1 Chapter 2 Chemistry of Life. 2 Outline Basic Chemistry – Atoms – Molecules and Compounds –...
-
Upload
clementine-carroll -
Category
Documents
-
view
215 -
download
1
Transcript of 1 Chapter 2 Chemistry of Life. 2 Outline Basic Chemistry – Atoms – Molecules and Compounds –...

1
Chapter 2
Chemistry of Life

2
Outline
• Basic Chemistry– Atoms– Molecules and Compounds– Chemical Reactions
• Properties of Water• Acids and Bases• Macromolecules• ATP

3
Basic Chemistry
• There are 92 naturally-occurring elements.– Over 90% of human body is composed of
four elements. (CHON)Carbon.Hydrogen.Oxygen.Nitrogen.

4
Atoms
• An atom is the smallest unit of matter that retains an element’s physical and chemical properties.– Positively-charged protons and neutral
neutrons are located in the nucleus.– Negatively-charged electrons orbit the
nucleus in shells.

5
Atoms
• An element’s atomic number is designated by its number of protons.
• An element’s atomic weight is designated by its protons and neutrons.

6
Elements and Atoms

7
Molecules and Compounds
• A molecule and compound is a group of atoms bonded together.

8
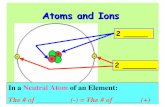
Ionic Reactions
• During an ionic reaction, atoms give up or take on an electron to stabilize their outer shells of the atom.
• Ions are particles that carry a positive (+) or negative (-) charge.– The attraction between oppositely
charged sodium ions and chloride ions forms an ionic bond.

9
Ionic “electron stripping” Reaction

10
Covalent “electron sharing” Reactions
• In covalent reactions, atoms share electrons in covalent bonds instead of losing or gaining them.– A single bond is formed when atoms
share a single pair of electrons.– A double bond is formed when atoms
share two pairs of electrons.– A triple bond is formed when atoms share
three pairs of electrons.

11
Covalent Reactions

12
Water and Living Things and a Third type of bond- Hydrogen bond
• The electrons in water spend more time circling the larger oxygen atom than the smaller hydrogen atom.– Water is a polar molecule with the oxygen
end being slightly negative and the hydrogen end being slightly positive.
A hydrogen bond occurs when a covalently bonded hydrogen is positive and is attracted to a negatively charged atom.

13
Hydrogen Bonding between Water Molecules

14
Some Properties of Water
• liquid at room temperature.
• solvent for polar molecules.
• cohesive.• temperature rises and
falls slowly.

15

16
Acids and Bases
• Acids break down in water and release hydrogen ions (H+).
• Bases/alkaline take up hydrogen ions (H+) or release hydroxide ions (OH-).– Buffers help keep the pH within normal
limits by taking up excess hydrogen ions or hydroxide ions.

17
Water can break down to form Ions (atoms with a + or – charge)

18
pH Scale
• The pH scale measures acidity or alkalinity of a solution.– Neutral = 7.– Acidic < 7.– Basic > 7.
Logarithmic Scale.

19
Log scale

20
The pH Scale

21
Molecules of Life
• Four categories of molecules are unique to cells.– Carbohydrates.– Lipids.– Proteins.– Nucleic Acids.
• Macromolecules are synthesized by a dehydration reaction, and degraded by a hydrolysis reaction.

22
pg.24a

23

24
Synthesis of larger product (macromolecule)

25
Breakdown of Molecule to simpler subunits

26
• Carbohydrates function for quick and short-term energy storage.– Monosaccharide (simple sugar).
Glucose.– Disaccharide.
Fructose.
Carbohydrates

27
Complex Carbohydrates
• Polysaccharides.– Starch (plants).– Glycogen (animals).– Cellulose (plant cell walls).

28
Glucose molecule in various forms

29
A Disacharride

30
Plant Polysaccharide

31
Animal polysaccharide

32
Lipids
• Lipids contain more energy per gram than any other biological molecule.– Do not dissolve in water.
Absence of polar groups.– Fats.
Animal origin, solid at room temperature.– Oils.
Plant origin, liquid at room temperature.

33
Synthesis and breakdown of Fat

34
Phospholipids and Steroids
• Phospholipids contain a phosphate head and fatty acid tails.– Polar head and non-polar tails.
Soluble in water.• Steroids are lipids with a backbone of four
fused carbon rings.– Estrogen and testosterone.

35
Another Example of a lipid

36
Cell membrane

37
Other lipid examples

38
Fig. 2.15bb

39
Emulsyfier
• Bile salts• Tween

40
Emulsyfication

41
Proteins
• Proteins are macromolecules with amino acid subunits.– An amino acid has a central carbon atom
bonded to a hydrogen and three groups.Peptide bond - Any bond joining two
amino acids. Polypeptide - Single amino acid
chain.

42
Building block of proteins

43
Fig. 2.16a

44
Peptide bond- bond between two amino acids

45
Levels of Protein Organization
• Primary Structure.– Linear sequence of amino acids.
• Secondary Structure.– Polypeptide takes on orientation in space.
• Tertiary Structure.– Final three-dimensional shape.
• Quaternary Structure.– Proteins with more than one polypeptide.

46

47
Nucleic Acids
• Nucleic acids are huge macromolecules composed of nucleotides. – A nucleotide is constructed of a
phosphate, a pentose sugar, and a nitrogenous base.
– Deoxyribonucleic acid (DNA).Double-stranded helix.
– Ribonucleic acid (RNA).Single stranded.

48
Building block of an Nucleic Acid- A Nucleotide

49
DNA Structure

50
(ATP) Adenosine Triphosphate
• ATP is the primary cellular energy carrier.– Energy currency of cells.– Breaks down to adenosine diphosphate
(ADP) and a molecule of inorganic phosphate, releasing energy to drive cellular metabolism.

51
Another nucleic acid example- ATP and ADP (cell energy)

52
Lecture Review
• Give an example of ionic bonding and explain it.• Give an example of covalent bonding and explain
it.• Relate the characteristics of water to its polarity
and hydrogen bonding.• List the four molecules of life and examples of
each.• Fat /triglyceride is composed of______ and
______.• The subunits of Proteins are _______ _______.• List some functions of proteins. What is a
polypeptide?• The subunits of Nucleic acids are ____________.