmacy Enacted by Chapter 280 2004 General Session
Agenda Board of Pharmacy
Collaborative Practice Agreement Discussion 100 PM - 200 PM
-~ Agenda Item Overview - I
Welcome and Introductions Paige Patterick and Carrie Dunford
Discuss the wording of 58-17b-l 02( 17) (17) Collaborative pharmacy practice agreement means a written and signed agreement between one or more pharmacists and one or more practitioners that provides for collaborative pharmacy practice for the purpose of drug therapy management of patients and prevention of disease of human subjects
Discuss the definition of Drug Therapy Management in R 156-17b-l 02 (19)
(J 9) Drug therapy management means the review of a drug therapy regimen of a patient by one or more pharmacists for the purpose of evaluating and rendering advice to one or more practitioners regarding adjustment of the regimen
Resources
NABP https llnabppharmacywp-contentuploads20 1607 ITFCollaborativePracticeAgreements AM95 Dec 1998 pdf
Operating Standards Drug therapy management R 156-1 7b-61 I
R 156-l7b-611 Operating Standards - Drug Therapy Management (I) In accordance with Subsections 58-17b-l 02( 17) and 58-l7b-60 I( I) decisions involving drug therapy management shall be made in the best interest of the patient Drug therapy management may include (a) implementing modifying and managing drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement (b) collecting and reviewing patient histories (c) obtaining and checking vital signs including pulse temperature blood pressure and respiration (d) ordering and evaluating the results of laboratory tests directly applicable to the drug therapy when perfolmed in accordance with approved protocols applicable to the practice setting and (e) such other patient care services as may be allowed by rule (2) For the purpose of promoting therapeutic appropriateness a pharmacist shall at the time of dispensing a prescription or a prescription drug order review the patients medication record Such review shall at a minimum identify clinically significant conditions situations or items such as (a) inappropriate drug utilization (b) therapeutic duplication (c) drug-disease contraindications (d) drug-drug interactions (e) incorrect drug dosage or duration of drug treatment (f) drug-allergy interactions and (g) clinical abuse or misuse (3) Upon identifying any clinically significant conditions situations or items listed in Subsection (2) above the pharmacist shall take appropriate steps to avoid or resolve the problem including consultation with the prescribing practitioner
Note In compliance with the Americans with Disabilities Act individuals needing special accommodations (including auxiliary communicative aids and services) during this meet ing should not ify Dave Taylor ADA Coordinator at least three working days prior to the meeting Division of Occupational amp Professional Licensing 160 East 300 South Salt Lake City Utah 84115 801-530-6628 or toll-free in Utah only 866-275-3675 Posted to Bulletin Board on 11-21-2017

(d) submit to a criminal backgrolUld check pursuant to Subsection 58-31 b-302(5) and Section R156-31 b-30 19 (3) ~ apph~anl who holds a current LPN license in an interstate compact state shall apply for a license within 90 days of
estabhshmg reSidency In Utah and complete all requirements pursuant to R 156-31 b-30 I a(2) (4) An applicant who has been licensed previously in Utah but whose license has expired or lapsed shall (a) if the applicant has not practiced as a nurse for up to five years document current compliance with the continuing competency
requirements as established in SubseL1ion R 156-31b-303(3)
(b) if the applicant has not practiced as a nurse for more than five years but less than eight years (I) pass the NCLEX-PN examination within 60 days following the date of application or (ii) successfully complete an approved re-entry program (c) if the applicant has not practiced as a nurse for more than eight years but less than 10 years (i) successfully complete an approved re-entry program and (ii) pass the NCLEX-PN examination within 60 days following the date of application or (d) if the applicant has not practiced as a nurse for 10 years or more comply with this Subsection (1) (5) An applicant who has been licensed in another state or country but whose license has expired or lapsed shall (a) comply with this Subsection (2)(b) and (b) comply with this Subsection (4) a~ applicable and (c) submit to a criminal background check pursuant to Subsection 58-31 b-302(5) and Section R156-31 b-30 I g
R156-31b-301b RN License- Education Examination and Experience Requirements (I) An applicant who has never obtained a license in any state or country shall (a) demonstrate that the applicant ha~ successfully completed an RN prelicensing education program that (i) meets the requirements of Section 58-31 b-60 I or (ii) is equivalent to an approved program under Section 58-3 I b-60 I (b) pass the NCLEX-RN examination purslant to Section R 156-31 b-30 I e and (c) submit to a climinal background check pursuant to Subsection 58-31 b-302(5) and Section R155-31 b-301g (2) An applicant who holds a current RN license issued by another country or state shall (a) demonstrate that the license issued by the other jurisdiction is current active and in good standing as of the date of
application (b)(i) demonstrate that the applicant has graduated from an RN prelicensing education program and (ii) if a foreign education program demonstrate that the program meets all requirements outlined in Section R 156-31 b-30 I d (c) pass the NCLEX-RN examination pursuant to Section RI56-31b-301e and (d) submit to a criminal backgrOlUld check pursuant to Subsection 58-31 b-302(5) and Sel-1ion R156-31 b-30 I g (3) An applicant who holds a current RN license in an interstate compact state shall apply for a license within 90 days of
establishing residency in Utah and complete all requirements pursuant to R156-31 b-301 b(2) (4) An applicant who has been licensed previously in Utah but whose license has expired or lapsed shall (a) if the applicant has not practiced as a nurse for up to five years document current compliance with the continuing competency
requirements as established in Subsection R 156-31 b-303(3) (b) if the applicant has not practiced as a nurse for more than five years but less than eight years (i) pass the NCLEX-RN examination within 60 days following the date of application or (ii) successfully complete an approved re-entry program (c) if the applicant has not practiced as a nurse for more than eight years but less than 10 years (i) successfully complete an approved re-entry program and (ii) pass theNCLEX-RN examination within 60 days following the date of application or (d) if the applicant ha~ not practiced as a nurse for 0 years or more comply with this Subsection (1) (5) An applicant who has been licensed in another state or country but whose license ha~ expired or lapsed shall (a) comply with this Subsection (2)(b) (b) comply with this Subsection (4) as applicable and (c) submit to a criminal background check pursuant to Subsection 58-31 b-302(5) and Section R156-3l b-30 I g
R156-31b-301c APRN Lkense - Education Examination and Experience Requirements (I) An applicant who is 110t currently and validly licensed alt an APRN in any state or country shall (a) demonstrate that the applicant holds a current active RN license in good standing (b) demonstrate that the applicant has successfully completelt an APRN prelicensing education program that meets the
requirements of Subsection 58-31b-601(1) and Subsection 58-31b-302(4)(e) (c) pass a national certitication examination consistent with the applicants educational specialty pursuant to Section R156-31 bshy
30 I e and administered by one of the following credentialing bodies (i) the American Nurses Credentialing Center Certification
4
PHARMACIST COLLABORATIVE PRACTICE AGREEMENTS
KEY ELEMENTS FOR LEGISLATIVE AND REGULATORY AUTHO RITY
A REPORT OF THE COLLABORATIVE PRACTICE WORKGROUP
CONVENED BY THE NATIONAL ALLIANCE OF STATE PHARMACY ASSOCIATIONS
PHARMACIST COLLABORATIVE PRACTICE AGREEMENTS KEY ELEMENTS FOR LEGISLATIVE AND REGULATORY AUTHORITY
PROJE CT OVERVIEW
Collaborative practice agreements (CPAs) create a formal practice relationship between pharmacists and other health care practitioners whereby the pharmacist assumes responsibility for specific patient care functions that are otherwise beyond their typical scope of practice but aligned with their education and training These patient care services can include initiation and modification of drug therapy The extent of the services authorized under the collaborative agreement depends on the states statutory and regulatory provisions for collaborative practice authority as well as the terms of the specific agreement between the pharmacist and other health care practitioners
State laws and regulations authorizing CPAs are highly variable Some states specify the practitioners
able to participate in CPAs restrict the services that may be provided under a CPA or include extensive
logistical barriers that limit the utility of such agreements
In their 2015 paper The Expanding Role of Pharmacists in a Transformed Health Care System the
National Governors Association (NGA) presented the following state policy considerations in regards to
collaborative practice provisions
bull Enact broad collaborative practice provisions that allow for specific provider functions to be
determined at the provider level rather than set in state statute or through regulation
bull Evaluate practice setting and drug therapy restrictions to determine whether pharmacists and
providers face disincentives that unnecessarily discourage collaborative arrangements
bull Examine whether CPAs unnecessarily dictate disease or patient specificity1
The National Alliance of State Pharmacy Associations (NASPAs) Executive Committee directed staff to
convene a workgroup to build upon the NGA recommendations with additional specificity The workgroup was charged with examining existing state CPA laws and regulations The workgroup was
tasked with developing recommendations for what elements of collaborative practice authority should
appropriately be defined under state law andor regulation and what elements are best left to be
determined between pharmacists and other practitioners when developing their specific collaborative
practice arrangement Using a modified Delphi method the Collaborative Practice Workgroup conducted
this work with two key questions in mind
bull Is this recommendation in the best interest of the patient receiving care under a collaborative
agreement
bull Is this recommendation aligned with pharmacists education and tra ining
The following is a report of the workgroups recommendations
1 Nati onal Governors Association The Expanding Role of Pharmacists in a Transformed Health Care System
JQ lwwwngaorgfiles llvesitesNGAfil esp d(20151 SOl Th eExp a ncli ngHo l e(jfPh~ rm cists~ Accessed
61515 1
WORKGROUP RECOMMENDATIONS The workgroup took the approach that rapid innovation in education training technology and evidence-based gUidelines necessitate a collaborative practice framework that is flexible and facilitates innovation in care delivery Thus the following statements include two levels of recommendations
1 Elements of collaborative practice authority that should be codified in state law andor state regulations and
2 Elements that are more appropriately determined by the parties at the practice level who voluntarily enter into a CPA and thus for which the laws and regulations should be silent
The workgroup views both levels of recommendations as needed and synergistic State law andor regulations if too restrictive can impede innovative team-based care models
COLLABORATIVE PRACTICE AGREEMENT PARTICIPANTS
RECOMMENDED ELEMENTS FOR INCLUSION IN STATE LAWS ANDOR REGULATIONS
Collaborative practice laws andor regulations should specify that
bull Any practitioner with prescriptive authority may collaborate with pharmacists using a CPA
bull CPAs may be between a single or multiple pharmacists and a single or multiple prescribers
bull CPAs may apply to a single patient multiple patients or patient populations as specified in the agreement
ELEMENTS THAT MAY BE DETERMINED AT THE PRACTITIONER LEVEL
Individual CPAs may address the below elements but state laws andor regulations should be silent
bull CPAs should specify which patient(s) andor patient population(s) can receive services under the agreement
bull Depending on the complexity of the services being provided under a CPA it may be appropriate for the pharmacist to have additional credentials or training beyond what is required for licensure
bull CPAs should specify which pharmacist(s) may provide services under the CPA A pharmacists practice setting should not be a barrier to their ability to enter into a CPA
COLLABORATIVE PRACTICE AGREEIVlEIlT AUTHORIZED SERVICES
RECOMMENDED ELEMENTS FOR INCLUSION IN STATE LAWS ANDOR REGULATIONS
Collaborative practice laws andor regulations should specify that
bull The initiation and modification of drug therapy may be authorized under a CPA with a prescriber
ELEMENTS THAT MAY BE DETERMINED AT THE PRACTITIONER LEVEL
Individual CPAs may address the below elements but state laws andor regulations should be silent
bull In some situations the use of an evidence-based protocol can ensure optimal care when pharmacists are initiating or modifying drug therapy and may be included in the CPA though they may not be needed or appropriate in others
2
bull Performing physical assessments as well as ordering performing or interpreting laboratory tests (eg CLiA-waived tests) may be included in a CPA to help identify or refer patients for services however a pharmacist may also perform these services without a CPA as these activities should fall within pharmacists standard scope of practice
bull Specific disease states may be included in a CPA at the participating practitioners discretion
COLLABORATIVE PRACTICE AGREEMENT REQUIREMENTS AND RESTRICTIONS
RECOMMENDED ELEMENTS FOR INCLUSION IN STATE LAWS ANDOR REGULATIONS
Collaborative practice laws andor regulations should specify that
bull All prescription drugs including controlled substances may be included within pharmacists collaborative practice authority
bull CPAs should be maintained by the pharmacist(s) and collaborating prescriber(s) and be available upon request or inspection
ELEMENTS THAT MAY BE DETERMINED AT THE PRACTITIONER LEVEL
Individual CPAs may address the below elements but state laws andor regulations should be silent
bull Pharmacist(s) and prescriber(s) may specify the level of patient involvement in the CPA Depending on the level of service elements such as informed consent written consent or optshyout provisions may be appropriate as determined by the parties to the agreement
bull Agreements should not be required to be sent to or approved by a state regulatory board or other agency such requirements create unnecessary paperwork burden and slow the efficiency of care delivery
bull Collaborating practitioners are encouraged to review andor renew their CPAs within a timeframe that is clinically appropriate
bull Collaborating practitioners should conform to evidence-based guidelines and the agreed upon process of care with regards to the documentation requirements and the collaborating practitioners responsibility for review of services provided under the agreement
bull Practitioners may consider liability insurance provisions and the appropriateness of articulating these in their voluntary agreement
bull It is the professional duty of all healthcare professionals to stay current in the clinical areas in which they practice If individual practitioners determine that continuing education requirements are appropriate for their clinical arrangement they may be specified in the agreement
3
APPENDIX A WORKGROUP PARTICIPANTS
The individuals listed below were appointed to participate in the Collaborative Practice Workgroup by one of two methods NASPA invited all Joint Commission of Pharmacy Practitioners (JCPP) member organizations CEOs to appoint a representative from their organization to participate (an invitation for an appointment was also extended to the National Association of Chain Drug Stores who currently is not a member of JCPP) It was recommended that professional affairs staff be considered for this work
State representatives were nominated by state pharmacy associations and appointed by the NASPA Executive Committee The selection process was intended to produce a group of participants who had experience with CPAs and practice in a variety of settings
Of note participants were only asked to represent their own opinions Participants from the national pharmacy associations were not acting as representatives of their organizations but rather as individuals whose experiences with their various memberships provide them with an informed perspective
Name StateNationa I OrganizationState
Alex Adams National NACDS
Jennifer Bacci State Pennsylvania
Lynette Bradley-Baker National AACP
Anne Burns National APhA
Carolyn Ha National NCPA
Julie Johnson State Minnesota
Sandra Leal State Arizona
Christine Lee-Wilson State Maryland
Dianne Miller State Michigan
Susan Oh National AMCP
Anthony Pudlo State Iowa
Kelly Ridgway State Arizona
Scotti Russell National NABP
Douglas Scheckel hoff National ASHP
L Michelle Vaughn State Alaska
Pete Vlasses National ACPE
Ed Webb National ACCP
Bryan Ziegler State South Carolina
4
APPENDIX B MODIFIED DELPHI METHOD
The goal of the workgroup was to reach consensus on each of the elements discussed To do this a modified Delphi method was used A survey was sent to all participants to collect their initial thoughts on each of the elements identified in currently existing collaborative practice authority laws andor regulations Participants were given the current variations of each element as a multiple-choice selection with the opportunity to answer in free form text if the desired option was not listed After completion of the survey the workgroup discussed all questions where consensus was not already reached via conference call The conference call discussions were structured to have a defined period of time for discussion followed by a summary of the current options being discussed and a roll call vote by each of the participants The item was included on the next survey if consensus was not reached on the conference call This process was repeated a total of three times before the group reached consensus on all items being considered See below for a diagram of the process used
1 Level-setting conference call
2 Distribution of survey with 3 weeks to complete
5
Task Force on
Collaborative Practice Agreements
Members Present
Sharlea M Leatherwood Chair (MO) Michael J Ayotte (VA) Winifred A Landis (IN) Jeffery Lindoo (NIN) C Ann Perry (GA) Charles R Young (MA)
Others Present
Joseph A Whaley Jr Executive Committee Liaison Carmen A Catizone NABP Executive DirectorSecretary Melissa Madigan NABP Staff
Introduction
The Task Force on Collaborative Practice Agreements (TFCPA) met December 3 1998 at the Marriott Suites Hotel in Rosemont Illinois The Task Force was established by the NABP Executive Corrunittee in response to recent increases in the number of prescribers and pharmacists entering into collaborative practice agreements and requests that boards of pharmacy define the elements of an appropriate collaborative practice agreement
Charge of the Task Force on Collaborative Practice Agreements
Task Force members reviewed their charge and proposing no changes accepted it as follows
Review the available literature and information pertaining to collaborative practice agreements between prescribers and pharmacists
bull Develop national model guidelines for writing and implementing uniform collaborative practice agreements that can be utilized by the state boards of pharmacy
TFCPA Recommendation 1
The Task Force on Collaborative Practice Agreements recorrunends to the Executive Committee and the Corrunittee on Law EnforcementLegislation that the following language be adopted for incorporation into the NABP Model State Pharmacy Act and Model Rules
Article I
Title Purpose Definition
Section 105 Definitions
(g) Collaborative Pharmacy Practice is that Practice of Pharmacy whereby a one or more Pharmacist~ has jointly agreed on a voluntary basis to work in conjunction with one or more Practitioners under protocol whereby the Pharmacist may perform certain patient care functions authorized by the Practitioner or Practitioners under certain specified conditions andor limitations
NATIONAL ASSOCIA nON OF BOARDS OF PHARMACYmiddot (P) 847391-4406 bull (F) 847391-4502 bull wwwnabpnel
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
(h) Collaborative Pharmacy Practice Agreement is a written and signed agreement between one or more Pharmacists and one or more Practitioners that provides for Collaborative Pharmacy Practice for the purpose of Drug Therapy Management of patients
(r) Drug Therapy Management means the review of Drug therapy regimen(s) of patients by one or more Pharmacists for the purpose of evaluating and rendering advice to one or more Practitioners regarding adjustment of the regimen Decisions involving Drug Therapy Management shall be made in the best interest of the patient Drug Therapy Management may include
(1) Implementing modifying and managing Drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement
(2) Collecting and reviewing patient Drug histories (3) Obtaining and checking vital signs including pulse temperature blood pressure
and respiration (4) Ordering and evaluating the results of laboratory tests directly relating to Drug
therapy when performed in accordance with approved protocols applicable to the practice setting and
(5) Such other patient care services as may be allowed by law
Pharmacists Scope of Practice Pursuant to the Collaborative Pharmacy Practice Agreement means those duties and limitations of duties placed upon one or more Pharmacists by the collaborating Practitioner or Practitioners the Board and applicable law and includes the limitations implied by the specialty practiced by the collaborating Practitioner or Practitioners
Model Rules for Pharmaceutical Care
Section 3 Pharmacy Practice
Collaborative Pharmacy Practice
(1) Collaborative Pharmacy Practice Agreement A Pharmacist planning to engage in Collaborative Pharmacy Practice shall have on file at his or her place of practice the written Collaborative Pharmacy Practice Agreement The existence and termination of such Agreement shall be reported to the Board and such Agreements shall be made available to the Board for review upon request The Agreement may allow the Pharmacist within the Pharmacists Scope of Practice Pursuant to the Collaborative Pharmacy Practice Agreement to conduct Drug Therapy Management activities approved by the Practitioner The collaboration that the Practitioner agrees to conduct with the Pharmacist must be within the scope of the Practitioners current practice Patients who receive services from one or more collaborating Pharmacists shall receive notification of receipt of such services
(2) Contents The Collaborative Pharmacy Practice Agreement shall include
(a) Identification of the Practitioner(s) and Pharmacist(s) who are parties to the Agreement
(b) The types of Drug Therapy Management decisions that the Pharmacist is allowed to make which may include
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpnet 2
Report of the Task Force on Collaborative Practice Agreements - NABP 9Smiddoth Annual Meeting Proceedings
(i) A detailed description of the types of diseases Drugs or Drug categories involved and the type of Drug Therapy Management allowed in each case
(ii) A detailed description of the methods procedures decision criteria and plan the Pharmacist is to follow when conducting Drug Therapy ~anagement and
(iii) A detailed description of the activities the Pharmacist is to follow in the course of conducting Drug Therapy Management including documentation of decisions made and a plan or appropriate mechanism for conununication feedback and reporting to the Practitioner concerning specific decisions made In addition to the Agreement documentation shall occur on the prescription record patient profile a separate log book or in some other appropriate system
(c) A method for the Practitioner to monitor compliance with the Agreement and clinical outcomes where Drug Therapy Management by the Pharmacist has occurred and to intercede where necessary
(d) A provision that allows the Practitioner to override a collaborative practice decision mad by the Pharmacist whenever he or she deems it necessary or appropriate
(e) A provision that allows either party to cancel the agreement by written notification
(f) An effective date and (g) Signatures of all collaborating Pharmacists and Practitioners who are party to the
agreement as well as the date of signing Amendments to a Collaborative Pharmacy Practice Agreement must be documented signed and dated
(3) Initiation of the Collaborative Pharmacy Practice Agreement The Collaborative Pharmacy Practice Agreement must be coupled with a medical order from the Practitioner to initiate Drug Therapy Management for any particular patient
(4) Documentation of Drug Therapy Management Docwnentation of Drug Therapy ~anagement must be kept as part of the patients permanent record and be readily available to other health care professionals providing care to that patient and who are authorized to received it Docwnentation of drug Therapy Management shall be considered Confidential Information
(5) Review At a minimum the written agreement shall be reviewed and renewed and if necessary revised every year
[NOTE the above new subsections would be inserted with sequential relettering of all subsequent subsections]
Background
Task Force members listened to a brief presentation by American College of Clinical Pharmacy (ACCP) Executive Director Robert Elenbaas and Immediate Past President Jerry Bauman regarding ACCPs position on collaborative practice agreements and later discussed their charge to develop national guidelines for writing and implementing uniform collaborative practice agreements for use by state boards of pharmacy They agreed that the best way to fulfill their charge was to create model regulations addressing the issue
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI3
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sb Annual Meeting Proceedings
To assist in developing the model regulations members looked to several sources of information including current state pharmacy laws and regulations as well as the information provided by Drs Elenbaas and Bauman The Task Force felt that the Idaho State Board of Pharmacys regulations on collaborative practice encompassed much of what they wanted to include in the model regulation therefore they were used as a blueprint
A brainstorming session brought issues and concerns regarding state regulation of collaborative pharmacy practice to the forefront Topics that members felt needed to be addressed in a model regulation included
Contents of collaborative pharmacy practice agreements
Practitioner override authority
Board approval of agreements
Phannacists documentation of care
Patient consentnotification
Confidentiality of patient information
Periodic review of agreement
Phannacist competencequalifications to provide drug therapy management services and
Quality assurance review of practice
Task Force members concurred on a majority of these topics and incorporated their determinations into the model recommendation Members agreed that certain key elements should be included in collaborative phannacy practice agreements so that such agreements may guide both the pharmacists and collaborating practitioners decisions and conduct Key elements incorporated included a description of the pharmacists activities a method describing a practitioner monitoring methods and a practitioner override clause
Members also agreed that it should not be necessary for boards of pharmacy to take on the responsibility for approving agreements or even requiring their submission but that boards should require parties to notify them of the existence of such agreements and to keep a copy at the practice site On this issue members concluded that the individual practitioners were the persons most qualified to approve the agreements noting that minimal government involvement in the practice of the phannacist and collaborating practitioner was the most desirable In order for the board to have some handle on the situation however it was determined that agreements should be made available for board inspection upon request Regarding he issue of pharmacist competence to provide drug therapy management services Task Force members concluded that the subject should not be addressed in the model regulations They agreed that he determination of competence should be left up to the individuals who are party to the agreement Members felt certain that most if not al1 collaborating practitioners would only delegate certain aspects of their patient care authority upon demonstration by the pharmacist of his or her abilities to provide such care Further members felt that the majority ofphannacists would not take on the responsibility for providing such care without being competent to do so
TFCP A Recommendation 2
The Task Force on Collaborative Practice Agreements recognized that the issue of quality care assurance is not still developing and at present cannot be addressed with regard to collaborative pharmacy practice The Task Force recommends that the NABP Executive Committee closely
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI4
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
follow this issue and should it be determined that language addressing the subject should be incorporated into the model regulation take further action at that time
Background
Task Force members were uncertain at this time of how to address the issue of quality care assurance Members noted that most states addressing collaborative pharmacy practice in their laws or regulations did not include a provision on this subject It was noted however that tat least one state placed the responsibility for quality assurance on the Principal pharmacist and practitioner and that ACCP urged that an appropriate body be responsible for such activities
Member postulated that collaborative practice activities would likely be subject to quality assurance review via physician peer-review processes Additionally they suggested that those parties practicing in institutional settings would follow the quality assurance policies guidelines andor requirements of the institution The Task Force felt it was premature to make a recommendation regarding those parties who practice in ambulatory settings and who may not be subject to quality assurance review Members observed that the work ofNABPs Task Force on Patient Outcomes Regulation should be reviewed before a recommendation was made
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406 (F) 847391-4502middot wwwnabpnet 5
58-1-107 Applicability -- Relationship to specific chapters under title The provisions of this chapter LJniformly apply to the administration and enforcement of this title However
unless expressly prohibited in this chapter any provision of this chapter may be supplemented or altered by specific chapters of this title
R1S6-1-107 Organization of Rules - Content Applicability and Relationship of Rules
(1) The rules and sections in Title R156 shall to the extent practicable follow the numbering and
organizational scheme of the chapters in Title 58
(2) Rule R156-1 shall contain general provisions applicable to the administration and enforcement of all
occupations and profeSSions regulated in Title 58
(3) The provisions of the other rules in Title R156 shall contain specific or unique provisions applicable to
particular occupations or professions
(4) Specific rules in Title R156 may supplement or alter Rule R156-1 unless expressly provided otherwise
in Rule R156-1
R1S6-17b-l04 Organization - Relationship to Rule R1S6-1
The organization of this rule and its relationship to Rule R156-1 is as described in Section R156-1-107
58-17b-50G Petitioning for reinstatement of licensure Any person whose license to practice pharmacy in this state has been revoked suspended or surrendered
voluntarily or by action of the division shall have the right at reasonable intervals to petition the division for reinstatement of such license Such petition shall be made in writing and in the form prescribed by the division Upon investigation and hearing the division may in its discretion grant or deny such petition or it may modify its driginal finding to reflect any circumstances that have changed sufficiently to warrant such modifications Themiddotdivision also at its discretion may require such person to pass an examination or examinations for reshyentry into the practice of pharmacy Enacted by Chapter 280 2004 General Session
Agenda Board of Pharmacy
Collaborative Practice Agreement Discussion 100 PM - 200 PM
-~ Agenda Item Overview - I
Welcome and Introductions Paige Patterick and Carrie Dunford
Discuss the wording of 58-17b-l 02( 17) (17) Collaborative pharmacy practice agreement means a written and signed agreement between one or more pharmacists and one or more practitioners that provides for collaborative pharmacy practice for the purpose of drug therapy management of patients and prevention of disease of human subjects
Discuss the definition of Drug Therapy Management in R 156-17b-l 02 (19)
(J 9) Drug therapy management means the review of a drug therapy regimen of a patient by one or more pharmacists for the purpose of evaluating and rendering advice to one or more practitioners regarding adjustment of the regimen
Resources
NABP https llnabppharmacywp-contentuploads20 1607 ITFCollaborativePracticeAgreements AM95 Dec 1998 pdf
Operating Standards Drug therapy management R 156-1 7b-61 I
R 156-l7b-611 Operating Standards - Drug Therapy Management (I) In accordance with Subsections 58-17b-l 02( 17) and 58-l7b-60 I( I) decisions involving drug therapy management shall be made in the best interest of the patient Drug therapy management may include (a) implementing modifying and managing drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement (b) collecting and reviewing patient histories (c) obtaining and checking vital signs including pulse temperature blood pressure and respiration (d) ordering and evaluating the results of laboratory tests directly applicable to the drug therapy when perfolmed in accordance with approved protocols applicable to the practice setting and (e) such other patient care services as may be allowed by rule (2) For the purpose of promoting therapeutic appropriateness a pharmacist shall at the time of dispensing a prescription or a prescription drug order review the patients medication record Such review shall at a minimum identify clinically significant conditions situations or items such as (a) inappropriate drug utilization (b) therapeutic duplication (c) drug-disease contraindications (d) drug-drug interactions (e) incorrect drug dosage or duration of drug treatment (f) drug-allergy interactions and (g) clinical abuse or misuse (3) Upon identifying any clinically significant conditions situations or items listed in Subsection (2) above the pharmacist shall take appropriate steps to avoid or resolve the problem including consultation with the prescribing practitioner
Note In compliance with the Americans with Disabilities Act individuals needing special accommodations (including auxiliary communicative aids and services) during this meet ing should not ify Dave Taylor ADA Coordinator at least three working days prior to the meeting Division of Occupational amp Professional Licensing 160 East 300 South Salt Lake City Utah 84115 801-530-6628 or toll-free in Utah only 866-275-3675 Posted to Bulletin Board on 11-21-2017

