100% texture and smoothness of their skin. 3.0 Expert Clinical Grader Evaluation -Monadic,...
Transcript of 100% texture and smoothness of their skin. 3.0 Expert Clinical Grader Evaluation -Monadic,...
v.0916877.777.2305 | osmosisskincare.com
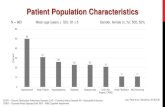
Independent, Monadic IRB-Reviewed Study of 56 people, 60% saw a reduction in skin imperfections and blemishes.
of test subjects had an improvement in complexion health, texture and smoothness of their skin.100%
CLINICAL STUDY
Four-Week Clinical Study Evaluates the Effects of
Harmonized Water on Facial Blemishes
100.00%
50.00%
0.00%PIH
Improvement
15.78%
OverallAppearance
25.63%
Active Lesions
29.70%
ComplexionHealth
30.87%
Texture / Smoothness
(Visual)
31.38%
Open and Closed
Comedones
49.20%
MEAN ACNE IMPROVEMENT IN 4 WEEKS
SKIN PERFECTION HARMONIZED WATER USE AFTER 4 WEEKS
Papules
80.00%
0%
10%
20%
30%
40%
50%
60%
70%62.9%54.3%
51.4%51.4%48.6%
60%
Saw a reduction of skin imperfections and blemishes
Felt the product prevented blemishes
Saw improved appearance in skin
Saw improvement in blemishes and skin complexion
Felt their skin tone was more even and clear
Felt their skin was smoother
7 .3.1 Procedure Summary Table
Procedures Washout Baseline Week4
Informed Consent and Medical X
Study Initiation and History Qualification Inclusion/Exclusion Criteria
X X reviewed
Compliance check, updated medical history, AE reportin!? X X
Dispense/ Collect Products D D
Suvvort Test
- Texture (visual)Expert Clinical - Global Acne Severity
X X Grading - Lesion count
- Overall Appearance
Photography Clarity 2D Research Ti (L, R, C
X X views)subwoup of 10
Image Analysis Acne Lesions X X
Consumer Perception Subjective Questionnaire X
8.0 Concomitant Medications and Products
The use of any topical cleansers and skin treatment products (other than those assigned during the study) on the
face was prohibited during the washout and study periods. This included, but was not limited to moisturizers,
serums, cleansers and medicated creams. Use of color-cosmetics which subjects were regularly using at time of
study enrollment was allowed during study period. No new cosmetics or personal care products were to be
introduced for the duration of the study. Use of certain other systemic and topical medications and treatments
products was prohibited during the study per Section 5.3.
9.0 Adverse Events
No adverse events were reported during the conduct of this study.
10.0 Institutional Review Board
IRB review was performed and approved.
11.0 Informed Consent
The informed consent process was completed prior to an individual's involvement in any study related activity.
The process was documented using a written informed consent form (ICF) conforming to FDA 21 CFR 50.25 (See
Appendix I Protocol, Section 11.0 and Appendix IV).
After review, two copies of the ICF were signed and dated by the individual and the Principal Investigator or his
designee administering the consent. One original copy was retained by IRSI and the other was given to the
individual.
12.0 Discontinuation of Study
The study was completed on schedule as per the clinical study protocol (and subsequent amendments).