PHARMACIST COLLABORATIVE PRACTICE AGREEMENTS
KEY ELEMENTS FOR LEGISLATIVE AND REGULATORY AUTHO RITY
A REPORT OF THE COLLABORATIVE PRACTICE WORKGROUP
CONVENED BY THE NATIONAL ALLIANCE OF STATE PHARMACY ASSOCIATIONS
PHARMACIST COLLABORATIVE PRACTICE AGREEMENTS KEY ELEMENTS FOR LEGISLATIVE AND REGULATORY AUTHORITY
PROJE CT OVERVIEW
Collaborative practice agreements (CPAs) create a formal practice relationship between pharmacists and other health care practitioners whereby the pharmacist assumes responsibility for specific patient care functions that are otherwise beyond their typical scope of practice but aligned with their education and training These patient care services can include initiation and modification of drug therapy The extent of the services authorized under the collaborative agreement depends on the states statutory and regulatory provisions for collaborative practice authority as well as the terms of the specific agreement between the pharmacist and other health care practitioners
State laws and regulations authorizing CPAs are highly variable Some states specify the practitioners
able to participate in CPAs restrict the services that may be provided under a CPA or include extensive
logistical barriers that limit the utility of such agreements
In their 2015 paper The Expanding Role of Pharmacists in a Transformed Health Care System the
National Governors Association (NGA) presented the following state policy considerations in regards to
collaborative practice provisions
bull Enact broad collaborative practice provisions that allow for specific provider functions to be
determined at the provider level rather than set in state statute or through regulation
bull Evaluate practice setting and drug therapy restrictions to determine whether pharmacists and
providers face disincentives that unnecessarily discourage collaborative arrangements
bull Examine whether CPAs unnecessarily dictate disease or patient specificity1
The National Alliance of State Pharmacy Associations (NASPAs) Executive Committee directed staff to
convene a workgroup to build upon the NGA recommendations with additional specificity The workgroup was charged with examining existing state CPA laws and regulations The workgroup was
tasked with developing recommendations for what elements of collaborative practice authority should
appropriately be defined under state law andor regulation and what elements are best left to be
determined between pharmacists and other practitioners when developing their specific collaborative
practice arrangement Using a modified Delphi method the Collaborative Practice Workgroup conducted
this work with two key questions in mind
bull Is this recommendation in the best interest of the patient receiving care under a collaborative
agreement
bull Is this recommendation aligned with pharmacists education and tra ining
The following is a report of the workgroups recommendations
1 Nati onal Governors Association The Expanding Role of Pharmacists in a Transformed Health Care System
JQ lwwwngaorgfiles llvesitesNGAfil esp d(20151 SOl Th eExp a ncli ngHo l e(jfPh~ rm cists~ Accessed
61515 1
WORKGROUP RECOMMENDATIONS The workgroup took the approach that rapid innovation in education training technology and evidence-based gUidelines necessitate a collaborative practice framework that is flexible and facilitates innovation in care delivery Thus the following statements include two levels of recommendations
1 Elements of collaborative practice authority that should be codified in state law andor state regulations and
2 Elements that are more appropriately determined by the parties at the practice level who voluntarily enter into a CPA and thus for which the laws and regulations should be silent
The workgroup views both levels of recommendations as needed and synergistic State law andor regulations if too restrictive can impede innovative team-based care models
COLLABORATIVE PRACTICE AGREEMENT PARTICIPANTS
RECOMMENDED ELEMENTS FOR INCLUSION IN STATE LAWS ANDOR REGULATIONS
Collaborative practice laws andor regulations should specify that
bull Any practitioner with prescriptive authority may collaborate with pharmacists using a CPA
bull CPAs may be between a single or multiple pharmacists and a single or multiple prescribers
bull CPAs may apply to a single patient multiple patients or patient populations as specified in the agreement
ELEMENTS THAT MAY BE DETERMINED AT THE PRACTITIONER LEVEL
Individual CPAs may address the below elements but state laws andor regulations should be silent
bull CPAs should specify which patient(s) andor patient population(s) can receive services under the agreement
bull Depending on the complexity of the services being provided under a CPA it may be appropriate for the pharmacist to have additional credentials or training beyond what is required for licensure
bull CPAs should specify which pharmacist(s) may provide services under the CPA A pharmacists practice setting should not be a barrier to their ability to enter into a CPA
COLLABORATIVE PRACTICE AGREEIVlEIlT AUTHORIZED SERVICES
RECOMMENDED ELEMENTS FOR INCLUSION IN STATE LAWS ANDOR REGULATIONS
Collaborative practice laws andor regulations should specify that
bull The initiation and modification of drug therapy may be authorized under a CPA with a prescriber
ELEMENTS THAT MAY BE DETERMINED AT THE PRACTITIONER LEVEL
Individual CPAs may address the below elements but state laws andor regulations should be silent
bull In some situations the use of an evidence-based protocol can ensure optimal care when pharmacists are initiating or modifying drug therapy and may be included in the CPA though they may not be needed or appropriate in others
2
bull Performing physical assessments as well as ordering performing or interpreting laboratory tests (eg CLiA-waived tests) may be included in a CPA to help identify or refer patients for services however a pharmacist may also perform these services without a CPA as these activities should fall within pharmacists standard scope of practice
bull Specific disease states may be included in a CPA at the participating practitioners discretion
COLLABORATIVE PRACTICE AGREEMENT REQUIREMENTS AND RESTRICTIONS
RECOMMENDED ELEMENTS FOR INCLUSION IN STATE LAWS ANDOR REGULATIONS
Collaborative practice laws andor regulations should specify that
bull All prescription drugs including controlled substances may be included within pharmacists collaborative practice authority
bull CPAs should be maintained by the pharmacist(s) and collaborating prescriber(s) and be available upon request or inspection
ELEMENTS THAT MAY BE DETERMINED AT THE PRACTITIONER LEVEL
Individual CPAs may address the below elements but state laws andor regulations should be silent
bull Pharmacist(s) and prescriber(s) may specify the level of patient involvement in the CPA Depending on the level of service elements such as informed consent written consent or optshyout provisions may be appropriate as determined by the parties to the agreement
bull Agreements should not be required to be sent to or approved by a state regulatory board or other agency such requirements create unnecessary paperwork burden and slow the efficiency of care delivery
bull Collaborating practitioners are encouraged to review andor renew their CPAs within a timeframe that is clinically appropriate
bull Collaborating practitioners should conform to evidence-based guidelines and the agreed upon process of care with regards to the documentation requirements and the collaborating practitioners responsibility for review of services provided under the agreement
bull Practitioners may consider liability insurance provisions and the appropriateness of articulating these in their voluntary agreement
bull It is the professional duty of all healthcare professionals to stay current in the clinical areas in which they practice If individual practitioners determine that continuing education requirements are appropriate for their clinical arrangement they may be specified in the agreement
3
APPENDIX A WORKGROUP PARTICIPANTS
The individuals listed below were appointed to participate in the Collaborative Practice Workgroup by one of two methods NASPA invited all Joint Commission of Pharmacy Practitioners (JCPP) member organizations CEOs to appoint a representative from their organization to participate (an invitation for an appointment was also extended to the National Association of Chain Drug Stores who currently is not a member of JCPP) It was recommended that professional affairs staff be considered for this work
State representatives were nominated by state pharmacy associations and appointed by the NASPA Executive Committee The selection process was intended to produce a group of participants who had experience with CPAs and practice in a variety of settings
Of note participants were only asked to represent their own opinions Participants from the national pharmacy associations were not acting as representatives of their organizations but rather as individuals whose experiences with their various memberships provide them with an informed perspective
Name StateNationa I OrganizationState
Alex Adams National NACDS
Jennifer Bacci State Pennsylvania
Lynette Bradley-Baker National AACP
Anne Burns National APhA
Carolyn Ha National NCPA
Julie Johnson State Minnesota
Sandra Leal State Arizona
Christine Lee-Wilson State Maryland
Dianne Miller State Michigan
Susan Oh National AMCP
Anthony Pudlo State Iowa
Kelly Ridgway State Arizona
Scotti Russell National NABP
Douglas Scheckel hoff National ASHP
L Michelle Vaughn State Alaska
Pete Vlasses National ACPE
Ed Webb National ACCP
Bryan Ziegler State South Carolina
4
APPENDIX B MODIFIED DELPHI METHOD
The goal of the workgroup was to reach consensus on each of the elements discussed To do this a modified Delphi method was used A survey was sent to all participants to collect their initial thoughts on each of the elements identified in currently existing collaborative practice authority laws andor regulations Participants were given the current variations of each element as a multiple-choice selection with the opportunity to answer in free form text if the desired option was not listed After completion of the survey the workgroup discussed all questions where consensus was not already reached via conference call The conference call discussions were structured to have a defined period of time for discussion followed by a summary of the current options being discussed and a roll call vote by each of the participants The item was included on the next survey if consensus was not reached on the conference call This process was repeated a total of three times before the group reached consensus on all items being considered See below for a diagram of the process used
1 Level-setting conference call
2 Distribution of survey with 3 weeks to complete
5
Task Force on
Collaborative Practice Agreements
Members Present
Sharlea M Leatherwood Chair (MO) Michael J Ayotte (VA) Winifred A Landis (IN) Jeffery Lindoo (NIN) C Ann Perry (GA) Charles R Young (MA)
Others Present
Joseph A Whaley Jr Executive Committee Liaison Carmen A Catizone NABP Executive DirectorSecretary Melissa Madigan NABP Staff
Introduction
The Task Force on Collaborative Practice Agreements (TFCPA) met December 3 1998 at the Marriott Suites Hotel in Rosemont Illinois The Task Force was established by the NABP Executive Corrunittee in response to recent increases in the number of prescribers and pharmacists entering into collaborative practice agreements and requests that boards of pharmacy define the elements of an appropriate collaborative practice agreement
Charge of the Task Force on Collaborative Practice Agreements
Task Force members reviewed their charge and proposing no changes accepted it as follows
Review the available literature and information pertaining to collaborative practice agreements between prescribers and pharmacists
bull Develop national model guidelines for writing and implementing uniform collaborative practice agreements that can be utilized by the state boards of pharmacy
TFCPA Recommendation 1
The Task Force on Collaborative Practice Agreements recorrunends to the Executive Committee and the Corrunittee on Law EnforcementLegislation that the following language be adopted for incorporation into the NABP Model State Pharmacy Act and Model Rules
Article I
Title Purpose Definition
Section 105 Definitions
(g) Collaborative Pharmacy Practice is that Practice of Pharmacy whereby a one or more Pharmacist~ has jointly agreed on a voluntary basis to work in conjunction with one or more Practitioners under protocol whereby the Pharmacist may perform certain patient care functions authorized by the Practitioner or Practitioners under certain specified conditions andor limitations
NATIONAL ASSOCIA nON OF BOARDS OF PHARMACYmiddot (P) 847391-4406 bull (F) 847391-4502 bull wwwnabpnel
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
(h) Collaborative Pharmacy Practice Agreement is a written and signed agreement between one or more Pharmacists and one or more Practitioners that provides for Collaborative Pharmacy Practice for the purpose of Drug Therapy Management of patients
(r) Drug Therapy Management means the review of Drug therapy regimen(s) of patients by one or more Pharmacists for the purpose of evaluating and rendering advice to one or more Practitioners regarding adjustment of the regimen Decisions involving Drug Therapy Management shall be made in the best interest of the patient Drug Therapy Management may include
(1) Implementing modifying and managing Drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement
(2) Collecting and reviewing patient Drug histories (3) Obtaining and checking vital signs including pulse temperature blood pressure
and respiration (4) Ordering and evaluating the results of laboratory tests directly relating to Drug
therapy when performed in accordance with approved protocols applicable to the practice setting and
(5) Such other patient care services as may be allowed by law
Pharmacists Scope of Practice Pursuant to the Collaborative Pharmacy Practice Agreement means those duties and limitations of duties placed upon one or more Pharmacists by the collaborating Practitioner or Practitioners the Board and applicable law and includes the limitations implied by the specialty practiced by the collaborating Practitioner or Practitioners
Model Rules for Pharmaceutical Care
Section 3 Pharmacy Practice
Collaborative Pharmacy Practice
(1) Collaborative Pharmacy Practice Agreement A Pharmacist planning to engage in Collaborative Pharmacy Practice shall have on file at his or her place of practice the written Collaborative Pharmacy Practice Agreement The existence and termination of such Agreement shall be reported to the Board and such Agreements shall be made available to the Board for review upon request The Agreement may allow the Pharmacist within the Pharmacists Scope of Practice Pursuant to the Collaborative Pharmacy Practice Agreement to conduct Drug Therapy Management activities approved by the Practitioner The collaboration that the Practitioner agrees to conduct with the Pharmacist must be within the scope of the Practitioners current practice Patients who receive services from one or more collaborating Pharmacists shall receive notification of receipt of such services
(2) Contents The Collaborative Pharmacy Practice Agreement shall include
(a) Identification of the Practitioner(s) and Pharmacist(s) who are parties to the Agreement
(b) The types of Drug Therapy Management decisions that the Pharmacist is allowed to make which may include
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpnet 2
Report of the Task Force on Collaborative Practice Agreements - NABP 9Smiddoth Annual Meeting Proceedings
(i) A detailed description of the types of diseases Drugs or Drug categories involved and the type of Drug Therapy Management allowed in each case
(ii) A detailed description of the methods procedures decision criteria and plan the Pharmacist is to follow when conducting Drug Therapy ~anagement and
(iii) A detailed description of the activities the Pharmacist is to follow in the course of conducting Drug Therapy Management including documentation of decisions made and a plan or appropriate mechanism for conununication feedback and reporting to the Practitioner concerning specific decisions made In addition to the Agreement documentation shall occur on the prescription record patient profile a separate log book or in some other appropriate system
(c) A method for the Practitioner to monitor compliance with the Agreement and clinical outcomes where Drug Therapy Management by the Pharmacist has occurred and to intercede where necessary
(d) A provision that allows the Practitioner to override a collaborative practice decision mad by the Pharmacist whenever he or she deems it necessary or appropriate
(e) A provision that allows either party to cancel the agreement by written notification
(f) An effective date and (g) Signatures of all collaborating Pharmacists and Practitioners who are party to the
agreement as well as the date of signing Amendments to a Collaborative Pharmacy Practice Agreement must be documented signed and dated
(3) Initiation of the Collaborative Pharmacy Practice Agreement The Collaborative Pharmacy Practice Agreement must be coupled with a medical order from the Practitioner to initiate Drug Therapy Management for any particular patient
(4) Documentation of Drug Therapy Management Docwnentation of Drug Therapy ~anagement must be kept as part of the patients permanent record and be readily available to other health care professionals providing care to that patient and who are authorized to received it Docwnentation of drug Therapy Management shall be considered Confidential Information
(5) Review At a minimum the written agreement shall be reviewed and renewed and if necessary revised every year
[NOTE the above new subsections would be inserted with sequential relettering of all subsequent subsections]
Background
Task Force members listened to a brief presentation by American College of Clinical Pharmacy (ACCP) Executive Director Robert Elenbaas and Immediate Past President Jerry Bauman regarding ACCPs position on collaborative practice agreements and later discussed their charge to develop national guidelines for writing and implementing uniform collaborative practice agreements for use by state boards of pharmacy They agreed that the best way to fulfill their charge was to create model regulations addressing the issue
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI3
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sb Annual Meeting Proceedings
To assist in developing the model regulations members looked to several sources of information including current state pharmacy laws and regulations as well as the information provided by Drs Elenbaas and Bauman The Task Force felt that the Idaho State Board of Pharmacys regulations on collaborative practice encompassed much of what they wanted to include in the model regulation therefore they were used as a blueprint
A brainstorming session brought issues and concerns regarding state regulation of collaborative pharmacy practice to the forefront Topics that members felt needed to be addressed in a model regulation included
Contents of collaborative pharmacy practice agreements
Practitioner override authority
Board approval of agreements
Phannacists documentation of care
Patient consentnotification
Confidentiality of patient information
Periodic review of agreement
Phannacist competencequalifications to provide drug therapy management services and
Quality assurance review of practice
Task Force members concurred on a majority of these topics and incorporated their determinations into the model recommendation Members agreed that certain key elements should be included in collaborative phannacy practice agreements so that such agreements may guide both the pharmacists and collaborating practitioners decisions and conduct Key elements incorporated included a description of the pharmacists activities a method describing a practitioner monitoring methods and a practitioner override clause
Members also agreed that it should not be necessary for boards of pharmacy to take on the responsibility for approving agreements or even requiring their submission but that boards should require parties to notify them of the existence of such agreements and to keep a copy at the practice site On this issue members concluded that the individual practitioners were the persons most qualified to approve the agreements noting that minimal government involvement in the practice of the phannacist and collaborating practitioner was the most desirable In order for the board to have some handle on the situation however it was determined that agreements should be made available for board inspection upon request Regarding he issue of pharmacist competence to provide drug therapy management services Task Force members concluded that the subject should not be addressed in the model regulations They agreed that he determination of competence should be left up to the individuals who are party to the agreement Members felt certain that most if not al1 collaborating practitioners would only delegate certain aspects of their patient care authority upon demonstration by the pharmacist of his or her abilities to provide such care Further members felt that the majority ofphannacists would not take on the responsibility for providing such care without being competent to do so
TFCP A Recommendation 2
The Task Force on Collaborative Practice Agreements recognized that the issue of quality care assurance is not still developing and at present cannot be addressed with regard to collaborative pharmacy practice The Task Force recommends that the NABP Executive Committee closely
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI4
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
follow this issue and should it be determined that language addressing the subject should be incorporated into the model regulation take further action at that time
Background
Task Force members were uncertain at this time of how to address the issue of quality care assurance Members noted that most states addressing collaborative pharmacy practice in their laws or regulations did not include a provision on this subject It was noted however that tat least one state placed the responsibility for quality assurance on the Principal pharmacist and practitioner and that ACCP urged that an appropriate body be responsible for such activities
Member postulated that collaborative practice activities would likely be subject to quality assurance review via physician peer-review processes Additionally they suggested that those parties practicing in institutional settings would follow the quality assurance policies guidelines andor requirements of the institution The Task Force felt it was premature to make a recommendation regarding those parties who practice in ambulatory settings and who may not be subject to quality assurance review Members observed that the work ofNABPs Task Force on Patient Outcomes Regulation should be reviewed before a recommendation was made
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406 (F) 847391-4502middot wwwnabpnet 5
58-1-107 Applicability -- Relationship to specific chapters under title The provisions of this chapter LJniformly apply to the administration and enforcement of this title However
unless expressly prohibited in this chapter any provision of this chapter may be supplemented or altered by specific chapters of this title
R1S6-1-107 Organization of Rules - Content Applicability and Relationship of Rules
(1) The rules and sections in Title R156 shall to the extent practicable follow the numbering and
organizational scheme of the chapters in Title 58
(2) Rule R156-1 shall contain general provisions applicable to the administration and enforcement of all
occupations and profeSSions regulated in Title 58
(3) The provisions of the other rules in Title R156 shall contain specific or unique provisions applicable to
particular occupations or professions
(4) Specific rules in Title R156 may supplement or alter Rule R156-1 unless expressly provided otherwise
in Rule R156-1
R1S6-17b-l04 Organization - Relationship to Rule R1S6-1
The organization of this rule and its relationship to Rule R156-1 is as described in Section R156-1-107
58-17b-50G Petitioning for reinstatement of licensure Any person whose license to practice pharmacy in this state has been revoked suspended or surrendered
voluntarily or by action of the division shall have the right at reasonable intervals to petition the division for reinstatement of such license Such petition shall be made in writing and in the form prescribed by the division Upon investigation and hearing the division may in its discretion grant or deny such petition or it may modify its driginal finding to reflect any circumstances that have changed sufficiently to warrant such modifications Themiddotdivision also at its discretion may require such person to pass an examination or examinations for reshyentry into the practice of pharmacy Enacted by Chapter 280 2004 General Session
Agenda Board of Pharmacy
Collaborative Practice Agreement Discussion 100 PM - 200 PM
-~ Agenda Item Overview - I
Welcome and Introductions Paige Patterick and Carrie Dunford
Discuss the wording of 58-17b-l 02( 17) (17) Collaborative pharmacy practice agreement means a written and signed agreement between one or more pharmacists and one or more practitioners that provides for collaborative pharmacy practice for the purpose of drug therapy management of patients and prevention of disease of human subjects
Discuss the definition of Drug Therapy Management in R 156-17b-l 02 (19)
(J 9) Drug therapy management means the review of a drug therapy regimen of a patient by one or more pharmacists for the purpose of evaluating and rendering advice to one or more practitioners regarding adjustment of the regimen
Resources
NABP https llnabppharmacywp-contentuploads20 1607 ITFCollaborativePracticeAgreements AM95 Dec 1998 pdf
Operating Standards Drug therapy management R 156-1 7b-61 I
R 156-l7b-611 Operating Standards - Drug Therapy Management (I) In accordance with Subsections 58-17b-l 02( 17) and 58-l7b-60 I( I) decisions involving drug therapy management shall be made in the best interest of the patient Drug therapy management may include (a) implementing modifying and managing drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement (b) collecting and reviewing patient histories (c) obtaining and checking vital signs including pulse temperature blood pressure and respiration (d) ordering and evaluating the results of laboratory tests directly applicable to the drug therapy when perfolmed in accordance with approved protocols applicable to the practice setting and (e) such other patient care services as may be allowed by rule (2) For the purpose of promoting therapeutic appropriateness a pharmacist shall at the time of dispensing a prescription or a prescription drug order review the patients medication record Such review shall at a minimum identify clinically significant conditions situations or items such as (a) inappropriate drug utilization (b) therapeutic duplication (c) drug-disease contraindications (d) drug-drug interactions (e) incorrect drug dosage or duration of drug treatment (f) drug-allergy interactions and (g) clinical abuse or misuse (3) Upon identifying any clinically significant conditions situations or items listed in Subsection (2) above the pharmacist shall take appropriate steps to avoid or resolve the problem including consultation with the prescribing practitioner
Note In compliance with the Americans with Disabilities Act individuals needing special accommodations (including auxiliary communicative aids and services) during this meet ing should not ify Dave Taylor ADA Coordinator at least three working days prior to the meeting Division of Occupational amp Professional Licensing 160 East 300 South Salt Lake City Utah 84115 801-530-6628 or toll-free in Utah only 866-275-3675 Posted to Bulletin Board on 11-21-2017

PHARMACIST COLLABORATIVE PRACTICE AGREEMENTS KEY ELEMENTS FOR LEGISLATIVE AND REGULATORY AUTHORITY
PROJE CT OVERVIEW
Collaborative practice agreements (CPAs) create a formal practice relationship between pharmacists and other health care practitioners whereby the pharmacist assumes responsibility for specific patient care functions that are otherwise beyond their typical scope of practice but aligned with their education and training These patient care services can include initiation and modification of drug therapy The extent of the services authorized under the collaborative agreement depends on the states statutory and regulatory provisions for collaborative practice authority as well as the terms of the specific agreement between the pharmacist and other health care practitioners
State laws and regulations authorizing CPAs are highly variable Some states specify the practitioners
able to participate in CPAs restrict the services that may be provided under a CPA or include extensive
logistical barriers that limit the utility of such agreements
In their 2015 paper The Expanding Role of Pharmacists in a Transformed Health Care System the
National Governors Association (NGA) presented the following state policy considerations in regards to
collaborative practice provisions
bull Enact broad collaborative practice provisions that allow for specific provider functions to be
determined at the provider level rather than set in state statute or through regulation
bull Evaluate practice setting and drug therapy restrictions to determine whether pharmacists and
providers face disincentives that unnecessarily discourage collaborative arrangements
bull Examine whether CPAs unnecessarily dictate disease or patient specificity1
The National Alliance of State Pharmacy Associations (NASPAs) Executive Committee directed staff to
convene a workgroup to build upon the NGA recommendations with additional specificity The workgroup was charged with examining existing state CPA laws and regulations The workgroup was
tasked with developing recommendations for what elements of collaborative practice authority should
appropriately be defined under state law andor regulation and what elements are best left to be
determined between pharmacists and other practitioners when developing their specific collaborative
practice arrangement Using a modified Delphi method the Collaborative Practice Workgroup conducted
this work with two key questions in mind
bull Is this recommendation in the best interest of the patient receiving care under a collaborative
agreement
bull Is this recommendation aligned with pharmacists education and tra ining
The following is a report of the workgroups recommendations
1 Nati onal Governors Association The Expanding Role of Pharmacists in a Transformed Health Care System
JQ lwwwngaorgfiles llvesitesNGAfil esp d(20151 SOl Th eExp a ncli ngHo l e(jfPh~ rm cists~ Accessed
61515 1
WORKGROUP RECOMMENDATIONS The workgroup took the approach that rapid innovation in education training technology and evidence-based gUidelines necessitate a collaborative practice framework that is flexible and facilitates innovation in care delivery Thus the following statements include two levels of recommendations
1 Elements of collaborative practice authority that should be codified in state law andor state regulations and
2 Elements that are more appropriately determined by the parties at the practice level who voluntarily enter into a CPA and thus for which the laws and regulations should be silent
The workgroup views both levels of recommendations as needed and synergistic State law andor regulations if too restrictive can impede innovative team-based care models
COLLABORATIVE PRACTICE AGREEMENT PARTICIPANTS
RECOMMENDED ELEMENTS FOR INCLUSION IN STATE LAWS ANDOR REGULATIONS
Collaborative practice laws andor regulations should specify that
bull Any practitioner with prescriptive authority may collaborate with pharmacists using a CPA
bull CPAs may be between a single or multiple pharmacists and a single or multiple prescribers
bull CPAs may apply to a single patient multiple patients or patient populations as specified in the agreement
ELEMENTS THAT MAY BE DETERMINED AT THE PRACTITIONER LEVEL
Individual CPAs may address the below elements but state laws andor regulations should be silent
bull CPAs should specify which patient(s) andor patient population(s) can receive services under the agreement
bull Depending on the complexity of the services being provided under a CPA it may be appropriate for the pharmacist to have additional credentials or training beyond what is required for licensure
bull CPAs should specify which pharmacist(s) may provide services under the CPA A pharmacists practice setting should not be a barrier to their ability to enter into a CPA
COLLABORATIVE PRACTICE AGREEIVlEIlT AUTHORIZED SERVICES
RECOMMENDED ELEMENTS FOR INCLUSION IN STATE LAWS ANDOR REGULATIONS
Collaborative practice laws andor regulations should specify that
bull The initiation and modification of drug therapy may be authorized under a CPA with a prescriber
ELEMENTS THAT MAY BE DETERMINED AT THE PRACTITIONER LEVEL
Individual CPAs may address the below elements but state laws andor regulations should be silent
bull In some situations the use of an evidence-based protocol can ensure optimal care when pharmacists are initiating or modifying drug therapy and may be included in the CPA though they may not be needed or appropriate in others
2
bull Performing physical assessments as well as ordering performing or interpreting laboratory tests (eg CLiA-waived tests) may be included in a CPA to help identify or refer patients for services however a pharmacist may also perform these services without a CPA as these activities should fall within pharmacists standard scope of practice
bull Specific disease states may be included in a CPA at the participating practitioners discretion
COLLABORATIVE PRACTICE AGREEMENT REQUIREMENTS AND RESTRICTIONS
RECOMMENDED ELEMENTS FOR INCLUSION IN STATE LAWS ANDOR REGULATIONS
Collaborative practice laws andor regulations should specify that
bull All prescription drugs including controlled substances may be included within pharmacists collaborative practice authority
bull CPAs should be maintained by the pharmacist(s) and collaborating prescriber(s) and be available upon request or inspection
ELEMENTS THAT MAY BE DETERMINED AT THE PRACTITIONER LEVEL
Individual CPAs may address the below elements but state laws andor regulations should be silent
bull Pharmacist(s) and prescriber(s) may specify the level of patient involvement in the CPA Depending on the level of service elements such as informed consent written consent or optshyout provisions may be appropriate as determined by the parties to the agreement
bull Agreements should not be required to be sent to or approved by a state regulatory board or other agency such requirements create unnecessary paperwork burden and slow the efficiency of care delivery
bull Collaborating practitioners are encouraged to review andor renew their CPAs within a timeframe that is clinically appropriate
bull Collaborating practitioners should conform to evidence-based guidelines and the agreed upon process of care with regards to the documentation requirements and the collaborating practitioners responsibility for review of services provided under the agreement
bull Practitioners may consider liability insurance provisions and the appropriateness of articulating these in their voluntary agreement
bull It is the professional duty of all healthcare professionals to stay current in the clinical areas in which they practice If individual practitioners determine that continuing education requirements are appropriate for their clinical arrangement they may be specified in the agreement
3
APPENDIX A WORKGROUP PARTICIPANTS
The individuals listed below were appointed to participate in the Collaborative Practice Workgroup by one of two methods NASPA invited all Joint Commission of Pharmacy Practitioners (JCPP) member organizations CEOs to appoint a representative from their organization to participate (an invitation for an appointment was also extended to the National Association of Chain Drug Stores who currently is not a member of JCPP) It was recommended that professional affairs staff be considered for this work
State representatives were nominated by state pharmacy associations and appointed by the NASPA Executive Committee The selection process was intended to produce a group of participants who had experience with CPAs and practice in a variety of settings
Of note participants were only asked to represent their own opinions Participants from the national pharmacy associations were not acting as representatives of their organizations but rather as individuals whose experiences with their various memberships provide them with an informed perspective
Name StateNationa I OrganizationState
Alex Adams National NACDS
Jennifer Bacci State Pennsylvania
Lynette Bradley-Baker National AACP
Anne Burns National APhA
Carolyn Ha National NCPA
Julie Johnson State Minnesota
Sandra Leal State Arizona
Christine Lee-Wilson State Maryland
Dianne Miller State Michigan
Susan Oh National AMCP
Anthony Pudlo State Iowa
Kelly Ridgway State Arizona
Scotti Russell National NABP
Douglas Scheckel hoff National ASHP
L Michelle Vaughn State Alaska
Pete Vlasses National ACPE
Ed Webb National ACCP
Bryan Ziegler State South Carolina
4
APPENDIX B MODIFIED DELPHI METHOD
The goal of the workgroup was to reach consensus on each of the elements discussed To do this a modified Delphi method was used A survey was sent to all participants to collect their initial thoughts on each of the elements identified in currently existing collaborative practice authority laws andor regulations Participants were given the current variations of each element as a multiple-choice selection with the opportunity to answer in free form text if the desired option was not listed After completion of the survey the workgroup discussed all questions where consensus was not already reached via conference call The conference call discussions were structured to have a defined period of time for discussion followed by a summary of the current options being discussed and a roll call vote by each of the participants The item was included on the next survey if consensus was not reached on the conference call This process was repeated a total of three times before the group reached consensus on all items being considered See below for a diagram of the process used
1 Level-setting conference call
2 Distribution of survey with 3 weeks to complete
5
Task Force on
Collaborative Practice Agreements
Members Present
Sharlea M Leatherwood Chair (MO) Michael J Ayotte (VA) Winifred A Landis (IN) Jeffery Lindoo (NIN) C Ann Perry (GA) Charles R Young (MA)
Others Present
Joseph A Whaley Jr Executive Committee Liaison Carmen A Catizone NABP Executive DirectorSecretary Melissa Madigan NABP Staff
Introduction
The Task Force on Collaborative Practice Agreements (TFCPA) met December 3 1998 at the Marriott Suites Hotel in Rosemont Illinois The Task Force was established by the NABP Executive Corrunittee in response to recent increases in the number of prescribers and pharmacists entering into collaborative practice agreements and requests that boards of pharmacy define the elements of an appropriate collaborative practice agreement
Charge of the Task Force on Collaborative Practice Agreements
Task Force members reviewed their charge and proposing no changes accepted it as follows
Review the available literature and information pertaining to collaborative practice agreements between prescribers and pharmacists
bull Develop national model guidelines for writing and implementing uniform collaborative practice agreements that can be utilized by the state boards of pharmacy
TFCPA Recommendation 1
The Task Force on Collaborative Practice Agreements recorrunends to the Executive Committee and the Corrunittee on Law EnforcementLegislation that the following language be adopted for incorporation into the NABP Model State Pharmacy Act and Model Rules
Article I
Title Purpose Definition
Section 105 Definitions
(g) Collaborative Pharmacy Practice is that Practice of Pharmacy whereby a one or more Pharmacist~ has jointly agreed on a voluntary basis to work in conjunction with one or more Practitioners under protocol whereby the Pharmacist may perform certain patient care functions authorized by the Practitioner or Practitioners under certain specified conditions andor limitations
NATIONAL ASSOCIA nON OF BOARDS OF PHARMACYmiddot (P) 847391-4406 bull (F) 847391-4502 bull wwwnabpnel
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
(h) Collaborative Pharmacy Practice Agreement is a written and signed agreement between one or more Pharmacists and one or more Practitioners that provides for Collaborative Pharmacy Practice for the purpose of Drug Therapy Management of patients
(r) Drug Therapy Management means the review of Drug therapy regimen(s) of patients by one or more Pharmacists for the purpose of evaluating and rendering advice to one or more Practitioners regarding adjustment of the regimen Decisions involving Drug Therapy Management shall be made in the best interest of the patient Drug Therapy Management may include
(1) Implementing modifying and managing Drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement
(2) Collecting and reviewing patient Drug histories (3) Obtaining and checking vital signs including pulse temperature blood pressure
and respiration (4) Ordering and evaluating the results of laboratory tests directly relating to Drug
therapy when performed in accordance with approved protocols applicable to the practice setting and
(5) Such other patient care services as may be allowed by law
Pharmacists Scope of Practice Pursuant to the Collaborative Pharmacy Practice Agreement means those duties and limitations of duties placed upon one or more Pharmacists by the collaborating Practitioner or Practitioners the Board and applicable law and includes the limitations implied by the specialty practiced by the collaborating Practitioner or Practitioners
Model Rules for Pharmaceutical Care
Section 3 Pharmacy Practice
Collaborative Pharmacy Practice
(1) Collaborative Pharmacy Practice Agreement A Pharmacist planning to engage in Collaborative Pharmacy Practice shall have on file at his or her place of practice the written Collaborative Pharmacy Practice Agreement The existence and termination of such Agreement shall be reported to the Board and such Agreements shall be made available to the Board for review upon request The Agreement may allow the Pharmacist within the Pharmacists Scope of Practice Pursuant to the Collaborative Pharmacy Practice Agreement to conduct Drug Therapy Management activities approved by the Practitioner The collaboration that the Practitioner agrees to conduct with the Pharmacist must be within the scope of the Practitioners current practice Patients who receive services from one or more collaborating Pharmacists shall receive notification of receipt of such services
(2) Contents The Collaborative Pharmacy Practice Agreement shall include
(a) Identification of the Practitioner(s) and Pharmacist(s) who are parties to the Agreement
(b) The types of Drug Therapy Management decisions that the Pharmacist is allowed to make which may include
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpnet 2
Report of the Task Force on Collaborative Practice Agreements - NABP 9Smiddoth Annual Meeting Proceedings
(i) A detailed description of the types of diseases Drugs or Drug categories involved and the type of Drug Therapy Management allowed in each case
(ii) A detailed description of the methods procedures decision criteria and plan the Pharmacist is to follow when conducting Drug Therapy ~anagement and
(iii) A detailed description of the activities the Pharmacist is to follow in the course of conducting Drug Therapy Management including documentation of decisions made and a plan or appropriate mechanism for conununication feedback and reporting to the Practitioner concerning specific decisions made In addition to the Agreement documentation shall occur on the prescription record patient profile a separate log book or in some other appropriate system
(c) A method for the Practitioner to monitor compliance with the Agreement and clinical outcomes where Drug Therapy Management by the Pharmacist has occurred and to intercede where necessary
(d) A provision that allows the Practitioner to override a collaborative practice decision mad by the Pharmacist whenever he or she deems it necessary or appropriate
(e) A provision that allows either party to cancel the agreement by written notification
(f) An effective date and (g) Signatures of all collaborating Pharmacists and Practitioners who are party to the
agreement as well as the date of signing Amendments to a Collaborative Pharmacy Practice Agreement must be documented signed and dated
(3) Initiation of the Collaborative Pharmacy Practice Agreement The Collaborative Pharmacy Practice Agreement must be coupled with a medical order from the Practitioner to initiate Drug Therapy Management for any particular patient
(4) Documentation of Drug Therapy Management Docwnentation of Drug Therapy ~anagement must be kept as part of the patients permanent record and be readily available to other health care professionals providing care to that patient and who are authorized to received it Docwnentation of drug Therapy Management shall be considered Confidential Information
(5) Review At a minimum the written agreement shall be reviewed and renewed and if necessary revised every year
[NOTE the above new subsections would be inserted with sequential relettering of all subsequent subsections]
Background
Task Force members listened to a brief presentation by American College of Clinical Pharmacy (ACCP) Executive Director Robert Elenbaas and Immediate Past President Jerry Bauman regarding ACCPs position on collaborative practice agreements and later discussed their charge to develop national guidelines for writing and implementing uniform collaborative practice agreements for use by state boards of pharmacy They agreed that the best way to fulfill their charge was to create model regulations addressing the issue
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI3
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sb Annual Meeting Proceedings
To assist in developing the model regulations members looked to several sources of information including current state pharmacy laws and regulations as well as the information provided by Drs Elenbaas and Bauman The Task Force felt that the Idaho State Board of Pharmacys regulations on collaborative practice encompassed much of what they wanted to include in the model regulation therefore they were used as a blueprint
A brainstorming session brought issues and concerns regarding state regulation of collaborative pharmacy practice to the forefront Topics that members felt needed to be addressed in a model regulation included
Contents of collaborative pharmacy practice agreements
Practitioner override authority
Board approval of agreements
Phannacists documentation of care
Patient consentnotification
Confidentiality of patient information
Periodic review of agreement
Phannacist competencequalifications to provide drug therapy management services and
Quality assurance review of practice
Task Force members concurred on a majority of these topics and incorporated their determinations into the model recommendation Members agreed that certain key elements should be included in collaborative phannacy practice agreements so that such agreements may guide both the pharmacists and collaborating practitioners decisions and conduct Key elements incorporated included a description of the pharmacists activities a method describing a practitioner monitoring methods and a practitioner override clause
Members also agreed that it should not be necessary for boards of pharmacy to take on the responsibility for approving agreements or even requiring their submission but that boards should require parties to notify them of the existence of such agreements and to keep a copy at the practice site On this issue members concluded that the individual practitioners were the persons most qualified to approve the agreements noting that minimal government involvement in the practice of the phannacist and collaborating practitioner was the most desirable In order for the board to have some handle on the situation however it was determined that agreements should be made available for board inspection upon request Regarding he issue of pharmacist competence to provide drug therapy management services Task Force members concluded that the subject should not be addressed in the model regulations They agreed that he determination of competence should be left up to the individuals who are party to the agreement Members felt certain that most if not al1 collaborating practitioners would only delegate certain aspects of their patient care authority upon demonstration by the pharmacist of his or her abilities to provide such care Further members felt that the majority ofphannacists would not take on the responsibility for providing such care without being competent to do so
TFCP A Recommendation 2
The Task Force on Collaborative Practice Agreements recognized that the issue of quality care assurance is not still developing and at present cannot be addressed with regard to collaborative pharmacy practice The Task Force recommends that the NABP Executive Committee closely
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI4
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
follow this issue and should it be determined that language addressing the subject should be incorporated into the model regulation take further action at that time
Background
Task Force members were uncertain at this time of how to address the issue of quality care assurance Members noted that most states addressing collaborative pharmacy practice in their laws or regulations did not include a provision on this subject It was noted however that tat least one state placed the responsibility for quality assurance on the Principal pharmacist and practitioner and that ACCP urged that an appropriate body be responsible for such activities
Member postulated that collaborative practice activities would likely be subject to quality assurance review via physician peer-review processes Additionally they suggested that those parties practicing in institutional settings would follow the quality assurance policies guidelines andor requirements of the institution The Task Force felt it was premature to make a recommendation regarding those parties who practice in ambulatory settings and who may not be subject to quality assurance review Members observed that the work ofNABPs Task Force on Patient Outcomes Regulation should be reviewed before a recommendation was made
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406 (F) 847391-4502middot wwwnabpnet 5
58-1-107 Applicability -- Relationship to specific chapters under title The provisions of this chapter LJniformly apply to the administration and enforcement of this title However
unless expressly prohibited in this chapter any provision of this chapter may be supplemented or altered by specific chapters of this title
R1S6-1-107 Organization of Rules - Content Applicability and Relationship of Rules
(1) The rules and sections in Title R156 shall to the extent practicable follow the numbering and
organizational scheme of the chapters in Title 58
(2) Rule R156-1 shall contain general provisions applicable to the administration and enforcement of all
occupations and profeSSions regulated in Title 58
(3) The provisions of the other rules in Title R156 shall contain specific or unique provisions applicable to
particular occupations or professions
(4) Specific rules in Title R156 may supplement or alter Rule R156-1 unless expressly provided otherwise
in Rule R156-1
R1S6-17b-l04 Organization - Relationship to Rule R1S6-1
The organization of this rule and its relationship to Rule R156-1 is as described in Section R156-1-107
58-17b-50G Petitioning for reinstatement of licensure Any person whose license to practice pharmacy in this state has been revoked suspended or surrendered
voluntarily or by action of the division shall have the right at reasonable intervals to petition the division for reinstatement of such license Such petition shall be made in writing and in the form prescribed by the division Upon investigation and hearing the division may in its discretion grant or deny such petition or it may modify its driginal finding to reflect any circumstances that have changed sufficiently to warrant such modifications Themiddotdivision also at its discretion may require such person to pass an examination or examinations for reshyentry into the practice of pharmacy Enacted by Chapter 280 2004 General Session
Agenda Board of Pharmacy
Collaborative Practice Agreement Discussion 100 PM - 200 PM
-~ Agenda Item Overview - I
Welcome and Introductions Paige Patterick and Carrie Dunford
Discuss the wording of 58-17b-l 02( 17) (17) Collaborative pharmacy practice agreement means a written and signed agreement between one or more pharmacists and one or more practitioners that provides for collaborative pharmacy practice for the purpose of drug therapy management of patients and prevention of disease of human subjects
Discuss the definition of Drug Therapy Management in R 156-17b-l 02 (19)
(J 9) Drug therapy management means the review of a drug therapy regimen of a patient by one or more pharmacists for the purpose of evaluating and rendering advice to one or more practitioners regarding adjustment of the regimen
Resources
NABP https llnabppharmacywp-contentuploads20 1607 ITFCollaborativePracticeAgreements AM95 Dec 1998 pdf
Operating Standards Drug therapy management R 156-1 7b-61 I
R 156-l7b-611 Operating Standards - Drug Therapy Management (I) In accordance with Subsections 58-17b-l 02( 17) and 58-l7b-60 I( I) decisions involving drug therapy management shall be made in the best interest of the patient Drug therapy management may include (a) implementing modifying and managing drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement (b) collecting and reviewing patient histories (c) obtaining and checking vital signs including pulse temperature blood pressure and respiration (d) ordering and evaluating the results of laboratory tests directly applicable to the drug therapy when perfolmed in accordance with approved protocols applicable to the practice setting and (e) such other patient care services as may be allowed by rule (2) For the purpose of promoting therapeutic appropriateness a pharmacist shall at the time of dispensing a prescription or a prescription drug order review the patients medication record Such review shall at a minimum identify clinically significant conditions situations or items such as (a) inappropriate drug utilization (b) therapeutic duplication (c) drug-disease contraindications (d) drug-drug interactions (e) incorrect drug dosage or duration of drug treatment (f) drug-allergy interactions and (g) clinical abuse or misuse (3) Upon identifying any clinically significant conditions situations or items listed in Subsection (2) above the pharmacist shall take appropriate steps to avoid or resolve the problem including consultation with the prescribing practitioner
Note In compliance with the Americans with Disabilities Act individuals needing special accommodations (including auxiliary communicative aids and services) during this meet ing should not ify Dave Taylor ADA Coordinator at least three working days prior to the meeting Division of Occupational amp Professional Licensing 160 East 300 South Salt Lake City Utah 84115 801-530-6628 or toll-free in Utah only 866-275-3675 Posted to Bulletin Board on 11-21-2017