Table 3.0 Expert Clinical Grader Evaluation - Monadic, comparison to Baseline
Placebo Waves Skin Harmony Harmonized Water
Mean Percent of Mean Percent of
Time Percent Subjects Percent Subjects P-ValueAssessment
Point n Improveme Showing P-Value n Mean± lmproveme Showing
Mean± SD TXvs. nt Improvemen TX vs. Bl SD nt Improvem
Bl From Bl t From Bl ent mean From Bl mean From Bl
Baseline 21 4.75 ±I.II 35 4.53 ±
Texture/ 0.43 Smoothness
27.05% 100% (Tactile) Week4 21 3.84 ± 1.17 16.31% 82.9%
<0.001* 35 3.31 ±
<0.001 * 0.74
Baseline 21 4.84 ± 1.10 35 4.77±
Texture/ 0.47 Smoothness
100% (Visual) Week4 21 3.73±1.15 19.69% 80.0%
<0.001* 35 3.28 ± 31.38%
<0.001 * 0.65
Baseline 21 4.80 ± 1.16 35 4.92 ±
Overall 0.56
Appearance Week4 21 4.04± 1.20
14.36% 71.4% <0.001* 35
3.69 ± 25.63% 95.2% <0.001•
0.88
Baseline 21 5.14 ± 0.98 35 5.20 ±
Complexion 0.78
Health 19.53% 82.9% 3.62 ± 30.87% 100% <0.001 * Week4 21 4.09 ± 1.32 <0.001 * 35
0.94
*Indicates a statistically significant improvement compared to baseline, p:50.05
Table 3.1 Expert Clinical Grader Evaluation - Product Comparison
Placebo Waves Skin Harmony
Assessment Time Harmonized Water P, Value
Point n
Mean Difference :I: SD n
Mean Difference :I: SD AvsB
From Bl From Bl
Texture/ Smoothness (Tactile) Week4 21 -0.91 ± 1.18 35 -1.21±0.61 0.020A
Texture/ Smoothness (Visual) Week4 21 -1.11 ± 1.24 35 -1.49 ± 0.52 0.01.8A
Overall Appearance Week4 21 -0.76 ± 0.90 35 -1.23 ± 0.60 0.022A
Complexion Health Week4 21 -1.04 ± 1.23 35 -1.58 ± 0.59 0.035" . .
Product Waves Skin Harmony Harmonized Water s1gn1f1cantly outperformed Product Placebo
Ta
ble
4.0
Le
sio
n C
ou
nt
-M
on
ad
ic,
co
mp
ari
son
to
Ba
seli
ne
Pla
ceb
o Per
cen
t of
Ass
essm
ent
Tim
e M
ean
Per
cen
t S
ubje
cts
n
Poin
t M
ean
±S
D
Red
uct
ion
S
how
ing
F
rom
BL
mean
R
edu
ctio
n
From
BL
IN
FL
AMMA
TO
RY
Bas
elin
e 21
6.5
7 ±
3.3
2
Pap
ule
s W
eek
4
21
6.0
5 ±
2.7
2
NR
5
4.3
%
Bas
elin
e P
ust
ule
s 21
3.
71 ±
3.7
7
Wee
k4
2
1
4.9
4±
3.9
4
NR
34
.3%
No
du
les
Bas
elin
e 21
0
.22
± 0
.65
Wee
k4
21
0.0
0±
0.0
0
100%
1
4.3
%
Bas
elin
e 21
0.2
5 ±
0.6
1 C
yst
s W
eek
4
21
0.4
0 ±
1.2
1
NR
1
1.4
%
Bas
elin
e 21
1
0.7
7 ±
5.7
8
To
tal
Infl
amm
ato
ry
Wee
k4
21
1
1.3
4±
6.1
1
NR
37.1
%
NO
N-I
NF
LA
MM
AT
OR
Y
Bas
elin
e 21
3.8
2 ±
4.3
6
Op
en C
om
edon
es
--
Wee
k4
21
3.0
5 ±
3.4
2
II
.II
%5
4.3
%
Bas
elin
e 21
2
.85
± 3.3
0
Clo
sed
Co
med
on
es
Wee
k4
2
1
2.6
5 ±
2.9
1
36
.56
%
42.9