WORKGROUP RECOMMENDATIONS The workgroup took the approach that rapid innovation in education training technology and evidence-based gUidelines necessitate a collaborative practice framework that is flexible and facilitates innovation in care delivery Thus the following statements include two levels of recommendations
1 Elements of collaborative practice authority that should be codified in state law andor state regulations and
2 Elements that are more appropriately determined by the parties at the practice level who voluntarily enter into a CPA and thus for which the laws and regulations should be silent
The workgroup views both levels of recommendations as needed and synergistic State law andor regulations if too restrictive can impede innovative team-based care models
COLLABORATIVE PRACTICE AGREEMENT PARTICIPANTS
RECOMMENDED ELEMENTS FOR INCLUSION IN STATE LAWS ANDOR REGULATIONS
Collaborative practice laws andor regulations should specify that
bull Any practitioner with prescriptive authority may collaborate with pharmacists using a CPA
bull CPAs may be between a single or multiple pharmacists and a single or multiple prescribers
bull CPAs may apply to a single patient multiple patients or patient populations as specified in the agreement
ELEMENTS THAT MAY BE DETERMINED AT THE PRACTITIONER LEVEL
Individual CPAs may address the below elements but state laws andor regulations should be silent
bull CPAs should specify which patient(s) andor patient population(s) can receive services under the agreement
bull Depending on the complexity of the services being provided under a CPA it may be appropriate for the pharmacist to have additional credentials or training beyond what is required for licensure
bull CPAs should specify which pharmacist(s) may provide services under the CPA A pharmacists practice setting should not be a barrier to their ability to enter into a CPA
COLLABORATIVE PRACTICE AGREEIVlEIlT AUTHORIZED SERVICES
RECOMMENDED ELEMENTS FOR INCLUSION IN STATE LAWS ANDOR REGULATIONS
Collaborative practice laws andor regulations should specify that
bull The initiation and modification of drug therapy may be authorized under a CPA with a prescriber
ELEMENTS THAT MAY BE DETERMINED AT THE PRACTITIONER LEVEL
Individual CPAs may address the below elements but state laws andor regulations should be silent
bull In some situations the use of an evidence-based protocol can ensure optimal care when pharmacists are initiating or modifying drug therapy and may be included in the CPA though they may not be needed or appropriate in others
2
bull Performing physical assessments as well as ordering performing or interpreting laboratory tests (eg CLiA-waived tests) may be included in a CPA to help identify or refer patients for services however a pharmacist may also perform these services without a CPA as these activities should fall within pharmacists standard scope of practice
bull Specific disease states may be included in a CPA at the participating practitioners discretion
COLLABORATIVE PRACTICE AGREEMENT REQUIREMENTS AND RESTRICTIONS
RECOMMENDED ELEMENTS FOR INCLUSION IN STATE LAWS ANDOR REGULATIONS
Collaborative practice laws andor regulations should specify that
bull All prescription drugs including controlled substances may be included within pharmacists collaborative practice authority
bull CPAs should be maintained by the pharmacist(s) and collaborating prescriber(s) and be available upon request or inspection
ELEMENTS THAT MAY BE DETERMINED AT THE PRACTITIONER LEVEL
Individual CPAs may address the below elements but state laws andor regulations should be silent
bull Pharmacist(s) and prescriber(s) may specify the level of patient involvement in the CPA Depending on the level of service elements such as informed consent written consent or optshyout provisions may be appropriate as determined by the parties to the agreement
bull Agreements should not be required to be sent to or approved by a state regulatory board or other agency such requirements create unnecessary paperwork burden and slow the efficiency of care delivery
bull Collaborating practitioners are encouraged to review andor renew their CPAs within a timeframe that is clinically appropriate
bull Collaborating practitioners should conform to evidence-based guidelines and the agreed upon process of care with regards to the documentation requirements and the collaborating practitioners responsibility for review of services provided under the agreement
bull Practitioners may consider liability insurance provisions and the appropriateness of articulating these in their voluntary agreement
bull It is the professional duty of all healthcare professionals to stay current in the clinical areas in which they practice If individual practitioners determine that continuing education requirements are appropriate for their clinical arrangement they may be specified in the agreement
3
APPENDIX A WORKGROUP PARTICIPANTS
The individuals listed below were appointed to participate in the Collaborative Practice Workgroup by one of two methods NASPA invited all Joint Commission of Pharmacy Practitioners (JCPP) member organizations CEOs to appoint a representative from their organization to participate (an invitation for an appointment was also extended to the National Association of Chain Drug Stores who currently is not a member of JCPP) It was recommended that professional affairs staff be considered for this work
State representatives were nominated by state pharmacy associations and appointed by the NASPA Executive Committee The selection process was intended to produce a group of participants who had experience with CPAs and practice in a variety of settings
Of note participants were only asked to represent their own opinions Participants from the national pharmacy associations were not acting as representatives of their organizations but rather as individuals whose experiences with their various memberships provide them with an informed perspective
Name StateNationa I OrganizationState
Alex Adams National NACDS
Jennifer Bacci State Pennsylvania
Lynette Bradley-Baker National AACP
Anne Burns National APhA
Carolyn Ha National NCPA
Julie Johnson State Minnesota
Sandra Leal State Arizona
Christine Lee-Wilson State Maryland
Dianne Miller State Michigan
Susan Oh National AMCP
Anthony Pudlo State Iowa
Kelly Ridgway State Arizona
Scotti Russell National NABP
Douglas Scheckel hoff National ASHP
L Michelle Vaughn State Alaska
Pete Vlasses National ACPE
Ed Webb National ACCP
Bryan Ziegler State South Carolina
4
APPENDIX B MODIFIED DELPHI METHOD
The goal of the workgroup was to reach consensus on each of the elements discussed To do this a modified Delphi method was used A survey was sent to all participants to collect their initial thoughts on each of the elements identified in currently existing collaborative practice authority laws andor regulations Participants were given the current variations of each element as a multiple-choice selection with the opportunity to answer in free form text if the desired option was not listed After completion of the survey the workgroup discussed all questions where consensus was not already reached via conference call The conference call discussions were structured to have a defined period of time for discussion followed by a summary of the current options being discussed and a roll call vote by each of the participants The item was included on the next survey if consensus was not reached on the conference call This process was repeated a total of three times before the group reached consensus on all items being considered See below for a diagram of the process used
1 Level-setting conference call
2 Distribution of survey with 3 weeks to complete
5
Task Force on
Collaborative Practice Agreements
Members Present
Sharlea M Leatherwood Chair (MO) Michael J Ayotte (VA) Winifred A Landis (IN) Jeffery Lindoo (NIN) C Ann Perry (GA) Charles R Young (MA)
Others Present
Joseph A Whaley Jr Executive Committee Liaison Carmen A Catizone NABP Executive DirectorSecretary Melissa Madigan NABP Staff
Introduction
The Task Force on Collaborative Practice Agreements (TFCPA) met December 3 1998 at the Marriott Suites Hotel in Rosemont Illinois The Task Force was established by the NABP Executive Corrunittee in response to recent increases in the number of prescribers and pharmacists entering into collaborative practice agreements and requests that boards of pharmacy define the elements of an appropriate collaborative practice agreement
Charge of the Task Force on Collaborative Practice Agreements
Task Force members reviewed their charge and proposing no changes accepted it as follows
Review the available literature and information pertaining to collaborative practice agreements between prescribers and pharmacists
bull Develop national model guidelines for writing and implementing uniform collaborative practice agreements that can be utilized by the state boards of pharmacy
TFCPA Recommendation 1
The Task Force on Collaborative Practice Agreements recorrunends to the Executive Committee and the Corrunittee on Law EnforcementLegislation that the following language be adopted for incorporation into the NABP Model State Pharmacy Act and Model Rules
Article I
Title Purpose Definition
Section 105 Definitions
(g) Collaborative Pharmacy Practice is that Practice of Pharmacy whereby a one or more Pharmacist~ has jointly agreed on a voluntary basis to work in conjunction with one or more Practitioners under protocol whereby the Pharmacist may perform certain patient care functions authorized by the Practitioner or Practitioners under certain specified conditions andor limitations
NATIONAL ASSOCIA nON OF BOARDS OF PHARMACYmiddot (P) 847391-4406 bull (F) 847391-4502 bull wwwnabpnel
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
(h) Collaborative Pharmacy Practice Agreement is a written and signed agreement between one or more Pharmacists and one or more Practitioners that provides for Collaborative Pharmacy Practice for the purpose of Drug Therapy Management of patients
(r) Drug Therapy Management means the review of Drug therapy regimen(s) of patients by one or more Pharmacists for the purpose of evaluating and rendering advice to one or more Practitioners regarding adjustment of the regimen Decisions involving Drug Therapy Management shall be made in the best interest of the patient Drug Therapy Management may include
(1) Implementing modifying and managing Drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement
(2) Collecting and reviewing patient Drug histories (3) Obtaining and checking vital signs including pulse temperature blood pressure
and respiration (4) Ordering and evaluating the results of laboratory tests directly relating to Drug
therapy when performed in accordance with approved protocols applicable to the practice setting and
(5) Such other patient care services as may be allowed by law
Pharmacists Scope of Practice Pursuant to the Collaborative Pharmacy Practice Agreement means those duties and limitations of duties placed upon one or more Pharmacists by the collaborating Practitioner or Practitioners the Board and applicable law and includes the limitations implied by the specialty practiced by the collaborating Practitioner or Practitioners
Model Rules for Pharmaceutical Care
Section 3 Pharmacy Practice
Collaborative Pharmacy Practice
(1) Collaborative Pharmacy Practice Agreement A Pharmacist planning to engage in Collaborative Pharmacy Practice shall have on file at his or her place of practice the written Collaborative Pharmacy Practice Agreement The existence and termination of such Agreement shall be reported to the Board and such Agreements shall be made available to the Board for review upon request The Agreement may allow the Pharmacist within the Pharmacists Scope of Practice Pursuant to the Collaborative Pharmacy Practice Agreement to conduct Drug Therapy Management activities approved by the Practitioner The collaboration that the Practitioner agrees to conduct with the Pharmacist must be within the scope of the Practitioners current practice Patients who receive services from one or more collaborating Pharmacists shall receive notification of receipt of such services
(2) Contents The Collaborative Pharmacy Practice Agreement shall include
(a) Identification of the Practitioner(s) and Pharmacist(s) who are parties to the Agreement
(b) The types of Drug Therapy Management decisions that the Pharmacist is allowed to make which may include
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpnet 2
Report of the Task Force on Collaborative Practice Agreements - NABP 9Smiddoth Annual Meeting Proceedings
(i) A detailed description of the types of diseases Drugs or Drug categories involved and the type of Drug Therapy Management allowed in each case
(ii) A detailed description of the methods procedures decision criteria and plan the Pharmacist is to follow when conducting Drug Therapy ~anagement and
(iii) A detailed description of the activities the Pharmacist is to follow in the course of conducting Drug Therapy Management including documentation of decisions made and a plan or appropriate mechanism for conununication feedback and reporting to the Practitioner concerning specific decisions made In addition to the Agreement documentation shall occur on the prescription record patient profile a separate log book or in some other appropriate system
(c) A method for the Practitioner to monitor compliance with the Agreement and clinical outcomes where Drug Therapy Management by the Pharmacist has occurred and to intercede where necessary
(d) A provision that allows the Practitioner to override a collaborative practice decision mad by the Pharmacist whenever he or she deems it necessary or appropriate
(e) A provision that allows either party to cancel the agreement by written notification
(f) An effective date and (g) Signatures of all collaborating Pharmacists and Practitioners who are party to the
agreement as well as the date of signing Amendments to a Collaborative Pharmacy Practice Agreement must be documented signed and dated
(3) Initiation of the Collaborative Pharmacy Practice Agreement The Collaborative Pharmacy Practice Agreement must be coupled with a medical order from the Practitioner to initiate Drug Therapy Management for any particular patient
(4) Documentation of Drug Therapy Management Docwnentation of Drug Therapy ~anagement must be kept as part of the patients permanent record and be readily available to other health care professionals providing care to that patient and who are authorized to received it Docwnentation of drug Therapy Management shall be considered Confidential Information
(5) Review At a minimum the written agreement shall be reviewed and renewed and if necessary revised every year
[NOTE the above new subsections would be inserted with sequential relettering of all subsequent subsections]
Background
Task Force members listened to a brief presentation by American College of Clinical Pharmacy (ACCP) Executive Director Robert Elenbaas and Immediate Past President Jerry Bauman regarding ACCPs position on collaborative practice agreements and later discussed their charge to develop national guidelines for writing and implementing uniform collaborative practice agreements for use by state boards of pharmacy They agreed that the best way to fulfill their charge was to create model regulations addressing the issue
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI3
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sb Annual Meeting Proceedings
To assist in developing the model regulations members looked to several sources of information including current state pharmacy laws and regulations as well as the information provided by Drs Elenbaas and Bauman The Task Force felt that the Idaho State Board of Pharmacys regulations on collaborative practice encompassed much of what they wanted to include in the model regulation therefore they were used as a blueprint
A brainstorming session brought issues and concerns regarding state regulation of collaborative pharmacy practice to the forefront Topics that members felt needed to be addressed in a model regulation included
Contents of collaborative pharmacy practice agreements
Practitioner override authority
Board approval of agreements
Phannacists documentation of care
Patient consentnotification
Confidentiality of patient information
Periodic review of agreement
Phannacist competencequalifications to provide drug therapy management services and
Quality assurance review of practice
Task Force members concurred on a majority of these topics and incorporated their determinations into the model recommendation Members agreed that certain key elements should be included in collaborative phannacy practice agreements so that such agreements may guide both the pharmacists and collaborating practitioners decisions and conduct Key elements incorporated included a description of the pharmacists activities a method describing a practitioner monitoring methods and a practitioner override clause
Members also agreed that it should not be necessary for boards of pharmacy to take on the responsibility for approving agreements or even requiring their submission but that boards should require parties to notify them of the existence of such agreements and to keep a copy at the practice site On this issue members concluded that the individual practitioners were the persons most qualified to approve the agreements noting that minimal government involvement in the practice of the phannacist and collaborating practitioner was the most desirable In order for the board to have some handle on the situation however it was determined that agreements should be made available for board inspection upon request Regarding he issue of pharmacist competence to provide drug therapy management services Task Force members concluded that the subject should not be addressed in the model regulations They agreed that he determination of competence should be left up to the individuals who are party to the agreement Members felt certain that most if not al1 collaborating practitioners would only delegate certain aspects of their patient care authority upon demonstration by the pharmacist of his or her abilities to provide such care Further members felt that the majority ofphannacists would not take on the responsibility for providing such care without being competent to do so
TFCP A Recommendation 2
The Task Force on Collaborative Practice Agreements recognized that the issue of quality care assurance is not still developing and at present cannot be addressed with regard to collaborative pharmacy practice The Task Force recommends that the NABP Executive Committee closely
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI4
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
follow this issue and should it be determined that language addressing the subject should be incorporated into the model regulation take further action at that time
Background
Task Force members were uncertain at this time of how to address the issue of quality care assurance Members noted that most states addressing collaborative pharmacy practice in their laws or regulations did not include a provision on this subject It was noted however that tat least one state placed the responsibility for quality assurance on the Principal pharmacist and practitioner and that ACCP urged that an appropriate body be responsible for such activities
Member postulated that collaborative practice activities would likely be subject to quality assurance review via physician peer-review processes Additionally they suggested that those parties practicing in institutional settings would follow the quality assurance policies guidelines andor requirements of the institution The Task Force felt it was premature to make a recommendation regarding those parties who practice in ambulatory settings and who may not be subject to quality assurance review Members observed that the work ofNABPs Task Force on Patient Outcomes Regulation should be reviewed before a recommendation was made
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406 (F) 847391-4502middot wwwnabpnet 5
58-1-107 Applicability -- Relationship to specific chapters under title The provisions of this chapter LJniformly apply to the administration and enforcement of this title However
unless expressly prohibited in this chapter any provision of this chapter may be supplemented or altered by specific chapters of this title
R1S6-1-107 Organization of Rules - Content Applicability and Relationship of Rules
(1) The rules and sections in Title R156 shall to the extent practicable follow the numbering and
organizational scheme of the chapters in Title 58
(2) Rule R156-1 shall contain general provisions applicable to the administration and enforcement of all
occupations and profeSSions regulated in Title 58
(3) The provisions of the other rules in Title R156 shall contain specific or unique provisions applicable to
particular occupations or professions
(4) Specific rules in Title R156 may supplement or alter Rule R156-1 unless expressly provided otherwise
in Rule R156-1
R1S6-17b-l04 Organization - Relationship to Rule R1S6-1
The organization of this rule and its relationship to Rule R156-1 is as described in Section R156-1-107
58-17b-50G Petitioning for reinstatement of licensure Any person whose license to practice pharmacy in this state has been revoked suspended or surrendered
voluntarily or by action of the division shall have the right at reasonable intervals to petition the division for reinstatement of such license Such petition shall be made in writing and in the form prescribed by the division Upon investigation and hearing the division may in its discretion grant or deny such petition or it may modify its driginal finding to reflect any circumstances that have changed sufficiently to warrant such modifications Themiddotdivision also at its discretion may require such person to pass an examination or examinations for reshyentry into the practice of pharmacy Enacted by Chapter 280 2004 General Session
Agenda Board of Pharmacy
Collaborative Practice Agreement Discussion 100 PM - 200 PM
-~ Agenda Item Overview - I
Welcome and Introductions Paige Patterick and Carrie Dunford
Discuss the wording of 58-17b-l 02( 17) (17) Collaborative pharmacy practice agreement means a written and signed agreement between one or more pharmacists and one or more practitioners that provides for collaborative pharmacy practice for the purpose of drug therapy management of patients and prevention of disease of human subjects
Discuss the definition of Drug Therapy Management in R 156-17b-l 02 (19)
(J 9) Drug therapy management means the review of a drug therapy regimen of a patient by one or more pharmacists for the purpose of evaluating and rendering advice to one or more practitioners regarding adjustment of the regimen
Resources
NABP https llnabppharmacywp-contentuploads20 1607 ITFCollaborativePracticeAgreements AM95 Dec 1998 pdf
Operating Standards Drug therapy management R 156-1 7b-61 I
R 156-l7b-611 Operating Standards - Drug Therapy Management (I) In accordance with Subsections 58-17b-l 02( 17) and 58-l7b-60 I( I) decisions involving drug therapy management shall be made in the best interest of the patient Drug therapy management may include (a) implementing modifying and managing drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement (b) collecting and reviewing patient histories (c) obtaining and checking vital signs including pulse temperature blood pressure and respiration (d) ordering and evaluating the results of laboratory tests directly applicable to the drug therapy when perfolmed in accordance with approved protocols applicable to the practice setting and (e) such other patient care services as may be allowed by rule (2) For the purpose of promoting therapeutic appropriateness a pharmacist shall at the time of dispensing a prescription or a prescription drug order review the patients medication record Such review shall at a minimum identify clinically significant conditions situations or items such as (a) inappropriate drug utilization (b) therapeutic duplication (c) drug-disease contraindications (d) drug-drug interactions (e) incorrect drug dosage or duration of drug treatment (f) drug-allergy interactions and (g) clinical abuse or misuse (3) Upon identifying any clinically significant conditions situations or items listed in Subsection (2) above the pharmacist shall take appropriate steps to avoid or resolve the problem including consultation with the prescribing practitioner
Note In compliance with the Americans with Disabilities Act individuals needing special accommodations (including auxiliary communicative aids and services) during this meet ing should not ify Dave Taylor ADA Coordinator at least three working days prior to the meeting Division of Occupational amp Professional Licensing 160 East 300 South Salt Lake City Utah 84115 801-530-6628 or toll-free in Utah only 866-275-3675 Posted to Bulletin Board on 11-21-2017

bull Performing physical assessments as well as ordering performing or interpreting laboratory tests (eg CLiA-waived tests) may be included in a CPA to help identify or refer patients for services however a pharmacist may also perform these services without a CPA as these activities should fall within pharmacists standard scope of practice
bull Specific disease states may be included in a CPA at the participating practitioners discretion
COLLABORATIVE PRACTICE AGREEMENT REQUIREMENTS AND RESTRICTIONS
RECOMMENDED ELEMENTS FOR INCLUSION IN STATE LAWS ANDOR REGULATIONS
Collaborative practice laws andor regulations should specify that
bull All prescription drugs including controlled substances may be included within pharmacists collaborative practice authority
bull CPAs should be maintained by the pharmacist(s) and collaborating prescriber(s) and be available upon request or inspection
ELEMENTS THAT MAY BE DETERMINED AT THE PRACTITIONER LEVEL
Individual CPAs may address the below elements but state laws andor regulations should be silent
bull Pharmacist(s) and prescriber(s) may specify the level of patient involvement in the CPA Depending on the level of service elements such as informed consent written consent or optshyout provisions may be appropriate as determined by the parties to the agreement
bull Agreements should not be required to be sent to or approved by a state regulatory board or other agency such requirements create unnecessary paperwork burden and slow the efficiency of care delivery
bull Collaborating practitioners are encouraged to review andor renew their CPAs within a timeframe that is clinically appropriate
bull Collaborating practitioners should conform to evidence-based guidelines and the agreed upon process of care with regards to the documentation requirements and the collaborating practitioners responsibility for review of services provided under the agreement
bull Practitioners may consider liability insurance provisions and the appropriateness of articulating these in their voluntary agreement
bull It is the professional duty of all healthcare professionals to stay current in the clinical areas in which they practice If individual practitioners determine that continuing education requirements are appropriate for their clinical arrangement they may be specified in the agreement
3
APPENDIX A WORKGROUP PARTICIPANTS
The individuals listed below were appointed to participate in the Collaborative Practice Workgroup by one of two methods NASPA invited all Joint Commission of Pharmacy Practitioners (JCPP) member organizations CEOs to appoint a representative from their organization to participate (an invitation for an appointment was also extended to the National Association of Chain Drug Stores who currently is not a member of JCPP) It was recommended that professional affairs staff be considered for this work
State representatives were nominated by state pharmacy associations and appointed by the NASPA Executive Committee The selection process was intended to produce a group of participants who had experience with CPAs and practice in a variety of settings
Of note participants were only asked to represent their own opinions Participants from the national pharmacy associations were not acting as representatives of their organizations but rather as individuals whose experiences with their various memberships provide them with an informed perspective
Name StateNationa I OrganizationState
Alex Adams National NACDS
Jennifer Bacci State Pennsylvania
Lynette Bradley-Baker National AACP
Anne Burns National APhA
Carolyn Ha National NCPA
Julie Johnson State Minnesota
Sandra Leal State Arizona
Christine Lee-Wilson State Maryland
Dianne Miller State Michigan
Susan Oh National AMCP
Anthony Pudlo State Iowa
Kelly Ridgway State Arizona
Scotti Russell National NABP
Douglas Scheckel hoff National ASHP
L Michelle Vaughn State Alaska
Pete Vlasses National ACPE
Ed Webb National ACCP
Bryan Ziegler State South Carolina
4
APPENDIX B MODIFIED DELPHI METHOD
The goal of the workgroup was to reach consensus on each of the elements discussed To do this a modified Delphi method was used A survey was sent to all participants to collect their initial thoughts on each of the elements identified in currently existing collaborative practice authority laws andor regulations Participants were given the current variations of each element as a multiple-choice selection with the opportunity to answer in free form text if the desired option was not listed After completion of the survey the workgroup discussed all questions where consensus was not already reached via conference call The conference call discussions were structured to have a defined period of time for discussion followed by a summary of the current options being discussed and a roll call vote by each of the participants The item was included on the next survey if consensus was not reached on the conference call This process was repeated a total of three times before the group reached consensus on all items being considered See below for a diagram of the process used
1 Level-setting conference call
2 Distribution of survey with 3 weeks to complete
5
Task Force on
Collaborative Practice Agreements
Members Present
Sharlea M Leatherwood Chair (MO) Michael J Ayotte (VA) Winifred A Landis (IN) Jeffery Lindoo (NIN) C Ann Perry (GA) Charles R Young (MA)
Others Present
Joseph A Whaley Jr Executive Committee Liaison Carmen A Catizone NABP Executive DirectorSecretary Melissa Madigan NABP Staff
Introduction
The Task Force on Collaborative Practice Agreements (TFCPA) met December 3 1998 at the Marriott Suites Hotel in Rosemont Illinois The Task Force was established by the NABP Executive Corrunittee in response to recent increases in the number of prescribers and pharmacists entering into collaborative practice agreements and requests that boards of pharmacy define the elements of an appropriate collaborative practice agreement
Charge of the Task Force on Collaborative Practice Agreements
Task Force members reviewed their charge and proposing no changes accepted it as follows
Review the available literature and information pertaining to collaborative practice agreements between prescribers and pharmacists
bull Develop national model guidelines for writing and implementing uniform collaborative practice agreements that can be utilized by the state boards of pharmacy
TFCPA Recommendation 1
The Task Force on Collaborative Practice Agreements recorrunends to the Executive Committee and the Corrunittee on Law EnforcementLegislation that the following language be adopted for incorporation into the NABP Model State Pharmacy Act and Model Rules
Article I
Title Purpose Definition
Section 105 Definitions
(g) Collaborative Pharmacy Practice is that Practice of Pharmacy whereby a one or more Pharmacist~ has jointly agreed on a voluntary basis to work in conjunction with one or more Practitioners under protocol whereby the Pharmacist may perform certain patient care functions authorized by the Practitioner or Practitioners under certain specified conditions andor limitations
NATIONAL ASSOCIA nON OF BOARDS OF PHARMACYmiddot (P) 847391-4406 bull (F) 847391-4502 bull wwwnabpnel
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
(h) Collaborative Pharmacy Practice Agreement is a written and signed agreement between one or more Pharmacists and one or more Practitioners that provides for Collaborative Pharmacy Practice for the purpose of Drug Therapy Management of patients
(r) Drug Therapy Management means the review of Drug therapy regimen(s) of patients by one or more Pharmacists for the purpose of evaluating and rendering advice to one or more Practitioners regarding adjustment of the regimen Decisions involving Drug Therapy Management shall be made in the best interest of the patient Drug Therapy Management may include
(1) Implementing modifying and managing Drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement
(2) Collecting and reviewing patient Drug histories (3) Obtaining and checking vital signs including pulse temperature blood pressure
and respiration (4) Ordering and evaluating the results of laboratory tests directly relating to Drug
therapy when performed in accordance with approved protocols applicable to the practice setting and
(5) Such other patient care services as may be allowed by law
Pharmacists Scope of Practice Pursuant to the Collaborative Pharmacy Practice Agreement means those duties and limitations of duties placed upon one or more Pharmacists by the collaborating Practitioner or Practitioners the Board and applicable law and includes the limitations implied by the specialty practiced by the collaborating Practitioner or Practitioners
Model Rules for Pharmaceutical Care
Section 3 Pharmacy Practice
Collaborative Pharmacy Practice
(1) Collaborative Pharmacy Practice Agreement A Pharmacist planning to engage in Collaborative Pharmacy Practice shall have on file at his or her place of practice the written Collaborative Pharmacy Practice Agreement The existence and termination of such Agreement shall be reported to the Board and such Agreements shall be made available to the Board for review upon request The Agreement may allow the Pharmacist within the Pharmacists Scope of Practice Pursuant to the Collaborative Pharmacy Practice Agreement to conduct Drug Therapy Management activities approved by the Practitioner The collaboration that the Practitioner agrees to conduct with the Pharmacist must be within the scope of the Practitioners current practice Patients who receive services from one or more collaborating Pharmacists shall receive notification of receipt of such services
(2) Contents The Collaborative Pharmacy Practice Agreement shall include
(a) Identification of the Practitioner(s) and Pharmacist(s) who are parties to the Agreement
(b) The types of Drug Therapy Management decisions that the Pharmacist is allowed to make which may include
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpnet 2
Report of the Task Force on Collaborative Practice Agreements - NABP 9Smiddoth Annual Meeting Proceedings
(i) A detailed description of the types of diseases Drugs or Drug categories involved and the type of Drug Therapy Management allowed in each case
(ii) A detailed description of the methods procedures decision criteria and plan the Pharmacist is to follow when conducting Drug Therapy ~anagement and
(iii) A detailed description of the activities the Pharmacist is to follow in the course of conducting Drug Therapy Management including documentation of decisions made and a plan or appropriate mechanism for conununication feedback and reporting to the Practitioner concerning specific decisions made In addition to the Agreement documentation shall occur on the prescription record patient profile a separate log book or in some other appropriate system
(c) A method for the Practitioner to monitor compliance with the Agreement and clinical outcomes where Drug Therapy Management by the Pharmacist has occurred and to intercede where necessary
(d) A provision that allows the Practitioner to override a collaborative practice decision mad by the Pharmacist whenever he or she deems it necessary or appropriate
(e) A provision that allows either party to cancel the agreement by written notification
(f) An effective date and (g) Signatures of all collaborating Pharmacists and Practitioners who are party to the
agreement as well as the date of signing Amendments to a Collaborative Pharmacy Practice Agreement must be documented signed and dated
(3) Initiation of the Collaborative Pharmacy Practice Agreement The Collaborative Pharmacy Practice Agreement must be coupled with a medical order from the Practitioner to initiate Drug Therapy Management for any particular patient
(4) Documentation of Drug Therapy Management Docwnentation of Drug Therapy ~anagement must be kept as part of the patients permanent record and be readily available to other health care professionals providing care to that patient and who are authorized to received it Docwnentation of drug Therapy Management shall be considered Confidential Information
(5) Review At a minimum the written agreement shall be reviewed and renewed and if necessary revised every year
[NOTE the above new subsections would be inserted with sequential relettering of all subsequent subsections]
Background
Task Force members listened to a brief presentation by American College of Clinical Pharmacy (ACCP) Executive Director Robert Elenbaas and Immediate Past President Jerry Bauman regarding ACCPs position on collaborative practice agreements and later discussed their charge to develop national guidelines for writing and implementing uniform collaborative practice agreements for use by state boards of pharmacy They agreed that the best way to fulfill their charge was to create model regulations addressing the issue
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI3
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sb Annual Meeting Proceedings
To assist in developing the model regulations members looked to several sources of information including current state pharmacy laws and regulations as well as the information provided by Drs Elenbaas and Bauman The Task Force felt that the Idaho State Board of Pharmacys regulations on collaborative practice encompassed much of what they wanted to include in the model regulation therefore they were used as a blueprint
A brainstorming session brought issues and concerns regarding state regulation of collaborative pharmacy practice to the forefront Topics that members felt needed to be addressed in a model regulation included
Contents of collaborative pharmacy practice agreements
Practitioner override authority
Board approval of agreements
Phannacists documentation of care
Patient consentnotification
Confidentiality of patient information
Periodic review of agreement
Phannacist competencequalifications to provide drug therapy management services and
Quality assurance review of practice
Task Force members concurred on a majority of these topics and incorporated their determinations into the model recommendation Members agreed that certain key elements should be included in collaborative phannacy practice agreements so that such agreements may guide both the pharmacists and collaborating practitioners decisions and conduct Key elements incorporated included a description of the pharmacists activities a method describing a practitioner monitoring methods and a practitioner override clause
Members also agreed that it should not be necessary for boards of pharmacy to take on the responsibility for approving agreements or even requiring their submission but that boards should require parties to notify them of the existence of such agreements and to keep a copy at the practice site On this issue members concluded that the individual practitioners were the persons most qualified to approve the agreements noting that minimal government involvement in the practice of the phannacist and collaborating practitioner was the most desirable In order for the board to have some handle on the situation however it was determined that agreements should be made available for board inspection upon request Regarding he issue of pharmacist competence to provide drug therapy management services Task Force members concluded that the subject should not be addressed in the model regulations They agreed that he determination of competence should be left up to the individuals who are party to the agreement Members felt certain that most if not al1 collaborating practitioners would only delegate certain aspects of their patient care authority upon demonstration by the pharmacist of his or her abilities to provide such care Further members felt that the majority ofphannacists would not take on the responsibility for providing such care without being competent to do so
TFCP A Recommendation 2
The Task Force on Collaborative Practice Agreements recognized that the issue of quality care assurance is not still developing and at present cannot be addressed with regard to collaborative pharmacy practice The Task Force recommends that the NABP Executive Committee closely
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI4
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
follow this issue and should it be determined that language addressing the subject should be incorporated into the model regulation take further action at that time
Background
Task Force members were uncertain at this time of how to address the issue of quality care assurance Members noted that most states addressing collaborative pharmacy practice in their laws or regulations did not include a provision on this subject It was noted however that tat least one state placed the responsibility for quality assurance on the Principal pharmacist and practitioner and that ACCP urged that an appropriate body be responsible for such activities
Member postulated that collaborative practice activities would likely be subject to quality assurance review via physician peer-review processes Additionally they suggested that those parties practicing in institutional settings would follow the quality assurance policies guidelines andor requirements of the institution The Task Force felt it was premature to make a recommendation regarding those parties who practice in ambulatory settings and who may not be subject to quality assurance review Members observed that the work ofNABPs Task Force on Patient Outcomes Regulation should be reviewed before a recommendation was made
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406 (F) 847391-4502middot wwwnabpnet 5
58-1-107 Applicability -- Relationship to specific chapters under title The provisions of this chapter LJniformly apply to the administration and enforcement of this title However
unless expressly prohibited in this chapter any provision of this chapter may be supplemented or altered by specific chapters of this title
R1S6-1-107 Organization of Rules - Content Applicability and Relationship of Rules
(1) The rules and sections in Title R156 shall to the extent practicable follow the numbering and
organizational scheme of the chapters in Title 58
(2) Rule R156-1 shall contain general provisions applicable to the administration and enforcement of all
occupations and profeSSions regulated in Title 58
(3) The provisions of the other rules in Title R156 shall contain specific or unique provisions applicable to
particular occupations or professions
(4) Specific rules in Title R156 may supplement or alter Rule R156-1 unless expressly provided otherwise
in Rule R156-1
R1S6-17b-l04 Organization - Relationship to Rule R1S6-1
The organization of this rule and its relationship to Rule R156-1 is as described in Section R156-1-107
58-17b-50G Petitioning for reinstatement of licensure Any person whose license to practice pharmacy in this state has been revoked suspended or surrendered
voluntarily or by action of the division shall have the right at reasonable intervals to petition the division for reinstatement of such license Such petition shall be made in writing and in the form prescribed by the division Upon investigation and hearing the division may in its discretion grant or deny such petition or it may modify its driginal finding to reflect any circumstances that have changed sufficiently to warrant such modifications Themiddotdivision also at its discretion may require such person to pass an examination or examinations for reshyentry into the practice of pharmacy Enacted by Chapter 280 2004 General Session
Agenda Board of Pharmacy
Collaborative Practice Agreement Discussion 100 PM - 200 PM
-~ Agenda Item Overview - I
Welcome and Introductions Paige Patterick and Carrie Dunford
Discuss the wording of 58-17b-l 02( 17) (17) Collaborative pharmacy practice agreement means a written and signed agreement between one or more pharmacists and one or more practitioners that provides for collaborative pharmacy practice for the purpose of drug therapy management of patients and prevention of disease of human subjects
Discuss the definition of Drug Therapy Management in R 156-17b-l 02 (19)
(J 9) Drug therapy management means the review of a drug therapy regimen of a patient by one or more pharmacists for the purpose of evaluating and rendering advice to one or more practitioners regarding adjustment of the regimen
Resources
NABP https llnabppharmacywp-contentuploads20 1607 ITFCollaborativePracticeAgreements AM95 Dec 1998 pdf
Operating Standards Drug therapy management R 156-1 7b-61 I
R 156-l7b-611 Operating Standards - Drug Therapy Management (I) In accordance with Subsections 58-17b-l 02( 17) and 58-l7b-60 I( I) decisions involving drug therapy management shall be made in the best interest of the patient Drug therapy management may include (a) implementing modifying and managing drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement (b) collecting and reviewing patient histories (c) obtaining and checking vital signs including pulse temperature blood pressure and respiration (d) ordering and evaluating the results of laboratory tests directly applicable to the drug therapy when perfolmed in accordance with approved protocols applicable to the practice setting and (e) such other patient care services as may be allowed by rule (2) For the purpose of promoting therapeutic appropriateness a pharmacist shall at the time of dispensing a prescription or a prescription drug order review the patients medication record Such review shall at a minimum identify clinically significant conditions situations or items such as (a) inappropriate drug utilization (b) therapeutic duplication (c) drug-disease contraindications (d) drug-drug interactions (e) incorrect drug dosage or duration of drug treatment (f) drug-allergy interactions and (g) clinical abuse or misuse (3) Upon identifying any clinically significant conditions situations or items listed in Subsection (2) above the pharmacist shall take appropriate steps to avoid or resolve the problem including consultation with the prescribing practitioner
Note In compliance with the Americans with Disabilities Act individuals needing special accommodations (including auxiliary communicative aids and services) during this meet ing should not ify Dave Taylor ADA Coordinator at least three working days prior to the meeting Division of Occupational amp Professional Licensing 160 East 300 South Salt Lake City Utah 84115 801-530-6628 or toll-free in Utah only 866-275-3675 Posted to Bulletin Board on 11-21-2017

APPENDIX A WORKGROUP PARTICIPANTS
The individuals listed below were appointed to participate in the Collaborative Practice Workgroup by one of two methods NASPA invited all Joint Commission of Pharmacy Practitioners (JCPP) member organizations CEOs to appoint a representative from their organization to participate (an invitation for an appointment was also extended to the National Association of Chain Drug Stores who currently is not a member of JCPP) It was recommended that professional affairs staff be considered for this work
State representatives were nominated by state pharmacy associations and appointed by the NASPA Executive Committee The selection process was intended to produce a group of participants who had experience with CPAs and practice in a variety of settings
Of note participants were only asked to represent their own opinions Participants from the national pharmacy associations were not acting as representatives of their organizations but rather as individuals whose experiences with their various memberships provide them with an informed perspective
Name StateNationa I OrganizationState
Alex Adams National NACDS
Jennifer Bacci State Pennsylvania
Lynette Bradley-Baker National AACP
Anne Burns National APhA
Carolyn Ha National NCPA
Julie Johnson State Minnesota
Sandra Leal State Arizona
Christine Lee-Wilson State Maryland
Dianne Miller State Michigan
Susan Oh National AMCP
Anthony Pudlo State Iowa
Kelly Ridgway State Arizona
Scotti Russell National NABP
Douglas Scheckel hoff National ASHP
L Michelle Vaughn State Alaska
Pete Vlasses National ACPE
Ed Webb National ACCP
Bryan Ziegler State South Carolina
4
APPENDIX B MODIFIED DELPHI METHOD
The goal of the workgroup was to reach consensus on each of the elements discussed To do this a modified Delphi method was used A survey was sent to all participants to collect their initial thoughts on each of the elements identified in currently existing collaborative practice authority laws andor regulations Participants were given the current variations of each element as a multiple-choice selection with the opportunity to answer in free form text if the desired option was not listed After completion of the survey the workgroup discussed all questions where consensus was not already reached via conference call The conference call discussions were structured to have a defined period of time for discussion followed by a summary of the current options being discussed and a roll call vote by each of the participants The item was included on the next survey if consensus was not reached on the conference call This process was repeated a total of three times before the group reached consensus on all items being considered See below for a diagram of the process used
1 Level-setting conference call
2 Distribution of survey with 3 weeks to complete
5
Task Force on
Collaborative Practice Agreements
Members Present
Sharlea M Leatherwood Chair (MO) Michael J Ayotte (VA) Winifred A Landis (IN) Jeffery Lindoo (NIN) C Ann Perry (GA) Charles R Young (MA)
Others Present
Joseph A Whaley Jr Executive Committee Liaison Carmen A Catizone NABP Executive DirectorSecretary Melissa Madigan NABP Staff
Introduction
The Task Force on Collaborative Practice Agreements (TFCPA) met December 3 1998 at the Marriott Suites Hotel in Rosemont Illinois The Task Force was established by the NABP Executive Corrunittee in response to recent increases in the number of prescribers and pharmacists entering into collaborative practice agreements and requests that boards of pharmacy define the elements of an appropriate collaborative practice agreement
Charge of the Task Force on Collaborative Practice Agreements
Task Force members reviewed their charge and proposing no changes accepted it as follows
Review the available literature and information pertaining to collaborative practice agreements between prescribers and pharmacists
bull Develop national model guidelines for writing and implementing uniform collaborative practice agreements that can be utilized by the state boards of pharmacy
TFCPA Recommendation 1
The Task Force on Collaborative Practice Agreements recorrunends to the Executive Committee and the Corrunittee on Law EnforcementLegislation that the following language be adopted for incorporation into the NABP Model State Pharmacy Act and Model Rules
Article I
Title Purpose Definition
Section 105 Definitions
(g) Collaborative Pharmacy Practice is that Practice of Pharmacy whereby a one or more Pharmacist~ has jointly agreed on a voluntary basis to work in conjunction with one or more Practitioners under protocol whereby the Pharmacist may perform certain patient care functions authorized by the Practitioner or Practitioners under certain specified conditions andor limitations
NATIONAL ASSOCIA nON OF BOARDS OF PHARMACYmiddot (P) 847391-4406 bull (F) 847391-4502 bull wwwnabpnel
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
(h) Collaborative Pharmacy Practice Agreement is a written and signed agreement between one or more Pharmacists and one or more Practitioners that provides for Collaborative Pharmacy Practice for the purpose of Drug Therapy Management of patients
(r) Drug Therapy Management means the review of Drug therapy regimen(s) of patients by one or more Pharmacists for the purpose of evaluating and rendering advice to one or more Practitioners regarding adjustment of the regimen Decisions involving Drug Therapy Management shall be made in the best interest of the patient Drug Therapy Management may include
(1) Implementing modifying and managing Drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement
(2) Collecting and reviewing patient Drug histories (3) Obtaining and checking vital signs including pulse temperature blood pressure
and respiration (4) Ordering and evaluating the results of laboratory tests directly relating to Drug
therapy when performed in accordance with approved protocols applicable to the practice setting and
(5) Such other patient care services as may be allowed by law
Pharmacists Scope of Practice Pursuant to the Collaborative Pharmacy Practice Agreement means those duties and limitations of duties placed upon one or more Pharmacists by the collaborating Practitioner or Practitioners the Board and applicable law and includes the limitations implied by the specialty practiced by the collaborating Practitioner or Practitioners
Model Rules for Pharmaceutical Care
Section 3 Pharmacy Practice
Collaborative Pharmacy Practice
(1) Collaborative Pharmacy Practice Agreement A Pharmacist planning to engage in Collaborative Pharmacy Practice shall have on file at his or her place of practice the written Collaborative Pharmacy Practice Agreement The existence and termination of such Agreement shall be reported to the Board and such Agreements shall be made available to the Board for review upon request The Agreement may allow the Pharmacist within the Pharmacists Scope of Practice Pursuant to the Collaborative Pharmacy Practice Agreement to conduct Drug Therapy Management activities approved by the Practitioner The collaboration that the Practitioner agrees to conduct with the Pharmacist must be within the scope of the Practitioners current practice Patients who receive services from one or more collaborating Pharmacists shall receive notification of receipt of such services
(2) Contents The Collaborative Pharmacy Practice Agreement shall include
(a) Identification of the Practitioner(s) and Pharmacist(s) who are parties to the Agreement
(b) The types of Drug Therapy Management decisions that the Pharmacist is allowed to make which may include
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpnet 2
Report of the Task Force on Collaborative Practice Agreements - NABP 9Smiddoth Annual Meeting Proceedings
(i) A detailed description of the types of diseases Drugs or Drug categories involved and the type of Drug Therapy Management allowed in each case
(ii) A detailed description of the methods procedures decision criteria and plan the Pharmacist is to follow when conducting Drug Therapy ~anagement and
(iii) A detailed description of the activities the Pharmacist is to follow in the course of conducting Drug Therapy Management including documentation of decisions made and a plan or appropriate mechanism for conununication feedback and reporting to the Practitioner concerning specific decisions made In addition to the Agreement documentation shall occur on the prescription record patient profile a separate log book or in some other appropriate system
(c) A method for the Practitioner to monitor compliance with the Agreement and clinical outcomes where Drug Therapy Management by the Pharmacist has occurred and to intercede where necessary
(d) A provision that allows the Practitioner to override a collaborative practice decision mad by the Pharmacist whenever he or she deems it necessary or appropriate
(e) A provision that allows either party to cancel the agreement by written notification
(f) An effective date and (g) Signatures of all collaborating Pharmacists and Practitioners who are party to the
agreement as well as the date of signing Amendments to a Collaborative Pharmacy Practice Agreement must be documented signed and dated
(3) Initiation of the Collaborative Pharmacy Practice Agreement The Collaborative Pharmacy Practice Agreement must be coupled with a medical order from the Practitioner to initiate Drug Therapy Management for any particular patient
(4) Documentation of Drug Therapy Management Docwnentation of Drug Therapy ~anagement must be kept as part of the patients permanent record and be readily available to other health care professionals providing care to that patient and who are authorized to received it Docwnentation of drug Therapy Management shall be considered Confidential Information
(5) Review At a minimum the written agreement shall be reviewed and renewed and if necessary revised every year
[NOTE the above new subsections would be inserted with sequential relettering of all subsequent subsections]
Background
Task Force members listened to a brief presentation by American College of Clinical Pharmacy (ACCP) Executive Director Robert Elenbaas and Immediate Past President Jerry Bauman regarding ACCPs position on collaborative practice agreements and later discussed their charge to develop national guidelines for writing and implementing uniform collaborative practice agreements for use by state boards of pharmacy They agreed that the best way to fulfill their charge was to create model regulations addressing the issue
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI3
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sb Annual Meeting Proceedings
To assist in developing the model regulations members looked to several sources of information including current state pharmacy laws and regulations as well as the information provided by Drs Elenbaas and Bauman The Task Force felt that the Idaho State Board of Pharmacys regulations on collaborative practice encompassed much of what they wanted to include in the model regulation therefore they were used as a blueprint
A brainstorming session brought issues and concerns regarding state regulation of collaborative pharmacy practice to the forefront Topics that members felt needed to be addressed in a model regulation included
Contents of collaborative pharmacy practice agreements
Practitioner override authority
Board approval of agreements
Phannacists documentation of care
Patient consentnotification
Confidentiality of patient information
Periodic review of agreement
Phannacist competencequalifications to provide drug therapy management services and
Quality assurance review of practice
Task Force members concurred on a majority of these topics and incorporated their determinations into the model recommendation Members agreed that certain key elements should be included in collaborative phannacy practice agreements so that such agreements may guide both the pharmacists and collaborating practitioners decisions and conduct Key elements incorporated included a description of the pharmacists activities a method describing a practitioner monitoring methods and a practitioner override clause
Members also agreed that it should not be necessary for boards of pharmacy to take on the responsibility for approving agreements or even requiring their submission but that boards should require parties to notify them of the existence of such agreements and to keep a copy at the practice site On this issue members concluded that the individual practitioners were the persons most qualified to approve the agreements noting that minimal government involvement in the practice of the phannacist and collaborating practitioner was the most desirable In order for the board to have some handle on the situation however it was determined that agreements should be made available for board inspection upon request Regarding he issue of pharmacist competence to provide drug therapy management services Task Force members concluded that the subject should not be addressed in the model regulations They agreed that he determination of competence should be left up to the individuals who are party to the agreement Members felt certain that most if not al1 collaborating practitioners would only delegate certain aspects of their patient care authority upon demonstration by the pharmacist of his or her abilities to provide such care Further members felt that the majority ofphannacists would not take on the responsibility for providing such care without being competent to do so
TFCP A Recommendation 2
The Task Force on Collaborative Practice Agreements recognized that the issue of quality care assurance is not still developing and at present cannot be addressed with regard to collaborative pharmacy practice The Task Force recommends that the NABP Executive Committee closely
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI4
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
follow this issue and should it be determined that language addressing the subject should be incorporated into the model regulation take further action at that time
Background
Task Force members were uncertain at this time of how to address the issue of quality care assurance Members noted that most states addressing collaborative pharmacy practice in their laws or regulations did not include a provision on this subject It was noted however that tat least one state placed the responsibility for quality assurance on the Principal pharmacist and practitioner and that ACCP urged that an appropriate body be responsible for such activities
Member postulated that collaborative practice activities would likely be subject to quality assurance review via physician peer-review processes Additionally they suggested that those parties practicing in institutional settings would follow the quality assurance policies guidelines andor requirements of the institution The Task Force felt it was premature to make a recommendation regarding those parties who practice in ambulatory settings and who may not be subject to quality assurance review Members observed that the work ofNABPs Task Force on Patient Outcomes Regulation should be reviewed before a recommendation was made
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406 (F) 847391-4502middot wwwnabpnet 5
58-1-107 Applicability -- Relationship to specific chapters under title The provisions of this chapter LJniformly apply to the administration and enforcement of this title However
unless expressly prohibited in this chapter any provision of this chapter may be supplemented or altered by specific chapters of this title
R1S6-1-107 Organization of Rules - Content Applicability and Relationship of Rules
(1) The rules and sections in Title R156 shall to the extent practicable follow the numbering and
organizational scheme of the chapters in Title 58
(2) Rule R156-1 shall contain general provisions applicable to the administration and enforcement of all
occupations and profeSSions regulated in Title 58
(3) The provisions of the other rules in Title R156 shall contain specific or unique provisions applicable to
particular occupations or professions
(4) Specific rules in Title R156 may supplement or alter Rule R156-1 unless expressly provided otherwise
in Rule R156-1
R1S6-17b-l04 Organization - Relationship to Rule R1S6-1
The organization of this rule and its relationship to Rule R156-1 is as described in Section R156-1-107
58-17b-50G Petitioning for reinstatement of licensure Any person whose license to practice pharmacy in this state has been revoked suspended or surrendered
voluntarily or by action of the division shall have the right at reasonable intervals to petition the division for reinstatement of such license Such petition shall be made in writing and in the form prescribed by the division Upon investigation and hearing the division may in its discretion grant or deny such petition or it may modify its driginal finding to reflect any circumstances that have changed sufficiently to warrant such modifications Themiddotdivision also at its discretion may require such person to pass an examination or examinations for reshyentry into the practice of pharmacy Enacted by Chapter 280 2004 General Session
Agenda Board of Pharmacy
Collaborative Practice Agreement Discussion 100 PM - 200 PM
-~ Agenda Item Overview - I
Welcome and Introductions Paige Patterick and Carrie Dunford
Discuss the wording of 58-17b-l 02( 17) (17) Collaborative pharmacy practice agreement means a written and signed agreement between one or more pharmacists and one or more practitioners that provides for collaborative pharmacy practice for the purpose of drug therapy management of patients and prevention of disease of human subjects
Discuss the definition of Drug Therapy Management in R 156-17b-l 02 (19)
(J 9) Drug therapy management means the review of a drug therapy regimen of a patient by one or more pharmacists for the purpose of evaluating and rendering advice to one or more practitioners regarding adjustment of the regimen
Resources
NABP https llnabppharmacywp-contentuploads20 1607 ITFCollaborativePracticeAgreements AM95 Dec 1998 pdf
Operating Standards Drug therapy management R 156-1 7b-61 I
R 156-l7b-611 Operating Standards - Drug Therapy Management (I) In accordance with Subsections 58-17b-l 02( 17) and 58-l7b-60 I( I) decisions involving drug therapy management shall be made in the best interest of the patient Drug therapy management may include (a) implementing modifying and managing drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement (b) collecting and reviewing patient histories (c) obtaining and checking vital signs including pulse temperature blood pressure and respiration (d) ordering and evaluating the results of laboratory tests directly applicable to the drug therapy when perfolmed in accordance with approved protocols applicable to the practice setting and (e) such other patient care services as may be allowed by rule (2) For the purpose of promoting therapeutic appropriateness a pharmacist shall at the time of dispensing a prescription or a prescription drug order review the patients medication record Such review shall at a minimum identify clinically significant conditions situations or items such as (a) inappropriate drug utilization (b) therapeutic duplication (c) drug-disease contraindications (d) drug-drug interactions (e) incorrect drug dosage or duration of drug treatment (f) drug-allergy interactions and (g) clinical abuse or misuse (3) Upon identifying any clinically significant conditions situations or items listed in Subsection (2) above the pharmacist shall take appropriate steps to avoid or resolve the problem including consultation with the prescribing practitioner
Note In compliance with the Americans with Disabilities Act individuals needing special accommodations (including auxiliary communicative aids and services) during this meet ing should not ify Dave Taylor ADA Coordinator at least three working days prior to the meeting Division of Occupational amp Professional Licensing 160 East 300 South Salt Lake City Utah 84115 801-530-6628 or toll-free in Utah only 866-275-3675 Posted to Bulletin Board on 11-21-2017