%
·-
To
tal
Bas
elin
e 2
1
6.6
8±
5.2
4
No
n-I
nfl
amm
ator
y
Wee
k4
21
5
.82 ±
4.3
2
5.1
3%
5
7.1
%
TO
TAL
LE
SIO
NS
Bas
elin
e 2
1
17.4
5 ±
8.9
9
Les
ion
Co
un
t T
ota
l W
eek
4
21
1
7.1
7±
7.6
1
NR
5
1.4
%
NR
=N
o R
ed
uct
ion
*In
dic
ate
s a
sta
tist
ica
lly s
ign
ific
an
t im
pro
vem
en
t co
mp
are
d t
o b
ase
line
, p
:50
.05
P-V
alu
en
TX
vs. BL
35
0.3
38
3
5
35
0.0
62
3
5
35
0.0
44*
35
35
0.4
83
35
35
0.4
65
35
I 35
0.1
45
35
35
0.7
11
35
35
0.2
80
35
35
0.8
16
35
Waves
Sk
in H
arm
on
y H
arm
on
ized
Wate
r
Mea
n P
erce
nt
Mea
n±
SD
R
edu
ctio
n
From
BL
mea
n
-
4.7
1 ±
2.2
3
4.0
4 ±
2.3
5
4.5
6%
2.6
1 ±
3.8
9
2.2
3 ±
3.9
1
7.8
9%
0.1
4±
0.4
7
0.0
0±
0.0
0
100%
0.0
0±
0.0
0
0.1
4±
0.4
7
NR
7.4
7 ±
4.5
3
6.4
2 ±
5.5
1
NR
2.76
± 4
.75
1.6
6 ±
2.6
1
48
.78
%
3.6
6±
2.7
2
1.7
6 ±
1.9
7
44.7
2%
6.4
2 ±
6.0
7
-
3.4
2 ±
3.6
5
49
.20
% --
13.9
0 ±
6.3
2
11
.85
± 6
.59
14
.65
%
Per
cen
t of
Su
bje
cts
Sh
ow
ing
R
edu
ctio
n
Fro
m B
L
63.3
%
69.0
%
9.5
%
NR
33.3
%
. -
42.9
%
71
.4%
71
.4%
66.7
%
P-V
alu
eT
Xv
s.
BL
0.1
94
0.0
58
0.1
86
0.1
86
0.1
67
- 0.0
97
0.00
3*
0.0
02*
0.0
25*
Ta
ble
4.1
Le
sio
n C
ou
nt
-P
rod
uct
Co
mp
ari
son
Pla
ceb
o
Waves
Sk
in H
arm
on
y
Ass
essm
ent
Tim
e H
arm
on
ized
Wate
r P
, V
alu
e P
oin
t n
M
ean
Diff
eren
ce :I:
SD
n
M
ean
Diff
eren
ce :I:
SD
A
vsB
From
BL
From
BL
IN
FL
AM
MA
TO
RY
Pap
ules
W
eek
4
21
-0
.51
± 3.1
235
-0
.64 ±
2.8
1
0.3
01
P
ustu
les
Wee
k4
2
1
1.2
2 ±
3.7
6 3
5
-0.4
1 ±
1.6
50.0
40
A
No
dule
s W
eek
4
21
-0
.22
± 0
.64
35
-0.1
4 ±
0.4
70
.573
C
yst
's
Wee
k4
2
1
0.1
4±
1.1
9 3
5
0.1
4 ±
0.4
7
1.0
00
T
ota
l In
flam
mat
ory
W
eek
4
21
0
.57 ±
4.5
7 3
5
-0.9
5 ±
3.0
40.0
10
A
NO
N-I
NF
LA
MM
AT
OR
Y
Ope
n C
om
edo
nes
Wee
k4
2
1
-0.7
7 ±
3.0
53
5
-1.0
9 ±
2.8
70
.69
3
Clo
sed
Co
med
ones
W
eek
4
21
-0
.20
±3.1
73
5
-1.9
0 ±
2.6
20.0
35
A
To
tal N
on-
Infl
amm
ato
ry
Wee
k4
2
1
-0.8
5 ±
4.6
23
5
-3.0
0 ±
3.8
20
.067
TO
TA
L L
ES
IO
NS
Les
ion
Co
unt T
ota
l W
eek
4
21
-0
.28
± 7
.21
35
-2
.04
± 3
.87
0.0
40
A
Pro
du
ct W
av
es
Ski
n H
arm
on
y H
arm
on
ize
d W
ate
r si
gn
ific
an
tly
ou
tpe
rfo
rme
d P
rod
uct
Pla
ceb
o
Ta
ble
4.2
Cla
rity
20
-M
on
ad
ic,
com
pa
riso
n t
o B
ase
lin
e
Mea
n P
erce
nt
Per
cen
t of S
ub
ject
s P
- Val
ue
Ass
essm
ent
Tim
e P
oin
t n
Mea
n:!:
SD
R
educt
ion
S
how
ing R
educt
ion
T
X v
s. B
L
Fro
m B
L m
ean
F
rom
BL
Bas
elin
e 1
4
2.6
7 ±
2.8
7
Act
ive
Les
ions
Wee
k4
1
4
1.7
3 ±
1.5
0
29.