APPENDIX B MODIFIED DELPHI METHOD
The goal of the workgroup was to reach consensus on each of the elements discussed To do this a modified Delphi method was used A survey was sent to all participants to collect their initial thoughts on each of the elements identified in currently existing collaborative practice authority laws andor regulations Participants were given the current variations of each element as a multiple-choice selection with the opportunity to answer in free form text if the desired option was not listed After completion of the survey the workgroup discussed all questions where consensus was not already reached via conference call The conference call discussions were structured to have a defined period of time for discussion followed by a summary of the current options being discussed and a roll call vote by each of the participants The item was included on the next survey if consensus was not reached on the conference call This process was repeated a total of three times before the group reached consensus on all items being considered See below for a diagram of the process used
1 Level-setting conference call
2 Distribution of survey with 3 weeks to complete
5
Task Force on
Collaborative Practice Agreements
Members Present
Sharlea M Leatherwood Chair (MO) Michael J Ayotte (VA) Winifred A Landis (IN) Jeffery Lindoo (NIN) C Ann Perry (GA) Charles R Young (MA)
Others Present
Joseph A Whaley Jr Executive Committee Liaison Carmen A Catizone NABP Executive DirectorSecretary Melissa Madigan NABP Staff
Introduction
The Task Force on Collaborative Practice Agreements (TFCPA) met December 3 1998 at the Marriott Suites Hotel in Rosemont Illinois The Task Force was established by the NABP Executive Corrunittee in response to recent increases in the number of prescribers and pharmacists entering into collaborative practice agreements and requests that boards of pharmacy define the elements of an appropriate collaborative practice agreement
Charge of the Task Force on Collaborative Practice Agreements
Task Force members reviewed their charge and proposing no changes accepted it as follows
Review the available literature and information pertaining to collaborative practice agreements between prescribers and pharmacists
bull Develop national model guidelines for writing and implementing uniform collaborative practice agreements that can be utilized by the state boards of pharmacy
TFCPA Recommendation 1
The Task Force on Collaborative Practice Agreements recorrunends to the Executive Committee and the Corrunittee on Law EnforcementLegislation that the following language be adopted for incorporation into the NABP Model State Pharmacy Act and Model Rules
Article I
Title Purpose Definition
Section 105 Definitions
(g) Collaborative Pharmacy Practice is that Practice of Pharmacy whereby a one or more Pharmacist~ has jointly agreed on a voluntary basis to work in conjunction with one or more Practitioners under protocol whereby the Pharmacist may perform certain patient care functions authorized by the Practitioner or Practitioners under certain specified conditions andor limitations
NATIONAL ASSOCIA nON OF BOARDS OF PHARMACYmiddot (P) 847391-4406 bull (F) 847391-4502 bull wwwnabpnel
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
(h) Collaborative Pharmacy Practice Agreement is a written and signed agreement between one or more Pharmacists and one or more Practitioners that provides for Collaborative Pharmacy Practice for the purpose of Drug Therapy Management of patients
(r) Drug Therapy Management means the review of Drug therapy regimen(s) of patients by one or more Pharmacists for the purpose of evaluating and rendering advice to one or more Practitioners regarding adjustment of the regimen Decisions involving Drug Therapy Management shall be made in the best interest of the patient Drug Therapy Management may include
(1) Implementing modifying and managing Drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement
(2) Collecting and reviewing patient Drug histories (3) Obtaining and checking vital signs including pulse temperature blood pressure
and respiration (4) Ordering and evaluating the results of laboratory tests directly relating to Drug
therapy when performed in accordance with approved protocols applicable to the practice setting and
(5) Such other patient care services as may be allowed by law
Pharmacists Scope of Practice Pursuant to the Collaborative Pharmacy Practice Agreement means those duties and limitations of duties placed upon one or more Pharmacists by the collaborating Practitioner or Practitioners the Board and applicable law and includes the limitations implied by the specialty practiced by the collaborating Practitioner or Practitioners
Model Rules for Pharmaceutical Care
Section 3 Pharmacy Practice
Collaborative Pharmacy Practice
(1) Collaborative Pharmacy Practice Agreement A Pharmacist planning to engage in Collaborative Pharmacy Practice shall have on file at his or her place of practice the written Collaborative Pharmacy Practice Agreement The existence and termination of such Agreement shall be reported to the Board and such Agreements shall be made available to the Board for review upon request The Agreement may allow the Pharmacist within the Pharmacists Scope of Practice Pursuant to the Collaborative Pharmacy Practice Agreement to conduct Drug Therapy Management activities approved by the Practitioner The collaboration that the Practitioner agrees to conduct with the Pharmacist must be within the scope of the Practitioners current practice Patients who receive services from one or more collaborating Pharmacists shall receive notification of receipt of such services
(2) Contents The Collaborative Pharmacy Practice Agreement shall include
(a) Identification of the Practitioner(s) and Pharmacist(s) who are parties to the Agreement
(b) The types of Drug Therapy Management decisions that the Pharmacist is allowed to make which may include
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpnet 2
Report of the Task Force on Collaborative Practice Agreements - NABP 9Smiddoth Annual Meeting Proceedings
(i) A detailed description of the types of diseases Drugs or Drug categories involved and the type of Drug Therapy Management allowed in each case
(ii) A detailed description of the methods procedures decision criteria and plan the Pharmacist is to follow when conducting Drug Therapy ~anagement and
(iii) A detailed description of the activities the Pharmacist is to follow in the course of conducting Drug Therapy Management including documentation of decisions made and a plan or appropriate mechanism for conununication feedback and reporting to the Practitioner concerning specific decisions made In addition to the Agreement documentation shall occur on the prescription record patient profile a separate log book or in some other appropriate system
(c) A method for the Practitioner to monitor compliance with the Agreement and clinical outcomes where Drug Therapy Management by the Pharmacist has occurred and to intercede where necessary
(d) A provision that allows the Practitioner to override a collaborative practice decision mad by the Pharmacist whenever he or she deems it necessary or appropriate
(e) A provision that allows either party to cancel the agreement by written notification
(f) An effective date and (g) Signatures of all collaborating Pharmacists and Practitioners who are party to the
agreement as well as the date of signing Amendments to a Collaborative Pharmacy Practice Agreement must be documented signed and dated
(3) Initiation of the Collaborative Pharmacy Practice Agreement The Collaborative Pharmacy Practice Agreement must be coupled with a medical order from the Practitioner to initiate Drug Therapy Management for any particular patient
(4) Documentation of Drug Therapy Management Docwnentation of Drug Therapy ~anagement must be kept as part of the patients permanent record and be readily available to other health care professionals providing care to that patient and who are authorized to received it Docwnentation of drug Therapy Management shall be considered Confidential Information
(5) Review At a minimum the written agreement shall be reviewed and renewed and if necessary revised every year
[NOTE the above new subsections would be inserted with sequential relettering of all subsequent subsections]
Background
Task Force members listened to a brief presentation by American College of Clinical Pharmacy (ACCP) Executive Director Robert Elenbaas and Immediate Past President Jerry Bauman regarding ACCPs position on collaborative practice agreements and later discussed their charge to develop national guidelines for writing and implementing uniform collaborative practice agreements for use by state boards of pharmacy They agreed that the best way to fulfill their charge was to create model regulations addressing the issue
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI3
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sb Annual Meeting Proceedings
To assist in developing the model regulations members looked to several sources of information including current state pharmacy laws and regulations as well as the information provided by Drs Elenbaas and Bauman The Task Force felt that the Idaho State Board of Pharmacys regulations on collaborative practice encompassed much of what they wanted to include in the model regulation therefore they were used as a blueprint
A brainstorming session brought issues and concerns regarding state regulation of collaborative pharmacy practice to the forefront Topics that members felt needed to be addressed in a model regulation included
Contents of collaborative pharmacy practice agreements
Practitioner override authority
Board approval of agreements
Phannacists documentation of care
Patient consentnotification
Confidentiality of patient information
Periodic review of agreement
Phannacist competencequalifications to provide drug therapy management services and
Quality assurance review of practice
Task Force members concurred on a majority of these topics and incorporated their determinations into the model recommendation Members agreed that certain key elements should be included in collaborative phannacy practice agreements so that such agreements may guide both the pharmacists and collaborating practitioners decisions and conduct Key elements incorporated included a description of the pharmacists activities a method describing a practitioner monitoring methods and a practitioner override clause
Members also agreed that it should not be necessary for boards of pharmacy to take on the responsibility for approving agreements or even requiring their submission but that boards should require parties to notify them of the existence of such agreements and to keep a copy at the practice site On this issue members concluded that the individual practitioners were the persons most qualified to approve the agreements noting that minimal government involvement in the practice of the phannacist and collaborating practitioner was the most desirable In order for the board to have some handle on the situation however it was determined that agreements should be made available for board inspection upon request Regarding he issue of pharmacist competence to provide drug therapy management services Task Force members concluded that the subject should not be addressed in the model regulations They agreed that he determination of competence should be left up to the individuals who are party to the agreement Members felt certain that most if not al1 collaborating practitioners would only delegate certain aspects of their patient care authority upon demonstration by the pharmacist of his or her abilities to provide such care Further members felt that the majority ofphannacists would not take on the responsibility for providing such care without being competent to do so
TFCP A Recommendation 2
The Task Force on Collaborative Practice Agreements recognized that the issue of quality care assurance is not still developing and at present cannot be addressed with regard to collaborative pharmacy practice The Task Force recommends that the NABP Executive Committee closely
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI4
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
follow this issue and should it be determined that language addressing the subject should be incorporated into the model regulation take further action at that time
Background
Task Force members were uncertain at this time of how to address the issue of quality care assurance Members noted that most states addressing collaborative pharmacy practice in their laws or regulations did not include a provision on this subject It was noted however that tat least one state placed the responsibility for quality assurance on the Principal pharmacist and practitioner and that ACCP urged that an appropriate body be responsible for such activities
Member postulated that collaborative practice activities would likely be subject to quality assurance review via physician peer-review processes Additionally they suggested that those parties practicing in institutional settings would follow the quality assurance policies guidelines andor requirements of the institution The Task Force felt it was premature to make a recommendation regarding those parties who practice in ambulatory settings and who may not be subject to quality assurance review Members observed that the work ofNABPs Task Force on Patient Outcomes Regulation should be reviewed before a recommendation was made
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406 (F) 847391-4502middot wwwnabpnet 5
58-1-107 Applicability -- Relationship to specific chapters under title The provisions of this chapter LJniformly apply to the administration and enforcement of this title However
unless expressly prohibited in this chapter any provision of this chapter may be supplemented or altered by specific chapters of this title
R1S6-1-107 Organization of Rules - Content Applicability and Relationship of Rules
(1) The rules and sections in Title R156 shall to the extent practicable follow the numbering and
organizational scheme of the chapters in Title 58
(2) Rule R156-1 shall contain general provisions applicable to the administration and enforcement of all
occupations and profeSSions regulated in Title 58
(3) The provisions of the other rules in Title R156 shall contain specific or unique provisions applicable to
particular occupations or professions
(4) Specific rules in Title R156 may supplement or alter Rule R156-1 unless expressly provided otherwise
in Rule R156-1
R1S6-17b-l04 Organization - Relationship to Rule R1S6-1
The organization of this rule and its relationship to Rule R156-1 is as described in Section R156-1-107
58-17b-50G Petitioning for reinstatement of licensure Any person whose license to practice pharmacy in this state has been revoked suspended or surrendered
voluntarily or by action of the division shall have the right at reasonable intervals to petition the division for reinstatement of such license Such petition shall be made in writing and in the form prescribed by the division Upon investigation and hearing the division may in its discretion grant or deny such petition or it may modify its driginal finding to reflect any circumstances that have changed sufficiently to warrant such modifications Themiddotdivision also at its discretion may require such person to pass an examination or examinations for reshyentry into the practice of pharmacy Enacted by Chapter 280 2004 General Session
Agenda Board of Pharmacy
Collaborative Practice Agreement Discussion 100 PM - 200 PM
-~ Agenda Item Overview - I
Welcome and Introductions Paige Patterick and Carrie Dunford
Discuss the wording of 58-17b-l 02( 17) (17) Collaborative pharmacy practice agreement means a written and signed agreement between one or more pharmacists and one or more practitioners that provides for collaborative pharmacy practice for the purpose of drug therapy management of patients and prevention of disease of human subjects
Discuss the definition of Drug Therapy Management in R 156-17b-l 02 (19)
(J 9) Drug therapy management means the review of a drug therapy regimen of a patient by one or more pharmacists for the purpose of evaluating and rendering advice to one or more practitioners regarding adjustment of the regimen
Resources
NABP https llnabppharmacywp-contentuploads20 1607 ITFCollaborativePracticeAgreements AM95 Dec 1998 pdf
Operating Standards Drug therapy management R 156-1 7b-61 I
R 156-l7b-611 Operating Standards - Drug Therapy Management (I) In accordance with Subsections 58-17b-l 02( 17) and 58-l7b-60 I( I) decisions involving drug therapy management shall be made in the best interest of the patient Drug therapy management may include (a) implementing modifying and managing drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement (b) collecting and reviewing patient histories (c) obtaining and checking vital signs including pulse temperature blood pressure and respiration (d) ordering and evaluating the results of laboratory tests directly applicable to the drug therapy when perfolmed in accordance with approved protocols applicable to the practice setting and (e) such other patient care services as may be allowed by rule (2) For the purpose of promoting therapeutic appropriateness a pharmacist shall at the time of dispensing a prescription or a prescription drug order review the patients medication record Such review shall at a minimum identify clinically significant conditions situations or items such as (a) inappropriate drug utilization (b) therapeutic duplication (c) drug-disease contraindications (d) drug-drug interactions (e) incorrect drug dosage or duration of drug treatment (f) drug-allergy interactions and (g) clinical abuse or misuse (3) Upon identifying any clinically significant conditions situations or items listed in Subsection (2) above the pharmacist shall take appropriate steps to avoid or resolve the problem including consultation with the prescribing practitioner
Note In compliance with the Americans with Disabilities Act individuals needing special accommodations (including auxiliary communicative aids and services) during this meet ing should not ify Dave Taylor ADA Coordinator at least three working days prior to the meeting Division of Occupational amp Professional Licensing 160 East 300 South Salt Lake City Utah 84115 801-530-6628 or toll-free in Utah only 866-275-3675 Posted to Bulletin Board on 11-21-2017

Task Force on
Collaborative Practice Agreements
Members Present
Sharlea M Leatherwood Chair (MO) Michael J Ayotte (VA) Winifred A Landis (IN) Jeffery Lindoo (NIN) C Ann Perry (GA) Charles R Young (MA)
Others Present
Joseph A Whaley Jr Executive Committee Liaison Carmen A Catizone NABP Executive DirectorSecretary Melissa Madigan NABP Staff
Introduction
The Task Force on Collaborative Practice Agreements (TFCPA) met December 3 1998 at the Marriott Suites Hotel in Rosemont Illinois The Task Force was established by the NABP Executive Corrunittee in response to recent increases in the number of prescribers and pharmacists entering into collaborative practice agreements and requests that boards of pharmacy define the elements of an appropriate collaborative practice agreement
Charge of the Task Force on Collaborative Practice Agreements
Task Force members reviewed their charge and proposing no changes accepted it as follows
Review the available literature and information pertaining to collaborative practice agreements between prescribers and pharmacists
bull Develop national model guidelines for writing and implementing uniform collaborative practice agreements that can be utilized by the state boards of pharmacy
TFCPA Recommendation 1
The Task Force on Collaborative Practice Agreements recorrunends to the Executive Committee and the Corrunittee on Law EnforcementLegislation that the following language be adopted for incorporation into the NABP Model State Pharmacy Act and Model Rules
Article I
Title Purpose Definition
Section 105 Definitions
(g) Collaborative Pharmacy Practice is that Practice of Pharmacy whereby a one or more Pharmacist~ has jointly agreed on a voluntary basis to work in conjunction with one or more Practitioners under protocol whereby the Pharmacist may perform certain patient care functions authorized by the Practitioner or Practitioners under certain specified conditions andor limitations
NATIONAL ASSOCIA nON OF BOARDS OF PHARMACYmiddot (P) 847391-4406 bull (F) 847391-4502 bull wwwnabpnel
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
(h) Collaborative Pharmacy Practice Agreement is a written and signed agreement between one or more Pharmacists and one or more Practitioners that provides for Collaborative Pharmacy Practice for the purpose of Drug Therapy Management of patients
(r) Drug Therapy Management means the review of Drug therapy regimen(s) of patients by one or more Pharmacists for the purpose of evaluating and rendering advice to one or more Practitioners regarding adjustment of the regimen Decisions involving Drug Therapy Management shall be made in the best interest of the patient Drug Therapy Management may include
(1) Implementing modifying and managing Drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement
(2) Collecting and reviewing patient Drug histories (3) Obtaining and checking vital signs including pulse temperature blood pressure
and respiration (4) Ordering and evaluating the results of laboratory tests directly relating to Drug
therapy when performed in accordance with approved protocols applicable to the practice setting and
(5) Such other patient care services as may be allowed by law
Pharmacists Scope of Practice Pursuant to the Collaborative Pharmacy Practice Agreement means those duties and limitations of duties placed upon one or more Pharmacists by the collaborating Practitioner or Practitioners the Board and applicable law and includes the limitations implied by the specialty practiced by the collaborating Practitioner or Practitioners
Model Rules for Pharmaceutical Care
Section 3 Pharmacy Practice
Collaborative Pharmacy Practice
(1) Collaborative Pharmacy Practice Agreement A Pharmacist planning to engage in Collaborative Pharmacy Practice shall have on file at his or her place of practice the written Collaborative Pharmacy Practice Agreement The existence and termination of such Agreement shall be reported to the Board and such Agreements shall be made available to the Board for review upon request The Agreement may allow the Pharmacist within the Pharmacists Scope of Practice Pursuant to the Collaborative Pharmacy Practice Agreement to conduct Drug Therapy Management activities approved by the Practitioner The collaboration that the Practitioner agrees to conduct with the Pharmacist must be within the scope of the Practitioners current practice Patients who receive services from one or more collaborating Pharmacists shall receive notification of receipt of such services
(2) Contents The Collaborative Pharmacy Practice Agreement shall include
(a) Identification of the Practitioner(s) and Pharmacist(s) who are parties to the Agreement
(b) The types of Drug Therapy Management decisions that the Pharmacist is allowed to make which may include
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpnet 2
Report of the Task Force on Collaborative Practice Agreements - NABP 9Smiddoth Annual Meeting Proceedings
(i) A detailed description of the types of diseases Drugs or Drug categories involved and the type of Drug Therapy Management allowed in each case
(ii) A detailed description of the methods procedures decision criteria and plan the Pharmacist is to follow when conducting Drug Therapy ~anagement and
(iii) A detailed description of the activities the Pharmacist is to follow in the course of conducting Drug Therapy Management including documentation of decisions made and a plan or appropriate mechanism for conununication feedback and reporting to the Practitioner concerning specific decisions made In addition to the Agreement documentation shall occur on the prescription record patient profile a separate log book or in some other appropriate system
(c) A method for the Practitioner to monitor compliance with the Agreement and clinical outcomes where Drug Therapy Management by the Pharmacist has occurred and to intercede where necessary
(d) A provision that allows the Practitioner to override a collaborative practice decision mad by the Pharmacist whenever he or she deems it necessary or appropriate
(e) A provision that allows either party to cancel the agreement by written notification
(f) An effective date and (g) Signatures of all collaborating Pharmacists and Practitioners who are party to the
agreement as well as the date of signing Amendments to a Collaborative Pharmacy Practice Agreement must be documented signed and dated
(3) Initiation of the Collaborative Pharmacy Practice Agreement The Collaborative Pharmacy Practice Agreement must be coupled with a medical order from the Practitioner to initiate Drug Therapy Management for any particular patient
(4) Documentation of Drug Therapy Management Docwnentation of Drug Therapy ~anagement must be kept as part of the patients permanent record and be readily available to other health care professionals providing care to that patient and who are authorized to received it Docwnentation of drug Therapy Management shall be considered Confidential Information
(5) Review At a minimum the written agreement shall be reviewed and renewed and if necessary revised every year
[NOTE the above new subsections would be inserted with sequential relettering of all subsequent subsections]
Background
Task Force members listened to a brief presentation by American College of Clinical Pharmacy (ACCP) Executive Director Robert Elenbaas and Immediate Past President Jerry Bauman regarding ACCPs position on collaborative practice agreements and later discussed their charge to develop national guidelines for writing and implementing uniform collaborative practice agreements for use by state boards of pharmacy They agreed that the best way to fulfill their charge was to create model regulations addressing the issue
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI3
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sb Annual Meeting Proceedings
To assist in developing the model regulations members looked to several sources of information including current state pharmacy laws and regulations as well as the information provided by Drs Elenbaas and Bauman The Task Force felt that the Idaho State Board of Pharmacys regulations on collaborative practice encompassed much of what they wanted to include in the model regulation therefore they were used as a blueprint
A brainstorming session brought issues and concerns regarding state regulation of collaborative pharmacy practice to the forefront Topics that members felt needed to be addressed in a model regulation included
Contents of collaborative pharmacy practice agreements
Practitioner override authority
Board approval of agreements
Phannacists documentation of care
Patient consentnotification
Confidentiality of patient information
Periodic review of agreement
Phannacist competencequalifications to provide drug therapy management services and
Quality assurance review of practice
Task Force members concurred on a majority of these topics and incorporated their determinations into the model recommendation Members agreed that certain key elements should be included in collaborative phannacy practice agreements so that such agreements may guide both the pharmacists and collaborating practitioners decisions and conduct Key elements incorporated included a description of the pharmacists activities a method describing a practitioner monitoring methods and a practitioner override clause
Members also agreed that it should not be necessary for boards of pharmacy to take on the responsibility for approving agreements or even requiring their submission but that boards should require parties to notify them of the existence of such agreements and to keep a copy at the practice site On this issue members concluded that the individual practitioners were the persons most qualified to approve the agreements noting that minimal government involvement in the practice of the phannacist and collaborating practitioner was the most desirable In order for the board to have some handle on the situation however it was determined that agreements should be made available for board inspection upon request Regarding he issue of pharmacist competence to provide drug therapy management services Task Force members concluded that the subject should not be addressed in the model regulations They agreed that he determination of competence should be left up to the individuals who are party to the agreement Members felt certain that most if not al1 collaborating practitioners would only delegate certain aspects of their patient care authority upon demonstration by the pharmacist of his or her abilities to provide such care Further members felt that the majority ofphannacists would not take on the responsibility for providing such care without being competent to do so
TFCP A Recommendation 2
The Task Force on Collaborative Practice Agreements recognized that the issue of quality care assurance is not still developing and at present cannot be addressed with regard to collaborative pharmacy practice The Task Force recommends that the NABP Executive Committee closely
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI4
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
follow this issue and should it be determined that language addressing the subject should be incorporated into the model regulation take further action at that time
Background
Task Force members were uncertain at this time of how to address the issue of quality care assurance Members noted that most states addressing collaborative pharmacy practice in their laws or regulations did not include a provision on this subject It was noted however that tat least one state placed the responsibility for quality assurance on the Principal pharmacist and practitioner and that ACCP urged that an appropriate body be responsible for such activities
Member postulated that collaborative practice activities would likely be subject to quality assurance review via physician peer-review processes Additionally they suggested that those parties practicing in institutional settings would follow the quality assurance policies guidelines andor requirements of the institution The Task Force felt it was premature to make a recommendation regarding those parties who practice in ambulatory settings and who may not be subject to quality assurance review Members observed that the work ofNABPs Task Force on Patient Outcomes Regulation should be reviewed before a recommendation was made
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406 (F) 847391-4502middot wwwnabpnet 5
58-1-107 Applicability -- Relationship to specific chapters under title The provisions of this chapter LJniformly apply to the administration and enforcement of this title However
unless expressly prohibited in this chapter any provision of this chapter may be supplemented or altered by specific chapters of this title
R1S6-1-107 Organization of Rules - Content Applicability and Relationship of Rules
(1) The rules and sections in Title R156 shall to the extent practicable follow the numbering and
organizational scheme of the chapters in Title 58
(2) Rule R156-1 shall contain general provisions applicable to the administration and enforcement of all
occupations and profeSSions regulated in Title 58
(3) The provisions of the other rules in Title R156 shall contain specific or unique provisions applicable to
particular occupations or professions
(4) Specific rules in Title R156 may supplement or alter Rule R156-1 unless expressly provided otherwise
in Rule R156-1
R1S6-17b-l04 Organization - Relationship to Rule R1S6-1
The organization of this rule and its relationship to Rule R156-1 is as described in Section R156-1-107
58-17b-50G Petitioning for reinstatement of licensure Any person whose license to practice pharmacy in this state has been revoked suspended or surrendered
voluntarily or by action of the division shall have the right at reasonable intervals to petition the division for reinstatement of such license Such petition shall be made in writing and in the form prescribed by the division Upon investigation and hearing the division may in its discretion grant or deny such petition or it may modify its driginal finding to reflect any circumstances that have changed sufficiently to warrant such modifications Themiddotdivision also at its discretion may require such person to pass an examination or examinations for reshyentry into the practice of pharmacy Enacted by Chapter 280 2004 General Session
Agenda Board of Pharmacy
Collaborative Practice Agreement Discussion 100 PM - 200 PM
-~ Agenda Item Overview - I
Welcome and Introductions Paige Patterick and Carrie Dunford
Discuss the wording of 58-17b-l 02( 17) (17) Collaborative pharmacy practice agreement means a written and signed agreement between one or more pharmacists and one or more practitioners that provides for collaborative pharmacy practice for the purpose of drug therapy management of patients and prevention of disease of human subjects
Discuss the definition of Drug Therapy Management in R 156-17b-l 02 (19)
(J 9) Drug therapy management means the review of a drug therapy regimen of a patient by one or more pharmacists for the purpose of evaluating and rendering advice to one or more practitioners regarding adjustment of the regimen
Resources
NABP https llnabppharmacywp-contentuploads20 1607 ITFCollaborativePracticeAgreements AM95 Dec 1998 pdf
Operating Standards Drug therapy management R 156-1 7b-61 I
R 156-l7b-611 Operating Standards - Drug Therapy Management (I) In accordance with Subsections 58-17b-l 02( 17) and 58-l7b-60 I( I) decisions involving drug therapy management shall be made in the best interest of the patient Drug therapy management may include (a) implementing modifying and managing drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement (b) collecting and reviewing patient histories (c) obtaining and checking vital signs including pulse temperature blood pressure and respiration (d) ordering and evaluating the results of laboratory tests directly applicable to the drug therapy when perfolmed in accordance with approved protocols applicable to the practice setting and (e) such other patient care services as may be allowed by rule (2) For the purpose of promoting therapeutic appropriateness a pharmacist shall at the time of dispensing a prescription or a prescription drug order review the patients medication record Such review shall at a minimum identify clinically significant conditions situations or items such as (a) inappropriate drug utilization (b) therapeutic duplication (c) drug-disease contraindications (d) drug-drug interactions (e) incorrect drug dosage or duration of drug treatment (f) drug-allergy interactions and (g) clinical abuse or misuse (3) Upon identifying any clinically significant conditions situations or items listed in Subsection (2) above the pharmacist shall take appropriate steps to avoid or resolve the problem including consultation with the prescribing practitioner
Note In compliance with the Americans with Disabilities Act individuals needing special accommodations (including auxiliary communicative aids and services) during this meet ing should not ify Dave Taylor ADA Coordinator at least three working days prior to the meeting Division of Occupational amp Professional Licensing 160 East 300 South Salt Lake City Utah 84115 801-530-6628 or toll-free in Utah only 866-275-3675 Posted to Bulletin Board on 11-21-2017

Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
(h) Collaborative Pharmacy Practice Agreement is a written and signed agreement between one or more Pharmacists and one or more Practitioners that provides for Collaborative Pharmacy Practice for the purpose of Drug Therapy Management of patients
(r) Drug Therapy Management means the review of Drug therapy regimen(s) of patients by one or more Pharmacists for the purpose of evaluating and rendering advice to one or more Practitioners regarding adjustment of the regimen Decisions involving Drug Therapy Management shall be made in the best interest of the patient Drug Therapy Management may include
(1) Implementing modifying and managing Drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement
(2) Collecting and reviewing patient Drug histories (3) Obtaining and checking vital signs including pulse temperature blood pressure
and respiration (4) Ordering and evaluating the results of laboratory tests directly relating to Drug
therapy when performed in accordance with approved protocols applicable to the practice setting and
(5) Such other patient care services as may be allowed by law
Pharmacists Scope of Practice Pursuant to the Collaborative Pharmacy Practice Agreement means those duties and limitations of duties placed upon one or more Pharmacists by the collaborating Practitioner or Practitioners the Board and applicable law and includes the limitations implied by the specialty practiced by the collaborating Practitioner or Practitioners
Model Rules for Pharmaceutical Care
Section 3 Pharmacy Practice
Collaborative Pharmacy Practice
(1) Collaborative Pharmacy Practice Agreement A Pharmacist planning to engage in Collaborative Pharmacy Practice shall have on file at his or her place of practice the written Collaborative Pharmacy Practice Agreement The existence and termination of such Agreement shall be reported to the Board and such Agreements shall be made available to the Board for review upon request The Agreement may allow the Pharmacist within the Pharmacists Scope of Practice Pursuant to the Collaborative Pharmacy Practice Agreement to conduct Drug Therapy Management activities approved by the Practitioner The collaboration that the Practitioner agrees to conduct with the Pharmacist must be within the scope of the Practitioners current practice Patients who receive services from one or more collaborating Pharmacists shall receive notification of receipt of such services
(2) Contents The Collaborative Pharmacy Practice Agreement shall include
(a) Identification of the Practitioner(s) and Pharmacist(s) who are parties to the Agreement
(b) The types of Drug Therapy Management decisions that the Pharmacist is allowed to make which may include
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpnet 2
Report of the Task Force on Collaborative Practice Agreements - NABP 9Smiddoth Annual Meeting Proceedings
(i) A detailed description of the types of diseases Drugs or Drug categories involved and the type of Drug Therapy Management allowed in each case
(ii) A detailed description of the methods procedures decision criteria and plan the Pharmacist is to follow when conducting Drug Therapy ~anagement and
(iii) A detailed description of the activities the Pharmacist is to follow in the course of conducting Drug Therapy Management including documentation of decisions made and a plan or appropriate mechanism for conununication feedback and reporting to the Practitioner concerning specific decisions made In addition to the Agreement documentation shall occur on the prescription record patient profile a separate log book or in some other appropriate system
(c) A method for the Practitioner to monitor compliance with the Agreement and clinical outcomes where Drug Therapy Management by the Pharmacist has occurred and to intercede where necessary
(d) A provision that allows the Practitioner to override a collaborative practice decision mad by the Pharmacist whenever he or she deems it necessary or appropriate
(e) A provision that allows either party to cancel the agreement by written notification
(f) An effective date and (g) Signatures of all collaborating Pharmacists and Practitioners who are party to the
agreement as well as the date of signing Amendments to a Collaborative Pharmacy Practice Agreement must be documented signed and dated
(3) Initiation of the Collaborative Pharmacy Practice Agreement The Collaborative Pharmacy Practice Agreement must be coupled with a medical order from the Practitioner to initiate Drug Therapy Management for any particular patient
(4) Documentation of Drug Therapy Management Docwnentation of Drug Therapy ~anagement must be kept as part of the patients permanent record and be readily available to other health care professionals providing care to that patient and who are authorized to received it Docwnentation of drug Therapy Management shall be considered Confidential Information
(5) Review At a minimum the written agreement shall be reviewed and renewed and if necessary revised every year
[NOTE the above new subsections would be inserted with sequential relettering of all subsequent subsections]
Background
Task Force members listened to a brief presentation by American College of Clinical Pharmacy (ACCP) Executive Director Robert Elenbaas and Immediate Past President Jerry Bauman regarding ACCPs position on collaborative practice agreements and later discussed their charge to develop national guidelines for writing and implementing uniform collaborative practice agreements for use by state boards of pharmacy They agreed that the best way to fulfill their charge was to create model regulations addressing the issue
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI3
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sb Annual Meeting Proceedings
To assist in developing the model regulations members looked to several sources of information including current state pharmacy laws and regulations as well as the information provided by Drs Elenbaas and Bauman The Task Force felt that the Idaho State Board of Pharmacys regulations on collaborative practice encompassed much of what they wanted to include in the model regulation therefore they were used as a blueprint
A brainstorming session brought issues and concerns regarding state regulation of collaborative pharmacy practice to the forefront Topics that members felt needed to be addressed in a model regulation included
Contents of collaborative pharmacy practice agreements
Practitioner override authority
Board approval of agreements
Phannacists documentation of care
Patient consentnotification
Confidentiality of patient information
Periodic review of agreement
Phannacist competencequalifications to provide drug therapy management services and
Quality assurance review of practice
Task Force members concurred on a majority of these topics and incorporated their determinations into the model recommendation Members agreed that certain key elements should be included in collaborative phannacy practice agreements so that such agreements may guide both the pharmacists and collaborating practitioners decisions and conduct Key elements incorporated included a description of the pharmacists activities a method describing a practitioner monitoring methods and a practitioner override clause
Members also agreed that it should not be necessary for boards of pharmacy to take on the responsibility for approving agreements or even requiring their submission but that boards should require parties to notify them of the existence of such agreements and to keep a copy at the practice site On this issue members concluded that the individual practitioners were the persons most qualified to approve the agreements noting that minimal government involvement in the practice of the phannacist and collaborating practitioner was the most desirable In order for the board to have some handle on the situation however it was determined that agreements should be made available for board inspection upon request Regarding he issue of pharmacist competence to provide drug therapy management services Task Force members concluded that the subject should not be addressed in the model regulations They agreed that he determination of competence should be left up to the individuals who are party to the agreement Members felt certain that most if not al1 collaborating practitioners would only delegate certain aspects of their patient care authority upon demonstration by the pharmacist of his or her abilities to provide such care Further members felt that the majority ofphannacists would not take on the responsibility for providing such care without being competent to do so
TFCP A Recommendation 2
The Task Force on Collaborative Practice Agreements recognized that the issue of quality care assurance is not still developing and at present cannot be addressed with regard to collaborative pharmacy practice The Task Force recommends that the NABP Executive Committee closely
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI4
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
follow this issue and should it be determined that language addressing the subject should be incorporated into the model regulation take further action at that time
Background
Task Force members were uncertain at this time of how to address the issue of quality care assurance Members noted that most states addressing collaborative pharmacy practice in their laws or regulations did not include a provision on this subject It was noted however that tat least one state placed the responsibility for quality assurance on the Principal pharmacist and practitioner and that ACCP urged that an appropriate body be responsible for such activities
Member postulated that collaborative practice activities would likely be subject to quality assurance review via physician peer-review processes Additionally they suggested that those parties practicing in institutional settings would follow the quality assurance policies guidelines andor requirements of the institution The Task Force felt it was premature to make a recommendation regarding those parties who practice in ambulatory settings and who may not be subject to quality assurance review Members observed that the work ofNABPs Task Force on Patient Outcomes Regulation should be reviewed before a recommendation was made
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406 (F) 847391-4502middot wwwnabpnet 5
58-1-107 Applicability -- Relationship to specific chapters under title The provisions of this chapter LJniformly apply to the administration and enforcement of this title However
unless expressly prohibited in this chapter any provision of this chapter may be supplemented or altered by specific chapters of this title
R1S6-1-107 Organization of Rules - Content Applicability and Relationship of Rules
(1) The rules and sections in Title R156 shall to the extent practicable follow the numbering and
organizational scheme of the chapters in Title 58
(2) Rule R156-1 shall contain general provisions applicable to the administration and enforcement of all
occupations and profeSSions regulated in Title 58
(3) The provisions of the other rules in Title R156 shall contain specific or unique provisions applicable to
particular occupations or professions
(4) Specific rules in Title R156 may supplement or alter Rule R156-1 unless expressly provided otherwise
in Rule R156-1
R1S6-17b-l04 Organization - Relationship to Rule R1S6-1
The organization of this rule and its relationship to Rule R156-1 is as described in Section R156-1-107
58-17b-50G Petitioning for reinstatement of licensure Any person whose license to practice pharmacy in this state has been revoked suspended or surrendered
voluntarily or by action of the division shall have the right at reasonable intervals to petition the division for reinstatement of such license Such petition shall be made in writing and in the form prescribed by the division Upon investigation and hearing the division may in its discretion grant or deny such petition or it may modify its driginal finding to reflect any circumstances that have changed sufficiently to warrant such modifications Themiddotdivision also at its discretion may require such person to pass an examination or examinations for reshyentry into the practice of pharmacy Enacted by Chapter 280 2004 General Session
Agenda Board of Pharmacy
Collaborative Practice Agreement Discussion 100 PM - 200 PM
-~ Agenda Item Overview - I
Welcome and Introductions Paige Patterick and Carrie Dunford
Discuss the wording of 58-17b-l 02( 17) (17) Collaborative pharmacy practice agreement means a written and signed agreement between one or more pharmacists and one or more practitioners that provides for collaborative pharmacy practice for the purpose of drug therapy management of patients and prevention of disease of human subjects
Discuss the definition of Drug Therapy Management in R 156-17b-l 02 (19)
(J 9) Drug therapy management means the review of a drug therapy regimen of a patient by one or more pharmacists for the purpose of evaluating and rendering advice to one or more practitioners regarding adjustment of the regimen
Resources
NABP https llnabppharmacywp-contentuploads20 1607 ITFCollaborativePracticeAgreements AM95 Dec 1998 pdf
Operating Standards Drug therapy management R 156-1 7b-61 I
R 156-l7b-611 Operating Standards - Drug Therapy Management (I) In accordance with Subsections 58-17b-l 02( 17) and 58-l7b-60 I( I) decisions involving drug therapy management shall be made in the best interest of the patient Drug therapy management may include (a) implementing modifying and managing drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement (b) collecting and reviewing patient histories (c) obtaining and checking vital signs including pulse temperature blood pressure and respiration (d) ordering and evaluating the results of laboratory tests directly applicable to the drug therapy when perfolmed in accordance with approved protocols applicable to the practice setting and (e) such other patient care services as may be allowed by rule (2) For the purpose of promoting therapeutic appropriateness a pharmacist shall at the time of dispensing a prescription or a prescription drug order review the patients medication record Such review shall at a minimum identify clinically significant conditions situations or items such as (a) inappropriate drug utilization (b) therapeutic duplication (c) drug-disease contraindications (d) drug-drug interactions (e) incorrect drug dosage or duration of drug treatment (f) drug-allergy interactions and (g) clinical abuse or misuse (3) Upon identifying any clinically significant conditions situations or items listed in Subsection (2) above the pharmacist shall take appropriate steps to avoid or resolve the problem including consultation with the prescribing practitioner
Note In compliance with the Americans with Disabilities Act individuals needing special accommodations (including auxiliary communicative aids and services) during this meet ing should not ify Dave Taylor ADA Coordinator at least three working days prior to the meeting Division of Occupational amp Professional Licensing 160 East 300 South Salt Lake City Utah 84115 801-530-6628 or toll-free in Utah only 866-275-3675 Posted to Bulletin Board on 11-21-2017