70%
78
.6%
0.0
37
*
Bas
elin
e 1
4
0.1
1 ±
0.1
7 P
apul
es
Wee
k4
14
0
.01
± 0
.05
8
0.0
0%
2
8.6
%
0.0
47
*
Bas
elin
e 1
4
0.0
9±0
.15
A
cne
Pust
ule
s W
eek
4
14
0
.01
± 0
.05
1
00
%
28
.6%
0
.13
6
Bas
elin
e 1
4
0.0
6 ±
0.1
2
..
Cy
sts
Wee
k4
1
4
0.0
3 ±
0.0
7
83
.33
%
21
.4%
0
.33
6 -
-·
Po
st I
nfla
mm
ato
ry H
yper
pig
men
tati
on
Bas
elin
e 1
4
12
.46
± 8
.55
Co
unt
Wee
k4
1
4
10
.51
± 7
.56
15
.78
%
92
.9%
<
0.0
01*
*In
dic
ate
s a
sta
tist
ica
lly
sig
nif
ica
nt
imp
rov
em
en
t co
mp
are
d t
o b
ase
lin
e,
ps
0.0
5
Table 5.0 Consumer Perception - Subjective Questionnaire - Week 4
Waves Skin Harmony Harmonized Water
Strongly Question n Agree
Agree
1. The test product made my skin 35
1 17 tone look more clear and even. (2.9%) (48.6%) 2. The test product decreased theappearance of discolorations caused
35 1 11
by imperfections and blemished on (2.9%) (31.4%) my facial skin. 3. The test product made my facial
35 9 13
skin feel smoother. (25.7%) (37.1%) 4. The test product made my facial
35 10 14
skin feel softer. (28.6%) (40.0%) 5. My facial skin appears less dull
2 13 and more radiant after using the test 35
(5.7%) (37.1) product.6. The test product helped improvemy facial skin imperfections and
35 2 15
blemished and gave homogenous (5.7%) (42.9%) complexion of my facial skin. 7. The test product improved the imperfection and blemished on my
1 10 facial skin, illuminating my 35
(2.9%) (28.6%) complexion with a fresh youthinfused glow.8. The test product game my skin a
35 0 9
flawless look. (0.0%) (25.7%) 9. The test product reduced the
35 3 13
number of lesions on my facial skin. (8.6%) (37.1%) 10. The test product reduced the
1 20 number of skin imperfections and 35
(2.9%) (57.1%) blemished on my facial skin. 11. It appears that the test productprevented the formation of new
35 4 13
lesions on my facial skin whiles using (11.4%) (37.1%) test product for four weeks. 12. It appears that the test product prevented the appearance of new
4 14 imperfections and blemishes on my 35 (11.4%) (40.0%)
skin while using the test product forfour weeks.13. The product improved the
35 2 13
quality of my facial skin. (5.7%) (37.1%) 14. The product improved the
35 5 14
overall appearance of my facial skin. (14.3%) (40.0%) 15. The test product did not irritate
13 16 my facial skin during the four week 35
(37.1%) (45.7%) treatment period.Bold/ Shaded = The majority of subjects responded favorably, >50%. A Waves Skin Harmony Harmonized Water Outperformed Placebo 6 Placebo Outperformed Waves Skin Harmony Harmonized Water
Strongly Neutral Disagree
Disagree
Response n (%) 9
7 (20.0%) 1
(25.7%) (2.9%)
11 11 1 (31.4%) (31.4%) (2.9%)
9 3 1 (25.7%) (8.6%) (2.9%)
6 4 (11.4%)
1 (17.1%) (2.9%)
13 1 (37.1%)
6 (17.1%) (2.9%)
9 7 (20.0%)
2 (25.7%) (5.7%)
13 2 (37.1%)
9 (25.7%) (5.7%)
12 10 4 (34.3%) {28.6%) (11.4%)
5 12 2 (14.3%) (34.3%) (5.7%)
1 11 2 (2.9%) (31.4%) (5.7%)
7 8 (22.9%)
3 (20.0%) (8.6%)
5 10 2 (14.3%) (28.6%) (5.7%)
13 6 (17.1%)
1 (37.1%) (2.9%)
6 9 (25.7%)
1 (17.1%) (2.9%)
3 0 3 (8.6%)
(8.6%) (0.0%)
Wilcoxon Percent
Responding Z-Score PrValue
Favorably
A vsB
51.4% -5.9522 <0.001A
34.3% -6.0308 <0.QQlA
62.9% -5.1520 <O.OOlA
68.6% -5.2820 <0.001A
42.9% -5.9683 <0.001A
48.6% -5.9833 <0.001A
31.4% -6.1540 <0.001A
25.7% -6.2064 <O.OOlA
45.7% -5.9232 <0.001A
60.0% -5.8924 <0.001A
48.6% -5.6088 <0.001A
51.4% -5.6542 <O.OOlA
42.9% -5.9833 <0.QQlA
54.3% -5.7115 <0.001A
82.9% -3.6842 <0.001A
IRSI, Inc. Protocol No. 39520SM0715.0 and .1
Draft Report Ver. 2.0