Report of the Task Force on Collaborative Practice Agreements - NABP 9Smiddoth Annual Meeting Proceedings
(i) A detailed description of the types of diseases Drugs or Drug categories involved and the type of Drug Therapy Management allowed in each case
(ii) A detailed description of the methods procedures decision criteria and plan the Pharmacist is to follow when conducting Drug Therapy ~anagement and
(iii) A detailed description of the activities the Pharmacist is to follow in the course of conducting Drug Therapy Management including documentation of decisions made and a plan or appropriate mechanism for conununication feedback and reporting to the Practitioner concerning specific decisions made In addition to the Agreement documentation shall occur on the prescription record patient profile a separate log book or in some other appropriate system
(c) A method for the Practitioner to monitor compliance with the Agreement and clinical outcomes where Drug Therapy Management by the Pharmacist has occurred and to intercede where necessary
(d) A provision that allows the Practitioner to override a collaborative practice decision mad by the Pharmacist whenever he or she deems it necessary or appropriate
(e) A provision that allows either party to cancel the agreement by written notification
(f) An effective date and (g) Signatures of all collaborating Pharmacists and Practitioners who are party to the
agreement as well as the date of signing Amendments to a Collaborative Pharmacy Practice Agreement must be documented signed and dated
(3) Initiation of the Collaborative Pharmacy Practice Agreement The Collaborative Pharmacy Practice Agreement must be coupled with a medical order from the Practitioner to initiate Drug Therapy Management for any particular patient
(4) Documentation of Drug Therapy Management Docwnentation of Drug Therapy ~anagement must be kept as part of the patients permanent record and be readily available to other health care professionals providing care to that patient and who are authorized to received it Docwnentation of drug Therapy Management shall be considered Confidential Information
(5) Review At a minimum the written agreement shall be reviewed and renewed and if necessary revised every year
[NOTE the above new subsections would be inserted with sequential relettering of all subsequent subsections]
Background
Task Force members listened to a brief presentation by American College of Clinical Pharmacy (ACCP) Executive Director Robert Elenbaas and Immediate Past President Jerry Bauman regarding ACCPs position on collaborative practice agreements and later discussed their charge to develop national guidelines for writing and implementing uniform collaborative practice agreements for use by state boards of pharmacy They agreed that the best way to fulfill their charge was to create model regulations addressing the issue
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI3
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sb Annual Meeting Proceedings
To assist in developing the model regulations members looked to several sources of information including current state pharmacy laws and regulations as well as the information provided by Drs Elenbaas and Bauman The Task Force felt that the Idaho State Board of Pharmacys regulations on collaborative practice encompassed much of what they wanted to include in the model regulation therefore they were used as a blueprint
A brainstorming session brought issues and concerns regarding state regulation of collaborative pharmacy practice to the forefront Topics that members felt needed to be addressed in a model regulation included
Contents of collaborative pharmacy practice agreements
Practitioner override authority
Board approval of agreements
Phannacists documentation of care
Patient consentnotification
Confidentiality of patient information
Periodic review of agreement
Phannacist competencequalifications to provide drug therapy management services and
Quality assurance review of practice
Task Force members concurred on a majority of these topics and incorporated their determinations into the model recommendation Members agreed that certain key elements should be included in collaborative phannacy practice agreements so that such agreements may guide both the pharmacists and collaborating practitioners decisions and conduct Key elements incorporated included a description of the pharmacists activities a method describing a practitioner monitoring methods and a practitioner override clause
Members also agreed that it should not be necessary for boards of pharmacy to take on the responsibility for approving agreements or even requiring their submission but that boards should require parties to notify them of the existence of such agreements and to keep a copy at the practice site On this issue members concluded that the individual practitioners were the persons most qualified to approve the agreements noting that minimal government involvement in the practice of the phannacist and collaborating practitioner was the most desirable In order for the board to have some handle on the situation however it was determined that agreements should be made available for board inspection upon request Regarding he issue of pharmacist competence to provide drug therapy management services Task Force members concluded that the subject should not be addressed in the model regulations They agreed that he determination of competence should be left up to the individuals who are party to the agreement Members felt certain that most if not al1 collaborating practitioners would only delegate certain aspects of their patient care authority upon demonstration by the pharmacist of his or her abilities to provide such care Further members felt that the majority ofphannacists would not take on the responsibility for providing such care without being competent to do so
TFCP A Recommendation 2
The Task Force on Collaborative Practice Agreements recognized that the issue of quality care assurance is not still developing and at present cannot be addressed with regard to collaborative pharmacy practice The Task Force recommends that the NABP Executive Committee closely
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI4
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
follow this issue and should it be determined that language addressing the subject should be incorporated into the model regulation take further action at that time
Background
Task Force members were uncertain at this time of how to address the issue of quality care assurance Members noted that most states addressing collaborative pharmacy practice in their laws or regulations did not include a provision on this subject It was noted however that tat least one state placed the responsibility for quality assurance on the Principal pharmacist and practitioner and that ACCP urged that an appropriate body be responsible for such activities
Member postulated that collaborative practice activities would likely be subject to quality assurance review via physician peer-review processes Additionally they suggested that those parties practicing in institutional settings would follow the quality assurance policies guidelines andor requirements of the institution The Task Force felt it was premature to make a recommendation regarding those parties who practice in ambulatory settings and who may not be subject to quality assurance review Members observed that the work ofNABPs Task Force on Patient Outcomes Regulation should be reviewed before a recommendation was made
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406 (F) 847391-4502middot wwwnabpnet 5
58-1-107 Applicability -- Relationship to specific chapters under title The provisions of this chapter LJniformly apply to the administration and enforcement of this title However
unless expressly prohibited in this chapter any provision of this chapter may be supplemented or altered by specific chapters of this title
R1S6-1-107 Organization of Rules - Content Applicability and Relationship of Rules
(1) The rules and sections in Title R156 shall to the extent practicable follow the numbering and
organizational scheme of the chapters in Title 58
(2) Rule R156-1 shall contain general provisions applicable to the administration and enforcement of all
occupations and profeSSions regulated in Title 58
(3) The provisions of the other rules in Title R156 shall contain specific or unique provisions applicable to
particular occupations or professions
(4) Specific rules in Title R156 may supplement or alter Rule R156-1 unless expressly provided otherwise
in Rule R156-1
R1S6-17b-l04 Organization - Relationship to Rule R1S6-1
The organization of this rule and its relationship to Rule R156-1 is as described in Section R156-1-107
58-17b-50G Petitioning for reinstatement of licensure Any person whose license to practice pharmacy in this state has been revoked suspended or surrendered
voluntarily or by action of the division shall have the right at reasonable intervals to petition the division for reinstatement of such license Such petition shall be made in writing and in the form prescribed by the division Upon investigation and hearing the division may in its discretion grant or deny such petition or it may modify its driginal finding to reflect any circumstances that have changed sufficiently to warrant such modifications Themiddotdivision also at its discretion may require such person to pass an examination or examinations for reshyentry into the practice of pharmacy Enacted by Chapter 280 2004 General Session
Agenda Board of Pharmacy
Collaborative Practice Agreement Discussion 100 PM - 200 PM
-~ Agenda Item Overview - I
Welcome and Introductions Paige Patterick and Carrie Dunford
Discuss the wording of 58-17b-l 02( 17) (17) Collaborative pharmacy practice agreement means a written and signed agreement between one or more pharmacists and one or more practitioners that provides for collaborative pharmacy practice for the purpose of drug therapy management of patients and prevention of disease of human subjects
Discuss the definition of Drug Therapy Management in R 156-17b-l 02 (19)
(J 9) Drug therapy management means the review of a drug therapy regimen of a patient by one or more pharmacists for the purpose of evaluating and rendering advice to one or more practitioners regarding adjustment of the regimen
Resources
NABP https llnabppharmacywp-contentuploads20 1607 ITFCollaborativePracticeAgreements AM95 Dec 1998 pdf
Operating Standards Drug therapy management R 156-1 7b-61 I
R 156-l7b-611 Operating Standards - Drug Therapy Management (I) In accordance with Subsections 58-17b-l 02( 17) and 58-l7b-60 I( I) decisions involving drug therapy management shall be made in the best interest of the patient Drug therapy management may include (a) implementing modifying and managing drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement (b) collecting and reviewing patient histories (c) obtaining and checking vital signs including pulse temperature blood pressure and respiration (d) ordering and evaluating the results of laboratory tests directly applicable to the drug therapy when perfolmed in accordance with approved protocols applicable to the practice setting and (e) such other patient care services as may be allowed by rule (2) For the purpose of promoting therapeutic appropriateness a pharmacist shall at the time of dispensing a prescription or a prescription drug order review the patients medication record Such review shall at a minimum identify clinically significant conditions situations or items such as (a) inappropriate drug utilization (b) therapeutic duplication (c) drug-disease contraindications (d) drug-drug interactions (e) incorrect drug dosage or duration of drug treatment (f) drug-allergy interactions and (g) clinical abuse or misuse (3) Upon identifying any clinically significant conditions situations or items listed in Subsection (2) above the pharmacist shall take appropriate steps to avoid or resolve the problem including consultation with the prescribing practitioner
Note In compliance with the Americans with Disabilities Act individuals needing special accommodations (including auxiliary communicative aids and services) during this meet ing should not ify Dave Taylor ADA Coordinator at least three working days prior to the meeting Division of Occupational amp Professional Licensing 160 East 300 South Salt Lake City Utah 84115 801-530-6628 or toll-free in Utah only 866-275-3675 Posted to Bulletin Board on 11-21-2017

Report of the Task Force on Collaborative Practice Agreements - NABP 9Sb Annual Meeting Proceedings
To assist in developing the model regulations members looked to several sources of information including current state pharmacy laws and regulations as well as the information provided by Drs Elenbaas and Bauman The Task Force felt that the Idaho State Board of Pharmacys regulations on collaborative practice encompassed much of what they wanted to include in the model regulation therefore they were used as a blueprint
A brainstorming session brought issues and concerns regarding state regulation of collaborative pharmacy practice to the forefront Topics that members felt needed to be addressed in a model regulation included
Contents of collaborative pharmacy practice agreements
Practitioner override authority
Board approval of agreements
Phannacists documentation of care
Patient consentnotification
Confidentiality of patient information
Periodic review of agreement
Phannacist competencequalifications to provide drug therapy management services and
Quality assurance review of practice
Task Force members concurred on a majority of these topics and incorporated their determinations into the model recommendation Members agreed that certain key elements should be included in collaborative phannacy practice agreements so that such agreements may guide both the pharmacists and collaborating practitioners decisions and conduct Key elements incorporated included a description of the pharmacists activities a method describing a practitioner monitoring methods and a practitioner override clause
Members also agreed that it should not be necessary for boards of pharmacy to take on the responsibility for approving agreements or even requiring their submission but that boards should require parties to notify them of the existence of such agreements and to keep a copy at the practice site On this issue members concluded that the individual practitioners were the persons most qualified to approve the agreements noting that minimal government involvement in the practice of the phannacist and collaborating practitioner was the most desirable In order for the board to have some handle on the situation however it was determined that agreements should be made available for board inspection upon request Regarding he issue of pharmacist competence to provide drug therapy management services Task Force members concluded that the subject should not be addressed in the model regulations They agreed that he determination of competence should be left up to the individuals who are party to the agreement Members felt certain that most if not al1 collaborating practitioners would only delegate certain aspects of their patient care authority upon demonstration by the pharmacist of his or her abilities to provide such care Further members felt that the majority ofphannacists would not take on the responsibility for providing such care without being competent to do so
TFCP A Recommendation 2
The Task Force on Collaborative Practice Agreements recognized that the issue of quality care assurance is not still developing and at present cannot be addressed with regard to collaborative pharmacy practice The Task Force recommends that the NABP Executive Committee closely
NATIONAL ASSOCIATION OF BOARDS OF PHARMACYmiddot (P) 847391-4406middot (F) 847391-4502middot wwwnabpneI4
Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
follow this issue and should it be determined that language addressing the subject should be incorporated into the model regulation take further action at that time
Background
Task Force members were uncertain at this time of how to address the issue of quality care assurance Members noted that most states addressing collaborative pharmacy practice in their laws or regulations did not include a provision on this subject It was noted however that tat least one state placed the responsibility for quality assurance on the Principal pharmacist and practitioner and that ACCP urged that an appropriate body be responsible for such activities
Member postulated that collaborative practice activities would likely be subject to quality assurance review via physician peer-review processes Additionally they suggested that those parties practicing in institutional settings would follow the quality assurance policies guidelines andor requirements of the institution The Task Force felt it was premature to make a recommendation regarding those parties who practice in ambulatory settings and who may not be subject to quality assurance review Members observed that the work ofNABPs Task Force on Patient Outcomes Regulation should be reviewed before a recommendation was made
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406 (F) 847391-4502middot wwwnabpnet 5
58-1-107 Applicability -- Relationship to specific chapters under title The provisions of this chapter LJniformly apply to the administration and enforcement of this title However
unless expressly prohibited in this chapter any provision of this chapter may be supplemented or altered by specific chapters of this title
R1S6-1-107 Organization of Rules - Content Applicability and Relationship of Rules
(1) The rules and sections in Title R156 shall to the extent practicable follow the numbering and
organizational scheme of the chapters in Title 58
(2) Rule R156-1 shall contain general provisions applicable to the administration and enforcement of all
occupations and profeSSions regulated in Title 58
(3) The provisions of the other rules in Title R156 shall contain specific or unique provisions applicable to
particular occupations or professions
(4) Specific rules in Title R156 may supplement or alter Rule R156-1 unless expressly provided otherwise
in Rule R156-1
R1S6-17b-l04 Organization - Relationship to Rule R1S6-1
The organization of this rule and its relationship to Rule R156-1 is as described in Section R156-1-107
58-17b-50G Petitioning for reinstatement of licensure Any person whose license to practice pharmacy in this state has been revoked suspended or surrendered
voluntarily or by action of the division shall have the right at reasonable intervals to petition the division for reinstatement of such license Such petition shall be made in writing and in the form prescribed by the division Upon investigation and hearing the division may in its discretion grant or deny such petition or it may modify its driginal finding to reflect any circumstances that have changed sufficiently to warrant such modifications Themiddotdivision also at its discretion may require such person to pass an examination or examinations for reshyentry into the practice of pharmacy Enacted by Chapter 280 2004 General Session
Agenda Board of Pharmacy
Collaborative Practice Agreement Discussion 100 PM - 200 PM
-~ Agenda Item Overview - I
Welcome and Introductions Paige Patterick and Carrie Dunford
Discuss the wording of 58-17b-l 02( 17) (17) Collaborative pharmacy practice agreement means a written and signed agreement between one or more pharmacists and one or more practitioners that provides for collaborative pharmacy practice for the purpose of drug therapy management of patients and prevention of disease of human subjects
Discuss the definition of Drug Therapy Management in R 156-17b-l 02 (19)
(J 9) Drug therapy management means the review of a drug therapy regimen of a patient by one or more pharmacists for the purpose of evaluating and rendering advice to one or more practitioners regarding adjustment of the regimen
Resources
NABP https llnabppharmacywp-contentuploads20 1607 ITFCollaborativePracticeAgreements AM95 Dec 1998 pdf
Operating Standards Drug therapy management R 156-1 7b-61 I
R 156-l7b-611 Operating Standards - Drug Therapy Management (I) In accordance with Subsections 58-17b-l 02( 17) and 58-l7b-60 I( I) decisions involving drug therapy management shall be made in the best interest of the patient Drug therapy management may include (a) implementing modifying and managing drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement (b) collecting and reviewing patient histories (c) obtaining and checking vital signs including pulse temperature blood pressure and respiration (d) ordering and evaluating the results of laboratory tests directly applicable to the drug therapy when perfolmed in accordance with approved protocols applicable to the practice setting and (e) such other patient care services as may be allowed by rule (2) For the purpose of promoting therapeutic appropriateness a pharmacist shall at the time of dispensing a prescription or a prescription drug order review the patients medication record Such review shall at a minimum identify clinically significant conditions situations or items such as (a) inappropriate drug utilization (b) therapeutic duplication (c) drug-disease contraindications (d) drug-drug interactions (e) incorrect drug dosage or duration of drug treatment (f) drug-allergy interactions and (g) clinical abuse or misuse (3) Upon identifying any clinically significant conditions situations or items listed in Subsection (2) above the pharmacist shall take appropriate steps to avoid or resolve the problem including consultation with the prescribing practitioner
Note In compliance with the Americans with Disabilities Act individuals needing special accommodations (including auxiliary communicative aids and services) during this meet ing should not ify Dave Taylor ADA Coordinator at least three working days prior to the meeting Division of Occupational amp Professional Licensing 160 East 300 South Salt Lake City Utah 84115 801-530-6628 or toll-free in Utah only 866-275-3675 Posted to Bulletin Board on 11-21-2017

Report of the Task Force on Collaborative Practice Agreements - NABP 9Sh Annual Meeting Proceedings
follow this issue and should it be determined that language addressing the subject should be incorporated into the model regulation take further action at that time
Background
Task Force members were uncertain at this time of how to address the issue of quality care assurance Members noted that most states addressing collaborative pharmacy practice in their laws or regulations did not include a provision on this subject It was noted however that tat least one state placed the responsibility for quality assurance on the Principal pharmacist and practitioner and that ACCP urged that an appropriate body be responsible for such activities
Member postulated that collaborative practice activities would likely be subject to quality assurance review via physician peer-review processes Additionally they suggested that those parties practicing in institutional settings would follow the quality assurance policies guidelines andor requirements of the institution The Task Force felt it was premature to make a recommendation regarding those parties who practice in ambulatory settings and who may not be subject to quality assurance review Members observed that the work ofNABPs Task Force on Patient Outcomes Regulation should be reviewed before a recommendation was made
NATIONAL ASSOCIATION OF BOARDS OF PHARMACY (P) 847391-4406 (F) 847391-4502middot wwwnabpnet 5
58-1-107 Applicability -- Relationship to specific chapters under title The provisions of this chapter LJniformly apply to the administration and enforcement of this title However
unless expressly prohibited in this chapter any provision of this chapter may be supplemented or altered by specific chapters of this title
R1S6-1-107 Organization of Rules - Content Applicability and Relationship of Rules
(1) The rules and sections in Title R156 shall to the extent practicable follow the numbering and
organizational scheme of the chapters in Title 58
(2) Rule R156-1 shall contain general provisions applicable to the administration and enforcement of all
occupations and profeSSions regulated in Title 58
(3) The provisions of the other rules in Title R156 shall contain specific or unique provisions applicable to
particular occupations or professions
(4) Specific rules in Title R156 may supplement or alter Rule R156-1 unless expressly provided otherwise
in Rule R156-1
R1S6-17b-l04 Organization - Relationship to Rule R1S6-1
The organization of this rule and its relationship to Rule R156-1 is as described in Section R156-1-107
58-17b-50G Petitioning for reinstatement of licensure Any person whose license to practice pharmacy in this state has been revoked suspended or surrendered
voluntarily or by action of the division shall have the right at reasonable intervals to petition the division for reinstatement of such license Such petition shall be made in writing and in the form prescribed by the division Upon investigation and hearing the division may in its discretion grant or deny such petition or it may modify its driginal finding to reflect any circumstances that have changed sufficiently to warrant such modifications Themiddotdivision also at its discretion may require such person to pass an examination or examinations for reshyentry into the practice of pharmacy Enacted by Chapter 280 2004 General Session
Agenda Board of Pharmacy
Collaborative Practice Agreement Discussion 100 PM - 200 PM
-~ Agenda Item Overview - I
Welcome and Introductions Paige Patterick and Carrie Dunford
Discuss the wording of 58-17b-l 02( 17) (17) Collaborative pharmacy practice agreement means a written and signed agreement between one or more pharmacists and one or more practitioners that provides for collaborative pharmacy practice for the purpose of drug therapy management of patients and prevention of disease of human subjects
Discuss the definition of Drug Therapy Management in R 156-17b-l 02 (19)
(J 9) Drug therapy management means the review of a drug therapy regimen of a patient by one or more pharmacists for the purpose of evaluating and rendering advice to one or more practitioners regarding adjustment of the regimen
Resources
NABP https llnabppharmacywp-contentuploads20 1607 ITFCollaborativePracticeAgreements AM95 Dec 1998 pdf
Operating Standards Drug therapy management R 156-1 7b-61 I
R 156-l7b-611 Operating Standards - Drug Therapy Management (I) In accordance with Subsections 58-17b-l 02( 17) and 58-l7b-60 I( I) decisions involving drug therapy management shall be made in the best interest of the patient Drug therapy management may include (a) implementing modifying and managing drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement (b) collecting and reviewing patient histories (c) obtaining and checking vital signs including pulse temperature blood pressure and respiration (d) ordering and evaluating the results of laboratory tests directly applicable to the drug therapy when perfolmed in accordance with approved protocols applicable to the practice setting and (e) such other patient care services as may be allowed by rule (2) For the purpose of promoting therapeutic appropriateness a pharmacist shall at the time of dispensing a prescription or a prescription drug order review the patients medication record Such review shall at a minimum identify clinically significant conditions situations or items such as (a) inappropriate drug utilization (b) therapeutic duplication (c) drug-disease contraindications (d) drug-drug interactions (e) incorrect drug dosage or duration of drug treatment (f) drug-allergy interactions and (g) clinical abuse or misuse (3) Upon identifying any clinically significant conditions situations or items listed in Subsection (2) above the pharmacist shall take appropriate steps to avoid or resolve the problem including consultation with the prescribing practitioner
Note In compliance with the Americans with Disabilities Act individuals needing special accommodations (including auxiliary communicative aids and services) during this meet ing should not ify Dave Taylor ADA Coordinator at least three working days prior to the meeting Division of Occupational amp Professional Licensing 160 East 300 South Salt Lake City Utah 84115 801-530-6628 or toll-free in Utah only 866-275-3675 Posted to Bulletin Board on 11-21-2017

58-1-107 Applicability -- Relationship to specific chapters under title The provisions of this chapter LJniformly apply to the administration and enforcement of this title However
unless expressly prohibited in this chapter any provision of this chapter may be supplemented or altered by specific chapters of this title
R1S6-1-107 Organization of Rules - Content Applicability and Relationship of Rules
(1) The rules and sections in Title R156 shall to the extent practicable follow the numbering and
organizational scheme of the chapters in Title 58
(2) Rule R156-1 shall contain general provisions applicable to the administration and enforcement of all
occupations and profeSSions regulated in Title 58
(3) The provisions of the other rules in Title R156 shall contain specific or unique provisions applicable to
particular occupations or professions
(4) Specific rules in Title R156 may supplement or alter Rule R156-1 unless expressly provided otherwise
in Rule R156-1
R1S6-17b-l04 Organization - Relationship to Rule R1S6-1
The organization of this rule and its relationship to Rule R156-1 is as described in Section R156-1-107
58-17b-50G Petitioning for reinstatement of licensure Any person whose license to practice pharmacy in this state has been revoked suspended or surrendered
voluntarily or by action of the division shall have the right at reasonable intervals to petition the division for reinstatement of such license Such petition shall be made in writing and in the form prescribed by the division Upon investigation and hearing the division may in its discretion grant or deny such petition or it may modify its driginal finding to reflect any circumstances that have changed sufficiently to warrant such modifications Themiddotdivision also at its discretion may require such person to pass an examination or examinations for reshyentry into the practice of pharmacy Enacted by Chapter 280 2004 General Session
Agenda Board of Pharmacy
Collaborative Practice Agreement Discussion 100 PM - 200 PM
-~ Agenda Item Overview - I
Welcome and Introductions Paige Patterick and Carrie Dunford
Discuss the wording of 58-17b-l 02( 17) (17) Collaborative pharmacy practice agreement means a written and signed agreement between one or more pharmacists and one or more practitioners that provides for collaborative pharmacy practice for the purpose of drug therapy management of patients and prevention of disease of human subjects
Discuss the definition of Drug Therapy Management in R 156-17b-l 02 (19)
(J 9) Drug therapy management means the review of a drug therapy regimen of a patient by one or more pharmacists for the purpose of evaluating and rendering advice to one or more practitioners regarding adjustment of the regimen
Resources
NABP https llnabppharmacywp-contentuploads20 1607 ITFCollaborativePracticeAgreements AM95 Dec 1998 pdf
Operating Standards Drug therapy management R 156-1 7b-61 I
R 156-l7b-611 Operating Standards - Drug Therapy Management (I) In accordance with Subsections 58-17b-l 02( 17) and 58-l7b-60 I( I) decisions involving drug therapy management shall be made in the best interest of the patient Drug therapy management may include (a) implementing modifying and managing drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement (b) collecting and reviewing patient histories (c) obtaining and checking vital signs including pulse temperature blood pressure and respiration (d) ordering and evaluating the results of laboratory tests directly applicable to the drug therapy when perfolmed in accordance with approved protocols applicable to the practice setting and (e) such other patient care services as may be allowed by rule (2) For the purpose of promoting therapeutic appropriateness a pharmacist shall at the time of dispensing a prescription or a prescription drug order review the patients medication record Such review shall at a minimum identify clinically significant conditions situations or items such as (a) inappropriate drug utilization (b) therapeutic duplication (c) drug-disease contraindications (d) drug-drug interactions (e) incorrect drug dosage or duration of drug treatment (f) drug-allergy interactions and (g) clinical abuse or misuse (3) Upon identifying any clinically significant conditions situations or items listed in Subsection (2) above the pharmacist shall take appropriate steps to avoid or resolve the problem including consultation with the prescribing practitioner
Note In compliance with the Americans with Disabilities Act individuals needing special accommodations (including auxiliary communicative aids and services) during this meet ing should not ify Dave Taylor ADA Coordinator at least three working days prior to the meeting Division of Occupational amp Professional Licensing 160 East 300 South Salt Lake City Utah 84115 801-530-6628 or toll-free in Utah only 866-275-3675 Posted to Bulletin Board on 11-21-2017

Agenda Board of Pharmacy
Collaborative Practice Agreement Discussion 100 PM - 200 PM
-~ Agenda Item Overview - I
Welcome and Introductions Paige Patterick and Carrie Dunford
Discuss the wording of 58-17b-l 02( 17) (17) Collaborative pharmacy practice agreement means a written and signed agreement between one or more pharmacists and one or more practitioners that provides for collaborative pharmacy practice for the purpose of drug therapy management of patients and prevention of disease of human subjects
Discuss the definition of Drug Therapy Management in R 156-17b-l 02 (19)
(J 9) Drug therapy management means the review of a drug therapy regimen of a patient by one or more pharmacists for the purpose of evaluating and rendering advice to one or more practitioners regarding adjustment of the regimen
Resources
NABP https llnabppharmacywp-contentuploads20 1607 ITFCollaborativePracticeAgreements AM95 Dec 1998 pdf
Operating Standards Drug therapy management R 156-1 7b-61 I
R 156-l7b-611 Operating Standards - Drug Therapy Management (I) In accordance with Subsections 58-17b-l 02( 17) and 58-l7b-60 I( I) decisions involving drug therapy management shall be made in the best interest of the patient Drug therapy management may include (a) implementing modifying and managing drug therapy according to the terms of the Collaborative Pharmacy Practice Agreement (b) collecting and reviewing patient histories (c) obtaining and checking vital signs including pulse temperature blood pressure and respiration (d) ordering and evaluating the results of laboratory tests directly applicable to the drug therapy when perfolmed in accordance with approved protocols applicable to the practice setting and (e) such other patient care services as may be allowed by rule (2) For the purpose of promoting therapeutic appropriateness a pharmacist shall at the time of dispensing a prescription or a prescription drug order review the patients medication record Such review shall at a minimum identify clinically significant conditions situations or items such as (a) inappropriate drug utilization (b) therapeutic duplication (c) drug-disease contraindications (d) drug-drug interactions (e) incorrect drug dosage or duration of drug treatment (f) drug-allergy interactions and (g) clinical abuse or misuse (3) Upon identifying any clinically significant conditions situations or items listed in Subsection (2) above the pharmacist shall take appropriate steps to avoid or resolve the problem including consultation with the prescribing practitioner
Note In compliance with the Americans with Disabilities Act individuals needing special accommodations (including auxiliary communicative aids and services) during this meet ing should not ify Dave Taylor ADA Coordinator at least three working days prior to the meeting Division of Occupational amp Professional Licensing 160 East 300 South Salt Lake City Utah 84115 801-530-6628 or toll-free in Utah only 866-275-3675 Posted to Bulletin Board on 11-21-2017










![Index [ ] · PDF fileIndex Microscope ... 31-33-03 31-31-40 7C9W120V 31-33-40, 31-32-14, 31-99-23 31-31-42 1649 ... Zeiss Types 310198 EFR 3800181730 76Z](https://static.fdocuments.in/doc/165x107/5a9e3e117f8b9a6a218c9c2b/index-microscope-31-33-03-31-31-40-7c9w120v-31-33-40-31-32-14-31-99-23.jpg)