April 28, 2016
19.2 Discussion
19.2.1 Enrollment and Demographics
At least 55 male and female subjects, ages 13 to 40 years old, were required to complete
study participation. The study completed with 56 male and female subjects with an age
range of 14 to 38 years old and an average age of 23.07 years old. The study population's
reported ethnicity was 57.1% Non-Hispanic or Latino and 42.9% Hispanic or Latino, while its
reported racial diversity was 42.9% White, 21.4% African American or Black, 7.1% each of
Multi-racial and "Other" and 21.4% No Response (Hispanic). Fitzpatrick Skin Types I-VI was
represented along with Combination, Dry, Normal and Oily Skin.
19.2.2 Expert Visual Grading
Analysis of results revealed statistically significant, improvement from Baseline in mean
scores for the appearance of facial skin's texture/smoothness (tactile and visual), overall
appearance, and complexion health after four weeks of test product use. Comparative data
with placebo group revealed statistically significant improvement compared to placebo for
the appearance of facial skin's texture/smoothness (tactile and visual), overall appearance,
and complexion health after four weeks of test product use
19.2.3 Lesion Counts
A statistically significant decrease (improvement) from Baseline was seen in the mean
number of closed comedones total non-inflammatory lesions and total lesion count after
four weeks of use. Comparative data with placebo group revealed statistically significant
improvement compared to placebo in the mean number of closed comedones, total non
inflammatory lesions. pustules and total lesion count after four weeks of use.
19.2.4 Subjective Questionnaire
After four weeks of use, the majority of subjects (>50%) responded favorably ("agree" or
"strongly agree") to the statements "The test product made my skin tone look more clear
and even", "The test product made my facial skin feel smoother", "The test product made
my facial skin feel softer", "The test product gave my skin a flawless look", "The test
product reduced the number of skin imperfections and blemishes on my facial skin", "It
appears that the test product prevented the formation of new lesions on my facial skin
while using [it] for four weeks", "It appears that the test product prevented the
appearance of new imperfections and blemishes on my skin while using [it] for four
weeks", "The product improved the quality of my facial skin", "The product improved the
overall appearance of my facial skin" and "The test product did not irritate my facial skin
during the four week treatment period". Additionally, the majority of questions were
responded significantly more favorable in those randomized to the active product compared
to those receiving placebo.
20.0 Conclusion
In conclusion, under the conditions of this study, use of Waves "Skin Harmony" Harmonized Water
#852543005277 led to significant reduction in lesions, as evidenced by results from lesion counts, and
subjective questionnaire results. Further, subjective questionnaire results showed that the majority of
CONFIDENTIAL Appendix I L
IRSI, Inc. Protocol No. 39520SM0715.0 and .1
Draft Report Ver. 2.0
April 28, 2016
subjects believed that test product use resulted in improvements in the appearance of facial skin,
prevention of formation of new blemishes and acne lesions, and that the test product did not irritate
facial skin. Furthermore these results were statistically significant compared to placebo.
CONFIDENTIAL Appendix! L